Hierarchical Porous P-Doped NiCo Alloy with α/ε Phase-Defect Synergy to Boost Alkaline HER Kinetics and Bifunctional Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Samples
2.3. Characterization
2.4. Electrochemical Measurements
3. Results and Discussion
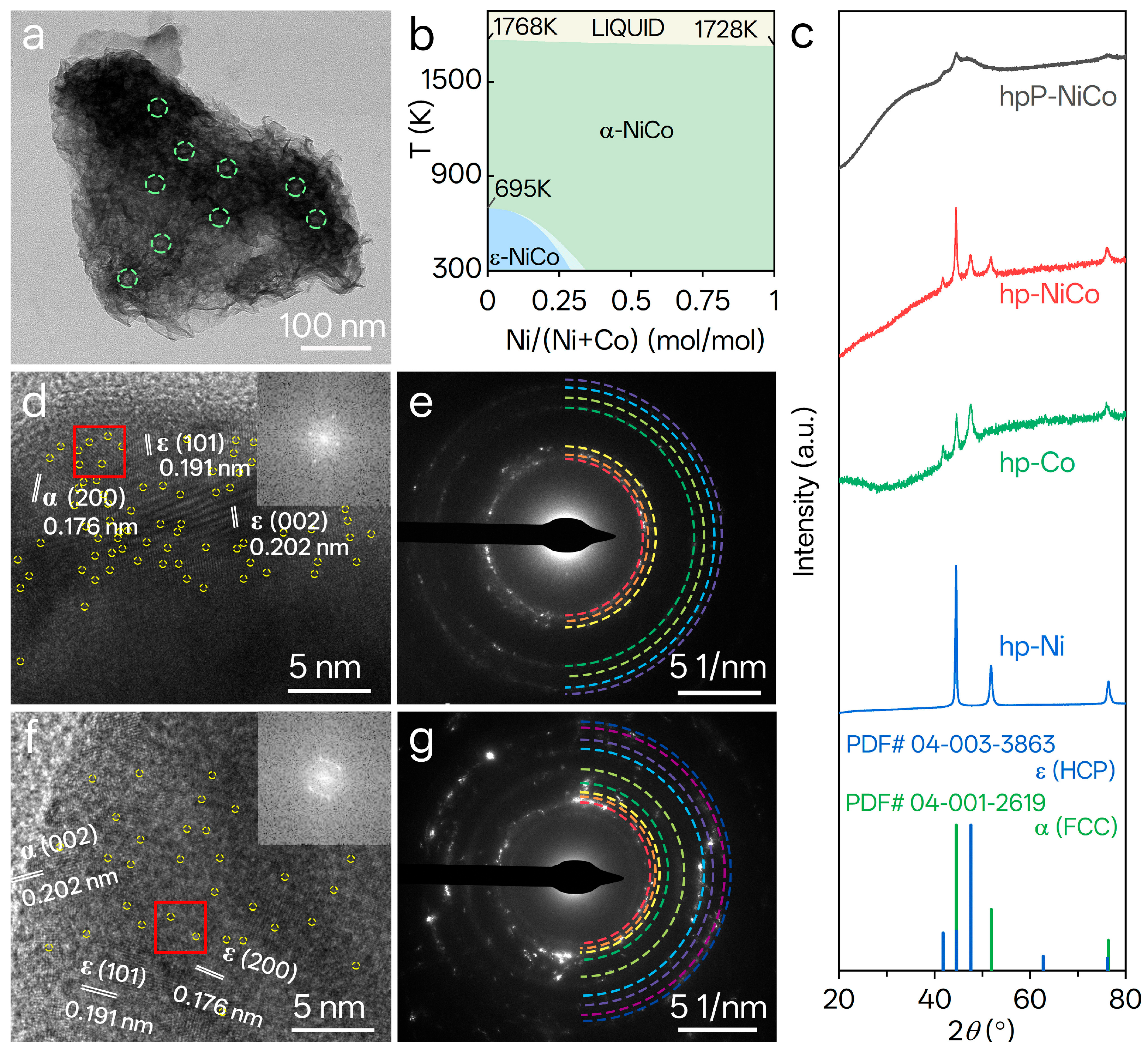
3.1. Morphological and Compositional Characteristics of Catalysts
3.2. Structural and Crystallinity Properties of Catalysts
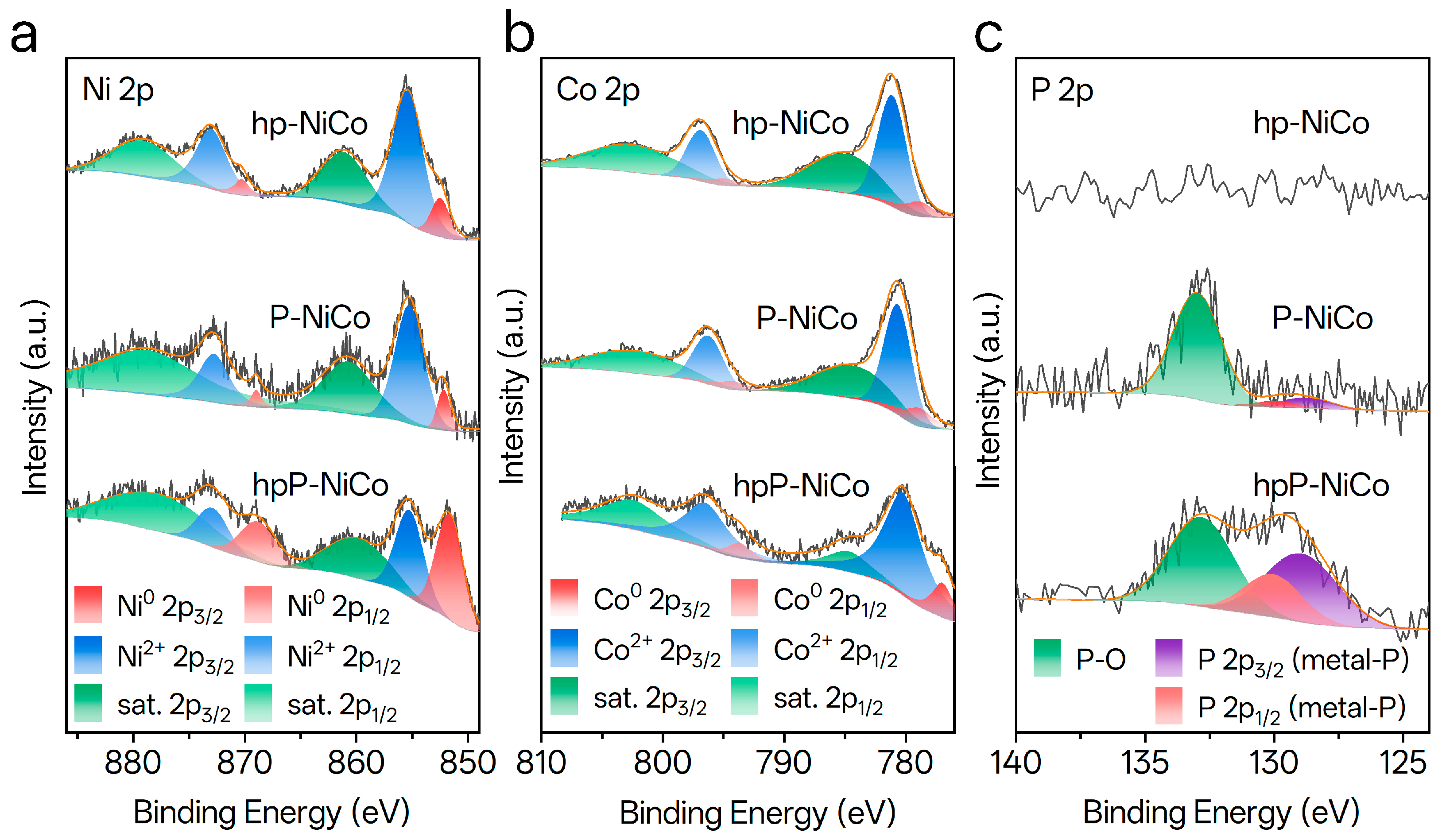
3.3. XPS Analysis of Chemical States in Catalysts
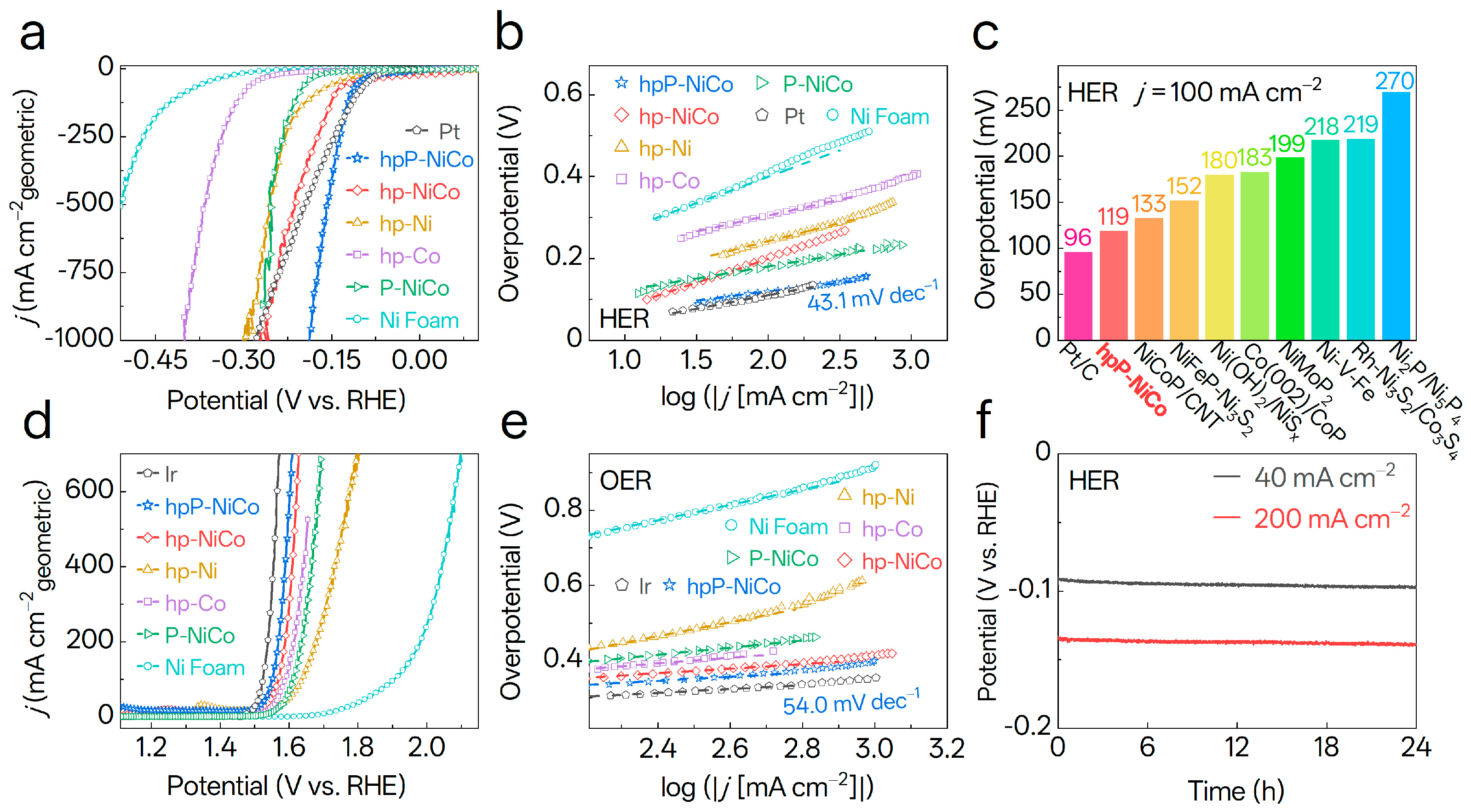
3.4. Electrocatalytic Performance of Catalysts Toward HER and OER
3.5. Mechanistic Insights into HER over NiCo-Based Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, W.; Shen, T.; Xing, C.; Sun, K.; Yan, Q.; Niu, W.; Yang, X.; Li, J.; Wei, C.; Wang, R.; et al. Ultrastable Supported Oxygen Evolution Electrocatalyst Formed by Ripening-Induced Embedding. Science 2025, 387, 791–796. [Google Scholar] [CrossRef]
- Sha, Q.; Wang, S.; Yan, L.; Feng, Y.; Zhang, Z.; Li, S.; Guo, X.; Li, T.; Li, H.; Zhuang, Z.; et al. 10,000-h-Stable Intermittent Alkaline Seawater Electrolysis. Nature 2025, 639, 360–367. [Google Scholar] [CrossRef]
- Li, G.; Han, G.; Wang, L.; Cui, X.; Moehring, N.K.; Kidambi, P.R.; Jiang, D.; Sun, Y. Dual Hydrogen Production from Electrocatalytic Water Reduction Coupled with Formaldehyde Oxidation via a Copper-Silver Electrocatalyst. Nat. Commun. 2023, 14, 525. [Google Scholar] [CrossRef]
- Yue, K.; Lu, R.; Gao, M.; Song, F.; Dai, Y.; Xia, C.; Mei, B.; Dong, H.; Qi, R.; Zhang, D.; et al. Polyoxometalated Metal-Organic Framework Superstructure for Stable Water Oxidation. Science 2025, 388, 430–436. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, Y.; Fu, N.; Mu, S.; Peng, J.; Liu, Y.; Zhang, G. Phase Engineering and Dispersion Stabilization of Cobalt Toward Enhanced Hydrogen Evolution. Small 2024, 20, 2310499. [Google Scholar] [CrossRef]
- Wang, H.; Qin, Y.; Wu, Y.; Qiu, Y.; Ling, L.; Fang, Q.; Wang, C.; Hu, L.; Gu, W.; Zhu, C. Pd Hydride Metallene Aerogels with Lattice Hydrogen Participation for Efficient Hydrogen Evolution Reaction. Nat. Commun. 2024, 15, 10289. [Google Scholar] [CrossRef]
- Chen, X.; Bi, M.; Yan, Q.; Fan, D.; Huang, B.; Sun, B.; Qin, C.; Chen, C.; Sun, D.; He, Q.; et al. Ce Single Atom-Engineered Amorphous/Crystalline Nanosheets for Enhanced Alkaline Water Electrolysis. Adv. Mater. 2025, e08893. [Google Scholar] [CrossRef]
