A Sweat Cortisol Sensor Based on Gold-Modified Molecularly Imprinted Polymer
Abstract
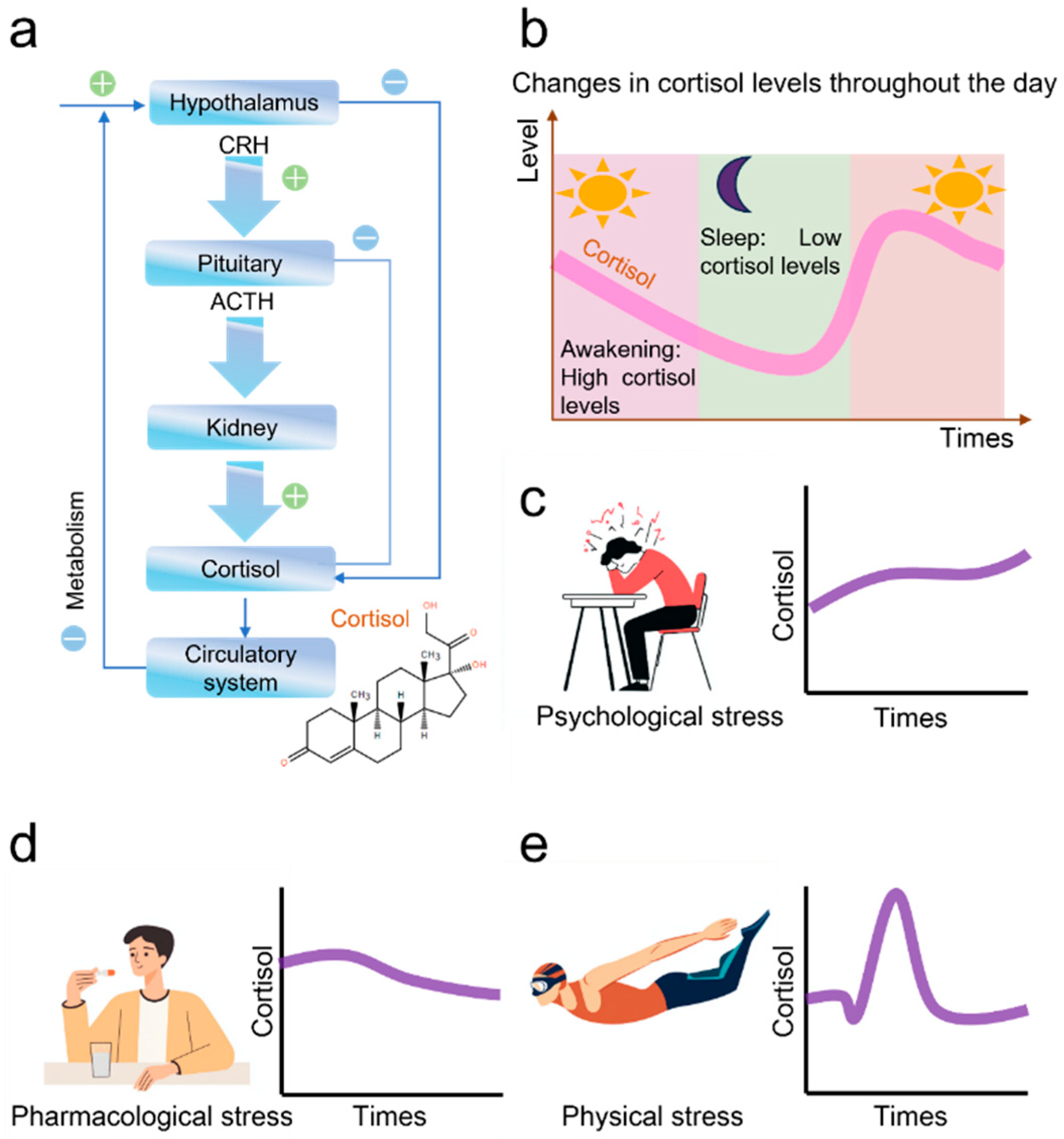
1. Introduction
2. Experimental
2.1. Materials
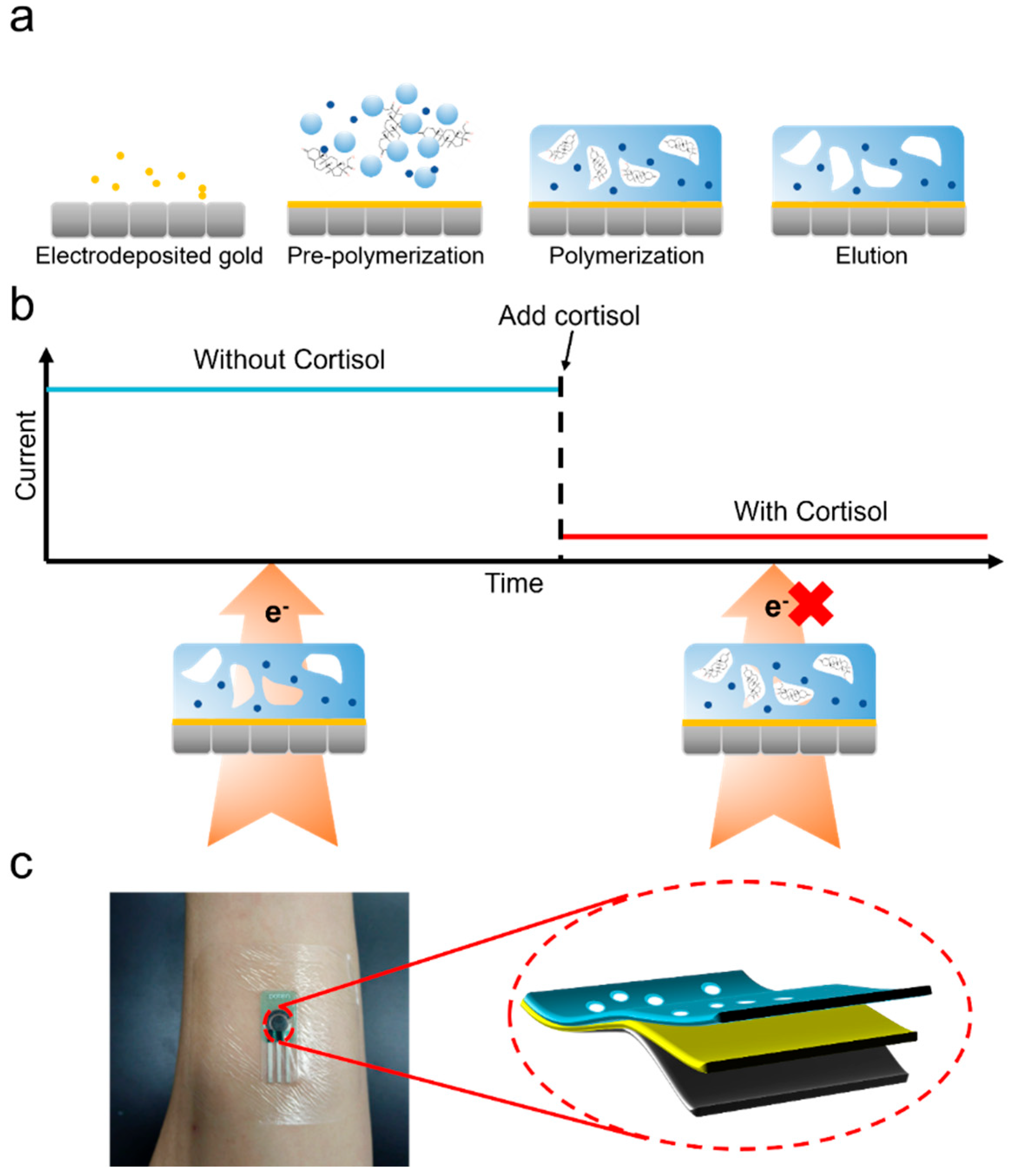
2.2. Electrodeposited Gold
