Solvent Annealing Influence of PEDOT on Its Electrochemical and Electrochromic Properties
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Electrochemical Tests and Polymerization
2.4. Spectroelectrochemical and Electrochromic Studies
3. Results and Discussion
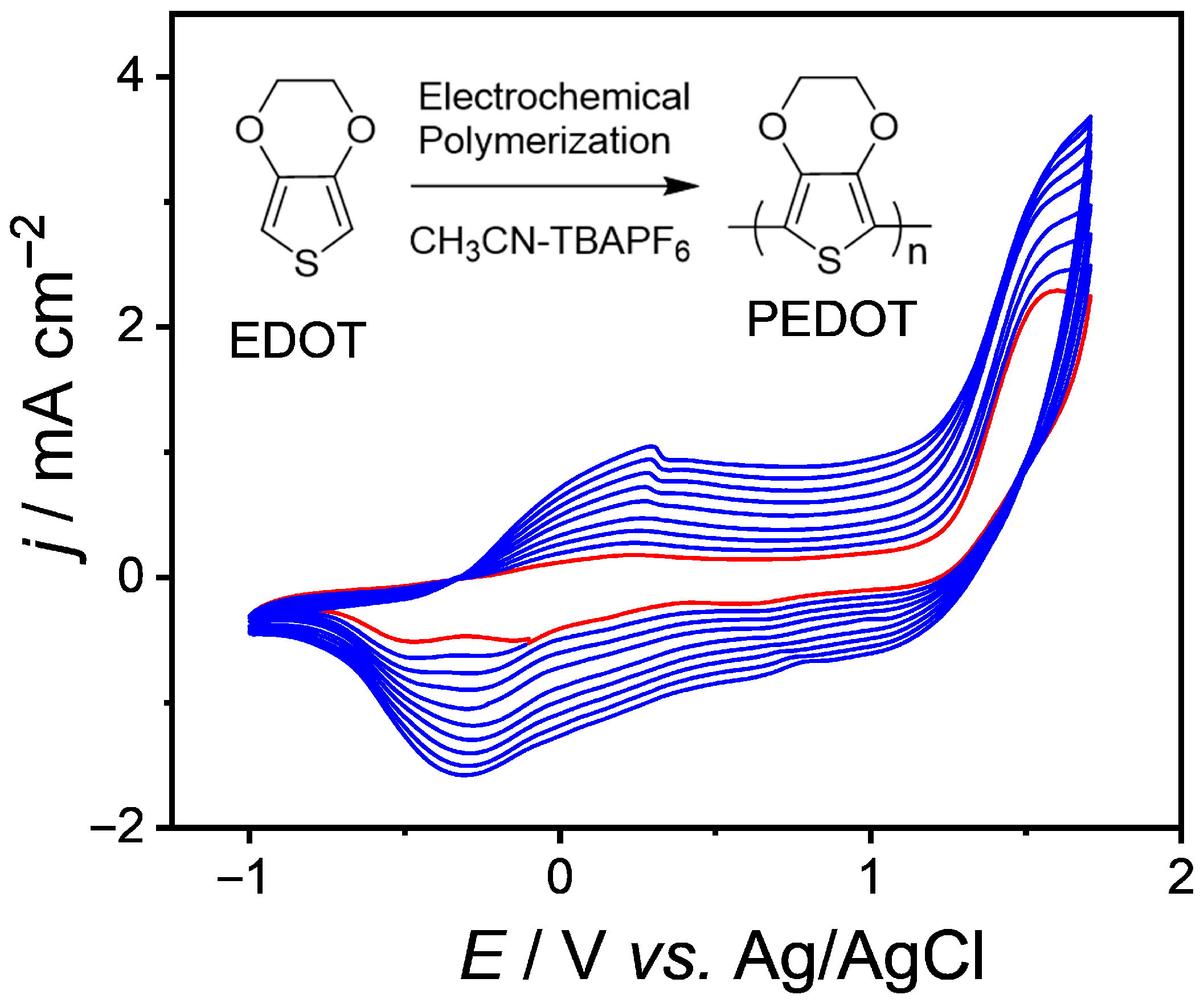
3.1. The Electrochemical Properties of EDOT
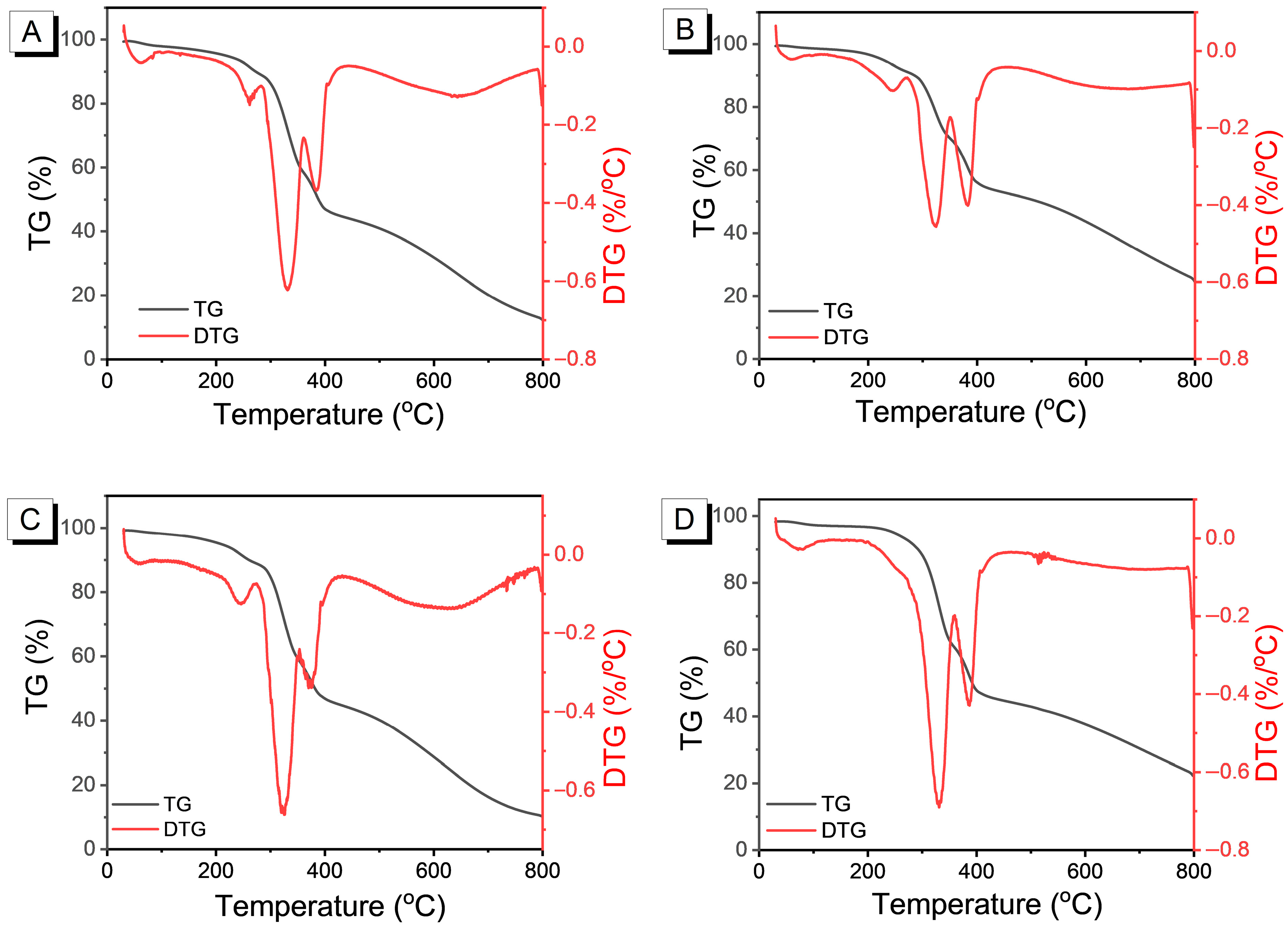
3.2. Thermal Analysis
3.3. Morphology
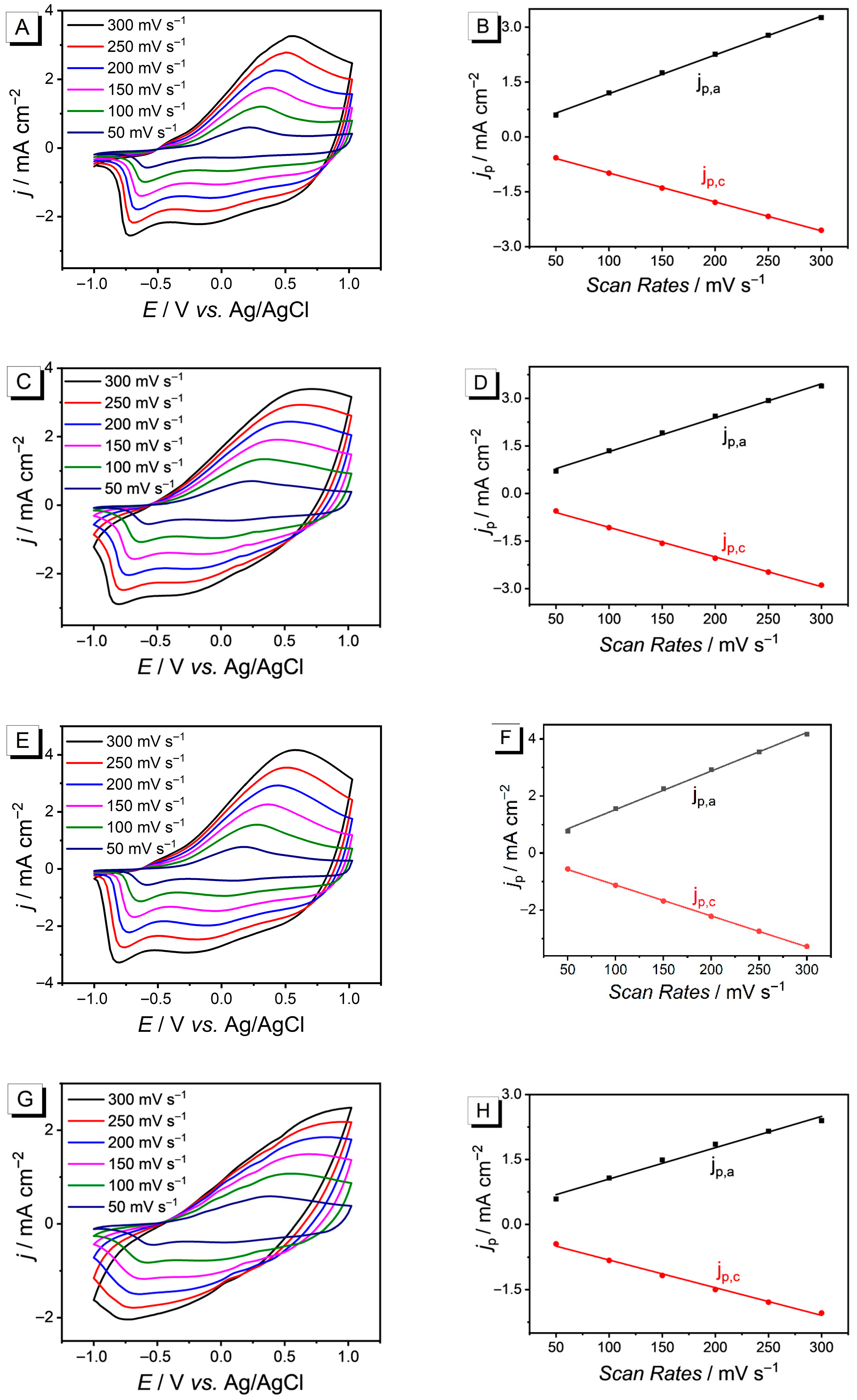
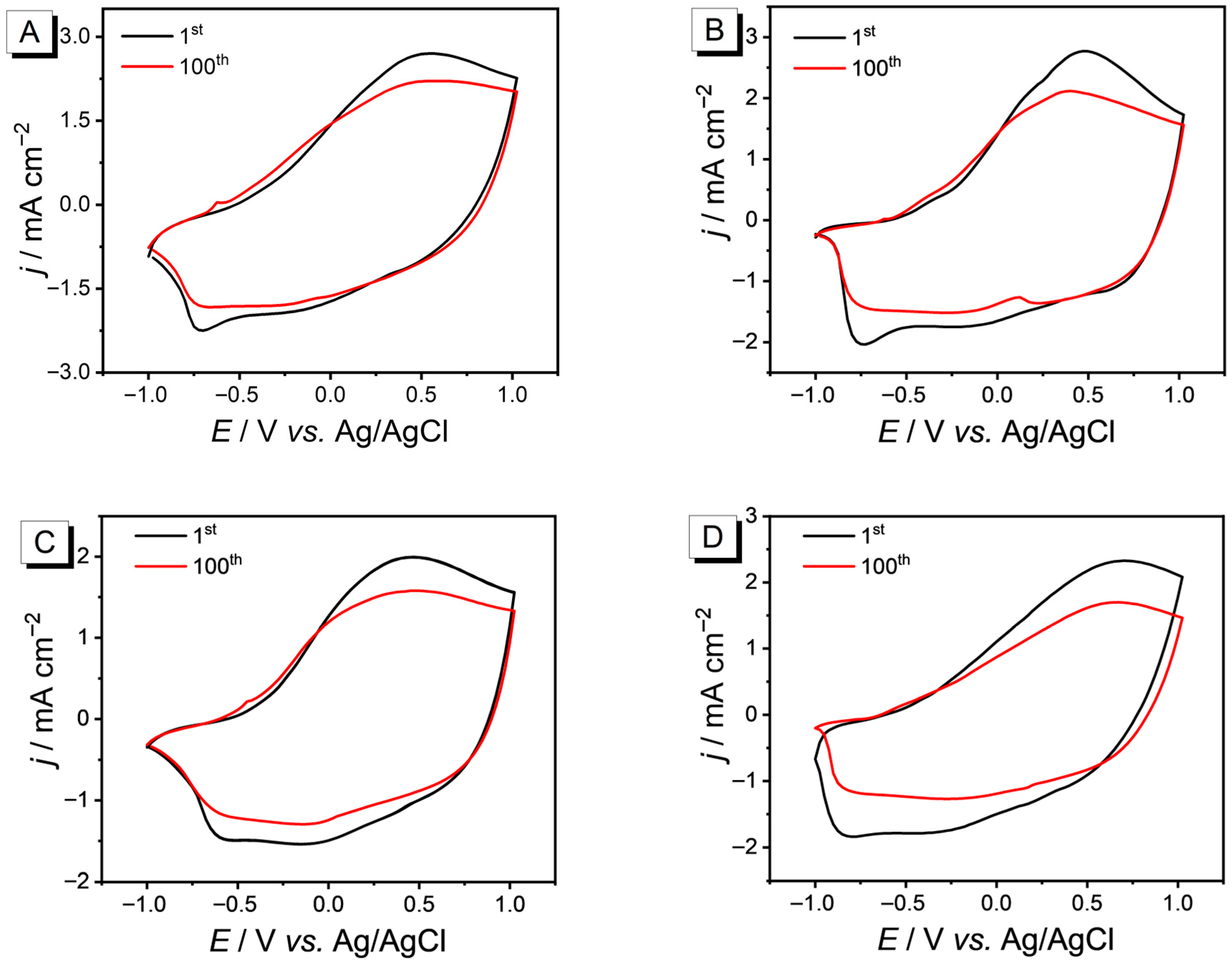
3.4. Redox Activity and Stability of PEDOT
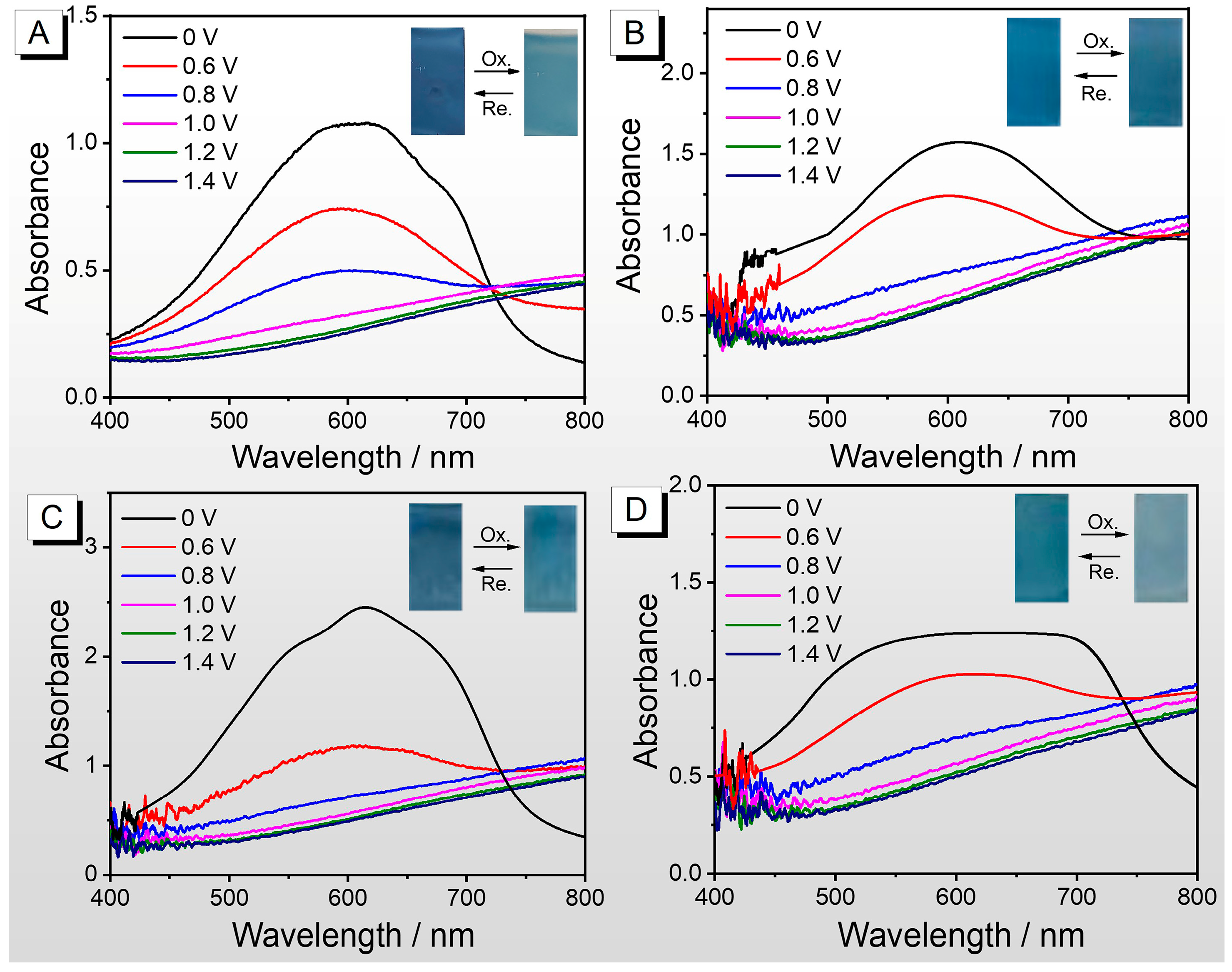
3.5. Electrochromic Performance
3.5.1. Spectroelectrochemistry
3.5.2. Dynamics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.; Wu, R.; Gao, Y.; Zhou, Y.; Zhu, J. Advances of Electrochemical and Electrochemiluminescent Sensors Based on Covalent Organic Frameworks. Nano-Micro Lett. 2024, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, H.; Zhao, Z.; Lin, P.; Yan, F. Flexible Organic Transistors for Biosensing: Devices and Applications. Adv. Mater. 2024, 36, 2300034. [Google Scholar] [CrossRef]
- Gu, C.; Jia, A.; Zhang, Y.; Zhang, S. Emerging Electrochromic Materials and Devices for Future Displays Click to Copy Article Link. Chem. Rev. 2022, 122, 14679–14721. [Google Scholar] [CrossRef]
