Non-Stoichiometric BaxMn0.7Cu0.3O3 Perovskites as Catalysts for CO Oxidation: Optimizing the Ba Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of BaxMn0.7Cu0.3O3 Samples
2.2. Activity Tests
3. Results and Discussion
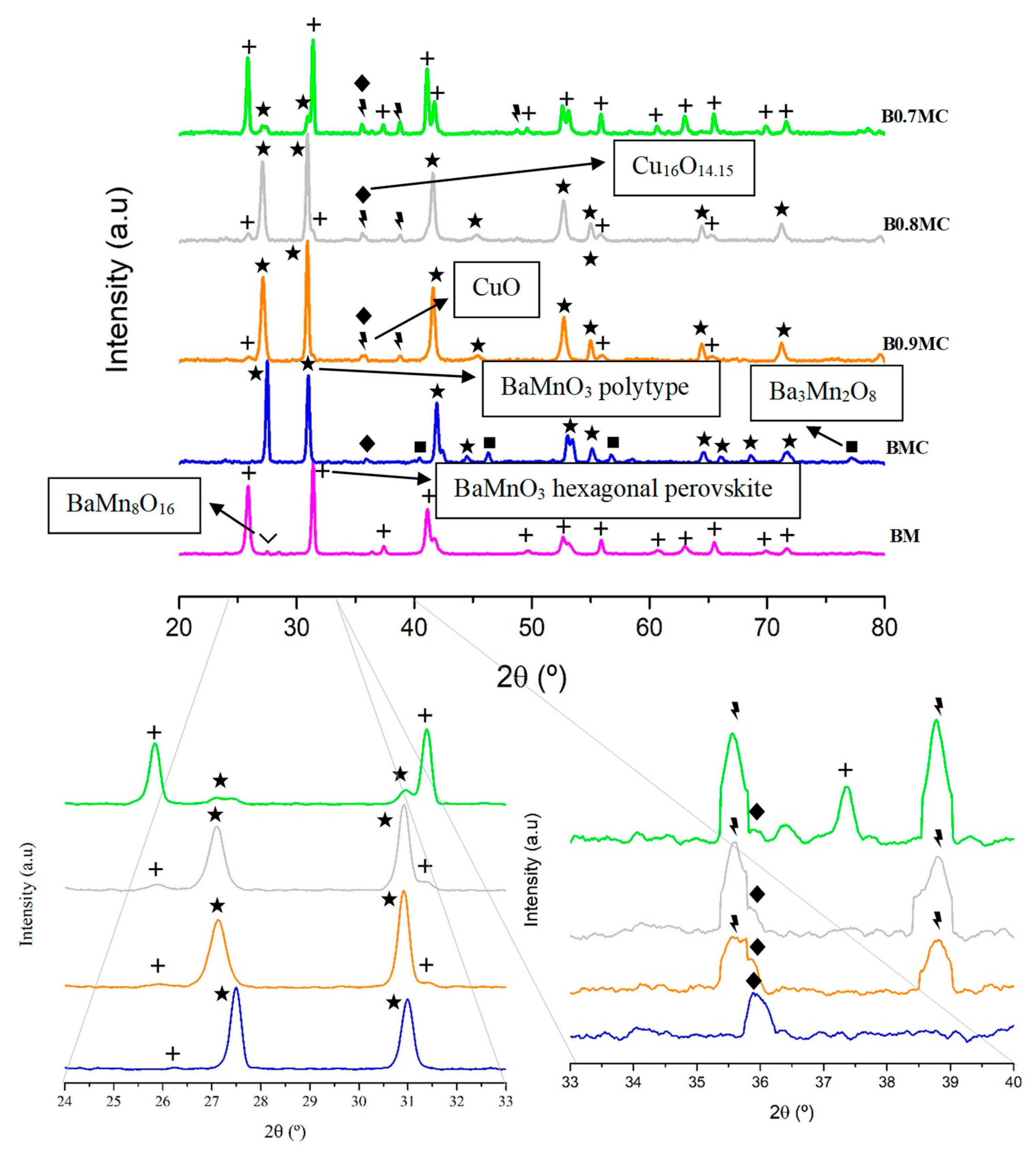
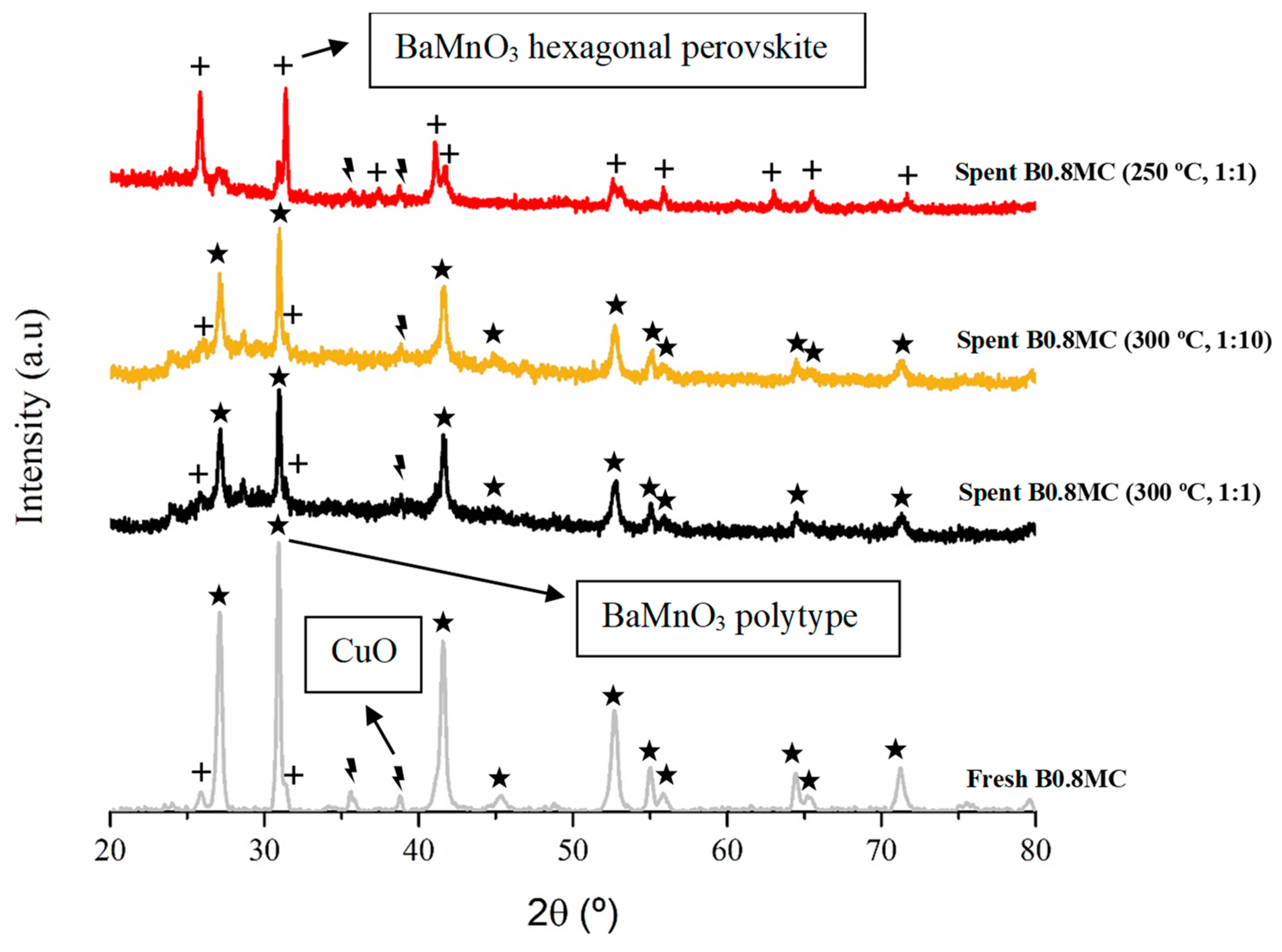
3.1. Chemical and Structural Properties
3.2. Surface Properties
3.3. Reducibility and Redox Properties