2.3. Prepare the Pre-Polymerization Solution
2.4. Electropolymerization and Elution
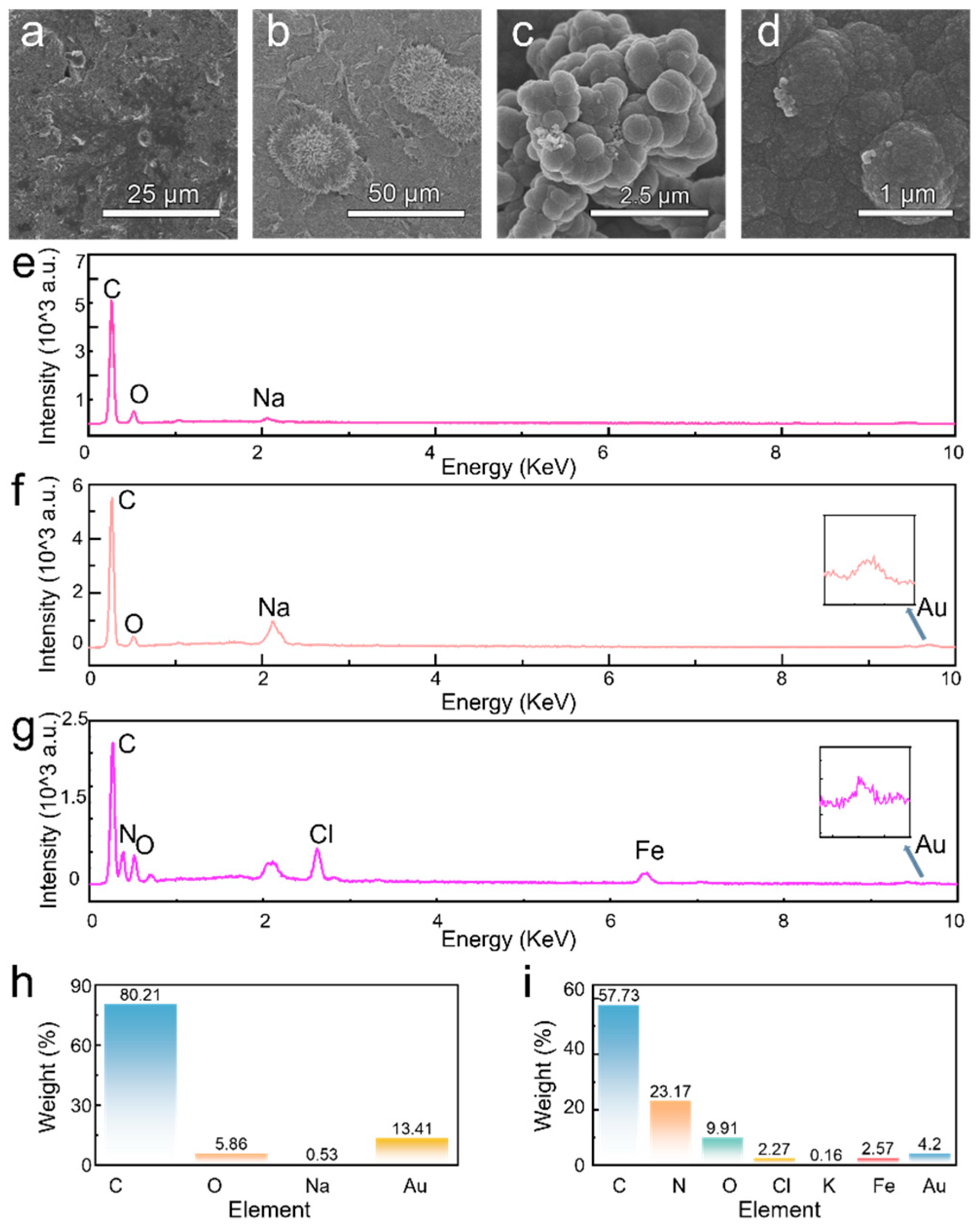
2.5. Characterization
2.6. Measurement Conditions
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, C.; Guo, L.; Li, Q.; Zhang, L. Interpretation on the report of global stroke data 2022. J. Diagn. Concepts Pract. 2023, 22, 238. [Google Scholar] [CrossRef]
- World Mental Health Today: Latest Data; World Health Organization: Geneva, Switzerland, 2025.