- Gao, Z.; Fang, C.; Gao, Y.; Yin, X.; Zhang, S.; Lu, J.; Wu, G.; Wu, H.; Xu, B. Hybrid Electromagnetic and Moisture Energy Harvesting Enabled by Ionic Diode Films. Nat. Commun. 2025, 16, 312. [Google Scholar] [CrossRef]
- Li, H.; Cao, J.; Wan, R.; Feig, V.R.; Tringides, C.M.; Xu, J.; Yuk, H.; Lu, B. PEDOTs-Based Conductive Hydrogels: Design, Fabrications, and Applications. Adv. Mater. 2025, 37, 2415151. [Google Scholar] [CrossRef] [PubMed]
- Petsagkourakis, I.; Kim, N.; Tybrandt, K.; Zozoulenko, I.; Crispin, X. Poly(3,4-ethylenedioxythiophene): Chemical Synthesis, Transport Properties, and Thermoelectric Devices. Adv. Electron. Mater. 2019, 5, 1800918. [Google Scholar] [CrossRef]
- Bravo-Plascencia, F.A.; Flores-Morales, M.P.; Kuhn, A.; Salinas, G.; Bernardo, A. Frontana-Uribe Fundamental Concepts of 3,4-Alkoxythiophene-based Polymeric Systems, from Synthesis to Applications. J. Mater. Chem. A 2025, 13, 27772–27793. [Google Scholar] [CrossRef]
- Anastasia, N.; KochevaKonstantin, V.; DeriabinAlexey, I.; VolkovAnna, V.; PonomarevaOleg, V.; LevinRegina, M.I. Flexible, Conductive, and Electrochromic Hybrids Based on Poly(3,4-ethylenedioxythiophene) and Polydimethylsiloxanes. ACS Appl. Polym. Mater. 2025, 7, 11873–11889. [Google Scholar] [CrossRef]
