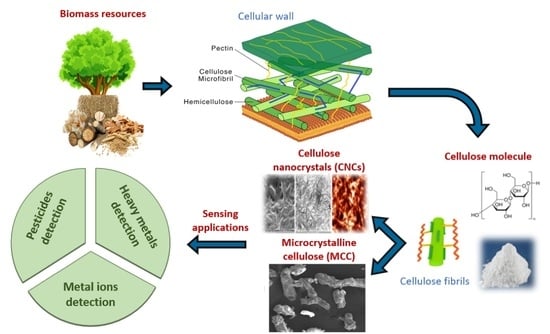
Sustainable Carbon Dots from Cellulose Precursors for Environmental Sensing: Recent Trends and Outlook
Abstract
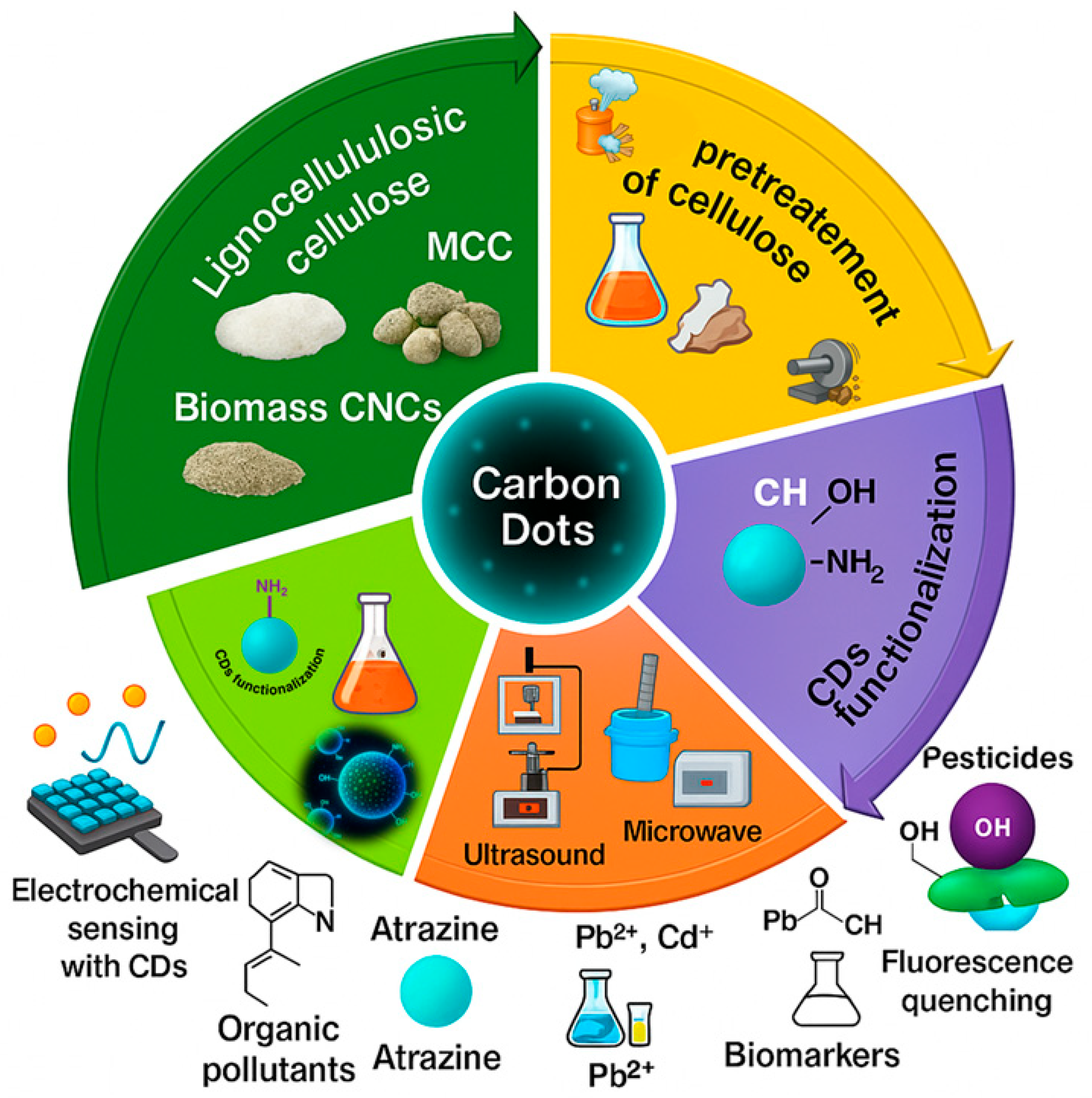
1. Introduction
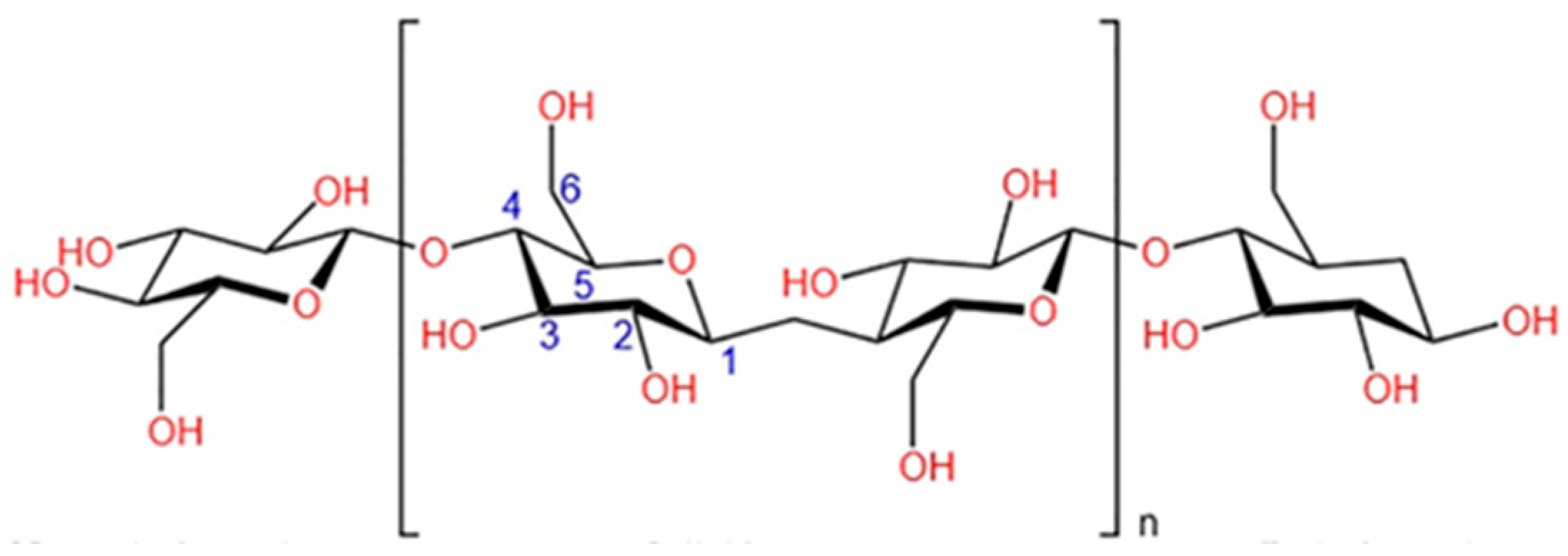
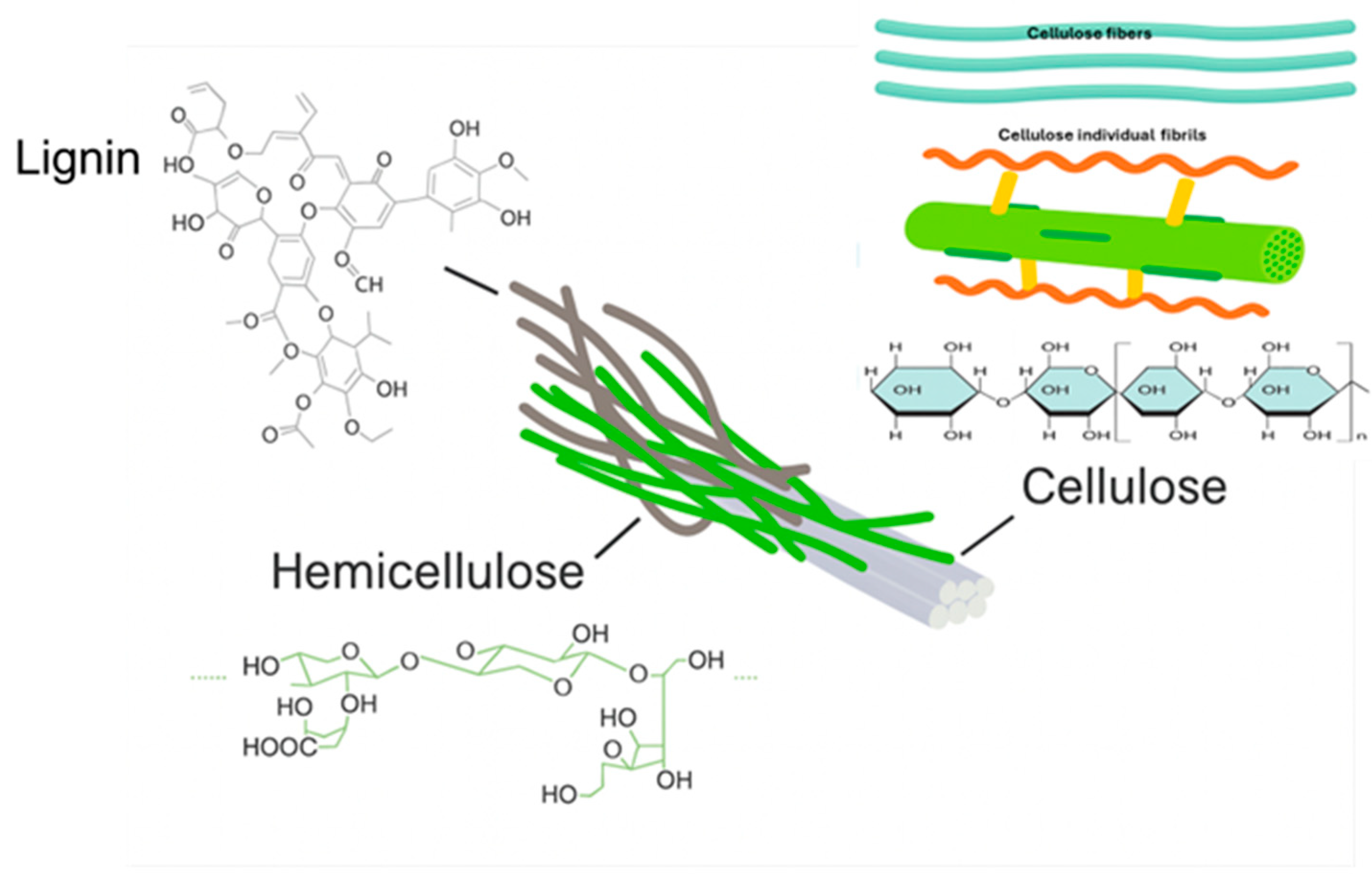
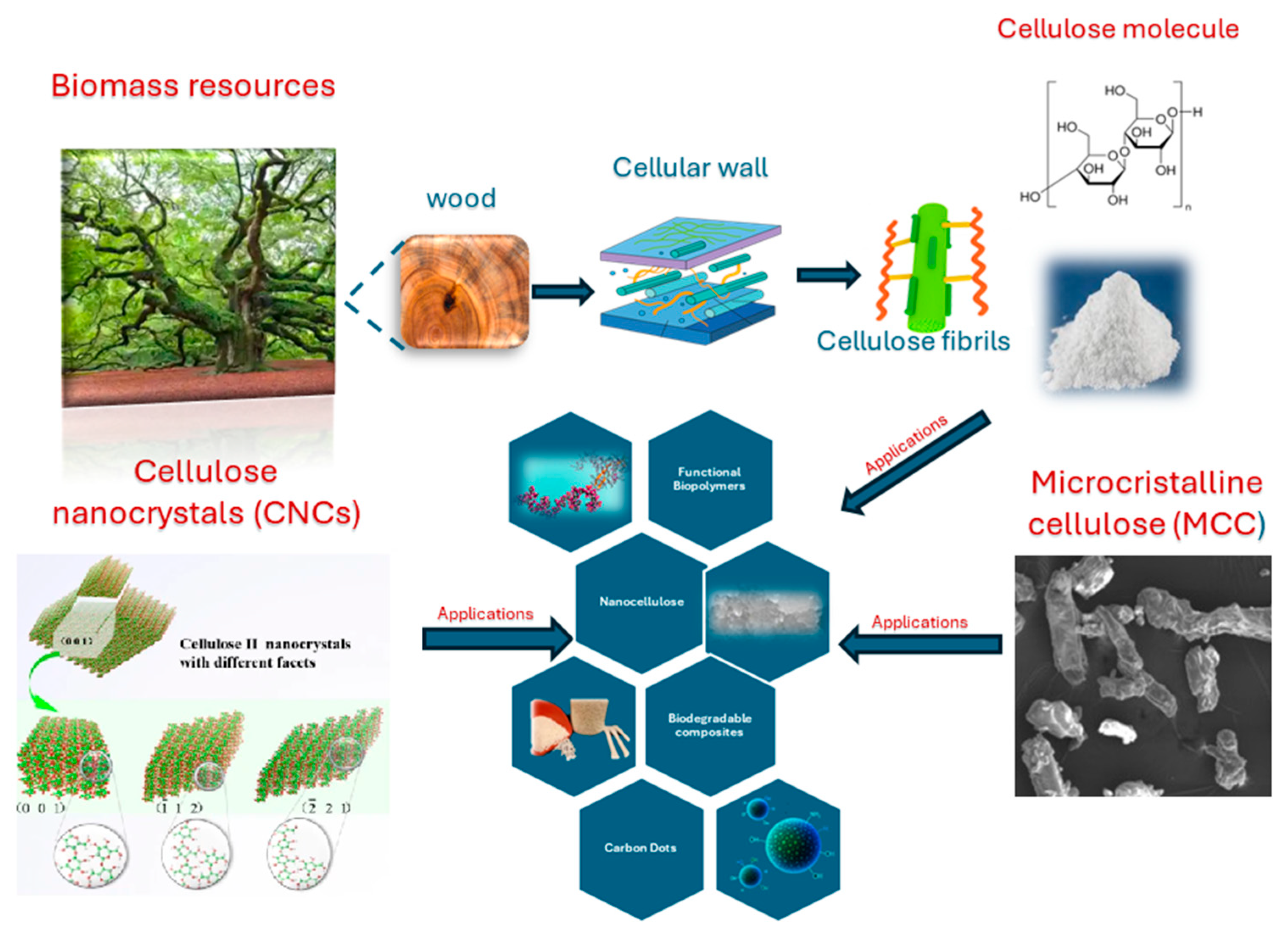
2. Cellulose as a Precursor for Sustainable CDs
2.1. Types and Sources of Cellulose
2.1.1. Microcrystalline Cellulose (MCC)
2.1.2. Nanocellulose
2.1.3. Waste Biomass
2.2. Structural Features Influencing the Carbonization of Cellulose
