Thermally Conductive Shape-Stabilized Phase Change Materials Enabled by Paraffin Wax and Nanoporous Structural Expanded Graphite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Paraffin Wax/Expanded Graphite Composite
2.2.1. Preparation of Expanded Graphite
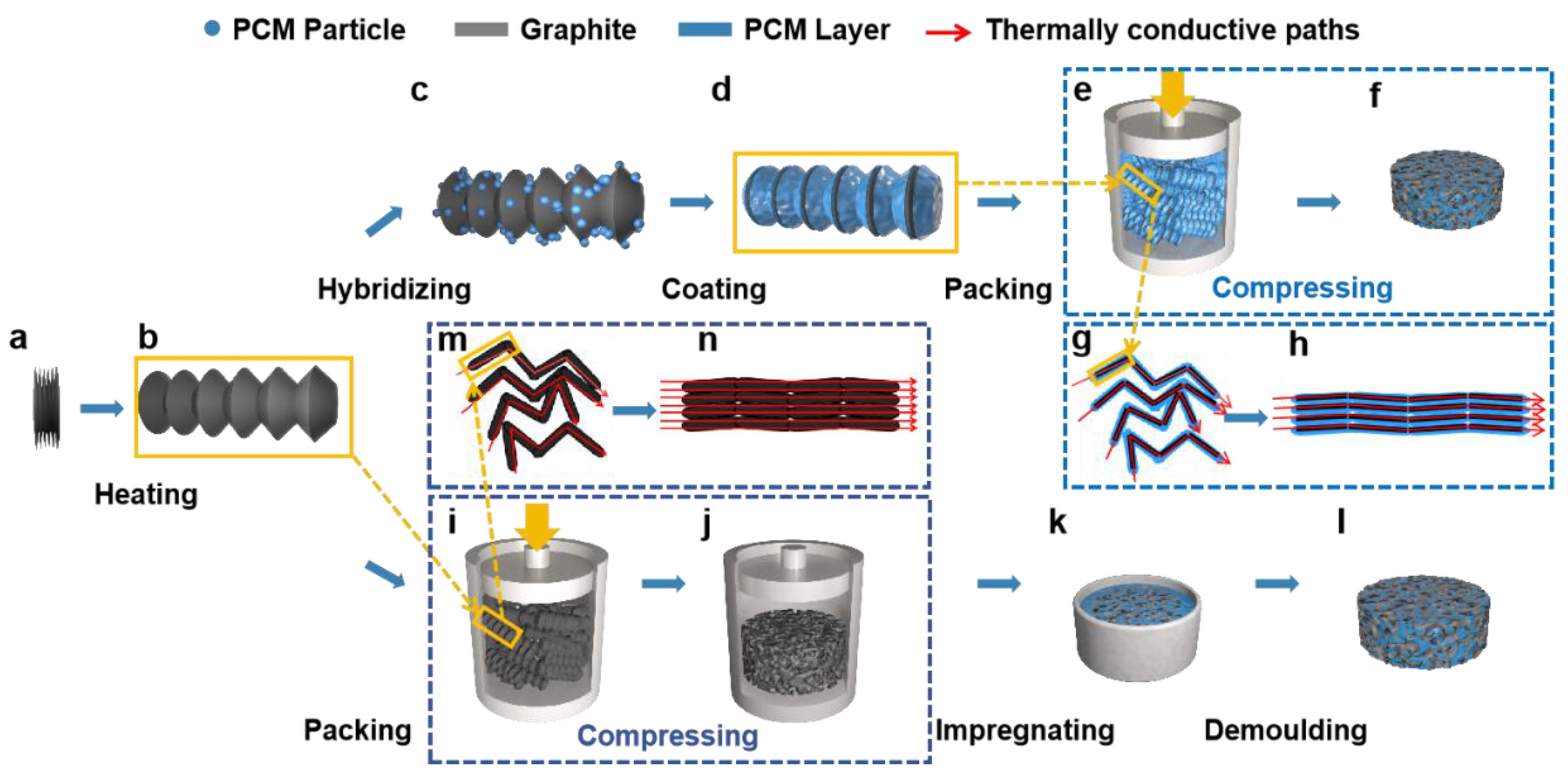
2.2.2. Preparation of PW/EG Composites by Pressure-Induced Method
2.2.3. Preparation of PW/EG Composites by Prefabricated Skeleton Method
2.3. Characterization
3. Results and Discussion
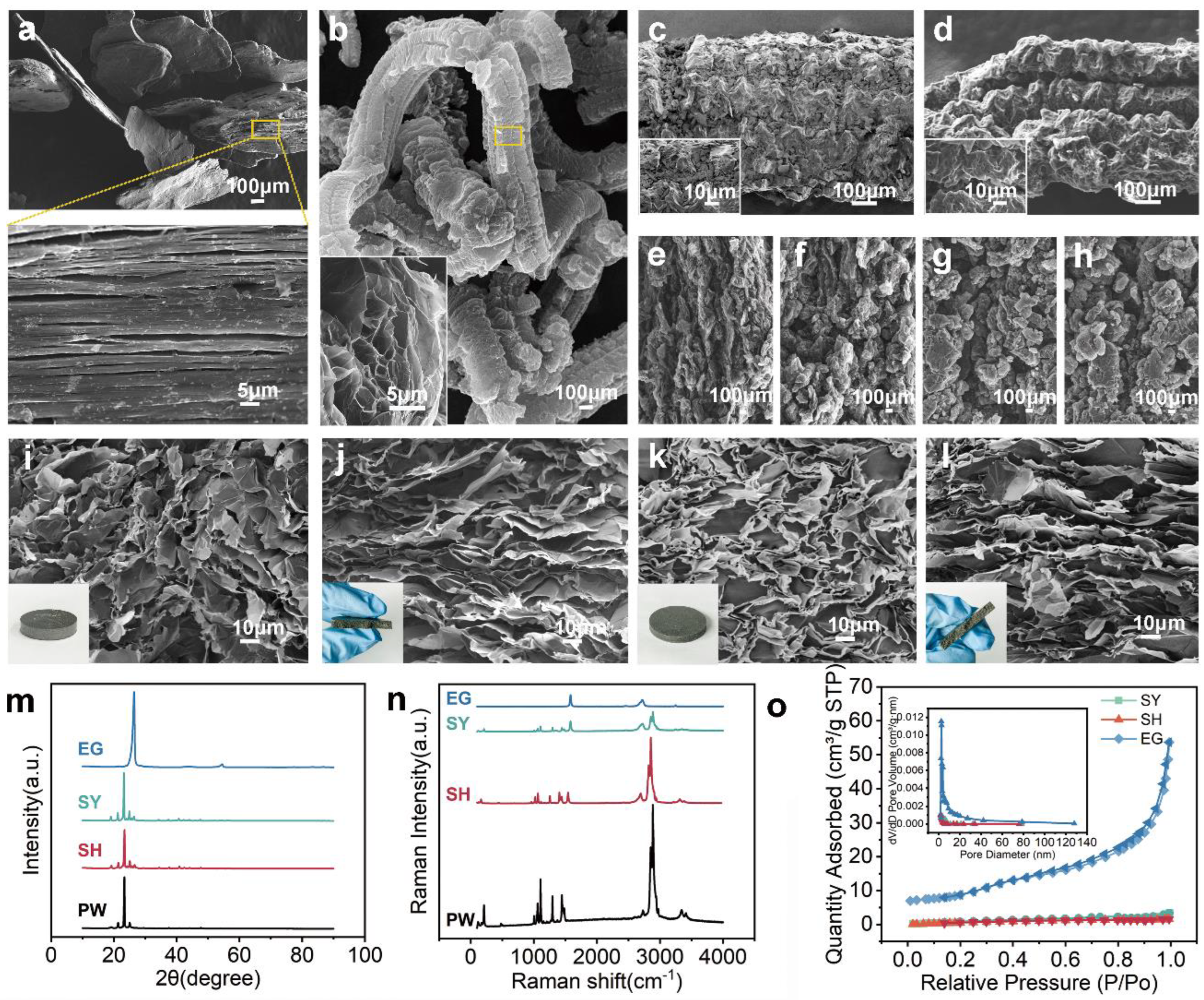
3.1. Characterizations of Morphology and Structure, Physical and Chemical Compatibility
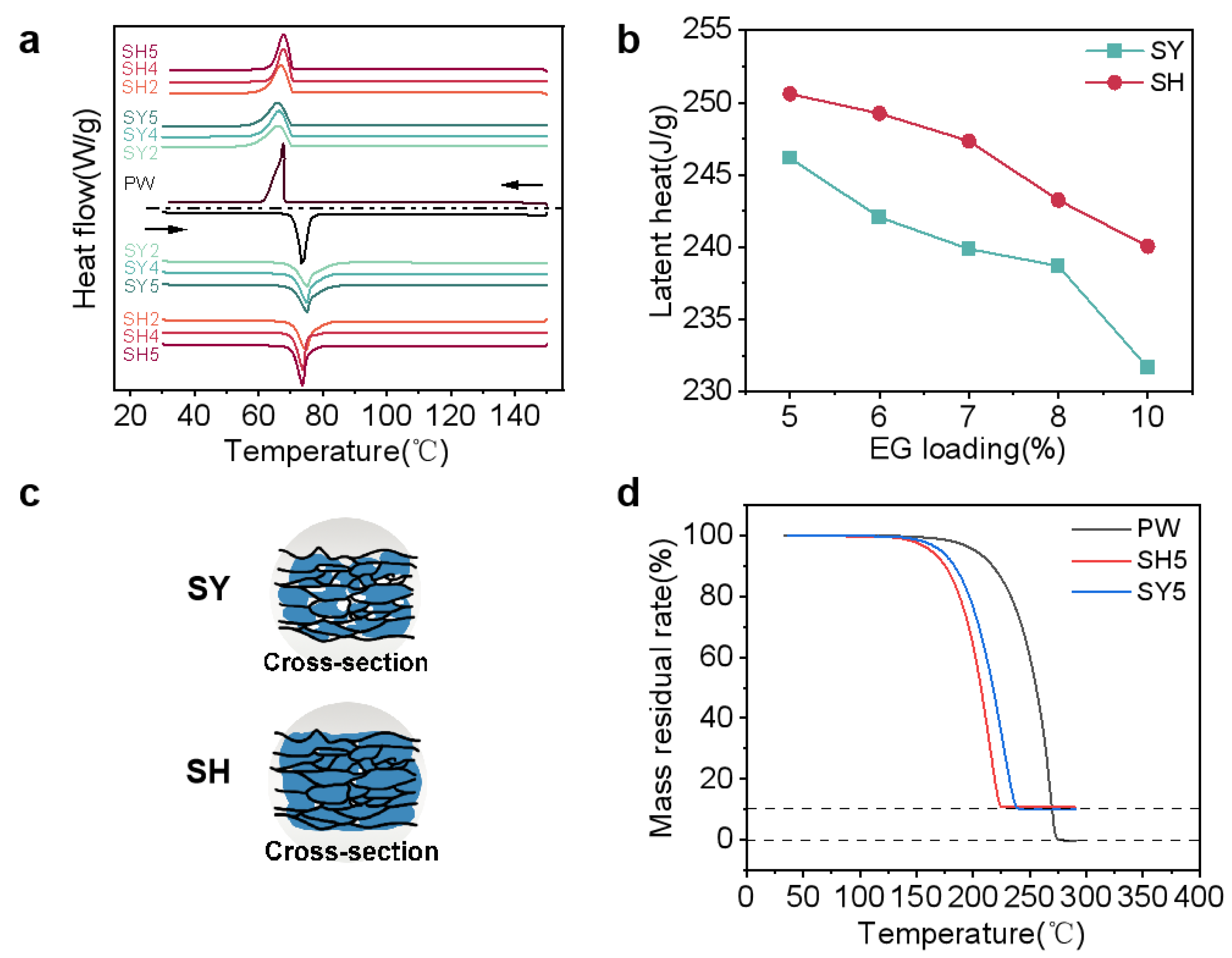
3.2. Phase Change Performance and Thermal Decomposition
3.3. Thermal Conductivity Enhancement
3.4. Shaped Stability and Leakage-Proof Performance
3.5. Thermal Management Test of Electronic Components
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bharathiraja, R.; Ramkumar, T.; Selvakumar, M.; Radhika, N. Thermal characteristics enhancement of Paraffin Wax Phase Change Material (PCM) for thermal storage applications. Renew. Energy 2024, 222, 119986. [Google Scholar] [CrossRef]
- Bianco, N.; Caliano, M.; Fragnito, A.; Iasiello, M.; Mauro, G.M.; Mongibello, L. Thermal analysis of micro-encapsulated phase change material (MEPCM)-based units integrated into a commercial water tank for cold thermal energy storage. Energy 2023, 266, 126479.1–126479.16. [Google Scholar] [CrossRef]
- Nematpourkeshteli, A.; Iasiello, M.; Langella, G.; Bianco, N. Using metal foam and nanoparticle additives with different fin shapes for PCM-based thermal storage in flat plate solar collectors. Therm. Sci. Eng. Prog. 2024, 52, 102690. [Google Scholar] [CrossRef]
- Wang, C.; Ouyang, Y.; Luo, Y.; Gao, X.; Gao, H.; Wang, G.; Shu, X. Review on Recent Advances in Phase Change Materials for Enhancing the Catalytic Process. Chin. J. Catal. 2024, 60, 128–157. [Google Scholar] [CrossRef]