- Chen, S.; Liang, L.; Zhang, Y.; Lin, K.; Yang, M.; Zhu, L.; Yang, X.; Zang, L.; Lu, B. PEDOT:PSS-based Electronic Materials: Preparation, Performance tuning, Processing, Applications, and Future Prospect. Prog. Polym. Sci. 2025, 166, 101990. [Google Scholar] [CrossRef]
- Doshi, S.; Forner, M.; Wang, P.; El Hadwe, S.; Jin, A.T.; Dijk, G.; Brinson, K.; Lim, J.; Dominguez-Alfaro, A.; Lim, C.; et al. Thermal Processing Creates Water-Stable PEDOT:PSS Films for Bioelectronics. Adv. Mater. 2025, 37, 2415827. [Google Scholar] [CrossRef]
- Lenar, N.; Piech, R.; Paczosa-Bator, B. Poly(3,4-ethylenedioxythiophene) and Poly(3-octylthiophene-2,5-diyl) Molecules as Composite Transducers in Potentiometric Sensors—Synthesis and Ap plication. Int. J. Mol. Sci. 2024, 25, 12381. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Berezkin, A.; Tejsner, T.; Posselt, D.; Smilgies, D.; Papadakis, C. Solvent Vapor Annealing of a Diblock Copolymer Thin Film with a Nonselective and a Selective Solvent: Importance of Pathway for the Morphological Changes. Macromol. Rapid Commun. 2020, 41, 2000150. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, T.; Chen, J. Pyridine Vapor Annealing Induced Reversible Switching Emission and Enhanced Electroluminescent Performance of Poly(fluorene-co-dibenzothiophene-S,S-dioxide). Electron. Mater. Lett. 2024, 20, 183–191. [Google Scholar] [CrossRef]