2.3. Advantages and Limitations of Cellulose as a Precursor for Carbon Dots
3. Synthesis Strategies
3.1. Hydrothermal and Solvothermal Methods
3.2. Microwave-Assisted Synthesis
3.3. Pyrolysis and Combustion Techniques
3.4. Ultrasound-Assisted and Other Green Methods
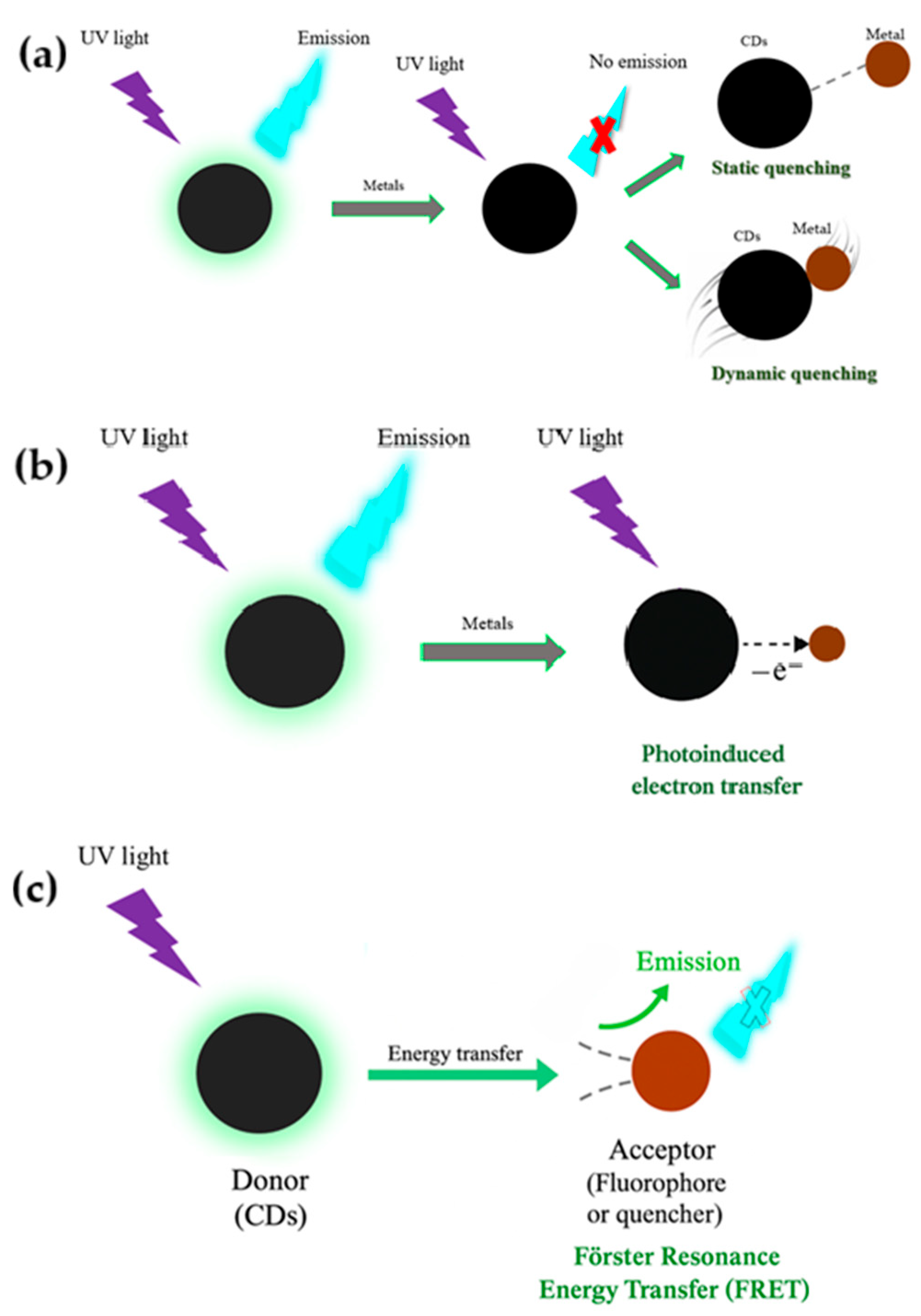
4. Carbon Dots Properties Relevant to Sensing and Sensing Mechanisms
- Förster Resonance Energy Transfer (FRET): A distance-dependent energy transfer process between a donor (CDs) and an acceptor fluorophore or quencher. Effective FRET requires spectral overlap and physical proximity (<10 nm) [84].
