Preparation of ZnCl2-Activated Magnetic Biochar and Its Performance in Removing Hexavalent Chromium from Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
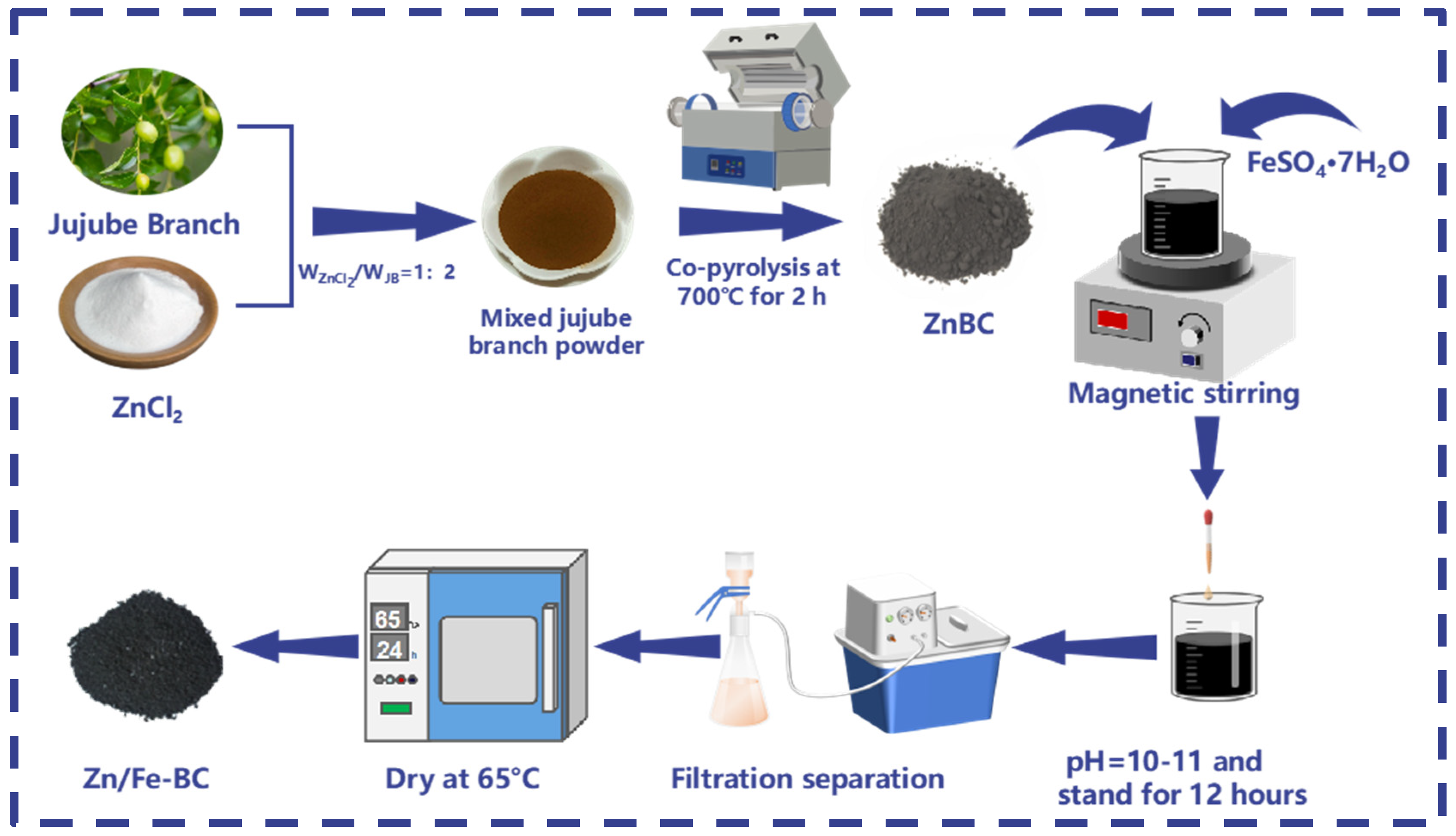
2.2. Preparation of Zn/Fe-BC
2.3. Characterization of Zn/Fe-BC
2.4. Experiments on Cr(VI) Adsorption
2.5. Adsorption Kinetics and Thermodynamics Study
2.6. Isotherm Models
3. Results and Discussion
3.1. Characterization of Biochar
3.1.1. BET Analysis
3.1.2. SEM Analysis
3.1.3. XRD and Raman Spectroscopy Analyses
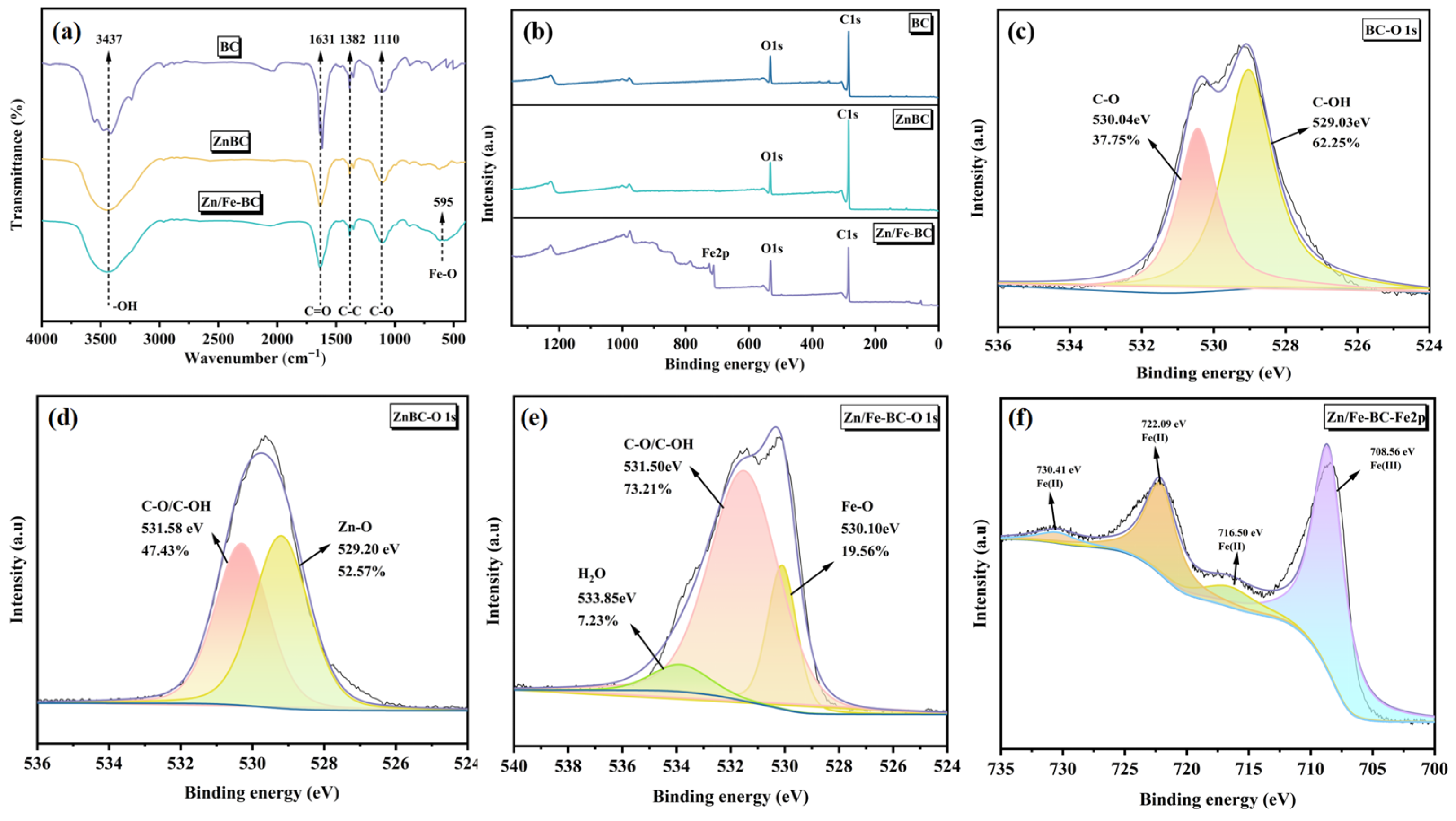
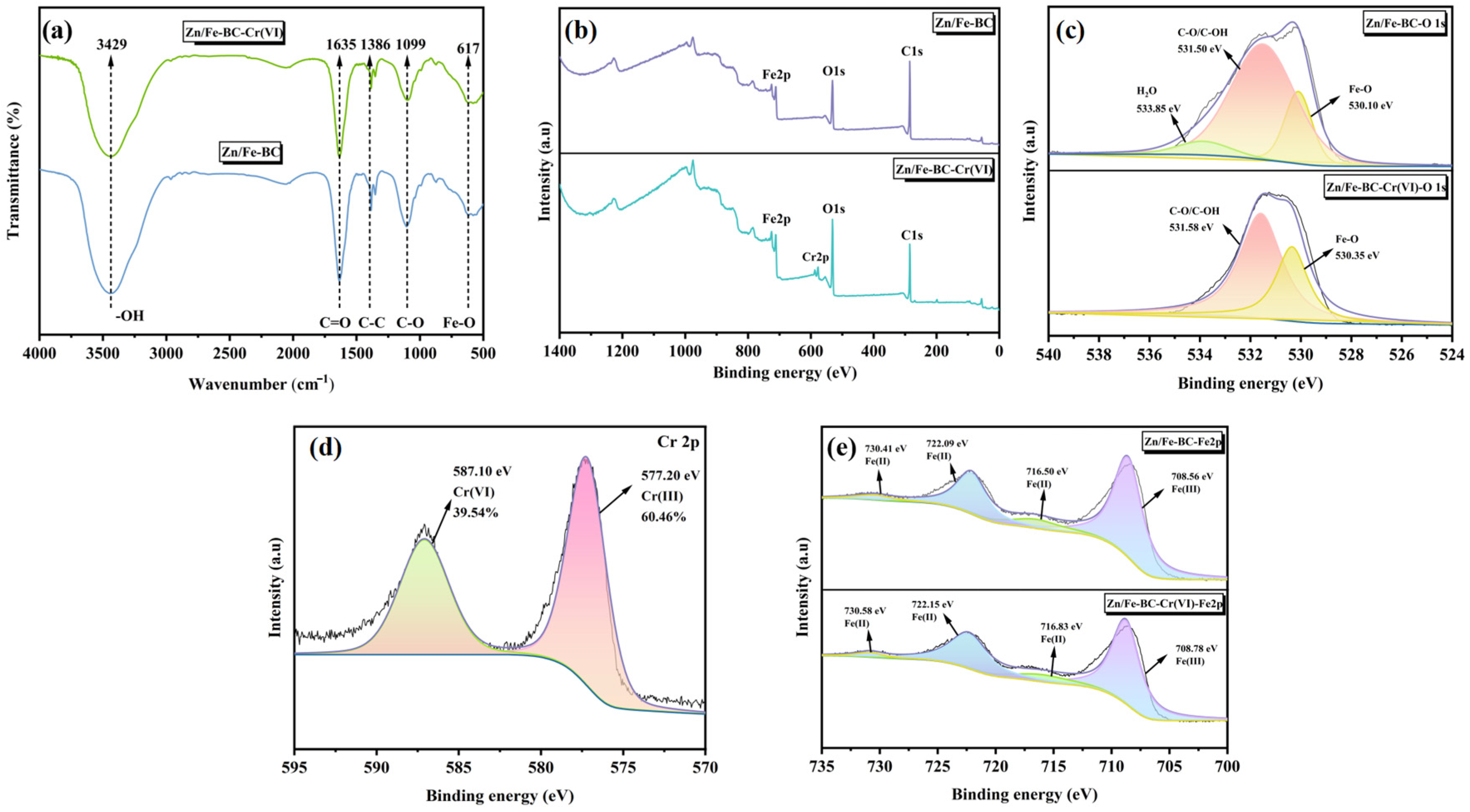
3.1.4. FT-IR and XPS Analyses
3.2. Cr(VI) Adsorption Experiments
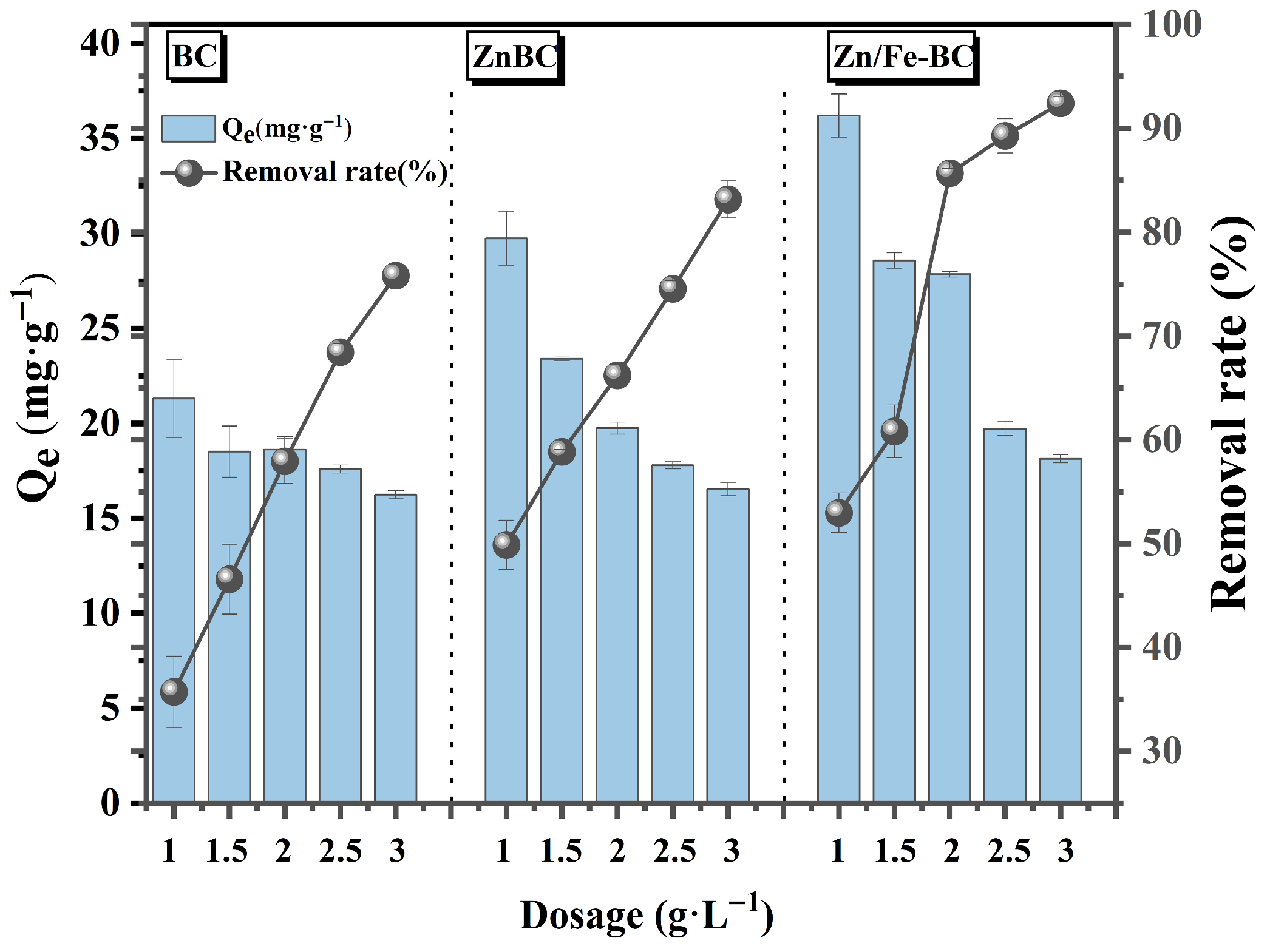
3.2.1. Effects of Adsorbent Dosage
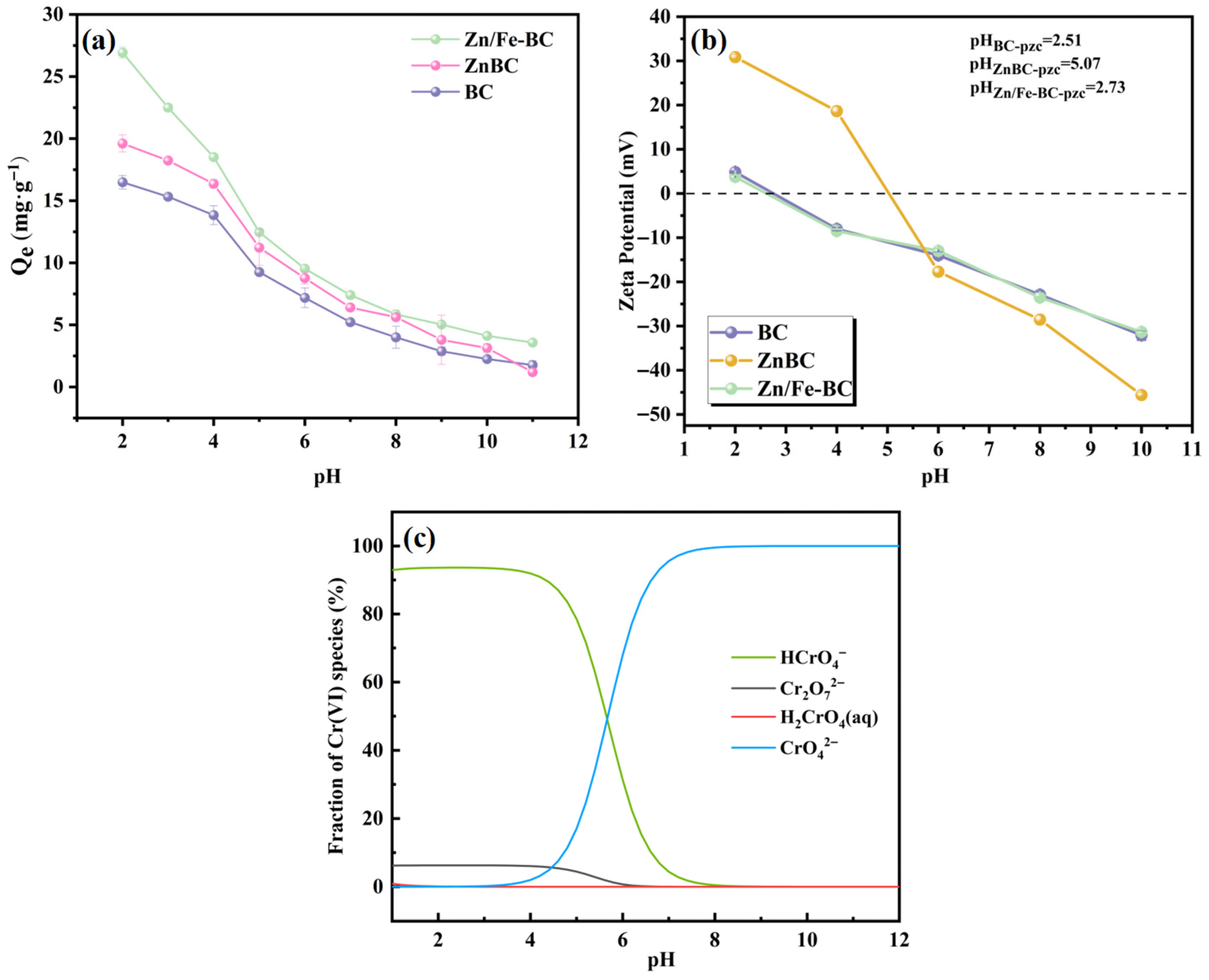
3.2.2. Effects of the Solution pH
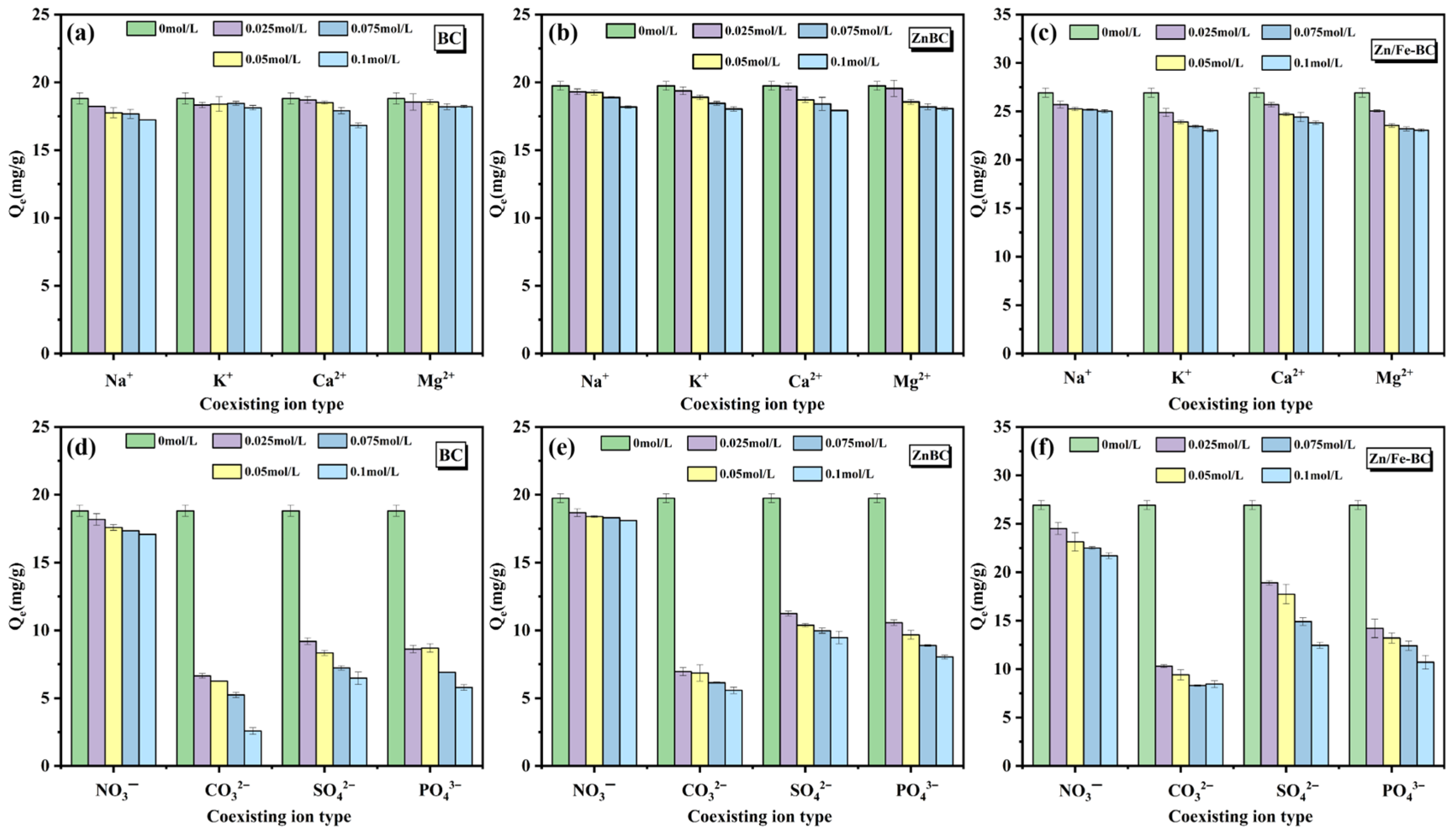
3.2.3. Coexisting Ion Effects
3.3. Adsorption Characteristics of Cr(VI) by BC, ZnBC, and Zn/Fe-BC
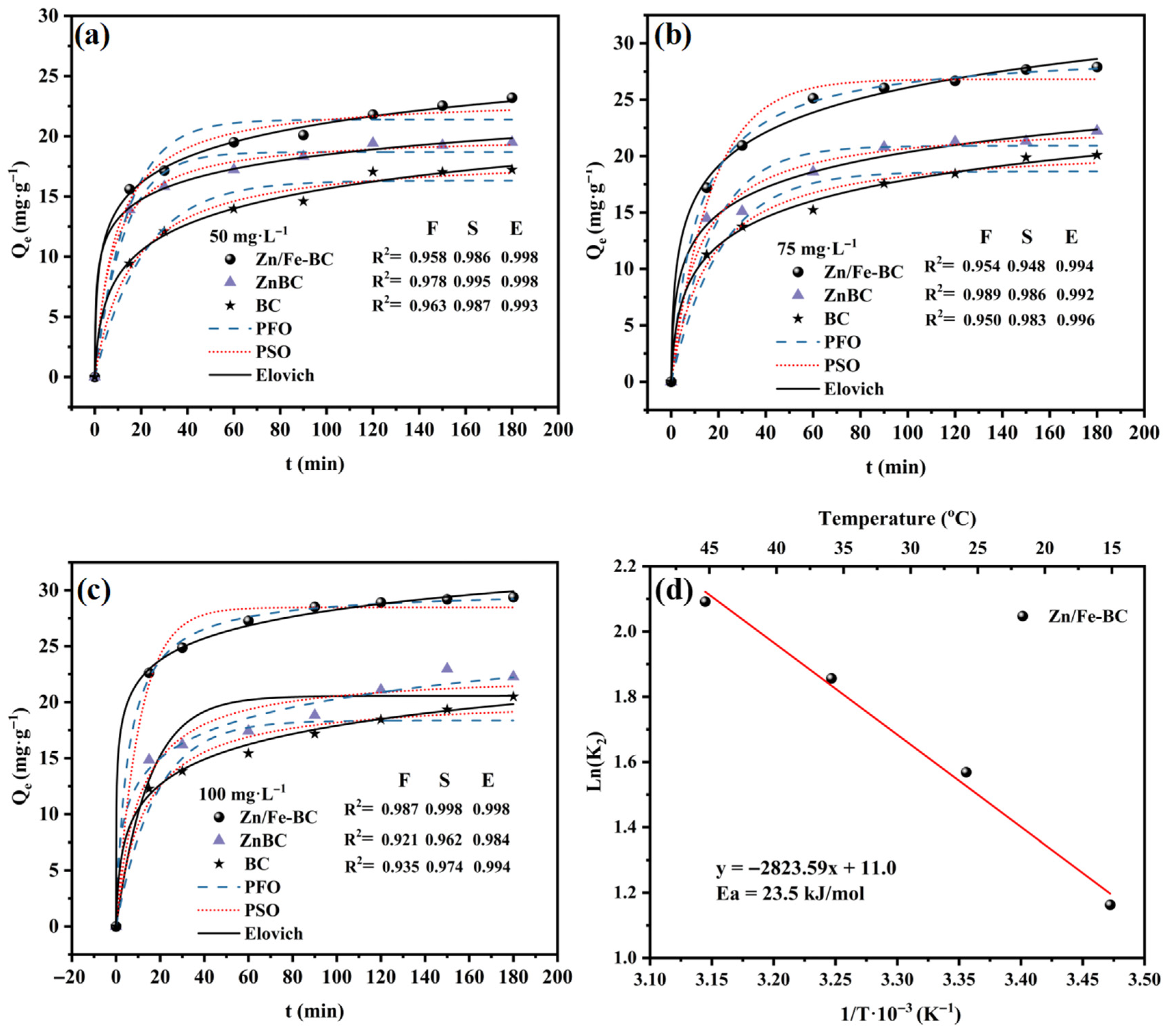
3.3.1. Adsorption Kinetics
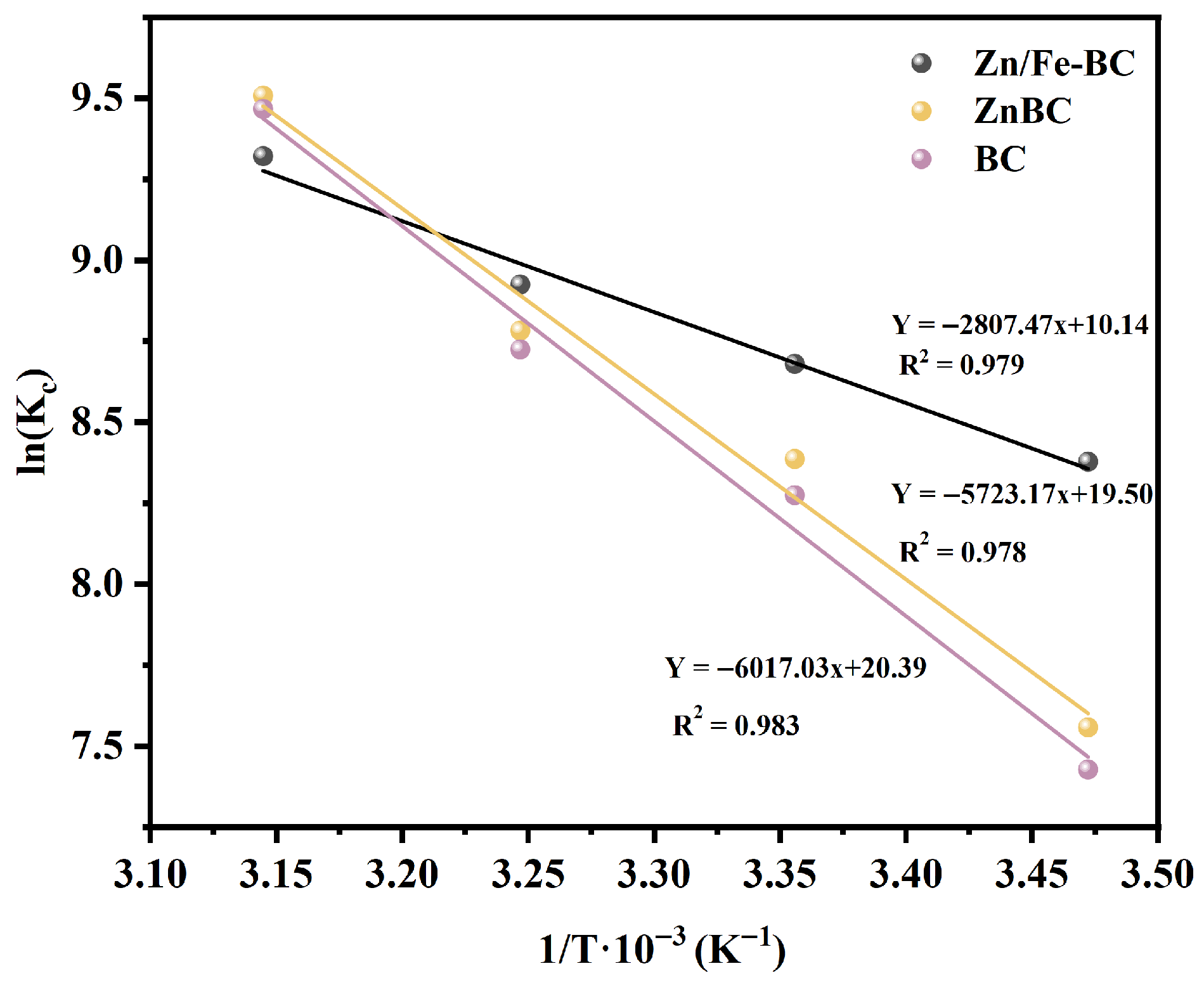
3.3.2. Adsorption Thermodynamics
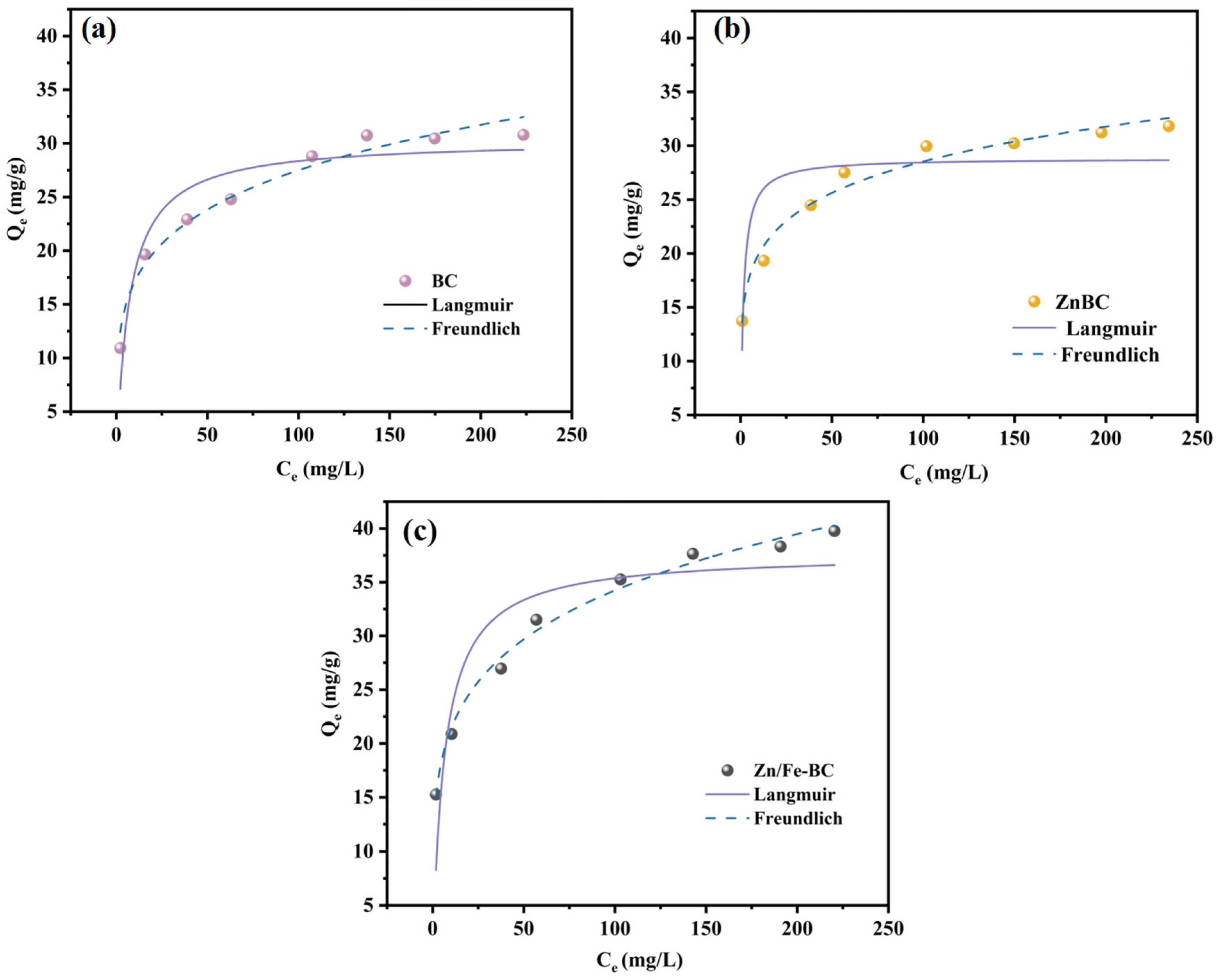
3.3.3. Adsorption Isotherms
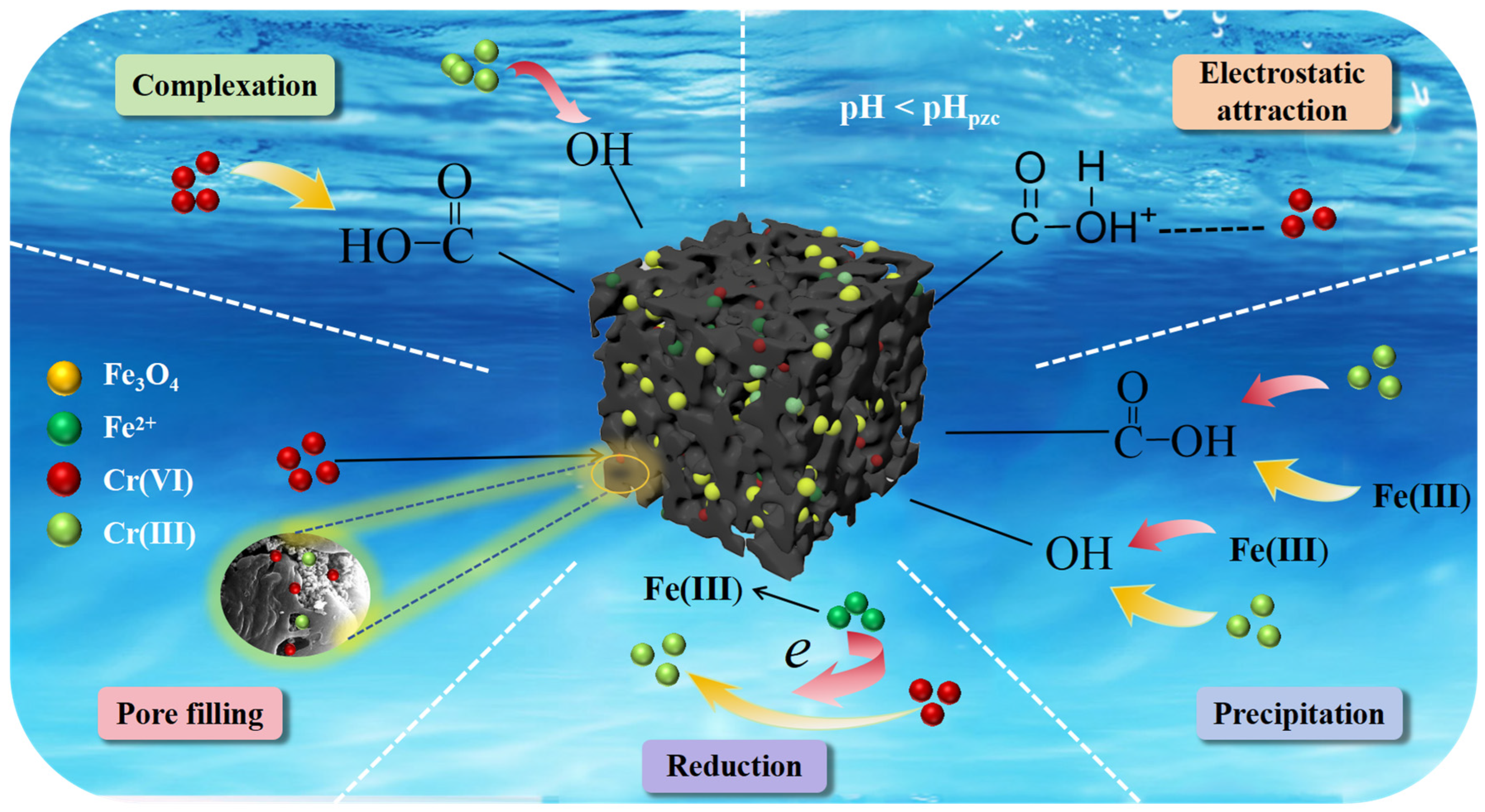
3.3.4. Cr(VI) Removal Mechanisms of Zn/Fe-BC
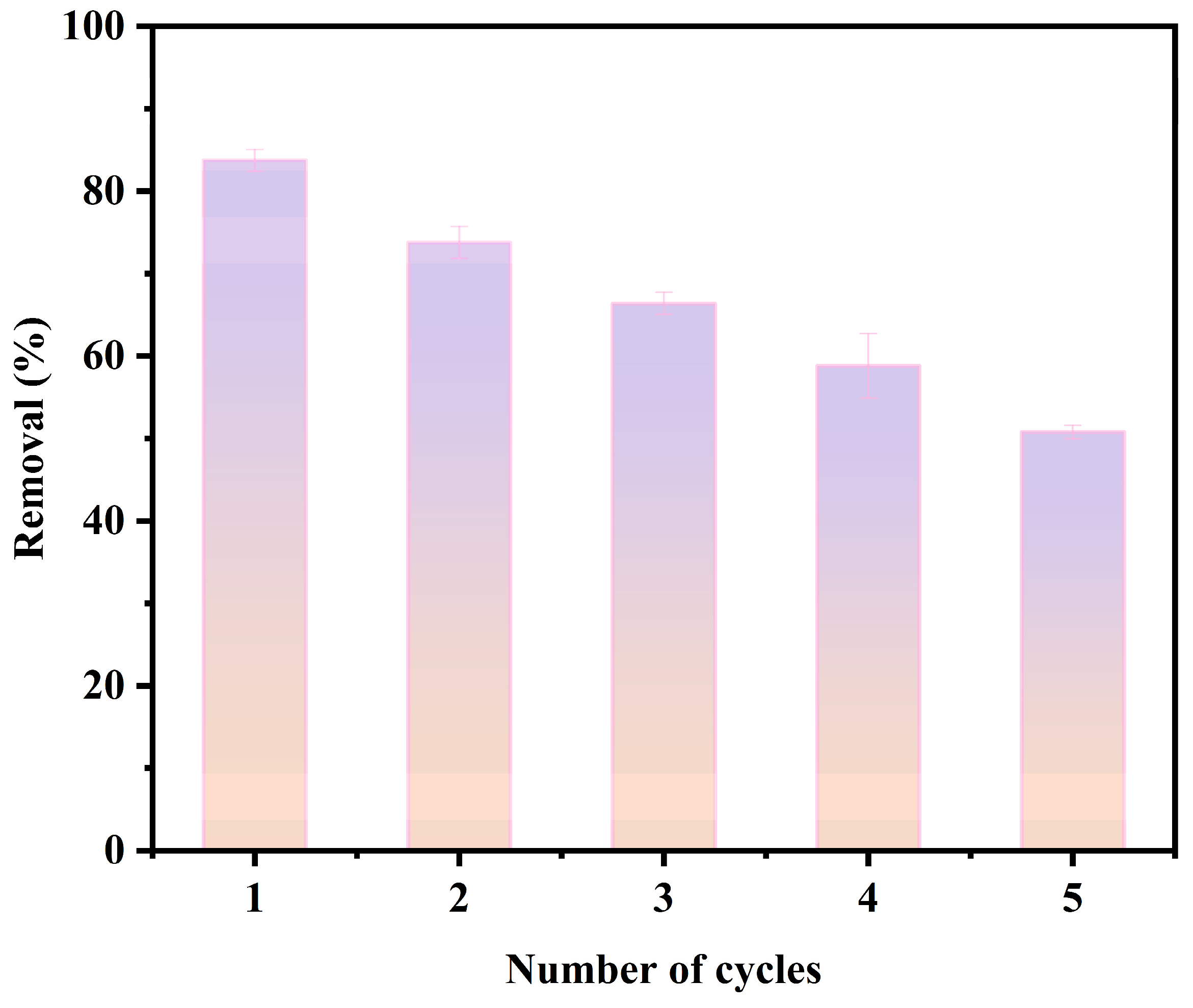
3.3.5. Reusability Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Shan, R.; Lu, L.; Yuan, H.; Jiang, H.; Zhang, Y.; Chen, Y. High-Efficiency Removal of Cr(VI) by Modified Biochar Derived from Glue Residue. J. Clean. Prod. 2020, 254, 119935. [Google Scholar] [CrossRef]
- Kurup, G.; Krishnan, N.; Vaishnav, M.R.; Roopak, A.R.; Nithya, K.; Sathish, A.; Sivamani, S.; Cheruvally, A.S. Competitive Adsorption Studies of MgFe2O4-Biochar Nanocomposites for the Removal of Chromium and Nickel Ions in Single and Binary Metal Ion System. Adsorption 2024, 30, 1805–1827. [Google Scholar] [CrossRef]
- Bharath, G.; Rambabu, K.; Banat, F.; Hai, A.; Arangadi, A.F.; Ponpandian, N. Enhanced Electrochemical Performances of Peanut Shell Derived Activated Carbon and Its Fe3O4 Nanocomposites for Capacitive Deionization of Cr(VI) Ions. Sci. Total Environ. 2019, 691, 713–726. [Google Scholar] [CrossRef]
