A Comprehensive Review of Niobium Nanoparticles: Synthesis, Characterization, Applications in Health Sciences, and Future Challenges
Abstract
1. Introduction
2. Synthesis and Characterization of Niobium Nanoparticles
2.1. Synthesis Methods
2.2. Characterization Techniques
2.3. Properties of Niobium Nanoparticles (Physicochemical)
2.4. Biocompatibility and Toxicity
2.4.1. In Vitro Studies
2.4.2. In Vivo Studies
2.5. Biodegradability and Clearance Mechanisms
3. Applications of Niobium in Health Sciences
3.1. Niobium in Drug Delivery Systems and Therapeutic Applications
3.2. Niobium as an Imaging Agent
3.3. Biosensing
4. Challenges and Future Perspectives
5. Author Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldberg, M.; Langer, R.; Jia, X. Nanostructured Materials for Applications in Drug Delivery and Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2007, 18, 241–268. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.A.; Saleh, A.M. Applications of Nanoparticle Systems in Drug Delivery Technology. Saudi Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, M.H.; Islam, M.A.; Hossain, N.; Al Mahmud, M.Z.; Rayhan, M.T.; Nishi, N.J.; Chowdhury, M.A. Recent Advances of Additive Manufacturing in Implant Fabrication—A Review. Appl. Surf. Sci. Adv. 2023, 18, 100462. [Google Scholar] [CrossRef]
- Nico, C.; Rino, L.; Matos, M.; Monteiro, R.; Costa, F.M.; Monteiro, T.; Graça, M.P.F. NbO/Nb2O5 Core–Shells by Thermal Oxidation. J. Eur. Ceram. Soc 2013, 33, 3077–3083. [Google Scholar] [CrossRef]
- Raba, A.M.; Bautista-Ruíz, J.; Joya, M.R. Synthesis and Structural Properties of Niobium Pentoxide Powders: A Comparative Study of the Growth Process. Mater. Res. 2016, 19, 1381–1387. [Google Scholar] [CrossRef]
- Borowski, T.; Zielińska, K.; Spychalski, M.; Adamczyk-Cieślak, B.; Żrodowski, Ł. Effect of Oxidation Temperature on the Properties of Niobium in View of Its Biomedical Applications. Surf. Coat. Technol. 2023, 473, 129911. [Google Scholar] [CrossRef]
- Dsouki, N.A.; de Lima, M.P.; Corazzini, R.; Gáscon, T.M.; Azzalis, L.A.; Junqueira, V.B.C.; Feder, D.; Fonseca, F.L.A. Cytotoxic, Hematologic and Histologic Effects of Niobium Pentoxide in Swiss Mice. J. Mater. Sci. Mater. Med. 2014, 25, 1301–1305. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing Nanotechnology to Improve Targeted Cancer Treatment: Overcoming Hurdles in Its Clinical Implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Lima, L.F.d.S.; Coelho, C.R.; Gomes, G.H.M.; Mohallem, N.D.S. Nb2O5/SiO2 Mesoporous Monoliths Synthetized by Sol–Gel Process Using Ammonium Niobate Oxalate Hydrate as Porogenic Agent. J. Sol-Gel Sci. Technol. 2020, 93, 168–174. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Meireles, A.; Nico, C.; Valente, M.A. Nb2O5 Nanosize Powders Prepared by Sol–Gel—Structure, Morphology and Dielectric Properties. J. Alloys Compd. 2013, 553, 177–182. [Google Scholar] [CrossRef]
- Ristić, M.; Popović, S.; Musić, S. Sol–Gel Synthesis and Characterization of Nb2O5 Powders. Mater. Lett. 2004, 58, 2658–2663. [Google Scholar] [CrossRef]
- Griesmar, P.; Papin, G.; Sanchez, C.; Livage, J. Sol-gel route to niobium pentoxide. Chem. Mater. 1991, 3, 335–339. [Google Scholar] [CrossRef]
- Ohtani, B.; Iwai, K.; Nishimoto, S.I.; Inui, T. Electrochromism of Niobium Oxide Thin Films Prepared by the Sol-Gel Process. J. Electrochem. Soc. 1994, 141, 2439. [Google Scholar] [CrossRef]
- Maček, M.; Orel, B. Electrochromism of Sol–Gel Derived Niobium Oxide Films. Sol. Energy Mater. Sol. Cells 1998, 54, 121–130. [Google Scholar] [CrossRef]
- Özer, N.; Rubin, M.D.; Lampert, C.M. Optical and Electrochemical Characteristics of Niobium Oxide Films Prepared by Sol-Gel Process and Magnetron Sputtering A Comparison. Sol. Energy Mater. Sol. Cells 1996, 40, 285–296. [Google Scholar] [CrossRef]
- Francisco, M.S.P.; Gushikem, Y. Synthesis and Characterization of SiO2–Nb2O5 Systems Prepared by the Sol–Gel Method: Structural Stability Studies. J. Mater. Chem. 2002, 12, 2552–2558. [Google Scholar] [CrossRef]
- Aronne, A.; Marenna, E.; Califano, V.; Fanelli, E.; Pernice, P.; Trifuoggi, M.; Vergara, A. Sol–Gel Synthesis and Structural Characterization of Niobium-Silicon Mixed-Oxide Nanocomposites. J. Sol-Gel Sci. Technol. 2007, 43, 193–204. [Google Scholar] [CrossRef]
- Joshi, B.N.; Yoon, H.; Hest, M.F.; Yoon, S.S. Niobium-doped titania photocatalyst film prepared via a nonaqueous sol-gel method. J. Am. Ceram. Soc. 2013, 96, 2623–2627. [Google Scholar] [CrossRef]
