1. Introduction
The integration of diverse functionalities onto microfluidic chips is key to realizing micro-total analysis systems such as organ-on-a-chip and lab-on-a-chip constructs [
1,
2,
3]. On-chip microfluidic systems incorporate multi-faceted technologies that mimic specific physiological environments and functionalities. For example, an organ-on-a-chip mimics the environment and functionality of a human organ [
1]. As a step in this direction, three-dimensional (3D) microfluidic cell culture systems serve as non-clinical human tissue models [
2]. Microfluidic systems are miniaturized systems that consume little reagent, support rapid chemical reactions, and are amenable to running parallel experiments. These advantages allow high-throughput testing and support the customization required for point-of-care applications [
3].
The selection of materials for large-scale manufacturing is a bottleneck in applying microfluidic systems to industrial applications [
3]. Microfluidic system injection molding is the most common fabrication method used, and 3D printing approaches are also widely utilized because they are accessible, more cost-effective than injection molding, and can be used with biocompatible materials. Currently, 3D printing is mostly used in microfluidic research for prototyping and preliminary studies [
3]. Increased access to high-performance laser equipment will particularly aid the adoption of optical 3D printing and accelerate the use of this technique on an industrial scale [
4].
Femtosecond laser direct writing (fs-LDW) is a versatile 3D optical printing approach with high spatial resolution and excellent 3D fabrication capability [
5,
6]. Two-photon polymerization processes using ultrafast lasers are often used to confine the processed regions in a 3D manner and reduce the effective spot size in transparent media due to multiphoton absorption [
7,
8]. Fs-LDW using a transparent precursor material that fills a glass microfluidic device can provide embedded functional micro-components or biomimetic structures within closed glass microchannels (‘ship-in-a-bottle’ integration), creating high-performance 3D biochips [
9].
Proteins dissolved in aqueous solvents are one example of a transparent medium for fs-LDW. Proper selection of the energy and photon intensity during optical printing can provide proteinaceous micro- and nanostructures that retain the native protein function. Applications such as micro-optics, pH actuation, cell culture scaffolds and enzymatic nanoreactors have been previously reported [
10]. Methacrylated protein was recently used to tune the Young’s modulus of a printed material by adjusting the photoresist composition in scaffolds for cell culture [
11]. Intricate 3D protein microstructures have been reported for precursor from unmodified protein and photosensitizer [
12,
13,
14] and pure unmodified protein [
15]. Although the fabrication mechanism using the pure protein precursor is not fully understood, we recently demonstrated that chemical crosslinking is involved [
15] similar to the case of using photosensitizers [
13,
16], and more recently we identified the amino acids that might aid the fabrication process, by comparing fabrication results obtained using commercially available homopeptides [
17]. Biocompatible applications using integrated microfluidic chips might particularly benefit from the use of pure protein precursors to prevent undesired leaching of photoactivators after fabrication, since photoactivators might reduce cell viability or cause other side effects [
18].
The integration of proteinaceous microstructures with photoactivators into microfluidic channels was previously demonstrated. An enzyme was incorporated as individual lines in a glass capillary and exhibited enzymatic activity. The activity of this miniaturized channel could be measured [
19]. Similarly, the integration of trypsin columns into a polydimethylsiloxane microfluidic channel confirmed its compatibility with classic microfluidic channels [
20]. Using antibody protein, the capture of living cells in a biochip was demonstrated [
21]. We demonstrated that refractive index matching enabled fs-LDW of 3D microstructures in closed microchannels via the ship-in-a-bottle integration technique [
22].
The integration of proteinaceous microstructures exhibiting pH sensitivity [
23] into microfluidic chips could have interesting applications. pH-sensitive proteinaceous devices have found utility in tunable micro-optics [
24], artificial musculoskeletal systems [
25] and shape-shifting structures [
26]. However, these studies were conducted using structures created in a dish or on a glass substrate, which obscured the spatial and temporal resolution of the pH measurements. The integration of microfluidic channels might help to control volume changes and buffer flow.
In this paper, we demonstrate the integration of photoactivator-free pH-sensitive pure 3D proteinaceous meshes in a closed microfluidic channel. As shown in
Figure 1a, the closed microchannel is filled with a pure, high-concentration protein precursor solution. Fs-LDW is used to fabricate proteinaceous meshes in the channels which are anchored to the top and bottom of the channel. The meshes allow accurate measurement of pH-dependent changes in the size of the mesh. The conditions used for fs-LDW fabrication are important for pH sensitivity. Using the chosen fabrication and precursor conditions, the relationship between pH and gap size allows manipulation of the gap size to adjust pH sensing, as indicated in
Figure 1b. The ship-in-a-bottle approach using pure protein allows pH control and should thus facilitate the development of active protein-based microdevices with practical applications in biomedicine.
