Physical Properties of New Silica-Based Denture Surface Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
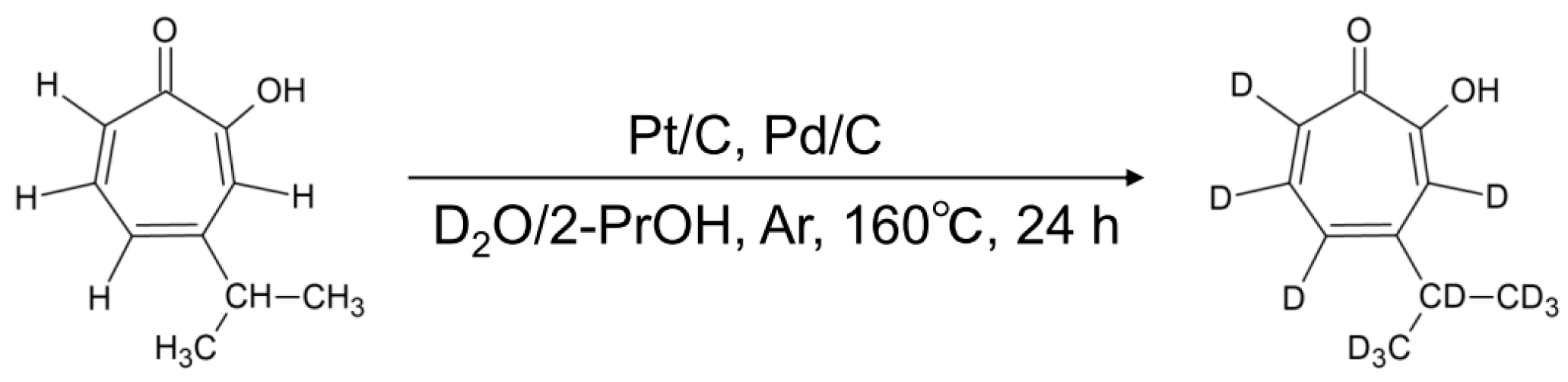
2.2. Synthesis of Deuterated Hinokitiol (Hinokitiol-d10)
2.3. Preparation of Silica–Resin and Hinokitiol-Containing Silica–Resin Layer Samples
2.4. Neutron Reflectivity Measurement
2.5. X-Ray Reflectivity Measurement
3. Results and Discussion
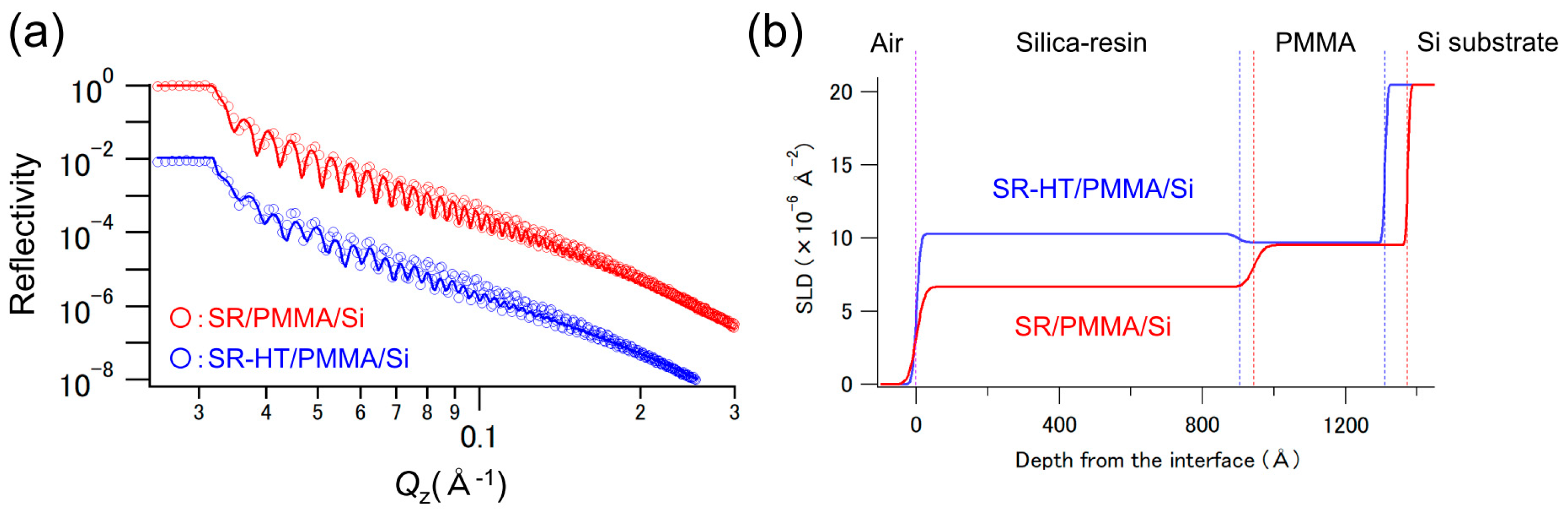
3.1. X-Ray Reflectivity Analysis of the SR/PMMA/Si and SR-HT/PMMA/Si Sample
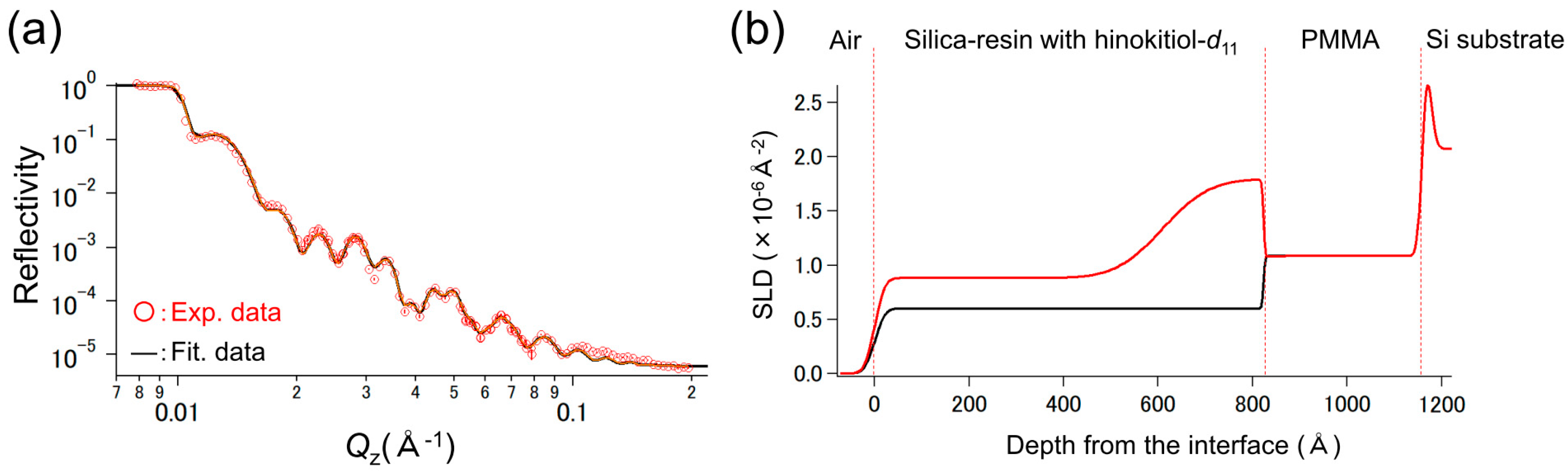
3.2. Neutron Reflectivity Analysis of the SR-HT/PMMA/Si Sample
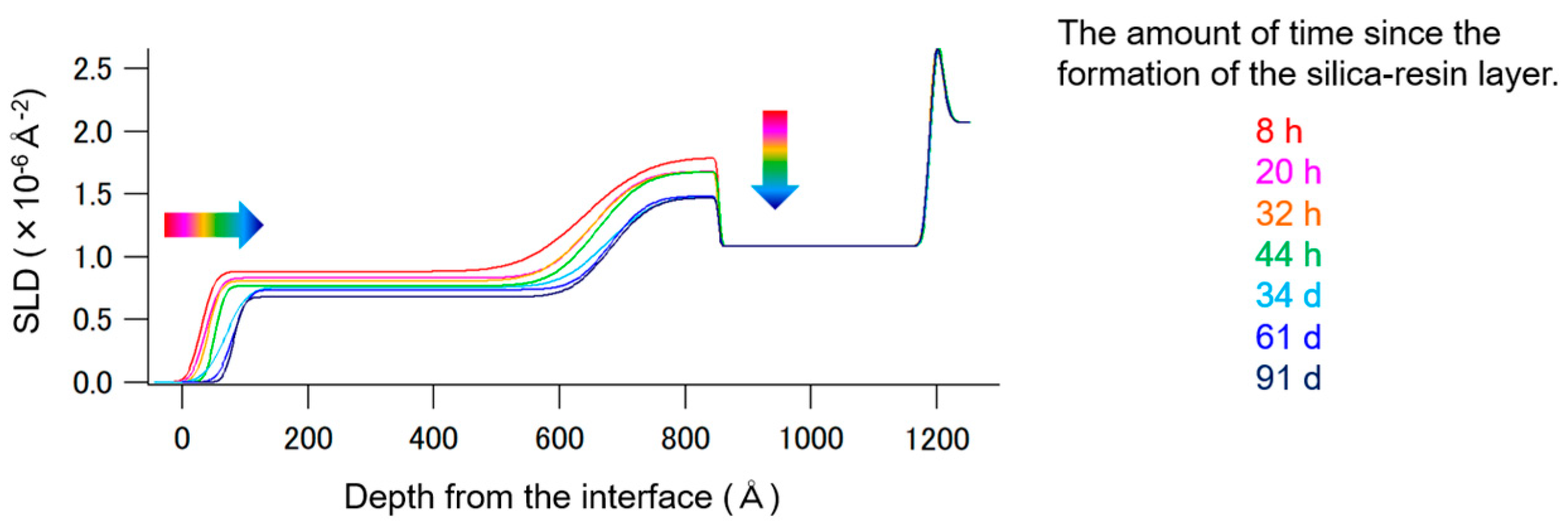
3.3. Studies on the Hinokitiol Sustained-Release System
3.4. Application in Dental Medicine and Stomatology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef]
- Nikawa, H.; Hamada, T.; Yamamoto, T. Denture plaque–past and recent concerns. J. Dent. 1998, 26, 299–304. [Google Scholar] [CrossRef]
- Salerno, C.; Pascale, M.; Contaldo, M.; Esposito, V.; Busciolano, M.; Milillo, L.; Guida, A.; Petruzzi, M.; Serpico, R. Candida-associated denture stomatitis. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e139–e143. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; López-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for Candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Rad. Endod. 2004, 98, 53–59. [Google Scholar] [CrossRef]
- Jackson, S.; Coulthwaite, L.; Loewy, Z.; Scallan, A.; Verran, J. Biofilm Development by Blastospores and Hyphae of Candida albicans on Abraded Denture Acrylic Resin Surfaces. J. Prosthet. Dent. 2014, 112, 988–993. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Walczak, K.; Schierz, G.; Basche, S.; Petto, C.; Boening, K.; Wieckiewicz, M. Antifungal and Surface Properties of Chitosan-Salts Modified PMMA Denture Base Material. Molecules 2020, 25, 5899. [Google Scholar] [CrossRef]
- Hirasawa, M.; Tsutsumi-Arai, C.; Takakusaki, K.; Oya, T.; Fueki, K.; Wakabayashi, N. Superhydrophilic Co-Polymer Coatings on Denture Surfaces Reduce Candida Albicans Adhesion—An in Vitro Study. Arch. Oral Biol. 2018, 87, 143–150. [Google Scholar] [CrossRef] [PubMed]