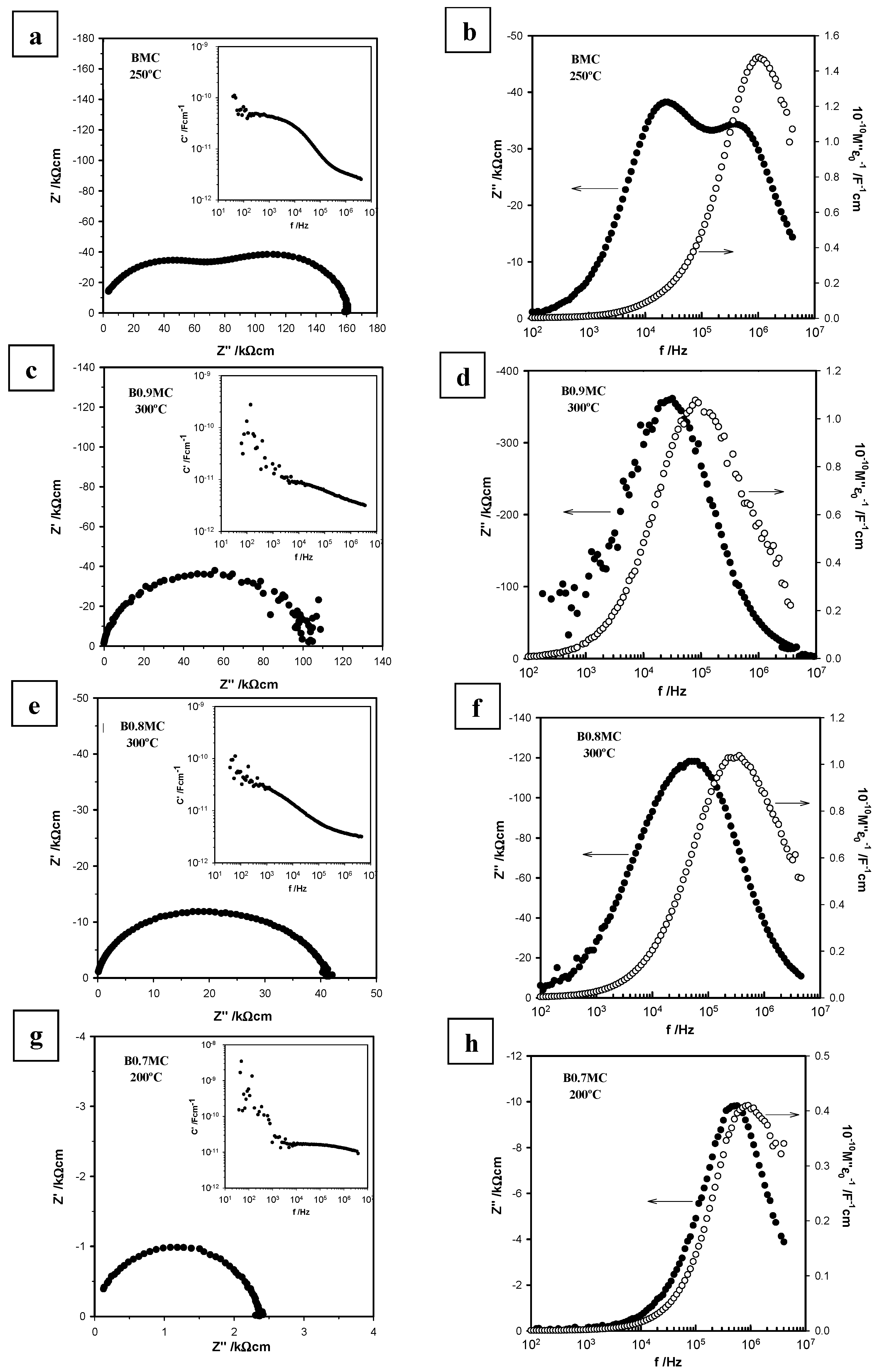
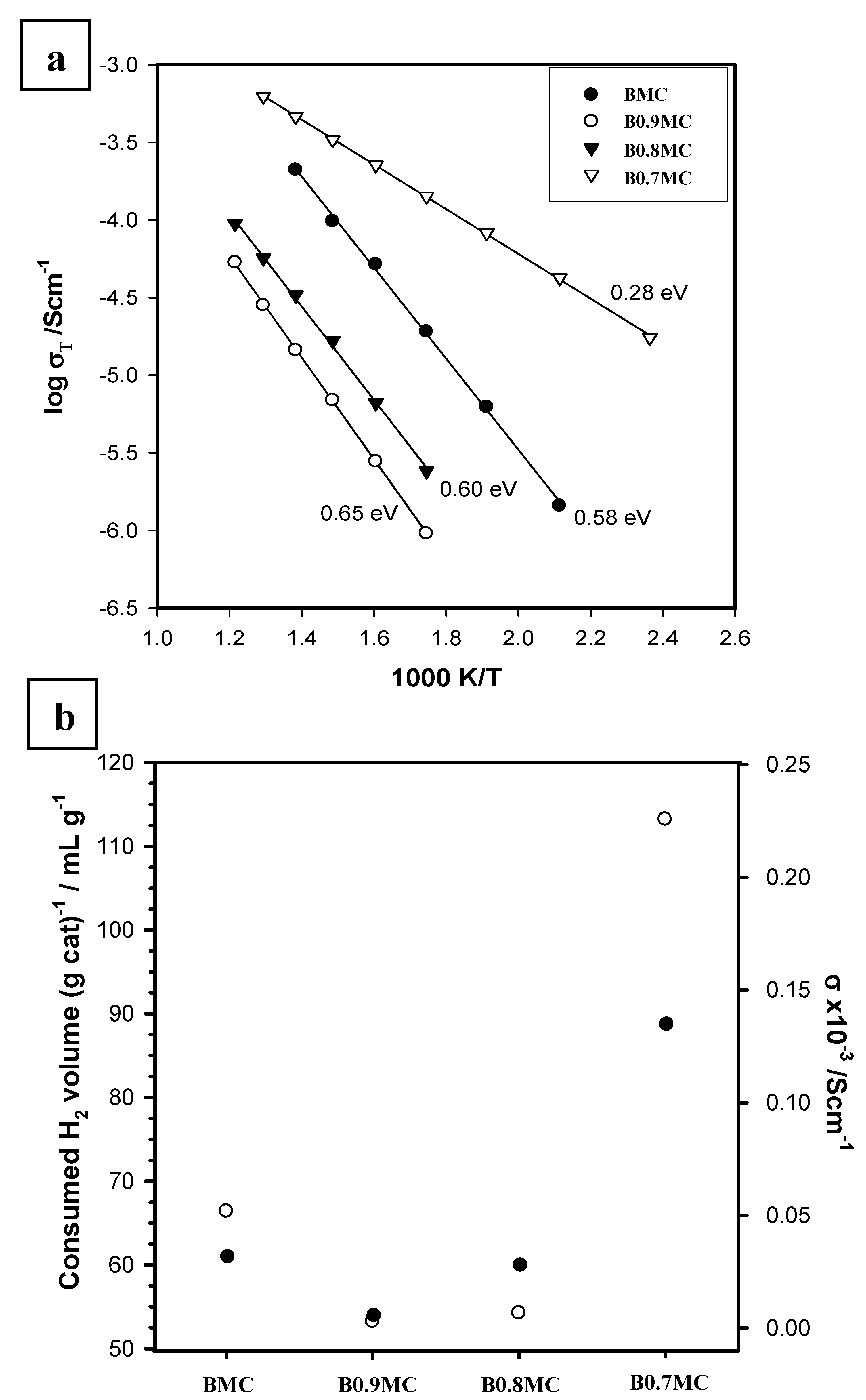
3.4. Electrical Properties
- (i)
- BMC and B0.7MC samples show higher conductivities as an almost unique crystalline phase (BaMnO3 polytype for BMC and hexagonal BaMnO3 for B0.7MC) is detected, presenting BMC having a higher resistance than B0.7MC, probably due to the contribution of the other minority phases and/or to the disorder degree of the polytype structure.