- Mohammadi, S.; Golobostanfard, M.R.; Abdizadeh, H. Synthesis and Characterization of Indium Niobium Oxide Thin Films via Sol–Gel Spin Coating Method. J. Mater. Sci. Technol. 2013, 29, 923–928. [Google Scholar] [CrossRef]
- Duta, M.; Predoana, L.; Calderon-Moreno, J.M.; Preda, S.; Anastasescu, M.; Marin, A.; Gartner, M. Nb-Doped TiO2 Sol–Gel Films for CO Sensing Applications. Mater. Sci. Semicond. Process. 2016, 42, 397–404. [Google Scholar] [CrossRef]
- Joya, M.R.; Barba Ortega, J.J.; Raba Paez, A.M.; Silva Filho, J.G.; Cavalcante Freire, P.D.T. Synthesis and Characterization of Nano-Particles of Niobium Pentoxide with Orthorhombic Symmetry. Metals 2017, 7, 142. [Google Scholar] [CrossRef]
- Jafari, H.; Sadeghzadeh, S.; Rabbani, M.; Rahimi, R. Effect of Nb on the Structural, Optical and Photocatalytic Properties of Al-Doped ZnO Thin Films Fabricated by the Sol-Gel Method. Ceram. Int. 2018, 44, 20170–20177. [Google Scholar] [CrossRef]
- Lucas, J.M.F.; Soreto Teixeira, S.; Gavinho, S.R.; Prezas, P.R.; Silva, C.C.; Sales, A.J.M.; Graça, M.P.F. Niobium Oxide Prepared by Sol–Gel Using Powder Coconut Water. J. Mater. Sci. Mater. Electron. 2019, 30, 11346–11353. [Google Scholar] [CrossRef]
- Baek, H.; Lee, C.; Choi, J.; Cho, J. Nonvolatile Memory Devices Prepared from Sol–Gel Derived Niobium Pentoxide Films. Langmuir 2013, 29, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Qiu, Z.; Lü, M.; Zhang, A.; Ma, Q. Preparation and Characterization of Porous Nb2O5 Nanoparticles. Mater. Res. Bull. 2008, 43, 1363–1368. [Google Scholar] [CrossRef]
- Fuchigami, T.; Kakimoto, K. Spiky Niobium Oxide Nanoparticles through Hydrothermal Synthesis. J. Mater. Res. 2017, 32, 3326–3332. [Google Scholar] [CrossRef]
- Santos, I.; Loureiro, L.H.; Silva, M.F.P.; Cavaleiro, A.M. Studies on the Hydrothermal Synthesis of Niobium Oxides. Polyhedron 2002, 21, 2009–2015. [Google Scholar] [CrossRef]
- Vega, M.R.O.; Consul, V.; Cadorin, M.; Arenas, L.T.; Aguzzoli, C.; Hübler, R.; Takimi, A.; de Fraga Malfatti, C. Niobium Oxide Nanorods Obtained by Hydrothermal Synthesis—Structure, Morphology, and Electrochemical Detection of Oxygen via Oxygen Reduction Reaction. Coatings 2023, 13, 1786. [Google Scholar] [CrossRef]
- Silva, R.M.; Noremberg, B.S.; Marins, N.H.; Milne, J.; Zhitomirsky, I.; Carreño, N.L. Microwave-Assisted Hydrothermal Synthesis and Electrochemical Characterization of Niobium Pentoxide/Carbon Nanotubes Composites. J. Mater. Res. 2019, 34, 592–599. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Yang, D.; Li, Y.; Liu, H.; Liu, P.; Zhao, H. Directly Hydrothermal Growth of Single Crystal Nb3O7(OH) Nanorod Film for High Performance Dye-Sensitized Solar Cells. Adv. Mater. 2012, 24, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Ma, X. Nb2O5-Carbon Core-Shell Nanocomposite as Anode Material for Lithium Ion Battery. J. Energy Chem. 2013, 22, 357–362. [Google Scholar] [CrossRef]
- Liu, M.; Xue, D. Large-Scale Fabrication of H2(H2O)Nb2O6 and Nb2O5 Hollow Microspheres. Mater. Res. Bull. 2010, 45, 333–338. [Google Scholar] [CrossRef][Green Version]
- Lopes, O.F.; Paris, E.C.; Ribeiro, C. Synthesis of Nb2O5 Nanoparticles through the Oxidant Peroxide Method Applied to Organic Pollutant Photodegradation: A Mechanistic Study. Appl. Catal. B Environ. 2014, 144, 800–808. [Google Scholar] [CrossRef]
- Wen, H.; Liu, Z.; Wang, J.; Yang, Q.; Li, Y.; Yu, J. Facile Synthesis of Nb2O5 Nanorod Array Films and Their Electrochemical Properties. Appl. Surf. Sci. 2011, 257, 10084–10088. [Google Scholar] [CrossRef]
- Liu, F.; Xue, D. Fabrication of Nb2O5 Nanotrees with Controlled Branching Degrees. Phys. Scr. 2010, 014074. [Google Scholar] [CrossRef]
- Zhao, Y.; Eley, C.; Hu, J.; Foord, J.S.; Ye, L.; He, H.; Tsang, S.C.E. Shape-Dependent Acidity and Photocatalytic Activity of Nb2O5 Nanocrystals with an Active TT (001) Surface. Angew. Chem.-Int. Ed. 2012, 51, 3846. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Dou, J.; Chen, L.; Lin, J.; Zeng, H.C. Antisolvent Precipitation for the Synthesis of Monodisperse Mesoporous Niobium Oxide Spheres as Highly Effective Solid Acid Catalysts. ChemCatChem 2012, 4, 1675–1682. [Google Scholar] [CrossRef]
- Ayudhya, S.K.N.; Soottitantawat, A.; Praserthdam, P.; Satayaprasert, C. Effect of Aging on the Properties of Mesoporous Niobium Oxide. Mater. Chem. Phys. 2008, 110, 387–392. [Google Scholar] [CrossRef]
- Luo, H.; Wei, M.; Wei, K. Synthesis of Nb2O5 Nanosheets and Its Electrochemical Measurements. Mater. Chem. Phys. 2010, 120, 6–9. [Google Scholar] [CrossRef]