- Chang, B.; Liu, X.; Zuo, S.; Ren, Y.; He, J.; Wang, D.; Lei, Y.; Hu, M.; Li, W.-L.; Khan, M.A.; et al. Dynamic Construction of a Durable Epitaxial Catalytic Layer for Industrial Alkaline Water Splitting. Nat. Commun. 2025, 16, 7959. [Google Scholar] [CrossRef]
- Chen, C.; Jin, H.; Wang, P.; Sun, X.; Jaroniec, M.; Zheng, Y.; Qiao, S.-Z. Local Reaction Environment in Electrocatalysis. Chem. Soc. Rev. 2024, 53, 2022–2055. [Google Scholar] [CrossRef]
- Fairhurst, A.R.; Snyder, J.; Wang, C.; Strmcnik, D.; Stamenkovic, V.R. Electrocatalysis: From Planar Surfaces to Nanostructured Interfaces. Chem. Rev. 2025, 125, 1332–1419. [Google Scholar] [CrossRef]
- Huang, H.; Xu, L.; Zuo, S.; Song, L.; Zou, C.; García-Melchor, M.; Li, Y.; Ren, Y.; Rueping, M.; Zhang, H. Manipulation of Oxidation States on Phase Boundary via Surface Layer Modification for Enhanced Alkaline Hydrogen Electrocatalysis. Adv. Mater. 2024, 36, 2405128. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-T.; Chen, L.; Wang, H.-Y.; Tian, W.-W.; Yuan, Z.-Y. Water Electrolysis for Hydrogen Production: From Hybrid Systems to Self-Powered/Catalyzed Devices. Energy Environ. Sci. 2024, 17, 49–113. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Y.; Liu, L.; Jiao, M.; Ye, C. Single-Atom Catalysts for Hydrogen Evolution Reaction: The Role of Supports, Coordination Environments, and Synergistic Effects. Nanomaterials 2025, 15, 1175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Xiang, N.; Wang, Y.-Q.; Ye, J.; Jin, Z.; Fu, L.; Chang, X.; Wang, D.; Xiao, H.; Xu, B. A Molecular Design Strategy to Enhance Hydrogen Evolution on Platinum Electrocatalysts. Nat. Energy 2025, 10, 725–736. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Chen, B.; Huang, W.-H.; Tang, Z.; Xu, M.; Yeh, M.-H.; Pao, C.-W.; Pu, M.; Yang, G.; et al. A-Site Cation Deficiency in Antiperovskites for Precisely Accelerating the Volmer Step of Alkaline Hydrogen Evolution. Adv. Energy Mater. 2025, 15, e03319. [Google Scholar] [CrossRef]
- Wan, C.; Zhang, Z.; Dong, J.; Xu, M.; Pu, H.; Baumann, D.; Lin, Z.; Wang, S.; Huang, J.; Shah, A.H.; et al. Amorphous Nickel Hydroxide Shell Tailors Local Chemical Environment on Platinum Surface for Alkaline Hydrogen Evolution Reaction. Nat. Mater. 2023, 22, 1022–1029. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Liang, Q.; Gao, Y.; Zou, X.; Zhou, X.; Liu, F.; Song, K.; Li, W.; She, J.; et al. Regulating the Electronic State of Pt via Architecting Symbiotic Oxide Support for Optimal Alkaline Hydrogen Evolution Reaction. eScience 2025, 100461. [Google Scholar] [CrossRef]
