Magnetic Hyperthermia with Iron Oxide Nanoparticles: From Toxicity Challenges to Cancer Applications
Abstract
1. Introduction
2. Strategies to Enhance Magnetic Heating Efficiency of Magnetic Nanoparticles
2.1. Formulation
2.2. Shape
2.3. Doping with Metallic Ions
2.4. Controlled Nanoscale Assembly of MNPs
2.5. Surface Functionalization
2.6. AFM Characteristics
2.7. Magnetic Nanoparticles for Combined Therapy and Imaging
3. Toxicity Issue, Biocompatibility, and Strategies to Improve Biocompatibility of Magnetic Nanomaterials
3.1. Toxicity Issues
3.2. Organic Coating
3.2.1. Synthetic Polymers
3.2.2. Natural Polymers
4. Combination of Magnetic Hyperthermia with Other Therapeutic Modalities
4.1. Combination with Other Therapeutic Methods
4.1.1. Chemotherapy
4.1.2. Radiotherapy
4.1.3. Immunotherapy
4.1.4. Role of Natural Compounds and Polymer-Based Carriers in MH
4.2. Experimental Studies of Biocompatibility and Oncologic Efficiency of IONPs In Vitro
4.2.1. In Vitro Cancer Models Used for Testing of IONPs
4.2.2. MNP Formulation
4.2.3. Efficiency, Side Effects
4.2.4. Type of Cell Death
- Apoptosis
- Necrosis
- Ferroptosis
4.3. Biocompatibility and Oncologic Efficiency of IONPs In Vivo
4.3.1. Biodistribution
4.3.2. Coating
4.3.3. Shape
4.3.4. Electrical Charge
4.3.5. IONPs Internalization
4.3.6. Immune Response Following IONPs Administration
4.3.7. Routes of Administration and Toxicity
4.3.8. Elimination
4.3.9. Combined Radiotherapy and MH
5. Clinical Translation and Studies
| Nanoparticles | Model | Main Results |
|---|---|---|
| IONPs with PEG coating/IONPs with PEI coating | SKOV-3 human ovarian cancer/RAW 264.7 murine macrophages | Cytotoxic effects by ROS production and apoptosis induction [185] |
| SPIONs loaded with curcumin, coated with poly (lactic-co-glycolic acid)-poly (ethylene glycol) di-block copolymer (PLGA-b-PEG) conjugated with glycine-arginine-glycine-aspartic acid-serine (GRGDS) | T98G-glioblastoma multiforme, fibroblast-like cell line | Induced cytotoxic effects increased by exposure to radiofrequency hyperthermia application [231] |
| IONPs | A549 human lung cancer cell line Staphylococcus aureus, Proteus vulgaris, Pseudomonas aeruginosa | Cytotoxic effect Antibacterial effect through ROS generation [232] |
| SPIONS functionalized with SDS and loaded with curcumin and coated with chitosan SPIONs-SDS-CU-CHIT | HeLAa human cervical cancer | Decreased viability in a dose and time related manner related to drug release in the medium [234] |
| Green iron nanoparticles (Rosemary-FeNPs) | 4T1 murine breast cancer C26 cancer cell lines | Cytotoxic effect against cancer cells, efficient intracellular delivery of the rosemary flavonoid components [236] |
| Bare superparamagnetic iron oxide nanoparticles (SPIONs) | Porcine aortic endothelial cells (PAEC) | ROS formation leads to morphological changes and forms actin stress fibers; blocking ROS formation by functionalization could increase medical applications [239] |
| IONPs coated with chitosan IONPs coated with polyvinyl alcohol (PVA) | Human fibroblasts | IONPs coated with chitosan induced mild toxicity, IONPs coated with PVA were well tolerated [240] |
| ferumoxytol carboxymethyldextran coating | mammary adeno carcinoma cells incubated with macrophages | Macrophages showed pro-inflammatory M1 phenotype upon ferumoxytol exposure Increased caspase -3 in mammary tumor cells [342] |
| IONPs loaded with LLY-507 (inhibitor of SMYD2), coated with PVA | A549 human non-small cell lung cancer cell line RBC- human | Efficient delivery of the SMYD2 inhibitor by the IONPs, dose dependent decrease in viability, hemolysis below 5% [343] |
| poly(ethylene glycol)-block-poly(lactic-co-glycolic acid) copolymer-encapsulated Fe3O4 superparticles (SPs), loaded with imiquimod (R837 a Toll-like receptor 7 agonist) | 4T1 triple-negative human breast cancer cells | Efficient photothermal ablation of 4T1 cells by apoptosis/necrosis upon PTT irradiation, efficient delivery of R837 in vivo against primary tumors to enhance immune response [344] |
| Fe3O4@PDA SPs | HeLa human cervical cancer cell line, mice bearing tumor (HeLa) | Biocompatible, increased efficacy of photothermal therapy against tumors in vivo [345] |
| IONPs—loaded with curcumin and coated with dextran CUR/DEX/Fe3O4-NPs | MCF-7 human breast cancer | Decreased cell viability in a dose and time related manner [346] |
| SPIONS functionalized with SDS and loaded with curcumin and coated with chitosan SPIONs-SDS-CU-CHIT | HeLAa human cervical cancer | Decreased viability in a dose and time related manner related to drug release in the medium [347] |
| PACLITAXEL | ||
|---|---|---|
| Nanoparticles | Model | Main Results |
| Multifunctional mesoporous silica nanoparticles (SPIONs) Surface modifications: Fluorescent dye molecules/Hydrophilic groups/Cancer-specific targeting ligands—folate (FA); Drugs: Camptothecin (CPT)/Paclitaxel (PTX) | Human pancreatic cancer cell lines: PANC-1, BxPC3, human foreskin fibroblasts (HFF) as control | Selective cytotoxicity; dual imaging capability; targeted drug delivery through ligands (FA) [229] |
| SPIONs coated with lauric acid and human serum albumin as carriers for paclitaxel (SPION-LA-HSA-Ptx) | Human breast cancer cell lines (T-47D, BT-474, MCF-7, and MDA-MB-231 cells) | High potential for magnetically targeted drug delivery in breast cancer Similar effects on human breast cancer as PTX alone [234] |
| SPION@Cs-PTX-PEG-FA SPIONs with paclitaxel (PTX)-loaded chitosan (Cs), polyethylene glycol (PEG), and receptors that target folate (FA) | WEHI-164: Mouse fibrosarcoma; MEF: Mouse embryonic fibroblast (normal) cell line | High nanoparticle stability, selective uptake, reduced systemic toxicity due to the FA receptors, apoptosis of cancer cells [238] |
| Fe3O4@LaF3:Ce3+,Tb3+/chi NPs bonded with Paclitaxel (PTX) | A549 human lung cancer cell line | Increased cell toxicity compared to free paclitaxel; efficient imaging (MRI and fluorescence imaging); reduced side effects [239] |
| MNPs coated with an amphiphilic polymer containing disulfide linkages (Hyaluronic Acid–disulfide bond–Polylactic Acid, HA-SS-PLA), loaded with PTX | HeLa cells human cervical cancer cell line) | Targeted delivery, through magnetism and redox response; improved cytotoxicity, and biocompatibility [348] |
| DOXORUBICIN | ||
| A54 peptide-functionalized poly(lactic-co-glycolic acid)-grafted dextran (A54-Dex-PLGA) micelles with DOX/SPIO | BEL-7402, HepG2 hepatic cancer | MNPs easy synthesis of SPIONs, low off-target distribution and toxicity; controlled drug release; dual imaging/therapy function [236] |
| Electro-spun fibers co-loaded with magnetic IONPs, cubic shaped loaded with doxorubicin | Mouse embryonic fibroblast cell line (NIH 3 T3 cells), DOX-sensitive HeLa-WT cervical cancer cells and the DOX-resistant MCF7 breast cancer cells | Hyperthermia combined with enhanced diffusion of DOX—effective oncotherapy [239] |
| Doxorubicin-loaded IONPs with surface coatings like trimethoxysilylpropyl-ethylenediamine triacetic acid (EDT) | MDCK-MDR1-GBM co-culture model | High DOX penetration through BBB; effective magnetic targeting and reduced systemic toxicity; possibly overcoming MDR cancer cells [241] |
| Fe3O4@MnO2@PPy nanocomposite loaded with DOX; Fe3O4 (Iron oxide) core; MnO2 (Manganese dioxide) shell; PPy (Polypyrrole) outer layer | Human hepatoma (HepG2) | Good magnetic targeting delivery and enhanced cancer toxicity improved PDT/photothermal therapy (PTT) reduced side effects and better tolerance to hypoxia induced by PDT/PTT [247] |
| IONP DOX: PEG-coated, doxorubicin-loaded nanoparticles | HeLa cells (human cervical cancer cell line) | Delivery of DOX directly into the cytoplasm trough macro pinocytosis and endocytosis; high biocompatibility [252] |
| PEG-coated Fe3O4 luteinizing hormone-releasing hormone (LHRH) ligand containing doxorubicin | A549 and MCF-7 cancer cells | Theranostic NP formulation using LHRH ligand with individual chemotherapy and thermotherapy, effective on both cell lines [349] |