- AlBin-Ameer, M.A.; Alsrheed, M.Y.; Aldukhi, I.A.; Matin, A.; Khan, S.Q.; Abualsaud, R.; Gad, M.M. Effect of protective coating on surface properties and Candida albicans adhesion to denture base materials. J. Prosthodont. 2020, 29, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, C.; Takakuda, K.; Wakabayashi, N. Reduction of Candida biofilm adhesion by incorporation of prereacted glass ionomer filler in denture base resin. J. Dent. 2016, 44, 37–43. [Google Scholar] [CrossRef]
- Tsutsumi-Arai, C.; Iwamiya, Y.; Hoshino, R.; Terada-Ito, C.; Sejima, S.; Akutsu-Suyama, K.; Shibayama, M.; Hiroi, Z.; Tokuyama-Toda, R.; Iwamiya, R.; et al. Surface Functionalization of Non-Woven Fabrics Using a Novel Silica-Resin Coating Technology: Antiviral Treatment of Non-Woven Fabric Filters in Surgical Masks. Int. J. Environ. Res. Public Health 2022, 19, 3639. [Google Scholar] [CrossRef]
- Iwamiya, Y.; Kawai, M. Tungsten-coated cloth for radiation shielding made with the SilicaTech® coating technique. In Proceedings of the 14th International Workshop on Spallation Materials Technology (JAPAN), Fukushima, Japan, 11–16 November 2020. [Google Scholar]
- Iwamiya, Y.; Kawai, M.; Nishio-Hamane, D.; Shibayama, M.; Hiroi, Z. Modern Alchemy: Making “Plastics” from Paper. Ind. Eng. Chem. Res. 2020, 60, 355–360. [Google Scholar] [CrossRef]
- Takakura, S.; Fujihara, N.; Saito, T.; Kudo, T.; Iinuma, Y.; Ichiyama, S. National surveillance of species in Japan and their susceptibility yo six antifungal agents including voriconazole and micafungin. J. Antimicrob. Chemother. 2004, 53, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Kawabe, K.; Suzuki, T.; Sano, K.; Ide, K.; Nishigaki, T.; Enoki, Y.; Taguchi, K.; Koike, H.; Kato, H.; et al. Species distribution of candidemia and their susceptibility in a single Japanese university hospital: Prior micafungin use affects the appearance of Candida parapsilosis and elevation of micafungin MICs in non-parapsilosis Candida Species. J. Fungi 2021, 7, 596. [Google Scholar] [CrossRef]