2. Materials and Methods
2.1. Protein Precursor Preparation and Free-Standing Fabrication
Bovine serum albumin (BSA) (pH 5.2 fraction, 017-21273 (Lot no. WTP3913), Fujifilm, Tokyo, Japan) was purchased as a lyophilized powder. The protein powder was weighed and dissolved in purified water or a 50% (w/w) glycerol−water solvent to 400 mg/mL (~6 mM) BSA.
We evaluated the pH -actuation of protein meshes unconstrained by a microchannel by fabricating meshes anchored onto cover glass without restricting the mesh itself. The precursor was drop-cast using 3 μL drops onto the same type of thin cover glass as used previously for channel bonding (120–170 μm-thick cover-glass; Matsunami, Osaka, Japan), then the cover slip was inverted to form a hanging drop to mimic the in-channel fabrication path from the fs laser objective lens, through air, to the thin cover glass and into the protein precursor.
2.2. Setup
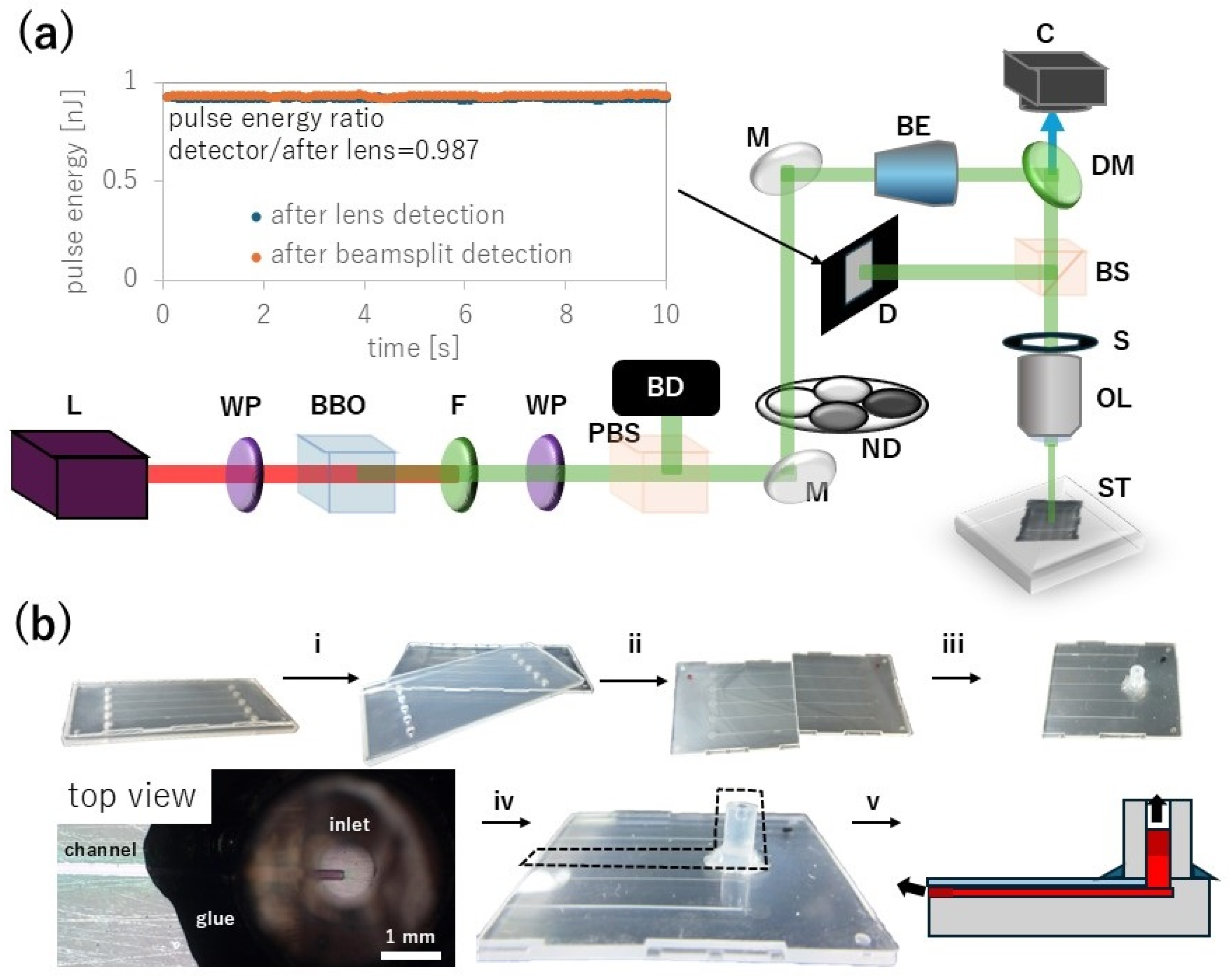
An fs laser (Spirit 1040-16-HE-SHG, Spectra-Physics, Milpitas, CA, USA) operated at a wavelength of 1040 nm, a pulse width of about 350 fs, and a repetition rate of 100 kHz was used as a light source. The near infrared (NIR) Gaussian beam was converted to 520 nm green laser light and the remaining NIR was filtered and removed by a bandpass filter, as shown in
Figure 2a. Proteins generally only strongly absorb light at wavelengths shorter than ultraviolet (UV) and thus the two-photon absorption process at the focus of the green laser pulse can excite proteins and form proteinaceous fine structures [
27]. Fs-LDW requires precise control of the laser pulse energy, and thus the pulse energy was monitored in real time through a 50:50 beam splitter cube with a PD300-MS microscope slide power sensor (Ophir, Jerusalem, Israel).