- Lin, G.; Dong, A.; Li, Z.; Li, W.; Cao, X.; Zhao, Y.; Wang, L.; Sun, L. An Interlayer Anchored NiMo/MoO2 Electrocatalyst for Hydrogen Evolution Reaction in Anion Exchange Membrane Water Electrolysis at High Current Density. Adv. Mater. 2025, 37, 2507525. [Google Scholar] [CrossRef]
- Chen, Z.W.; Li, J.; Ou, P.; Huang, J.E.; Wen, Z.; Chen, L.; Yao, X.; Cai, G.; Yang, C.C.; Singh, C.V.; et al. Unusual Sabatier Principle on High Entropy Alloy Catalysts for Hydrogen Evolution Reactions. Nat. Commun. 2024, 15, 359. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Mohmad, A.R.; Wang, Y.; Fullon, R.; Song, X.; Zhao, F.; Bozkurt, I.; Augustin, M.; Santos, E.J.G.; Shin, H.S.; et al. Ultrahigh-Current-Density Niobium Disulfide Catalysts for Hydrogen Evolution. Nat. Mater. 2019, 18, 1309–1314. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Sun, M.; Zhou, J.; Hao, F.; Zhang, Q.; Ye, C.; Wang, X.; Xu, Z.; Wa, Q.; et al. Controlled Synthesis of Unconventional Phase Alloy Nanobranches for Highly Selective Electrocatalytic Nitrite Reduction to Ammonia. Angew. Chem. Int. Ed. 2024, 63, e202402841. [Google Scholar] [CrossRef]
- Wu, T.; Han, M.-Y.; Xu, Z.J. Size Effects of Electrocatalysts: More than a Variation of Surface Area. ACS Nano 2022, 16, 8531–8539. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Ren, B.; Wang, X.; Tan, L.; Dong, S.; Xiong, H.; Gao, R.; Luo, D.; Duan, X.; Zhu, N.; et al. Constructing a Localized Buffer Interlayer to Elevate High-Rate CO2-to-C2 Electrosynthesis. J. Am. Chem. Soc. 2025, 147, 18110–18121. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Geng, S.; Li, M.; Qiu, L.; Wu, F.; He, L.; Sheng, J.; Zhou, X.; Chen, Z.; Luo, M.; et al. Synergetic Oxidized Mg and Mo Sites on Amorphous Ru Metallene Boost Hydrogen Evolution Electrocatalysis. Adv. Mater. 2025, 37, 2501230. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, S.; Yao, S.; Ji, K.; Wu, J.; Bai, W.; Wang, G.; Yang, J.; Wang, J.; Zhang, F.; et al. Spatial-Confinement-Driven Bubble Self-Management for Compact, High-Performance Water Electrolyzers. Nano Lett. 2025, 25, 12025–12034. [Google Scholar] [CrossRef]
- Yang, X.; Shen, H.; Xiao, X.; Li, Z.; Liang, H.; Chen, S.; Sun, Y.; Jiang, B.; Wen, G.; Wang, S.; et al. Regulating Interfacial H2O Activity and H2 Bubbles by Core/Shell Nanoarrays for 800 h Stable Alkaline Seawater Electrolysis. Adv. Mater. 2025, 37, e2416658. [Google Scholar] [CrossRef]