- Emeema, J.; Murali, G.; Reddi, B.V.; Mangesh, V.L. Investigations on Paraffin Wax/CQD Composite Phase Change Material—Improved Latent Heat and Thermal Stability. J. Energy Storage 2024, 85, 1.1–1.11. [Google Scholar] [CrossRef]
- Nishad, S. Thermal Energy Storage Materials Designed from Recycled Tetra Pak Waste and Paraffin Waxes with Enhanced Photothermal Conversion Efficiencies. Energy Built Environ. 2024, in press. [Google Scholar] [CrossRef]
- Yuan, K.; Shi, J.; Aftab, W.; Qin, M.; Usman, A.; Zhou, F.; Lv, Y.; Gao, S.; Zou, R. Engineering the Thermal Conductivity of Functional Phase-Change Materials for Heat Energy Conversion, Storage, and Utilization. Adv. Funct. Mater. 2020, 30, 1904228.1–1904228.31. [Google Scholar] [CrossRef]
- Liu, Z. Preparation and Characterization of Microencapsulated Phase Change Materials Containing Inorganic Hydrated Salt with Silica Shell for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2019, 200, 110004. [Google Scholar] [CrossRef]
- Azizi, Y.; Sadrameli, S.M. Thermal Management of a LiFePO4 Battery Pack at High Temperature Environment Using a Composite of Phase Change Materials and Aluminum Wire Mesh Plates. Energy Convers. Manag. 2016, 128, 294–302. [Google Scholar] [CrossRef]
- Pan, M. Cutting Copper Fiber/Paraffin Composite Phase Change Material Discharging Experimental Study Based on Heat Dissipation Capability of Li-Ion Battery. Renew. Energy 2017, 114 Pt B, 408–422. [Google Scholar] [CrossRef]
- Rehman, T. Copper Foam/PCMs Based Heat Sinks: An Experimental Study for Electronic Cooling Systems. Int. J. Heat Mass Transf. 2018, 127 Pt C, 381–393. [Google Scholar] [CrossRef]