- Sakagami, T.; Kawano, T.; Yamashita, K.; Yamada, E.; Fujino, N.; Kaeriyama, M.; Fukuda, Y.; Nomura, N.; Mitsuyama, J.; Suematsu, H.; et al. Antifungal susceptibility trend and analysis of resistance mechanism for Candida species isolated from bloodstream at a Japanese university hospital. J. Infect. Chemother. 2019, 25, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Srimaneepong, V.; Thanamee, T.; Wattanasirmkit, K.; Muangsawat, S.; Matangkasombut, O. Efficacy of low-molecular weight chitosan against Candida albicans biofilm on polymethyl methacrylate resin. Aust. Dent. J. 2021, 66, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, X.; Falk, S.P.; Mowery, B.P.; Karlsson, A.J.; Weisblum, B.; Palecek, S.P.; Masters, K.S.; Gellman, S.H. Structure-Activity Relationships among Antifungal Nylon-3 Polymers: Identification of Materials Active against Drug-Resistant Strains of Candida albicans. J. Am. Chem. Soc. 2014, 136, 4333–4342. [Google Scholar] [CrossRef]
- Martini Garcia, I.; Becker Rodrigues, S.; Rodrigues Gama, M.E.; Branco Leitune, V.C.; Melo, M.A.; Mezzomo Collares, F. Guanidine derivative inhibits C. albicans biofilm growth on denture liner without promote loss of materials’ resistance. Bioact. Mater. 2020, 5, 228–232. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Silva, S.; Negri, M.; de Camargo, E.R.; Oliveira, R.; Barbosa, D.B.; Henriques, M. Silver colloidal nanoparticles: Antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling 2011, 27, 711–719. [Google Scholar] [CrossRef]
- Guisbiers, G.; Lara, H.; Mendoza-Cruz, R.; Naranjo, G.; Vincent, B.A.; Peralta, X.G.; Nash, K.L. Inhibition of Candida albicans biofilm by pure selenium nanoparticles synthesized by pulsed laser ablation in liquids. Nanomedicine 2017, 13, 1095–1103. [Google Scholar] [CrossRef]
- Roozbehani, N.; Golfeshan, F.; Pakshir, K.; Doorandishan, M.; Jassbi, A.R.; Mosaddad, S.A. Chemical Composition and Effectiveness of Ocimum basilicum L. Extracts on the Adhesion of Candida albicans and C. dubliniensis on Acrylic Surfaces of RemovableOrthodontic Appliances. Biointerface Res. Appl. Chem. 2021, 11, 9477–9489. [Google Scholar]
