Peroxydisulfate Activation by Pyrolysis Products of Iron Grinding Sludge and Polyethylene Glycol for Methylene Blue Degradation: Mechanism and Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of ISPEG
2.3. Method of Analysis
2.4. Experimental Procedures
3. Results and Discussion
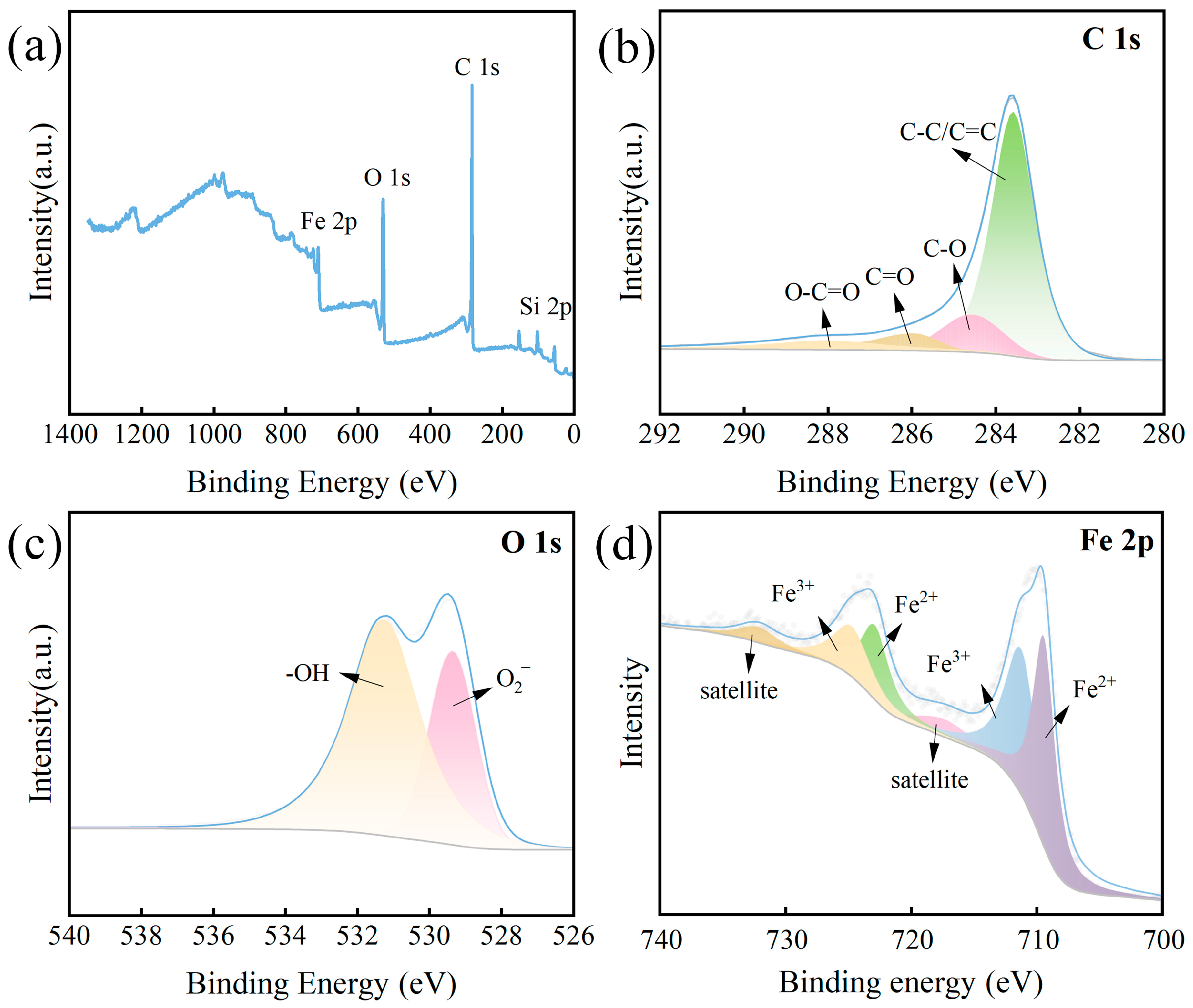
3.1. Characterization
3.2. Degradation of MB by ISPEG
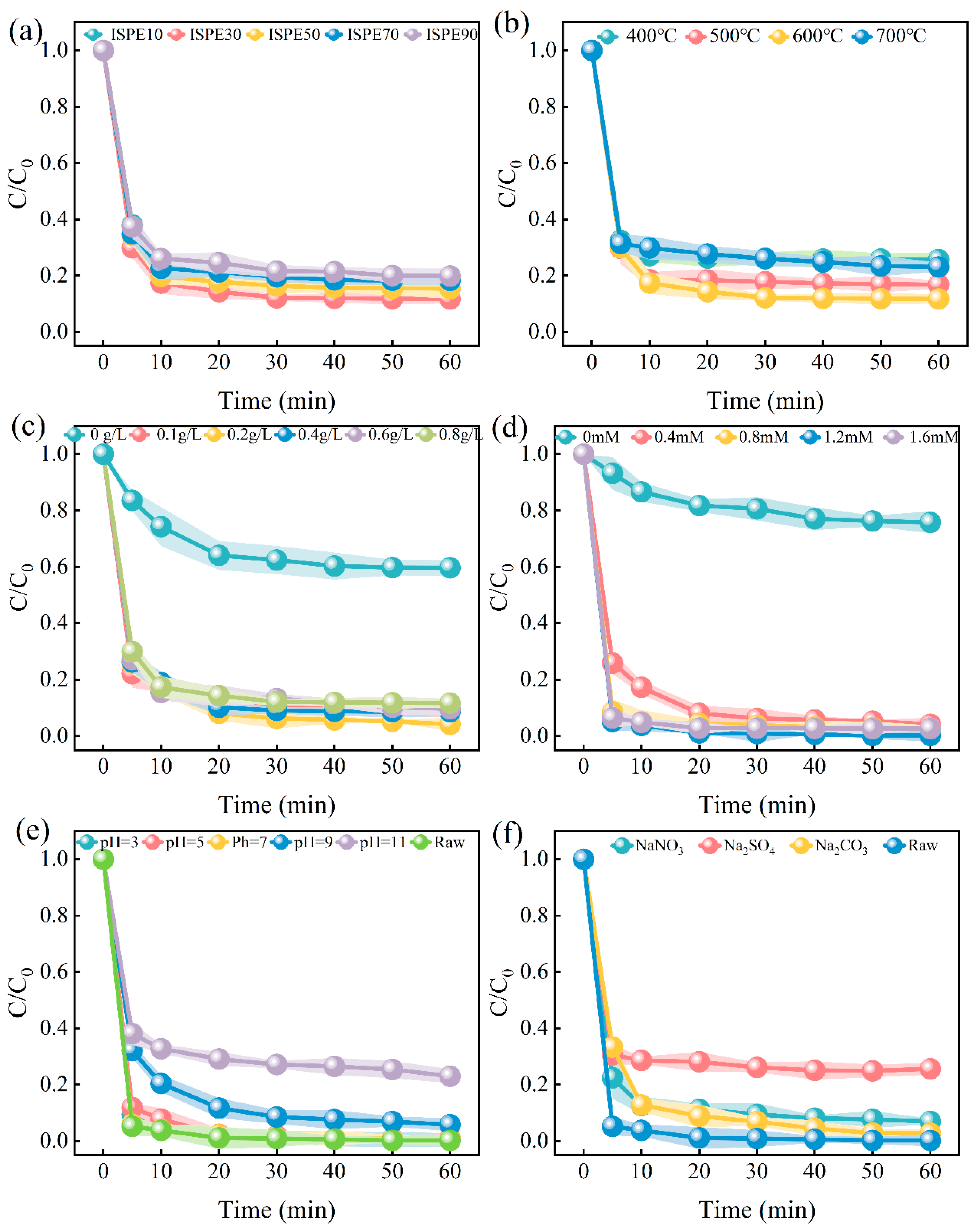
3.2.1. Effects of Different Ratios
3.2.2. Effects of Different Pyrolysis Temperatures
3.2.3. Effects of ISPEG Dosages
3.2.4. Effects of PDS Concentration
3.2.5. Effects of pH
3.2.6. Effects of Anions
3.3. Reusability and Stability
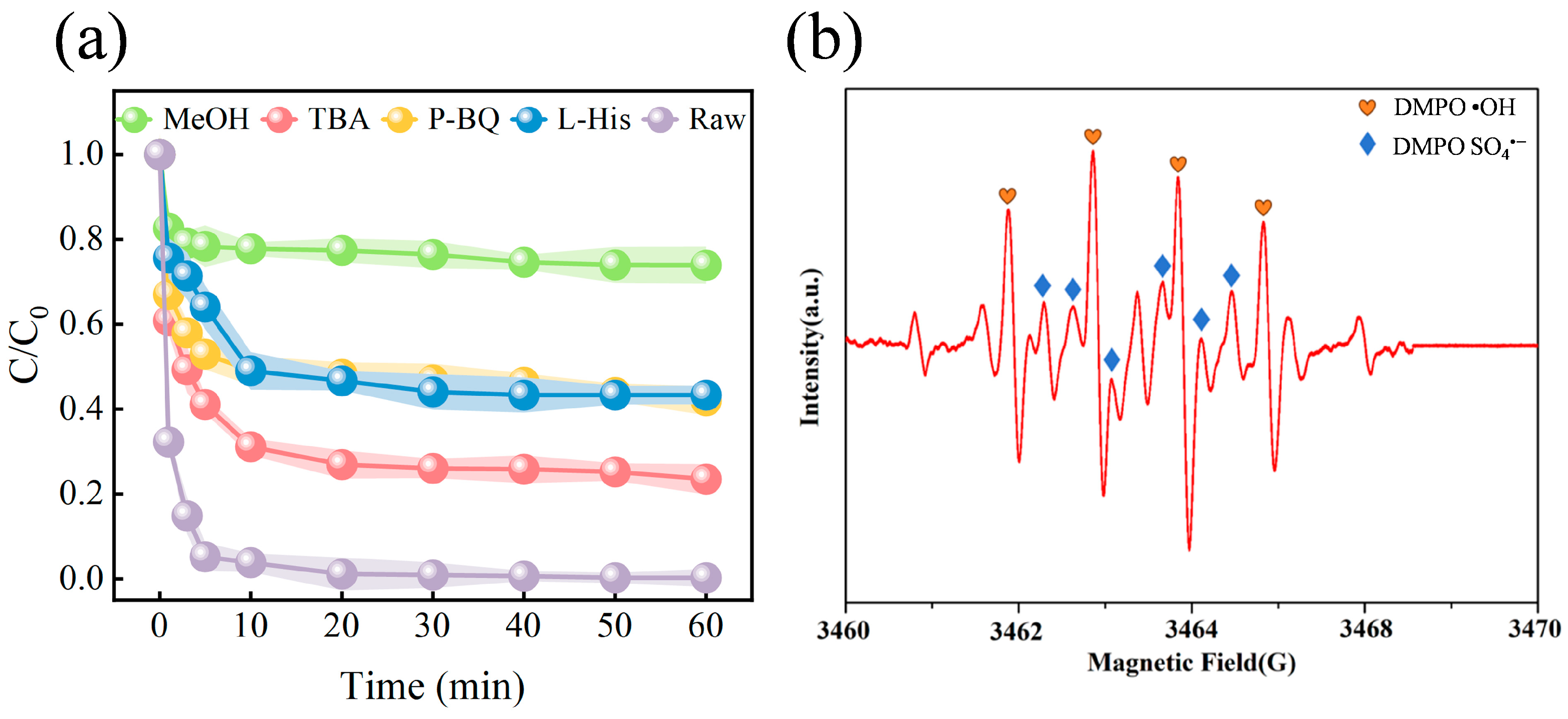
3.4. Identification of Reactive Oxygen Species and Degradation Mechanisms
3.5. Degradation Pathway of MB
3.6. MB Degradation in Actual Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, C.Q.; Huang, R.; Sun, R.R.; Yang, J.P.; Sillanpää, M. A review on persulfates activation by functional biochar for organic contaminants removal: Synthesis, characterizations, radical determination, and mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Kang, Z.Y.; Jia, X.G.; Zhang, Y.C.; Kang, X.X.; Ge, M.; Liu, D.; Wang, C.; He, Z. A Review on Application of Biochar in the Removal of Pharmaceutical Pollutants through Adsorption and Persulfate-Based AOPs. Sustainability 2022, 14, 10128. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; García-Cañibano, C.; Lepistö, R.J.; Encinas, Á.; Pellinen, J.; Marugán, J. Intensification of UV-C tertiary treatment: Disinfection and removal of micropollutants by sulfate radical based Advanced Oxidation Processes. J. Hazard. Mater. 2019, 372, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Xiao, S.; Lin, Y.; Yu, P.; Zhong, M.-e.; Yang, L.; Wang, H.; Su, L.; Liao, C.; Zhou, Y.; et al. Attapulgite-supported nano-Fe0/peroxymonsulfate for quinclorac removal: Performance, mechanism and degradation pathway. Chem. Eng. J. 2019, 360, 104–114. [Google Scholar] [CrossRef]