- Cheng, G. 3D Graphene Paraffin Composites Based on Sponge Skeleton for Photo Thermal Conversion and Energy Storage. Appl. Therm. Eng. 2020, 178, 115560. [Google Scholar] [CrossRef]
- Kumar, K. Experimental Investigation of Graphene-Paraffin Wax Nanocomposites for Thermal Energy Storage. Mater. Today 2019, 18, 5158–5163. [Google Scholar] [CrossRef]
- Cui, X.; Cheng, X.; Xu, H.; Li, B.; Zhu, J. Enhancement of Thermophysical Coefficients in Nanofluids: A Simulation Study. Int. J. Mod. Phys. B 2020, 34, 2050222. [Google Scholar] [CrossRef]
- Zou, D.; Ma, X.; Liu, X.; Zheng, P.; Hu, Y. Thermal Performance Enhancement of Composite Phase Change Materials (PCM) Using Graphene and Carbon Nanotubes as Additives for the Potential Application in Lithium-Ion Power Battery. Int. J. Heat Mass Transf. 2018, 120, 33–41. [Google Scholar] [CrossRef]
- Zhang, S. Experimental Investigations of Alum/Expanded Graphite Composite Phase Change Material for Thermal Energy Storage and Its Compatibility with Metals. Energy 2018, 161, 508–516. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Fang, G.-Y. Synthesis and Characteristics of Form-Stable n-Octadecane/Expanded Graphite Composite Phase Change Materials. Appl. Phys. A 2010, 100, 1143–1148. [Google Scholar] [CrossRef]
- Xia, L.; Zhang, P.; Wang, R.Z. Preparation and Thermal Characterization of Expanded Graphite/Paraffin Composite Phase Change Material. Carbon 2010, 48, 2538–2548. [Google Scholar] [CrossRef]
- Fang, G.; Li, H.; Chen, Z.; Liu, X. Preparation and Characterization of Stearic Acid/Expanded Graphite Composites as Thermal Energy Storage Materials. Energy 2010, 35, 4622–4626. [Google Scholar] [CrossRef]