- (ii)
- For B0.9MC and B0.8MC samples, the polytype and the hexagonal perovskite structures coexist, causing the observed increase of the resistivity respect to BMC and B0.7MC.
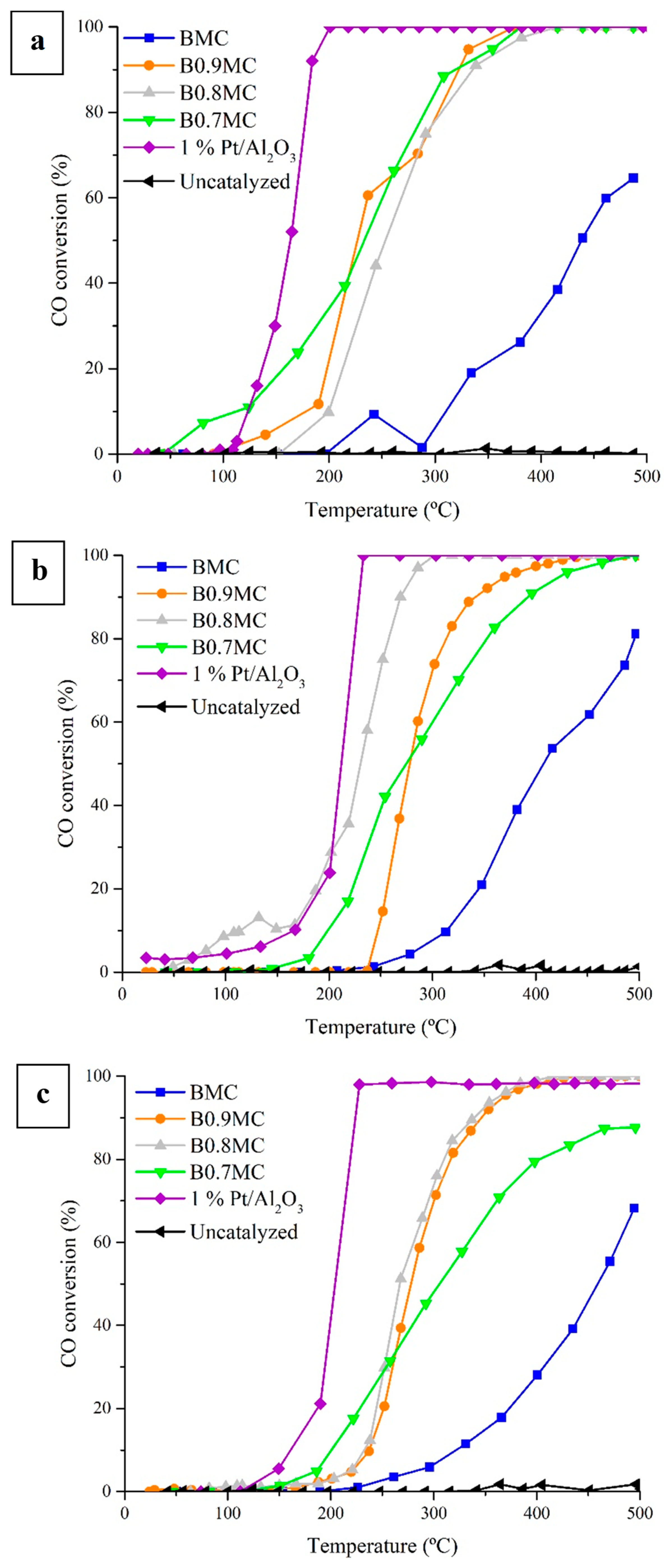
3.5. Catalytic Activity
4. Conclusions
- The Ba content in BxMC perovskites formulations determines the Cu distribution and, consequently, the structure of the samples.