- Li, D.H.; Zhang, S.X.; Li, S.N.; Tang, J.C.; Hua, T.; Li, F. Mechanism of the application of single-atom catalyst-activated PMS/PDS to the degradation of organic pollutants in water environment: A review. J. Clean. Prod. 2023, 397, 136468. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, S.; Zhong, H.; Yan, M.; Yang, X. Activation of persulfates by carbonaceous materials: A review. Chem. Eng. J. 2021, 418, 129297. [Google Scholar] [CrossRef]
- Lu, J.; Lu, Q.; Di, L.; Zhou, Y.; Zhou, Y. Iron-based biochar as efficient persulfate activation catalyst for emerging pollutants removal: A review. Chin. Chem. Lett. 2023, 34, 108357. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Wang, F.; Wang, C.-C.; Wang, P.; Fu, H.; Zhao, C. Photocatalysis activation of peroxodisulfate over the supported Fe3O4 catalyst derived from MIL-88A(Fe) for efficient tetracycline hydrochloride degradation. Chem. Eng. J. 2021, 426, 131927. [Google Scholar] [CrossRef]
- Li, X.; Rykov, A.I.; Zhang, B.; Zhang, Y.; Wang, J. Graphene encapsulated Fe x Co y nanocages derived from metal–organic frameworks as efficient activators for peroxymonosulfate. Catal. Sci. Technol. 2016, 6, 7486–7494. [Google Scholar] [CrossRef]
- He, L.; Wu, D.; Rong, H.; Li, M.; Tong, M.; Kim, H. Influence of Nano- and Microplastic Particles on the Transport and Deposition Behaviors of Bacteria in Quartz Sand. Environ. Sci. Technol. 2018, 52, 11555–11563. [Google Scholar] [CrossRef]
- Zhang, K.-X.; Song, C.; Zhao, S.; Yan, Z.; Feng, L.-J.; Wang, S.-G. AOPs enhance the migration of polystyrene nanoparticles in saturated quartz sand. Environ. Sci. Process. Impacts 2021, 23, 1509–1515. [Google Scholar] [CrossRef]