- Inner Filter Effect (IFE): Analytes may absorb the excitation or emission light of CDs, leading to apparent quenching without direct interaction [85].
- Static and Dynamic Quenching: Static quenching involves the formation of a non-fluorescent complex between the CD and analyte, whereas dynamic quenching results from collisional encounters [74].
- Photoinduced Electron Transfer: Occurs when excited-state CDs transfer electrons to an electron-deficient analyte, modulating the emission signal [76].
- Dexter energy transfer (DET): happens when the donor and acceptor molecules are so close that their electron orbitals overlap, allowing an electron exchange. Because this overlap is essential, DET only works at sub-nanometer distances and is typical in tightly packed molecular systems [76].
- Surface energy transfer (SET): occurs when an excited fluorophore interacts with the conduction electrons of a nearby metal surface or nanoparticle. In-stead of orbital overlap, it relies on electromagnetic coupling, and its efficiency decreases with the fourth power of distance, making it important in plasmonic and nanoscale sensors [76].
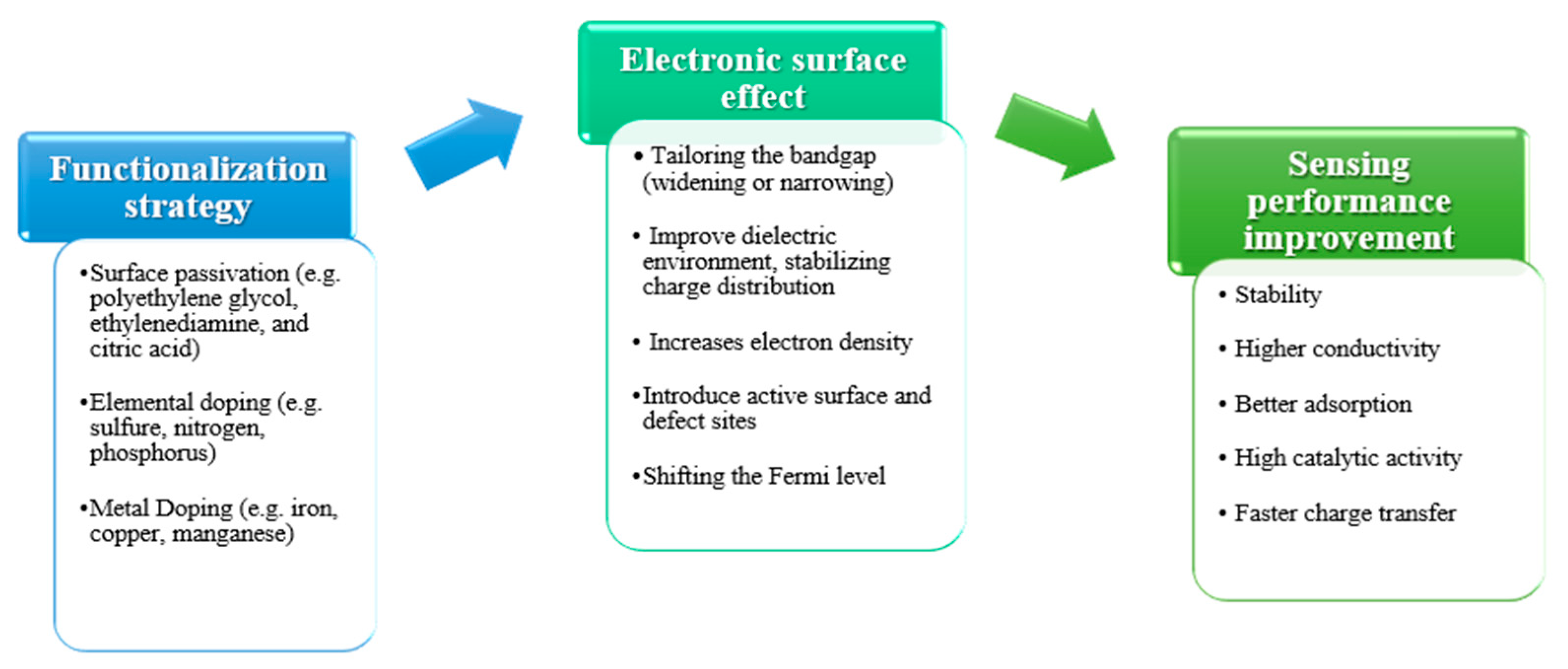
5. Functionalization and Property Tuning
5.1. Surface Passivation
5.2. Elemental Doping
5.3. Metal Doping and Composite Formation
5.4. Tailoring for Target-Specific Sensing
6. Environmental Sensing Applications
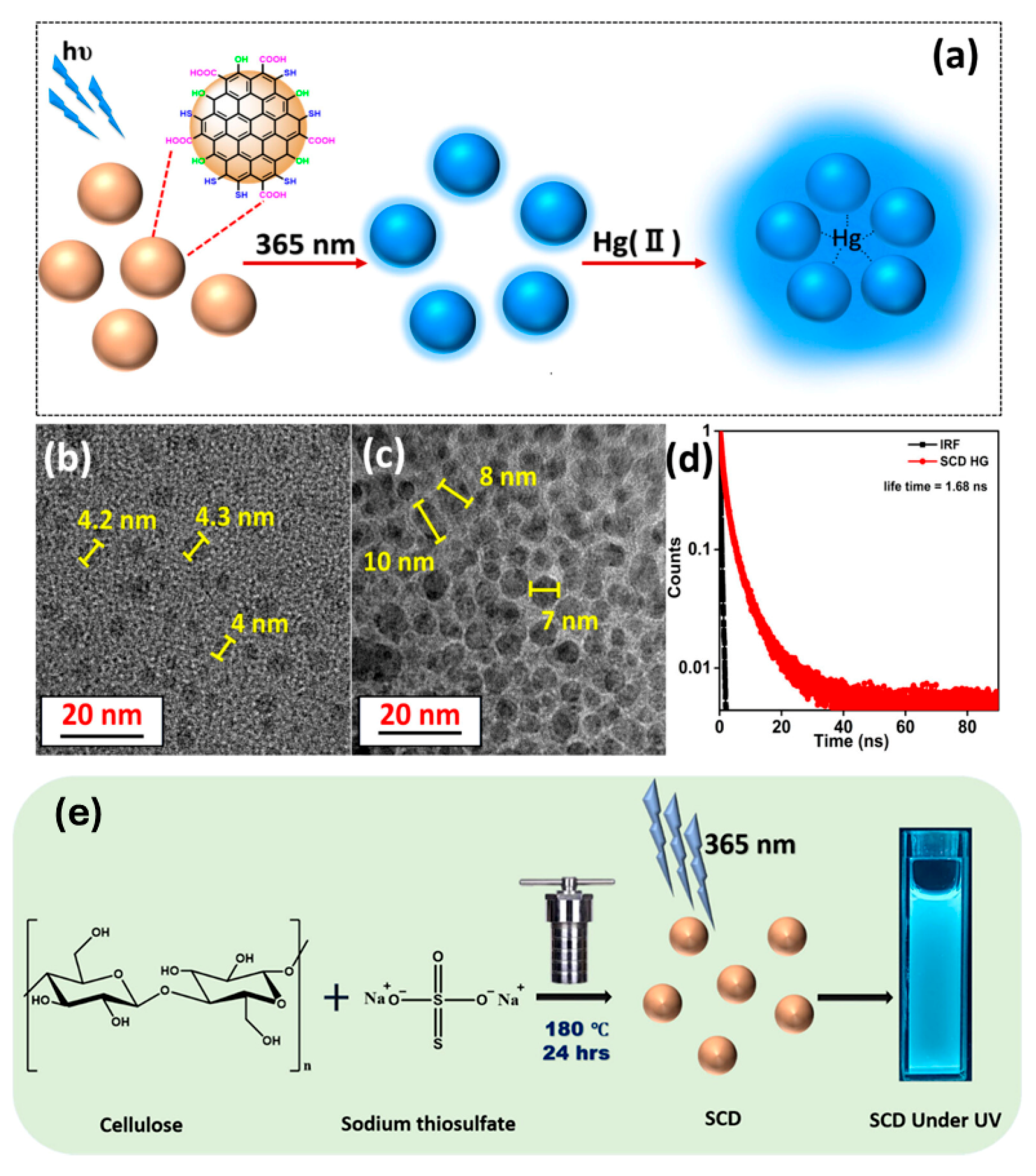
6.1. Detection of Heavy Metals
6.2. Detection of Pesticides
6.3. Detection of Other Pollutants
7. Challenges, Limitations and Future Perspectives
Funding
Data Availability Statement
Conflicts of Interest
References
- Magagula, L.P.; Masemola, C.M.; Ballim, M.A.; Tetana, Z.N.; Moloto, N.; Linganiso, E.C. Lignocellulosic Biomass Waste-Derived Cellulose Nanocrystals and Carbon Nanomaterials: A Review. Int. J. Mol. Sci. 2022, 23, 4310. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, D.; Li, Y.; Ma, X.; Li, J. Green Carbon Quantum Dots from Sustainable Lignocellulosic Biomass and Its Application in the Detection of Fe3+. Cellulose 2022, 29, 367–378. [Google Scholar] [CrossRef]
- Gan, J.; Chen, L.; Chen, Z.; Zhang, J.; Yu, W.; Huang, C.; Wu, Y.; Zhang, K. Lignocellulosic Biomass-Based Carbon Dots: Synthesis Processes, Properties, and Applications. Small 2023, 19, 2304066. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.S.; Brandão, P.; Duarte, S.O.D.; da Silveira, I.B.; Leite, M.d.F.; Gonçalves, M.P.; Borsagli, F.G.L.M.; Fonte, P. Sustainable Carbon Dots Loaded into Carboxymethylcellulose Based Hydrogels for Uterine Cancer Bioimaging. Pharmaceutics 2024, 16, 1500. [Google Scholar] [CrossRef]
- Li, M.; Zhang, P.; Mao, J.; Li, J.; Zhang, Y.; Xu, B.; Zhou, J.; Cao, Q.; Xiao, H. Construction of Cellulose-Based Hybrid Hydrogel Beads Containing Carbon Dots and Their High Performance in the Adsorption and Detection of Mercury Ions in Water. J. Environ. Manag. 2024, 359, 121076. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, A. Carbon Quantum Dots: Synthesis, Properties and Applications. J. Mater. Chem. C 2014, 2, 6921–6939. [Google Scholar] [CrossRef]
- Sheshmani, S.; Mardali, M.; Shokrollahzadeh, S.; Bide, Y.; Tarlani, R. Synthesis, Optical, and Photocatalytic Properties of Cellulose-Derived Carbon Quantum Dots. Sci. Rep. 2025, 15, 19027. [Google Scholar] [CrossRef]
- Tan, X.W.; Romainor, A.N.B.; Chin, S.F.; Ng, S.M. Carbon Dots Production via Pyrolysis of Sago Waste as Potential Probe for Metal Ions Sensing. J. Anal. Appl. Pyrolysis 2014, 105, 157–165. [Google Scholar] [CrossRef]
- Quraishi, S.; Plappert, S.; Ungerer, B.; Taupe, P.; Gindl-Altmutter, W.; Liebner, F. Preparation and Characterization of Bacterial Cellulose-Carbon Dot Hybrid Nanopaper for Potential Sensing Applications. Appl. Sci. 2018, 9, 107. [Google Scholar] [CrossRef]
- Thanjavur, N.; Kim, Y.-J. Illuminating Pollutants: The Role of Carbon Dots in Environmental Sensing. Chemosensors 2025, 13, 241. [Google Scholar] [CrossRef]
- Fan, J.; Kang, L.; Cheng, X.; Liu, D.; Zhang, S. Biomass-Derived Carbon Dots and Their Sensing Applications. Nanomaterials 2022, 12, 4473. [Google Scholar] [CrossRef] [PubMed]
- Hindi, S.S.; Sabir, J.S.M.; Dawoud, U.M.; Ismail, I.M.; Asiry, K.A.; Mirdad, Z.M.; Abo-Elyousr, K.A.; Shiboob, M.H.; Gabal, M.A.; Albureikan, M.O.I.; et al. Nanocellulose-Based Passivated-Carbon Quantum Dots (P-CQDs) for Antimicrobial Applications: A Practical Review. Polymers 2023, 15, 2660. [Google Scholar] [CrossRef]
- Zhao, S.; Song, X.; Chai, X.; Zhao, P.; He, H.; Liu, Z. Green Production of Fluorescent Carbon Quantum Dots Based on Pine Wood and Its Application in the Detection of Fe3+. J. Clean. Prod. 2020, 263, 121561. [Google Scholar] [CrossRef]
- Qi, H.; Teng, M.; Liu, M.; Liu, S.; Li, J.; Yu, H.; Teng, C.; Huang, Z.; Liu, H.; Shao, Q.; et al. Biomass-Derived Nitrogen-Doped Carbon Quantum Dots: Highly Selective Fluorescent Probe for Detecting Fe3+ Ions and Tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef]
- Li, R.; Liang, F.; Hu, X.; Bian, H.; Deng, C.; Seidi, F.; Zhang, B.; Xiao, H.; Liu, Y. A Versatile Cellulose Nanocrystal—Carbon Dots Architecture: Preparation and Environmental/Biological Applications. Carbohydr. Polym. 2022, 298, 120073. [Google Scholar] [CrossRef] [PubMed]
