Abstract
The intensification of industrialization and increasing energy consumption have led to elevated emissions of hazardous gases such as NO, NO2, and SO2, making their efficient capture and removal crucial for environmental remediation. In this work, first-principles calculations were employed to systematically investigate the adsorption behavior of these gases on single-atom-decorated (Sc, Ti, and V) 1T-ZrS2 monolayers. The results indicate that the transition metal atoms preferentially occupy the hexagonal hollow sites of ZrS2, forming an approximately octahedral coordination field and exhibiting characteristic d-orbital splitting. During gas adsorption, the decorated systems exhibit pronounced metal-to-adsorbate charge donation and strong d-p hybridization, indicative of strong chemisorption. Notably, Ti-ZrS2 exhibits the strongest adsorption toward NO2, inducing partial molecular dissociation and suggesting catalytic activity, whereas Sc- and V-decorated systems predominantly maintain molecular adsorption. Recovery time calculations indicate that the adsorption processes are comparatively stable, making these systems suitable for gas capture and pollution abatement. Overall, single-atom decoration provides an effective strategy to modulate the electronic structure and gas interactions of ZrS2, highlighting its potential as an efficient gas scavenger for NO, NO2, and SO2.
1. Introduction
With the intensification of industrialization and energy consumption, the emission of harmful gases such as nitrogen oxides NOx (NO, NO2) and sulfur dioxide (SO2) into the atmosphere has continued to increase. These gaseous pollutants originate primarily from industrial combustion processes, vehicle exhaust emissions, and agricultural activities, which not only cause acid rain and photochemical smog but also pose serious threats to human health [1,2,3,4,5]. Therefore, developing efficient and stable gas sensing and capture materials is crucial for environmental remediation and the protection of human health [6,7,8,9].
Among various candidate materials, two-dimensional (2D) materials have attracted extensive attention owing to their high specific surface area, tunable electronic structures, and abundant active sites [10,11,12,13,14]. In particular, transition metal dichalcogenides (TMDCs), featuring layered crystal structures, adjustable band gaps, and excellent chemical stability, are considered ideal materials for the adsorption and immobilization of toxic gases [15,16,17,18,19,20,21,22]. In recent years, numerous studies have focused on gas capture applications based on monolayer TMDCs. For example, Cho et al. systematically investigated the adsorption behaviors of toxic gases such as O3, SO2, and SO3 on MoS2 monolayers through combined experimental and theoretical approaches, demonstrating the great potential of MoS2 for gas capture [23]. Similarly, Scardamaglia et al. experimentally synthesized WS2 monolayers and demonstrated their high-sensitivity detection capability toward NO2 molecules [24]. As a representative layered TMDC, ZrS2 has been successfully fabricated in various nanostructured forms, exhibiting good structural stability and semiconducting characteristics [25,26,27]. Benefiting from its excellent optical, thermal, and electronic properties, ZrS2 has been widely regarded as a promising candidate material for multifunctional applications [28,29,30]. For instance, Li et al. incorporated ZrS2 into erbium-doped fiber lasers, achieving high-performance dual-wavelength Q-switched pulse outputs [31].
Extensive first-principles studies have demonstrated that single-atom modification can significantly tune the surface reactivity and electronic properties of TMDCs [32,33,34,35,36,37,38]. For example, Zhang et al. systematically investigated Co-, Pd-, and Pt-decorated MoS2 monolayers and found that, compared with pristine MoS2, these systems exhibit remarkably enhanced adsorption capabilities toward small molecules such as NO2 and NH3 [39]. This enhancement mainly arises from the strong hybridization between the metal d orbitals and the molecular orbitals of the adsorbates. Likewise, Ni-decorated WSe2 monolayers exhibit significant modulation of electronic structures during CO and HCHO adsorption, indicating their potential as resistive-type gas sensing materials [40]. Pt- and Au-modified ZrSe2 systems have also shown improved chemical adsorption and gas-sensing performances [41]. Furthermore, several studies have extended the single-atom decoration strategy to ZrS2, revealing that transition metal decoration can significantly improve its gas adsorption and sensing behavior [42,43,44]. Notably, recent experimental advances have enabled the synthesis of several single-atom-modified TMDCs [45,46], which demonstrates the feasibility of preparing single-atom-decorated ZrS2.
Motivated by this, we employ first-principles calculations to systematically investigate the adsorption behavior of NO, NO2, and SO2 molecules on Sc-, Ti-, and V-decorated 1T-ZrS2 monolayers (1T denotes the trigonal phase of TMDCs with octahedral coordination). Sc, Ti, and V were selected as decorating atoms because they are located close to Zr in the periodic table and possess covalent radii slightly smaller than that of Zr, which may facilitate the experimental doping or surface decoration of ZrS2. Moreover, recent experimental studies have demonstrated that Sc-, Ti-, and V-doped MoS2 exhibit exceptional performance in photocatalytic nitrogen reduction [47], giant photoluminescence [48], and electrocatalytic hydrogen generation [49], respectively, motivating the hypothesis that these elements may also enhance gas adsorption upon decorating ZrS2. We comprehensively analyze the influence of different adatom species on adsorption strength, charge transfer, geometric configuration, and potential dissociative adsorption behavior. In addition, binding energies, adsorption energies, density of states (DOS), crystal orbital Hamilton population (COHP), and electron localization function (ELF) analyses are performed to elucidate the underlying interaction mechanisms. Our results reveal that decoration with Sc, Ti, and V significantly enhances the chemisorption of NO, NO2, and SO2 on ZrS2, demonstrating excellent gas capture performance and highlighting single-atom–decorated 1T-ZrS2 as a promising gas scavenger for environmental remediation.
2. Computational Details
All density functional theory (DFT) calculations were performed using the Vienna ab initio Simulation Package (VASP5.4.4) [50,51,52,53]. The generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) functional was employed to describe the exchange-correlation energy [54,55]. Spin polarization was included in all calculations. The DFT-D3 method was applied to account for van der Waals interactions between the ZrS2 monolayer and the adsorbed gas molecules in the transition-metal-decorated systems [56]. The plane-wave cutoff energy was set to 600 eV. In the subsequent calculations, a 4 × 4 × 1 ZrS2 supercell containing 16 Zr atoms and 32 S atoms was adopted, as shown in Figure 1a. The Brillouin zone was sampled using a 3 × 3 × 1 Γ-centered k-point mesh [57], while a denser 6 × 6 × 1 k-point mesh was employed for the density of states (DOS) calculations to improve accuracy. The band structure and density of states of pristine ZrS2 were calculated, showing a band gap of 1.21 eV with the GGA functional and 1.8 eV with the HSE06 hybrid functional, consistent with literature reports [58,59]. To avoid spurious interactions between periodic images, a vacuum spacing of 20 Å was introduced. Geometry optimization was considered converged when the maximum Hellmann-Feynman force on any atom fell below 0.01 eV/Å. During self-consistent-field (SCF) and electronic-property calculations, the energy convergence threshold was set to 1 × 10−6 eV.
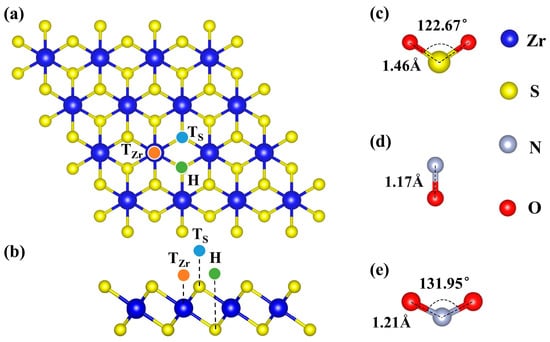
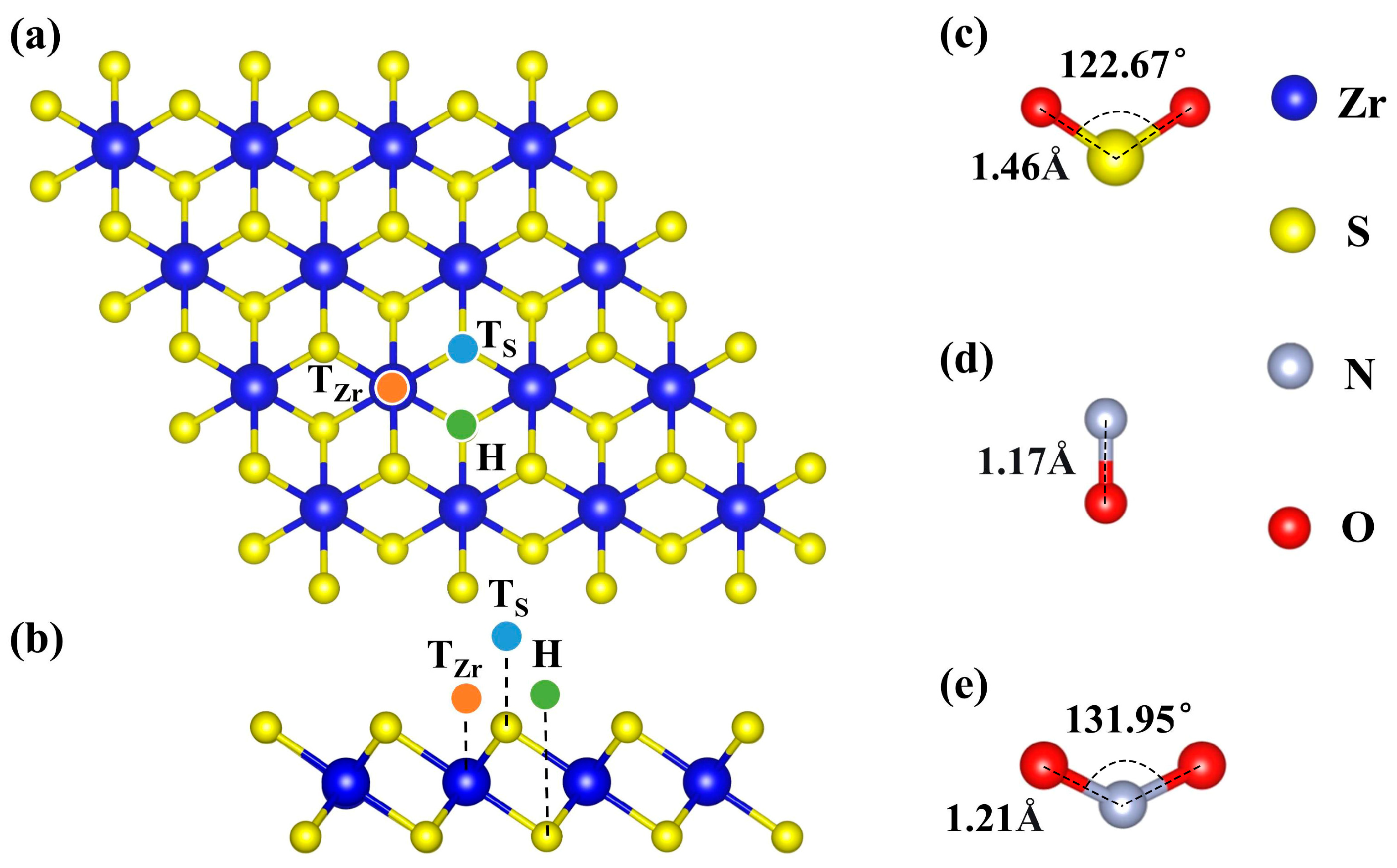
Figure 1.
Optimized structures. (a,b) top and side views of initial ZrS2 monolayer, respectively, and (c–e) Gas molecules SO2, NO, and NO2, respectively.
To identify the most stable adsorption configuration, the binding energy (Ebind) of a transition metal (TM) adatom on the ZrS2 surface was calculated using the following equation:
where , and are the total energy of TM-ZrS2 monolayer, TM atoms, and intrinsic ZrS2 monolayer, respectively. In addition, Eads was estimated to describe the adsorption between gas molecules and substrate, and the formula is as follows:
where Egas-substrate, Egas, and Esubstrate represent the total energy of the adsorbed system, the energy of the gas molecules, and the energy of the substrate, respectively. A negative Eads represents an energetically favorable adsorption process, whereas a positive Eads corresponds to an energetically unfavorable one. A larger absolute value of the binding energy (Ebind) or adsorption energy (Eads) indicates stronger binding or adsorption and, consequently, a more stable system.
All subsequent computational post-processing was performed using VASPKIT [60], and the results were visualized with VESTA [61]. To further analyze bonding characteristics, the LOBSTER program [62] was employed to carry out the crystal orbital Hamilton population (COHP) methodology [63]. The Bader charge decomposition was carried out using the efficient algorithm developed by Henkelman and co-workers, in which the core charges were incorporated into the partitions [64,65,66,67]. In this analysis, the electron density was interpolated onto a refined grid of 240 × 240 × 320, and the number of electrons in the vacuum region was confirmed to be zero. To characterize the degree of electron localization and bonding nature, the electron localization function (ELF) [68,69] was employed, and its topological analysis was carried out using the CP2K2022.2 [70] package in conjunction with the Multiwfn3.8 program [71,72]. Additionally, to ensure that the projected density of states (PDOS) analysis was consistent with the crystal-field splitting reported in the previous studies [73,74], the qvasp script [75] was used to rotate the Cartesian coordinates so that each axis aligned along the Zr-S bond direction and corresponded with the octahedral framework surrounding the Zr atoms, thereby ensuring the accuracy of the subsequent electronic-structure analysis.