- The amount of Mn(IV) and of oxygen vacancies (defects) increases as the Ba content decreases, and B0.8MC exhibits an enrichment of reduced copper species on the surface.
- In the presence of Cu, a Mn-Cu synergistic effect is observed, which promotes the reducibility and the oxygen emission. However, this synergistic effect decreases with the Ba content, due to the different location of Cu into the perovskite network.
- The reducibility trend during the H2-TPR characterization tests appears to be directly linked to the total conductivity of perovskites.
- All BxMC perovskites tested are active for CO oxidation, showing B0.8MC the best catalytic performance, which is the closest to that of 1% Pt/Al2O3 reference sample. The outstanding catalytic performance of B0.8MC is mainly related to the presence of a high Mn(IV)/Mn(III) ratio and the highest amount of oxygen vacancies and of reduced copper species on surface.
- A correlation is observed between the conductivity, the crystalline structure, and the reducibility of the samples, which is, in turn, related to their catalytic performance.
- The stability of B0.8MC during the isothermal reaction depends on the temperature and on the reactant atmosphere composition (CO and O2 proportion). Thus, the reaction temperature determines the distribution of copper species in the structure and the stability is highly dependent on the proportion of reactants, with the preservation of the reduced copper species (Cu(I) and Cu(0)) on the surface in the most reductant atmosphere composition (CO:O2 1:1) being the key factor, as these oxidation states are more effective for CO and O2 activation than Cu(II).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhong, Q.; Huang, Y.; Shen, H.; Chen, Y.; Chen, H.; Huang, T.; Zeng, E.Y.; Tao, S. Global estimates of carbon monoxide emissions from 1960 to 2013. Environ. Sci. Pollut. Res. 2017, 24, 864–873. [Google Scholar] [CrossRef]
- Siegmund, T.; Gollmer, C.; Horstmann, N.; Kaltschmitt, M. Carbon monoxide (CO) and particulate matter (PM) emissions during the combustion of wood pellets in a small-scale combustion unit—Influence of aluminum-(silicate-)based fuel additivation. Fuel Process. Technol. 2024, 262, 108111. [Google Scholar] [CrossRef]
- Savioli, G.; Gri, N.; Ceresa, I.F.; Piccioni, A.; Zanza, C.; Longhitano, Y.; Ricevuti, G.; Daccò, M.; Esposito, C.; Candura, S.M. Carbon monoxide poisoning: From occupational health to emergency medicine. J. Clin. Med. 2024, 13, 2466. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.Y.; Long, B.; Koyfman, A. Clinical chameleons: An emergency medicine focused review of carbon monoxide poisoning. Intern. Emerg. Med. 2018, 13, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cui, Y.; Zhu, M.; Fu, Y.; Sun, Y.; Meng, Z.; Peng, B.; Nie, L. Incorporation of Ag on stable Pt/CeO2 for low-temperature active and high-temperature stable CO oxidation catalyst. Mol. Catal. 2024, 553, 113704. [Google Scholar] [CrossRef]
- van Spronsen, M.A.; Frenken, J.W.M.; Groot, I.M.N. Surface science under reaction conditions: CO oxidation on Pt and Pd model catalysts. Chem. Soc. Rev. 2017, 46, 4347–4374. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Cha, X.; Huang, Z.; Hu, S.; Xu, K.; Cai, D.; Xiao, J.; Zhan, G. Green fabrication of hierarchically-structured Pt/bio-CeO2 nanocatalysts using natural pollen templates for low-temperature CO oxidation. Mol. Catal. 2022, 524, 112251. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, J.; Liu, T.; Xu, M.; Dong, Y.; Wang, C.A. Constructing morphologically stable supported noble metal catalysts in heterogeneous catalysis: Mechanisms and strategies. Nano Energy 2024, 129, 109975. [Google Scholar] [CrossRef]
- Chai, Y.; Shang, W.; Li, W.; Wu, G.; Dai, W.; Guan, N.; Li, L. Noble metal particles confined in zeolites: Synthesis, characterization, and applications. Adv. Sci. 2019, 6, 1900299. [Google Scholar] [CrossRef]
- Sidorowicz, A.; Yigit, N.; Wicht, T.; Stöger-Pollach, M.; Concas, A.; Orrù, R.; Cao, G.; Rupprechter, G. Microalgae-derived Co3O4 nanomaterials for catalytic CO oxidation. RSC Adv. 2024, 14, 4575–4586. [Google Scholar] [CrossRef]
- Zedan, A.F.; Mohamed, A.T.; El-Shall, M.S.; AlQaradawi, S.Y.; AlJaber, A.S. Tailoring the reducibility and catalytic activity of CuO nanoparticles for low temperature CO oxidation. RSC Adv. 2018, 8, 19499–19511. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Zhao, M.; Xu, S.; Wang, M.; Cao, J.; Zhang, Q.; Chen, J. Engineering defective Mnδ+ -OV active structure in α-MnO2 for efficient low-temperature CO oxidation. Appl. Surf. Sci. 2024, 670, 160602. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Arun, P.S.; Ranjith, B.P.; Shibli, S.M.A. Control of carbon monoxide (CO) from automobile exhaust by a dealuminated zeolite supported regenerative MnCo2O4 catalyst. Environ. Sci. Technol. 2013, 47, 2746–2753. [Google Scholar] [CrossRef]
- Zheng, X.C.; Wu, S.H.; Wang, S.P.; Wang, S.R.; Zhang, S.M.; Huang, W.P. The preparation and catalytic behavior of copper-cerium oxide catalysts for low-temperature carbon monoxide oxidation. Appl. Catal. A Gen. 2005, 283, 217–223. [Google Scholar] [CrossRef]
- Taylor, S.H.; Hutchings, G.J.; Mirzaei, A.A. The preparation and activity of copper zinc oxide catalysts for ambient temperature carbon monoxide oxidation. Catal. Today 2003, 84, 113–119. [Google Scholar] [CrossRef]