- Shadabroo, M.; Abdizadeh, H.; Golobostanfard, M. Dimethyl Sulfoxide Vapor-Assisted Cs2AgBiBr6 Homogenous Film Deposition for Solar Cell Application. ACS Appl. Energy Mater. 2021, 4, 6797–6805. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, G. Controlling the Pore Size of Mesoporous Carbon Thin Films through Thermal and Solvent Annealing. Small 2017, 13, 1603107. [Google Scholar] [CrossRef]
- Jung, B.; Kim, K.; Eom, Y.; Kim, W. High-Pressure Solvent Vapor Annealing with a Benign Solvent to Rapidly Enhance the Performance of Organic Photovoltaics. ACS Appl. Mater. Interfaces 2015, 7, 13342–13349. [Google Scholar] [CrossRef]
- Khan, H.; Li, R.; Ren, Y.; Chen, L.; Payne, M.; Bhansali, U.; Smilgies, D.; Anthony, J.; Amassian, A. Solvent Vapor Annealing in the Molecular Regime Drastically Improves Carrier Transport in Small-Molecule Thin-Film Transistors. ACS Appl. Mater. Interfaces 2013, 5, 7, 2325–2330. [Google Scholar] [CrossRef]
- Xiao, Z.; Dong, Q.; Bi, C.; Shao, Y.; Yuan, Y.; Huang, J. Solvent Annealing of Perovskite-Induced Crystal Growth for Photovoltaic-Device Effi ciency Enhancement. Adv. Mater. 2014, 26, 6503–6509. [Google Scholar] [CrossRef]
- Huang, J.; Yang, C.; Hsu, C.; Chen, C.; Lin, L.; Wang, R.; Ho, K.; Chu, C. Solvent-Annealing-Induced Self-Organization of Poly(3-hexylthiophene), a High-Performance Electrochromic Material. ACS Appl. Mater. Interfaces 2009, 1, 2821–2828. [Google Scholar] [CrossRef]