- Preethi, J.; Vigneshwaran, S.; Meenakshi, S. Performance of Chitosan Engraved Iron and Lanthanum Mixed Oxyhydroxide for the Detoxification of Hexavalent Chromium. Int. J. Biol. Macromol. 2019, 130, 491–498. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, X.; Cen, J.; Shi, P.; Fan, J.; Min, Y.; Xu, Q. A Novel Path to Prepare Fe/Al–Layered Double Hydroxide Nanosheets by Sacrificial Double Anodes for the Treatment of Cr-Containing Wastewater. J. Colloid Interface Sci. 2019, 542, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Hanif, A.; Ali, S.; Hanif, M.A.; Rashid, U.; Bhatti, H.N.; Asghar, M.; Alsalme, A.; Giannakoudakis, D.A. A Novel Combined Treatment Process of Hybrid Biosorbent–Nanofiltration for Effective Pb(II) Removal from Wastewater. Water 2021, 13, 3316. [Google Scholar] [CrossRef]
- Mi, B.; Wang, Y. Performance and Mechanism of Porous Carbons Derived from Biomass as Adsorbent for Removal of Cr(VI). Processes 2024, 12, 2229. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.; Liu, D.; Li, T.; Yu, G. Preparation of Aminated Cross-Linked Chitosan Beads for Efficient Adsorption of Hexavalent Chromium. Int. J. Biol. Macromol. 2019, 139, 352–360. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Hassani, A.; Bhatnagar, A. Facile Hydrothermal Synthesis of Novel Fe-Cu Layered Double Hydroxide/Biochar Nanocomposite with Enhanced Sonocatalytic Activity for Degradation of Cefazolin Sodium. J. Hazard. Mater. 2020, 381, 120742. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, N.; Liu, P.; Liu, Y.Y.; Lei, Y. Molecular-Level Investigation on Removal Mechanisms of Aqueous Hexavalent Chromium by Pine Needle Biochar. Arab. J. Chem. 2023, 16, 104966. [Google Scholar] [CrossRef]
- Dahiya, A.; Bhardwaj, A.; Rani, A.; Arora, M.; Babu, J.N. Reduced and Oxidized Rice Straw Biochar for Hexavalent Chromium Adsorption: Revisiting the Mechanism of Adsorption. Heliyon 2023, 9, e21735. [Google Scholar] [CrossRef]
- Choi, K.; Yong Lee, S.; Kim, H.; Bong Lee, K.; Choi, J.W.; Jung, K.W. Mechanistic Insights into the Simultaneous Removal of As(V) and Cr(VI) Oxyanions by a Novel Hierarchical Corolla-like MnO2-Decorated Porous Magnetic Biochar Composite: A Combined Experimental and Density Functional Theory Study. Appl. Surf. Sci. 2022, 578, 151991. [Google Scholar] [CrossRef]
- Zou, Y.; Yang, W.; Chen, Y.; Zhang, J.; Wang, B.; Niazi, N.K.; Yang, L.; Wang, S.; Zhou, H.; Wu, P. Efficient Removal of Tetracycline from Wastewater via KHCO3-Activated Biochar: Characterization, Performance, and Mechanism. J. Water Process Eng. 2024, 68, 106450. [Google Scholar] [CrossRef]