| OTHERS | ||
| Magnetic IONPs/temozolomide | SD3, G-16, G-302, GL-261 cell lines | Combined hyperthermia using IONPs with temozolomide and radiation showed synergistic anti-glioblastoma effects [208] |
| SPIONs- PLGA core/poly(N-isopropylacrylamide)-carboxymethyl chitosan shell with NU7441/Gemcitabine | A549 and H460 lung cancer cells | Approach for simultaneous radiotherapy and chemotherapy, Folate receptor targeting increased specific uptake [248] |
| SPIONs (PVA/LDH-coated and PEG/LDH-coated) with Sorafenib | HepG2 human hepatoma/3T3 mouse fibroblast cell line | Strong SP behavior; enhanced anticancer activity and selectivity; minimal side effects [249] |
| Magnetic-core silica nanoparticles with nano valves and loaded with cucurbituril | MDA-MB-231 breast cancer cells | Targeted delivery using a nano valve system and hyperthermia [350] |
| Fe-NP2 coated with PEI conjugated with cisplatin (IV) prodrug | Human ovarian carcinoma A2780 cells/cisplatin-resistant A2780DDP cells | Efficient drug delivery overcoming cisplatin resistance through unique internalization pathway of NPs/increased production of ROS [351] |
| Phospholipid-modified Pt(IV) prodrug-loaded IONP-filled micelles | B16-F10 melanoma cells | Redox-triggered release of cisplatin, ferroptosis of melanoma cells, lower concentration threshold, lymphatic delivery [352] |
| Nanoflowers MoS2@Fe3O4- loaded with ICG/Pt(IV) indocyanine green (ICG) and platinum (IV) prodrugs {c,c,t-Pt(NH3)2Cl2(OOCCH2CH2COOH)2} | I.929 fibroblasts, HeLa, H22 tumor-bearing Balb/c mice | Biocompatible, theranostics bioimaging capabilities and laser-induced cytotoxicity [353] |
| Fe(Salen) nanoparticles with μ-oxo N,N′-bis (salicylidene) ethylene diamine | tongue cancer VX2 (rabbit), HSC-3 (human), and OSC-19 (human) | Hyperthermia-guided, temperature stable cytotoxic effects, even at low concentrations [354] |
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef]
- Chabner, B.A.; Roberts, T.G., Jr. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Winterford, C.M.; Harmon, B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994, 73, 2013–2026. [Google Scholar] [CrossRef] [PubMed]
- Moyer, H.R.; Delman, K.A. The role of hyperthermia in optimizing tumor response to regional therapy. Int. J. Hyperth. 2008, 24, 251–261. [Google Scholar] [CrossRef]
- Baronzio, G.F.; Hager, E.D. Hyperthermia in Cancer Treatment: A Primer; Springer: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Cavaliere, R.; Ciocatto, E.C.; Giovanella, B.C.; Heidelberger, C.; Johnson, R.O.; Margottini, M.; Mondovi, B.; Moricca, G.; Rossi-Fanelli, A. Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer 1967, 20, 1351–1381. [Google Scholar] [CrossRef]
- Falk, M.H.; Issels, R.D. Hyperthermia in oncology. Int. J. Hyperth. 2001, 17, 1–18. [Google Scholar] [CrossRef]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Chichel, A.; Skowronek, J.; Kubaszewska, M.; Kanikowski, M. Hyperthermia—Description of a method and a review of clinical applications. Rep. Pract. Oncol. Radiother. 2007, 12, 267–275. [Google Scholar] [CrossRef]
- Kampinga, H.H. Cell biological effects of hyperthermia alone or combined with radiation or drugs: A short introduction to newcomers in the field. Int. J. Hyperth. 2006, 22, 191–196. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, F.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field-induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.K.; Medal, R.; Shorey, W.D.; Hanselman, R.C.; Parrott, J.C.; Taylor, C.B. Selective inductive heating of lymph nodes. Ann. Surg. 1957, 146, 596–606. [Google Scholar] [CrossRef]
- Huang, Y.; Hsu, J.C.; Koo, H.; Cormode, D.P. Repurposing ferumoxytol: Diagnostic and therapeutic applications of an FDA-approved nanoparticle. Theranostics 2022, 12, 796–816. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Pineiro, Y.; Vargas, Z.; Rivas, J.; López-Quintela, M.A. Iron oxide based nanoparticles for magnetic hyperthermia strategies in biological applications. Eur. J. Inorg. Chem. 2015, 2015, 4495–4509. [Google Scholar] [CrossRef]
- Gneveckow, U.; Jordan, A.; Scholz, R.; Brüss, V.; Waldöfner, N.; Ricke, J.; Feussner, A.; Hildebrandt, B.; Rau, B.; Wust, P. Description and characterization of the novel hyperthermia- and thermoablation-system MFH 300F for clinical magnetic fluid hyperthermia. Med. Phys. 2004, 31, 1444–1451. [Google Scholar] [CrossRef]
- van Landeghem, F.K.; Maier-Hauff, K.; Jordan, A.; Hoffmann, K.T.; Gneveckow, U.; Scholz, R.; Thiesen, B.; Brück, W.; von Deimling, A. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials 2009, 30, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Egea-Benavente, D.; Ovejero, J.G.; Morales, M.D.P.; Barber, D.F. Understanding MNPs behaviour in response to AMF in biological milieus and the effects at the cellular level: Implications for a rational design that drives magnetic hyperthermia therapy toward clinical implementation. Cancers 2021, 13, 4583. [Google Scholar] [CrossRef] [PubMed]
- Kwok, M.K.Y.; Maley, C.C.J.; Dworkin, A.; Hattersley, S.; Southern, P.; Pankhurst, Q.A. Nonspecific eddy current heating in magnetic field hyperthermia. Appl. Phys. Lett. 2023, 122, 240502. [Google Scholar] [CrossRef]
- Pilpilidis, K.; Tsanidis, G.; Rouni, M.A.; Markakis, J.; Samaras, T. Revisiting the safety limit in magnetic nanoparticle hyperthermia: Insights from eddy current induced heating. Phys. Med. Biol. 2025, 70, 035001. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia-biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Xu, C.; Sun, S. New forms of superparamagnetic nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2013, 65, 732–743. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Wang, Y.; Zhu, W.; Li, G.; Ma, X.; Zhang, Y.; Chen, S.; Tiwari, S.; Shi, K.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Etemadi, H.; Plieger, P.G. Magnetic fluid hyperthermia based on magnetic nanoparticles: Physical characteristics, historical perspective, clinical trials, technological challenges, and recent advances. Adv. Therap. 2020, 3, 2000061. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Wong, L.H.; Schumann, C.; Sabei, F.Y.; Korzun, T.; Li, X.; Hansen, M.N.; Dhagat, P.; Moses, A.S.; Taratula, O.; et al. Biocompatible nanoclusters with high heating efficiency for systemically delivered magnetic hyperthermia. ACS Nano 2019, 13, 6383–6395. [Google Scholar] [CrossRef] [PubMed]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Tromsdorf, U.I.; Bruns, O.T.; Salmen, S.C.; Beisiegel, U.; Weller, H. A highly effective, nontoxic T1 MR contrast agent based on ultrasmall PEGylated iron oxide nanoparticles. Nano Lett. 2009, 9, 4434–4440. [Google Scholar] [CrossRef]
- Rui, Y.-P.; Liang, B.; Hu, F.; Xu, J.; Peng, Y.-F.; Yin, P.-H.; Duan, Y.; Zhang, C.; Gu, H. Ultra-large-scale production of ultrasmall superparamagnetic iron oxide nanoparticles for T1-weighted MRI. RSC Adv. 2016, 6, 22575–22585. [Google Scholar] [CrossRef]
- Figuerola, A.; Di Corato, R.; Manna, L.; Pellegrino, T. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol. Res. 2010, 62, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.P.; Gazeau, F.; Wilhelm, C. Intracellular heating of living cells through Néel relaxation of magnetic nanoparticles. Eur. Biophys. J. 2008, 37, 223–228. [Google Scholar] [CrossRef]
- Jeun, M.; Lee, S.; Kang, J.K.; Tomitaka, A.; Kang, K.W.; Kim, Y.I.; Takemura, Y.; Chung, K.-W.; Kwak, J.; Bae, S. Physical limits of pure superparamagnetic Fe3O4 nanoparticles for a local hyperthermia agent in nanomedicine. Appl. Phys. Lett. 2012, 100, 092406. [Google Scholar] [CrossRef]
- Gonzales-Weimuller, M.; Zeisberger, M.; Krishnan, K.M. Size-dependent heating rates of iron oxide nanoparticles for magnetic fluid hyperthermia. J. Magn. Magn. Mater. 2009, 321, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Dutz, S.; Neeb, A.; Cato, A.C.B.; Zeisberger, M. Magnetic heating effect of nanoparticles with different sizes and size distributions. J. Magn. Magn. Mater. 2013, 328, 80–85. [Google Scholar] [CrossRef]