- Yang, T.; Dong, A.; Liao, W.; Zhang, X.; Ma, Y.; Che, L.; Gao, H. Facile Engineering of CoS@NiS Heterostructures for Efficient Oxygen Evolution Reaction. Nanomaterials 2025, 15, 1216. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, A.; Gao, H.; Wang, G.; Yin, Y.; Che, L.; Gao, H. Boosting Oxygen Evolution Reaction Catalyzed by Transition Metal Carbides. Nanomaterials 2025, 15, 1319. [Google Scholar] [CrossRef]
- Li, Y.; Dou, Z.; Pan, Y.; Zhao, H.; Yao, L.; Wang, Q.; Zhang, C.; Yue, Z.; Zou, Z.; Cheng, Q.; et al. Crystalline Phase Engineering to Modulate the Interfacial Interaction of the Ruthenium/Molybdenum Carbide for Acidic Hydrogen Evolution. Nano Lett. 2024, 24, 5705–5713. [Google Scholar] [CrossRef]
- Geng, S.; Ji, Y.; Su, J.; Hu, Z.; Fang, M.; Wang, D.; Liu, S.; Li, L.; Li, Y.; Chen, J.-M.; et al. Homogeneous Metastable Hexagonal Phase Iridium Enhances Hydrogen Evolution Catalysis. Adv. Sci. 2023, 10, 2206063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Ji, Y.; Liu, X.; Su, D.; Zhuang, Z.; Chang, Y.-C.; Pao, C.-W.; Shao, Q.; Hu, Z.; et al. Atomic-Thick Metastable Phase RhMo Nanosheets for Hydrogen Oxidation Catalysis. Nat. Commun. 2023, 14, 1761. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Chen, K.; Tong, Y.; Lin, C.; Zheng, X.; Chen, P. Phosphorus-Modulated Cobalt Nanosheets with Confined Metal Defects for Enhanced Kinetics in Nitrite-Glycerol Co-Electrolysis. Adv. Funct. Mater. 2025, 35, 2506735. [Google Scholar] [CrossRef]
- Xu, J.; Shao, G.; Tang, X.; Lv, F.; Xiang, H.; Jing, C.; Liu, S.; Dai, S.; Li, Y.; Luo, J.; et al. Frenkel-Defected Monolayer MoS2 Catalysts for Efficient Hydrogen Evolution. Nat. Commun. 2022, 13, 2193. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Sun, Y.; You, B. Dynamic Electrodeposition on Bubbles: An Effective Strategy toward Porous Electrocatalysts for Green Hydrogen Cycling. Acc. Chem. Res. 2023, 56, 1421–1432. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Strmcnik, D.; Lopes, P.P.; Markovic, N.M. Energy and Fuels from Electrochemical Interfaces. Nat. Mater. 2017, 16, 57–69. [Google Scholar] [CrossRef]
- Huang, J.; Xu, X.; Yan, Y.; Zheng, Y.; Yao, Y.; Li, Z.; Yan, Y.; Hui, K.N.; Zou, J.; Liu, M. Facile Microwave Synthesis of Kilogram-Scale Electrocatalysts with Nanocarbons Bridged Cobalt Active Sites for Enhanced Oxygen Electrocatalysis. Adv. Energy Mater. 2025, 15, 2500360. [Google Scholar] [CrossRef]