- Passos Zattar, A.P.; Paulo de Mesquita, J.; Pereira, F.V. Luminescent Carbon Dots Obtained from Cellulose and Their Applications as Sensors for Metal Ions. Mater. Chem. Phys. 2022, 290, 126633. [Google Scholar] [CrossRef]
- Huang, H.; Ge, H.; Ren, Z.; Huang, Z.; Xu, M.; Wang, X. Controllable Synthesis of Biocompatible Fluorescent Carbon Dots From Cellulose Hydrogel for the Specific Detection of Hg2+. Front. Bioeng. Biotechnol. 2021, 9, 617097. [Google Scholar] [CrossRef] [PubMed]
- Magagula, L.P.; Masemola, C.M.; Motaung, T.E.; Moloto, N.; Linganiso-Dziike, E.C. Synthesis of Cellulose-Based Fluorescent Carbon Dots for the Detection of Fe(III) in Aqueous Solutions. Processes 2025, 13, 257. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Li, Y.; Li, J. Cellulose Nanocrystals Composites with Excellent Thermal Stability and High Tensile Strength for Preparing Flexible Resistance Strain Sensors. Carbohydr. Polym. Technol. Appl. 2022, 3, 100214. [Google Scholar] [CrossRef]
- Akar, T.; Alim, S.; Meltem, G.; Sayin, F.; Tunali Akar, S. A Novel Sustainable and Eco-Friendly Biosourced Hybrid Sorbent for Toxic Pb2+ Decontamination: Nano Metal Oxide Functionalized Salt-Tolerant Plant Biomass. J. Clean. Prod. 2024, 439, 140838. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, D.; Xu, Q. Emerging dissolving strategy of cellulose nanomaterial for flexible electronics sensors in wearable devices: A review. Cellulose 2024, 31, 27–60. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of Recent Research into Cellulosic Whiskers, Their Properties and Their Application in Nanocomposite Field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef]
- Zhou, X.; Hao, Y.; Zhang, X.; He, X.; Zhang, C. Cellulose-Based Polymers. Phys. Sci. Rev. 2023, 8, 2001–2048. [Google Scholar] [CrossRef]
- Abidi, N. Cellulose Macromolecule as a Source for Advanced Materials Preparation. Mater. Today Proc. 2021, 45, 7473–7476. [Google Scholar] [CrossRef]
- He, W.; Wu, F.; Hong, H. Extraction, Utilization, Functional Modification, and Application of Cellulose and Its Derivatives. J. Renew. Mater. 2025, 13, 1707–1763. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Trache, D.; Hussin, M.H.; Hui Chuin, C.T.; Sabar, S.; Fazita, M.R.N.; Taiwo, O.F.A.; Hassan, T.M.; Haafiz, M.K.M. Microcrystalline Cellulose: Isolation, Characterization and Bio-Composites Application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Ioelovich, M. Cellulose as a Nanostructured Polymer: A Short Review. Bioresources 2008, 3, 1403–1418. [Google Scholar] [CrossRef]
- Magalhães, S.; Fernandes, C.; Pedrosa, J.F.S.; Alves, L.; Medronho, B.; Ferreira, P.J.T.; Rasteiro, M.d.G. Eco-Friendly Methods for Extraction and Modification of Cellulose: An Overview. Polymers 2023, 15, 3138. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. Engl. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, C.; Chang, P.R.; Cao, X.; Anderson, D.P. Bionanocomposites Based on Pea Starch and Cellulose Nanowhiskers Hydrolyzed from Pea Hull Fibre: Effect of Hydrolysis Time. Carbohydr. Polym. 2009, 76, 607–615. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green Composites from Sustainable Cellulose Nanofibrils: A Review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Chen, W.; Li, Q.; Wang, Y.; Yi, X.; Zeng, J.; Yu, H.; Liu, Y.; Li, J. Comparative Study of Aerogels Obtained from Differently Prepared Nanocellulose Fibers. ChemSusChem 2014, 7, 154–161. [Google Scholar] [CrossRef]
- Uetani, K.; Yano, H. Nanofibrillation of Wood Pulp Using a High-Speed Blender. Biomacromolecules 2011, 12, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Wågberg, L.; Decher, G.; Norgren, M.; Lindström, T.; Ankerfors, M.; Axnäs, K. The Build-Up of Polyelectrolyte Multilayers of Microfibrillated Cellulose and Cationic Polyelectrolytes. Langmuir 2008, 24, 784–795. [Google Scholar] [CrossRef]
- Abe, K.; Yano, H. Cellulose Nanofiber-based Hydrogels with High Mechanical Strength. Cellulose 2012, 19, 1907–1912. [Google Scholar] [CrossRef]
- Abe, K.; Yano, H. Formation of Hydrogels from Cellulose Nanofibers. Carbohydr. Polym. 2011, 85, 733–737. [Google Scholar] [CrossRef]
- Prasad, B.R.; Padhi, R.K.; Ghosh, G. A Review on Key Pretreatment Approaches for Lignocellulosic Biomass to Produce Biofuel and Value-Added Products. Int. J. Environ. Sci. Technol. 2023, 20, 6929–6944. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of Lignocellulosic Materials for Ethanol Production: A Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Leng, C.; Li, K.; Tian, Z.; Si, Y.; Huang, H.; Li, J.; Liu, J.; Huang, W.-Q.; Li, K. Theoretical study of cellulose II nanocrystals with different exposed facets. Sci. Rep. 2021, 11, 21871. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, T.; Mehmood, S.; Irshad, M.; Anwar, Z.; Afroz, A.; Zeeshan, N.; Rashid, U.; Sughra, K. Advances in Lignocellulosic Biotechnology: A Brief Review on Lignocellulosic Biomass and Cellulases. Adv. Biosci. Biotechnol. 2014, 5, 246–251. [Google Scholar] [CrossRef]
- Chaudhari, Y.B.; Várnai, A.; Sørlie, M.; Horn, S.J.; Eijsink, V.G.H. Engineering Cellulases for Conversion of Lignocellulosic Biomass. Protein Eng. Des. Sel. 2023, 36, gzad002. [Google Scholar] [CrossRef]