- Lévy, M.; Wilhelm, C.; Siaugue, J.-M.; Horner, O.; Bacri, J.-C.; Gazeau, F. Magnetically induced hyperthermia: Size-dependent heating power of γ-Fe2O3 nanoparticles. J. Phys. Condens. Matter 2008, 20, 204133. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Wilhelm, C.; Servais, J.; Ménager, C.; Bacri, J.-C.; Gazeau, F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef]
- Gazeau, F.; Lévy, M.; Wilhelm, C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomedicine 2008, 3, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 109, 083921. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Martinez, L.M.; Khurshid, H.; Garaio, E.; Garcia, J.A.; Phan, M.H.; Srikanth, H. Improving the heating efficiency of iron oxide nanoparticles by tuning their shape and size. J. Phys. Chem. C 2018, 122, 2367–2381. [Google Scholar] [CrossRef]
- Chen, R.; Christiansen, M.G.; Anikeeva, P. Maximizing hysteretic losses in magnetic ferrite nanoparticles via model-driven synthesis and materials optimization. ACS Nano 2013, 7, 8990–9000. [Google Scholar] [CrossRef]
- Mohapatra, J.; Zeng, F.; Elkins, K.; Xing, M.; Ghimire, M.; Yoon, S.; Mishra, S.R.; Liu, J.P. Size-dependent magnetic and inductive heating properties of Fe3O4 nanoparticles: Scaling laws across the superparamagnetic size. Phys. Chem. Chem. Phys. 2018, 20, 12879–12887. [Google Scholar] [CrossRef]
- Tong, S.; Xiang, J.; Zheng, C.; Gao, J.; Bao, G. Size-dependent heating of magnetic iron oxide nanoparticles. ACS Nano 2017, 11, 6808–6816. [Google Scholar] [CrossRef]
- Serantes, D.; Baldomir, D.; Martinez-Boubeta, C.; Simeonidis, K.; Angelakeris, M.; Natividad, E.; Castro, M.; Mediano, A.; Chen, D.-X.; Sanchez, A.; et al. Influence of dipolar interactions on hyperthermia properties of ferromagnetic particles. J. Appl. Phys. 2010, 108, 073918. [Google Scholar] [CrossRef]
- Salas, G.; Camarero, J.; Cabrera, D.; Takacs, H.; Varela, M.; Ludwig, R.; Dähring, H.; Hilger, I.; Miranda, R.; Morales, M.P.; et al. Modulation of magnetic heating via dipolar magnetic interactions in monodisperse and crystalline iron oxide nanoparticles. J. Phys. Chem. C 2014, 118, 19985–19994. [Google Scholar] [CrossRef]
- Coral, D.F.; Mendoza Zélis, P.; Marciello, M.; Morales, M.P.; Craievich, A.; Sánchez, F.H.; Fernández van Raap, M.B. Effect of nanoclustering and dipolar interactions in heat generation for magnetic hyperthermia. Langmuir 2016, 32, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Schaller, V.; Wahnstrom, G.; Sanz-Velasco, A.; Gustafsson, S.; Olsson, E.; Enoksson, P.; Johansson, C. Effective magnetic moment of magnetic multicore nanoparticles. Phys. Rev. B 2009, 80, 092406. [Google Scholar] [CrossRef]
- Tadica, M.; Kralj, S.; Jagodic, M.; Hanzel, D.; Makovec, D. Magnetic properties of novel superparamagnetic iron oxide nanoclusters and their peculiarity under annealing treatment. Appl. Surf. Sci. 2014, 322, 255–264. [Google Scholar] [CrossRef]
- Ganesan, V.; Lahiri, B.B.; Louis, C.; Philip, J.; Damodaran, S.P. Size-controlled synthesis of superparamagnetic magnetite nanoclusters for heat generation in an alternating magnetic field. J. Mol. Liq. 2019, 281, 315–323. [Google Scholar] [CrossRef]
- Stolarczyk, J.K.; Deak, A.; Brougham, D.F. Nanoparticle clusters: Assembly and control over internal order, current capabilities, and future potential. Adv. Mater. 2016, 28, 5400–5424. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Zhang, L.; Colvin, V.L.; Zhang, Q.; Bao, G. Synthesis and application of magnetic nanocrystal clusters. Ind. Eng. Chem. Res. 2022, 61, 7613–7625. [Google Scholar] [CrossRef]
- Antone, A.J.; Sun, Z.; Bao, Y. Preparation and application of iron oxide nanoclusters. Magnetochemistry 2019, 5, 45. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Iacovita, C.; Florea, A.; Dudric, R.; Pall, E.; Moldovan, A.; Tetean, R.; Stiufiuc, R.; Lucaciu, C. Small versus large iron oxide magnetic nanoparticles: Hyperthermia and cell uptake properties. Molecules 2016, 21, 1357. [Google Scholar] [CrossRef]
- Sakellari, D.; Brintakis, K.; Kostopoulou, A.; Myrovali, E.; Simeonidis, K.; Lappas, A.; Angelakeris, M. Ferrimagnetic nanocrystal assemblies as versatile magnetic particle hyperthermia mediators. Mater. Sci. Eng. C 2016, 58, 187–193. [Google Scholar] [CrossRef]
- Hemery, G.; Keyes, A.C.; Garaio, E.; Rodrigo, I.; Garcia, J.A.; Plazaola, F.; Garanger, E.; Sandre, O. Tuning sizes, morphologies, and magnetic properties of monocore versus multicore iron oxide nanoparticles through the controlled addition of water in the polyol synthesis. Inorg. Chem. 2017, 56, 8232–8243. [Google Scholar] [CrossRef]
- Iacovita, C.; Dudric, R.; Vomir, M.; Ersen, O.; Donnio, B.; Gallani, J.L.; Rastei, M.V. Imaging large iron-oxide nanoparticle clusters by field-dependent magnetic force microscopy. J. Phys. Chem. C 2021, 125, 24001–24010. [Google Scholar] [CrossRef]
- Bertuit, E.; Neveu, S.; Abou-Hassan, A. High temperature continuous flow syntheses of iron oxide nanoflowers using the polyol route in a multi-parametric millifluidic device. Nanomaterials 2022, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Hugounenq, P.; Levy, M.; Alloyeau, D.; Lartigue, L.; Dubois, E.; Cabuil, V.; Ricolleau, C.; Roux, S.; Wilhelm, C.; Gazeau, F.; et al. Iron oxide monocrystalline nanoflowers for highly efficient magnetic hyperthermia. J. Phys. Chem. C 2012, 116, 15702–15712. [Google Scholar] [CrossRef]
- Gavilán, H.; Sánchez, E.H.; Brollo, M.E.F.; Asín, L.; Moerner, K.K.; Frandsen, C.; Lázaro, F.J.; Serna, C.J.; Veintemillas-Verdaguer, S.; Morales, M.P.; et al. Formation mechanism of maghemite nanoflowers synthesized by a polyol-mediated process. ACS Omega 2017, 2, 7172–7184. [Google Scholar] [CrossRef] [PubMed]
- Storozhuk, L.; Besenhard, M.O.; Mourdikoudis, S.; LaGrow, A.P.; Lees, M.R.; Tung, L.D.; Gavriilidis, A.; Thanh, N.T.K. Stable Iron Oxide Nanoflowers with Exceptional Magnetic Heating Efficiency: Simple and Fast Polyol Synthesis. ACS Appl. Mater. Interfaces 2021, 13, 45870–45880. [Google Scholar] [CrossRef]
- Lartigue, L.; Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Lévy, M.; Bacri, J.C.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F. Cooperative Organization in Iron Oxide Multi-Core Nanoparticles Potentiates Their Efficiency as Heating Mediators and MRI Contrast Agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Lee, S.; Song, D.Y.; Kang, S.; Shin, T.H.; Choi, J.S. Hyperthermia Effect of Nanoclusters Governed by Interparticle Crystalline Structures. ACS Omega 2021, 6, 31161–31167. [Google Scholar] [CrossRef]
- Amorim, C.O. A compendium of magnetic nanoparticle essentials: A comprehensive guide for beginners and experts. Pharmaceutics 2025, 17, 137. [Google Scholar] [CrossRef]
- Noh, S.-H.; Na, W.; Jang, J.-T.; Lee, J.-H.; Lee, E.J.; Moon, S.H.; Lim, Y.; Shin, J.-S.; Cheon, J. Nanoscale magnetism control via surface and exchange anisotropy for optimized ferrimagnetic hysteresis. Nano Lett. 2012, 12, 3716–3721. [Google Scholar] [CrossRef]
- Chang, F.; Davies, G.-L. From 0D to 2D: Synthesis and bio-application of anisotropic magnetic iron oxide nanomaterials. Prog. Mater. Sci. 2024, 144, 101267. [Google Scholar] [CrossRef]
- Elsayed, W.E.M.; Al-Hazmi, F.S.; Memesh, L.S.; Bronstein, L.M. A Novel Approach for Rapid Green Synthesis of Nearly Mono-Disperse Iron Oxide Magnetic Nanocubes with Remarkable Surface Magnetic Anisotropy Density for Enhancing Hyperthermia Performance. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 239–245. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Riedinger, A.; Nitti, S.; Pugliese, G.; Marras, S.; Genovese, A.; Materia, M.E.; Lefevre, C.; Manna, L.; Pellegrino, T. One Pot Synthesis of Monodispersed Water Soluble Iron Oxide Nanocrystals with High Values of the Specific Absorption Rate. J. Mater. Chem. B 2014, 2, 4426–4434. [Google Scholar] [CrossRef] [PubMed]