3. Results and Discussion
3.1. Geometry and Electronic Structure of the 1T-ZrS2 Monolayer Decorated with Sc, Ti, and V Single Atoms
As shown in Figure 1, we optimized the structures of the pristine ZrS2 monolayer and of the NO, NO2, and SO2 molecules. The optimized results indicate that the N-O bond length in the NO molecule is 1.16 Å, while both NO2 and SO2 adopt a bent (V-shaped) geometry, with N-O and S-O bond lengths of 1.22 Å and 1.48 Å, and O-N-O and O-S-O bond angles of 134.56° and 117.24°, respectively, consistent with previous reports [40,76,77]. The optimized lattice parameters of the ZrS2 monolayer are a = b = 3.69 Å, with a Zr-S bond length of 2.58 Å, in good agreement with prior studies [78,79]. Three representative sites were considered as candidate decoration sites: atop Zr (TZr), atop S (TS), and the hollow site (H), as shown in Figure 1a,b. Previous studies (Table 1) have shown that the adsorption of these three gases on pristine ZrS2 is relatively weak [44,77,80], which is unfavorable for gas sensing or capture. Accordingly, we next systematically investigated ZrS2 decorated with single TM atoms to enhance adsorption strength.

Table 1.
Adsorption energies (Eads) and charge transfer (Qt) of three characteristic gases on ZrS2 monolayer.
Accordingly, we assessed Sc-, Ti-, and V-decoration of 1T-ZrS2 across three candidate adsorption sites (Figure 1) and examined their effects on atomic geometry and electronic properties. The results show that when a TM atom is placed at the sulfur top site (TS site), it relaxes to a nearby hollow site (H site), indicating that this configuration is energetically unstable. Therefore, subsequent analyses focused on the Zr top site (TZr site) and the hollow site (H site). As summarized in Table 2, all three transition metals prefer the hollow site, where the adsorption energy is the lowest (most negative), corroborating that the H site is the most stable adsorption site on the ZrS2 monolayer. This conclusion is consistent with previous theoretical studies [73,74].

Table 2.
Adsorption positions (TZr: TM adsorbed above a Zr atom, hollow: TM atom adsorbed above the lower-layer S atom), distance between the TM atom (TM = Sc, Ti, V) and its nearest S atom (d), binding energies (Ebind), Bader charge transfer (Qt), and magnetic moments (Mtot) of TM-decorated ZrS2.
The dynamical stability of all TM-decorated ZrS2 systems was evaluated by calculating their phonon dispersion relations. As shown in Figure S1, using the Sc-decorated ZrS2 system as a representative case, no imaginary frequencies were observed across the Brillouin zone, indicating that the hollow-site configuration is dynamically stable. These results further support selecting the hollow site as the most stable configuration for subsequent structural and electronic analyses.
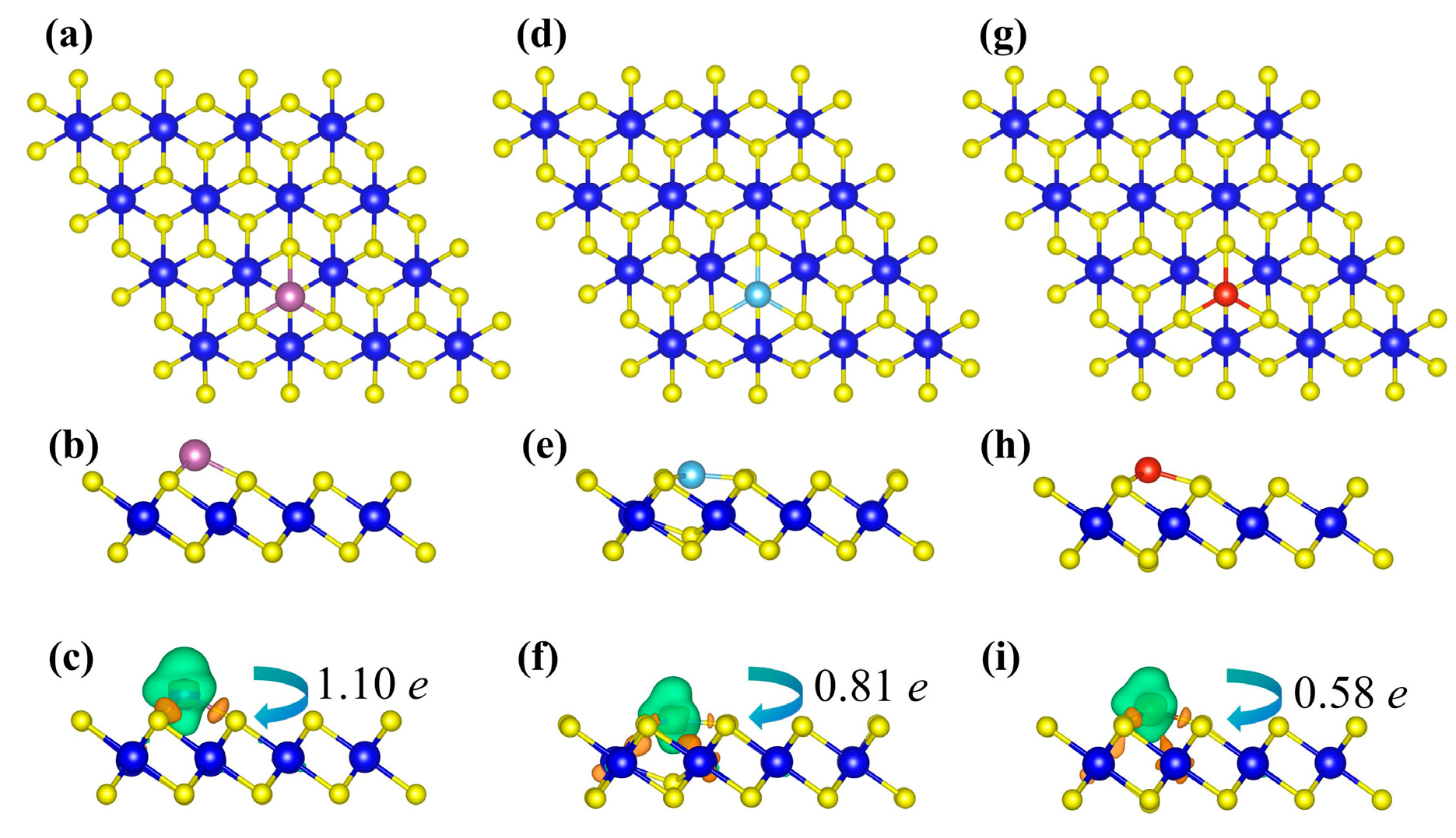
Figure 2 shows the optimized structures of TM-decorated ZrS2 monolayers at the hollow (H) sites, corresponding to the most stable adsorption sites discussed above. Upon TM decoration, only minor distortions of the hexagonal lattice are observed, and each TM atom forms stable TM-S bonds with neighboring S atoms. The bond lengths between the TM dopant atoms (Sc, Ti, and V) and their nearest neighboring S atoms are 2.38 Å, 2.29 Å, and 2.25 Å, respectively. Additionally, the Zr-S bonds adjacent to the dopants are noticeably elongated relative to the pristine value of 2.57 Å, reaching 2.70 Å, 2.79 Å, and 2.65 Å, respectively. Notably, in the Ti-decorated system, the Ti atom slightly embeds into the substrate lattice, causing the underlying S atom to move upward toward Ti. This is consistent with the most negative binding energy among the three, indicating a strong chemical adsorption between the Ti atom and the substrate. Bader analysis shows a net electron transfer from the adatom to ZrS2: Sc, Ti, and V donate 1.10 e, 0.81 e, and 0.58 e, respectively. To visualize the charge redistribution, charge density difference (CDD) maps are plotted (Figure 2c,f,i).
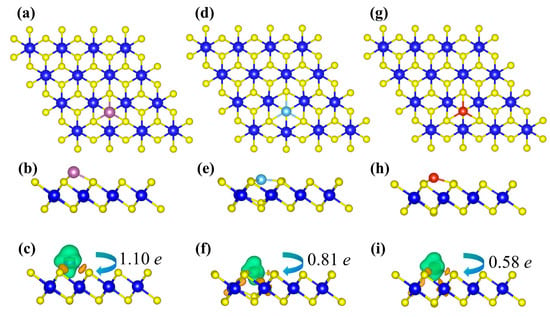
Figure 2.