- Advances in the Valorization of Lignocellulosic Materials by Biotechnology: An Overview: BioResources. Available online: https://bioresources.cnr.ncsu.edu/ (accessed on 15 September 2025).
- Chai, Y.D.; Pang, Y.L.; Lim, S.; Chong, W.C.; Lai, C.W.; Abdullah, A.Z. Recent Progress on Tailoring the Biomass-Derived Cellulose Hybrid Composite Photocatalysts. Polymers 2022, 14, 5244. [Google Scholar] [CrossRef]
- Rhim, Y.-R.; Zhang, D.; Fairbrother, D.H.; Wepasnick, K.A.; Livi, K.J.; Bodnar, R.J.; Nagle, D.C. Changes in Electrical and Microstructural Properties of Microcrystalline Cellulose as Function of Carbonization Temperature. Carbon 2010, 48, 1012–1024. [Google Scholar] [CrossRef]
- Long, S.-Y.; Liu, J.-L.; Zhou, L.-Q.; Lv, W.-D.; Xian, X.-Q.; Tang, P.-D.; Du, Q.-S. Study on the Microcrystal Cellulose and the Derived 2D Graphene and Relative Carbon Materials. Sci. Rep. 2023, 13, 23063. [Google Scholar] [CrossRef] [PubMed]
- Plachy, T.; Kutalkova, E.; Skoda, D.; Holcapkova, P. Transformation of Cellulose via Two-Step Carbonization to Conducting Carbonaceous Particles and Their Outstanding Electrorheological Performance. Int. J. Mol. Sci. 2022, 23, 5477. [Google Scholar] [CrossRef]
- Bindhu, D.; Ling, J.; Misnon, I.I.; Yang, C.-C.; Sreekala, C.N.O.; Jose, R. Tetrahedrally Crystallized Carbon from Biowaste-Derived Microcrystalline Cellulose. ACS Sustain. Resour. Manag. 2024, 1, 2128–2135. [Google Scholar] [CrossRef]
- Ma, L.-L.; Liu, W.-J.; Hu, X.; Lam, P.K.S.; Zeng, J.R.; Yu, H.-Q. Ionothermal Carbonization of Biomass to Construct Sp2/Sp3 Carbon Interface in N-Doped Biochar as Efficient Oxygen REDUCTION electrocatalysts. Chem. Eng. J. 2020, 400, 125969. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, M.; Yu, L.; Liu, X.; Wang, J.; Li, N.; Li, L.; Zhou, C. Enhanced Cellular Viability and Osteogenic Activity in Oxygen-Self-Generating and Magnetically Responsive Alginate Microgels as Advanced Cell Carriers. Biomater. Adv. 2025, 170, 214198. [Google Scholar] [CrossRef] [PubMed]
- Ayisha Naziba, T.; Praveen Kumar, D.; Karthikeyan, S.; Sriramajayam, S.; Djanaguiraman, M.; Sundaram, S.; Ghamari, M.; Prasada Rao, R.; Ramakrishna, S.; Ramesh, D. Biomass Derived Biofluorescent Carbon Dots for Energy Applications: Current Progress and Prospects. Chem. Rec. 2024, 24, e202400030. [Google Scholar] [CrossRef]
- Dua, S.; Kumar, P.; Pani, B.; Kaur, A.; Khanna, M.; Bhatt, G. Stability of Carbon Quantum Dots: A Critical Review. RSC Adv. 2023, 13, 13845–13861. [Google Scholar] [CrossRef]
- Cai, D.; Zhong, X.; Xu, L.; Xiong, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Biomass-Derived Carbon Dots: Synthesis, Modification and Application in Batteries. Chem. Sci. 2025, 16, 4937–4970. [Google Scholar] [CrossRef] [PubMed]
- Varadharajan, S.; Vasanthan, K.S.; Mathur, V.; Hariperumal, N.; Mazumder, N. Green Synthesis and Multifaceted Applications: Challenges and Innovations in Carbon Dot Nanocomposites. Discov. Nano 2024, 19, 205. [Google Scholar] [CrossRef]
- Huang, Z.; Ren, L. Large Scale Synthesis of Carbon Dots and Their Applications: A Review. Molecules 2025, 30, 774. [Google Scholar] [CrossRef]
- Rani, R.; Kumar, V.; Rizzolio, F. Fluorescent Carbon Nanoparticles in Medicine for Cancer Therapy: An Update. ACS Med. Chem. Lett. 2018, 9, 4–5. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S. Recent Trends and Advancements in Green Synthesis of Biomass-Derived Carbon Dots. Eng 2024, 5, 2223–2263. [Google Scholar] [CrossRef]
- García-Salcedo, Á.J.; Giraldo-Pinto, L.Á.; Márquez-Castro, D.J.; Tirado-Mejía, L. Influence of Synthesis Parameters on the Optical Properties of Carbon Dots. Carbon Trends 2024, 17, 100403. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, H.; Mi, R.; Min, X.; Fang, M.; Wu, X.; Huang, Z.; Liu, Y. Biomass-Derived Carbon Dots: Preparation, Properties, and Applications. Nanomaterials 2025, 15, 1279. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, C.; Feng, J.; Zhang, L.; Hou, Q.; Ji, X. Enhancing Photocatalytic Destruction of Lignin via Cellulose Derived Carbon Quantum Dots/g-C3N4 Heterojunctions. Int. J. Biol. Macromol. 2024, 260, 129587. [Google Scholar] [CrossRef]
- Bavya, V.; Rajan, T.P.D.; Suresh, K.I. Design of Fluorescence Enhancing Sensor for Mercury Detection via Bamboo Cellulose-Derived Carbon Dots. Langmuir 2025, 41, 1333–1343. [Google Scholar] [CrossRef]
- Rani, Y.; Km, M.P.; Tripathi, P. Curcumin-Derived (3-Aminopropyl)trimethoxysilane-Functionalized Carbon Quantum Dots: A Fluorometric and Colorimetric Nanoprobe for Picric Acid Detection, Antioxidant Activity, and Liposome Encapsulation. ACS Appl. Mater. Interfaces 2024, 16, 68936–68949. [Google Scholar] [CrossRef]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-Assisted Synthesis of Carbon Dots and Their Applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Koteswararao, D.; Bheemudu, P.; Prameela, K. Microwave-Assisted Solid-Phase Synthesis of N-Doped Carbon Dots for the Catalytic Reduction of Malachite Green and Crystal Violet Dyes. React. Kinet. Mech. Catal. 2025. [Google Scholar] [CrossRef]