2.3. Microchannel Preparation
A microfluidic chip was prepared using a commercial product, a Microfluidic Kit Natural Flow Chip Type III (Richell, Toyama City, Japan), as shown in
Figure 2b. The product comes with a 1 mm-thick plastic cover. The working distance of the lens can accommodate this thickness but the plastic cover is slightly cloudy white, reducing the focus of the laser on the microfluidic channels. Therefore, we separated the plastic cover into two components. With scissors, we cut the channel in half to increase test samples, then we wiped the elastomer components with ethanol to remove adhesive residue, and subjected them to O
2 plasma treatment for 60 s in an O
2 plasma device (SP-0400, Strex, Osaka, Japan). Then, the simultaneously O
2 plasma-treated 120–170 μm-thick cover-glass (Matsunami, Osaka, Japan) was immediately firmly bonded to the microchannel. Further, a 5 mm-long silicon tube (part of the kit) with an inner diameter of 1 mm was bonded on top of the channel inlet.
After confirming proper alignment between tube and channel inlet, we used 5 min super glue for glass surfaces and enhanced the tube to channel bond. Then, we treated the channel with 30 s O2 plasma to prepare for filling the microfluidic chip with precursor. Less than 10 μL of 400 mg/mL protein precursor was pipetted into the inlet per chip. The precursor was sufficiently viscous to provide the desired fs-LDW results but not so viscous as to prevent its flow into the channel due to capillary action, and thus the channel was filled without the use of an external pump.
For several hours after the channel was filled, fs-LDW fabrication cannot be executed reliably because the evaporation from the inlet and outlet and capillary flow along the walls of the channel cause precursor solution to move inside the channel. Especially at slow fabrication speed such as our designated fabrication speed of 5 μm/s across our chosen channel dimensions of 100 × 50 μm2, already fabricated parts of the mesh will dislodge or detach before the whole mesh can be finished. Therefore, we stored the protein-solution-filled channels for at least 1 day at room temperature and approximately 40% relative humidity, so that evaporation significantly reduced upon the formation of solidified protein plugs at the inlet and outlet while retaining protein precursor liquid in the inner channel.
Due to reduced evaporation, precursor flow inside the channel halts nearly completely when a few millimeters away from the inlet or outlet. We found that after 1 day, this condition of minimal flow is reached and fs-LDW fabrication can be executed with high reliability. The necessary storage time is likely dependent on precursor composition, channel dimensions and inlet/outlet geometry, with room temperature and relative humidity playing a secondary role. After fs-LDW, the solidified protein plugs were gently rehydrated with buffer solution and all of the excess protein precursor rinsed by repeatedly adding buffer to the inlet.
2.4. Buffer Exchange
We used the following standard pH solutions: Phosphate buffer pH 6, carbonate pH standard solution (pH 10), pH 4.01, pH 9.18 and pH 6.86 buffers from Fujifilm Wako Chemical (Osaka, Japan) and pH standard solutions (technical type) of pH 5 and pH 8 from Hanna Instruments (Woonsocket, RI, USA). Fluorescein sodium salt (F6377-1006, Sigma-Aldrich, Tokyo, Japan) fluorescent dye was used to determine that the speed of gravitation flow was approximately 30 μm/s in the center of the channel (
Figure S1 in the Supplementary Information).