- Hu, J.; Shen, D.; Wu, S.; Xiao, R. Insight into the Effect of ZnCl2 on Analytical Pyrolysis Behavior of Cellulolytic Enzyme Corn Stover Lignin. J. Anal. Appl. Pyrolysis 2017, 127, 444–450. [Google Scholar] [CrossRef]
- Ahmad Wani, A.; Shahadat, M.; Wazed Ali, S.; Ziauddin Ahammad, S.; Kashif Uddin, M. Recent Advances and Future Perspectives of Polymer-Based Magnetic Nanomaterials for Detection and Removal of Radionuclides: A Review. J. Mol. Liq. 2022, 365, 119976. [Google Scholar] [CrossRef]
- Ding, K.; Zhou, X.; Hadiatullah, H.; Lu, Y.; Zhao, G.; Jia, S.; Zhang, R.; Yao, Y. Removal Performance and Mechanisms of Toxic Hexavalent Chromium (Cr(VI)) with ZnCl2 Enhanced Acidic Vinegar Residue Biochar. J. Hazard. Mater. 2021, 420, 126551. [Google Scholar] [CrossRef]
- Zheng, Z.; Duan, X.; Tie, J. One-Pot Synthesis of a Magnetic Zn/Iron-Based Sludge/Biochar Composite for Aqueous Cr(VI) Adsorption. Environ. Technol. Innov. 2022, 28, 102661. [Google Scholar] [CrossRef]
- Huang, Y.; Chu, H.; Wang, D.; Hui, S. Performance and Mechanism of Benzene Adsorption on ZnCl2 One-Step Modified Corn Cob Biochar. Environ. Sci. Pollut. Res. 2024, 31, 15209–15222. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, J.; Liu, J.; Liu, B.; Chen, G. Preparation and Application of Magnetic Biochar in Water Treatment: A Critical Review. Sci. Total Environ. 2020, 711, 134847. [Google Scholar] [CrossRef]
- Sinha, R.; Kumar, R.; Abhishek, K.; Shang, J.; Bhattacharya, S.; Sengupta, S.; Kumar, N.; Singh, R.K.; Mallick, J.; Kar, M.; et al. Single-Step Synthesis of Activated Magnetic Biochar Derived from Rice Husk for Hexavalent Chromium Adsorption: Equilibrium Mechanism, Kinetics, and Thermodynamics Analysis. Groundw. Sustain. Dev. 2022, 18, 100796. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Huang, W.; Lu, J.; Luo, S.; Czech, B.; Li, T.; Wang, H. Capture-Reduction Mechanism for Promoting Cr(VI) Removal by Sulfidated Microscale Zerovalent Iron/Sulfur-Doped Graphene-like Biochar Composite. Carbon Res. 2023, 2, 11. [Google Scholar] [CrossRef]
- Campos, A.F.C.; de Oliveira, H.A.L.; da Silva, F.N.; da Silva, F.G.; Coppola, P.; Aquino, R.; Mezzi, A.; Depeyrot, J. Core-Shell Bimagnetic Nanoadsorbents for Hexavalent Chromium Removal from Aqueous Solutions. J. Hazard. Mater. 2019, 362, 82–91. [Google Scholar] [CrossRef]