- Li, W.; Ni, Z.; Akdim, O.; Liu, T.; Zhu, B.; Kuang, P.; Yu, J. Dual Active Site Engineering in Porous NiW Bimetallic Alloys for Enhanced Alkaline Hydrogen Evolution Reaction. Adv. Mater. 2025, 37, 2503742. [Google Scholar] [CrossRef]
- Patel, S.B.; Wang, J.; Chen, X.; Wu, Y.; Acevedo-Esteves, R.; Evans-Lutterodt, K.; Headrick, R.L.; Zhou, G. Redox-Induced Microstructure and Phase Dynamics in Nickel: Insights from In Situ Synchrotron X-Ray Diffraction. J. Am. Chem. Soc. 2025, 147, 27651–27663. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Z.-W.; Gu, K.; Chen, C.; Liu, Y.; Wei, X.; Singh, C.V.; Wang, S. Hexagonal Cobalt Nanosheets for High-Performance Electrocatalytic NO Reduction to NH3. J. Am. Chem. Soc. 2023, 145, 6899–6904. [Google Scholar] [CrossRef]
- Khan, A.; Wei, D.; Wang, Z.; Su, X.; Wang, J.; Alam, S.; Wang, L.; Wu, R.; Maloletnev, A.S.; Yang, C. MOF-Derived Nickel−Cobalt Bimetal Oxide Nanostructures as a Cooperative Catalyst for the Reduction of 4-Nitrophenol. J. Chem. Technol. Biotechnol. 2021, 96, 697–703. [Google Scholar] [CrossRef]
- Sun, C.B.; Guo, M.W.; Siwal, S.S.; Zhang, Q.B. Efficient Hydrogen Production via Urea Electrolysis with Cobalt Doped Nickel Hydroxide-Riched Hybrid Films: Cobalt Doping Effect and Mechanism Aspect. J. Catal. 2020, 381, 454–461. [Google Scholar] [CrossRef]
- Wu, J.; Ge, X.; Li, Z.; Cao, D.; Xiao, J. Highly Dispersed NiCoP Nanoparticles on Carbon Nanotubes Modified Nickel Foam for Efficient Electrocatalytic Hydrogen Production. Electrochim. Acta 2017, 252, 101–108. [Google Scholar] [CrossRef]
- Han, X.; Li, S.; Zhu, X.; An, H.; Zhao, X.; Wang, Y. Influence of Noble Metals on the Catalytic Performance of Ni/TiO2 for Ethanol Guerbet Condensation. React. Kinet. Mech. Catal. 2020, 131, 919–933. [Google Scholar] [CrossRef]
- Liu, Y.; Song, C.; Wang, Y.; Cao, W.; Lei, Y.; Feng, Q.; Chen, Z.; Liang, S.; Xu, L.; Jiang, L. Rational Designed Co@N-Doped Carbon Catalyst for High-Efficient H2S Selective Oxidation by Regulating Electronic Structures. Chem. Eng. J. 2020, 401, 126038. [Google Scholar] [CrossRef]
- Wang, X.; Tian, X.; Duan, X.; Wu, C.; Pei, W.; Wang, K.; Yuan, S.; Wang, Q. Hyperbranched Co2P Nanocrystals with 3D Morphology for Hydrogen Generation in Both Alkaline and Acidic Media. RSC Adv. 2019, 9, 20612–20617. [Google Scholar] [CrossRef]