- Du, L.; Wang, W.; Yan, H.; Wang, X.; Jin, Z.; Song, Q.; Si, R.; Jia, C. Copper-ceria sheets catalysts: Effect of copper species on catalytic activity in CO oxidation reaction. J. Rare Earths 2017, 35, 1186–1196. [Google Scholar] [CrossRef]
- Wang, W.W.; Yu, W.Z.; Du, P.P.; Xu, H.; Jin, Z.; Si, R.; Ma, C.; Shi, S.; Jia, C.J.; Yan, C.H. Crystal plane effect of ceria on supported copper oxide cluster catalyst for CO oxidation: Importance of metal-support interaction. ACS Catal. 2017, 7, 1313–1329. [Google Scholar] [CrossRef]
- Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. A review on the preparation, characterization and potential application of perovskites as adsorbents for wastewater treatment. Chemosphere 2020, 244, 125474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite oxides: Preparation, characterizations, and applications in heterogeneous catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Zhu, T.; Li, D.; Wang, H.; Zhu, X. Oxygen storage characteristics and redox behaviors of lanthanum perovskite oxides with transition metals in the B-sites. Energy Fuels 2023, 37, 9419–9433. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in oxygen carrier development of methane-based chemical-looping reforming: A review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef]
- Zhao, K.; He, F.; Huang, Z.; Zheng, A.; Li, H.; Zhao, Z. La1-xSrxFeO3 perovskites as oxygen carriers for the partial oxidation of methane to syngas. Chin. J. Catal. 2014, 35, 1196–1205. [Google Scholar] [CrossRef]
- Valderrama, G.; Goldwasser, M.R.; de Navarro, C.U.; Tatibouët, J.M.; Barrault, J.; Batiot-Dupeyrat, C.; Martínez, F. Dry reforming of methane over Ni perovskite type oxides. Catal. Today 2005, 107–108, 785–791. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Li, Y.; Chen, M.; Jiang, L.; Tian, D.; Li, K. Perovskites as oxygen storage materials for chemical looping partial oxidation and reforming of methane. Phys. Chem. Chem. Phys. 2024, 26, 1516. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xu, X.; Chen, G.; Shao, Z. Perovskite oxides as electrocatalysts for water electrolysis: From crystalline to amorphous. Carbon Energy 2024, 6, e595. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Guan, D.; Lin, Z.; Hu, Z.; Ni, M.; Huang, H.; Bhatelia, T.; Jiang, S.P.; Shao, Z. New nanocomposites derived from cation-nonstoichiometric Bax(Co, Fe, Zr, Y)O3-δ as efficient electrocatalysts for water oxidation in alkaline solution. ACS Mater. Lett. 2024, 6, 2985–2994. [Google Scholar] [CrossRef]
- Díaz-Verde, A.; Torregrosa-Rivero, V.; Illán-Gómez, M.J. Copper catalysts supported on barium deficient perovskites for CO oxidation reaction. Top. Catal. 2023, 66, 895–907. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Copper doped BaMnO3 perovskite catalysts for NO oxidation and NO2-assisted diesel soot removal. RSC Adv. 2017, 7, 35228–35238. [Google Scholar] [CrossRef]
- Díaz-Verde, Á.; Illán-Gómez, M.J. Enhancing the performance of BaxMnO3 (x = 1, 0.9, 0.8 and 0.7) perovskites as catalysts for CO oxidation by decreasing the Ba content. Nanomaterials 2024, 14, 1334. [Google Scholar] [CrossRef] [PubMed]
- Karoui, L.; Smari, M.; Mnasri, T. The effect of the gelation temperature on the structural, magnetic and magnetocaloric properties of perovskite nanoparticles manufactured using the sol-gel method. RSC Adv. 2024, 14, 11456–11469. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Structural and morphological alterations induced by cobalt substitution in LaMnO3 perovskites. J. Colloid Interface Sci. 2019, 556, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.A.U.; Ikram, M. Structural stability improvement, Williamson Hall analysis and band-gap tailoring through A-site Sr doping in rare earth based double perovskite La2NiMnO6. Rare Met. 2019, 38, 805–813. [Google Scholar] [CrossRef]
- Guan, D.; Zhong, J.; Xu, H.; Huang, Y.C.; Hu, Z.; Chen, B.; Zhang, Y.; Ni, M.; Xu, X.; Zhou, W.; et al. A universal chemical-induced tensile strain tuning strategy to boost oxygen-evolving electrocatalysis on perovskite oxides. Appl. Phys. Rev. 2022, 9, 011422. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, L.; Ma, R.; Chen, Y.; Guan, Q.; Li, W. CuO nano-island anchored Pt catalyst for CO preferential oxidation in H2-rich stream. Chem. Eng. J. 2024, 495, 153295. [Google Scholar] [CrossRef]
- Giménez-Mañogil, J.; García-García, A. Identifying the nature of copper entities over ceria-based supports to promote diesel soot combustion: Synergistic effects. Appl. Catal. A Gen. 2017, 542, 226–239. [Google Scholar] [CrossRef]
- dos Santos Veiga, E.L.; Beltrán-Mir, H.; Vendrell, X.; Llorca, J.; Cordoncillo, E. Impedance spectroscopy: A useful technique to unveil the mechanism of a CO oxidation reaction. J. Am. Ceram. Soc. 2023, 107, 321–333. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Johnson, W.B. Fundamentals of Impedance Spectroscopy; Wiley: Hoboken, NJ, USA, 2005; pp. 1–26. [Google Scholar] [CrossRef]
- Lvovich, V.F. Impedance Spectroscopy: Applications to Electrochemical and Dielectric Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Irvine, J.T.S.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by impedance spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- West, A.R. Solid state Chemistry and Its Applications, 2nd ed., John Wiley & Sons: Oxford, UK, 2022.