- Ye, J.Y.; Wan, X.N.; Li, Y.Y.; Kong, L.L. A Research of Psychiatrists Experiencing Death by Suicide in Psychopaths. Adv. Clin. Med. 2023, 13, 7232–7239. [Google Scholar] [CrossRef]
- Ma, Y.S. Research on the mental health of contemporary college students from the perspective of higher education level stratification. Jinan Shandong Univ. 2022, 1, 10–20. [Google Scholar] [CrossRef]
- Shchaslyvyi, A.Y.; Antonenko, S.V.; Telegeev, G.D. Comprehensive Review of Chronic Stress Pathways and the Efficacy of Behavioral Stress Reduction Programs (BSRPs) in Managing Diseases. Int. J. Environ. Res. Public Health 2024, 21, 1077. [Google Scholar] [CrossRef] [PubMed]
- Torrente-Rodríguez, R.M.; Tu, J.; Yang, Y.; Min, J.; Wang, M.; Song, Y.; Yu, Y.; Xu, C.; Ye, C.; IsHak, W.W.; et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter 2020, 2, 921–937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, F.; Huang, N.; Yan, W.; Zhang, B.; Zhang, C.; Peng, K.; Guo, J. Assessment of Factors Associated with Mental Well-Being Among Chinese Youths at Individual, School, and Province Levels. JAMA Netw. Open 2023, 6, e2324025. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, X.; Wang, Y.; Peng, K.; Ma, Y. Unraveling the Relationship Between Teachers’ and Students’ Mental Health: A One-to-One Matched Analysis. J. Exp. Educ. 2024, 93, 136–148. [Google Scholar] [CrossRef]
- Bao, H.; Liu, Y.Y. Research progress on the etiology and diagnostic methods of depression. Adv. Clin. Med. 2023, 13, 5641–5645. [Google Scholar] [CrossRef]
- Sitorus, H.P.; Silitonga, M. The Role of cortisol in the stress response. Int. J. Ecophysiol. 2025, 7, 48–58. [Google Scholar] [CrossRef]
- Singh, N.K.; Chung, S.; Chang, A.-Y.; Wang, J.; Hall, D.A. A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer. Biosens. Bioelectron. 2023, 227, 115097. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, Y. A disposable printed liquid gate graphene field effect transistor for a salivary cortisol test. ACS Sens. 2021, 6, 3024–3031. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, H.; Ye, H.; Chen, Z.; Jaffrezic-Renault, N.; Guo, Z. An ultrasensitive aptamer-antibody sandwich cortisol sensor for the noninvasive monitoring of stress state. Biosens. Bioelectron. 2021, 190, 113451. [Google Scholar] [CrossRef]
- Leitão, C.; Leal-Junior, A.; Almeida, A.R.; Pereira, S.O.; Costa, F.M.; Pinto, J.L.; Marques, C. Cortisol AuPd plasmonic unclad POF biosensor. Biotechnol. Rep. 2021, 29, e00587. [Google Scholar] [CrossRef] [PubMed]