- Sangkarak, S.; Kittipongvises, S.; Kitkaew, D.; Chaveanghong, S.; Ittisupornrat, S.; Phetrak, A.; Lohwacharin, J. Influence of the Iron-Oxide Mass Fractions of Magnetic Powdered Activated Carbon on Its Hexavalent Chromium Adsorption Performance in Water. Chemosphere 2024, 364, 142997. [Google Scholar] [CrossRef] [PubMed]
- Wani, I.; Kushvaha, V.; Garg, A.; Kumar, R.; Naik, S.; Sharma, P. Review on Effect of Biochar on Soil Strength: Towards Exploring Usage of Biochar in Geo-Engineering Infrastructure. Biomass Convers. Biorefin. 2022, 7, 1–32. [Google Scholar] [CrossRef]
- Guo, N.; Lv, X.; Yang, Q.; Xu, X.; Song, H. Effective Removal of Hexavalent Chromium from Aqueous Solution by ZnCl2 Modified Biochar: Effects and Response Sequence of the Functional Groups. J. Mol. Liq. 2021, 334, 116149. [Google Scholar] [CrossRef]
- Liang, M.; Ding, Y.; Zhang, Q.; Wang, D.; Li, H.; Lu, L. Removal of Aqueous Cr(VI) by Magnetic Biochar Derived from Bagasse. Sci. Rep. 2020, 10, 21473. [Google Scholar] [CrossRef]
- Hoang, L.P.; Nguyen, T.M.P.; Van, H.T.; Yılmaz, M.; Hoang, T.K.; Nguyen, Q.T.; Vi, T.M.H.; Nga, L.T.Q. Removal of Tetracycline from Aqueous Solution Using Composite Adsorbent of ZnAl Layered Double Hydroxide and Bagasse Biochar. Environ. Technol. Innov. 2022, 28, 102914. [Google Scholar] [CrossRef]
- Isaac, R.; Siddiqui, S. Sequestration of Ni(II) and Cu(II) Using FeSO4 Modified Zea Mays Husk Magnetic Biochar: Isotherm, Kinetics, Thermodynamic Studies and RSM. J. Hazard. Mater. Adv. 2022, 8, 100162. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, J.; Xiao, Z.; Liu, J.; Zhang, J.; Zhu, B.; You, X.; Ni, F.; Ao, T.; Tan, Y. A Novel N Self-Doped Porous Biochar Synthesized by KHCO3-Activated Chicken Feather for the Remediation of Tetracycline-Contaminated Water and Soil. Water Air Soil Pollut. 2024, 235, 133. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, X.; Chen, N.; Cui, X.; Yu, H.; Feng, Y.; Xing, D.; He, W. Efficient Removal of Hexavalent Chromium from Wastewater Using a Novel Sodium Alginate-Biochar Composite Adsorbent. J. Water Process Eng. 2024, 64, 105655. [Google Scholar] [CrossRef]
- Chen, T.W.; Rajaji, U.; Chen, S.M.; Al Mogren, M.M.; Hochlaf, M.; Al Harbi, S.D.A.; Ramalingam, R.J. A Novel Nanocomposite with Superior Electrocatalytic Activity: A Magnetic Property Based ZnFe2O4 Nanocubes Embellished with Reduced Graphene Oxide by Facile Ultrasonic Approach. Ultrason. Sonochem. 2019, 57, 116–124. [Google Scholar] [CrossRef]