- Guiotto, M.; Pacella, M.; Perin, G.; Iovino, A.; Michelon, N.; Natile, M.M.; Glisenti, A.; Canu, P. Washcoating vs. Direct synthesis of LaCoO3 on monoliths for environmental applications. Appl. Catal. A Gen. 2015, 499, 146–157. [Google Scholar] [CrossRef]
- Park, D.C.; Moon, S.; Song, J.H.; Kim, H.; Lee, E.; Lim, Y.H.; Kim, D.H. Widening the operating window of Pt/ZSM-5 catalysts for efficient NOx removal in H2-SCR: Insights from thermal aging. Catal. Today 2024, 425, 114318. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Ding, J.; Zhang, Y.; Jia, J.; Sun, T. Catalytic oxidation of VOCs over SmMnO3 perovskites: Catalyst synthesis, change mechanism of active species, and degradation path of toluene. Inorg. Chem. 2019, 58, 14275–14283. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Du, Y.; Deng, J.; Zhang, L.; Zhao, Z. Lysine-aided PMMA-templating preparation and high performance of three-dimensionally ordered microporous LaMnO3 with mesoporous walls for the catalytic combustion of toluene. Appl. Catal. B Environ. 2012, 119–120, 20–31. [Google Scholar] [CrossRef]
- Xia, C.; Wang, X.; He, C.; Qi, R.; Zhu, D.; Lu, R.; Li, F.M.; Chen, Y.; Chen, S.; You, B.; et al. Highly selective electrocatalytic CO2 conversion to tailored products through precise regulation of hydrogenation and C-C coupling. J. Am. Chem. Soc. 2024, 146, 20530–20538. [Google Scholar] [CrossRef] [PubMed]
- Ulyanov, A.N.; Yu, S.C.; Yang, D.S. Mn-site-substituted lanthanum manganites: Destruction of electron pathway and local structure effects on Curie temperatura. J. Magn. Magn. Mater. 2004, 282, 303–306. [Google Scholar] [CrossRef]
- Ghezali, N.; Díaz-Verde, A.; Illán-Gómez, M.J. Ba0.9A0.1MnO3 (A = Ce, La, Mg) perovskite-type mixed oxides: Effect of partial substitution of Ba on the catalytic performance for the oxidation of CO in simulated automobile exhaust conditions. Crystals 2024, 14, 191. [Google Scholar] [CrossRef]
- Abdelmoneim, A.; Abdel-wahab, M.S.; Lee, J.K.; Hameed, M.M.A.; Thamer, B.M.; Al-Enizi, A.M.; Alkhalifah, R.I.; Tawfik, W.Z. High performance of nanostructured Cu2O-based photodetectors grown on a Ti/Mo metallic substrate. Catalysts 2024, 13, 1145. [Google Scholar] [CrossRef]
- Andersson, S.L.T. Catalytic oxidation of toluene over Y-Ba-Cu perovskites: Activity and x-ray photoelectron spectroscopy studies. J. Chem. Soc. Faraday Trans. 1992, 88, 83–90. [Google Scholar] [CrossRef]
- Yuan, B.; Liu, X.; Cai, X.; Fang, X.; Liu, J.; Wu, M.; Zhu, Q. Preparation of zinc and cerium or both doped Cu2O photoelectric material via hydrothermal method. Sol. Energy 2020, 196, 74–79. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Yang, K.; Tang, W.; Liu, H.; Yang, J.; Yue, R.; Chen, Y. Effects of cerium incorporation on the catalytic oxidation of benzene over flame-made perovskite La1-xCexMnO3 catalysts. Particuology 2015, 19, 60–68. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, W.; Lai, S.; Chen, X. Integral structured Co-Mn composite oxides grown on interconnected Ni foam for catalytic toluene oxidation. RSC Adv. 2019, 9, 6533–6541. [Google Scholar] [CrossRef] [PubMed]
- Mane, R.; Kim, H.; Han, K.; Kim, H.; Lee, S.S.; Roh, H.S.; Lee, C.; Jeon, Y. Important factors of the A-site deficient Mn perovskites design affecting the CO oxidation activity. Catal. Today 2024, 425, 114347. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The influence of nonstoichiometry on LaMnO3 perovskite for catalytic NO oxidation. Appl. Catal. B Environ. 2013, 134–135, 251–257. [Google Scholar] [CrossRef]
- Batis, N.H.; Delichere, P.; Batis, H. Physicochemical and catalytic properties in methane combustion of La1-xCaxMnO3±y (0 ≤ x ≤ 1; -0.04 ≤ y ≤ 0.24) perovskite-type oxide. Appl. Catal. A Gen. 2005, 282, 173–180. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Relationship between catalytic deactivation and physicochemical properties of LaMnO3 perovskite catalyst during catalytic oxidation of vinyl chloride. Appl. Catal. B Environ. 2016, 186, 173–183. [Google Scholar] [CrossRef]
- Shim, K.; Kim, H.W.; Park, S.; Seo, K.D.; Kim, C.Y.; Lee, J.B.; Bae, J.S.; Kim, H.J. A MnS/MnO-coated S,N-doped carbon anode obtained from a Mn(II)-coordinated polymer for long-cycle life Li-ion batteries. Mater. Adv. 2024, 5, 3014–3021. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Cerrato, J.M.; Hochella, M.F.; Knocke, W.R.; Dietrich, A.M.; Cromer, T.F. Use of XPS to identify the oxidation state of Mn in solid surfaces of filtration media oxide samples from drinking water treatment plants. Environ. Sci. Technol. 2010, 44, 5881–5886. [Google Scholar] [CrossRef]
- Qahtan, T.F.; Owolabi, T.O.; Saleh, T.A. X-ray photoelectron spectroscopy of surface-treated TiO2 mesoporous film by 500eV argon ion beam. J. Mol. Liq. 2024, 393, 123556. [Google Scholar] [CrossRef]
- Yang, J.; Hu, S.; Fang, Y.; Hoang, S.; Li, L.; Yang, W.; Liang, Z.; Wu, J.; Hu, J.; Xiao, W.; et al. Oxygen vacancy promoted O2 activation over perovskite oxide for low-temperature CO oxidation. ACS Catal. 2019, 9, 9751–9763. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; López-Rodríguez, S.; Villar-García, I.J.; Pérez-Dieste, V.; Bueno-López, A.; González-Velasco, J.R. Molecular elucidation of CO2 methanation over a highly active, selective and stable LaNiO3/CeO2-derived catalyst by in situ FTIR and NAP-XPS. Appl. Catal. B Environ. 2024, 342, 123367. [Google Scholar] [CrossRef]
- Koch, G.; Hävecker, M.; Teschner, D.; Carey, S.J.; Wang, Y.; Kube, P.; Hetaba, W.; Lunkenbein, T.; Auffermann, G.; Timpe, O.; et al. Surface conditions that constrain alkane oxidation on perovskites. ACS Catal. 2020, 10, 7007–7020. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Xing, J.; Zhang, M.; Liao, R.; Wang, C.; Hua, Y.; Ji, H. Novel synergistically effects of palladium-iron bimetal and manganese carbonate carrier for catalytic oxidation of formaldehyde at room temperature. J. Colloid Interface Sci. 2024, 656, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Y.; Li, R.L.; Wu, H.L.; Zhang, S.F.; Chai, M.W.; Shen, X.X.; Hong, M.; Lin, H. Selective removal of Cu2+ ion in aqueous solution by poly (acrylic acid/acrylamide) hydrogel. Chin. J. Anal. Chem. 2020, 48, e20098–e20106. [Google Scholar] [CrossRef]
- Wu, K.; Lu, Y.; Liu, Y.; Shen, M.; Debliquy, M.; Zhang, C. Synthesis and acetone sensing properties of copper (Cu2+) substituted zinc ferrite hollow micro-nanospheres. Ceram. Int. 2020, 46, 28835–28843. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; López-Suárez, F.E.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Tailoring the properties of BaTi0.8Cu0.2O3 catalyst selecting the synthesis method. Appl. Catal. A Gen. 2016, 519, 7–15. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; López-Suárez, F.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. BaTi0.8Cu0.2O3 catalysts for NO oxidation and NOx storage: Effect of synthesis method. Top. Catal. 2017, 60, 220–224. [Google Scholar] [CrossRef]
- LaSurface Database. Available online: http://www.lasurface.com/accueil/ (accessed on 10 September 2024).