- Liang, J.; Zhong, W.; Ying, L.; Yang, W.; Peng, J.; Cao, Y. The Effects of Solvent Vapor Annealing on the Performance of Blue Polymer Light-emitting Diodes. Org. Electron. 2015, 1, 6. [Google Scholar] [CrossRef]
- Neo, W.; Ye, Q.; Shi, Z.; Chua, S.; Xu, J. Control of Morphology and Performance of Diketopyrrolopyrrole-based Electrochromic Polymers using Solvent Vapor Annealing. Polym. Res. 2018, 25, 68. [Google Scholar] [CrossRef]
- Tusy, C.; Jiang, K.; Peng, K.; Huang, L.; Xia, J. Effect of Monomers’ Structure on Self-acid Assisted Polycondensation for the Synthesis of Poly(3,4-ethylenedioxythiophene) and Homopolythiophene. Polym. Chem. 2015, 6, 1014–1022. [Google Scholar] [CrossRef]
- Khodakarimi, S.; Hekhmatshoar, M.; Nasiri, M.; Khaleghi Moghaddam, M.; Abbasi, F. Effects of Process and Post-process Treatments on the Electrical Conductivity of the PEDOT:PSS Films. J. Mater. Sci. Mater. Electron. 2016, 27, 1278–1285. [Google Scholar] [CrossRef]
- Ergun, E.; Hacioglu, S. Electrochromic Insight on Electro-Deposited Copolymers of Push-Pull Conjugated Systems with 3,4-Ethylenedioxythiophene (EDOT) and Other Conjugated Units. Chemistryselect 2023, 8, e202303262. [Google Scholar] [CrossRef]