- Yan, D.; Dou, S.; Tao, L.; Liu, Z.; Liu, Z.; Huo, J.; Wang, S. Electropolymerized Supermolecule Derived N, P Co-Doped Carbon Nanofiber Networks as a Highly Efficient Metal-Free Electrocatalyst for the Hydrogen Evolution Reaction. J. Mater. Chem. A 2016, 4, 13726–13730. [Google Scholar] [CrossRef]
- Su, L.; Chen, J.; Yang, F.; Li, P.; Jin, Y.; Luo, W.; Chen, S. Electric-Double-Layer Origin of the Kinetic pH Effect of Hydrogen Electrocatalysis Revealed by a Universal Hydroxide Adsorption-Dependent Inflection-Point Behavior. J. Am. Chem. Soc. 2023, 145, 12051–12058. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Su, Y.; Tan, H.; Wang, Y.; Huang, Q.; Wang, H.; Wang, J.; Kubo, M.; Ni, Z.; Kong, Y.; et al. Atomically Dispersed Co–P Moieties via Direct Thermal Exfoliation for Alkaline Hydrogen Electrosynthesis. J. Am. Chem. Soc. 2025, 147, 3994–4004. [Google Scholar] [CrossRef]
- Li, Z.; Wang, K.; Tan, X.; Liu, X.; Wang, G.; Xie, G.; Jiang, L. Defect-Enriched Multistage Skeleton Morphology Ni-Fe-P-Ni3S2 Heterogeneous Catalyst on Ni Foam for Efficient Overall Water Splitting. Chem. Eng. J. 2021, 424, 130390. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Liu, W.; Gui, T.; Xiao, L.; Liu, Q.; Ding, J.; Hu, G.; Liu, X. Partial Sulfidation Engineering of a Ni(OH)2/NiSx Heterostructure Towards Sulfion Oxidation-Assisted Seawater Splitting. Mater. Chem. Front. 2025, 9, 2658–2667. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Xia, S.; Fu, X.; Liu, Z. Enhanced Hydrogen Evolution Performance of Co/CoP in Electrolysis of Water by Interfacial Regulation of Crystal Plane Orientation. Int. J. Hydrogen Energy 2024, 67, 1106–1116. [Google Scholar] [CrossRef]
- Wang, X.-D.; Chen, H.-Y.; Xu, Y.-F.; Liao, J.-F.; Chen, B.-X.; Rao, H.-S.; Kuang, D.-B.; Su, C.-Y. Self-Supported NiMoP2 Nanowires on Carbon Cloth as an Efficient and Durable Electrocatalyst for Overall Water Splitting. J. Mater. Chem. A 2017, 5, 7191–7199. [Google Scholar] [CrossRef]
- Fan, H.; Chen, W.; Chen, G.; Huang, J.; Song, C.; Du, Y.; Li, C.; Ostrikov, K.K. Plasma-Heteroatom-Doped Ni-V-Fe Trimetallic Phospho-Nitride as High-Performance Bifunctional Electrocatalyst. Appl. Catal. B 2020, 268, 118440. [Google Scholar] [CrossRef]