- Mugo, S.M.; Alberkant, J. Flexible molecularly imprinted electrochemical sensor for cortisol monitoring in sweat. Anal. Bioanal. Chem. 2020, 412, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, H.; He, S.; Yang, H.; Liu, K.; Duan, G.; Jiang, S. Advanced electrospun nanofibers as bifunctional electrocatalysts for flexible metal-air (O2) batteries: Opportunities and challenges. Mater. Des. 2022, 214, 110406. [Google Scholar] [CrossRef]
- Wang, L.; Snihirova, D.; Deng, M.; Vaghefinazari, B.; Xu, W.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L. Sustainable aqueous metal-air batteries: An insight into electrolyte system. Energy Storage Mater. 2022, 52, 573–597. [Google Scholar] [CrossRef]
- Karuppaiah, G.; Lee, M.-H.; Bhansali, S.; Manickam, P. Electrochemical sensors for cortisol detection: Principles, designs, fabrication, and characterization. Biosens. Bioelectron. 2023, 239, 115600. [Google Scholar] [CrossRef]
- Yulianti, E.S.; Rahman, S.F.; Whulanza, Y. Molecularly imprinted polymer-based sensor for electrochemical detection of cortisol. Biosensors 2022, 12, 1090. [Google Scholar] [CrossRef]
- Lavignac, N.; Allender, C.J.; Brain, K.R. Current status of molecularly imprinted polymers as alternatives to antibodies in sorbent assays. Anal. Chim. Acta 2004, 510, 139–145. [Google Scholar] [CrossRef]
- Bedwell, T.S.; Whitcombe, M.J. Analytical applications of MIPs in diagnostic assays: Future perspectives. Anal. Bioanal. Chem. 2016, 408, 1735–1751. [Google Scholar] [CrossRef]
- Martin, J.A.; Chávez, J.L.; Chushak, Y.; Chapleau, R.R.; Hagen, J.; Kelley-Loughnane, N. Tunable stringency aptamer selection and gold nanoparticle assay for detection of cortisol. Anal. Bioanal. Chem. 2014, 406, 4637–4647. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Moore, J.A.; Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N.; Chou, C.-F.; Swami, N.S. Aptamer-functionalized nanoparticles for surface immobilization-free electrochemical detection of cortisol in a microfluidic device. Biosens. Bioelectron. 2016, 78, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.; Pandiaraj, M.; Bhansali, S.; Ponpandian, N.; Viswanathan, C. Carbon fiber based electrochemical sensor for sweat cortisol measurement. Sci. Rep. 2019, 9, 403. Available online: https://www.x-mol.com/paperRedirect/971433 (accessed on 23 March 2024). [CrossRef]
- Manickam, P.; Pasha, S.K.; Snipes, S.A.; Bhansali, S. A reusable electrochemical biosensor for monitoring of small molecules (cortisol) using molecularly imprinted polymers. J. Electrochem. Soc. 2016, 164, B54. [Google Scholar] [CrossRef]
- Madhu, S.; Anthuuvan, A.J.; Ramasamy, S.; Manickam, P.; Bhansali, S.; Nagamony, P.; Chinnuswamy, V. ZnO nanorod integrated flexible carbon fibers for sweat cortisol detection. ACS Appl. Electron. Mater. 2020, 2, 499–509. [Google Scholar] [CrossRef]