- Furletti, V.F.; Teixeira, I.P.; Obando-Pereda, G.; Mardegan, R.C.; Sartoratto, A.; Figueira, G.M.; Duarte, R.M.T.; Rehder, V.L.G.; Duarte, M.C.T.; Höfling, J.F. Action of Coriandrum sativum L. Essential Oil upon Oral Candida albicans Biofilm Formation. Evid Based Complement Altern. Med. 2011, 2011, 985832. [Google Scholar] [CrossRef]
- Narayanan, V.S.; Muddaiah, S.; Shashidara, R.; Sudheendra, U.S.; Deepthi, N.C.; Samaranayake, L. Variableantifungalactivityof curcumin against planktonic and biofilm phase of different candida species. Indian J. Dent. Res. 2020, 31, 145–148. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, M.R.C.; Maciel, P.P.; Castellano, L.R.C.; Bonan, P.R.F.; Alves, D.D.N.; de Medeiros, A.C.D.; de Castro, R.D. Efficacy of essential oil of cinnamon for the treatment of oral candidiasis: A randomized trial. Spec. Care Dent. 2021, 41, 349–357. [Google Scholar] [CrossRef]
- Syahmina, A.; Usuki, T. Ionic Liquid-Assisted Extraction of Essential Oils from Thujopsis dolobrata (Hiba). ACS Omega 2020, 5, 29618–29622. [Google Scholar] [CrossRef]
- Domon, H.; Hiyoshi, T.; Maekawa, T.; Yonezawa, D.; Tamura, H.; Kawabata, S.; Yanagihara, K.; Kimura, O.; Kunitomo, E.; Terao, Y. Antibacterial activity of hinokitiol against both antibiotic-resistant and-susceptible pathogenic bacteria that predominate in the oral cavity and upper airways. Microbiol. Immunol. 2019, 63, 213–222. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, M.W.; Choi, J.S.; Lee, S.G.; Park, J.Y.; Kim, S.W. Inhibitory activity of hinokitiol against biofilm formation in fluconazole-resistant Candida species. PLoS ONE 2017, 12, e0171244. [Google Scholar] [CrossRef]
- Komaki, N.; Watanabe, T.; Ogasawara, A.; Sato, N.; Mikami, T.; Matsumoto, T. Antifungal mechanism of hinokitiol against Candida albicans. Biol. Pharm. Bull. 2008, 31, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Magori, N.; Fujita, T.; Kumamoto, E. Hinokitiol inhibits compound action potentials in the frog sciatic nerve. Eur. J. Pharmacol. 2018, 819, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Yamato, M.; Hashigaki, K.; Sakai, J.; Takeuchi, Y.; Tsukagoshi, S.; Tashiro, T.; Tsuruo, T. Synthesis and antitumor activity of tropolone derivatives. J. Med. Chem. 1987, 30, 1245–1248. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, J.H.; Lee, Y.J.; Park, S.Y. SIRT1, a class III histone deacetylase, regulates LPS-induced inflammation in human keratinocytes and mediates the anti-inflammatory effects of hinokitiol. J. Invest. Dermatol. 2017, 137, 1257–1266. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, S.; Miao, Q.; Jin, K.; Lou, L.; Ye, X.; Xi, Y.; Ye, J. Protective role of hinokitiol against H2O2-induced injury in human corneal epithelium. Curr. Eye Res. 2017, 42, 47–53. [Google Scholar] [CrossRef]
- Trust, T.J.; Coombs, R.W. Antibacterial activity of β-thujaplicin. Can. J. Microbiol. 1973, 19, 1341–1346. [Google Scholar] [CrossRef]
- van der Kamp, B.J. Effects of heartwood inhabiting fungi on thujaplicin content and decay resistance of western redcedar (Thuja-Plicata Donn). Wood Fiber Sci. 1986, 18, 421–427. [Google Scholar]
- Shih, Y.-H.; Chang, K.-W.; Hsia, S.-M.; Yu, C.-C.; Fuh, L.-J.; Chi, T.-Y.; Shieh, T.-M. In vitro antimicrobial and anticancer potential of hinokitiol against oral pathogens and oral cancer cell lines. Microbiol. Res. 2013, 168, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-H.; Hsia, S.-M.; Wu, C.-H.; Ko, S.-Y.; Chen, M.Y.; Shih, Y.-H.; Shieh, T.-M.; Chuang, L.-C.; Wu, C.-Y. Evaluation of the antibacterial potential of liquid and vapor phase phenolic essential oil compounds against oral microorganisms. PLoS ONE 2016, 11, e0163147. [Google Scholar] [CrossRef]
- Osawa, K.; Matsumoto, T.; Maruyama, T. Studies of the antibacterial activity of plant extracts and their constituents against periodontopathic bacteria. Bull. Tokyo Dent. Coll. 1990, 31, 17–21. [Google Scholar] [PubMed]
- Sakaguchi, H. Treatment and prevention of oral candidiasis in elderly patients. Med. Mycol. J. 2017, 58, J43–J49. [Google Scholar] [CrossRef]
- Iha, K.; Suzuki, N.; Yoneda, M.; Takeshita, T.; Hirofuji, T. Effect of mouth cleaning with hinokitiol-containing gel on oral malodor: A randomized, open-label pilot study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 433–439. [Google Scholar] [CrossRef]