Panels (a,d,g) and (b,e,h) show the top and side views of the Sc-, Ti-, and V-decorated ZrS2 systems, respectively. Panels (c,f,i) present the corresponding charge density difference diagrams. In these diagrams, orange and green isosurfaces denote charge accumulation and charge depletion, respectively, with corresponding isovalues of 0.007 e/Å3, 0.009 e/Å3, and 0.008 e/Å3 for the Sc-, Ti-, and V-ZrS2 systems.
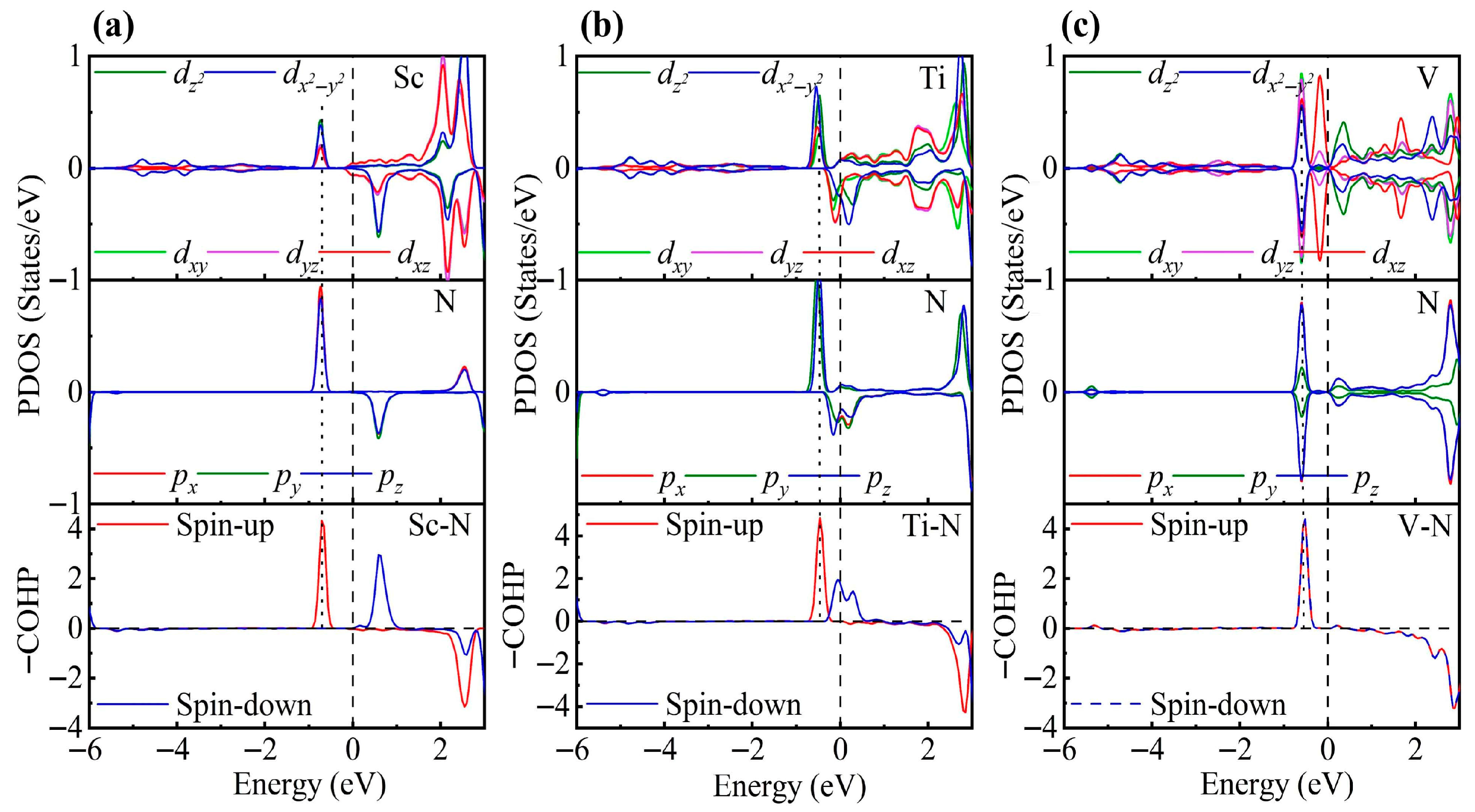
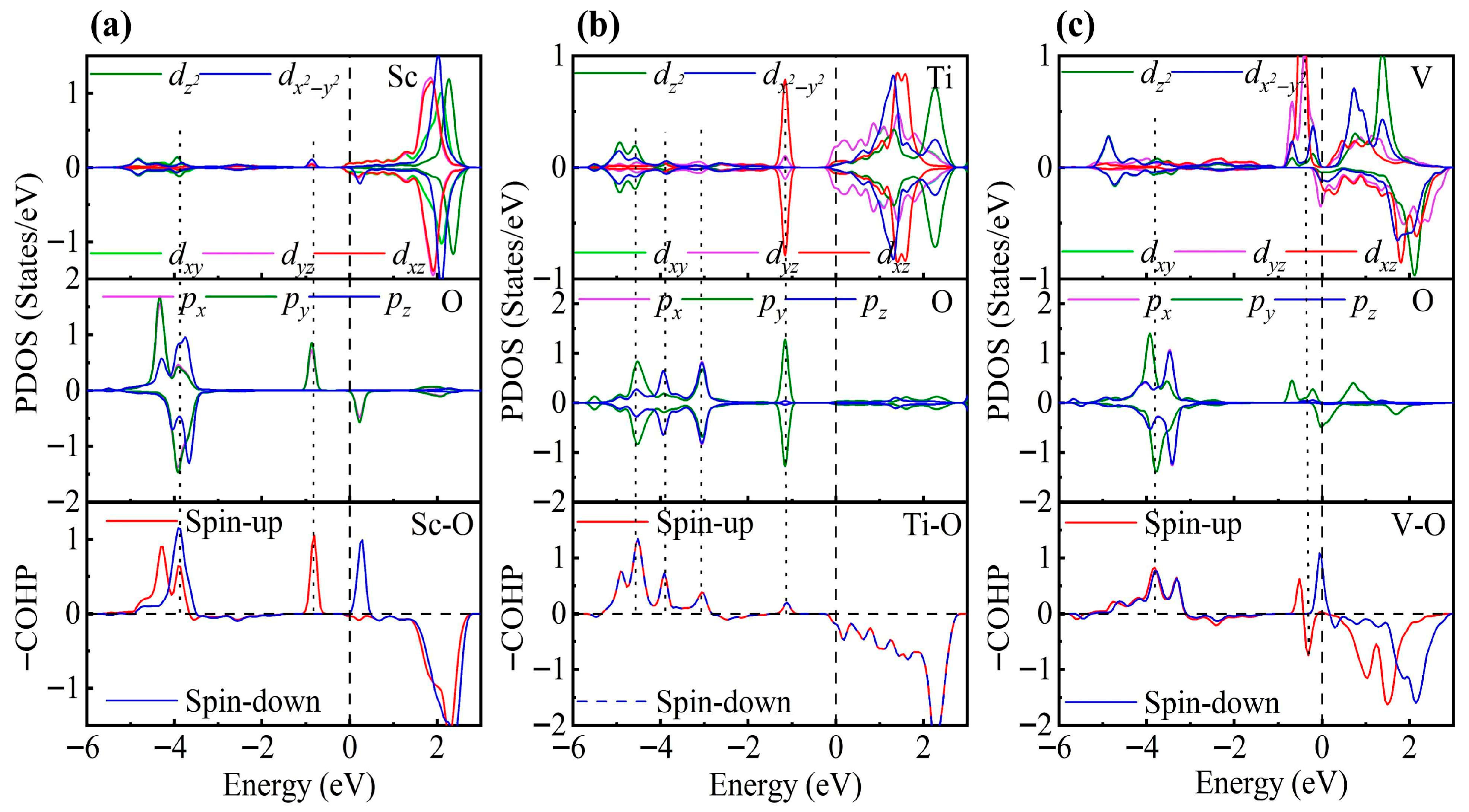
To gain a deeper understanding of TM-modified ZrS2, we further analyze the PDOS. In the 1T phase, each Zr atom is coordinated by six S atoms, forming an octahedral coordination environment. Under this crystal field, the Zr d orbitals split into eg and t2g manifolds. When a TM atom is adsorbed on the ZrS2 surface, it binds to the three nearest-neighbor S atoms. Although the other three coordination positions remain vacant, the TM atom still experiences an approximately octahedral crystal field close to Oh symmetry and thus exhibits similar orbital splitting [78,79]. To test this hypothesis, we rotated the Cartesian axes to the local octahedral orientation (x, y and z aligned with the Zr-S bonds) and calculated the PDOS to substantiate this interpretation.
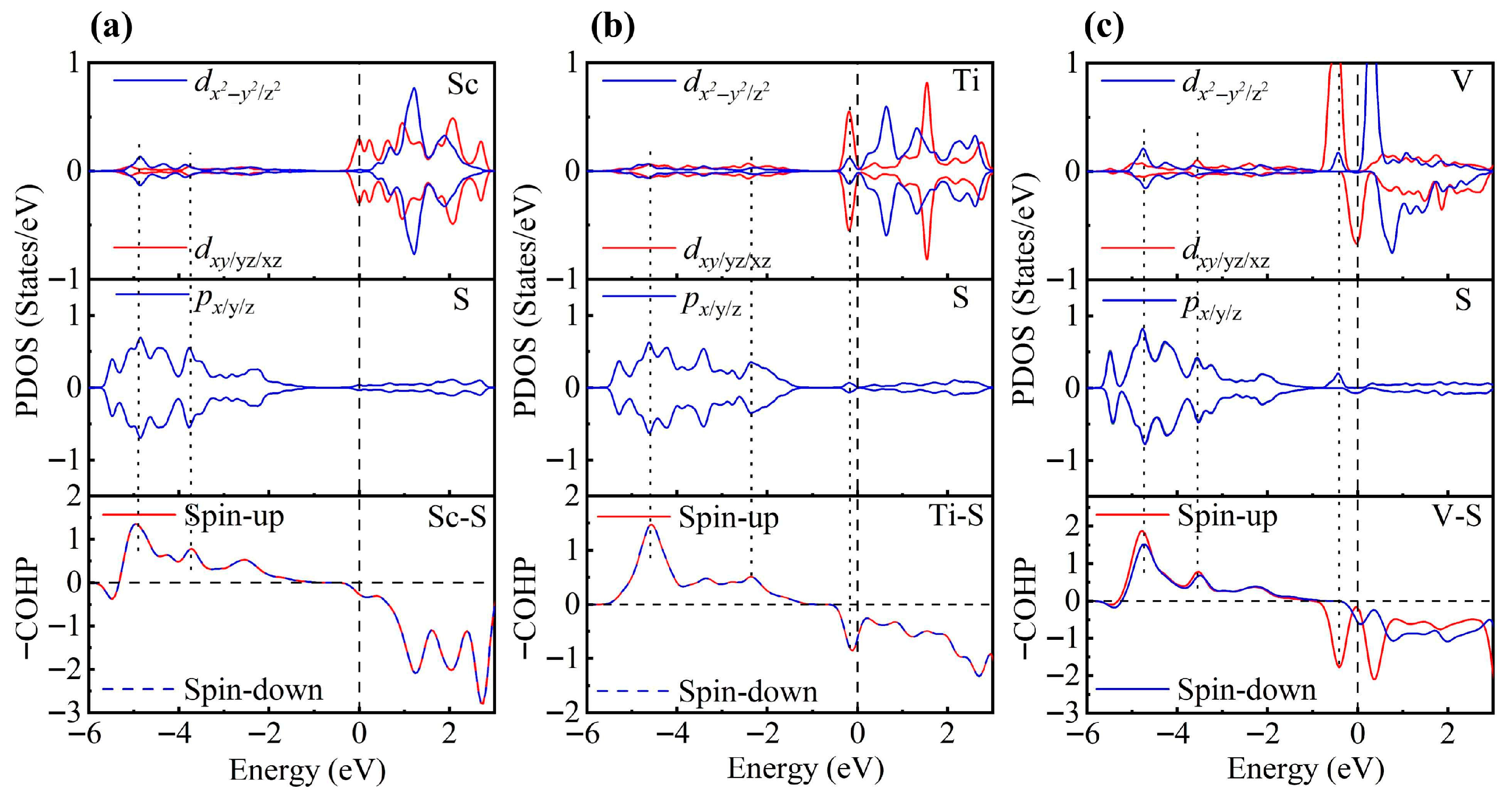
As shown in Figure 3, the PDOS of the TM-decorated systems shows the characteristic octahedral crystal field splitting: dxy, dyz and dxz form a t2g-like set, whereas and constitute an eg-like set, as expected. Closer examination of the PDOS for the Sc-decorated system reveals hybridization at −3.85 eV and −4.96 eV. This system exhibits no significant spin splitting, and its total magnetic moment is 0 . In the Ti-decorated system, Ti d orbitals and S p orbitals exhibited hybridization at −2.47 eV and −4.56 eV, and pronounced hybridization was also present near the Fermi level (around −0.26 eV). The total magnetic moment is 0 In contrast, the V-decorated system shows hybridization between V d orbitals and S p orbitals at −3.55 eV and −4.86 eV, along with pronounced hybridization near the Fermi level. Unlike the Sc- and Ti-decorated systems, the V-decorated system exhibited clear spin polarization, with a total magnetic moment of 0.94 originating mainly from unpaired V 3d electrons. As seen in the figure, hybridization near the Fermi level in TM-decorated systems is dominated by t2g orbitals, whereas eg orbitals contribute less. Moreover, with increasing d-electron count of the TM atom, the occupancy of d orbitals near the Fermi level increased, and the hybridization strength of both t2g and eg orbitals was enhanced.
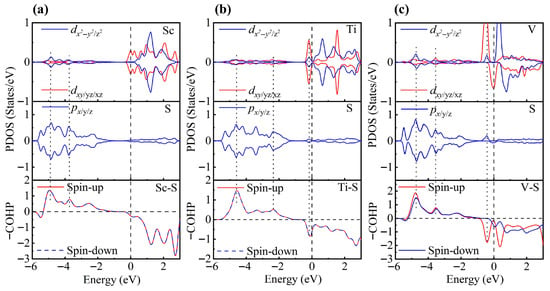
Figure 3.
PDOS and COHP of (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2 monolayers. DOS showing orbital degeneracy between TM dxy/dyz/dxz and S px/py/pz orbitals, as well as additional degeneracy of and orbitals below the Fermi level.
To gain deeper insight into the interaction between TM atoms and the substrate, we calculated the COHP between TM atoms and neighboring S atoms. In the COHP analysis, the Fermi level is set to 0 eV, with positive values representing bonding states and negative values representing antibonding states. The integrated COHP (ICOHP) below the Fermi level quantifies bond strength, so that more negative values (i.e., larger absolute values) indicate stronger bonding, thereby reflecting the relative strength of atomic interactions. As shown in Figure 3, the V-decorated system exhibits pronounced antibonding states near the Fermi level due to its larger number of d electrons, whereas the Sc- and Ti-decorated systems are dominated by bonding states below the Fermi level, with fewer antibonding contributions. Consequently, the V-decorated system has the smallest ICOHP absolute value, corresponding to the weakest adsorption strength. Further analysis reveals that the Sc-decorated system not only shows antibonding states at the Fermi level but also features an additional antibonding peak around −5.46 eV; in contrast, the Ti-decorated system exhibits antibonding states only near the Fermi level. As a result, the Ti-decorated system presents the largest ICOHP absolute value, corresponding to the strongest adsorption and the most significant structural distortion. Specifically, the ICOHP values of the V-, Sc-, and Ti-decorated systems are −4.53 eV, −4.78 eV, and −4.98 eV, respectively, in excellent agreement with the corresponding adsorption energy trends.