- Iacovita, C.; Stiufiuc, R.; Radu, T.; Florea, A.; Stiufiuc, G.; Dutu, A.; Mican, S.; Tetean, R.; Lucaciu, C.M. Polyethylene glycol-mediated synthesis of cubic iron oxide nanoparticles with high heating power. Nanoscale Res. Lett. 2015, 10, 391. [Google Scholar] [CrossRef]
- Freis, B.; Ramirez, M.D.L.A.; Kiefer, C.; Harlepp, S.; Iacovita, C.; Henoumont, C.; Affolter-Zbaraszczuk, C.; Meyer, F.; Mertz, D.; Boos, A.; et al. Effect of the size and shape of dendronized iron oxide nanoparticles bearing a targeting ligand on MRI, magnetic hyperthermia, and photothermia properties—From suspension to in vitro studies. Pharmaceutics 2023, 15, 1104. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso, J.; Martinez, L.M.; Khurshid, H.; Garaio, E.; Garcia, J.A.; Phan, M.H.; Srikanth, H. Enhanced magnetic hyperthermia in iron oxide nano-octopods: Size and anisotropy effects. J. Phys. Chem. C 2016, 120, 8370–8379. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, Y.; Fang, J.; Zhang, H.; Peng, E.; Liu, X.; Xiao, W.; Ding, J. Size dependent magnetic hyperthermia of octahedral Fe3O4 nanoparticles. RSC Adv. 2015, 5, 76764–76771. [Google Scholar] [CrossRef]
- Singh, P.; Duraisamy, K.; Raitmayr, C.; Sharma, K.S.; Korzun, T.; Singh, K.; Moses, A.S.; Yamada, K.; Grigoriev, V.; Demessie, A.A.; et al. Precision-Engineered Cobalt-Doped Iron Oxide Nanoparticles: From Octahedron Seeds to Cubical Bipyramids for Enhanced Magnetic Hyperthermia. Adv. Funct. Mater. 2025, 35, 2414719. [Google Scholar] [CrossRef]
- Das, R.; Alonso, J.; Porshokouh, Z.N.; Kalappatti, V.; Torres, D.; Phan, M.-H.; Garaio, E.; Garcia, J.A.; Llamazares Sanchez, J.L.; Srikanth, H. Tunable high aspect ratio iron oxide nanorods for enhanced hyperthermia. J. Phys. Chem. C 2016, 120, 10086–10093. [Google Scholar] [CrossRef]
- Geng, S.; Yang, H.; Ren, X.; Liu, Y.; He, S.; Zhou, J.; Su, N.; Li, Y.; Xu, C.; Zhang, X.; et al. Anisotropic Magnetite Nanorods for Enhanced Magnetic Hyperthermia. Chem. Asian J. 2016, 11, 2996–3000. [Google Scholar] [CrossRef]
- Sugumaran, P.J.; Yang, Y.; Wang, Y.; Liu, X.; Ding, J. Influence of the aspect ratio of iron oxide nanorods on hysteresis-loss-mediated magnetic hyperthermia. ACS Appl. Bio Mater. 2021, 4, 4809–4820. [Google Scholar] [CrossRef]
- Nemati, Z.; Salili, S.M.; Alonso, J.; Ataie, A.; Das, A.; Phan, M.H.; Srikanth, H. Superparamagnetic iron oxide nanodiscs for hyperthermia therapy. Does size matter? J. Alloys Compd. 2017, 714, 709–714. [Google Scholar] [CrossRef]
- Dias, C.S.B.; Hanchuk, T.D.M.; Wender, H.; Shigeyosi, W.T.; Kobarg, J.; Rossi, A.L.; Tanaka, M.N.; Cardoso, M.B.; Garcia, F. Shape tailored magnetic nanorings for intracellular hyperthermia cancer therapy. Sci. Rep. 2017, 7, 14633. [Google Scholar] [CrossRef] [PubMed]
- Iacovita, C.; Fizeșan, I.; Pop, A.; Scorus, L.; Dudric, R.; Stiufiuc, G.; Vedeanu, N.; Tetean, R.; Loghin, F.; Stiufiuc, R.; et al. In vitro intracellular hyperthermia of iron oxide magnetic nanoparticles, synthesized at high temperature by a polyol process. Pharmaceutics 2020, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Cotin, G.; Perton, F.; Blanco-Andujar, C.; Pichon, B.; Mertz, D.; Begin-Colin, S. Design of anisotropic iron-oxide-based nanoparticles for magnetic hyperthermia. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Fratila, R.M., de la Fuente, J.M., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 41–60. [Google Scholar]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.-W.; Seo, J.-W.; Jang, J.-T.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S.; et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef]
- Jang, J.-T.; Nah, H.; Lee, J.-H.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical enhancements of MRI contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 1234–1238. [Google Scholar] [CrossRef]
- Vamvakidis, K.; Sakellari, D.; Angelakeris, M.; Dendrinou-Samara, C. Size and compositionally controlled manganese ferrite nanoparticles with enhanced magnetization. J. Nanopart. Res. 2015, 15, 1743. [Google Scholar] [CrossRef]
- Sabale, S.; Jadhav, V.; Khot, V.; Zhu, X.; Xin, M.; Chen, H. Superparamagnetic MFe2O4 (M = Ni, Co, Zn, Mn) nanoparticles: Synthesis, characterization, induction heating and cell viability studies for cancer hyperthermia applications. J. Mater. Sci. Mater. Med. 2015, 26, 127. [Google Scholar] [CrossRef] [PubMed]
- Casula, M.F.; Conca, E.; Bakaimi, I.; Sathya, A.; Materia, M.E.; Casu, A.; Falqui, A.; Sogne, E.; Pellegrino, T.; Kanaras, A.G. Manganese doped-iron oxide nanoparticle clusters and their potential as agents for magnetic resonance imaging and hyperthermia. Phys. Chem. Chem. Phys. 2016, 18, 16848–16855. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Yang, Y.; Xiao, W.; Li, Z.; Xue, D.; Li, F.; Ding, J. Synthesis of nonstoichiometric zinc ferrite nanoparticles with extraordinary room temperature magnetism and their diverse applications. J. Mater. Chem. C 2013, 1, 2875–2885. [Google Scholar] [CrossRef]
- Srivastava, M.; Alla, S.K.; Meena, S.S.; Gupta, N.; Mandala, R.K.; Prasad, N.K. ZnxFe3−xO4 (0.01 ≤ x ≤ 0.8) nanoparticles for controlled magnetic hyperthermia application. New J. Chem. 2018, 42, 7144–7153. [Google Scholar] [CrossRef]
- He, S.; Zhang, H.; Liu, Y.; Su, F.; Yu, X.; Li, X.; Zhang, L.; Wang, L.; Mao, K.; Wang, G.; et al. Maximizing specific loss power for magnetic hyperthermia by hard-soft mixed ferrites. Small 2018, 14, 1800135. [Google Scholar] [CrossRef]
- Jang, J.-t.; Lee, J.; Seon, J.; Ju, E.; Kim, M.; Kim, Y.I.; Kim, M.G.; Takemura, Y.; Arbab, A.S.; Kang, K.W.; et al. Giant magnetic heat induction of magnesium-doped γ-Fe2O3 superparamagnetic nanoparticles for completely killing tumors. Adv. Mater. 2018, 30, 1704362. [Google Scholar] [CrossRef] [PubMed]
- Saville, S.L.; Qi, B.; Baker, J.; Stone, R.; Camley, R.E.; Livesey, K.L.; Ye, L.; Crawford, T.M.; Mefford, O.T. The Formation of Linear Aggregates in Magnetic Hyperthermia: Implications on Specific Absorption Rate and Magnetic Anisotropy. J. Colloid. Interface Sci. 2014, 424, 141–151. [Google Scholar] [CrossRef]
- Morales, I.; Costo, R.; Mille, N.; Carrey, J.; Hernando, A.; de la Presa, P. Time-Dependent AC Magnetometry and Chain Formation in Magnetite: The Influence of Particle Size, Initial Temperature and the Shortening of the Relaxation Time by the Applied Field. Nanoscale Adv. 2021, 3, 5801–5812. [Google Scholar] [CrossRef]
- Mille, N.; De Massi, D.; Faure, S.; Asensio, J.M.; Chaudret, B.; Carrey, J. Probing dynamics of nanoparticle chains formation during magnetic hyperthermia using time-dependent high-frequency hysteresis loops. Appl. Phys. Lett. 2021, 119, 022407. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Silvestri, N.; Fernandez-Cabada, T.; Marinaro, F.; Fernandes, S.; Fiorito, S.; Miscuglio, M.; Serantes, D.; Ruta, S.; Livesey, K.; et al. Exploiting Unique Alignment of Cobalt Ferrite Nanoparticles, Mild Hyperthermia, and Controlled Intrinsic Cobalt Toxicity for Cancer Therapy. Adv. Mater. 2020, 32, e2003712. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Afonso, Y.; Ruta, S.; Páez-Rodríguez, A.; van Zanten, T.S.; Gleadhall, S.; Fratila, R.M.; Moros, M.; del Puerto Morales, M.; Satoh, A.; Chantrell, R.W.; et al. Reversible Alignment of Nanoparticles and Intracellular Vesicles during Magnetic Hyperthermia Experiments. Adv. Funct. Mater. 2024, 34, 2405334. [Google Scholar] [CrossRef]
- Hergt, R.; Hiergeist, R.; Zeisberger, M.; Schüler, D.; Heyen, U.; Hilger, I.; Kaiser, W.A. Magnetic Properties of Bacterial Magnetosomes as Potential Diagnostic and Therapeutic Tools. J. Magn. Magn. Mater. 2005, 293, 80–86. [Google Scholar] [CrossRef]
- Alphandery, E.; Faure, S.; Raison, L.; Duguet, E.; Howse, P.A.; Bazylinski, D.A. Heat Production by Bacterial Magnetosomes Exposed to an Oscillating Magnetic Field. J. Phys. Chem. C 2011, 115, 18–22. [Google Scholar] [CrossRef]
- Gandia, D.; Gandarias, L.; Rodrigo, I.; Robles-García, J.; Das, R.; Garaio, E.; García, J.Á.; Phan, M.-H.; Srikanth, H.; Orue, I.; et al. Unlocking the Potential of Magnetotactic Bacteria as Magnetic Hyperthermia Agents. Small 2019, 15, 1902626. [Google Scholar] [CrossRef]