- Yan, J.; Han, X.; Dang, Z.; Li, J.; He, X. Thermal-Conductivity-Enhancing Copper-Plated Expanded Graphite/Paraffin Composite for Highly Stable Phase-Change Materials. ChemPhysChem 2023, 24, e202300320. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, S.; Wei, X.; Liu, Z.; Guo, Q.; Shi, J.; Liu, L. Heat transfer enhancement of paraffin wax using compressed expanded natural graphite for thermal energy storage. Carbon 2010, 48, 300–304. [Google Scholar] [CrossRef]
- Li, Y.; Yan, H.; Yang, F.; Li, S.; Jiang, C.; Xie, L. Paraffin Wax–Expanded Graphite Composite Phase Change Materials with Enhancing Thermal Conductivity and Thermal Stability for Thermal Energy Storage. Chem. Sel. 2024, 9, e202404879. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Gong, Y.; Chen, M. A Novel Paraffin Wax/Expanded Graphite/Bacterial Cellulose Powder Phase Change Materials for the Dependable Battery Safety Management. Batterie 2024, 10, 363. [Google Scholar] [CrossRef]
- Yu, X.K.; Tao, Y.B. Preparation and characterization of paraffin/expanded graphite composite phase change materials with high thermal conductivity. Int. J. Heat Mass Transf. 2022, 198, 123433. [Google Scholar] [CrossRef]
- Shi, T.; Zhang, X.; Qiao, J.; Wu, X.; Chen, G.; Leng, G.; Lin, F.; Min, X.; Huang, Z. Preparation and Characterization of Composite Phase Change Materials Based on Paraffin and Carbon Foams Derived from Starch. Polymer 2021, 212, 123143. [Google Scholar] [CrossRef]
- Hou, B.; Sun, H.; Peng, T.; Zhang, X.; Ren, Y. Rapid Preparation of Expanded Graphite at Low Temperature. New Carbon Mater. 2020, 35, 262–268. [Google Scholar] [CrossRef]
- Deng, R.; Chu, F.; Yu, H.; Kwofie, F.; Qian, M.; Zhou, Y.; Wu, F. Electrochemical Performance of Expanded Graphite Prepared from Anthracite via a Microwave Method. Fuel Process. Technol. 2022, 227, 107100. [Google Scholar] [CrossRef]