- Luo, H.; Wei, M.; Wei, K. Synthesis of Nb2O5 Nanorods by a Soft Chemical Process. J. Nanomater. 2009, 2009, 758353. [Google Scholar] [CrossRef]
- Hu, W.; Zhao, Y.; Liu, Z.; Zhu, Y. NbS2/Nb2O5 nanocables. Nanotechnology 2007, 18, 095605. [Google Scholar] [CrossRef]
- Wei, M.; Qi, Z.M.; Ichihara, M.; Zhou, H. Synthesis of single-crystal niobium pentoxide nanobelts. Acta Mater. 2008, 56, 2488–2494. [Google Scholar] [CrossRef]
- Kominami, H.; Oki, K.; Kohno, M.; Onoue, S.I.; Kera, Y.; Ohtani, B. Novel Solvothermal Synthesis of Niobium (V) Oxide Powders and Their Photocatalytic Activity in Aqueous Suspensions. J. Mater. Chem. 2001, 11, 604–609. [Google Scholar] [CrossRef]
- Buha, J.; Arčon, D.; Niederberger, M.; Djerdj, I. Solvothermal and Surfactant-Free Synthesis of Crystalline Nb2O5, Ta2O5, HfO2, and Co-Doped HfO2 Nanoparticles. Phys. Chem. Chem. Phys. 2010, 12, 15537–15543. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Liu, Z.; Tian, D.; Zhang, S.; Zhao, Y.; Yao, K. Morphological Evolution of Nb2O5 in a Solvothermal Reaction: From Nb2O5 Grains to Nb2O5 Nanorods and Hexagonal Nb2O5 Nanoplatelets. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2009, 24, 245–248. [Google Scholar] [CrossRef]
- Prado, N.T.; Oliveira, L.C. Nanostructured Niobium Oxide Synthetized by a New Route Using Hydrothermal Treatment: High Efficiency in Oxidation Reactions. Appl. Catal. B Environ. 2017, 205, 481–488. [Google Scholar] [CrossRef]
- Prado, N.T.; Souza, T.E.; Machado, A.R.T.; Souza, P.P.; Monteiro, R.S.; Oliveira, L.C. Enhanced Catalytic Activity for Fructose Conversion on Nanostructured Niobium Oxide after Hydrothermal Treatment: Effect of Morphology and Porous Structure. J. Mol. Catal. A Chem. 2016, 422, 23–34. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Huang, G.; Zhang, Z.; Han, L.; Song, W.; Zhang, Y. Facile Synthesis of Urchin-like Hierarchical Nb2O5 Nanospheres with Enhanced Visible Light Photocatalytic Activity. J. Alloys Compd. 2017, 728, 19–28. [Google Scholar] [CrossRef]
- Liu, X.; Liu, G.; Chen, H.; Ma, J.; Zhang, R. Facile Synthesis of Nb2O5 Nanobelts Assembled from Nanorods and Their Applications in Lithium Ion Batteries. J. Phys. Chem. Solids 2017, 111, 8–11. [Google Scholar] [CrossRef]
- Salim, E.T.; Ismail, R.A.; Halbos, H.T. Deposition Geometry Effect on Structural, Morphological and Optical Properties of Nb2O5 Nanostructure Prepared by Hydrothermal Technique. Appl. Phys. A 2020, 126, 891. [Google Scholar] [CrossRef]
- Zu, D.; Song, H.; Wang, Y.; Chao, Z.; Li, Z.; Wang, G.; Ma, J. One-Pot In-Situ Hydrothermal Synthesis of CdS/Nb2O5/Nb2C Heterojunction for Enhanced Visible-Light-Driven Photodegradation. Appl. Catal. B Environ. 2020, 277, 119140. [Google Scholar] [CrossRef]
- Salim, E.T.; Ismail, R.A.; Halbos, H.T. Growth of Nb2O5 Film Using Hydrothermal Method: Effect of Nb Concentration on Physical Properties. Mater. Res. Express 2019, 6, 116429. [Google Scholar] [CrossRef]
- Tayyab, M.; Liu, Y.; Liu, Z.; Pan, L.; Xu, Z.; Yue, W.; Zhang, J. One-Pot in-Situ Hydrothermal Synthesis of Ternary In2S3/Nb2O5/Nb2C Schottky/S-Scheme Integrated Heterojunction for Efficient Photocatalytic Hydrogen Production. J. Colloid Interface Sci. 2022, 628, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.; Brandalise, R.N.; Savaris, M. Biomaterials: Characteristics and Properties. Eng. Biomater. 2017, 5–15. [Google Scholar] [CrossRef]
- Nivedita, L.R.; Haubert, A.; Battu, A.K.; Ramana, C.V. Correlation between Crystal Structure, Surface/Interface Microstructure, and Electrical Properties of Nanocrystalline Niobium Thin Films. Nanomaterials 2020, 10, 1287. [Google Scholar] [CrossRef]
- Ücker, C.L.; Gularte, L.T.; Fernandes, C.D.; Goetzke, V.; Moreira, E.C.; Raubach, C.W.; Moreira, M.L.; Cava, S.S. Investigation of the Properties of Niobium Pentoxide for Use in Dye-sensitized Solar Cells. J. Am. Ceram. Soc. 2019, 102, 1884–1892. [Google Scholar] [CrossRef]
- Moreto, J.A.; Gelamo, R.V.; Da Silva, M.V.; Steffen, T.T.; De Oliveira, C.J.F.; De Almeida Buranello, P.A.; Pinto, M.R. New Insights of Nb2O5-Based Coatings on the 316L SS Surfaces: Enhanced Biological Responses. J. Mater. Sci. Mater. Med. 2021, 32, 25. [Google Scholar] [CrossRef]
- Rani, R.A.; Zoolfakar, A.S.; O’Mullane, A.P.; Austin, M.W.; Kalantar-Zadeh, K. Thin Films and Nanostructures of Niobium Pentoxide: Fundamental Properties, Synthesis Methods and Applications. J. Mater. Chem. A 2016, 2, 15683–15703. [Google Scholar] [CrossRef]