- Zhao, Z.; Rinaldi, C. Magnetization dynamics and energy dissipation of interacting magnetic nanoparticles in alternating magnetic fields with and without a static bias field. J. Phys. Chem. C 2018, 122, 21018–21030. [Google Scholar] [CrossRef]
- Serantes, D.; Simeonidis, K.; Angelakeris, M.; Chubykalo-Fesenko, O.; Marciello, M.; del Puerto Morales, M.; Baldomir, D.; Martinez-Boubeta, C. Multiplying magnetic hyperthermia response by nanoparticle assembling. J. Phys. Chem. C 2014, 118, 5927–5934. [Google Scholar] [CrossRef]
- Myrovali, E.; Maniotis, N.; Makridis, A.; Terzopoulou, A.; Ntomprougkidis, V.; Simeonidis, K.; Sakellari, D.; Kalogirou, O.; Samaras, T.; Salikhov, R.; et al. Arrangement at the nanoscale: Effect on magnetic particle hyperthermia. Sci. Rep. 2016, 6, 37934. [Google Scholar] [CrossRef] [PubMed]
- Iacovita, C.; Florea, A.; Scorus, L.; Pall, E.; Dudric, R.; Moldovan, A.I.; Stiufiuc, R.; Tetean, R.; Lucaciu, C.M. Hyperthermia, cytotoxicity and cellular uptake properties of manganese and zinc ferrite magnetic nanoparticles synthesized by a polyol-mediated process. Nanomaterials 2019, 9, 1489. [Google Scholar] [CrossRef]
- Freis, B.; Kiefer, C.; Ramirez, M.d.l.A.; Harlepp, S.; Mertz, D.; Pichon, B.; Iacovita, C.; Laurent, S.; Begin, S. Defects or No Defects? Or How to Design 20–25 nm Spherical Iron Oxide Nanoparticles to Harness Both Magnetic Hyperthermia and Photothermia. Nanoscale 2024, 16, 20542. [Google Scholar] [CrossRef]
- Sanz, B.; Cabreira-Gomes, R.; Torres, T.E.; Valdés, D.P.; Lima, E., Jr.; De Biasi, E.; Zysler, R.D.; Ibarra, M.R.; Goya, G.F. Low-dimensional assemblies of magnetic MnFe2O4 nanoparticles and direct in vitro measurements of enhanced heating driven by dipolar interactions: Implications for magnetic hyperthermia. ACS Appl. Nano Mater. 2020, 3, 8719–8731. [Google Scholar] [CrossRef]
- Petru, A.-E.; Iacovita, C.; Fizeșan, I.; Dudric, R.; Crestin, I.-V.; Lucaciu, C.M.; Loghin, F.; Kiss, B. Evaluating manganese-doped magnetic nanoflowers for biocompatibility and in vitro magnetic hyperthermia efficacy. Pharmaceutics 2025, 17, 384. [Google Scholar] [CrossRef] [PubMed]
- Ranoo, S.; Lahiri, B.B.; Philip, J. Enhancement in field-induced heating efficiency of TMAOH coated superparamagnetic Fe3O4 nanoparticles by texturing under a static bias field. J. Magn. Magn. Mater. 2019, 498, 166138. [Google Scholar] [CrossRef]
- Lucaciu, C.M.; Nitica, S.; Fizesan, I.; Filip, L.; Bilteanu, L.; Iacovita, C. Enhanced magnetic hyperthermia performance of zinc ferrite nanoparticles under a parallel and a transverse bias DC magnetic field. Nanomaterials 2022, 12, 3578. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Fan, D.D.; Ding, J. Optimization of Surface Coating on Fe3O4 Nanoparticles for High Performance Magnetic Hyperthermia Agents. J. Mater. Chem. 2012, 22, 8235–8244. [Google Scholar] [CrossRef]
- Castellanos-Rubio, I.; Rodrigo, I.; Olazagoitia-Garmendia, A.; Arriortua, O.; Gil de Muro, I.; Garitaonandia, J.S.; Bilbao, J.R.; Fdez-Gubieda, M.L.; Plazaola, F.; Orue, I.; et al. Highly Reproducible Hyperthermia Response in Water, Agar, and Cellular Environment by Discretely PEGylated Magnetite Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 27917–27929. [Google Scholar] [CrossRef]
- Angotzi, M.S.; Mameli, V.; Khanal, S.; Veverka, M.; Vejpravova, J.; Cannas, C. Effect of Different Molecular Coatings on the Heating Properties of Maghemite Nanoparticles. Nanoscale Adv. 2022, 4, 408. [Google Scholar] [CrossRef] [PubMed]
- Mornet, S.; Vasseur, S.; Grasset, F.; Duguet, E. Magnetic Nanoparticle Design for Medical Diagnosis and Therapy. J. Mater. Chem. 2004, 14, 2161–2175. [Google Scholar] [CrossRef]
- Ovejero, J.G.; Morales, I.; De La Presa, P.; Mille, N.; Carrey, J.; Garcia, M.A.; Hernando, A.; Herrasti, P. Hybrid nanoparticles for magnetic and plasmonic hyperthermia. Phys. Chem. Chem. Phys. 2018, 20, 24065–24073. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Balaji, G.; Weber, A.; Uppu, R.M.; Kumar, C.S. Influence of Gold Nanoshell on Hyperthermia of Super Paramagnetic Iron Oxide Nanoparticles (SPIONs). J. Phys. Chem. C Nanomater. Interfaces 2010, 114, 19194–19201. [Google Scholar] [CrossRef]
- Guardia, P.; Nitti, S.; Materia, M.E.; Pugliese, G.; Yaacoub, N.; Greneche, J.-M.; Lefevre, C.; Manna, L.; Pellegrino, T. Gold-Iron Oxide Dimers for Magnetic Hyperthermia: The Key Role of Chloride Ions in the Synthesis to Boost the Heating Efficiency. J. Mater. Chem. B 2017, 5, 4587–4594. [Google Scholar] [CrossRef]
- Guerrero-Martínez, A.; Pérez-Juste, J.; Liz-Marzán, L.M. Recent progress on silica coating of nanoparticles and related nanomaterials. Adv. Mater. 2010, 22, 1182–1195. [Google Scholar] [CrossRef]
- Mamun, A.; Rumi, K.M.J.U.; Das, H.; Hoque, S.M. Synthesis, properties and applications of silica-coated magnetite nanoparticles: A review. Nano 2021, 16, 2130005. [Google Scholar] [CrossRef]
- Nitica, S.; Fizesan, I.; Dudric, R.; Barbu-Tudoran, L.; Pop, A.; Loghin, F.; Vedeanu, N.; Lucaciu, C.M.; Iacovita, C. A fast, reliable oil-in-water microemulsion procedure for silica coating of ferromagnetic Zn ferrite nanoparticles capable of inducing cancer cell death in vitro. Biomedicines 2022, 10, 1647. [Google Scholar] [CrossRef]
- Gao, Z.; Ring, H.L.; Sharma, A.; Namsrai, B.; Tran, N.; Finger, E.B.; Garwood, M.; Haynes, C.; Bischof, J.C. Preparation of scalable silica-coated iron oxide nanoparticles for nanowarming. Adv. Sci. 2020, 7, 1901624. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, J.; Liu, Y.; Zhong, R.; Yu, X.; Liu, Q.; Zhang, L.; Lv, C.; Mao, K.; Tang, P. The cell uptake properties and hyperthermia performance of Zn0.5Fe2.5O4/SiO2 nanoparticles as magnetic hyperthermia agents. R. Soc. Open Sci. 2020, 7, 191139. [Google Scholar] [CrossRef] [PubMed]
- Horny, M.-C.; Gamby, J.; Dupuis, V.; Siaugue, J.-M. Magnetic hyperthermia on γ-Fe2O3@SiO2 core-shell nanoparticles for miRNA 122 detection. Nanomaterials 2021, 11, 149. [Google Scholar] [CrossRef]
- Villanueva, A.; de la Presa, P.; Alonso, J.M.; Rueda, T.; Martínez, A.; Crespo, P.; Morales, M.D.P.; Gonzalez-Fernandez, M.A.; Valdés, J.; Rivero, G. Hyperthermia HeLa cell treatment with silica-coated manganese oxide nanoparticles. J. Phys. Chem. C 2010, 114, 1976–1981. [Google Scholar] [CrossRef]
- Iacovita, C.; Fizesan, I.; Nitica, S.; Florea, A.; Barbu-Tudoran, L.; Dudric, R.; Pop, A.; Vedeanu, N.; Crisan, O.; Tetean, R.; et al. Silica coating of ferromagnetic iron oxide magnetic nanoparticles significantly enhances their hyperthermia performances for efficiently inducing cancer cell death in vitro. Pharmaceutics 2021, 13, 2026. [Google Scholar] [CrossRef]
- Lei, S.; He, J.; Gao, P.; Wang, Y.; Hui, H.; An, Y.; Tian, J. Magnetic particle imaging-guided hyperthermia for precise treatment of cancer: Review, challenges, and prospects. Mol. Imaging Biol. 2023, 25, 1020–1033. [Google Scholar] [CrossRef]
- Oskoui, P.R.; Rezvani, M. Revolution in cancer treatment methods: Perspective review of factors affecting the final results of nanoparticles used in magnetic fluid hyperthermia. Health Sci. Rev. 2025, 14, 100212. [Google Scholar] [CrossRef]
- Herrero de la Parte, B.; Rodrigo, I.; Gutierrez-Basoa, J.; Iturrizaga Correcher, S.; Mar Medina, C.; Echevarria-Uraga, J.J.; Garcia, J.A.; Plazaola, F.; Garcia-Alonso, I. Proposal of new safety limits for in vivo experiments of magnetic hyperthermia antitumor therapy. Cancers 2022, 14, 3084. [Google Scholar] [CrossRef]
- Shah, R.R.; Davis, T.P.; Glover, A.L.; Nikles, D.E.; Brazel, C.S. Impact of Magnetic Field Parameters and Iron Oxide Nanoparticle Properties on Heat Generation for Use in Magnetic Hyperthermia. J. Magn. Magn. Mater. 2015, 387, 96–106. [Google Scholar] [CrossRef]
- Lahiri, B.B.; Muthukumaran, T.; Philip, J. Magnetic hyperthermia in phosphate coated iron oxide nanofluids. J. Magn. Magn. Mater. 2016, 407, 101–113. [Google Scholar] [CrossRef]