- Wei, Q.; Xu, L.; Tang, Z.; Xu, Z.; Xie, C.; Guo, L.; Li, W. High-Performance Expanded Graphite from Flake Graphite by Microwave-Assisted Chemical Intercalation Process. J. Ind. Eng. Chem. 2023, 122, 562–572. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.; Tong, Z.; Chao, J.; Zhai, T.; Xu, J.; Yan, T.; Wu, M.; Xu, Z.; Bao, H.; et al. High-Performance Thermally Conductive Phase Change Composites by Large-Size Oriented Graphite Sheets for Scalable Thermal Energy Harvesting. Adv. Mater. 2019, 31, 1905099. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Cheng, X.; Li, Y.; Shi, D.; Wang, X.; Zhong, H. Enhancement of Ceramic Foam Modified Hierarchical Al2O3@expanded Graphite on Thermal Properties of 1-Octadecanol Phase Change Materials. J. Energy Storage 2019, 26, 101025. [Google Scholar] [CrossRef]
- Chen, W.; Liang, X.; Wang, S.; Ding, Y.; Gao, X.; Zhang, Z.; Fang, Y. SiO2 Hydrophilic Modification of Expanded Graphite to Fabricate Form-Stable Ternary Nitrate Composite Room Temperature Phase Change Material for Thermal Energy Storage. Chem. Eng. J. 2021, 413, 127549. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A.; Kaygusuz, K. Thermal Conductivity Improvement of Stearic Acid Using Expanded Graphite and Carbon Fiber for Energy Storage Applications. Renew. Energy 2007, 32, 2201–2210. [Google Scholar] [CrossRef]
- Wu, M. Thermally Conductive and Form-Stable Phase Change Composite for Building Thermal Management. Energy 2022, 239 Pt A2, 121938.1–121938.9. [Google Scholar]
- Fang, Y.; Ding, Y.; Tang, Y.; Liang, X.; Jin, C.; Wang, S.; Gao, X.; Zhang, Z. Thermal Properties Enhancement and Application of a Novel Sodium Acetate Trihydrate-Formamide/Expanded Graphite Shape-Stabilized Composite Phase Change Material for Electric Radiant Floor Heating. Appl. Therm. Eng. 2019, 150, 1177–1185. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Gan, J.; Zhu, F.-R.; Yu, S.-B.; Xiao, Z.-L.; Yan, W.-P.; Zhu, L.; Liu, Z.-Q.; Sun, L.