- Huang, B.; Huang, D.; Zheng, Q.; Yan, C.; Feng, J.; Gao, H.; Fu, H.; Liao, Y. Enhanced Adsorption Capacity of Tetracycline on Porous Graphitic Biochar with an Ultra-Large Surface Area. RSC Adv. 2023, 13, 10397–10407. [Google Scholar] [CrossRef] [PubMed]
- Truong, Q.M.; Nguyen, T.B.; Chen, C.W.; Chen, W.H.; Bui, X.T.; Dong, C. Di KHCO3-Activated High Surface Area Biochar Derived from Brown Algae: A Case Study for Efficient Adsorption of Cr(VI) in Aqueous Solution. Environ. Res. 2024, 247, 118227. [Google Scholar] [CrossRef]
- Zhong, H.; Huang, W.; Wei, Y.; Yang, X.; Jiang, C.; Liu, H.; Zhang, W.; Liang, C.; Dai, L.; Xu, X. Facile Constructing Hierarchical Fe3O4@C Nanocomposites as Anode for Superior Lithium-Ion Storage. Batteries 2023, 9, 403. [Google Scholar] [CrossRef]
- Kohzadi, H.; Soleiman-Beigi, M. XPS and Structural Studies of Fe3O4-PTMS-NAS@Cu as a Novel Magnetic Natural Asphalt Base Network and Recoverable Nanocatalyst for the Synthesis of Biaryl Compounds. Sci. Rep. 2021, 11, 24508. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tan, Z.; Shi, S.; Zhang, S.; Yang, S.; Zhang, X.; Gao, P.; Zhang, Y. Preparation of Cellulose-Grafted Acrylic Acid Stabilized Jujube Branch Biochar-Supported Nano Zero-Valent Iron Composite for Cr(VI) Removal from Water. Nanomaterials 2025, 15, 441. [Google Scholar] [CrossRef]
- Li, K.; Xu, W.; Song, H.; Bi, F.; Li, Y.; Jiang, Z.; Tao, Y.; Qu, J.; Zhang, Y. Superior Reduction and Immobilization of Cr(VI) in Soil Utilizing Sulfide Nanoscale Zero-Valent Iron Supported by Phosphoric Acid-Modified Biochar: Efficiency and Mechanism Investigation. Sci. Total Environ. 2024, 907, 168133. [Google Scholar] [CrossRef]
- Wang, H.; Liu, R.; Chen, Q.; Mo, Y.; Zhang, Y. Biochar-Supported Starch/Chitosan-Stabilized Nano-Iron Sulfide Composites for the Removal of Lead Ions and Nitrogen from Aqueous Solutions. Bioresour. Technol. 2022, 347, 126700. [Google Scholar] [CrossRef]
- Liu, L.; Sun, P.; Chen, Y.; Li, X.; Zheng, X. Distinct Chromium Removal Mechanisms by Iron-Modified Biochar under Varying PH: Role of Iron and Chromium Speciation. Chemosphere 2023, 331, 138796. [Google Scholar] [CrossRef]
- Hao, J.; Cui, Z.; Liang, J.; Ma, J.; Ren, N.; Zhou, H.; Xing, D. Sustainable Efficient Utilization of Magnetic Porous Biochar for Adsorption of Orange G and Tetracycline: Inherent Roles of Adsorption and Mechanisms. Environ. Res. 2024, 252, 118834. [Google Scholar] [CrossRef]