- Guillen-Bas, E.; Agrisuelas, J.; Garcia-Jareno, J.; Vicente, F. Altered Electrochemistry of Poly(3,4-ethylenedioxythiophene) after Activation of the Inserted Cobalt Ions. J. Electroanal. Chem. 2024, 968, 118440. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Q.; Luo, X.; Ma, H.; Zheng, W.; Yu, J.; Zhang, Z.; Zhang, K.; Qu, K.; Yang, R.; et al. Low-cost Fabrication of High-performance Fluorinated Polythiophene-based Vis–NIR Electrochromic Devices toward Deformable Display and Camouflage. Chem. Mater. 2022, 34, 9923–9933. [Google Scholar] [CrossRef]
- Sanviti, M.; Mester, L.; Hillenbrand, R.; Martínez-Tong, A. Solvent-structured PEDOT:PSS Surfaces: Fabrication Strategies and Nanoscale Properties. Polymer 2022, 246, 124723. [Google Scholar] [CrossRef]
- Wu, Y.; Zou, Y.; Yang, H.; Li, Y.; Li, H.; Cui, C.; Li, Y. Achieving over 9.8% Efficiency in Nonfullerene Polymer Solar Cells by Environmentally Friendly Solvent Processing. ACS Appl. Mater. Interfaces 2017, 9, 37078–37086. [Google Scholar] [CrossRef]
- Zheng, X.; Miao, L.; Song, Z.; Du, W.; Zhu, D.; Lv, Y.; Li, L.; Gan, L.; Liu, M. In Situ Nanoarchitecturing of Conjugated Polyamide Network-derived Carbon Cathodes toward High Energy-power Zn-ion Capacitors. J. Mater. Chem. A 2022, 10, 611–621. [Google Scholar] [CrossRef]
- Minaksh, M.; Nallathamby, K.; Mitchell, D. Electrochemical Characterization of an Aqueous Lithium Rechargeable Battery: The Effect of CeO2 Additions to the MnO2 Cathode. J. Alloys Compd. 2009, 479, 87–90. [Google Scholar] [CrossRef]