- He, Y.; Tang, C.; Ren, Y.; Yuan, B.; Li, L.; You, T.; Chen, X. Better Together: Synergistic Enhancement of AuNPs and Bifunctional Monomers in a Dual-Channel Molecularly Imprinting Electrochemical Sensor for Simultaneous Detection of Diuron and Thidiazuron. Anal. Chem. 2025, 97, 7869–7878. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qin, W.; Li, X.X.; Feng, L.; Gu, C.; Chen, J.; Tian, Z.; Chen, J.; Yang, M.; Qiao, H.; et al. Molecularly Imprinted Electrochemical Sensor for Epinephrine Based on the Composite of Graphene and Gold Particles. Chem. Reag. 2022, 44, 872–879. [Google Scholar] [CrossRef]
- Tang, W.; Yin, L.; Sempionatto, J.R.; Moon, J.; Teymourian, H.; Wang, J. Touch-based stressless cortisol sensing. Adv. Mater. 2021, 33, 2008465. [Google Scholar] [CrossRef] [PubMed]
- Aryan, Z.; Khajehsharifi, H.; Shahrokhian, S. A sensitive platform based on molecularly imprinted polymer modified with AuNPs-ultrathin/graphitic-C3N4 for voltammetric determination of hippuric acid. Diam. Relat. Mater. 2025, 154, 112083. [Google Scholar] [CrossRef]
- Lasia, A. Modern Aspects of Electrochemistry; Springer: Boston, MA, USA, 2002; pp. 143–248. [Google Scholar]
- Laviron, E.J.J. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Upasham, S.; Tanak, A.; Jagannath, B.; Prasad, S. Development of ultra-low volume, multi-bio fluid, cortisol sensing platform. Sci. Rep. 2018, 8, 16745. [Google Scholar] [CrossRef]
- Richard, Y.A.; Koventhan, C.; Lo, A.-Y.; Lincy, S.A.; Dharuman, V.; Huang, Y.-J. Hydrothermally Synthesized Erbium Oxide/Cobalt Oxide Nanoflowers for Electrochemical Sensing of 4-Nitroaniline in Environmental Samples. ACS Appl. Nano Mater. 2025, 8, 12561–12573. [Google Scholar] [CrossRef]
- Richard, Y.A.; Lincy, S.A.; Lo, A.-Y.; Koventhan, C.; Dharuman, V.; Piraman, S. Electrochemical investigation of an antipyretic drug in plant extracts and environmental samples at the O-MWCNT/CuO nanostructure modified glassy carbon electrode. Environ. Sci. Nano 2025, 12, 909–923. [Google Scholar] [CrossRef]
- Singh, N.K.; Chung, S.; Sveiven, M.; Hall, D.A. Cortisol detection in undiluted human serum using a sensitive electrochemical structure-switching aptamer over an antifouling nanocomposite layer. ACS Omega 2021, 6, 27888–27897. [Google Scholar] [CrossRef]
- Dalirirad, S.; Steckl, A.J. Aptamer-based lateral flow assay for point of care cortisol detection in sweat. Sens. Actuators B Chem. 2019, 283, 79–86. [Google Scholar] [CrossRef]