- Ohara, H.; Odanaka, K.; Shiine, M.; Hayasaka, M. Antimicrobial effect of oral care gel containing hinokitiol and 4-isopropyl-3-methylphenol against intraoral pathogenic microorganisms. PLoS ONE 2023, 18, e0283295. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi-Arai, C.; Akutsu-Suyama, K.; Iwamiya, Y.; Terada-Ito, C.; Hiroi, Z.; Shibayama, M.; Satomura, K. Antimicrobial surface processing of polymethyl methacrylate denture base resin using a novel silica-based coating technology. Clin. Oral Invest. 2022, 27, 1043–1053. [Google Scholar] [CrossRef]
- Russell, T.P. X-Ray and Neutron Reflectivity for the Investigation of Polymers. Mater. Sci. Rep. 1990, 5, 171–271. [Google Scholar] [CrossRef]
- Delcea, M.; Helm, C.A. X-ray and Neutron Reflectometry of Thin Films at Liquid Interfaces. Langmuir 2019, 35, 8519–8530. [Google Scholar] [CrossRef]
- Darwish, T.A.; Smith, A.R.G.; Gentle, I.R.; Burn, P.L.; Luks, E.; Moraes, G.; Gillon, M.; Holden, P.J.; James, M. Deuteration of molecules for neutron reflectometry on organic light-emitting diode thin films. Tetrahedron Lett. 2012, 53, 931–935. [Google Scholar] [CrossRef]
- Akutsu-Suyama, K.; Cagnes, M.; Tamura, K.; Kanaya, T.; Darwish, T.A. Controlled deuterium labelling of imidazolium ionic liquids to probe the fine structure of the electrical double layer using neutron reflectometry. Phys. Chem. Chem. Phys. 2019, 21, 17512–17516. [Google Scholar] [CrossRef] [PubMed]
- Akutsu-Suyama, K.; Park, K.; Takakura, R.; Tamura, K.; Cagnes, M.; Darwish, T.A.; Yamada, T.; Sawama, Y.; Sajiki, H. Metal catalyzed H–D Exchange methods using D2O as a deuterium source: A comparative study in different sealed devices. JPS Conf. Proc. 2021, 33, 011150. [Google Scholar] [CrossRef]
- Takeda, M.; Yamazaki, D.; Soyama, K.; Maruyama, R.; Hayashida, H.; Asaoka, H.; Yamazaki, T.; Kubota, M.; Aizawa, K.; Arai, M.; et al. Current Status of a New Polarized Neutron Reflectometer at the Intense Pulsed Neutron Source of the Materials and Life Science Experimental Facility (MLF) of J-PARC. Chin. J. Phys. 2012, 50, 161–170. [Google Scholar]