- Kerroum, M.A.A.; Iacovita, C.; Baaziz, W.; Ihiawakrim, D.; Rogez, G.; Benaissa, M.; Lucaciu, C.M.; Ersen, O. Quantitative analysis of the specific absorption rate dependence on the magnetic field strength in ZnxFe3−xO4 nanoparticles. Int. J. Mol. Sci. 2020, 21, 7775. [Google Scholar] [CrossRef]
- Castellanos-Rubio, I.; Arriortua, O.; Iglesias-Rojas, D.; Barón, A.; Rodrigo, I.; Marcano, L.; Garitaonandia, J.S.; Orue, I.; Fdez-Gubieda, M.L.; Insausti, M. A milestone in the chemical synthesis of Fe3O4 nanoparticles: Unreported bulklike properties lead to a remarkable magnetic hyperthermia. Chem. Mater. 2021, 33, 8693–8704. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Christiansen, M.G.; Sourakov, A.; Mohr, A.; Matsumoto, Y.; Okada, S.; Jasanoff, A.; Anikeeva, P. High-Performance Ferrite Nanoparticles through Nonaqueous Redox Phase Tuning. Nano Lett. 2016, 16, 1345–1351. [Google Scholar] [CrossRef]
- Dennis, C.L.; Krycka, K.L.; Borchers, J.A.; Desautels, R.D.; van Lierop, J.; Huls, N.F.; Jackson, A.J.; Gruettner, C.; Ivkov, R. Internal Magnetic Structure of Nanoparticles Dominates Time-Dependent Relaxation Processes in a Magnetic Field. Adv. Funct. Mater. 2015, 25, 4300–4311. [Google Scholar] [CrossRef]
- Ghazi, R.; Ibrahim, T.K.; Nasir, J.A.; Gai, S.; Ali, G.; Boukhris, I.; Rehman, Z. Iron oxide based magnetic nanoparticles for hyperthermia, MRI and drug delivery applications: A review. RSC Adv. 2025, 15, 11587–11616. [Google Scholar] [CrossRef] [PubMed]
- Kostevšek, N. A Review on the Optimal Design of Magnetic Nanoparticle-Based T2 MRI Contrast Agents. Magnetochemistry 2020, 6, 11. [Google Scholar] [CrossRef]
- Tegafaw, T.; Liu, S.; Ahmad, M.Y.; Saidi, A.K.A.A.; Zhao, D.; Liu, Y.; Nam, S.-W.; Chang, Y.; Lee, G.H. Magnetic Nanoparticle-Based High-Performance Positive and Negative Magnetic Resonance Imaging Contrast Agents. Pharmaceutics 2023, 15, 1745. [Google Scholar] [CrossRef]
- Pöselt, E.; Kloust, H.; Tromsdorf, U.; Janschel, M.; Hahn, C.; Maßlo, C.; Weller, H. Relaxivity optimization of a PEGylated iron-oxide-based negative magnetic resonance contrast agent for T2-weighted spin–echo imaging. ACS Nano 2012, 6, 1619–1624. [Google Scholar] [CrossRef]
- Smolensky, E.D.; Park, H.-Y.E.; Zhou, Y.; Marjanska, M.; Botta, M.; Pierre, V. Scaling laws at the nano size: The effect of particle size and shape on the magnetism and relaxivity of iron oxide nanoparticle contrast agents. J. Mater. Chem. B 2013, 1, 2818–2828. [Google Scholar] [CrossRef]
- Lee, N.; Choi, Y.; Lee, Y.; Park, M.; Moon, W.K.; Choi, S.H.; Hyeon, T. Water-dispersible ferrimagnetic iron oxide nanocubes with extremely high r2 relaxivity for highly sensitive in vivo MRI of tumors. Nano Lett. 2012, 12, 3127–3131. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tan, M.; Fang, J.; Wang, S.; Guo, Y.; Cao, Z.; Xie, T.; Xu, K.; Zhao, Z.; Zhang, W. Cubic Zinc-Doped Iron Oxide Nanoparticles with Poly(Ethylene Glycol) or Sodium Citrate Surface Coatings for Tumor Imaging. ACS Appl. Nano Mater. 2024, 7, 7543–7554. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, Z.; Bao, J.; Wang, Z.; Hu, J.; Chi, X.; Ni, K.; Wang, R.; Chen, X.; Chen, Z.; et al. Octapod iron oxide nanoparticles as high-performance T2 contrast agents for magnetic resonance imaging. Nat. Commun. 2013, 4, 2266. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef] [PubMed]
- Kasparis, G.; Sangnier, A.P.; Wang, L.; Efstathiou, C.; LaGrow, A.P.; Sergides, A.; Wilhelm, C.; Thanh, N.T.K. Zn doped iron oxide nanoparticles with high magnetization and photothermal efficiency for cancer treatment. J. Mater. Chem. B 2023, 11, 787–801. [Google Scholar] [CrossRef]
- Das, P.; Salvioni, L.; Malatesta, M.; Vurro, F.; Mannucci, S.; Gerosa, M.; Rizzuto, M.A.; Tullio, C.; Degrassi, A.; Colombo, M.; et al. Colloidal polymer-coated Zn-doped iron oxide nanoparticles with high relaxivity and specific absorption rate for efficient magnetic resonance imaging and magnetic hyperthermia. J. Colloid. Interface Sci. 2020, 579, 186–194. [Google Scholar] [CrossRef]
- Roca, A.G.; Veintemillas-Verdaguer, S.; Port, M.; Robic, C.; Serna, C.J.; Morales, M.P. Effect of nanoparticle and aggregate size on the relaxometric properties of MR contrast agents based on high quality magnetite nanoparticles. J. Phys. Chem. B 2009, 113, 7033–7039. [Google Scholar] [CrossRef]
- Paquet, C.; de Haan, H.W.; Leek, D.M.; Lin, H.Y.; Xiang, B.; Tian, G.; Kell, A.; Simard, B. Clusters of superparamagnetic iron oxide nanoparticles encapsulated in a hydrogel: A particle architecture generating a synergistic enhancement of the T2 relaxation. ACS Nano 2011, 5, 3104–3112. [Google Scholar] [CrossRef]
- LaConte, L.E.; Nitin, N.; Zurkiya, O.; Caruntu, D.; O’Connor, C.J.; Hu, X.; Bao, G. Coating thickness of magnetic iron oxide nanoparticles affects R2 relaxivity. J. Magn. Reson. Imag. 2007, 26, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.L.; Laurent, S.; Rocha, J.; Roch, A.; Delville, M.H.; Mornet, S.; Carlos, L.D.; Elst, L.V.; Muller, R.N.; Geraldes, C.F.G.C. Relaxometric studies of γ-Fe2O3@SiO2 core shell nanoparticles: When the coating matters. J. Phys. Chem. C 2012, 116, 2285. [Google Scholar] [CrossRef]
- Duan, H.; Kuang, M.; Wang, X.; Wang, Y.A.; Mao, H.; Nie, S. Reexamining the effects of particle size and surface chemistry on the magnetic properties of iron oxide nanocrystals: New insights into spin disorder and proton relaxivity. J. Phys. Chem. C 2008, 112, 8127–8131. [Google Scholar] [CrossRef]
- Walter, A.; Billotey, C.; Garofalo, A.; Ulhaq-Bouillet, C.; Lefèvre, C.; Taleb, J.; Laurent, S.; Elst, L.V.; Muller, R.N.; Lartigue, L.; et al. Mastering the shape and composition of dendronized iron oxide nanoparticles to tailor magnetic resonance imaging and hyperthermia. Chem. Mater. 2014, 26, 5252–5264. [Google Scholar] [CrossRef]
- Basly, B.; Felder-Flesch, D.; Perriat, P.; Billotey, C.; Taleb, J.; Pourroy, G.; Bégin-Colin, S. Dendronized iron oxide nanoparticles as contrast agents for MRI. Chem. Commun. 2010, 46, 985–987. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Villanova, J.; Ines, D.M.; Chen, J.; Lee, S.S.; Xiao, Z.; Guo, X.; Dunn, J.A.; Stueber, D.D.; Decuzzi, P.; et al. Sensitive T2 MRI Contrast Agents from the Rational Design of Iron Oxide Nanoparticle Surface Coatings. J. Phys. Chem. C 2023, 127, 1057–1070. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef]
- Pablico-Lansigan, M.H.; Situ, S.F.; Samia, A.C. Magnetic particle imaging: Advancements and perspectives for real-time in vivo monitoring and image-guided therapy. Nanoscale 2013, 5, 4040–4055. [Google Scholar] [CrossRef]
- Bauer, L.M.; Situ, S.F.; Griswold, M.A.; Samia, A.C. Magnetic Particle Imaging Tracers: State-of-the-Art and Future Directions. J. Phys. Chem. Lett. 2015, 6, 2509–2517. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.W.; Chandrasekharan, P.; Fellows, B.D.; Arrizabalaga, I.R.; Yu, E.; Olivo, M.; Conolly, S.M. Magnetic Particle Imaging: An Emerging Modality with Prospects in Diagnosis, Targeting and Therapy of Cancer. Cancers 2021, 13, 5285. [Google Scholar] [CrossRef]
- Bauer, L.M.; Situ, S.F.; Griswold, M.A.; Samia, A.C. High-performance iron oxide nanoparticles for magnetic particle imaging—Guided hyperthermia (hMPI). Nanoscale 2016, 8, 12162–12169. [Google Scholar] [CrossRef]
- Song, G.; Chen, M.; Zhang, Y.; Cui, L.; Qu, H.; Zheng, X.; Wintermark, M.; Liu, Z.; Rao, J. Janus Iron Oxides @ Semiconducting Polymer Nanoparticle Tracer for Cell Tracking by Magnetic Particle Imaging. Nano Lett. 2018, 18, 182–189. [Google Scholar] [CrossRef]
- Rahmer, J.; Wirtz, D.; Bontus, C.; Borgert, J.; Gleich, B. Interactive Magnetic Catheter Steering With 3-D Real-Time Feedback Using Multi-Color Magnetic Particle Imaging. IEEE Trans. Med. Imaging 2017, 36, 1449–1456. [Google Scholar] [CrossRef]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided heating in Vivo using Gradient Fields for Arbitrary Localization of magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef]