- Gan, J.-C.; Jiang, Z.-F.; Fang, K.-M.; Li, X.-S.; Zhang, L.; Feng, J.-J.; Wang, A.-J. Low Rh Doping Accelerated HER/OER Bifunctional Catalytic Activities of Nanoflower-like Ni-Co Sulfide for Greatly Boosting Overall Water Splitting. J. Colloid Interface Sci. 2025, 677, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wei, X.; Yu, Y.; Sun, D.; Qu, Y.; Wen, J.; Yuan, X.; Su, Q.; Meng, F.; Du, G.; et al. In-Situ Construct Ni2P/Ni5P4 Heterostructured Electrocatalyst through Controllable Ni2P Phase Transition for Enhanced HER Performance. J. Mater. Sci. Technol. 2026, 242, 306–316. [Google Scholar] [CrossRef]
- Sundaram, M.M.; Appadoo, D. Traditional Salt-in-Water Electrolyte vs. Water-in-Salt Electrolyte with Binary Metal Oxide for Symmetric Supercapacitors: Capacitive vs. Faradaic. Dalton Trans. 2020, 49, 11743–11755. [Google Scholar] [CrossRef]
- Kulandaivalu, S.; Hussein, M.Z.; Jaafar, A.M.; Abdah, M.A.A.M.; Azman, N.H.N.; Sulaiman, Y. A Simple Strategy to Prepare a Layer-by-Layer Assembled Composite of Ni–Co LDHs on Polypyrrole/rGO for a High Specific Capacitance Supercapacitor. RSC Adv. 2019, 9, 40478–40486. [Google Scholar] [CrossRef]
- Fominykh, K.; Tok, G.C.; Zeller, P.; Hajiyani, H.; Miller, T.; Döblinger, M.; Pentcheva, R.; Bein, T.; Fattakhova-Rohlfing, D. Rock Salt Ni/Co Oxides with Unusual Nanoscale-Stabilized Composition as Water Splitting Electrocatalysts. Adv. Funct. Mater. 2017, 27, 1605121. [Google Scholar] [CrossRef]
- Deabate, S.; Fourgeot, F.; Henn, F. X-Ray Diffraction and Micro-Raman Spectroscopy Analysis of New Nickel Hydroxide Obtained by Electrodialysis. J. Power Sources 2000, 87, 125–136. [Google Scholar] [CrossRef]
- Shieh, S.R.; Duffy, T.S. Raman Spectroscopy of Co(OH)2 at High Pressures: Implications for Amorphization and Hydrogen Repulsion. Phys. Rev. B 2002, 66, 134301. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Zhang, Y.; Xu, Y.; Shang, S.; Yin, Y. Ni–Co Catalyst Derived from Layered Double Hydroxides for Dry Reforming of Methane. Int. J. Hydrogen Energy 2015, 40, 16115–16126. [Google Scholar] [CrossRef]
- Gao, X.; Lu, K.; Chen, J.; Min, J.; Zhu, D.; Tan, M. NiCoP–CoP Heterostructural Nanowires Grown on Hierarchical Ni Foam as a Novel Electrocatalyst for Efficient Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2021, 46, 23205–23213. [Google Scholar] [CrossRef]
- Xue, M.; Bao, Y.; Xu, X.; Liao, L.; Li, P.; Zhang, H.; Li, D.; Wei, B.; Duo, S. Eco-Friendly and Large-Scale Fabrication of NiMoN/Ni(OH)2/NF as Highly Efficient and Stable Alkaline Hydrogen Evolution Reaction Electrode. Int. J. Hydrogen Energy 2024, 82, 655–661. [Google Scholar] [CrossRef]