2.5. Data Analysis
The data are presented as the mean and standard deviation for n samples (number of physical samples). Mesh gap sizes were observed using a bright-field microscope (BX53, Evident, Tokyo, Japan) and analyzed with ImageJ (version1.54j, National Institutes of Health, Bethesda, MD, USA) by manual read-out. We attempted to automate the analysis (see
Supplementary Information Table S1), but unfortunately it was very difficult due to contrast differences between images and due to meshes bending in and out of the image focus.
Free-standing microstructures were imaged with a 20× magnification lens immersed in the respective buffer. Scale bar distortion due to liquid immersion was evaluated using fixed dimensions embedded into the cover glass surface and the obtained corrective factors were applied to the image measurements. The 20× images without lens immersion had a scale of 155 pixels to 50 μm, whereas the buffer-immersed images had a scale of 155 pixels to 41.67 μm.
The laser scanning fluorescence images were reconstructed into 3D format using an FV3000 laser scanning microscope (Evident, Tokyo, Japan) and the integrated SV31-SW software v.2.6.1. Images were obtained with an NA = 0.85 40× lens using 405 and 488 nm excitation lasers to excite the autofluorescence of cross-linked proteinaceous materials.
4. Discussion
Pure proteinaceous meshes were integrated into a microchannel using the fs-LDW approach and the effect of buffer pH on the mesh gap size was evaluated. Within our fabrication window and the pH range tested, the mesh gap size increased mostly monotonically with increasing pH. Monitoring changes in the gap size enabled real-time sensing of the pH of the liquid buffer flowing in the microfluidic channel. The ability to control the gap size of a protein mesh by changing the pH of the surrounding buffer might allow cells and particles to be filtered using microfluidic devices.
These characteristics of pure 3D proteinaceous microstructures are distinctly different from those of microstructures reported previously. Specifically, two extreme pH values were previously required to activate switching states [
25,
26]—either pH 5 and pH 13 [
25] or pH 5 and pH 11 [
26]—or monotonic regimes were not identified by lack of selection of fabrication conditions [
23]. Our findings of increasing swelling with higher pH are matching the reports for BSA pH responsiveness reported in [
23] and [
25,
26], where our specific superiority is due to the selected fabrication conditions that utilize a narrow pH range to produce a monotonic relationship between the change in the gap size and the pH value.
In our pH exchange experiment, we utilize microchannel flow to precisely deliver the buffer to the mesh using a simple setup with gravitation and capillary flow. Deformation of the microstructure begins almost instantaneously upon changing the pH of the environment. More accurate timing to compensate for a possible sub-second delay would require the use of microfluidic pumps, but this would be challenging because we removed the plastic cover and replaced it with bonded glass, complicating the attachment of microfluidic tubing to reliably sustain pressure controlled by a microfluidic pump.
The literature demonstrates that pH-actuation of proteinaceous microstructures is likely due to charged surface residues affecting the expansion or contraction of the proteinaceous mesh [
23,
25]. More specifically, we suggest that surface charges and charged residues might affect the protein hydration shell (PHS). In contrast to photopolymer molecules, protein molecules are surrounded by a hydration shell comprising static and dynamical layers of water molecules. Such hydration shells are controlled by the surface properties of the protein and are also strongly influenced by the size and geometric shape of the protein [
29].
All previous reports of proteinaceous microstructures consider the relationship between some fabrication conditions and microstructure swelling and shrinkage. Notably, Lee et al. investigated the effect of fabrication conditions on stiffness in pH response [
26]. Some reports used hatching layer distance [
26] or step length [
25], which required maintaining the other parameters such as beam spot size. Total accumulated fluence might be a great candidate to compare results among different laboratories and laser fabrication setups. Our results are in agreement with [
26] that less densely cross-linked networks swell and shrink more and are less mechanically robust.
A slower response time was observed when pH 10 buffer was used in comparison to when pH 4 was applied to the mesh. This can be explained by the response of the PHS to osmotic pressure, electrostatic repulsion, and molecule packing density. When the mesh is initially exposed to a lower pH environment, such as pH 4, the mesh experiences a lower osmotic pressure compared to that in a higher pH environment such as pH 10 [
29]. Similarly, in a lower pH environment, amino acid residues are protonated and electrostatic repulsion is weaker than in a higher pH environment with deprotonated amino acid residues. Lastly, when pH 4 is used as the beginning state, the molecular network is condensed, slowing the diffusion of buffer ions, even though the diameter of pH 10 ions is smaller than that of lower pH ions.