- Bulte, J.W.M. Superparamagnetic iron oxides as MPI tracers: A primer and review of early applications. Adv. Drug Deliv. Rev. 2019, 138, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Velazquez-Albino, A.C.; Imhoff, E.D.; Rinaldi-Ramos, C.M. Advances in engineering nanoparticles for magnetic particle imaging (MPI). Sci. Adv. 2025, 11, eado7356. [Google Scholar] [CrossRef] [PubMed]
- Häfeli, U.O.; Riffle, J.S.; Harris-Shekhawat, L.; Carmichael-Baranauskas, A.; Mark, F.; Dailey, J.P.; Bardenstein, D. Cell uptake and in vitro toxicity of magnetic nanoparticles suitable for drug delivery. Mol. Pharm. 2009, 6, 1417–1428. [Google Scholar] [CrossRef]
- Weissleder, R.; Stark, D.D.; Engelstad, B.L.; Bacon, B.R.; Compton, C.C.; White, D.L.; Jacobs, P.; Lewis, J. Superparamagnetic iron oxide: Pharmacokinetics and toxicity. AJR Am. J. Roentgenol. 1989, 152, 167–173. [Google Scholar] [CrossRef]
- Mejías, R.; Delgado, A.; Herrero, V.; García, M.L.; Martín, C.; Rico, P.; Morales, M.D.P.; Barber, D.F. Long term biotransformation and toxicity of dimercaptosuccinic acid-coated magnetic nanoparticles support their use in biomedical applications. J. Control Release 2013, 171, 225–233. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Alam, J.; Khan, M.A.M.; Ali, D.; Alarafi, S. Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr. Pharm. Des. 2013, 19, 6681–6690. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Wahab, R.; Saquib, Q.; Ahmad, J.; Farshori, N.N.; Al-Sheddi, E.S.; Al-Oqail, M.M.; Al-Massarani, S.M.; Al-Khedhairy, A.A. Iron Oxide Nanoparticles Induced Cytotoxicity, Oxidative Stress, Cell Cycle Arrest, and DNA Damage in Human Umbilical Vein Endothelial Cells. J. Trace Elem. Med. Biol. 2023, 80, 127302. [Google Scholar] [CrossRef]
- Wan, J.; Ren, H.; Wang, J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc. Neurol. 2019, 4, 93–95. [Google Scholar] [CrossRef]
- Kruszewski, M. Labile iron pool: The main determinant of cellular response to oxidative stress. Mutat. Res. 2003, 531, 81–92. [Google Scholar] [CrossRef]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and polymer polyethylene glycol (PEG) coating reduce both 5 and 30 nm iron oxide nanoparticle cytotoxicity in 2D and 3D cell culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, C.; Cuschieri, A.; Wang, L. The cytotoxicity of polycationic iron oxide nanoparticles: Common endpoint assays and alternative approaches for improved understanding of cellular response mechanism. J. Nanobiotechnol. 2012, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Kut, C.; Zhang, Y.; Hedayati, M.; Zhou, H.; Cornejo, C.; Bordelon, D.; Mihalic, J.; Wabler, M.; Burghardt, E.; Gruettner, C.; et al. Preliminary Study of Injury From Heating Systemically Delivered, Nontargeted Dextran–Superparamagnetic Iron Oxide Nanoparticles in Mice. Nanomedicine 2012, 7, 1697–1711. [Google Scholar] [CrossRef]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall Iron Oxide Nanoparticles Cause Significant Toxicity by Specifically Inducing Acute Oxidative Stress to Multiple Organs. Part. Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef]
- Levy, M.; Luciani, N.; Alloyeau, D.; Elgrabli, D.; Deveaux, V.; Pechoux, C.; Chat, S.; Wang, G.; Vats, N.; Gendron, F.; et al. Long term in vivo biotransformation of iron oxide nanoparticles. Biomaterials 2011, 32, 3988–3999. [Google Scholar] [CrossRef]
- Curcio, A.; Van de Walle, A.; Péchoux, C.; Abou-Hassan, A.; Wilhelm, C. In vivo assimilation of CuS, iron oxide and iron oxide@CuS nanoparticles in mice: A 6-month follow-up study. Pharmaceutics 2022, 14, 179. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Padín-González, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the Role and Impact of Poly (Ethylene Glycol) (PEG) on Nanoparticle Formulation: Implications for COVID-19 Vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef]
- Gaballa, S.; Naguib, Y.; Mady, F.; Khaled, K. Polyethylene glycol: Properties, applications, and challenges. J. Adv. Biomed. Pharm. Sci. 2024, 7, 26–36. [Google Scholar] [CrossRef]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, Distribution, Clearance, and Toxicity of Iron Oxide Nanoparticles with Different Sizes and Coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef]
- Mahmood, H.S.; Habubi, N.F. Structural, mechanical and magnetic properties of PVA-PVP: Iron oxide nanocomposite. Appl. Phys. A 2022, 128, 956. [Google Scholar] [CrossRef]
- Baldea, I.; Petran, A.; Florea, A.; Sevastre-Berghian, A.; Nenu, I.; Filip, G.A.; Cenariu, M.; Radu, M.T.; Iacovita, C. Magnetic Nanoclusters Stabilized with Poly [3,4-Dihydroxybenzhydrazide] as Efficient Therapeutic Agents for Cancer Cells Destruction. Nanomaterials 2023, 13, 933. [Google Scholar] [CrossRef]
- Petran, A.; Suciu, M.; Baldea, I.; Boca, S.; Pana, O.; Leoștean, C.; Dan, M.; Bunge, A. One-pot synthesis and biological assessment of fluorescent magnetite clusters coated with polydopamine and -analogues. Appl. Surf. Sci. 2025, 711, 164028. [Google Scholar] [CrossRef]
- Escobar Zapata, E.V.; Martínez Pérez, C.A.; Rodríguez González, C.A.; Castro Carmona, J.S.; Quevedo Lopez, M.A.; García-Casillas, P.E. Adherence of paclitaxel drug in magnetite chitosan nanoparticles. J. Alloys Compd. 2012, 536, S441–S444. [Google Scholar] [CrossRef]
- Hejjaji, E.M.A.; Smith, A.M.; Morris, G.A. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int. J. Biol. Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. High impact of in situ dextran coating on biocompatibility, stability and magnetic properties of iron oxide nanoparticles. Mater. Sci. Eng. C 2017, 75, 947–956. [Google Scholar] [CrossRef]
- Predoi, D.; Balas, M.; Badea, M.A.; Ciobanu, S.C.; Buton, N.; Dinischiotu, A. Dextran-Coated Iron Oxide Nanoparticles Loaded with 5-Fluorouracil for Drug-Delivery Applications. Nanomaterials 2023, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Spirou, S.V.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic Hyperthermia and Radiation Therapy: Radiobiological Principles and Current Practice. Nanomaterials 2018, 8, 401. [Google Scholar] [CrossRef]
- Issels, R.D.; Lindner, L.H.; Verweij, J.; Wust, P.; Reichardt, P.; Schem, B.C.; Abdel-Rahman, S.; Daugaard, S.; Salat, C.; Wendtner, C.-M.; et al. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: A randomised phase 3 multicentre study. Lancet Oncol. 2010, 11, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Yagawa, Y.; Tanigawa, K.; Kobayashi, Y.; Yamamoto, M. Cancer immunity and therapy using hyperthermia with immunotherapy, radiotherapy, chemotherapy, and surgery. J. Cancer Metastasis Treat. 2017, 3, 218–230. [Google Scholar] [CrossRef]
- Gautier, J.; Allard-Vannier, E.; Munnier, E.; Soucé, M.; Chourpa, I. Recent advances in theranostic nanocarriers of doxorubicin based on iron oxide and gold nanoparticles. J. Control. Release 2013, 169, 48–61. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Wang, Q.; Zhang, Z.; Liu, J.; Zhang, C.; Shi, J. Recent Progress on High-Z Metal-Based Nanomaterials for Cancer Radiosensitization. Chin. J. Org. Chem. 2023, 41, 2545–2556. [Google Scholar] [CrossRef]
- Mirzayans, R. Changing the Landscape of Solid Tumor Therapy from Apoptosis-Promoting to Apoptosis-Inhibiting Strategies. Curr. Issues Mol. Biol. 2024, 46, 5379–5396. [Google Scholar] [CrossRef]
- Mirzayans, R. Anastasis and Other Apoptosis-Related Prosurvival Pathways Call for a Paradigm Shift in Oncology: Significance of Deintensification in Treating Solid Tumors. Int. J. Mol. Sci. 2025, 26, 1881. [Google Scholar] [CrossRef]
- Ergün, S.; Aslan, S.; Demir, D.; Kayaoğlu, S.; Saydam, M.; Keleş, Y.; Kolcuoğlu, D.; Taşkurt Hekim, N.; Güneş, S. Beyond Death: Unmasking the Intricacies of Apoptosis Escape. Mol. Diagn. Ther. 2024, 28, 403–423. [Google Scholar] [CrossRef]