- Soares, M.; Leite, S.; Nico, C.; Peres, M.; Fernandes, A.; Graça, M.; Matos, M.; Monteiro, R.; Monteiro, T.; Costa, F. Effect of Processing Method on Physical Properties of Nb2O5. J. Eur. Ceram. Soc. 2011, 31, 501–506. [Google Scholar] [CrossRef]
- Çetinörgü-Goldenberg, E.; Klemberg-Sapieha, J.; Martinu, L. Effect of Postdeposition Annealing on the Structure, Composition, and the Mechanical and Optical Characteristics of Niobium and Tantalum Oxide Films. Appl. Opt. 2012, 51, 6498. [Google Scholar] [CrossRef]
- Yao, D.D.; Rani, R.A.; O’Mullane, A.P.; Kalantar-Zadeh, K.; Ou, J.Z. High Performance Electrochromic Devices Based on Anodized Nanoporous Nb2O5. J. Phys. Chem. C 2013, 118, 476–481. [Google Scholar] [CrossRef]
- Pawlicka, A.; Atik, M.; Aegerter, M. Synthesis of Multicolor Nb2O5 Coatings for Electrochromic Devices. Thin Solid Film. 1997, 301, 236–241. [Google Scholar] [CrossRef]
- Mitterhuber, L.; Kraker, E.; Defregger, S. Structure Function Analysis of Temperature-Dependent Thermal Properties of NM-Thin Nb2O5. Energies 2019, 12, 610. [Google Scholar] [CrossRef]
- Hota, M.K.; Bera, M.K.; Maiti, C.K. Flexible Metal–Insulator–Metal Capacitors on Polyethylene Terephthalate Plastic Substrates. Semicond. Sci. Technol. 2012, 27, 105001. [Google Scholar] [CrossRef]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of Nanoparticles in Biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Huynh, K.-H.; Pham, X.-H.; Kim, J.; Lee, S.H.; Chang, H.; Rho, W.-Y.; Jun, B.-H. Synthesis, Properties, and Biological Applications of Metallic Alloy Nanoparticles. Int. J. Mol. Sci. 2020, 21, 5174. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Ealia, S.A.M.; Saravanakumar, M.P. A Review on the Classification, Characterisation, Synthesis of Nanoparticles and Their Application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Yagublu, V.; Karimova, A.; Hajibabazadeh, J.; Reissfelder, C.; Muradov, M.; Bellucci, S.; Allahverdiyev, A. Overview of Physicochemical Properties of Nanoparticles as Drug Carriers for Targeted Cancer Therapy. J. Funct. Biomater. 2022, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ozcan, S. Green Nanomaterials: On Track for a Sustainable Future. Nano Today 2015, 10, 417–420. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Olaya, J.J.; Ramírez, C.; Rodil, S.E. Biocompatibility of niobium coatings. Coatings 2011, 1, 72–87. [Google Scholar] [CrossRef]
- Chézeau, L.; Tchinda, A.; Pierson, G.; Bravetti, P.; Ferrari, L.; Joubert, O.; Zaiou, M.; Rihn, B.H. In vitro Molecular study of Titanium-Niobium Alloy biocompatibility. Biomedicines 2022, 10, 1898. [Google Scholar] [CrossRef] [PubMed]
- Senocak, T.C.; Ezirmik, K.V.; Aysin, F.; Ozek, N.S.; Cengiz, S. Niobium-Oxynitride Coatings for Biomedical Applications: Its Antibacterial Effects and in-Vitro Cytotoxicity. Mater. Sci. Eng. C Biomim. Mater. Sens. Syst. 2021, 120, 111662. [Google Scholar] [CrossRef] [PubMed]
- Carli Schardosim, R.F.; Cardozo, T.R.; Souza, A.P.; Seeber, A.; Flores, W.H.; Lehmann, M.; Dihl, R.R. Cyto—Genotoxicity of Crystalline and Amorphous Niobium (V) Oxide Nanoparticles in CHO-K1 Cells. Toxicol. Res. 2022, 11, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Mestieri, L.B.; Gomes-Cornélio, A.L.; Rodrigues, E.M.; Faria, G.; Guerreiro-Tanomaru, J.M.; Tanomaru-Filho, M. Cytotoxicity and Bioactivity of Calcium Silicate Cements Combined with Niobium Oxide in Different Cell Lines. Braz. Dent. J. 2017, 28, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.O.A.; Mariani, F.E.; Leite, N.B.; Gelamo, R.V.; Aoki, I.V.; de Siervo, A.; Pinto, H.C.; Moreto, J.A. Niobium and Carbon Nanostructured Coatings for Corrosion Protection of the 316 L Stainless Steel. Mater. Chem. Phys. 2024, 312, 128610. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; Carvalho, S.M.; Silva, A.R.; Ciminelli, V.S.; Mansur, H.S. Niobium-Doped Hydroxyapatite Bioceramics: Synthesis, Characterization and In Vitro Cytocompatibility. Materials 2015, 8, 4191–4209. [Google Scholar] [CrossRef]
- Yassin, M.T.; Al-Otibi, F.O.; Al-Sahli, S.A.; El-Wetidy, M.S.; Mohamed, S. Metal Oxide Nanoparticles as Efficient Nanocarriers for Targeted Cancer Therapy: Addressing Chemotherapy-Induced Disabilities. Cancers 2024, 16, 4234. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, H. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef]
- Li, J.; Xie, Q.; Ma, R.; Li, Y.; Yuan, J.; Ren, M.; Li, H.; Wang, J.; Lu, D.; Xu, Z. Recent Progress on the Synergistic Antitumor Effect of a Borneol-Modified Nanocarrier Drug Delivery System. Front. Med. 2021, 8, 750170. [Google Scholar] [CrossRef]