3.2. Adsorption of NO on TM-Decorated 1T-ZrS2 Monolayers
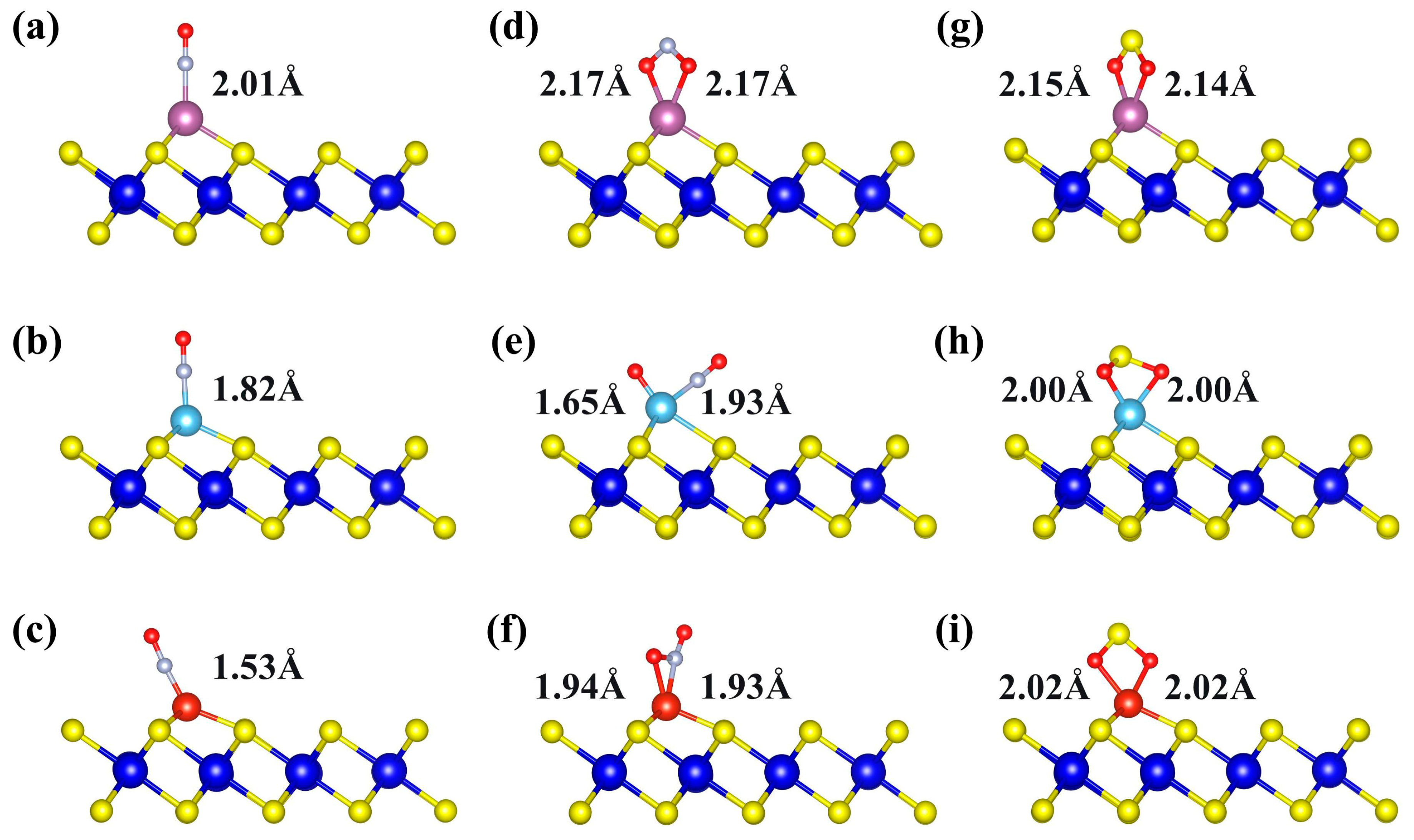
To gain deeper insight into the influence of TM decoration on the gas-adsorption capability, the adsorption behaviors of NO, NO2, and SO2 molecules on TM-decorated ZrS2 monolayers were systematically investigated. As illustrated in Figure 4a–c, the NO molecule binds through the N atom to Sc-, Ti-, and V-decorated ZrS2, with TM-N bond lengths of 2.01, 1.82, and 1.53 Å, respectively. After adsorption, the N-O bond length of NO is elongated relative to its optimized value of 1.17 Å, increasing to 1.20, 1.20, and 1.19 Å, respectively. This elongation indicates that the interaction with the TM weakens the intrinsic N–O bond.
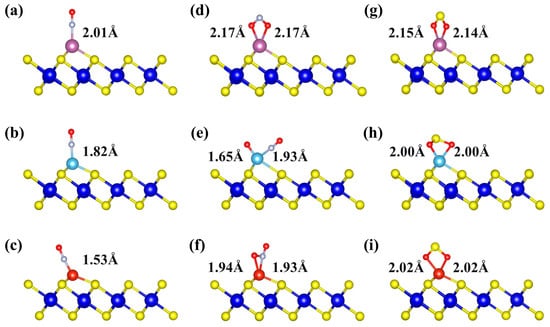
Figure 4.
The most stable adsorption structures of three gases on Sc-, Ti-, and V-decorated ZrS2 monolayers: (a–c) NO adsorption on Sc-, Ti-, and V-ZrS2, respectively; (d–f) NO2 adsorption on Sc-, Ti-, and V-ZrS2, respectively; and (g–i) SO2 adsorption on Sc-, Ti-, and V-ZrS2, respectively.
To elucidate the adsorption mechanism, we analyzed the PDOS. As shown in Figure 5, for NO adsorbed on Sc-ZrS2, a pronounced orbital hybridization appears at approximately −0.83 eV below the Fermi level, with contributions from both the t2g and eg orbitals. The COHP results indicate bonding interactions in this energy region, and the calculated adsorption energy is −2.10 eV. For the Ti-decorated system, in addition to the hybridization between the spin-up orbitals of Ti-d and N-p states at −0.5 eV, a spin-up bonding state is also present at the Fermi level. This enhances the adsorption, yielding an adsorption energy of −2.47 eV. For the V-decorated system, bonding states appear in both spin up and down channels at approximately −0.76 eV, which further strengthens the adsorption and results in an adsorption energy of −2.89 eV. Moreover, the adsorption strength tends to increase with increasing d-electron count, which is likely due to enhanced interaction between the N p orbitals and the TM d orbitals. Compared with the adsorption energy on the pristine ZrS2 monolayer (–0.298 eV) [44], the adsorption energies on TM-decorated ZrS2 are significantly enhanced, indicating that TM decoration plays a crucial role in improving the adsorption capability of ZrS2.
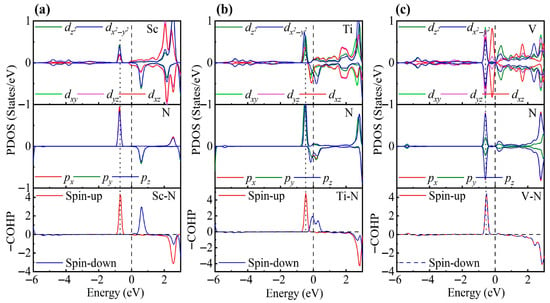
Figure 5.
PDOS and COHP for NO adsorption on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2 monolayers. The DOS reveals partial degeneracy of the TM dxy/dyz/dxz orbitals and the N px/py/pz orbitals.
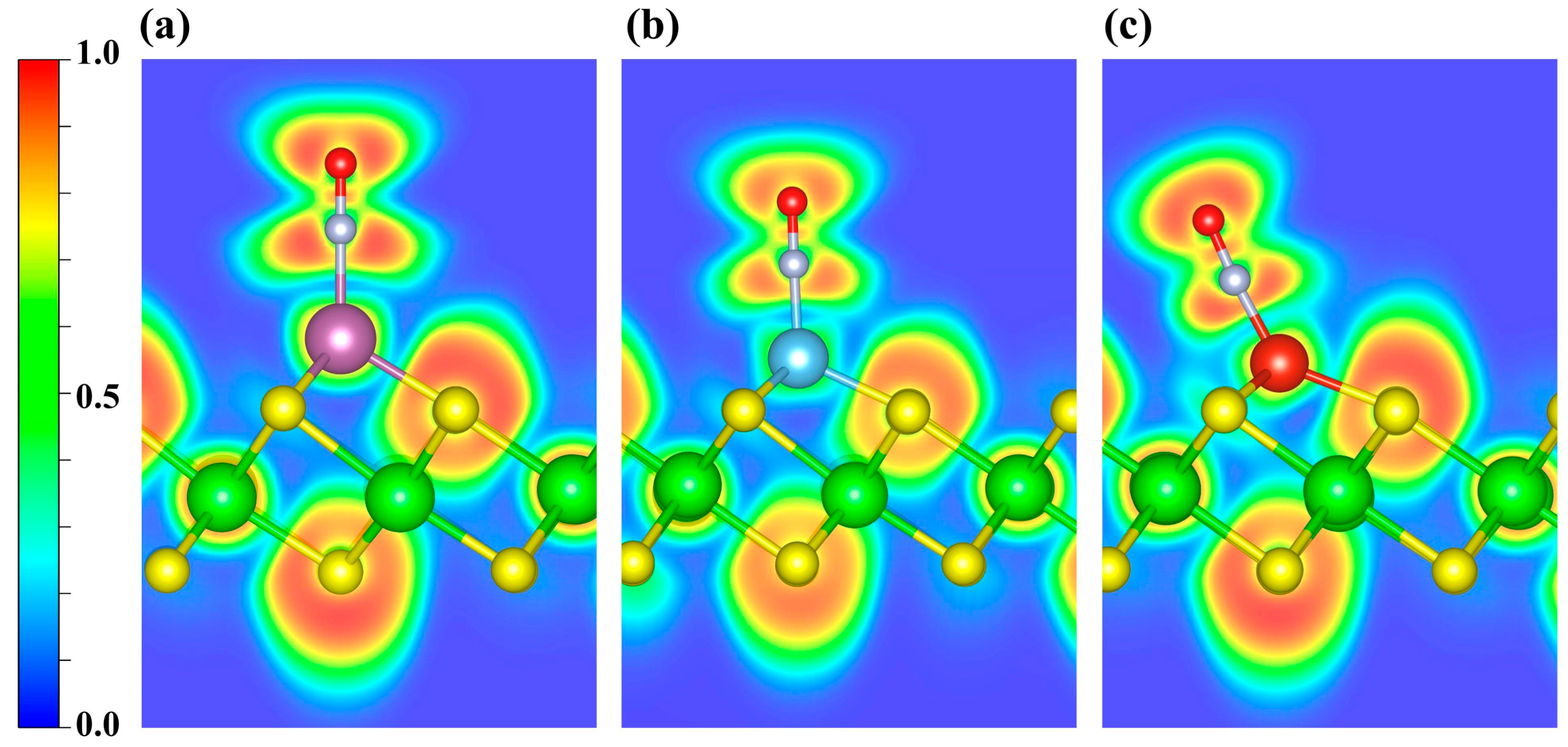
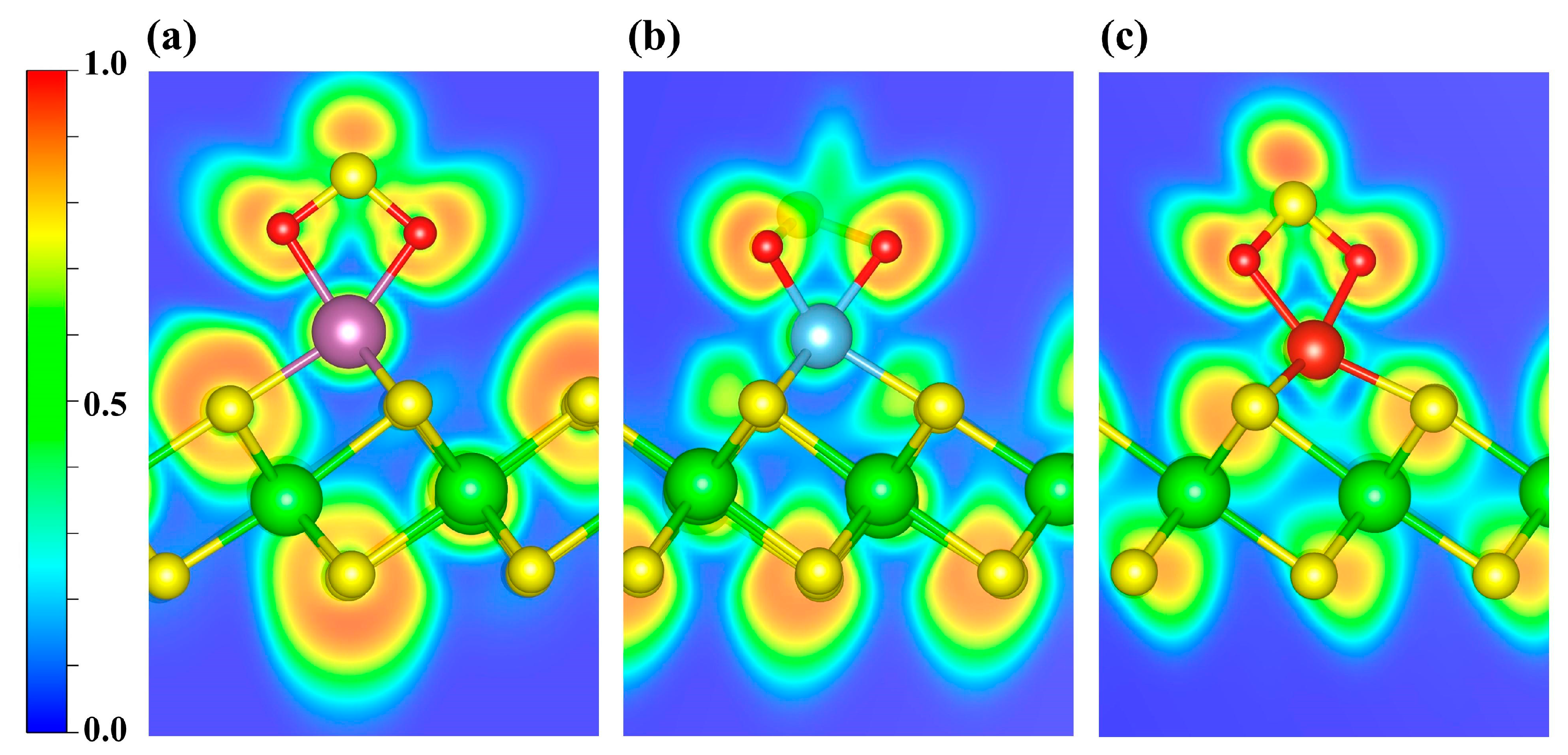
As shown in Figure S2, the CDD indicates electron depletion around the transition metal atoms and accumulation on the NO molecule. Further Bader charge analysis reveals charge transfers of 0.47 e, 0.51 e, and 0.48 e to the NO molecule in the Sc-, Ti-, and V-decorated systems, respectively. To elucidate the bonding between NO and the TM atom, we further performed ELF calculations (Figure 6). ELF ranges from 0 to 1: values near 1 denote highly localized electron pairs (covalent bonds or lone pairs); values around 0.5 indicate uniform electron gas; and values near 0 denote very weak localization or very low electron density. As shown, the ELF values are relatively high around the NO molecule, indicating strong electron localization, whereas the regions near the decorated metal atoms exhibit slightly weaker electron localization. Furthermore, the ELF topological analysis (Figure S3a–c) identifies multiple (3, −3) critical points (CPs; ELF attractors) in the N-TM interaction region that lie off the N-TM internuclear line. Moreover, considering the torus-shaped ELF around N (Figure S3d–f), together with the previously discussed PDOS/COHP signatures of orbital hybridization, the NO-TM interaction is likely governed primarily by π-type character.