- Song, H.; Tsang, D.C.; Kwon, G.; Kwon, E.E.; Cho, D.-W. Coupling carbon dioxide and magnetite for the enhanced thermolysis of polyvinyl chloride. Sci. Total Environ. 2019, 696, 133951. [Google Scholar] [CrossRef]
- Kwon, G.; Cho, D.-W.; Wang, H.; Bhatnagar, A.; Song, H. Valorization of plastics and paper mill sludge into carbon composite and its catalytic performance for acarbon material consisted of the multi-layerzo dye oxidation. J. Hazard. Mater. 2020, 398, 123173. [Google Scholar] [CrossRef]
- Ma, D.; Yang, Y.; Liu, B.; Xie, G.; Chen, C.; Ren, N.; Xing, D. Zero-valent iron and biochar composite with high specific surface area via K2FeO4 fabrication enhances sulfadiazine removal by persulfate activation. Chem. Eng. J. 2021, 408, 127992. [Google Scholar] [CrossRef]
- Großwendt, F.; Bürk, V.; Kopanka, B.; Jäger, S.; Pollak, S.; Leich, L.; Röttger, A.; Petermann, M.; Weber, S. A novel powder-metallurgical eco-friendly recycling process for tool steel grinding sludge. J. Clean. Prod. 2023, 392, 136329. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Z.; Shi, W.; Zhang, B.; Li, W.; Cui, F.; Lens, P.N.L. Evolution of the sludge mineral composition enhances operation performance of the aerobic granular sludge reactor coupled with iron electrolysis. J. Clean. Prod. 2021, 295, 126394. [Google Scholar] [CrossRef]
- Yao, Z.; Seong, H.J.; Jang, Y.-S. Environmental toxicity and decomposition of polyethylene. Ecotoxicol. Environ. Saf. 2022, 242, 113933. [Google Scholar] [CrossRef]
- Jin, H.-Y.; He, Z.-W.; Ren, Y.-X.; Zou, Z.-S.; Tang, C.-C.; Zhou, A.-J.; Liu, W.; Li, Z.; Wang, A. Revealing the roles of biochar derived from iron-rich fermented sludge residue in anaerobic digestion. Chem. Eng. J. 2024, 481, 148376. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, C.; Zhang, Y.; Yu, G.; Xiong, Y.; Zhou, L.; Liu, Y.; Chi, T.; Wang, G.; Su, Y. Degradation of oxytetracycline by magnetic MOFs heterojunction photocatalyst with persulfate: High stability and wide range. Environ. Sci. Pollut. Res. 2022, 29, 30019–30029. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Sun, C.; Sun, J.Q.; Zhou, R. Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process. Sep. Purif. Technol. 2015, 142, 182–188. [Google Scholar] [CrossRef]
- Criveanu, A.; Dumitrache, F.; Fleaca, C.; Gavrila-Florescu, L.; Lungu, I.; Morjan, I.P.; Socoliuc, V.; Prodan, G. Chitosan-coated iron oxide nanoparticles obtained by laser pyrolysis. Appl. Surf. Sci. Adv. 2023, 15, 100405. [Google Scholar] [CrossRef]
- Meng, F.; Song, M.; Song, B.; Wei, Y.; Cao, Q.; Cao, Y. Enhanced degradation of Rhodamine B via α-Fe2O3 microspheres induced persulfate to generate reactive oxidizing species. Chemosphere 2020, 243, 125322. [Google Scholar] [CrossRef] [PubMed]
- Pu, M.; Wan, J.; Zhang, F.; Brusseau, M.L.; Ye, D.; Niu, J. Insight into degradation mechanism of sulfamethoxazole by metal-organic framework derived novel magnetic Fe@C composite activated persulfate. J. Hazard. Mater. 2021, 414, 125598. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. 2004, 116, 607–611. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Feng, H.; Zou, J.; Wang, J.; Feng, C.; Zhu, X.; Ouyang, X.; et al. Hierarchical porous biochar from shrimp shell for persulfate activation: A two-electron transfer path and key impact factors. Appl. Catal. B Environ. 2020, 260, 118160. [Google Scholar] [CrossRef]
- Lu, L.; Ai, Z.; Li, J.; Zheng, Z.; Li, Q.; Zhang, L. Synthesis and Characterization of Fe−Fe2O3 Core−Shell Nanowires and Nanonecklaces. Cryst. Growth Des. 2007, 7, 459–464. [Google Scholar] [CrossRef]