- Shen, M.; Zhao, Z.; Chen, J.; Su, Y.; Wang, J.; Wang, X. Effects of calcium substitute in LaMnO3 perovskites for NO catalytic oxidation. Rare Earths 2013, 31, 119–123. [Google Scholar] [CrossRef]
- Sarshar, Z.; Kaliaguine, S. Reduction kinetics of perovskite-based oxygen carriers for chemical looping combustion. Ind. Eng. Chem. Res. 2013, 52, 6946–6955. [Google Scholar] [CrossRef]
- Santamaria, A.; Méndez-A, C.; Gallego, J. LaMn1-xCoxO3 perovskite-like doped and impregnated with Cu for the enhancement of toluene total oxidation. Appl. Surf. Sci. 2024, 669, 160587. [Google Scholar] [CrossRef]
- Kobayashi, M.; Naniwa, S.; Goto, K.; Matsuo, H.; Iguchi, S.; Tanaka, T.; Teramura, K. Promoting effect of Pd nanoparticles on SrTi0.8Mn0.2O3 in the reverse water-gas shift reaction via the Mars-Van Krevelen mechanism. ChemCatChem 2024, 16, e202400871. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Salari, D.; Niaei, A.; Oskoui, S.A. Physical-chemical property and activity evaluation of LaB0.5Co0.5O3 (B = Cr, Mn, Cu) and LaMnxCo1-xO3 (x = 0.1, 0.25, 0.5) nano perovskites in VOC combustion. J. Ind. Eng. Chem. 2013, 19, 1903–1909. [Google Scholar] [CrossRef]
- Tarjomannejad, A.; Niaei, A.; Farzi, A.; Salari, D.; Zonouz, P.R. Catalytic oxidation of CO over LaMn1-xBxO3 (B = Cu, Fe) perovskite-type oxides. Catal. Letters 2016, 146, 1544–1551. [Google Scholar] [CrossRef]
- Buciuman, F.C.; Patcas, F.; Hahn, T. A spillover approach to oxidation catalysts over copper and manganese mixed oxides. Chem. Eng. Process. Process Intensif. 1999, 38, 563–569. [Google Scholar] [CrossRef]
- Wei, G.; Zhang, Q.; Zhang, D.; Wang, J.; Tang, T.; Wang, H.; Liu, X.; Song, Z.; Ning, P. The influence of annealing temperature on copper-manganese catalyst towards the catalytic combustion of toluene: The mechanism study. Appl. Surf. Sci. 2019, 497, 143777. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Y.; Niu, X.; Yuan, F.; Fu, H. Preparation of La-Mn-O perovskite catalyst by microwave irradiation method and its application to methane combustion. Catal. Letters 2010, 135, 152–158. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.; Qin, Y.; Fu, Q.; Sun, H.; Duan, X. Revealing the highly catalytic performance of spinel CoMn2O4 for toluene oxidation: Involvement and replenishment of oxygen species using in situ designed-TP techniques. ACS Catal. 2019, 9, 6698–6710. [Google Scholar] [CrossRef]
- Natesakhawat, S.; Popczun, E.J.; Baltrus, J.P.; Wang, K.; Serna, P.; Liu, S.; Meyer, R.; Lekse, J.W. Investigation of AFeO3 (A = Ba, Sr) perovskites for the oxidative dehydrogenation of light alkanes under chemical looping conditions. ChemPlusChem 2024, 89, e202300596. [Google Scholar] [CrossRef] [PubMed]
- García-García, F.J.; Sayagués, M.J.; Gotor, F.J. A novel, simple and highly efficient route to obtain PrBaMn2O5+δ double perovskite: Mechanochemical synthesis. Nanomaterials 2021, 11, 380. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Huang, T.J.; Tsai, D.H. CO oxidation behavior of copper and copper oxides. Catal. Letters 2003, 87, 173–178. [Google Scholar] [CrossRef]
- Jernigan, G.G.; Somorjai, G.A. Carbon monoxide oxidation over three different oxidation states of copper: Metallic copper, copper (I) oxide, and copper (II) oxide—A surface science and kinetic study. J. Catal. 1994, 147, 567–577. [Google Scholar] [CrossRef]
- Liu, T.; Yao, Y.; Wei, L.; Shi, Z.; Han, L.; Yuan, H.; Li, B.; Dong, L.; Wang, F.; Sun, C. Preparation and evaluation of copper-manganese oxide as high-efficiency catalyst for CO oxidation and NO reduction by CO. J. Phys. Chem. C 2017, 121, 12757–12770. [Google Scholar] [CrossRef]
- Hao, Y.; Mihaylov, M.; Ivanova, E.; Hadjiivanov, K.; Hnözinger, H.; Gates, B.C. CO oxidation catalyzed by gold supported on MgO: Spectroscopic identification of carbonate-like species bonded to gold during catalyst deactivation. J. Catal. 2009, 261, 137–149. [Google Scholar] [CrossRef]
- Jansson, J.; Palmqvist, A.E.C.; Fridell, E.; Skoglundh, M.; Österlund, L.; Thormählen, P.; Langer, V. On the catalytic activity of Co3O4 in low-temperature CO oxidation. J. Catal. 2002, 211, 387–397. [Google Scholar] [CrossRef]
- Saavedra, J.; Powell, C.; Panthi, B.; Pursell, C.J.; Chandler, B.D. CO oxidation over Au/TiO2 catalyst: Pretreatment effects, catalyst deactivation, and carbonates production. J. Catal. 2013, 307, 37–47. [Google Scholar] [CrossRef]
- Yang, X.; Sun, K.; Sun, W.; Ma, M.; Ren, R.; Qiao, J.; Wang, Z.; Zhen, S.; Xu, C. Surface reconstruction of defective SrTi0.7Cu0.2Mo0.1O3-δ perovskite oxide induced by in-situ copper nanoparticle exsolution for high-performance direct CO2 electrolysis. J. Eur. Ceram. Soc. 2023, 43, 3414–3420. [Google Scholar] [CrossRef]
- NIST XPS Database. Available online: https://srdata.nist.gov/xps/ (accessed on 18 September 2024).