Our time observations differed from those recently published elsewhere that measured protein shrinkage and swelling through the displacement of attached polymer components [
25]. This group worked with pH 1 and pH 13 solutions, which are quite extreme pH values that denature BSA, given that BSA denatures at pH 3 and pH 11 [
30], which might contribute to the differences from our findings since denaturation would change the tertiary structure and thus the surface charges. They reported several response times depending on their specific device design, each with very high repeatability. In our experiment, about 1–2 s of standard deviation existed even when using the same device, and leakage and a flow variation within the microfluidic channel that might further cause a negative effect with each new buffer exchange. Ref. [
25] reported that the response time of pH 5 was very similar to that of pH 13 for two different designs, one design with 1.4–1.5 s and the other with circa 1 s each. For a third design, they reported about 3 s for pH 5 while less than 2 s for pH 13. In contrast, we observed the 3 times longer response time for pH 10. The trend of longer time for pH 10 was observed throughout different experiments. As proposed in the previous paragraph, the relationship between pH 4 and pH 10 can be explained via PHS. The other groups reported different results due to use of extreme pH, or because pH 5 is close to the isoelectric point for BSA which creates a different dynamic of surface charges in response to pH changes. Our results might differ from previous works due to microfluidic flow, interplay with surface charges of the elastomer or the use of standard solutions of buffers.
The highly pH-sensitive meshes with a low crosslink density used in the current study allow us to determine the pH from the gap size, because the gap size changes greatly in response to changes in the pH values in the environment. The error of those deformations resulting in gap size changes is approximately 3%, allowing pH determinations with at least integer accuracy. Improved gap size analysis methods to compensate for mesh irregularities and out-of-plane bending might provide finer resolution.
We demonstrated ship-in-a-bottle integration in 100 × 50 μm
2 channels in a 3D manner. Furthermore, we showed that the relationship between gap size and pH matched the results obtained using free-standing structures. Slight differences observed between the two approaches appear to be due to the microfluidic channel slightly restricting mesh gap expansion at higher pH values, leading to a reduction to 13% from the expected 24% at pH 10. Channel restrictions can further increase the risk of entanglement during swelling and straining of the meshes, which might break the anchorage in channel walls during shrinkage. In
Table S5, we show examples of detached and well-attached meshes. When properly attached, the mesh can withstand repeated use and rough treatment. It is not fully clear what improves anchorage, but O
2 plasma treatment and embedded fabrication into the channel elastomer seem to have contributed to the attachment.
Our results demonstrate an approach to 3D fabrication to anchor proteinaceous meshes into microfluidic channels, supporting increased fabrication versatility compared to previous reports [
19,
20,
21,
22]. Our findings also show the potential applicability of integrated proteinaceous 3D structures for creating cell culture microenvironments to support the controlled growth of cell cultures or tissues on-chip. The scalability to commercial or industrial needs is not yet achieved, but with higher power lasers, it will become possible to compensate for the slow fabrication speeds of 5 μm/s to increase throughput by a parallel processing scheme using multi-spots.
Meshes for use as filtration layers with pH-sensitive gap sizes should probably be generated using a 2 nJ laser power setting rather than 1.5 nJ. Control of the gap size by the external pH is possible without compromising the robustness of the mesh required for filtration applications. The gap size can be changed in the range from −12 to +24 percent of the original size. An average gap of 10 μm encompasses the range from 8.8 to 12.4 μm. Choosing a smaller gap, such as 5 μm, could allow filtering of 4.4 to 6.2 μm-sized particles. However, a pH sensing application benefits from fabrication at 1.5 nJ because the gap size change in response to individual pH values is wider and the monotonic relationship is more pronounced, which increases the sensitivity. A greater change in response might be easier to trace by microscopic observation, and the resolution of the image is less affected.
This study was conducted entirely using BSA only. BSA can be considered the standard protein because it is prominently featured nearly always for laser 3D printing of protein [
10]. The reason why BSA is so popular is that it is commercially available at an economic price, easily cross-links due to beneficial amino acids, and is quite easy to handle because of very basic storing and safety measures. In the comprehensive study of chemical responsiveness of 3D printed proteinaceous microstructures [
23], it was demonstrated that three different protein types (BSA, avidin and lysozyme) had different pH responsiveness profiles (e.g., at specific pH values, BSA microstructures swell, while avidin microstructures shrink). Other protein types likely have other pH-dependencies, and as long as one can identify a monotonic relationship between the size change and pH, the concept of this work is transferable.