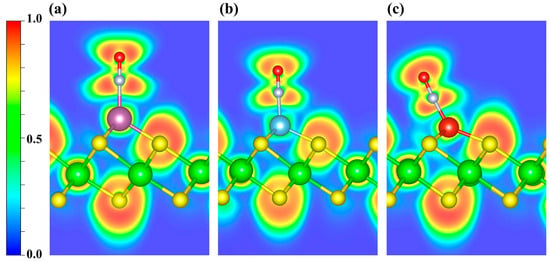
Figure 6.
ELF distributions of NO adsorbed on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2 systems. The plots highlight the ELF features around the adsorption sites. Blue and red denote ELF values of 0 and 1, respectively.
Furthermore, changes in the magnetic properties were observed upon NO adsorption. For the Sc- and Ti-decorated systems, NO adsorption induces net magnetic moments of 1.96 and 0.59 , respectively. The reduced net moment in the Ti-decorated system relative to Sc is attributed to partial occupation of spin-down states in the occupied region. For the V-decorated system, NO adsorption reduces the magnetic moment from 0.94 to a negligible value, rendering the system effectively nonmagnetic.
3.3. Adsorption of NO2 on TM-Decorated 1T-ZrS2 Monolayers
To elucidate the interaction of NO2 with Sc-, Ti-, and V-decorated ZrS2 monolayers, we first analyzed the optimized adsorption geometries (Figure 4d–f). The results show that NO2 is molecularly adsorbed on Sc-ZrS2 and V-ZrS2, whereas a dissociative adsorption final state is stabilized on Ti-ZrS2. In the molecular state, NO2 binds in a bidentate fashion: O, O-chelation on Sc and O, N-chelation on V. In contrast, on Ti the adsorbate splits into O and NO fragments: one O forms a Ti-O bond, the N atom of the NO fragment forms a Ti-N bond, and the remaining O stays bonded to N within the NO fragment. Representative bond lengths are Sc-O ≈ 2.17 Å (two bonds), Ti-N = 1.93 Å, Ti-O = 1.65 Å, V-N = 1.92 Å, and V-O = 1.94 Å, consistent with the above configurations.
As summarized in Table 3, NO2 binds most strongly on the Ti-decorated system, with an adsorption energy of −3.63 eV, substantially larger in magnitude than on Sc (−3.08 eV) and V (−2.70 eV). As shown in Figure S4, the CDD indicates that during NO2 adsorption, electron depletion occurs around the TM atoms, while electron accumulation develops on the molecule, facilitating bond formation between NO2 and the TM atom. Further Bader charge analysis reveals that Sc and V transfer 0.67 e and 0.56 e to NO2, respectively, whereas Ti exhibits the largest charge transfer of 1.08 e, nearly twice that of V. This pronounced electron redistribution weakens the intramolecular N-O bonds, providing favorable thermodynamic conditions for NO2 dissociation. Moreover, the adsorption energies of NO2 on all three TM-decorated systems are significantly higher than that on the pristine ZrS2 monolayer (−0.16 eV), and the corresponding charge transfers are also markedly larger than that for NO2 adsorption on pristine ZrS2 (0.04 e) [77], further demonstrating that TM decoration can substantially enhance the adsorption capability of ZrS2.

Table 3.
Adsorption energy (Eads), charge transfer (Qₜ), adsorption distance (D), gas molecular bond length (d), total magnetic moment (Mₜₒₜ), and ICOHP for the adsorption of three representative gases (NO, NO2, and SO2) on Sc-, Ti-, and V-decorated ZrS2 monolayers.
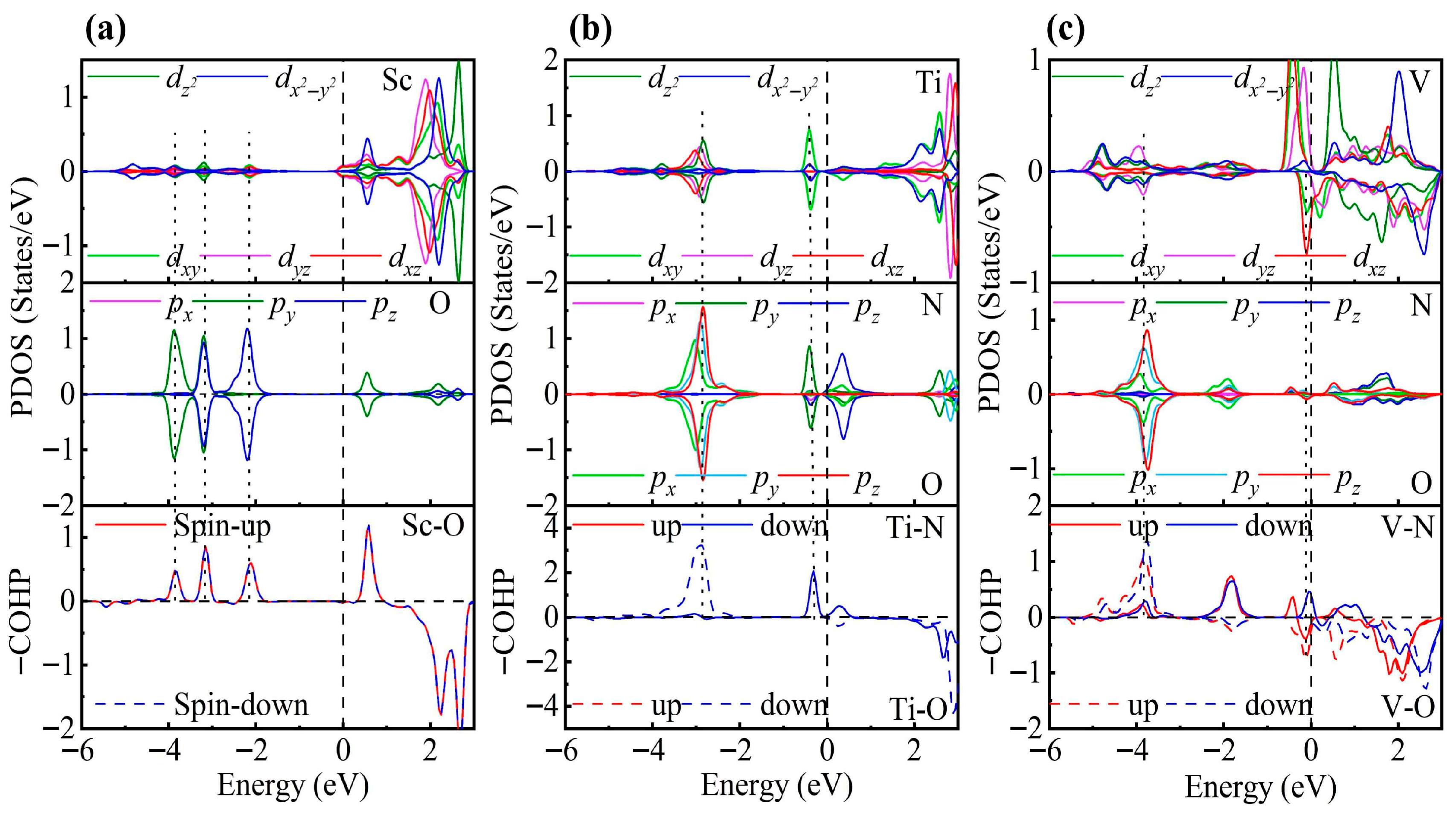
Electronic structure analysis clearly reveals that TM decoration significantly enhances the gas adsorption capability of ZrS2. As shown in Figure 7, in the Ti-decorated system, the Ti 3d states strongly hybridize with the N-p and O-p orbitals near the Fermi level: the Ti eg states hybridize with N-py at −0.36 eV, and the Ti state couples with O-p at −2.74 eV, forming stable bonding states. Such strong d–p interactions promote electron transfer from the Ti atom to NO2, effectively populating the N-O antibonding orbital, thereby weakening the N-O bond and driving its dissociation. In contrast, the Sc-decorated system exhibits weak d-p hybridization only at deeper energy levels (−2.05, −3.18, and −3.90 eV), with nearly no contribution near the Fermi level, whereas the V-decorated system shows limited hybridization with O-py/z orbitals around −3.87 eV and only weak interaction near the Fermi level. Consequently, NO2 remains in a molecular adsorption state on Sc- and V-decorated surfaces but undergoes dissociative adsorption on the Ti-decorated surface.
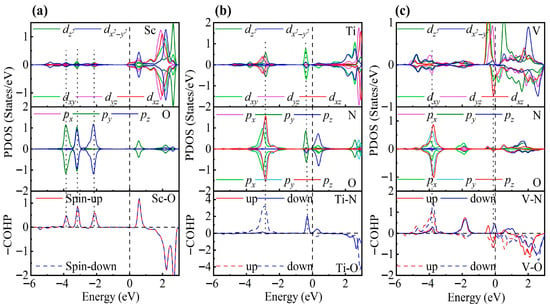
Figure 7.
PDOS and COHP for NO2 adsorption on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2 monolayers. The DOS reveals partial degeneracy between the TM dxy/dyz/dxz orbitals and the px/py/pz orbitals of N and O.
COHP analysis further substantiates this trend. All three TM-decorated systems enhance the orbital hybridization between NO2 and the substrate, but the effect is strongest for Ti. Both Ti-O and Ti-N bonds show pronounced bonding contributions near the Fermi level, with more negative −ICOHP values and larger integrated bonding areas, which together stabilize the dissociative adsorption state. By contrast, Sc exhibits relatively weak bonding peaks, and V even displays antibonding features at the Fermi level, which weakens the metal-ligand interactions. Mechanistically, the cooperative Ti-O/Ti-N bonding channels facilitate electron transfer from the intrinsic N-O bonding orbital to the newly formed metal-ligand bonds, thereby weakening the internal N-O interaction and promoting cleavage. For Sc and V, insufficient d-p overlap or higher d-orbital occupation prevents the formation of such strong multicenter bonding.