- Laiju, A.R.; Sarkar, S. A Novel Hybrid Ferrous Sulfide Impregnated Anion Exchanger for Trace Removal of Hexavalent Chromium from Contaminated Water. Chemosphere 2022, 305, 135369. [Google Scholar] [CrossRef]
- Dong, L.; Liang, J.; Li, Y.; Hunang, S.; Wei, Y.; Bai, X.; Jin, Z.; Zhang, M.; Qu, J. Effect of Coexisting Ions on Cr(VI) Adsorption onto Surfactant Modified Auricularia Auricula Spent Substrate in Aqueous Solution. Ecotoxicol. Environ. Saf. 2018, 166, 390–400. [Google Scholar] [CrossRef]
- Li, Z.; Dong, J.; Azi, F.; Feng, X.; Ge, Z.; Yang, S.; Sun, Y.; Guan, X.; Dong, M. Mechanism of Cr(VI) Removal by Polyphenols-Rich Bacterial Cellulose Gel Produced from Fermented Wine Pomace. npj Clean Water 2024, 7, 21. [Google Scholar] [CrossRef]
- Luo, M.; Huang, C.; Chen, F.; Chen, C.; Li, H. Removal of Aqueous Cr(VI) Using Magnetic-Gelatin Supported on Brassica-Straw Biochar. J. Dispers. Sci. Technol. 2021, 42, 1710–1722. [Google Scholar] [CrossRef]
- Tytłak, A.; Oleszczuk, P.; Dobrowolski, R. Sorption and Desorption of Cr(VI) Ions from Water by Biochars in Different Environmental Conditions. Environ. Sci. Pollut. Res. 2015, 22, 5985–5994. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, X.; Hang, J.; Li, Z.; Zhong, C.; Sun, A.; Li, J.; Xu, S. Oxidative Magnetization of Biochar at Relatively Low Pyrolysis Temperature for Efficient Removal of Different Types of Pollutants. Bioresour. Technol. 2023, 387, 129572. [Google Scholar] [CrossRef]
- Qu, Q.; Guo, X.; Shao, Z.; Wang, X.; Zhu, M.; Qiu, L. Adsorption Performance and Mechanism of Fe-Loaded Biochar Derived from Waste Zanthoxylum Branch for Removing Cr(VI) from Aqueous Solution. Biomass Convers. Biorefin. 2024, 14, 10201–10215. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of Mechanism Involved in Adsorption of Pb(II) onto Lobeira Fruit (Solanum Lycocarpum) Using Langmuir, Freundlich and Temkin Isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, Q.; Gao, B.; Li, M.; Fan, Z.; Sang, W.; Hao, H.; Wei, X. Removal Mechanisms of Cr(VI) and Cr(III) by Biochar Supported Nanosized Zero-Valent Iron: Synergy of Adsorption, Reduction and Transformation. Environ. Pollut. 2020, 265, 115018. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Zhang, Y.; Yang, J.; Shen, W.; Yang, S.; Quan, Z.; Liu, B.; Yuan, Z.; Zhang, Y. Thermodynamic Restrictions Determine Ammonia Tolerance of Functional Floras during Anaerobic Digestion. Bioresour. Technol. 