-X.; Cao, Z. Tetradecanol/Expanded Graphite Composite Form-Stable Phase Change Material for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2014, 127, 122–128. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, Y.; Zhang, N.; Cao, X.; Liu, C. Preparation and Properties of Myristic–Palmitic–Stearic Acid/Expanded Graphite Composites as Phase Change Materials for Energy Storage. Sol. Energy 2014, 99, 259–266. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Wang, X.; Cao, X.; Yang, X.; Hu, S. Preparation and Characterization of Lauric–Myristic–Palmitic Acid Ternary Eutectic Mixtures/Expanded Graphite Composite Phase Change Material for Thermal Energy Storage. Chem. Eng. J. 2013, 231, 214–219. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, J.; An, Q.; Zhao, D.; Meng, F.; Liu, X. Experiments on Thermal Performance of Erythritol/Expanded Graphite in a Direct Contact Thermal Energy Storage Container. Appl. Therm. Eng. 2017, 113, 858–866. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K.; Kahwash, F.; Makhkamova, I. Enhancing Thermal Conductivity of Paraffin Wax 53–57 °C Using Expanded Graphite. Sol. Energy Mater. Sol. Cells 2019, 200, 110026. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, F.; Zhang, B.; Shen, M.; Zhang, Y. Preparation of Coconut Oil/Aluminum Nitride/Expanded Graphite Composite Phase Change Materials with High Thermal Conductivity and Stable Shape for Thermal Energy Storage. Chin. J. Chem. Eng. 2024, 76, S1004954124003045. [Google Scholar] [CrossRef]
- Zhelezny, V. Experimental Study of Phase Transition Heat of Composite Thermal Energy Storage Materials Paraffin Wax/Expanded Graphite. J. Energy Storage 2024, 77, 110174.1–110174.15. [Google Scholar] [CrossRef]
- Lin, J. Enhancing the Solar Absorption Capacity of Expanded Graphite-Paraffin Wax Composite Phase Change Materials by Introducing Carbon Nanotubes Additives. Surf. Interfaces 2022, 30, 101871. [Google Scholar] [CrossRef]