- Hasan, M.; Baheerathan, B.; Sutradhar, S.; Shahbandinejad, R.; Rakshit, S.; Kozinski, J.; Li, D.; Hu, Y.; Kang, K. Microwave-Assisted Synthesis of Biomass-Derived N-Doped Carbon Dots for Metal Ion Sensing. Carbon Res. 2025, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dev, K.; Bhardwaj, S.; Ramakanth, D.; Singh, K.R.; Poluri, K.M.; Ghosh, K.; Maji, P.K. Biodegradable Cellulose Nanocrystal Composites Doped with Carbon Dots for Packaging and Anticounterfeiting Applications. Nanoscale 2025, 17, 904–918. [Google Scholar] [CrossRef]
- Lee, W.; Ko, S. Synthesis and Characterization of Lignocellulose-Based Carbon Quantum Dots (CQDs) and Their Antimicrobial and Antioxidant Functionalities. Molecules 2025, 30, 667. [Google Scholar] [CrossRef]
- Chen, M.; Zhai, J.; An, Y.; Li, Y.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Liu, C.; Lin, X. Solvent-Free Pyrolysis Strategy for the Preparation of Biomass Carbon Dots for the Selective Detection of Fe3+ Ions. Front. Chem. 2022, 10, 940398. [Google Scholar] [CrossRef] [PubMed]
- Marpongahtun; Andriayani; Muis, Y.; Gea, S.; Amaturrahim, S.A.; Attaurrazaq, B.; Daulay, A. Synthesis of Nitrogen-Doped Carbon Dots from Nanocrystalline Cellulose by Pyrolysis Method as Hg2+ Detector. Int. J. Technol. 2023, 14, 219–231. [Google Scholar] [CrossRef]
- Zaib, M.; Arshad, A.; Khalid, S.; Shahzadi, T. One Pot Ultrasonic Plant Mediated Green Synthesis of Carbon Dots and Their Application Invisible Light Induced Dye Photocatalytic Studies: A Kinetic Approach. Int. J. Environ. Anal. Chem. 2023, 103, 5063–5081. [Google Scholar] [CrossRef]
- Tang, Y.; Shang, S.; Yang, J.; Lü, B.; Peng, F.; Huan, W.; Bian, J. Synthesis and Application of Biomass-Based Carbon Dots in Deep Eutectic Solvent Systems. Ind. Crops Prod. 2025, 224, 120440. [Google Scholar] [CrossRef]
- Noun, F.; Jury, E.A.; Naccache, R. Elucidating the Quenching Mechanism in Carbon Dot-Metal Interactions–Designing Sensitive and Selective Optical Probes. Sensors 2021, 21, 1391. [Google Scholar] [CrossRef]
- van Dam, B.; Nie, H.; Ju, B.; Marino, E.; Paulusse, J.M.J.; Schall, P.; Li, M.; Dohnalová, K. Excitation-Dependent Photoluminescence from Single-Carbon Dots. Small 2017, 13, 1702098. [Google Scholar] [CrossRef]
- Jose, J.; Rangaswamy, M.; Shamnamol, G.K.; Greeshma, K.P. A Short Review on Natural Precursors-Plant-Based Fluorescent Carbon Dots for the Targeted Detection of Metal Ions. Sustain. Chem. Environ. 2024, 7, 100114. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent Advances in Graphene Quantum Dots for Sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Gan, Z.; Xu, H.; Hao, Y. Mechanism for Excitation-Dependent Photoluminescence from Graphene Quantum Dots and Other Graphene Oxide Derivates: Consensus, Debates and Challenges. Nanoscale 2016, 8, 7794–7807. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.B.; Hossain, S.M.; Pramanick, A.K.; Ray, M. Excitation Dependence and Independence of Photoluminescence in Carbon Dots and Graphene Quantum Dots: Insights into the Mechanism of Emission. Nanoscale 2021, 13, 16662–16671. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Yang, Y.; Wang, B.; Chang, J.; Tang, Z.; Yang, B.; Lu, S. Insights into Photoluminescence Mechanisms of Carbon Dots: Advances and Perspectives. Sci. Bull. 2021, 66, 839–856. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence Mechanism of Carbon Dots: Triggering High-Color-Purity Red Fluorescence Emission Through Edge Amino Protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef]
- Bressi, V.; Celesti, C.; Ferlazzo, A.; Len, T.; Moulaee, K.; Neri, G.; Luque, R.; Espro, C. Waste-Derived Carbon Nanodots for Fluorimetric and Simultaneous Electrochemical Detection of Heavy Metals in Water. Environ. Sci. Nano 2024, 11, 1245–1258. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The Quenching of the Fluorescence of Carbon Dots: A Review on Mechanisms and Applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Melle, S.; Calderón, O.G.; Laurenti, M.; Mendez-Gonzalez, D.; Egatz-Gómez, A.; López-Cabarcos, E.; Cabrera-Granado, E.; Díaz, E.; Rubio-Retama, J. Förster Resonance Energy Transfer Distance Dependence from Upconverting Nanoparticles to Quantum Dots. J. Phys. Chem. C 2018, 122, 18751–18758. [Google Scholar] [CrossRef]
- Kumar Panigrahi, S.; Kumar Mishra, A. Inner Filter Effect in Fluorescence Spectroscopy: As a Problem and As a Solution. J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100318. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, N.; Huang, J.; Wang, S.; Qing, T.; Zhang, P.; Feng, B. N, S Co-Doped Carbon Dots for Fluorescent and Colorimetric Dual-Mode Detection of Co(II) in Actual Water. J. Ind. Eng. Chem. 2024, 137, 122–131. [Google Scholar] [CrossRef]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue Luminescent Graphene Quantum Dots and Graphene Oxide Prepared by Tuning the Carbonization Degree of Citric Acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, Q.; Wang, L.; Zhang, J.; Song, Y.; Jin, H.; Zhang, K.; Sun, H.; Wang, H.; Yang, B. Highly Photoluminescent Carbon Dots for Multicolor Patterning, Sensors, and Bioimaging. Angew. Chem. Int. Ed. 2013, 52, 3953–3957. [Google Scholar] [CrossRef]
- Wang, F.; Xie, Z.; Zhang, H.; Liu, C.; Zhang, Y. Highly Luminescent Organosilane-Functionalized Carbon Dots. Adv. Funct. Mater. 2011, 21, 1027–1031. [Google Scholar] [CrossRef]