- Ming, S.; Li, Z.; Yi, R.; Zhao, J.; Lin, K.; Zhang, H. Influence of Cross-link Density on Electrochromic Properties of Conjugated Polymer. ACS Appl. Polym. Mater. 2024, 6, 7161–7170. [Google Scholar] [CrossRef]
- Jackson, S.; Kingsford, R.; Collins, G.; Bischak, C. Crystallinity Determines Ion Injection Kinetics and Local Ion Density in Organic Mixed Conductors. Chem. Mater. 2023, 35, 5392–5400. [Google Scholar] [CrossRef]
- Lin, K.; Liang, H.; Zheng, Y.; Hu, R.; Chen, H.; Wu, Z.; Zhang, X.; Xie, X.; Wang, Y.; Jiang, Q.; et al. Unraveling the Main Chain Effects of Fused Thiophene Conjugated Polymers in Electrochromism. Soft Sci. 2021, 1, 12. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.; Xu, Y.; Han, Y. Fibrillar Morphology of Derivatives of Poly(3-alkylthiophene)s by Solvent Vapor Annealing: Effects of Conformational Transition and Conjugate Length. Phys. Chem. B 2013, 117, 5996–6006. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Ji, S.; Liu, C.; Craig, G.; Nealey, P. Directed Self-assembly of Solvent-vapor-induced Non-bulk Block Copolymer Morphologies on Nanopatterned Substrates. Soft Matter 2016, 12, 2914–2922. [Google Scholar] [CrossRef] [PubMed]


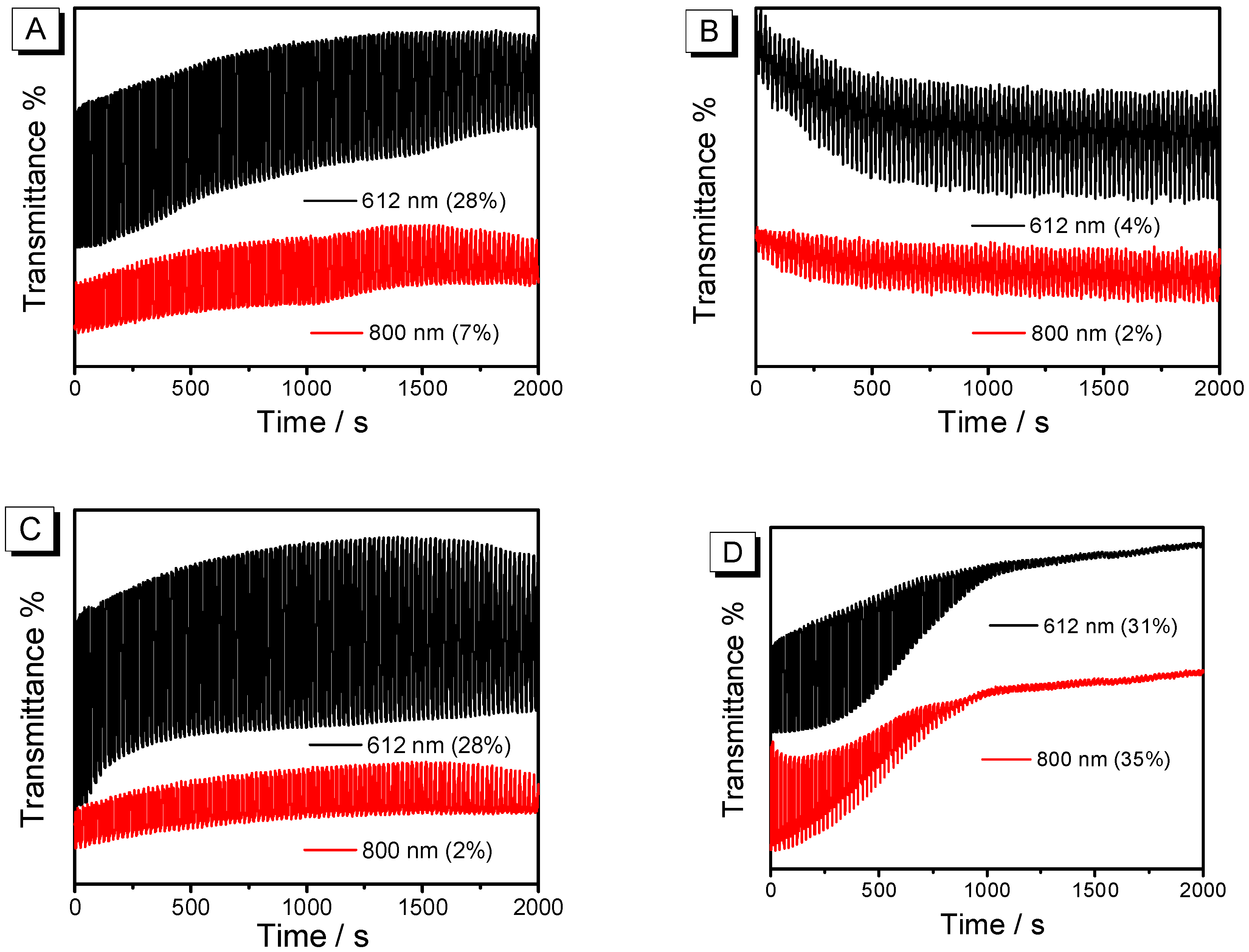

| PEDOT | Wavelength | ∆T (%) | Response Time (s) | CE (cm2 C−1) | ||