2024, 391, 129919. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, A.; Lu, J.; Niu, X.; Jiang, M.; Ma, Y.; Liu, X.; Li, M. Adsorption Mechanism of Hexavalent Chromium on Biochar: Kinetic, Thermodynamic, and Characterization Studies. ACS Omega 2020, 5, 27323–27331. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Cao, N.; Sun, D.; Zhang, Z.; Wang, Y.; Wen, Y.; Yang, Y.; Lyu, T. Sustainable Chromium (VI) Removal from Contaminated Groundwater Using Nano-Magnetite-Modified Biochar via Rapid Microwave Synthesis. Molecules 2021, 26, 103. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Bian, Y.; Zhi, F.; Hou, X.; Lv, C.; Hu, Q. Enhanced Adsorption Capacity of Cellulose Hydrogel Based on Corn Stalk for Pollutants Removal and Mechanism Exploration. J. Clean. Prod. 2022, 375, 134130. [Google Scholar] [CrossRef]
- Abilio, T.E.; Soares, B.C.; José, J.C.; Milani, P.A.; Labuto, G.; Carrilho, E.N.V.M. Hexavalent chromium removal from water: Adsorption properties of in natura and magnetic nanomodified sugarcane bagasse. Environ. Sci. Pollut. Res. 2021, 28, 24816–24829. [Google Scholar] [CrossRef]
- Wang, K.; Sun, Y.; Tang, J.; He, J.; Sun, H. Aqueous Cr(VI) removal by a novel ball milled Fe0-biochar composite: Role of biochar electron transfer capacity under high pyrolysis temperature. Chemosphere 2020, 241, 125044. [Google Scholar] [CrossRef]
- Elfeky, S.A.; Mahmoud, S.A.; Youssef, A.F. Applications of CTAB modified magnetic nanoparticles for removal of chromium (VI) from contaminated water. J. Adv. Res. 2017, 8, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Liu, G.; Wang, G.; Liang, W.; Peng, C.; Zhang, W.; Lin, K.; Yang, J. Exploring different mechanisms of biochars in removing hexavalent chromium: Sorption, reduction and electron shuttle. Bioresour. Technol. 2021, 337, 125382. [Google Scholar] [CrossRef] [PubMed]
- Prabu, D.; Senthil Kumar, P.; Senthil Rathi, B.; Sathish, S.; Vijai Anand, K.; Aravind Kumar, J.; Mohammed Osama, B.; Silambarasan, P. Feasibility of magnetic nano adsorbent impregnated with activated carbon from animal bone waste: Application for the chromium (VI) removal. Environ. Res. 2022, 203, 111813. [Google Scholar] [CrossRef]
- Masuku, M.; Nure, J.F.; Atagana, H.I.; Hlongwa, N.; Nkambule, T.T. The development of zinc-doped nickel ferrite nano-adsorbent for the adsorption of chromium (VI) from wastewater. J. Water Process Eng. 2024, 64, 105587. [Google Scholar] [CrossRef]