| Sample | Nomenclature | BET Surface Area (m2 g−1) | Nominal Cu (wt %) |
|---|---|---|---|
| BaMn0.7Cu0.3O3 | BMC | 1 | 8 |
| Ba0.9Mn0.7Cu0.3O3 | B0.9MC | 6 | 8 |
| Ba0.8Mn0.7Cu0.3O3 | B0.8MC | 7 | 9 |
| Ba0.7Mn0.7Cu0.3O3 | B0.7MC | 4 | 9 |
| Sample | Cell Parameters (Å) 1 | Perovskite Average Crystal Size (nm) | (110) Interplanar Spacing (Å) | Lattice Strain | |
|---|---|---|---|---|---|
| a | c | ||||
| BM | 5.7 | 4.8 | 24.8 | 2.85 | 3.0·10−4 |
| BMC | 5.8 | 4.3 | 57.8 | 2.88 | 2.1·10−3 |
| B0.9MC | 5.8 | 4.3 | 29.5 | 2.89 | 1.1·10−3 |
| B0.8MC | 5.8 | 4.4 | 81.6 | 2.89 | 3.9·10−3 |
| B0.7MC | 5.7 | 4.8 | 53.3 | 2.85 | 1.6·10−3 |
| Sample | Hexagonal BaMnO3 (%) | Polytype BaMnO3 (%) | CuO/Cu16O14.15 (%) | Ba3Mn2O8/BaMn8O16 (%) |
|---|---|---|---|---|
| BM | 98 | - | - | 2 |
| BMC | - | 96 | 3 | 1 |
| B0.9MC | 3 | 93 | 4 | - |
| B0.8MC | 8 | 85 | 7 | - |
| B0.7MC | 82 | 8 | 10 | - |
| Sample | Mn(IV)/Mn(III) | OL/(Ba + Mn + (Cu)) (Nominal) |
|---|---|---|
| BM | 0.2 | 1.2 (1.5) |
| BMC | 0.3 | 0.8 (1.5) |
| B0.9MC | 0.8 | 0.9 (1.6) |
| B0.8MC | 1.0 | 0.9 (1.7) |
| B0.7MC | 1.1 | 1.0 (1.8) |
| Sample | Cu/(Ba + Mn + Cu) (Nominal) | Surface Cu (%) 1 | Cusat/Cu2p | Cusi/Cuwi | Cu 2p3/2 BE (eV) | Cu LMM KE (eV) |
|---|---|---|---|---|---|---|
| BMC | 0.14 (0.15) | 93 | 0.6 | 2.7 | 932.28 | 918.12 |
| B0.9MC | 0.09 (0.16) | 56 | 0.5 | 2.0 | 932.88 | 917.62 |
| B0.8MC | 0.08 (0.17) | 47 | 0.5 | 1.4 | 933.68 | 917.52 |
| B0.7MC | 0.07 (0.18) | 39 | 0.4 | 1.2 | 933.28 | 917.72 |
| Sample | T50% (°C) | ||
|---|---|---|---|
| 0.1% CO/1% O2/He | 1% CO/1% O2/He | 1% CO/10% O2/He | |
| BMC | 438 | 408 | 459 |
| B0.9MC | 227 | 278 | 278 |
| B0.8MC | 253 | 231 | 267 |
| B0.7MC | 233 | 275 | 306 |
| 1% Pt/Al2O3 | 164 | 212 | 204 |
| Atmosphere 1:1 300 °C | Atmosphere 1:10 300 °C | Atmosphere 1:1 250 °C | ||||
|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | Cycle 1 | Cycle 2 | |
| Deactivation (%) 1 | 8 | 1 | 19 | 8 | 14 | 13 |
| CO specific activity (5 h) 2 | 0.4 | 0.3 | 0.3 | |||
| Sample | Mn(IV)/Mn(III) | Cu/(Ba + Mn + Cu) | OL/(Ba + Mn + Cu) | BaCO3/BaL 1 |
|---|---|---|---|---|
| Fresh | 1.0 | 0.08 | 1.12 | 0.2 |
| Used (300 °C, 1:1) | 0.6 | 0.07 | 0.14 | 0.7 |
| Used (300 °C, 1:10) | 0.6 | 0.06 | 0.17 | 0.4 |
| Used (250 °C, 1:1) | 0.6 | 0.09 | 0.20 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Verde, Á.; dos Santos Veiga, E.L.; Beltrán-Mir, H.; Illán-Gómez, M.J.; Cordoncillo-Cordoncillo, E. Non-Stoichiometric BaxMn0.7Cu0.3O3 Perovskites as Catalysts for CO Oxidation: Optimizing the Ba Content. Nanomaterials 2025, 15, 103. https://doi.org/10.3390/nano15020103
Díaz-Verde Á, dos Santos Veiga EL, Beltrán-Mir H, Illán-Gómez MJ, Cordoncillo-Cordoncillo E. Non-Stoichiometric BaxMn0.7Cu0.3O3 Perovskites as Catalysts for CO Oxidation: Optimizing the Ba Content. Nanomaterials. 2025; 15(2):103. https://doi.org/10.3390/nano15020103
Chicago/Turabian StyleDíaz-Verde, Álvaro, Emerson Luiz dos Santos Veiga, Héctor Beltrán-Mir, María José Illán-Gómez, and Eloísa Cordoncillo-Cordoncillo. 2025. "Non-Stoichiometric BaxMn0.7Cu0.3O3 Perovskites as Catalysts for CO Oxidation: Optimizing the Ba Content" Nanomaterials 15, no. 2: 103. https://doi.org/10.3390/nano15020103
APA StyleDíaz-Verde, Á., dos Santos Veiga, E. L., Beltrán-Mir, H., Illán-Gómez, M. J., & Cordoncillo-Cordoncillo, E. (2025). Non-Stoichiometric BaxMn0.7Cu0.3O3 Perovskites as Catalysts for CO Oxidation: Optimizing the Ba Content. Nanomaterials, 15(2), 103. https://doi.org/10.3390/nano15020103