- Dutta, S.; Han, H.; Je, M.; Choi, H.; Kwon, J.; Park, K.; Indra, A.; Kim, K.M.; Paik, U.; Song, T. Chemical and Structural Engineering of Transition Metal Boride towards Excellent and Sustainable Hydrogen Evolution Reaction. Nano Energy 2020, 67, 104245. [Google Scholar] [CrossRef]
- Zhou, P.; Xing, D.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Qin, X.; Zhang, X.; Dai, Y.; Huang, B. Accelerated Electrocatalytic Hydrogen Evolution on Non-Noble Metal Containing Trinickel Nitride by Introduction of Vanadium Nitride. J. Mater. Chem. A 2019, 7, 5513–5521. [Google Scholar] [CrossRef]
- Zhou, N.; Hu, H.; Chai, X.; Liu, R.; Pei, Z.; Liang, S.; Hu, L.; Yang, L.; Hu, K.; Huang, Z.; et al. Synergistic Heterointerface Engineering of Co9S8/Ni(OH)2 Hierarchical Nanosheets for Remarkable Overall Water Splitting. Int. J. Hydrogen Energy 2024, 63, 114–122. [Google Scholar] [CrossRef]
- Belhani, I.; Bouasla, C.; Meliani, M.H.; Mahdi, M.; Suleiman, R.K.; Laid Rekbi, F.M.; Saleh, T.A. Enhanced Electrocatalytic Activity of Ni-Mn-Co-Fe Alloys for Efficient Hydrogen and Oxygen Evolution Reactions: A Study on the Effects of Electrodeposition Parameters. Renew. Energy 2024, 237, 121688. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Zhang, M.; Shi, M.; Yao, Y.; Liu, Y.; Zhou, J.; Cao, Y.; Li, Z.; Liu, M.; Wang, X.; et al. Hierarchical Porous P-Doped NiCo Alloy with α/ε Phase-Defect Synergy to Boost Alkaline HER Kinetics and Bifunctional Activity. Nanomaterials 2025, 15, 1562. https://doi.org/10.3390/nano15201562
Yang L, Zhang M, Shi M, Yao Y, Liu Y, Zhou J, Cao Y, Li Z, Liu M, Wang X, et al. Hierarchical Porous P-Doped NiCo Alloy with α/ε Phase-Defect Synergy to Boost Alkaline HER Kinetics and Bifunctional Activity. Nanomaterials. 2025; 15(20):1562. https://doi.org/10.3390/nano15201562
Chicago/Turabian StyleYang, Lun, Meng Zhang, Mengran Shi, Yi Yao, Ying Liu, Jianqing Zhou, Yi Cao, Zhong Li, Meifeng Liu, Xiuzhang Wang, and et al. 2025. "Hierarchical Porous P-Doped NiCo Alloy with α/ε Phase-Defect Synergy to Boost Alkaline HER Kinetics and Bifunctional Activity" Nanomaterials 15, no. 20: 1562. https://doi.org/10.3390/nano15201562
APA StyleYang, L., Zhang, M., Shi, M., Yao, Y., Liu, Y., Zhou, J., Cao, Y., Li, Z., Liu, M., Wang, X., Gan, Z., Zhang, H., Chang, S., Zhou, G., & Shan, Y. (2025). Hierarchical Porous P-Doped NiCo Alloy with α/ε Phase-Defect Synergy to Boost Alkaline HER Kinetics and Bifunctional Activity. Nanomaterials, 15(20), 1562. https://doi.org/10.3390/nano15201562