- Vervald, A.M.; Laptinskiy, K.A.; Khmeleva, M.Y.; Dolenko, T.A. Toward Carbon Dots from Citric Acid and Ethylenediamine, Part 1: Structure, Optical Properties, Main Luminophore at Different Stages of Synthesis. Carbon Trends 2025, 19, 100452. [Google Scholar] [CrossRef]
- Chen, H.; Wu, B.; Ji, S.; Dang, L.; Wei, H.; Wang, Z. Nitrogen-Doped Carbon Dots with High Quantum Yield for the Detection of Fe3+ and Antibacterial Property. Mater. Today Commun. 2025, 47, 113021. [Google Scholar] [CrossRef]
- Tian, Y.; Yue, Y. Phosphor Doped Carbon Dots with High Photoluminescence and Stability Towards pH and Cr(VI) Sensors. Microchem. J. 2024, 207, 112046. [Google Scholar] [CrossRef]
- Venkatesh, Y.; Naidu, P.V.S.; Ramanjaneyulu, M.; Rao, P.A.; Kundrapu, D.B. Synthesis of Multifunctional Sulfur-Nitrogen Co-Doped Carbon Quantum Dots via Facile One-Pot Microwave-Assisted Synthesis: Applications on Antioxidant, Antimicrobial Activities, and Fe3+ Ion Sensing. J. Nanoparticle Res. 2025, 27, 62. [Google Scholar] [CrossRef]
- Jian, K.; Men, W.; Miao, C.; Du, Y.; Yang, H.; Zhao, X. Fe-Doped Carbon Dots with Enhanced Fluorescence: Facilitating Cu2+ Detection and Urea Electro-Oxidation. Talanta 2025, 292, 127942. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, H.; Wan, L.; Gao, H.; Liu, S.; Liu, Y. The Fluorescent and Colorimetric Dual-Response Sensor Based on Carbon Dots Doped with Nitrogen and Sulfur for Detecting Copper Ions. Carbon Lett. 2024, 34, 1155–1164. [Google Scholar] [CrossRef]
- Mondal, T.K.; Kapuria, A.; Miah, M.; Saha, S.K. Solubility Tuning of Alkyl Amine Functionalized Carbon Quantum Dots for Selective Detection of Nitroexplosive. Carbon 2023, 209, 117972. [Google Scholar] [CrossRef]
- Gupta, A.; Chaudhary, A.; Mehta, P.; Dwivedi, C.; Khan, S.; Verma, N.C.; Nandi, C.K. Nitrogen-Doped, Thiol-Functionalized Carbon Dots for Ultrasensitive Hg(II) Detection. Chem. Commun. 2015, 51, 10750–10753. [Google Scholar] [CrossRef]
- Tian, F.; Ma, G.; Zhao, X.; Gao, J.; Zhang, J.; Suo, H.; Zhao, C. The Influence of Carboxyl-Functionalized Carbon Dots on Ethanol Selectivity in Gas Sensing. Chemosensors 2023, 11, 370. [Google Scholar] [CrossRef]
- Du, Z.; Chen, H.; Guo, X.; Qin, L.; Lin, D.; Huo, L.; Yao, Y.; Zhang, Z. Mechanism and Industrial Application Feasibility Analysis on Microwave-Assisted Rapid Synthesis of Amino-Carboxyl Functionalized Cellulose for Enhanced Heavy Metal Removal. Chemosphere 2021, 268, 128833. [Google Scholar] [CrossRef]
- Li, X.; Shi, Q.; Li, M.; Song, N.; Xiao, Y.; Xiao, H.; James, T.D.; Feng, L. Functionalization of Cellulose Carbon Dots with Different Elements (N, B and S) for Mercury Ion Detection and Anti-Counterfeit Applications. Chin. Chem. Lett. 2024, 35, 109021. [Google Scholar] [CrossRef]
- Fan, J.; Kang, L.; Gao, J.; Cheng, X.; Zhang, Q.; Wu, Y. Preparation of Microcrystalline Cellulose-Derived Carbon Dots as a Sensor for Fe3+ Detection. Coatings 2023, 13, 1979. [Google Scholar] [CrossRef]
- Gao, S.; Wang, X.; Xu, N.; Lian, H.; Xu, L.; Zhang, W.; Xu, C. From Coconut Petiole Residues to Fluorescent Carbon Dots via a Green Hydrothermal Method for Fe3+ Detection. Cellulose 2021, 28, 1647–1661. [Google Scholar] [CrossRef]
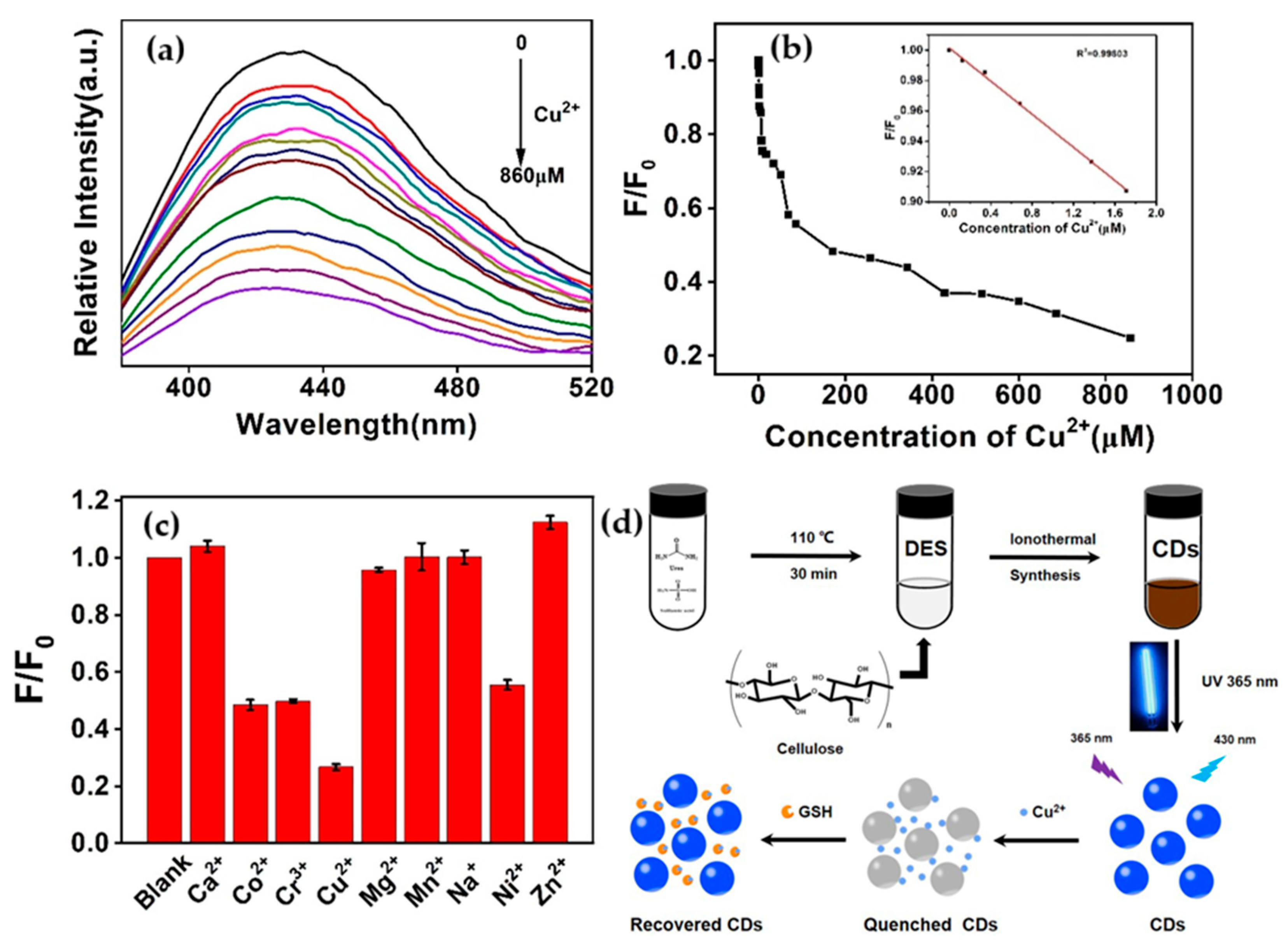
- Wang, Y.; Feng, M.; He, B.; Chen, X.; Zeng, J.; Sun, J. Ionothermal Synthesis of Carbon Dots from Cellulose in Deep Eutectic Solvent: A Sensitive Probe for Detecting Cu2+ and Glutathione with “Off-On” Pattern. Appl. Surf. Sci. 2022, 599, 153705. [Google Scholar] [CrossRef]
- Abdullah Issa, M.; Abidin, Z.Z.; Sobri, S.; Rashid, S.; Adzir Mahdi, M.; Azowa Ibrahim, N.; Pudza, M.Y. Facile Synthesis of Nitrogen-Doped Carbon Dots from Lignocellulosic Waste. Nanomaterials 2019, 9, 1500. [Google Scholar] [CrossRef]
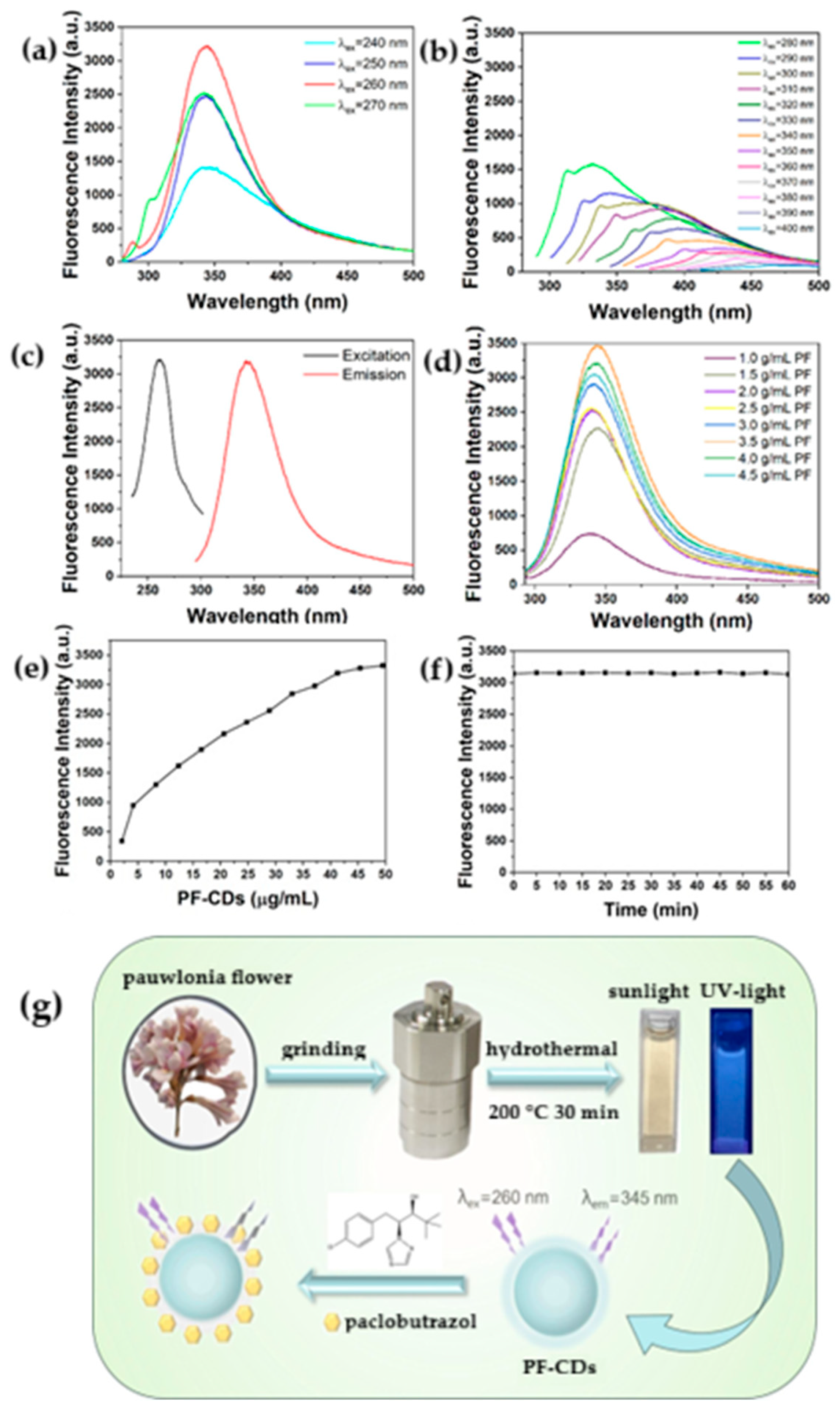
- Zhang, Y.; Dong, P.; Sun, N.; Wang, X.; She, X.; Xu, Y.; Wang, Q.; Zhang, Y. Green Synthesis of Paulownia Flower-Derived Carbon Dots for Sensitive Fluorescent Detection of Paclobutrazol. Food Chem. 2025, 487, 144748. [Google Scholar] [CrossRef]
- Alfi, A.A.; Alamrani, N.A.; Azher, O.A.; Snari, R.M.; Abumelha, H.M.; Al-Ahmed, Z.A.; El-Metwaly, N.M. Development of Carbon Dots Sensor Dipstick from Sugarcane Bagasse Agricultural Waste Toward All-Cellulose-Derived Tetracycline Sensor. J. Mater. Res. Technol. 2022, 19, 4697–4707. [Google Scholar] [CrossRef]
- Meng, X.; Wang, D.; Shen, F.; Hou, J. Preparation of Cellulose-Derived Carbon Dots and Their Applications in Removal and Detection of Silver Ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 343, 126514. [Google Scholar] [CrossRef] [PubMed]
- Cailotto, S.; Amadio, E.; Facchin, M.; Selva, M.; Pontoglio, E.; Rizzolio, F.; Riello, P.; Toffoli, G.; Benedetti, A.; Perosa, A. Carbon Dots from Sugars and Ascorbic Acid: Role of the Precursors on Morphology, Properties, Toxicity, and Drug Uptake. ACS Med. Chem. Lett. 2018, 9, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Mate, N.; Nabeela, K.; Mobin, S.M. A Carbon Dot Anchored Bacterial Cellulose Hybrid Platform as a Fluorescent Sensor and Photocatalytic Remover of Pharmaceuticals. J. Mater. Chem. C 2025, 13, 4691–4701. [Google Scholar] [CrossRef]
| Features | Advantages | Limitations | Ref. |
|---|---|---|---|
| Abundance & Renewability |