- Li, X. Melting and Solidification Performance of Latent Heat Thermal Energy Storage System under Flip Condition. Int. J. Heat Mass Transf. 2025, 236, 126370. [Google Scholar] [CrossRef]
- Kabir, M. Design and Development of a PCM-Based Two-Phase Heat Exchanger Manufactured Additively for Spacecraft Thermal Management Systems. Int. J. Heat Mass Transf. 2021, 180, 121782. [Google Scholar] [CrossRef]
- Peng, H. Melting Behavior and Heat Transfer Performance of Gallium for Spacecraft Thermal Energy Storage Application. Energy 2021, 228, 120575.1–120575.14. [Google Scholar] [CrossRef]
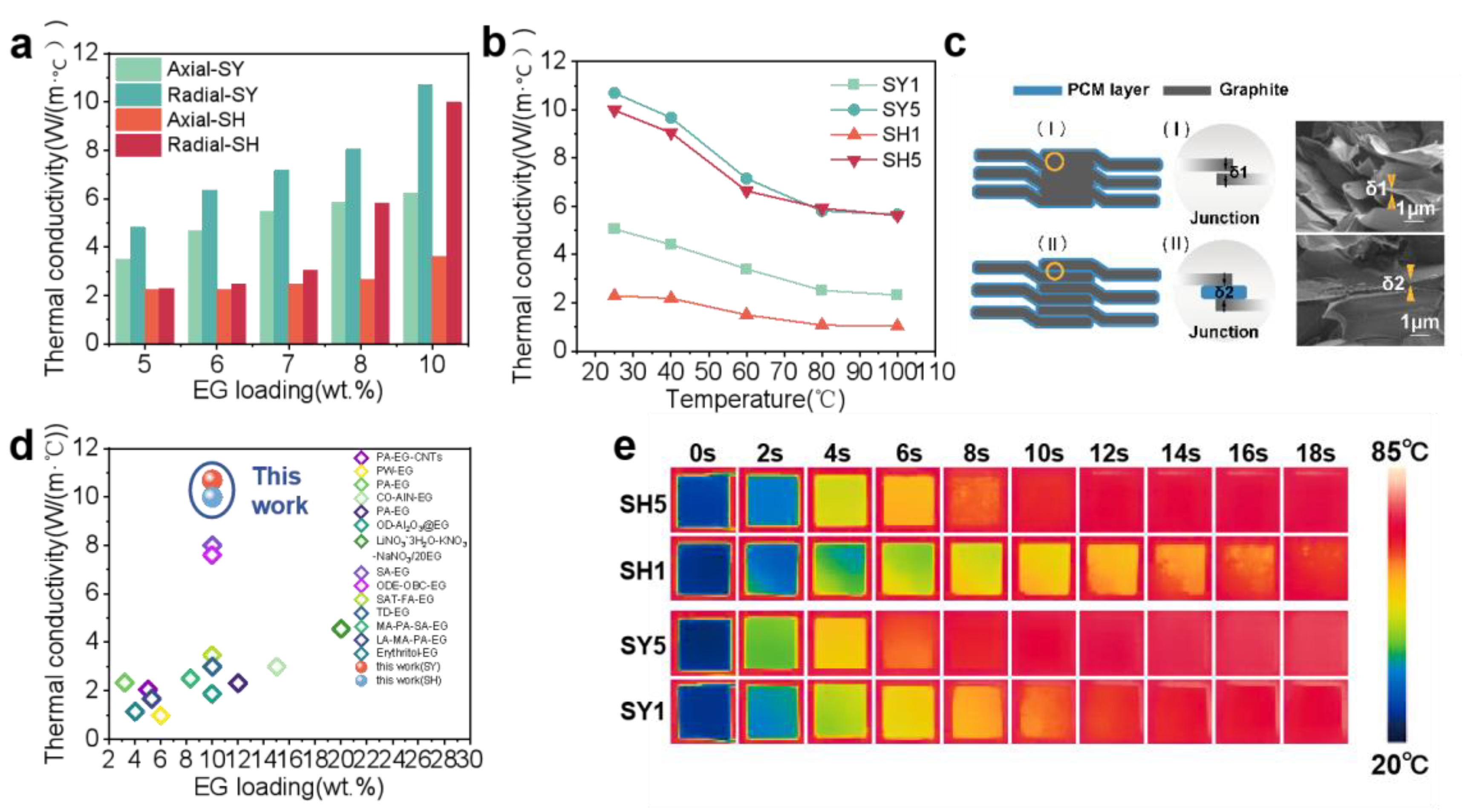
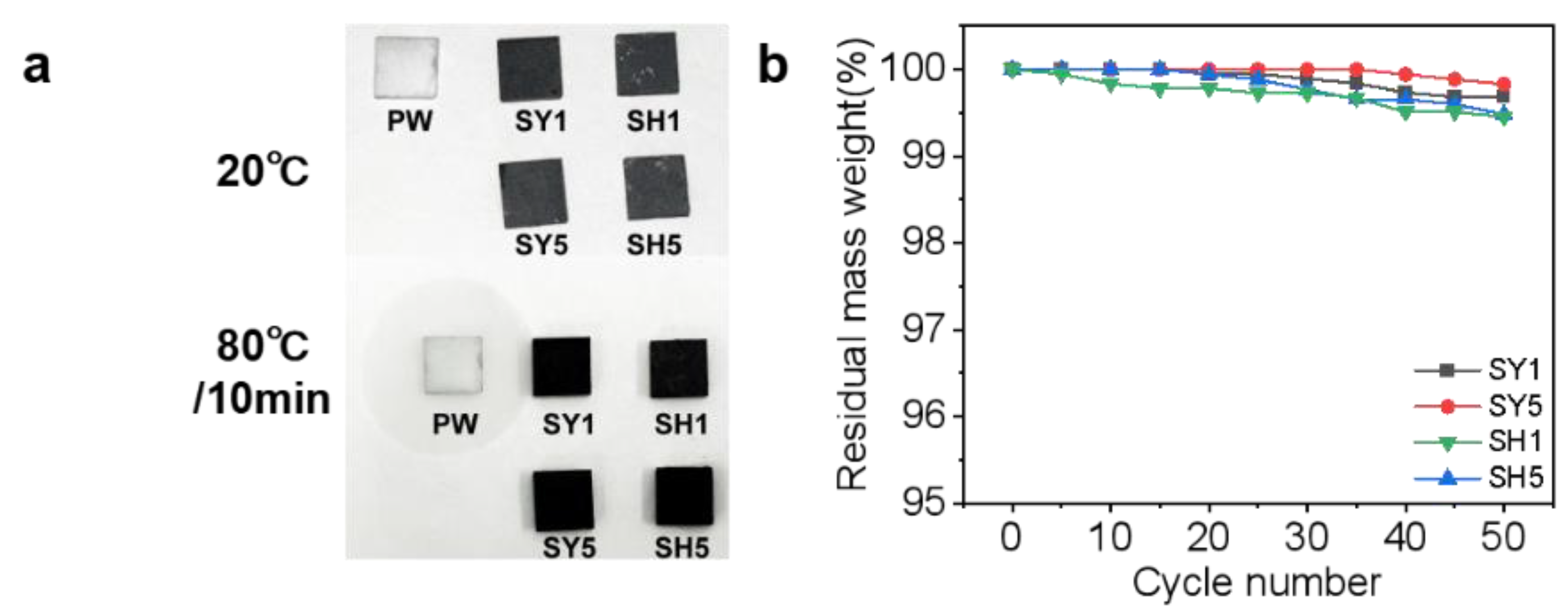
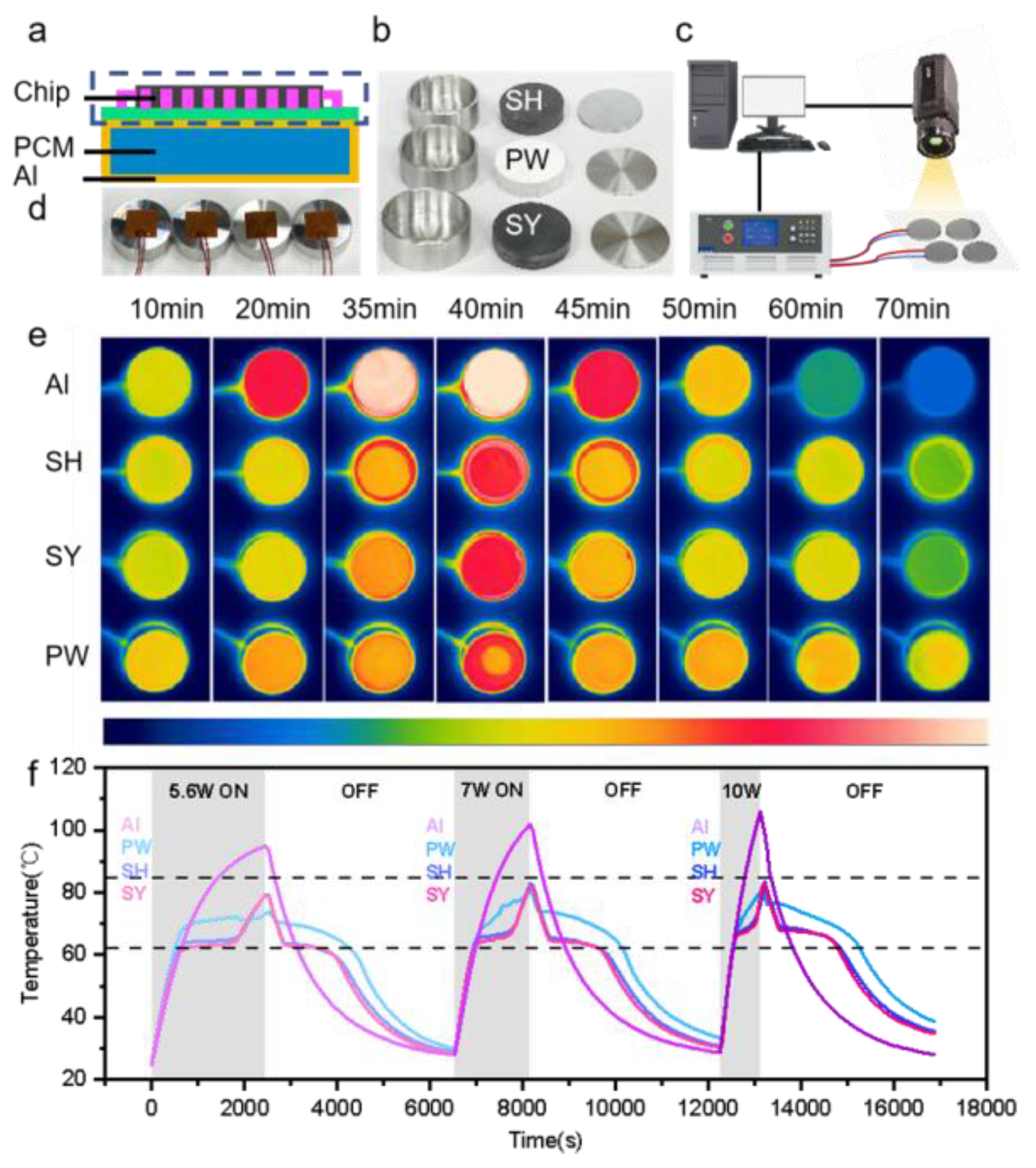
| References | Articles | Loading (wt.%) | Thermal Conductivity W/(m·K)) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Vacuum impregnation | Yan (2023) [20] | 80PW-14EG-6CPEG | 4.94 | High impregnation rate, less leakage | Complex process, difficult to handle high-temperature PCMs |
| Zhong (2009) [21] | 92PW-8EG | 8 | |||
| Li (2024) [22] | 90PW-10EG | 2.74 | |||
| Melt blending | Zhao (2024) [23] | 90PW-5EG-5BC | 1.27 | Simple process, low cost | Incomplete impregnation |
| Yu (2022) [24] | 92PW-8EG | 2.29 | |||
| Xia (2010) [18] | 90PE-10EG | 3.83 |
| Samples | SH1 | SH2 | SH3 | SH4 | SH5 |
|---|---|---|---|---|---|
| Density (g/cm3) | 0.988 | 1.009 | 1.028 | 1.047 | 1.103 |
| Mass fraction (wt.%) | 5 | 6 | 7 | 8 | 10 |
| Samples | SY1 | SY2 | SY3 | SY4 | SY5 |
|---|---|---|---|---|---|
| Density (g/cm3) | 0.946 | 0.960 | 0.972 | 0.987 | 1.013 |
| Mass fraction (wt.%) | 5 | 6 | 7 | 8 | 10 |
| Samples | Tm (°C) | ΔHm/ΔHcal (J/g) | Ts (°C) | ΔHs/ΔHcal (J/g) |
|---|---|---|---|---|
| PW | 73.40 | 260.85/260.85 | 67.68 | 256.63/256.63 |