- Yuan, R.; Huang, Y.; Chan, L.; He, D.; Chen, T. Engineering EHD1-Targeted Natural Borneol Nanoemulsion Potentiates Therapeutic Efficacy of Gefitinib Against Nonsmall Lung Cancer. ACS Appl. Mater. Interfaces 2020, 12, 45714–45727. [Google Scholar] [CrossRef] [PubMed]
- Lei, D.-X.; Peng, C.-H.; Peng, S.-Y.; Jiang, X.-C.; Wu, Y.-L.; Shen, H.-W. Safe Upper Limit of Intermittent Hepatic Inflow Occlusion for Liver Resection in Cirrhotic Rats. World J. Gastroenterol. 2001, 7, 713. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Nordberg, G. The Effects of Nanoparticles on the Renal System. Crit. Rev. Toxicol. 2016, 46, 490–560. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.; Verma, Y.; Rani, V.; Rana, S.V.S. Renal Toxicity of Nanoparticles of Cadmium Sulphide in Rat. Chemosphere 2018, 193, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Geng, S.; Shao, J.; Luo, G.; Liu, Q.; Wang, J.; Chen, Y.; Chu, P.K.; Li, Z.; Yu, X. Niobium Diselenide Nanosheets: An Emerging Biodegradable Nanoplatform for Efficient Cancer Phototheranostics in the NIR-II Window. Adv. Healthc. Mater. 2022, 11, 2202126. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, X.; Li, J.; Liu, Z.; Cheng, L. Inorganic Nanomaterials with Rapid Clearance for Biomedical Applications. Chem. Soc. Rev. 2021, 50, 8669–8742. [Google Scholar] [CrossRef] [PubMed]
- Havelikar, U.; Ghorpade, K.B.; Kumar, A.; Patel, A.; Singh, M.; Banjare, N.; Gupta, P.N. Comprehensive Insights into Mechanism of Nanotoxicity, Assessment Methods and Regulatory Challenges of Nanomedicines. Discov. Nano 2024, 19, 165. [Google Scholar] [CrossRef]
- Lin, X.; Li, Z.; Du, S.; Wang, Q.; Guan, Y.; Cheng, G.; Hong, H.; Li, J.; Chen, X.; Chen, T. Occam’s Razor-Inspired Nb2C Delivery Platform Potentiates Breast Cancer Therapy and Inhibits Lung Metastasis. Chem. Eng. J. 2023, 464, 142732. [Google Scholar] [CrossRef]
- Heidari, A.; Schmitt, K.; Henderson, M.; Besana, E. Classification of Drug Delivery System of Niobium Nanoparticles in Human Gum Cancer Gum Cells, Tissues and Tumors Treatment under Synchrotron Radiation. Dent. Oral Maxillofac. Res. 2020, 6, 1–17. [Google Scholar] [CrossRef]
- Alizadeh, M.; Yadollahi, B. A Niobium Polyoxometalate–Folic Acid Conjugate as a Hybrid Drug for Cancer Therapeutics. New J. Chem. 2022, 46, 18199–18206. [Google Scholar] [CrossRef]
- Baffou, G.; Cichos, F.; Quidant, R. Applications and Challenges of Thermoplasmonics. Nat. Mater. 2020, 19, 946–958. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Cheng, G.; Guo, J.; Du, S.; Qiu, J.; Wang, C.; Li, C.; Yang, X.; Chen, T.; et al. Tailored Hydrogel Delivering Niobium Carbide Boosts ROS-Scavenging and Antimicrobial Activities for Diabetic Wound Healing. Small 2022, 18, 2201300. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Song, Z.; Du, T.; Du, X. Antimicrobial Materials Based on Photothermal Action and Their Application in Wound Treatment. Burn. Trauma 2024, 12, tkae046. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I. The Role of Niobium Component in Heterogeneous Catalysts. Catal. Today 2017, 285, 211–225. [Google Scholar] [CrossRef]
- Pleskunov, P.; Prysiazhnyi, V.; Nikitin, D.; Košutová, T.; Cieslar, M.; Gordeev, I.; Krtouš, Z.; Ali-Ogly, S.; Šomvársky, J.; Protsak, M.; et al. Magnetron-Sputtered Niobium Nanoparticles for Molecular Imaging of Brain Tissues through Surface-Assisted Laser Desorption/Ionization Mass Spectrometry. ACS Appl. Nano Mater. 2022, 5, 12865–12875. [Google Scholar] [CrossRef]
- Yan, J.; Wang, X.; Wang, Q.; Sun, X. Novel MRI-Compatible Zr–Mo–Nb Alloys with Superior Mechanical Performance, High Corrosion Resistance and Good Cytocompatibility for Biomedical Applications. J. Mater. Res. Technol. 2024, 30, 794–806. [Google Scholar] [CrossRef]
- Radchenko, V.; Hauser, H.; Eisenhut, M.; Vugts, D.J.; Van Dongen, G.A.M.S.; Roesch, F. 90Nb—A Potential PET Nuclide: Production and Labeling of Monoclonal Antibodies. Radiochim. Acta 2012, 100, 857–864. [Google Scholar] [CrossRef]
- Nassar, E.J.; Moscardini, S.B.; Lechevallier, S.; Vereslt, M.; Borak, B.; Rocha, L.A. Niobium Oxide Doped with Tm3+ and Gd3+ Ions for Multimodal Imaging in Biology. J. Sol-Gel Sci. Technol. 2020, 93, 546–553. [Google Scholar] [CrossRef]
- Ravel, B.; Kropf, A.J.; Yang, D.; Wang, M.; Topsakal, M.; Lu, D.; Stennett, M.C.; Hyatt, N.C. Nonresonant Valence-to-Core X-Ray Emission Spectroscopy of Niobium. Phys. Rev. B 2018, 97, 125139. [Google Scholar] [CrossRef]