- Cai, W.; Dionysiou, D.D.; Fu, F.; Tang, B. CTAB–intercalated molybdenum disulfide nanosheets for enhanced simultaneous removal of Cr (VI) and Ni (II) from aqueous solutions. J. Hazard. Mater. 2020, 396, 122728. [Google Scholar] [CrossRef]
- Dai, X.; Luo, Y.; Deng, J.; Wen, J.; He, Y.; Yuan, Y.; Wang, Y. Ultra-efficient removal of aqueous hexavalent chromium by activated biochar nanoparticles derived from squid ink. Environ. Res. 2024, 263, 120185. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Chen, Z.; Chen, X.; Zhong, Z. Investigation of the adsorption process of chromium (VI) ions from petrochemical wastewater using nanomagnetic carbon materials. Nanomaterials 2022, 12, 3815. [Google Scholar] [CrossRef] [PubMed]
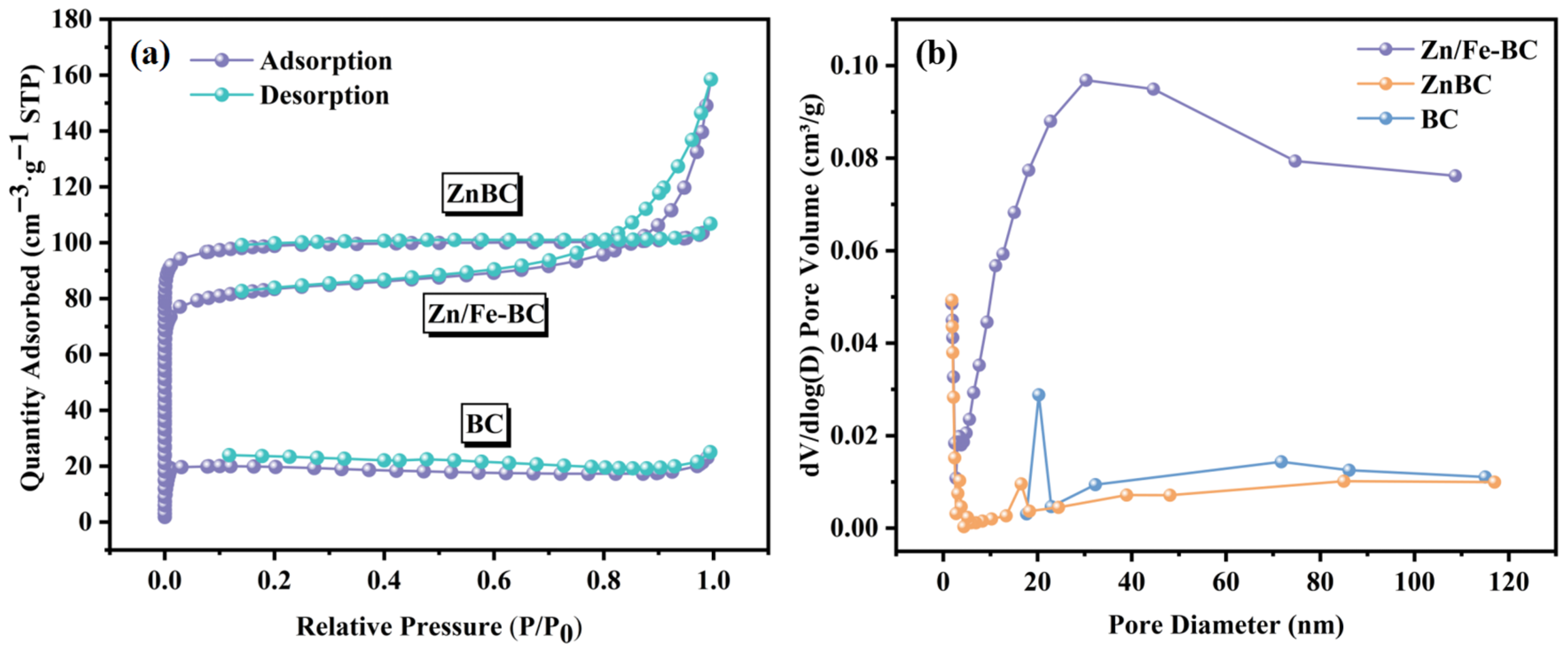


| Sample | SBET (m2/g) | Vt (cm3/g) | Vmicro (cm3/g) | DP (nm) |
|---|---|---|---|---|
| BC | 78.6 | 0.04 | 0.03 | 1.89 |
| ZnBC | 375.9 | 0.17 | 0.14 | 1.63 |
| Zn/Fe-BC | 269.3 | 0.24 | 0.11 | 1.90 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm/(mg/g) | KL | R2 | KF | 1/n | R2 | |
| BC | 30.312 | 0.144 | 0.870 | 10.62 | 0.207 | 0.969 |
| ZnBC | 28.829 | 0.727 | 0.660 | 13.95 | 0.155 | 0.974 |
| Zn/Fe-BC | 37.658 | 0.154 | 0.786 | 13.26 | 0.206 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.; Tan, Z.; Yan, Y.; Yang, M.; Han, S.; Yang, C.; Li, S.; Zhang, Y. Preparation of ZnCl2-Activated Magnetic Biochar and Its Performance in Removing Hexavalent Chromium from Water. Nanomaterials 2025, 15, 1586. https://doi.org/10.3390/nano15201586
Gao P, Tan Z, Yan Y, Yang M, Han S, Yang C, Li S, Zhang Y. Preparation of ZnCl2-Activated Magnetic Biochar and Its Performance in Removing Hexavalent Chromium from Water. Nanomaterials. 2025; 15(20):1586. https://doi.org/10.3390/nano15201586
Chicago/Turabian StyleGao, Pingqiang, Zhe Tan, Yonghao Yan, Min Yang, Shuai Han, Chen Yang, Shuai Li, and Yan Zhang. 2025. "Preparation of ZnCl2-Activated Magnetic Biochar and Its Performance in Removing Hexavalent Chromium from Water" Nanomaterials 15, no. 20: 1586. https://doi.org/10.3390/nano15201586
APA StyleGao, P., Tan, Z., Yan, Y., Yang, M., Han, S., Yang, C., Li, S., & Zhang, Y. (2025). Preparation of ZnCl2-Activated Magnetic Biochar and Its Performance in Removing Hexavalent Chromium from Water. Nanomaterials, 15(20), 1586. https://doi.org/10.3390/nano15201586