- Sakasai, K.; Satoh, S.; Seya, T.; Nakamura, T.; Toh, K.; Yamagishi, H.; Soyama, K.; Yamazaki, D.; Maruyama, R.; Oku, T.; et al. Materials and Life Science Experimental Facility at the Japan Proton Accelerator Research Complex III: Neutron Devices and Computational and Sample Environments. Quantum Beam Sci. 2017, 1, 10. [Google Scholar] [CrossRef]
- Nelson, A. Co-refinement of multiple-contrast neutron/X-ray reflectivity data using MOTOFIT. J. Appl. Crystallogr. 2006, 39, 273–276. [Google Scholar] [CrossRef]
- Kumada, T.; Miura, D.; Akutsu-Suyama, K.; Ohishi, K.; Morikawa, T.; Kawamura, Y.; Suzuki, J.; Oku, T.; Torikai, N.; Niizeki, T. Structure analysis of a buried interface between organic and porous inorganic layers using spin-contrast-variation neutron reflectivity. J. Appl. Crystallogr. 2022, 55, 1147–1153. [Google Scholar] [CrossRef]
- Hair, M.L.; Hertl, W. Adsorption on Hydroxylated Silica Surfaces. J. Phys. Chem. 1969, 73, 4269–4276. [Google Scholar] [CrossRef]
- Asay, D.B.; Barnette, A.L.; Kim, S.H. Effects of surface chemistry on structure and thermodynamics of water layers at solid−vapor interfaces. J. Phys. Chem. C 2008, 113, 2128–2133. [Google Scholar] [CrossRef]
- Leed, E.A.; Pantano, C.G. Computer modeling of water adsorption on silica and silicate glass fracture surfaces. J. Non-Cryst. Solids 2003, 325, 48–60. [Google Scholar] [CrossRef]
- Park, D.; Hong, S.H.; Kim, K.M.; Lee, C.H. Adsorption Equilibria and Kinetics of Silica Gel for N2O, O2, N2, and CO2. Sep. Purif. Technol. 2020, 251, 117326. [Google Scholar] [CrossRef]
- Chiang, J.; Birla, S.; Bedoya, M.; Jones, D.; Subbiah, J.; Brace, C.L. Modeling and validation of microwave ablations with internal vaporization. IEEE Trans. Biomed. Eng. 2015, 62, 657–663. [Google Scholar] [CrossRef]
- Custelcean, R.; Williams, N.J.; Garrabrant, K.A.; Agullo, P.; Brethomé, F.M.; Martin, H.J.; Kidder, M.K. Direct Air Capture of CO2 with Aqueous Amino Acids and Solid Bis-Iminoguanidines (BIGs). Ind. Eng. Chem. Res. 2019, 58, 23338–23346. [Google Scholar] [CrossRef]
- Micheau, C.; Ueda, Y.; Akutsu-Suyama, K.; Bourgeois, D.; Motokawa, R. Deuterated malonamide synthesis for fundamental research on solvent extraction systems. Solvent Extr. Ion Exch. 2023, 41, 221–240. [Google Scholar] [CrossRef]