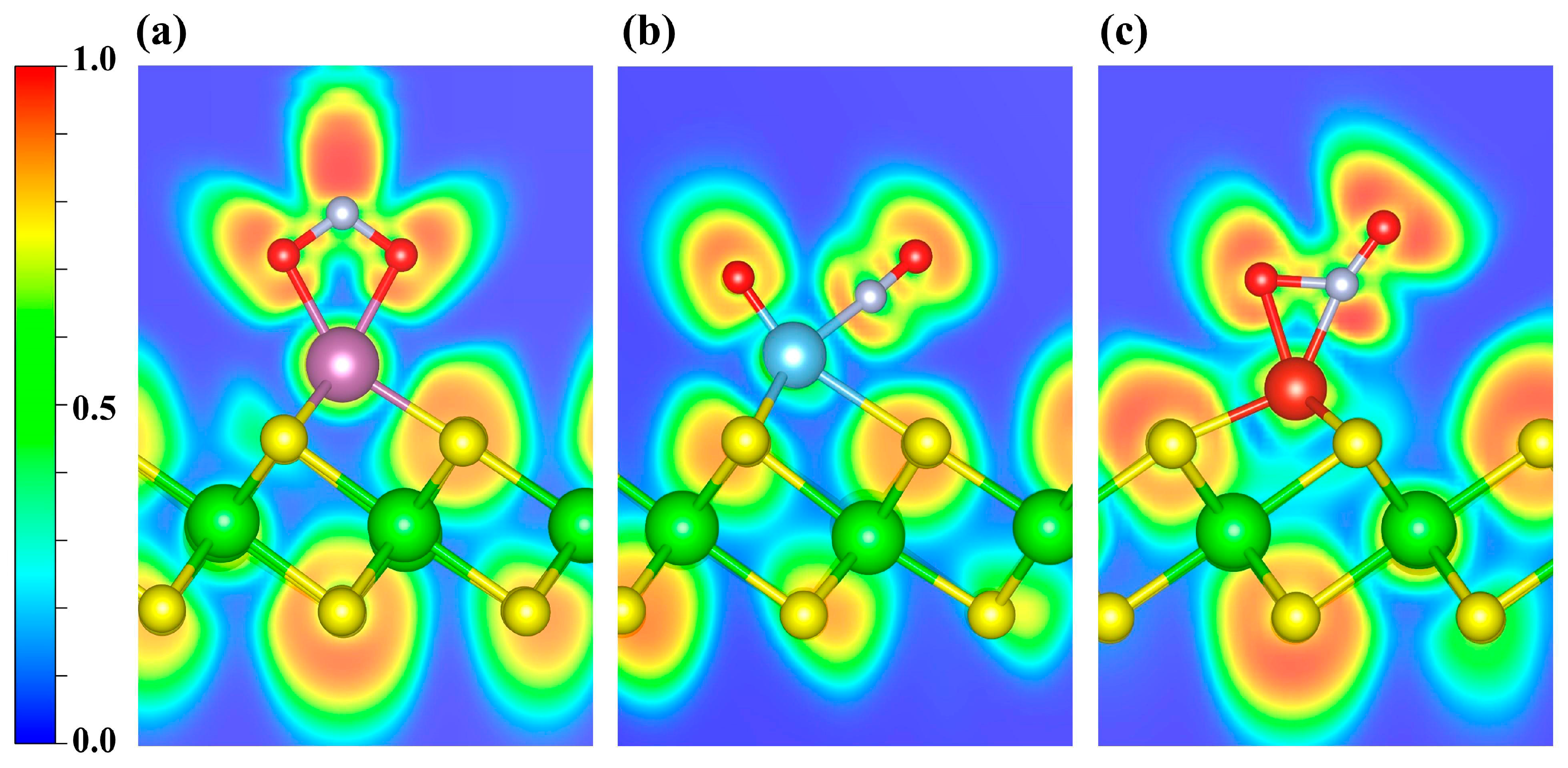
In Figure 8, the ELF maps display pronounced localization around the N and O atoms of NO2. To further investigate the bonding interactions, an ELF topological analysis was carried out (Figure S5a–c). It is revealed that the (3, −3) CPs between Sc and the two O atoms lie along their respective internuclear axes (Figure S5a), indicating that both Sc-O bonds are consistent with σ-dominated covalent bonds. However, similar to the case of NO, in the Ti- and V-decorated systems the (3, −3) CPs for TM-O and TM-N lie off the internuclear axis (Figure S5b,c). Taken together with the previously discussed PDOS/COHP evidence for orbital hybridization, the interactions are likely governed primarily by π-type character.
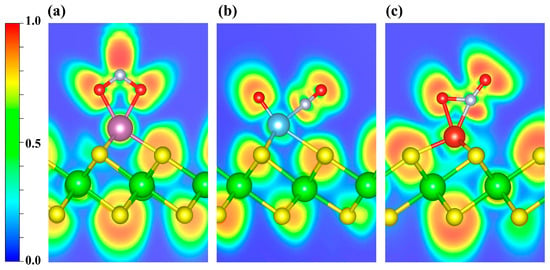
Figure 8.
ELF distributions of NO2 adsorbed on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2 systems. The plots highlight the ELF features around the adsorption sites. Blue and red denote ELF values of 0 and 1, respectively.
3.4. Adsorption of SO2 on TM-Decorated 1T-ZrS2 Monolayers
As shown in Figure 4g–i, SO2 molecules are stably adsorbed on Sc-, Ti- and V-decorated ZrS2 monolayers via bonds formed between the two oxygen atoms and the TM atoms. The bond lengths are 2.15 Å and 2.14 Å for Sc-O, 2.00 Å for Ti-O, and 2.02 Å for V-O, indicating a gradual shortening as the atomic number of the TM atom increases. Conventionally, shorter bond lengths are expected to correspond to stronger adsorption. However, the adsorption energies show the opposite trend, with the absolute values decreasing in sequence: Sc, −2.28 eV; Ti, −2.21 eV; V, −1.63 eV. This finding indicates that bond-length reduction alone does not determine adsorption strength, and the adsorption behavior is primarily governed by the electronic structure and orbital interactions of the system.
The CDD and Bader charge analysis further indicate that transition metal modification significantly enhances the adsorption of SO2 on the ZrS2 surface (Figure S6). During adsorption, the CDD shows electron depletion around the transition metal atoms, while electron accumulation occurs on the SO2 molecule, resulting in a stable chemical interaction between the molecule and the TM atom. Bader charge analysis reveals that 0.71 e and 0.73 e are transferred to SO2 in the Sc- and Ti-decorated systems, respectively, while the charge transfer is slightly lower in the V-decorated system, at 0.63 e. Consistently, the ICOHP calculations show that the Ti-O interaction is the strongest (−3.24 eV), while the interactions in the Sc and V systems are relatively weaker (−2.54 eV and −2.61 eV, respectively), as listed in Table 3. In contrast, the adsorption energy of SO2 on pristine ZrS2 is only −0.52 eV, with a charge transfer of merely 0.01 e [77], significantly lower than those of the TM-modified systems.
The PDOS and COHP analyses further support this conclusion (Figure 9). In the Sc-modified system, a pronounced hybridization between Sc d and O py orbitals is observed at −0.86 eV, corresponding to a spin-up bonding state, while at −3.90 eV, Sc d and O p orbitals also exhibit significant hybridization, forming both spin-up and spin-down bonding states. The absence of notable antibonding states near the Fermi level ensures the stability of adsorption. In the Ti-modified system, Ti t2g orbitals hybridize with O p orbitals at −1.05 eV, and stronger Ti d-O p hybridizations are observed at −3.08 eV, −3.91 eV, and −4.45 eV, with the largest COHP area at −4.45 eV. Despite the presence of weak antibonding states near the Fermi level, the bonding states dominate, resulting in strong adsorption. In contrast, the V-modified system exhibits pronounced occupation of antibonding states near the Fermi level, mainly involving t2g orbitals, with the strongest V d-O p hybridization at −3.82 eV. The increased d-electron count reduces the covalent contribution, explaining the significantly lower adsorption energy compared to the Sc- and Ti-modified systems.
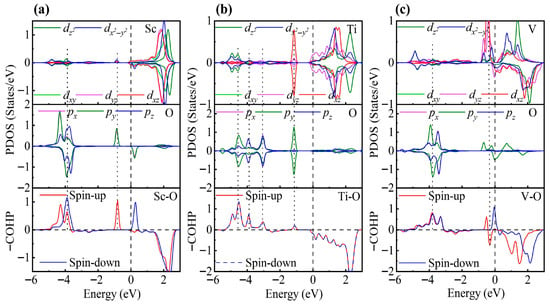
Figure 9.
PDOS and COHP for SO2 adsorption on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2 monolayers. The DOS reveals partial degeneracy of the TM dxy/dyz/dxz orbitals and the O px/py/pz orbitals.
Figure 10 shows the ELF maps for SO2 adsorbed on Sc-, Ti-, and V-decorated surfaces, in which the regions around the S and O atoms exhibit pronounced electron localization. An ELF topological analysis (Figure S7a–c) shows that, in the Sc- and V-decorated systems, the (3, −3) CPs lie along the TM-O bond direction (Figure S7a,c), which is consistent with a predominantly σ-type covalent character. In contrast, for SO2 adsorption on the Ti-decorated ZrS2, the (3, −3) CPs are located outside the bonding axis (Figure S7b). However, given the relatively strong electron localization in the region between Ti and O (Figure 10b and Figure S7e), together with the PDOS/COHP analyses discussed above, we infer that a covalent component also contributes to the Ti-O interaction.
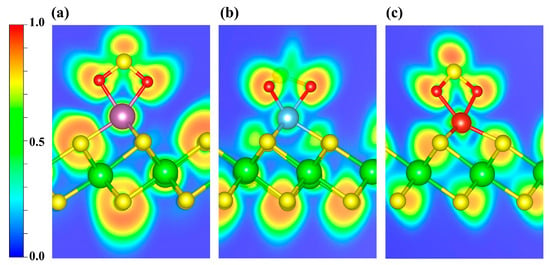
Figure 10.
ELF distributions of SO2 adsorbed on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2 systems. The plots highlight the ELF features around the adsorption sites. Blue and red denote ELF values of 0 and 1, respectively.
Notably, SO2 adsorption modifies the bonding characteristics and significantly affects the magnetic properties of the systems. For Sc-decorated ZrS2, which is nonmagnetic prior to adsorption, a magnetic moment of 0.97 appears after SO2 adsorption (Table 3). DOS analysis indicates that the observed moment primarily arises from the SO2 molecule, as the O s and py orbitals near the Fermi level show preferential occupation by spin up electrons, inducing local spin polarization. In contrast, V decorated ZrS2 has a magnetic moment of 0.94 before adsorption, which increases markedly to 2.96 after SO2 adsorption. The DOS results indicate that this enhancement mainly arises from strong hybridization between V dxz and dyz orbitals and O py orbitals. This observation further confirms that the strong interaction between SO2 and transition metal decorated ZrS2 is crucial for tuning the magnetic properties of the system.
3.5. Gas Sensing Properties of TM-Decorated 1T-ZrS2 Monolayers
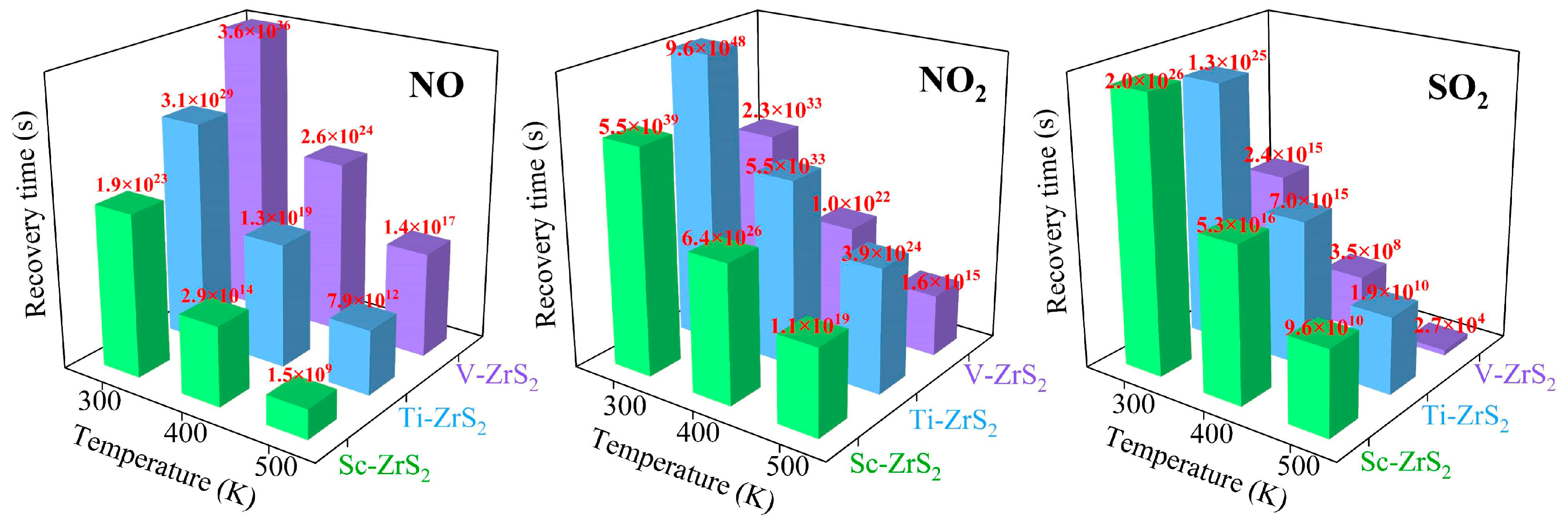
The recovery time is an important parameter for gas sensors, which is directly related to the adsorption properties of the material and the sensor’s reusability [81]. The recovery time affects the sensor response speed and also determines its stability and reliability in practical applications. As shown in Figure 11, ZrS2 monolayers decorated with TM (Sc, Ti and V) show significantly different recovery times for NO, NO2 and SO2. The recovery time (τ) was estimated using the van’t Hoff-Arrhenius relation within the framework of transition state theory, as expressed by [82]:
where ν0 is the attempt frequency (typically on the order of 1 × 1012 s−1 [83]), Eads is the adsorption energy, kB is the Boltzmann constant (8.617 × 10−5 eV·K−1), and T is the absolute temperature. Here, the recovery times are calculated at three different temperatures: 300 K, 400 K, and 500 K.
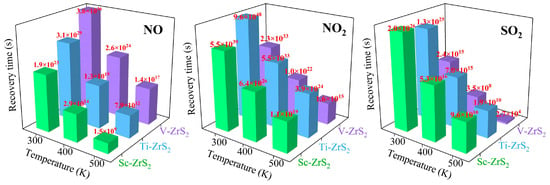
Figure 11.