| SY1 | 73.92 | 246.17/247.81 | 67.62 | 242.53/243.80 |
| SY2 | 74.08 | 242.07/245.20 | 66.32 | 238.04/241.23 |
| SY3 | 74.68 | 239.88/242.59 | 66.26 | 230.32/238.67 |
| SY4 | 74.14 | 238.70/239.98 | 66.44 | 229.20/236.10 |
| SY5 | 74.18 | 231.67/234.77 | 66.66 | 225.48/230.99 |
| SH1 | 75.85 | 250.60/247.81 | 66.26 | 246.91/243.80 |
| SH2 | 74.95 | 249.25/245.20 | 66.95 | 245.55/241.23 |
| SH3 | 74.27 | 247.34/242.59 | 67.48 | 234.88/238.67 |
| SH4 | 73.92 | 243.26/239.98 | 67.71 | 233.93/236.10 |
| SH5 | 73.72 | 240.06/234.77 | 67.82 | 231.98/230.99 |
| Samples | BET Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|
| SY5 | 2.1882 | 6.100 × 10−3 |
| SH5 | 2.1090 | 3.895 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Huang, S.; Jin, Z.; Xie, Z.; Guo, H.; Xie, H. Thermally Conductive Shape-Stabilized Phase Change Materials Enabled by Paraffin Wax and Nanoporous Structural Expanded Graphite. Nanomaterials 2025, 15, 110. https://doi.org/10.3390/nano15020110
Zhao Y, Huang S, Jin Z, Xie Z, Guo H, Xie H. Thermally Conductive Shape-Stabilized Phase Change Materials Enabled by Paraffin Wax and Nanoporous Structural Expanded Graphite. Nanomaterials. 2025; 15(2):110. https://doi.org/10.3390/nano15020110
Chicago/Turabian StyleZhao, Yilin, Shuhui Huang, Zhaoguo Jin, Zhongnan Xie, Hong Guo, and Haofeng Xie. 2025. "Thermally Conductive Shape-Stabilized Phase Change Materials Enabled by Paraffin Wax and Nanoporous Structural Expanded Graphite" Nanomaterials 15, no. 2: 110. https://doi.org/10.3390/nano15020110
APA StyleZhao, Y., Huang, S., Jin, Z., Xie, Z., Guo, H., & Xie, H. (2025). Thermally Conductive Shape-Stabilized Phase Change Materials Enabled by Paraffin Wax and Nanoporous Structural Expanded Graphite. Nanomaterials, 15(2), 110. https://doi.org/10.3390/nano15020110