- Waheed, A.; Zaid, M.H.M.; Shahid, F.; Razipwee, M.F.M.; Shahidan, M.A. Mediator-Free Electrochemical Biosensor Based on Carboxyl-Functionalized Niobium Carbide for Sensitive Ammonium Determination. Microchem. J. 2025, 208, 112408. [Google Scholar] [CrossRef]
- Barzegar, M. Niobium Incorporation in 2D MoSe2 for Lung Cancer Biomarkers Detection: The First-Principle Study of Sensitivity Improvement. Comput. Theor. Chem. 2023, 1225, 114169. [Google Scholar] [CrossRef]
- Peng, Y.; Lin, C.; Tang, M.; Yang, L.; Yang, Y.; Liu, J.; Huang, Z.; Li, Z. Niobium Pentoxide Ultra-Thin Nanosheets: A Photocatalytic Degradation and Recyclable Surface-Enhanced Raman Scattering Substrate. Appl. Surf. Sci. 2020, 509, 145376. [Google Scholar] [CrossRef]
- Heydari, S.; Haghayegh, G.H. Application of Nanoparticles in Quartz Crystal Microbalance Biosensors. J. Sens. Technol. 2014, 4, 47365. [Google Scholar] [CrossRef]
- Egbuna, C.; Parmar, V.K.; Jeevanandam, J.; Ezzat, S.M.; Patrick-Iwuanyanwu, K.C.; Adetunji, C.O.; Khan, J.; Onyeike, E.N.; Uche, C.Z.; Akram, M.; et al. Toxicity of Nanoparticles in Biomedical Application: Nanotoxicology. J. Toxicol. 2021, 2021, 9954443. [Google Scholar] [CrossRef] [PubMed]
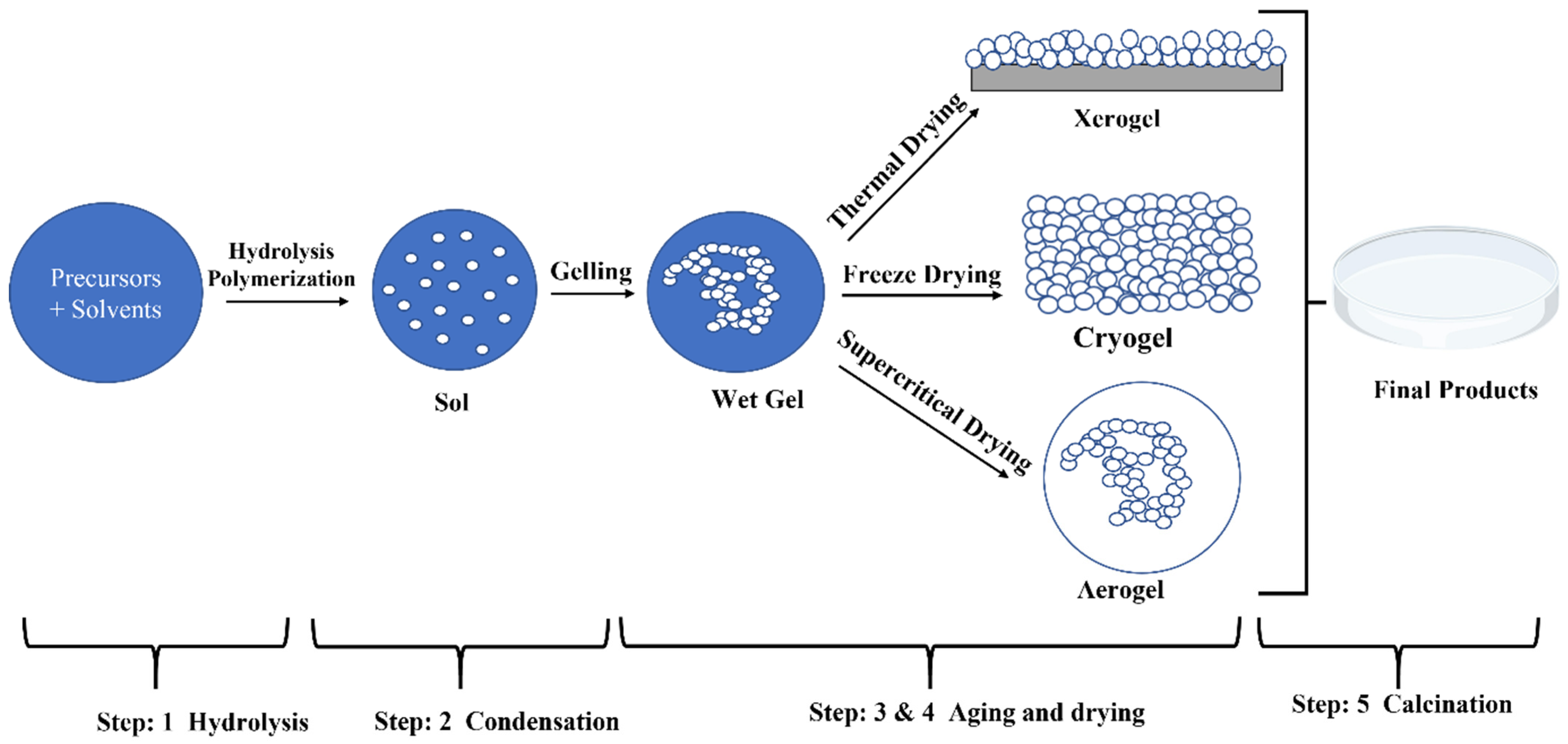
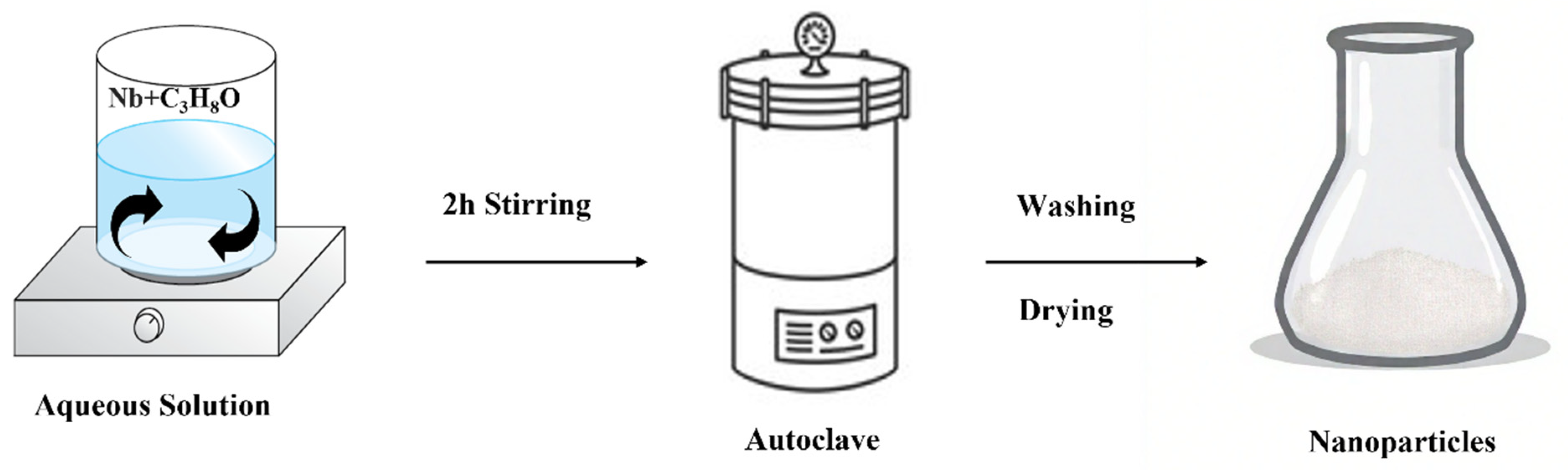
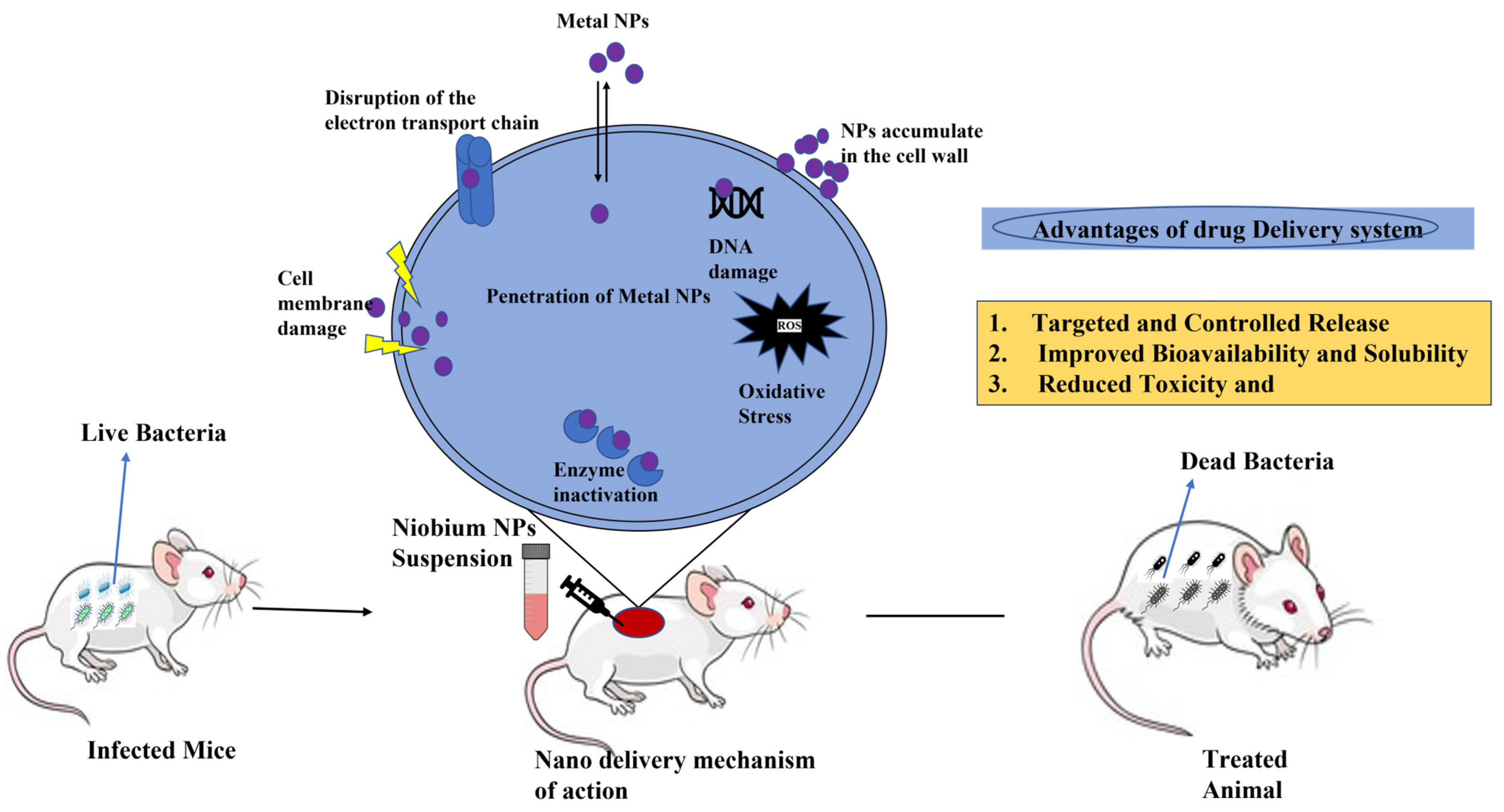
| Solution | Time (h) | Temp. °C | Morphology | Crystal Phase | Dimensions (nm) | Applications |
|---|---|---|---|---|---|---|
| Niobium alkoxides (Nb(OR)5) with acetic acid [13] | 4 | 80 | Xerogels and powders | Monolithic, tetragonal when heated above 500 °C | N/A | Nb2O5 powders were synthesized |
| Niobium oxide (Nbx) with ethanol and HCL [14] | 24 | 25 | Xerogels and powders | Amorphous | N/A | Electrochromic material |
| NbCl5 with propanol and acetic acid [15] | 1 | 300–500 | Xerogel films | Amorphous at 300 °C and pseudo-hexagonal at 500 °C | N/A | Electrochemical reversibility and dip coating |
| NbCl5 with ethanol and acetic acid [16] | 2 | 450 | Thin Films | Amorphous | T: 83–178 | Counter electrode, particularly in nickel oxide devices. |
| NbCl5 with ethanol [17] | 24 | 25 | Silica aerogel | Amorphous | N/A | N/A |
| Niobium chloride, NbCl5, with tetraethoxysilane [18] | 48 | 25 | Dried gels | Amorphous | N/A | N/A |
| NbCl5 with citric acid, ethylene glycol, deionized water, hydrogen peroxide [11] | 12 | 25 | Transparent and yellow gel | Below 600 °C, amorphous Orthorhombic when calcined at 900 °C | T: 50, when heated T: >200 nm | Capacitors |
| Niobium ethoxide with acetic acid [19] | 24 | 300 | Thin Films | Tetragonal | N/A | Photocatalytic activity |
| Anhydrous NbCl5 with InCl3 and acetylacetone [20] | 1 | 85 | Thin Films | Cubic bixbyite | T: 15–25 | Highest optical transmittance, conductive films |
| Niobium ethoxide with tetra-ethyl-ortho-titanate and ethanol [21] | 1 | 450 | Thin Films | Amorphous to crystalline | T: 100–370 | CO sensing applications |
| Niobium ethoxide with NH4OH [22] | 2 | 25 | Nanoparticles | Orthorhombic and hexagonal | D: 1–100 | N/A |
| Niobium chloride (NbCl5) with aluminum chloride hexahydrate [23] | 1 | 70 | Nanorods and thin films | Hexagonal | N/A | Photocatalytic Activities |
| Niobium chloride (NbCl5) with hydrogen peroxide and coconut water powder [24] | 136 | 25 | Powder | Orthorhombic and monoclinic when heated at 1000 °C | N/A | Dielectric constant |
| Niobium ethoxide with isopropyl alcohol [25] | 2 | 180 | Thin Films | Polycrystalline and amorphous structure | N/A | Fabrication of unipolarswitching memory devices |
| Nb2O5 powder with hydrofluoric acid [26] | 2 | 100 | Nanoparticles | Polycrystalline | N/A | N/A |
| Solution | Time (h) and Tem. °C | Morphology | Crystal Phase | Size (nm) | Application |
|---|---|---|---|---|---|
| NbCl5 in HCl [31] | 24 and 210 | Nanorods | Monoclinic after being calcined at 450 °C | D: 22 L: 230 | Lithium-ion batteries and dye-sensitized solar cells |
| Ammonium niobate oxalate hydrate, sucrose and HCl in deionized water [32] | 12 and 180 | Nanocomposites | Pseudo-hexagonal | D: 25–29 | Highly active and stable catalyst for electrochemical reactions |
| Nb2O5, LiOH and NH3.H2O in H2O2 [33] | 24 and 240 | Hollow microspheres | Pseudo-hexagonal after being calcined at 500 °C | D: 1000–2000 | Catalysis, drug carriers, and gas sensors |
| Ammonium niobate oxalate and hydrogen peroxide distilled water [34] | 2–24 and 100–175 | Nanoparticle | Orthorhombic | D: 9–35 | Photoactive degradation of pollutants |