|---|---|---|---|---|---|---|
| (nm) | Oxidation | Reduction | Oxidation | Reduction | ||
| Without post-treatment | 612 nm | 28% | 1.1 s | 1.4 s | 64 | 93 |
| Treated with CB | 612 nm | 4% | -- | -- | -- | -- |
| Treated with THF | 612 nm | 28% | 1.1 s | 2 s | 113 | 184 |
| Treated with DMF | 612 nm | 31% | 1.2 s | 1.9 s | 69 | 135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, K.; Jiang, Y.; Chen, Q.; Kong, W.; Luo, R.; Zhou, Q.; Liu, H. Solvent Annealing Influence of PEDOT on Its Electrochemical and Electrochromic Properties. Nanomaterials 2025, 15, 1620. https://doi.org/10.3390/nano15211620
Lin K, Jiang Y, Chen Q, Kong W, Luo R, Zhou Q, Liu H. Solvent Annealing Influence of PEDOT on Its Electrochemical and Electrochromic Properties. Nanomaterials. 2025; 15(21):1620. https://doi.org/10.3390/nano15211620
Chicago/Turabian StyleLin, Kaiwen, Yuying Jiang, Qinran Chen, Wangdaiqi Kong, Ruiyu Luo, Qianhui Zhou, and Hao Liu. 2025. "Solvent Annealing Influence of PEDOT on Its Electrochemical and Electrochromic Properties" Nanomaterials 15, no. 21: 1620. https://doi.org/10.3390/nano15211620
APA StyleLin, K., Jiang, Y., Chen, Q., Kong, W., Luo, R., Zhou, Q., & Liu, H. (2025). Solvent Annealing Influence of PEDOT on Its Electrochemical and Electrochromic Properties. Nanomaterials, 15(21), 1620. https://doi.org/10.3390/nano15211620