- Ai, Z.H.; Gao, Z.T.; Zhang, L.Z.; He, W.W.; Yin, J.J. Core–shell structure dependent reactivity of Fe@ Fe2O3 nanowires on aerobic degradation of 4-chlorophenol. Environ. Sci. Technol. 2013, 47, 5344–5352. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.S.; Li, C.; Guo, J.Q.; Sun, C.M. Zero-valent iron loaded on N-doped biochar fabricated by one-step pyrolysis of K2FeO4 and coffee grounds as a persulfate activator for Bisphenol A degradation. Process Saf. Environ. Prot. 2023, 170, 328–338. [Google Scholar] [CrossRef]
- Qin, J.H.; Wang, X.; Deng, M.J.; Li, H.S.; Lin, C.X. Red mud-biochar composites (co-pyrolyzed red mud-plant materials): Characteristics and improved efficacy on the treatment of acidic mine water and trace element-contaminated soils. Sci. Total Environ. 2022, 844, 157062. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, G.; Huang, B.; Cai, N.; Guo, P.; Chen, L. Preparation and application of a novel biochar-supported red mud catalyst: Active sites and catalytic mechanism. J. Hazard. Mater. 2021, 408, 124802. [Google Scholar] [CrossRef]
- Chen, Y.; Su, R.; Xu, F.; Ma, M.; Wang, Y.; Ma, D.; Li, Q. Oxygen-containing functional groups in Fe3O4@three-dimensional graphene nanocomposites for enhancing H2O2 production and orientation to 1O2 in electro-Fenton. J. Hazard. Mater. 2024, 470, 134162. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhou, G.; Liu, S.; Ang, H.M.; Tadé, M.O.; Wang, S. Nano-Fe0 Encapsulated in Microcarbon Spheres: Synthesis, Characterization, and Environmental Applications. ACS Appl. Mater. Interfaces 2012, 4, 6235–6241. [Google Scholar] [CrossRef]
- Fan, X.; Lin, Q.; Zheng, J.; Fu, H.; Xu, K.; Liu, Y.; Ma, Y.; He, J. Peroxydisulfate activation by nano zero-valent iron graphitized carbon materials for ciprofloxacin removal: Effects and mechanism. J. Hazard. Mater. 2022, 437, 129392. [Google Scholar] [CrossRef]
- Jonidi Jafari, A.; Kakavandi, B.; Jaafarzadeh, N.; Rezaei Kalantary, R.; Ahmadi, M.; Akbar Babaei, A. Fenton-like catalytic oxidation of tetracycline by AC@Fe3O4 as a heterogeneous persulfate activator: Adsorption and degradation studies. J. Ind. Eng. Chem. 2017, 45, 323–333. [Google Scholar] [CrossRef]