| Niobium foil with ammonium fluoride [35] | 24–144 and 150 | Nanorods | Orthorhombic for duration > 48 h | D: 50–100 | Nano-scaled sensors, optoelectronic devices |
| Lithium hydroxide in HF acid [36] | 20–40 and 150–200 | Nano-trees | Pseudo-hexagonal | D: 30–500 | UV sensors |
| Ammonium niobate oxalate hydrate and oleic acid in triethylamine [37] | 2–6 and 180 | Nanorods | Pseudo-hexagonal | D: 5–20 L: 100–500 | Photoactive degradation of pollutants |
| Ammonium niobate oxalate hydrate in distilled water [37] | 1 and 580 | Nanospheres | Pseudo-hexagonal | D: 20–50 | Photoactive degradation of pollutants |
| Niobium ethoxide, diethylene glycol and acetone in water [38] | 4–12 and 180 | Mesoporous spheres | Pseudo-hexagonal | D: 400–500 | Highly effective solid acid catalysts |
| NbCl5 in ethanol mixed with triblock copolymer dissolved in distilled water [39] | 24 and 110 | Mesoporous | Orthorhombic after being calcined at 600 °C | N/A | N/A |
| NbO2 powder in distilled water and ethanol containing 1 M urea [40] | 72–720 and 130 | Nanosheets | Orthorhombic and monoclinic | T: 3–5 | High reversible charge/discharge capacity and cycling stability |
| Nb powder in distilled water [41] | 72–720 and 200 | Nanorods | Orthorhombic | D: 50 | An efficient material synthesized without catalyst |
| NbCl5 and ethanol in cyclohexanol [42] | 8–90 and 200–240 | Nanocables and nanorods | Orthorhombic | D: 50–80 L: >1000 | High-performance optoelectrical devices |
| Nb powder in urea [43] | 24–336 and 170–200 | Nanobelts | N/A | W: ~60 T ~15 | Lithium-ion batteries and dye-sensitized solar cells |
| Niobium Penta butoxide in toluene [44] | 2 and 300 | Nano powders | Pseudo-hexagonal | L: <80 | Photocatalytic dehydro-genation of methanol in an aqueous solution under deaerated conditions |
| NbCl5 in anhydrous benzyl alcohol [45] | 72 and 250 | Nanoparticles | Pseudo-hexagonal | D: 18–35 | High-rate-performance supercapacitor |
| NbCl5 and ethanol in cyclohexanol [46] | 8–90 and 200–240 | Nanograins and nanoparticles | Orthorhombic for T > 225 °C | D: 50–80 L: >1000 | Catalysis and its structure–activity relationships |
| Oxalic acid and hydrogen peroxide [47] | 4–12 and 150–220 | Nanorods and nanospheres | Octahedral | L: 5 | Used as nano catalyst |
| Oxalic acid and hydrogen peroxide [48] | 4–12 and 150–220 | Nanospheres and mesoporous | Monoclinic | D: 6 | Used as nano catalyst |
| Aluminum niobium oxalate with deionized water and ethanol [49] | 24 and 180 | Nanorods and nanospheres | Orthorhombic after being calcined at 600 °C | D: 300–500 D: 5–10 L: 100 | Higher photocatalytic activities (organic pollutants, dye sensitized and solar cells) |
| Nb2O5 with isopropanol [50] | 48 and 180 | Nanobelts | Pseudo-hexagonal | D: 100–200 | Energy storage electrodes |
| Nb powder with distilled water [51] | 72 and 180 | Flake, Nanorods, Springs | Polycrystalline | L: 20–100 | Nano-scaled sensor, solar cells |
| Ammonium oxalate with Nb2AlC [52] | 21 and 180 | Nanorods | Hexagonal and orthorhombic | D: 20–50 L: 70–150 | Catalyst and water treatments |
| Nb with distilled water [53] | 72 and 150 | Nanoflakes | Orthorhombic | D: 46–53 | High-performance optoelectrical devices |
| Nb2AlC with Hydrofluoric acid [54] | 20 and 190 | Nanorods | Monoclinic | L: 60–180 | Photocatalyst |
| Niobium with distilled water [29] | 20–40 and 130–150 | Nanorods | Octahedral | D: 20–50 | Biomedicine and environmental monitoring |
| Property | Niobium Pentoxide Nanoparticles (Nb2O5NPs) [11] | Gold Nanoparticles (AuNPs) [66,67] | Silica Nanoparticles (SiO2NPs) [68] | Silver Nanoparticles (AgNPs) [69] | Zinc Oxide Nanoparticles (ZnONPs) [70] | Carbon-Based Nanoparticles [69,71] | Titanium Dioxide Nanoparticles (TiO2) [68,72] |
|---|---|---|---|---|---|---|---|
| Chemical Composition | Niobium and oxygen | Pure gold | Silicon dioxide | Pure silver | Zinc and oxygen | Carbon (e.g., fullerenes, graphene) | Titanium and oxygen |
| Size Range (nm) | 10–200 nm | 1–100 nm | 5–500 nm | 1–100 nm | 10–200 nm | Varies (e.g., graphene sheets, carbon dots) | 1–100 nm |
| Morphology | Crystalline or amorphous | Spherical, rods, others | Spherical, mesoporous, others | Spherical, triangular, others | Spherical, rod-shaped, others | Spherical, tubular (e.g., nanotubes), sheets | Spherical, rods, others |
| Surface Modification | Functionalized with organic groups, metals, or polymers | Thiol groups, PEGylation, antibodies | Functionalized with silanes, organic groups, or biomolecules | Citrate, PEGylation, antibodies | Organic molecules, polymers | Functionalized with various chemical groups | Coatings, doping with metals or non-metals |