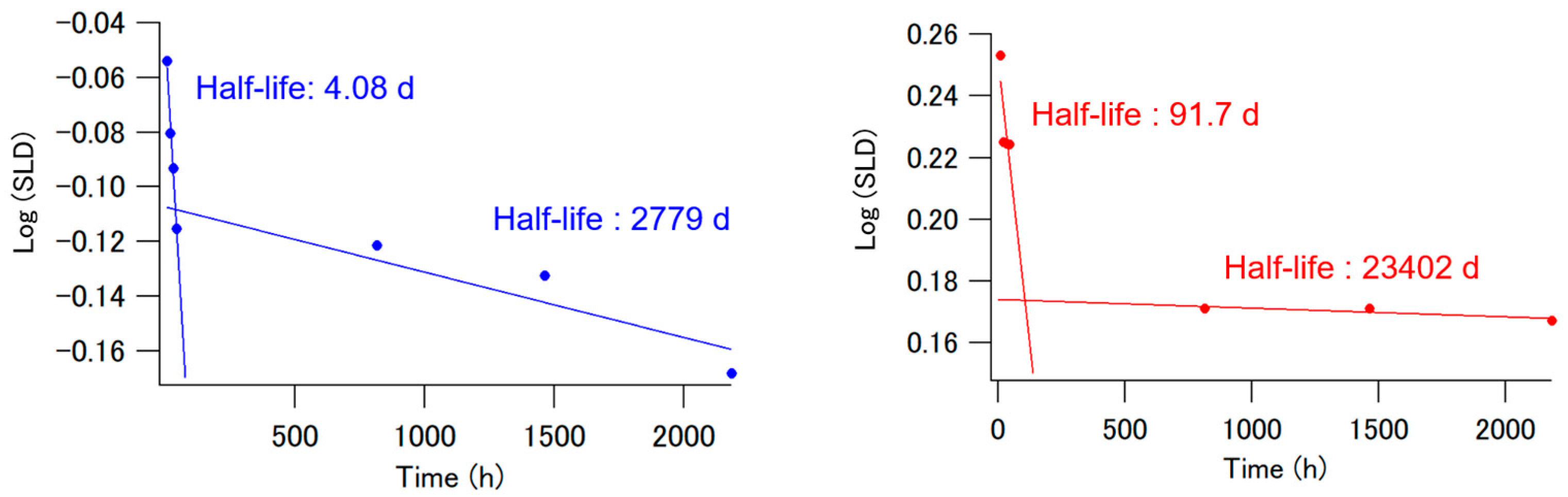
| Desorption Rate Constant | Half-Lives | |
|---|---|---|
| Fast hinokitiol-desorption reaction at the surface (kfs) | 1.6 × 10−2 h−1 | 4.08 d |
| Slow hinokitiol-desorption reaction at the surface (kss) | 2.4 × 10−5 h−1 | 2779 d |
| Fast hinokitiol-desorption reaction at the interface (kfi) | 7.2 × 10−4 h−1 | 91.7 d |
| Slow hinokitiol-desorption reaction at the interface (ksi) | 2.8 × 10−6 h−1 | 23,402 d |
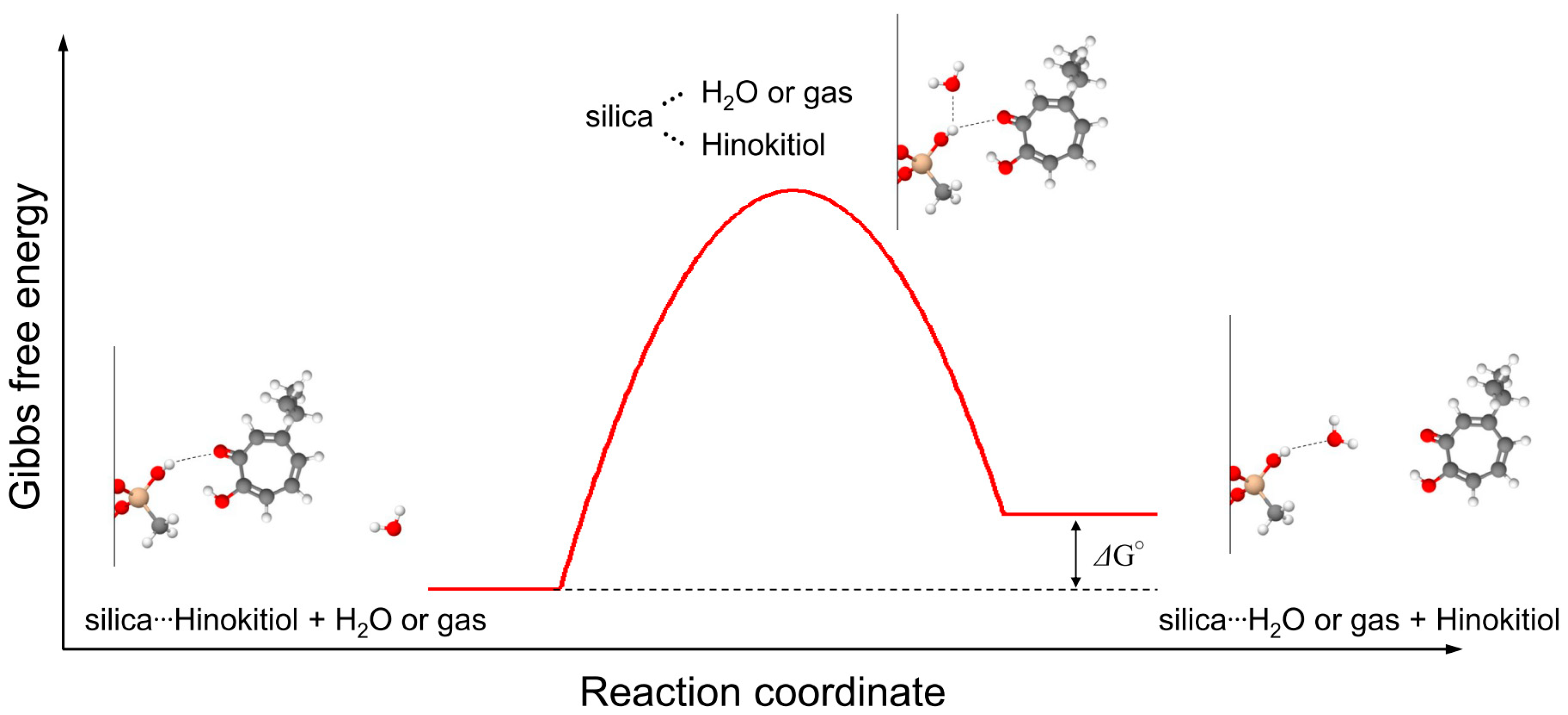
| ΔG° | |
|---|---|
| ΔG° for the fast hinokitiol-desorption reaction at the surface (ΔG°fs) | 10.0 kJ mol−1 |
| ΔG° for the slow hinokitiol-desorption reaction at the surface (ΔG°ss) | 25.9 kJ mol−1 |
| ΔG° for the fast hinokitiol-desorption reaction at the interface (ΔG°fi) | 17.6 kJ mol−1 |
| ΔG° for the slow hinokitiol-desorption reaction at the interface (ΔG°si) | 31.1 kJ mol−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akutsu-Suyama, K.; Tokuyama-Toda, R.; Tsutsumi-Arai, C.; Terada-Ito, C.; Iwamiya, Y.; Hiroi, Z.; Shibayama, M.; Satomura, K. Physical Properties of New Silica-Based Denture Surface Coating. Nanomaterials 2025, 15, 1652. https://doi.org/10.3390/nano15211652
Akutsu-Suyama K, Tokuyama-Toda R, Tsutsumi-Arai C, Terada-Ito C, Iwamiya Y, Hiroi Z, Shibayama M, Satomura K. Physical Properties of New Silica-Based Denture Surface Coating. Nanomaterials. 2025; 15(21):1652. https://doi.org/10.3390/nano15211652
Chicago/Turabian StyleAkutsu-Suyama, Kazuhiro, Reiko Tokuyama-Toda, Chiaki Tsutsumi-Arai, Chika Terada-Ito, Yoko Iwamiya, Zenji Hiroi, Mitsuhiro Shibayama, and Kazuhito Satomura. 2025. "Physical Properties of New Silica-Based Denture Surface Coating" Nanomaterials 15, no. 21: 1652. https://doi.org/10.3390/nano15211652
APA StyleAkutsu-Suyama, K., Tokuyama-Toda, R., Tsutsumi-Arai, C., Terada-Ito, C., Iwamiya, Y., Hiroi, Z., Shibayama, M., & Satomura, K. (2025). Physical Properties of New Silica-Based Denture Surface Coating. Nanomaterials, 15(21), 1652. https://doi.org/10.3390/nano15211652