- Saidani, A.; Boudraa, R.; Fendi, K.; Benouadah, L.; Benabbas, A.; Djermoune, A.; Salvestrini, S.; Bollinger, J.-C.; Alayyaf, A.A.; Mouni, L. Effect of Calcination Temperature on the Photocatalytic Activity of Precipitated ZnO Nanoparticles for the Degradation of Rhodamine B Under Different Light Sources. Water 2025, 17, 32. [Google Scholar] [CrossRef]
- Wu, X.; Li, T.; Wang, R.; Zhang, Y.; Liu, W.; Yuan, L. One-pot green synthesis of Zero-Valent iron particles supported on N-Doped porous carbon for efficient removal of organic pollutants via Persulfate Activation: Low iron leaching and degradation mechanism. Sep. Purif. Technol. 2021, 279, 119768. [Google Scholar] [CrossRef]
- Guo, M.; Han, Y.; Gao, H.; Xu, Z.; Wang, D.; Peng, Z.; Hou, H. Red mud-based biochar activated peroxydisulfate under visible light for tetracycline removal: Synergistic effect of FeAl2O4 photocatalysis. Sep. Purif. Technol. 2024, 328, 125078. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Liu, Z.; Wang, S.; Zhang, X.; Xu, B.; Yu, P.; Xu, Y.; Sun, Y. Effective removal of tetracycline from water by catalytic peroxymonosulfate oxidation over Co@MoS2: Catalytic performance and degradation mechanism. Sep. Purif. Technol. 2022, 294, 121139. [Google Scholar] [CrossRef]
- Zheng, M.M.; Li, Y.H.; Cao, M.H.; Guo, Y.; Qiu, G.; Tu, S.; Xiong, S.; Fang, D. Amino acid promoted oxidation of atrazine by Fe3O4/persulfate. Heliyon 2024, 10, e23371. [Google Scholar] [CrossRef]
- Jin, Z.A.; Zhao, X.T.; Zhang, M.; Li, Y.; Guo, J.; Lan, Y.; Chen, C. Waste self-heating bag derived CoFe2O4 composite enhances peroxymonosulfate activation: Performance, mechanism, and adaptability under high-salinity conditions. J. Water Process Eng. 2024, 60, 105221. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, K.; Cui, M.; Liu, T.; Chen, X.; Chen, Y.; Nie, X.; Xu, Z.; Li, C.-X. Insight into the degradation of tetracycline hydrochloride by non-radical-dominated peroxymonosulfate activation with hollow shell-core Co@NC: Role of cobalt species. Sep. Purif. Technol. 2022, 289, 120662. [Google Scholar] [CrossRef]
- Li, B.; Li, C.-X.; Wang, Y.; Xu, W.; Cui, K.; Zhan, X.; Deng, R.; Zhang, X. In-situ preparation of yeast-supported Fe0@Fe2O3 as peroxymonosulfate activator for enhanced degradation of tetracycline hydrochloride. Chemosphere 2023, 324, 138340. [Google Scholar] [CrossRef]
- Huang, Q.L.; Chen, C.J.; Zhao, X.L.; Bu, X.Y.; Liao, X.F.; Fan, H.; Gao, W.; Hu, H.; Zhang, Y.; Huang, Z. Malachite green degradation by persulfate activation with CuFe2O4@biochar composite: Efficiency, stability and mechanism. J. Environ. Chem. Eng. 2021, 9, 105800. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, X.; Ma, F.; Wang, L.; Duan, X.; Wang, S. Catalytic Removal of Aqueous Contaminants on N-Doped Graphitic Biochars: Inherent Roles of Adsorption and Nonradical Mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef]
- Liao, L.; Yue, H.; Cui, Y. Crosslink Polymerization Kinetics and Mechanism of Hydrogels Composed of Acrylic Acid and 2-Acrylamido-2-methylpropane Sulfonic Acid. Chin. J. Chem. Eng. 2011, 19, 285–291. [Google Scholar] [CrossRef]
- Tian, L.; Liu, S.-S.; Jiang, X.-H.; Chen, L.-S.; Wu, S.-L.; Xiao, W.-J.; Fan, J.-P.; Wu, D.-S.; Zou, J.-P. Selective oxidation of diclofenac sodium with different electronegative moieties via coexisting SO4− and OH. Sci. Total Environ. 2021, 782, 146857. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, J.; Yuan, S.; Ge, B.; Du, H.; Qu, C.; Chen, H.; Wu, C.; Li, W.; Zhang, Y. Degradation of octane using an efficient and stable core-shell Fe3O4@C during Fenton processes: Enhanced mass transfer, adsorption and catalysis. Appl. Surf. Sci. 2020, 515, 146083. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced photocatalytic degradation of methylene blue and methyl orange by ZnO:Eu nanoparticles. Appl. Catal. B Environ. 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–157. [Google Scholar] [CrossRef]





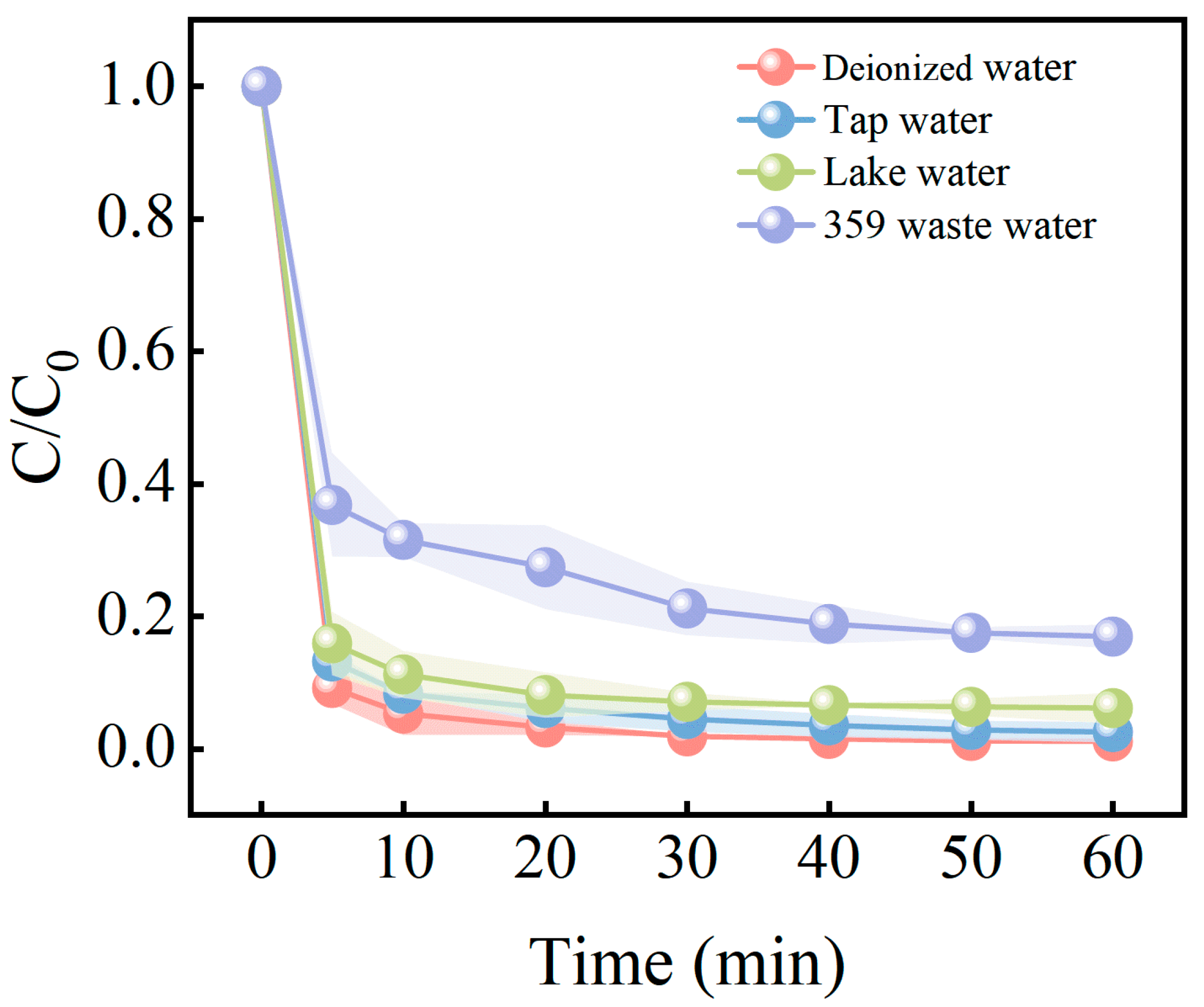
| Water Sources | CODcr (mg/L) | pH | Conductivity (μS/cm) | MB Concentration (mg/L) |
|---|---|---|---|---|
| Lake water | 41 | 7.25 | 187 | Not detected |
| Tap water | 4.53 | 7.12 | 191 | Not detected |
| 359 dye wastewater | 5845 | 10.1 | 17,209 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.-F.; Liu, H.-L.; Han, Y.; Shi, T.; Wang, D.-J.; Chen, X. Peroxydisulfate Activation by Pyrolysis Products of Iron Grinding Sludge and Polyethylene Glycol for Methylene Blue Degradation: Mechanism and Performance. Nanomaterials 2025, 15, 1585. https://doi.org/10.3390/nano15201585
Kong D-F, Liu H-L, Han Y, Shi T, Wang D-J, Chen X. Peroxydisulfate Activation by Pyrolysis Products of Iron Grinding Sludge and Polyethylene Glycol for Methylene Blue Degradation: Mechanism and Performance. Nanomaterials. 2025; 15(20):1585. https://doi.org/10.3390/nano15201585
Chicago/Turabian StyleKong, De-Feng, Hui-Lai Liu, Yi Han, Ting Shi, De-Jin Wang, and Xing Chen. 2025. "Peroxydisulfate Activation by Pyrolysis Products of Iron Grinding Sludge and Polyethylene Glycol for Methylene Blue Degradation: Mechanism and Performance" Nanomaterials 15, no. 20: 1585. https://doi.org/10.3390/nano15201585
APA StyleKong, D.-F., Liu, H.-L., Han, Y., Shi, T., Wang, D.-J., & Chen, X. (2025). Peroxydisulfate Activation by Pyrolysis Products of Iron Grinding Sludge and Polyethylene Glycol for Methylene Blue Degradation: Mechanism and Performance. Nanomaterials, 15(20), 1585. https://doi.org/10.3390/nano15201585