| Optical Properties | High refractive index, photoluminescent | Surface plasmon resonance (SPR) | Transparent, adjustable refractive index | Strong SPR, antimicrobial | UV absorption, photoluminescent | Fluorescence (e.g., carbon dots), high surface area | UV absorption, photo catalytic activity |
| Electrical Properties | High dielectric constant, resistive switching | Conductive, plasmonic effects | Insulator | Conductive | Semi-conductor | Conductive (graphene), semi-conducting (carbon dots) | Semi-conductor |
| Thermal Stability | High | Moderate | High | Moderate | High | High | High |
| Biocompatibility | Moderate; dependent on surface functionalization | High; excellent for biological applications | High; generally inert | Variable; size- and concentration-dependent | Generally good; antimicrobial properties | High (e.g., graphene oxide), variable for others | Generally good; dependent on surface properties |
| Toxicity | Low; surface-dependent | Low; size- and concentration-dependent | Generally low | Potential cytotoxicity; dose-dependent | Low; antimicrobial properties | Low; dependent on form and functionalization | Low; dependent on crystal structure and surface properties |
| Key Applications | Catalysis, electrochromic devices, gas sensors, photocatalysis, batteries | Biomedical imaging, drug delivery, photothermal therapy, sensors | Drug delivery, imaging, coatings, catalysis, chromatography | Antimicrobial agents, biosensing, medical imaging | Sunscreens, antibacterial agents, photocatalysis, sensors | Drug delivery, imaging, electronics (e.g., graphene) | Photocatalysis, UV blockers, sensors, biomedical applications |
| Unique Features | High photocatalytic activity, ion storage capabilities, high refractive index | Strong SPR for optical applications, high specificity with functionalization | High surface area (e.g., mesoporous), high chemical stability | Potent antimicrobial activity, strong SPR | Antimicrobial, UV-blocking properties, semi-conductor behavior | Exceptional mechanical strength (graphene), tunable electronic properties | Photocatalytic efficiency, UV absorption, chemical stability |
| Synthesis Methods | Sol–gel, hydro- thermal, precipitation | Chemical reduction, seed-mediated growth | Stöber process, sol–gel methods | Chemical reduction, photo-chemical methods | Sol–gel, hydro-thermal, precipitation | Chemical vapor deposition (CVD), arc discharge, laser ablation | Sol–gel, hydro-thermal, chemical vapor deposition |
| Challenges | Limited commercial availability, complex surface functionalization | Cost of gold, stability in solutions | Aggregation in some environments, limited thermal conductivity | Potential cytotoxicity, stability under various conditions | Photocatalytic activity leading to ROS generation, potential cytotoxicity | Production scalability (e.g., graphene), potential cytotoxicity | Control over crystal phase, potential environmental impact |
| Cost | Moderate | High | Low to moderate | Moderate | Low | Variable (e.g., graphene can be expensive) | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, M.U.; Rudokaite, A.; da Silva, A.M.H.; Kirsnyte-Snioke, M.; Stirke, A.; Melo, W.C.M.A. A Comprehensive Review of Niobium Nanoparticles: Synthesis, Characterization, Applications in Health Sciences, and Future Challenges. Nanomaterials 2025, 15, 106. https://doi.org/10.3390/nano15020106
Khalid MU, Rudokaite A, da Silva AMH, Kirsnyte-Snioke M, Stirke A, Melo WCMA. A Comprehensive Review of Niobium Nanoparticles: Synthesis, Characterization, Applications in Health Sciences, and Future Challenges. Nanomaterials. 2025; 15(2):106. https://doi.org/10.3390/nano15020106
Chicago/Turabian StyleKhalid, Muhammad Usman, Austeja Rudokaite, Alessandro Marcio Hakme da Silva, Monika Kirsnyte-Snioke, Arunas Stirke, and Wanessa C. M. A. Melo. 2025. "A Comprehensive Review of Niobium Nanoparticles: Synthesis, Characterization, Applications in Health Sciences, and Future Challenges" Nanomaterials 15, no. 2: 106. https://doi.org/10.3390/nano15020106
APA StyleKhalid, M. U., Rudokaite, A., da Silva, A. M. H., Kirsnyte-Snioke, M., Stirke, A., & Melo, W. C. M. A. (2025). A Comprehensive Review of Niobium Nanoparticles: Synthesis, Characterization, Applications in Health Sciences, and Future Challenges. Nanomaterials, 15(2), 106. https://doi.org/10.3390/nano15020106