|
| [26,42] |
| Eco-Friendliness |
|
| [42,53] |
| Chemical Functional Groups |
|
| [26,54] |
| Biocompatibility |
|
| [11,52] |
| Versatility |
|
| [55,56] |
| Carbon Yield |
|
| [57,58] |
| Reproducibility |
|
| [7,54] |
| Method | Description | Advantages | Critical Issues | Ref. |
|---|---|---|---|---|
| Hydrothermal Solvothermal | Heating cellulose suspensions (150–300 °C) in sealed autoclave; solvothermal involves aqueous–organic solvents |
|
| [75,76] |
| Microwave-Assisted | Rapid heating of cellulose or derivatives under microwave irradiation (300–900 W, 5–20 min) |
|
| [65,69] |
| Pyrolysis/Combustion | Thermal decomposition (300–600 °C) in inert atmosphere (pyrolysis) or open-air (combustion) |
|
| [70,71] |
| Ultrasound-Assisted | Cavitation bubbles create local high T/P, degrading cellulose |
|
| [72,74] |
| Other green methods (e.g., DES, enzymatic hydrolysis) | Deep eutectic solvents (DES) or enzymes under mild T (~110 °C) |
|
| [73] |
| Functionalization Strategy | Effect on Properties | Applications in Electrochemical/Sensing | (Ref.) |
|---|---|---|---|
| Surface Passivation (PEG, EDA, Citric Acid) | Reduces surface traps, increases fluorescence, improves stability and dispersibility. | Enhanced electrochemical stability in aqueous media; better signal reproducibility in biosensing platforms. | [88,89,90] |
| N-Doping | Introduces electron-rich sites, increases QY, improves electron transfer. | Sensitive detection of electron-deficient analytes; enhanced electrochemical current response. | [91] |
| P-Doping | Increases binding affinity to certain metal ions; stabilizes charge transfer. | Selective sensing of metal ions; improved redox activity. | [92] |
| S-Doping and N, S Co-Doping | Introduces mid-gap states; red-shifted emission; generates redox-active sites. | Enhanced antioxidant and electrochemical sensing; tunable emission for multiplexed detection. | [93] |
| Fe-Doping | Creates additional catalytic active sites; improves charge transfer kinetics. | Electrochemical detection of Cu2+; catalytic degradation of urea. | [94] |
| Cu-Doping | Enhances dual-mode sensing via FRET and inner filter effect; improves selectivity. | Dual-mode fluorescence + electrochemical sensing of Cu2+. | [95] |
| Surface Functionalization with Amine Groups (-NH2) | Facilitates electron transfer via hydrogen bonding and chelation with nitroaromatics. | Electrochemical/fluorescence detection of nitroaromatic explosives. | [96] |
| Surface Functionalization with Thiol Groups (-SH) | Strong chelation with Hg2+; improves quenching efficiency. | Ultrasensitive detection of Hg2+ via fluorescence and electrochemical methods. | [97] |
| Surface Carboxyl Groups (-COOH) | Improves dispersibility and electron transfer via hydrogen bonding. | Electrochemical sensing of amines and nitro compounds; improved biocompatibility. | [99,100] |
| Field | Analyte | Source | Doping | Detection Range (µM) | Detection Limit (µM) | Real Sample | Ref. |
|---|---|---|---|---|---|---|---|
| Heavy metals | Hg2+ | Cellulose Hydrogel | Oxygen, Nitrogen, Sulfur Doping | 0.2–100 | ~0.2 | - | [17] |
| Hg2+ | Carboxymethyl Nanocellulose | Amine, Nitrogen Doping | 0–100 | 8.29 | - | [100] | |
| Hg2+ | Bamboo Cellulose | None | 5 × 10−4–1 × 10−3 | 5.16 × 10−3 | Tap water Industry sample | [10] | |
| Fe3+ | Microcrystalline Cellulose | Nitrogen Doping (Polyethylenimine) | 0–250.72 | 3.76 | Tap and pond water | [101] | |
| Fe3+ | Coconut Petiole Residues | None | 5–200 | 2.3 | Tap and lake water | [102] | |
| Heavy metals | Cu2+ | Cellulose (via Ionothermal Approach) | Nitrogen & Sulfur Doping | 0–1.7 | 0.0234 | - | [103] |
| Cu2+ | Lignocellulosic Waste (Oil Palm) | Nitrogen Doping (Carboxymethylcellulose & Polyethylenimine) | 1–30 | 0.93 | Real water samples | [104] | |
| Pesticide | Paclobutrazol | Paulownia Flower (Rich in Cellulose) | None | 2.65–63.83 | 0.017 | Apple juice samples | [105] |
| Other pollutants | Tetracycline (Antibiotic) | Sugarcane Bagasse (Rich in Cellulose) | Nitrogen Doping | 0–110 | 0.01 | - | [106] |
| Silver Ions (Ag+) | Cellulose (carboxymethyl cellulose) | None | 0–200 | 0.01 | Tap water, Yitong river water, South lake sample water | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bressi, V.; Belhaj, J.; Zribi, R.; Khiari, R.; Espro, C. Sustainable Carbon Dots from Cellulose Precursors for Environmental Sensing: Recent Trends and Outlook. Nanomaterials 2025, 15, 1649. https://doi.org/10.3390/nano15211649
Bressi V, Belhaj J, Zribi R, Khiari R, Espro C. Sustainable Carbon Dots from Cellulose Precursors for Environmental Sensing: Recent Trends and Outlook. Nanomaterials. 2025; 15(21):1649. https://doi.org/10.3390/nano15211649
Chicago/Turabian StyleBressi, Viviana, Jihene Belhaj, Rayhane Zribi, Ramzi Khiari, and Claudia Espro. 2025. "Sustainable Carbon Dots from Cellulose Precursors for Environmental Sensing: Recent Trends and Outlook" Nanomaterials 15, no. 21: 1649. https://doi.org/10.3390/nano15211649
APA StyleBressi, V., Belhaj, J., Zribi, R., Khiari, R., & Espro, C. (2025). Sustainable Carbon Dots from Cellulose Precursors for Environmental Sensing: Recent Trends and Outlook. Nanomaterials, 15(21), 1649. https://doi.org/10.3390/nano15211649