The calculated recovery time (τ) for the desorption of NO, NO2, and SO2 molecules from Sc-, Ti-, and V-decorated ZrS2 monolayers at various temperatures (300 K to 500 K).
The computational results show that at room temperature (300 K) the shortest recovery time calculated for NO desorption from TM decorated ZrS2 is 1.9 × 1023 s, which is a consequence of the high adsorption energy of NO on the substrate (see Table 3). This result indicates that TM decorated ZrS2 can effectively capture NO, but NO is difficult to desorb from this substrate within a short time, which makes the material unsuitable for reversible gas sensing in typical sensor applications. Raising the operating temperature reduces the recovery time; however, even at 400 K and 500 K the desorption remains slow compared with time scales relevant for practical sensing.
For NO2 and SO2, the shortest recovery times are observed in the V-decorated ZrS2 system at 500 K, being 1.6 × 1015 s and 2.7 × 104 s, respectively; these values correspond approximately to 5.07 × 107 years for NO2 and 7.5 h for SO2. Such long recovery times, particularly the extremely long value for NO2, indicate that V-decorated ZrS2 is unsuitable for reversible gas sensing but may have potential for gas capture or storage applications.
It is worth noting that using the desorption barrier instead of the adsorption energy may yield a more rigorous estimation of recovery time. However, for the systems studied here, the desorption barrier obtained via CI-NEB and the adsorption energy differ by only about 0.1 eV. A similar difference has been reported for CO adsorption on Pt(111) [84]. Therefore, substituting the desorption barrier into the van’t Hoff-Arrhenius relation does not alter the overall conclusions regarding the gas-capture capability of ZrS2. Consequently, employing the adsorption energy as an approximation for the desorption barrier is a reasonable and widely adopted approach for estimating recovery behavior.
Compared with the adsorption of NO, NO2, and SO2 on pristine ZrS2, the recovery times of these gases on the transition-metal-decorated structures are significantly prolonged (see Table S1), indicating that the gas desorption process becomes more difficult after decoration. Overall, TM-decorated ZrS2 exhibits predominantly irreversible adsorption and slow desorption for all three gases, resulting in poor reusability and rendering the material unsuitable for reversible gas sensing under typical operating conditions. However, the long recovery times indicate strong adsorption, suggesting that TM-decorated ZrS2 has potential for gas capture and environmental remediation. Notably, Ti-decorated ZrS2 shows catalytic activity toward NO2, where the adsorbed molecule undergoes surface dissociation, implying its potential for catalytic degradation or conversion of harmful gases. Therefore, beyond conventional sensing applications, TM-decorated ZrS2 may find use in gas capture, storage, environmental cleanup, and catalytic processes. Future studies should focus on optimizing the adsorption–desorption kinetics to balance strong gas capture with controllable desorption, facilitating practical applications in industrial and environmental contexts.
4. Conclusions
In summary, we investigated the adsorption of NO, NO2, and SO2 on Sc-, Ti-, and V-decorated 1T-ZrS2 by first-principles calculations. The decorating metals preferentially occupy the hexagonal hollow site of ZrS2 and generate an approximately octahedral coordination environment, giving rise to characteristic d-orbital splitting. Transition-metal decoration markedly strengthens gas adsorption; notably, Ti decoration yields the strongest interaction with NO2 (Eads = −3.63 eV) and drives dissociative adsorption, indicating catalytic activation of NO2 on Ti-decorated ZrS2. Electronic structure analyses indicate pronounced metal-to-adsorbate electron donation and robust hybridization between metal d and molecular p orbitals, which together underpin the stability of the adsorbed states. Within the investigated temperature range, Sc-, Ti-, and V-decorated ZrS2 exhibit relatively long recovery times for NO, NO2, and SO2, indicating strong adsorption stability and high gas-capture capability. From a practical perspective, Sc, Ti, and V are earth-abundant and less toxic compared with certain heavy metals, making them promising candidates for environmentally sustainable modification of ZrS2. Nevertheless, future work should assess their environmental stability before large-scale applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15211653/s1, Figure S1. The calculated phonon dispersion of Sc-decorated 1T-ZrS2, evidencing the dynamical stability of the structure. Figure S2. The top and side views of the charge density difference for NO adsorbed on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2 systems. In these diagrams, orange and green isosurfaces denote charge accumulation and charge depletion, respectively, with an isovalue of 0.008 e/Å3. Figure S3. Topological analyses of the ELF for NO adsorption on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2. The purple dots represent (3, −3) critical points (CPs), corresponding to local maxima in the ELF. Only the CPs near the decorated atoms and the gas molecule are shown. The chemical bonds were generated by the analysis software, and some bonding representations may contain visualization errors. (d–f) depict the corresponding 3D isosurface representations of the ELF, where n denotes the isosurface value. Figure S4. The top and side views of the charge density difference for NO2 adsorbed on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2 systems. In these diagrams, orange and green isosurfaces denote charge accumulation and charge depletion, respectively, with an isovalue of 0.008 e/Å3. Figure S5. Topological analyses of the ELF for NO2 adsorption on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2. The purple dots represent (3, −3) critical points (CPs), corresponding to local maxima in the ELF. Only the CPs near the decorated atoms and the gas molecule are shown. The chemical bonds were generated by the analysis software, and some bonding representations may contain visualization errors. (d–f) depict the corresponding 3D isosurface representations of the ELF, where n denotes the isosurface value. Figure S6. The top and side views of the charge density difference for SO2 adsorbed on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2 systems. In these diagrams, orange and green isosurfaces denote charge accumulation and charge depletion, respectively, with an isovalue of 0.008 e/Å3. Figure S7. Topological analyses of the ELF for SO2 adsorption on (a) Sc-, (b) Ti-, and (c) V-decorated ZrS2. The purple dots represent (3, −3) critical points (CPs), corresponding to local maxima in the ELF. Only the CPs near the decorated atoms and the gas molecule are shown. The chemical bonds were generated by the analysis software, and some bonding representations may contain visualization errors. (d–f) depict the corresponding 3D isosurface representations of the ELF, where n denotes the isosurface value. Table S1. The recovery times (τ) of NO, NO2, and SO2 adsorbed on pristine ZrS2, with T denoting the adsorption temperature (300, 400, 500 K).
Author Contributions
X.W. and J.Z. (Jiaqi Zhang) implemented the computational study, conducted DFT calculations and analyses, prepared the figures, and wrote the first draft. X.L., Y.L., F.L. and Y.X. contributed to formal analysis, validation, and visualization. P.W., J.Z. (Jinjuan Zhang) and G.W. conceived the idea and supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by the Natural Science Foundation of Shandong Province (Nos. ZR2021QA031, ZR2024QA213), the National Natural Science Foundation of China (No. 12504282), the Postdoctoral Fellowship Program of CPSF (No. GZC20240951), and Qingdao Postdoctoral Project Funding (No. QDBSH20240102115).
Data Availability Statement
The data is available on the request from corresponding author.
Conflicts of Interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Jion, M.M.F.; Jannat, J.N.; Mia, M.Y.; Ali, M.A.; Islam, M.S.; Ibrahim, S.M.; Pal, S.C.; Islam, A.; Sarker, A.; Malafaia, G.; et al. A Critical Review and Prospect of NO2 and SO2 Pollution over Asia: Hotspots, Trends, and Sources. Sci. Total Environ. 2023, 876, 162851. [Google Scholar] [CrossRef]
- Lewis, A.; Edwards, P. Validate Personal Air-Pollution Sensors. Nature 2016, 535, 29–31. [Google Scholar] [CrossRef]
- Yang, J.; Sun, R.; Bao, X.; Liu, J.; Ng, J.W.; Tang, B.; Liu, Z. Enhancing Selectivity of Two-Dimensional Materials-Based Gas Sensors. Adv. Funct. Mater. 2025, 35, 2420393. [Google Scholar] [CrossRef]
- Feng, Q.; Huang, B.; Li, X. Graphene-Based Heterostructure Composite Sensing Materials for Detection of Nitrogen-Containing Harmful Gases. Adv. Funct. Mater. 2021, 31, 2104058. [Google Scholar] [CrossRef]
- Tyagi, D.; Wang, H.; Huang, W.; Hu, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent Advances in Two-Dimensional-Material-Based Sensing Technology toward Health and Environmental Monitoring Applications. Nanoscale 2020, 12, 3535–3559. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, G.; Zhang, X.; Tang, J. Rh-Doped MoSe2 as a Toxic Gas Scavenger: A First-Principles Study. Nanoscale Adv. 2019, 1, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Guo, Y.; Zhu, T.; Zheng, Y. Adsorption Species Distribution and Multicomponent Adsorption Mechanism of SO2, NO, and CO2 on Commercial Adsorbents. Energy Fuels 2017, 31, 11026–11033. [Google Scholar] [CrossRef]
- Zheng, R.; Qiu, L.; Zhou, X.; Liu, X.; Wang, Q.; Wang, C.; Zhu, T.; Chang, H. Research Progress in CO2 and NOx Adsorption by Two-Dimensional Materials. Catal. Sci. Technol. 2025, 15, 5950–5973. [Google Scholar] [CrossRef]
- Joshi, M.; Ren, X.; Lin, T.; Joshi, R. Mechanistic Insights into Gas Adsorption on 2D Materials. Small 2025, 21, 2406706. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, C.; Shen, L. Polar Materials for Photocatalytic Applications: A Critical Review. Interdiscip. Mater. 2024, 3, 530–564. [Google Scholar] [CrossRef]
- Pawar, K.K.; Kumar, A.; Mirzaei, A.; Kumar, M.; Kim, H.W.; Kim, S.S. 2D Nanomaterials for Realization of Flexible and Wearable Gas Sensors: A Review. Chemosphere 2024, 352, 141234. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Li, Y.; Yang, A.; Tan, X.; Chu, J.; Zhang, Y.; Yan, Y.; Tang, J.; Yuan, H.; Zhang, X.; et al. Advances in 2D Materials Based Gas Sensors for Industrial Machine Olfactory Applications. ACS Sens. 2024, 9, 2728–2776. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Wang, S.; Li, H.; Li, M.; Chen, T.; Xiao, X.; Wang, Y. Recent Advances in MoS2-Based Nanomaterial Sensors for Room-Temperature Gas Detection: A Review. Sens. Diagn. 2023, 2, 361–381. [Google Scholar] [CrossRef]
- Hou, W.; Mi, H.; Peng, R.; Peng, S.; Zeng, W.; Zhou, Q. First-Principle Insight into Ga-Doped MoS2 for Sensing SO2, SOF2 and SO2F2. Nanomaterials 2021, 11, 314. [Google Scholar] [CrossRef]
- Kumar, S.; Meng, G.; Mishra, P.; Tripathi, N.; Bannov, A.G. A Systematic Review on 2D MoS2 for Nitrogen Dioxide (NO2) Sensing at Room Temperature. Mater. Today Commun. 2023, 34, 105045. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, N.; Kumar, M. Strategy and Future Prospects to Develop Room-Temperature-Recoverable NO2 Gas Sensor Based on Two-Dimensional Molybdenum Disulfide. Nano Micro Lett. 2021, 13, 38. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, Y.; Zheng, L.; Yang, C.; Pinna, N.; Liu, X.; Zhang, J. MoS2 van Der Waals p-n Junctions Enabling Highly Selective Room-Temperature NO2 Sensor. Adv. Funct. Mater. 2020, 30, 2000435. [Google Scholar] [CrossRef]
- Kumar, R.; Zheng, W.; Liu, X.; Zhang, J.; Kumar, M. MoS2-Based Nanomaterials for Room-Temperature Gas Sensors. Adv. Mater. Technol. 2020, 5, 1901062. [Google Scholar] [CrossRef]
- Pham, T.; Li, G.; Bekyarova, E.; Itkis, M.E.; Mulchandani, A. MoS2-Based Optoelectronic Gas Sensor with Sub-Parts-per-Billion Limit of NO2 Gas Detection. ACS Nano 2019, 13, 3196–3205. [Google Scholar] [CrossRef]