- Ganguly, A.; Lin, K.C.; Muthukumar, S.; Prasad, S. Autonomous, real-time monitoring electrochemical aptasensor for circadian tracking of cortisol hormone in sub-microliter volumes of passively eluted human sweat. ACS Sens. 2020, 6, 63–72. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, S.; Ning, Q.; Li, T.; Xu, H.; Sun, Q.; Cui, D.; Wang, K. Dual-signal readout paper-based wearable biosensor with a 3D origami structure for multiplexed analyte detection in sweat. Microsyst. Nanoeng. 2023, 9, 36. [Google Scholar] [CrossRef]
- Abu Shama, N.; Aşır, S.; Göktürk, I.; Yılmaz, F.; Türkmen, D.; Denizli, A. Electrochemical detection of cortisol by silver nanoparticle-modified molecularly imprinted polymer-coated pencil graphite electrodes. ACS Omega 2023, 8, 29202–29212. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Vasudev, A.; Arya, S.K.; Pasha, S.K.; Bhansali, S. Recent advances in cortisol sensing technologies for point-of-care application. Biosens. Bioelectron. 2014, 53, 499–512. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Z. Electrochemical detection of cortisol in sweat for sports performance analysis using graphene oxide-silicon carbide nanocomposites. Microchim. Acta 2025, 192, 631. [Google Scholar] [CrossRef]
- Yang, S.; Shi, Z.; Ma, Z.; Li, Z.; Wei, G.; Švorc, Ľ.; Zhu, Z. Ti-Cu BMOFs based molecularly imprinted electrochemical sensor for non-invasive cortisol detection in sweat. Microchem. J. 2025, 115497. [Google Scholar] [CrossRef]

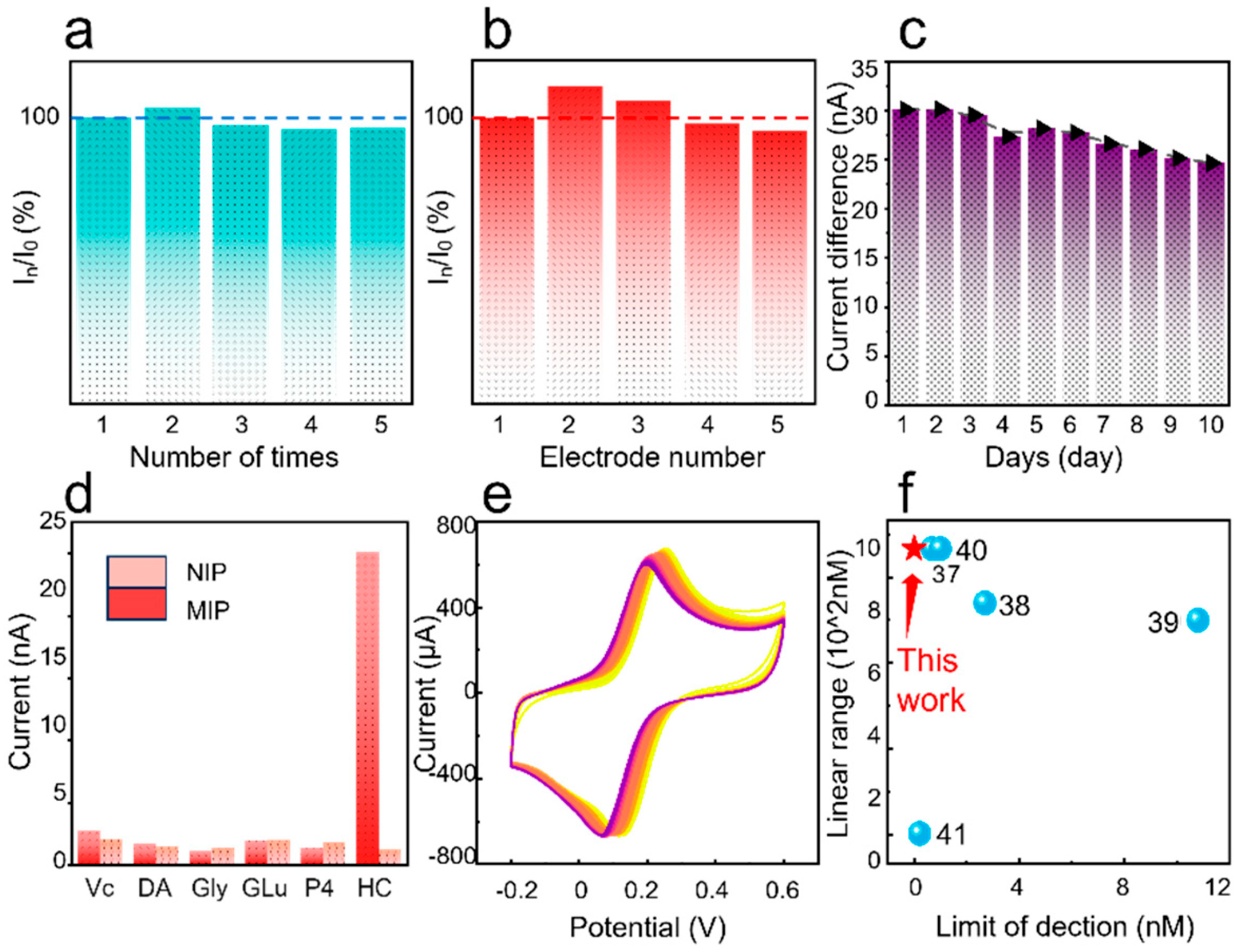
| Sensing Platform | Sensing Molecule | Linear Range | LOD | References |
|---|---|---|---|---|
| Ag@AgO/PANI | Anti-Cmab | 1 pM–1 μM | 0.64 pM | [42] |
| MIP-PPy-SPCE | MIP | 1 pM–10 μM | 1 pM | [25] |
| MIP-PPy-SPCE | MIP | 1 nM–1 μM | 0.9 nM | [29] |
| Au@PDMS | Anti-Cmab | 1 pM–1 μM | 0.2 pM | [11] |
| GO-SiC | Anti-Cmab | 100 fg/mL–1 μg/mL | 90 fg/mL | [43] |
| Ti-Cu BMOFs/MIPs | MIP | 0.05 nM–1 μM | 37 pM | [44] |
| MIP-PPy-Au@SPCE | MIP | 1 fM–1 μM | 0.4112 fM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Xie, G.; Li, J.; Yuan, H.; Su, Y. A Sweat Cortisol Sensor Based on Gold-Modified Molecularly Imprinted Polymer. Nanomaterials 2025, 15, 1654. https://doi.org/10.3390/nano15211654
Liu Z, Xie G, Li J, Yuan H, Su Y. A Sweat Cortisol Sensor Based on Gold-Modified Molecularly Imprinted Polymer. Nanomaterials. 2025; 15(21):1654. https://doi.org/10.3390/nano15211654
Chicago/Turabian StyleLiu, Ziyu, Guangzhong Xie, Jing Li, Hong Yuan, and Yuanjie Su. 2025. "A Sweat Cortisol Sensor Based on Gold-Modified Molecularly Imprinted Polymer" Nanomaterials 15, no. 21: 1654. https://doi.org/10.3390/nano15211654
APA StyleLiu, Z., Xie, G., Li, J., Yuan, H., & Su, Y. (2025). A Sweat Cortisol Sensor Based on Gold-Modified Molecularly Imprinted Polymer. Nanomaterials, 15(21), 1654. https://doi.org/10.3390/nano15211654