- Lau, T.H.M.; Lu, X.; Kulhavý, J.; Wu, S.; Lu, L.; Wu, T.-S.; Kato, R.; Foord, J.S.; Soo, Y.-L.; Suenaga, K.; et al. Transition Metal Atom Doping of the Basal Plane of MoS2 Monolayer Nanosheets for Electrochemical Hydrogen Evolution. Chem. Sci. 2018, 9, 4769–4776. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Kumar, M. High Performance NO2 Sensor Using MoS2 Nanowires Network. Appl. Phys. Lett. 2018, 112, 53502. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, D.; Ding, Z.; Ma, K. Strain-Tunable Gas Sensing Properties of Ag- and Au-Doped SnSe2 Monolayers for the Detection of NO, NO2, SO2, H2S and HCN. Nanomaterials 2025, 15, 1454. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Hahm, M.G.; Choi, M.; Yoon, J.; Kim, A.R.; Lee, Y.-J.; Park, S.-G.; Kwon, J.-D.; Kim, C.S.; Song, M.; et al. Charge-Transfer-Based Gas Sensing Using Atomic-Layer MoS2. Sci. Rep. 2015, 5, 8052. [Google Scholar] [CrossRef] [PubMed]
- Scardamaglia, M.; Casanova-Cháfer, J.; Temperton, R.; Annanouch, F.E.; Mohammadpour, A.; Malandra, G.; Das, A.; Alagh, A.; Arbouch, I.; Montoisy, L.; et al. Operando Investigation of WS2 Gas Sensors: Simultaneous Ambient Pressure X-ray Photoelectron Spectroscopy and Electrical Characterization in Unveiling Sensing Mechanisms during Toxic Gas Exposure. ACS Sens. 2024, 9, 4079–4088. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, Y.; Wang, X.; Feng, Q.; Qiao, S.; Wen, W.; Chen, Y.; Cui, M.; Zhang, J.; Cai, C.; et al. Controlled Synthesis of ZrS2 Monolayer and Few Layers on Hexagonal Boron Nitride. J. Am. Chem. Soc. 2015, 137, 7051–7054. [Google Scholar] [CrossRef]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. High-Yield Preparation of Single- and Few-Layer Transition Metal Dichalcogenides by Electrochemical Lithium Intercalation and Exfoliation. Angew. Chem. Int. Ed. 2011, 50, 11093–11097. [Google Scholar] [CrossRef]
- Tian, Y.; Li, W.; Zhou, J.; Chen, X.; Zhang, H.; Xie, L.; Li, S.; Wang, C. Epitaxial Growth of Large-Area ZrS2 Thin Films on Sapphire for High-Performance Photodetectors. Nano Res. 2022, 15, 5678–5686. [Google Scholar] [CrossRef]
- Turcicova, H.; Novak, O.; Muzik, J.; Stepankova, D.; Smrz, M.; Mocek, T. Laser Induced Damage Threshold (LIDT) of β-Barium Borate (BBO) and Cesium Lithium Borate (CLBO)—Overview. Opt. Laser Technol. 2022, 149, 107876. [Google Scholar] [CrossRef]
- Ma, J.; Li, S.; Huang, X.; Jiang, J.; Xu, T.; Liu, T. All Optic-Fiber Waveguide-Coupled SPR Sensor for CRP Sensing Based on Dielectric Layer and Poly-Dopamine. Photonic Sens. 2025, 15, 250224. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, G.; Wang, G.; Feng, Z.; Li, Q.; Zhang, H.; Li, Y.; Liu, C. Pressure-induced Enhancement and Retainability of Optoelectronic Properties in Layered Zirconium Disulfide. Small 2024, 20, 2400216. [Google Scholar] [CrossRef]
- Li, L.; Lv, R.; Wang, J.; Chen, Z.; Wang, H.; Liu, S.; Ren, W.; Liu, W.; Wang, Y. Optical Nonlinearity of ZrS2 and Applications in Fiber Laser. Nanomaterials 2019, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Pan, H.; Mei, H.; Liu, X.; Lu, G.; Lou, C.; Li, Z.; Zhang, J. Emerging Single Atom Catalysts in Gas Sensors. Chem. Soc. Rev. 2022, 51, 7260–7280. [Google Scholar] [CrossRef] [PubMed]
- Chu, T.; Rong, C.; Zhou, L.; Mao, X.; Zhang, B.; Xuan, F. Progress and Perspectives of Single-Atom Catalysts for Gas Sensing. Adv. Mater. 2023, 35, 2206783. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Wu, J.; Zhang, Z.; Liao, Q.; Kang, Z.; Zhang, Y. Single-Atom Engineering to Ignite 2D Transition Metal Dichalcogenide Based Catalysis: Fundamentals, Progress, and Beyond. Chem. Rev. 2022, 122, 1273–1348. [Google Scholar] [CrossRef]
- Yong, Y.; Gao, R.; Yuan, X.; Zhao, Z.; Hu, S.; Kuang, Y. Gas Sensing and Capturing Based on the C7N6 Monolayer with and without Metal Decoration: A First-Principles Investigation. Appl. Surf. Sci. 2022, 591, 153129. [Google Scholar] [CrossRef]
- Liu, K.; Lin, L.; Wang, Y. Adsorption and Sensing Properties of ZrSe2 Monolayer Decorated with Transition Metal for CO2, NO2 and SO2 Gases: First-Principles Calculations. Mater. Today Commun. 2023, 36, 106698. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, K.; Xie, K.; Wang, P.; Lin, L.; Su, L. Adsorption of Toxic and Harmful Gas NO2 and SO2 on TM (Fe, Co and Ni) Decorated ZrSe2 Monolayer: A DFT Study. Mater. Today Commun. 2024, 39, 108483. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Li, W.; Ren, Q.-Y.; Li, J.-Z.; Xu, W.; Xu, J. First-Principles Study on Adsorption of Gas Molecules by Metal Sc Modified Ti2CO2. Acta Phys. Sin. 2024, 73, 73101. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.; Zhu, K.; Shen, J.; Xu, Y.; Chen, D.; Wang, P. Adsorption of NO2 and NH3 on Single-Atom (Co, Pd, Pt)-Decorated 2H-MoS2 Monolayer: A DFT Study. Results Phys. 2023, 51, 106694. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wei, Z.; Yuan, T.; Cui, H. Single Ni Atom-Dispersed WSe2 Monolayer for Sensing Typical Fault Gases in Dry-Type Transformers: A First-Principles Study. ACS Omega 2023, 8, 47067–47074. [Google Scholar] [CrossRef]
- Liu, K.; Lin, L.; Xie, K.; Shi, P.; Xu, D. Adsorption and Gas-Sensing Performance of the Small-Molecule Gas on ZrSe2 Monolayers: A First-Principles Study. Langmuir 2023, 39, 8879–8888. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, D.; Xie, K.; Shi, P.; Shen, Y.; Lin, L. Theoretical Calculation of Dissolved Gas in Transformer Oil Using the Gas Sensitive Properties of Sc- and Ti-Modified ZrS2. Langmuir 2024, 40, 24576–24584. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Bi, M.; Lei, S.; He, X.; Hu, X.; He, J.; Jiang, T. Adsorption of SF6 Decomposition Gases (H2S, SO2 and SOF2) on TM (Pd and Pt) Modified Monolayer ZrS2: A DFT Study. Comput. Theor. Chem. 2024, 1236, 114586. [Google Scholar] [CrossRef]
- Lin, X.-Q.; Zhang, X.; Qin, Y.-Y.; Yao, Y.-G. Tuning the Gas Sensing Properties of ZrS2 Monolayers via Pt Decoration: Insights from DFT Simulations. Langmuir 2025, 41, 6801–6815. [Google Scholar] [CrossRef]
- Guang, Q.; Huang, B.; Li, X. Au-Decorated WS2 Microflakes Based Sensors for Selective Ammonia Detection at Room Temperature. Chemosensors 2022, 10, 9. [Google Scholar] [CrossRef]
- Park, J.; Mun, J.; Shin, J.-S.; Kang, S.-W. Highly Sensitive Two-Dimensional MoS2 Gas Sensor Decorated with Pt Nanoparticles. R. Soc. Open Sci. 2018, 5, 181462. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Z.; Xia, S. Sc-Doped Sulfur Vacancy MoS2 Photocatalytic Nitrogen Reduction to Ammonia: Experimental and Theoretical Investigation. Inorg. Chem. 2025, 64, 15736–15747. [Google Scholar] [CrossRef]
- Ma, Z.; Ren, C.; Wu, Y.; Qiu, H.; Liu, H.; Hu, Z.; Wu, Y. Dopant-induced Giant Photoluminescence of Monolayer MoS2 by Chemical Vapor Transport. Adv. Mater. Interfaces 2022, 9, 2200431. [Google Scholar] [CrossRef]
- Sahoo, K.R.; Guha, A.; Bawari, S.; Sharma, R.; Maity, D.; Narayanan, T.N. Basal Plane Activation of MoS2 by the Substitutional Doping of Vanadium toward Electrocatalytic Hydrogen Generation. ACS Appl. Energy Mater. 2022, 5, 11263–11270. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, W.; Yang, W. Describing van Der Waals Interaction in Diatomic Molecules with Generalized Gradient Approximations: The Role of the Exchange Functional. J. Chem. Phys. 1997, 107, 7921–7925. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Lau, K.W.; Cocchi, C.; Draxl, C. Electronic and Optical Excitations of Two-Dimensional ZrS2 and HfS2 and Their Heterostructure. Phys. Rev. Mater. 2019, 3, 74001. [Google Scholar] [CrossRef]
- Li, Y.; Kang, J.; Li, J. Indirect-to-Direct Band Gap Transition of the ZrS2 Monolayer by Strain: First-Principles Calculations. RSC Adv. 2014, 4, 7396. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A User-Friendly Interface Facilitating High-Throughput Computing and Analysis Using VASP Code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A Tool to Extract Chemical Bonding from Plane-wave Based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef]
- Dronskowski, R.; Bloechl, P.E. Crystal Orbital Hamilton Populations (COHP): Energy-Resolved Visualization of Chemical Bonding in Solids Based on Density-Functional Calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Clarendon Press: Oxford, UK, 1994. [Google Scholar]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef] [PubMed]
- Sanville, E.; Kenny, S.D.; Smith, R.; Henkelman, G. An Improved Grid-Based Algorithm for Bader Charge Allocation. J. Comput. Chem. 2007, 28, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A Fast and Robust Algorithm for Bader Decomposition of Charge Density. Comput. Mater. Sci. 2006, 36, 254–360. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A Simple Measure of Electron Localization in Atomic and Molecular Systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of Chemical Bonds Based on Topological Analysis of Electron Localization Functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An Electronic Structure and Molecular Dynamics Software Package—Quickstep: Efficient and Accurate Electronic Structure Calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- Lu, T. A Comprehensive Electron Wavefunction Analysis Toolbox for Chemists, Multiwfn. J. Chem. Phys. 2024, 161, 82503. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Karbalaee Aghaee, A.; Belbasi, S.; Hadipour, H. Ab Initio Calculation of the Effective Coulomb Interactions in MX2 (M = Ti, V, Cr, Mn, Fe, Co, Ni; X = S, Se, Te): Intrinsic Magnetic Ordering and Mott Phase. Phys. Rev. B 2022, 105, 115115. [Google Scholar] [CrossRef]
- Hu, T.; Li, Z.; Hu, M.; Wang, J.; Hu, Q.; Li, Q.; Wang, X. Chemical Origin of Termination-Functionalized MXenes: Ti3C2T2 as a Case Study. J. Phys. Chem. C 2017, 121, 19254–19261. [Google Scholar] [CrossRef]
- Yi, W.; Tang, G.; Chen, X.; Yang, B.; Liu, X. Qvasp: A Flexible Toolkit for VASP Users in Materials Simulations. Comput. Phys. Commun. 2020, 257, 107535. [Google Scholar] [CrossRef]
- Han, Y. An Evaluation for Geometries, Formation Enthalpies, and Dissociation Energies of Diatomic and Triatomic (C, H, N, O), NO3, and HNO3 Molecules from the PAW DFT Method with PBE and optB88-vdW Functionals. AIP Adv. 2022, 12, 120501. [Google Scholar] [CrossRef]
- Zhu, M.-Q.; Wang, X.-F.; Vasilopoulos, P. Transition Metal-Doped ZrS2 Monolayer as Potential Gas Sensor for CO2, SO2, and NO2: Density Functional Theory and Non-Equilibrium Green’s Functions Analysis. J. Phys. D Appl. Phys. 2025, 58, 135306. [Google Scholar] [CrossRef]
- Xin, Q.; Zhao, X.; Ma, X.; Wu, N.; Liu, X.; Wei, S. Electronic Structure in 1T-ZrS2 Monolayer by Strain. Phys. E Low-Dimens. Syst. Nanostructures 2017, 93, 87–91. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Wang, T.; Wei, S.; Yang, L. Electronic Structures and Optical Properties of ZrS2 Monolayer by N- and P-Type Doping. J. Alloys Compd. 2018, 748, 798–803. [Google Scholar] [CrossRef]
- Raya, S.S.; Ansari, A.S.; Shong, B. Molecular Adsorption of NH3 and NO2 on Zr and Hf Dichalcogenides (S, Se, Te) Monolayers: A Density Functional Theory Study. Nanomaterials 2020, 10, 1215. [Google Scholar] [CrossRef]
- Chen, Y.; Gui, Y.; Chen, X. Adsorption and Gas-Sensing Properties of C2H4, CH4, H2, H2O on Metal Oxides (CuO, NiO) Modified SnS2 Monolayer: A DFT Study. Results Phys. 2021, 28, 104680. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.-H.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving Gas Sensing Properties of Graphene by Introducing Dopants and Defects: A First-Principles Study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef]
- Peng, S.; Cho, K.; Qi, P.; Dai, H. Ab Initio Study of CNT NO2 Gas Sensor. Chem. Phys. Lett. 2004, 387, 271–276. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Z.; Wang, D.; Wang, H.-F.; Hu, P. First-Principles Determination of CO Adsorption and Desorption on Pt (111) in the Free Energy Landscape. J. Phys. Chem. C 2018, 122, 21478–21483. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).