- Corsi, F.; Capradossi, F.; Pelliccia, A.; Briganti, S.; Bruni, E.; Traversa, E.; Torino, F.; Reichle, A.; Ghibelli, L. Apoptosis as Driver of Therapy-Induced Cancer Repopulation and Acquired Cell-Resistance (CRAC): A Simple In Vitro Model of Phoenix Rising in Prostate Cancer. Int. J. Mol. Sci. 2022, 23, 1152. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Khatib, S.A.; Ma, L.; Dang, H.; Forgues, M.; Chung, J.-Y.; Ylaya, K.; Hewitt, S.M.; Chaisaingmongkol, J.; Rucchirawat, M.; Wang, X.W. Single-cell biology uncovers apoptotic cell death and its spatial organization as a potential modifier of tumor diversity in HCC. Hepatology 2022, 76, 599–611. [Google Scholar] [CrossRef]
- Park, W.-Y.; Gray, J.M.; Holewinski, R.J.; Andresson, T.; So, J.Y.; Carmona-Rivera, C.; Hollander, M.C.; Yang, H.H.; Lee, M.; Kaplan, M.J.; et al. Apoptosis-Induced Nuclear Expulsion in Tumor Cells Drives S100a4-Mediated Metastatic Outgrowth through the RAGE Pathway. Nat. Cancer 2023, 4, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fang, J.; Chen, J. Tumor cell senescence response produces aggressive variants. Cell Death Discov. 2017, 3, 17049. [Google Scholar] [CrossRef]
- Rivera, D.; Bouras, A.; Mattioli, M.; Anastasiadou, M.; Pacentra, A.C.; Pelcher, O.; Koziel, C.; Schupper, A.J.; Chanenchuk, T.; Carlton, H.; et al. Magnetic Hyperthermia Therapy Enhances the Chemoradiosensitivity of Glioblastoma. Sci. Rep. 2025, 15, 10532. [Google Scholar] [CrossRef] [PubMed]
- Piehler, S.; Dähring, H.; Grandke, J.; Göring, J.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Courty, J.; Latorre, A.; Somoza, Á.; et al. Iron Oxide Nanoparticles as Carriers for DOX and Magnetic Hyperthermia after Intratumoral Application into Breast Cancer in Mice: Impact and Future Perspectives. Nanomaterials 2020, 10, 1016. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jain, S.; Sahoo, S.K. Magnetic nanoparticles for amalgamation of magnetic hyperthermia and chemotherapy: An approach towards enhanced attenuation of tumor. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110695. [Google Scholar] [CrossRef]
- Lin, W.; Xie, X.; Yang, Y.; Fu, X.; Liu, H.; Yang, Y.; Deng, J. Thermosensitive magnetic liposomes with doxorubicin cell-penetrating peptides conjugate for enhanced and targeted cancer therapy. Drug Deliv. 2016, 23, 3436–3443. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and Safety of Intratumoral Thermotherapy Using Magnetic Iron-Oxide Nanoparticles Combined with External Beam Radiotherapy on Patients with Recurrent Glioblastoma Multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Foo, C.Y.; Munir, N.; Kumaria, A.; Akhtar, Q.; Bullock, C.J.; Narayanan, A.; Fu, R.Z. Medical Device Advances in the Treatment of Glioblastoma. Cancers 2022, 14, 5341. [Google Scholar] [CrossRef]
- Yan, B.; Liu, C.; Wang, S.; Li, H.; Jiao, J.; Lee, W.S.V.; Zhang, S.; Hou, Y.; Hou, Y.; Ma, X.; et al. Magnetic hyperthermia induces effective and genuine immunogenic tumor cell death with respect to exogenous heating. J. Mater. Chem. B 2022, 10, 5364–5374. [Google Scholar] [CrossRef]
- Song, Q.; Javid, A.; Zhang, G.; Li, Y. Applications of Magnetite Nanoparticles in Cancer Immunotherapies: Present Hallmarks and Future Perspectives. Front. Immunol. 2021, 12, 701485. [Google Scholar] [CrossRef]
- Chao, Y.; Chen, G.; Liang, C.; Xu, J.; Dong, Z.; Han, X.; Wang, C.; Liu, Z. Iron Nanoparticles for Low-Power Local Magnetic Hyperthermia in Combination with Immune Checkpoint Blockade for Systemic Antitumor Therapy. Nano Lett. 2019, 19, 4287–4296. [Google Scholar] [CrossRef] [PubMed]
- Laskar, A.; Eilertsen, J.; Li, W.; Yuan, X.M. SPION primes THP1 derived M2 macrophages towards M1-like macrophages. Biochem. Biophys. Res. Commun. 2013, 441, 737–742. [Google Scholar] [CrossRef]
- Yan, B.; Wang, S.; Liu, C.; Wen, N.; Li, H.; Zhang, Y.; Wang, H.; Xi, Z.; Lv, Y.; Fan, H.; et al. Engineering magnetic nano-manipulators for boosting cancer immunotherapy. J. Nanobiotechnol. 2022, 20, 547. [Google Scholar] [CrossRef] [PubMed]
- Carter, T.J.; Agliardi, G.; Lin, F.Y.; Ellis, M.; Jones, C.; Robson, M.; Richard-Londt, A.; Southern, P.; Lythgoe, M.; Zaw Thin, M.; et al. Potential of Magnetic Hyperthermia to Stimulate Localized Immune Activation. Small 2021, 17, e2005241. [Google Scholar] [CrossRef]
- Macedo, J.B.; Bueno, J.N.S.; Kanunfre, C.C.; Miranda, J.R.A.; Bakuzis, A.F.; Ferrari, P.C. Polymer-Functionalized Magnetic Nanoparticles for Targeted Quercetin Delivery: A Potential Strategy for Colon Cancer Treatment. Pharmaceutics 2025, 17, 467. [Google Scholar] [CrossRef]
- Hernández, R.; Sacristán, J.; Asín, L.; Torres, T.E.; Ibarra, M.R.; Goya, G.F.; Mijangos, C. Magnetic Hydrogels Derived from Polysaccharides with Improved Specific Power Absorption: Potential Devices for Remotely Triggered Drug Delivery. J. Phys. Chem. B 2010, 114, 12002–12007. [Google Scholar] [CrossRef]
- Javid, A.; Ahmadian, S.; Saboury, A.A.; Kalantar, S.M.; Rezaei-Zarchi, S. Chitosan-Coated Superparamagnetic Iron Oxide Nanoparticles for Doxorubicin Delivery: Synthesis and Anticancer Effect Against Human Ovarian Cancer Cells. Chem. Biol. Drug Des. 2013, 81, 784–792. [Google Scholar] [CrossRef]
- Javid, A.; Ahmadian, S.; Saboury, A.A.; Kalantar, S.M.; Rezaei-Zarchi, S.; Shahzad, S. Biocompatible APTES-PEG Modified Magnetite Nanoparticles: Effective Carriers of Antineoplastic Agents to Ovarian Cancer. Appl. Biochem. Biotechnol. 2014, 173, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Thong, P.Q.; Thu Huong, L.T.; Tu, N.D.; My Nhung, H.T.; Khanh, L.; Manh, D.H.; Nam, P.H.; Phuc, N.X.; Alonso, J.; Qiao, J.; et al. Multifunctional Nanocarriers of Fe3O4@PLA-PEG/Curcumin for MRI, Magnetic Hyperthermia and Drug Delivery. Nanomedicine 2022, 17, 1677–1693. [Google Scholar] [CrossRef] [PubMed]
- Sallem, F.; Haji, R.; Vervandier-Fasseur, D.; Nury, T.; Maurizi, L.; Boudon, J.; Lizard, G.; Millot, N. Elaboration of Trans-Resveratrol Derivative-Loaded Superparamagnetic Iron Oxide Nanoparticles for Glioma Treatment. Nanomaterials 2019, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Refai, H.; El Sayed, N.; Rashed, L.A.; Mousa, M.R.; Zewail, M. Superparamagnetic Iron Oxide Loaded Chitosan Coated Bilosomes for Magnetic Nose to Brain Targeting of Resveratrol. Int. J. Pharm. 2021, 610, 121244. [Google Scholar] [CrossRef]
- Boztepe, C.; Daskin, M.; Erdogan, A. Synthesis of Magnetic Responsive Poly(NIPAAm-co-VSA)/Fe3O4 IPN Ferrogels and Modeling Their Deswelling and Heating Behaviors under AMF by Using Artificial Neural Networks. React. Funct. Polym. 2022, 173, 105219. [Google Scholar] [CrossRef]
- Ndong, C.; Toraya-Brown, S.; Kekalo, K.; Baker, I.; Gerngross, T.; Fiering, S.; Griswold, K. Antibody-Mediated Targeting of Iron Oxide Nanoparticles to the Folate Receptor Alpha Increases Tumor Cell Association In Vitro and In Vivo. Int. J. Nanomed. 2015, 10, 2595–2617. [Google Scholar] [CrossRef]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, H.; Huang, J.; Qian, W.; Martinson, D.E.; Ji, B.; Li, Y.; Wang, Y.A.; Yang, L.; Mao, H. Probing and Enhancing Ligand-Mediated Active Targeting of Tumors Using Sub-5 nm Ultrafine Iron Oxide Nanoparticles. Theranostics 2020, 10, 2479–2494. [Google Scholar] [CrossRef]
- Jiang, W.; Xie, H.; Ghoorah, D.; Shang, Y.; Shi, H.; Liu, F.; Yang, X.; Xu, H. Conjugation of Functionalized SPIONs with Transferrin for Targeting and Imaging Brain Glial Tumors in Rat Model. PLoS ONE 2012, 7, e37376. [Google Scholar] [CrossRef]
- Senturk, F.; Cakmak, S.; Kocum, I.C.; Gumusderelioglu, M.; Ozturk, G.G. GRGDS-Conjugated and Curcumin-Loaded Magnetic Polymeric Nanoparticles for the Hyperthermia Treatment of Glioblastoma Cells. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 622, 126648. [Google Scholar] [CrossRef]
- Das, S.; Diyali, S.; Vinothini, G.; Perumalsamy, B.; Balakrishnan, G.; Ramasamy, T.; Dharumadurai, D.; Biswas, B. Synthesis, Morphological Analysis, Antibacterial Activity of Iron Oxide Nanoparticles and the Cytotoxic Effect on Lung Cancer Cell Line. Heliyon 2020, 6, e04953. [Google Scholar] [CrossRef] [PubMed]
- Lugert, S.; Unterweger, H.; Mühlberger, M.; Janko, C.; Draack, S.; Ludwig, F.; Eberbeck, D.; Alexiou, C.; Friedrich, R.P. Cellular Effects of Paclitaxel-Loaded Iron Oxide Nanoparticles on Breast Cancer Using Different 2D and 3D Cell Culture Models. IJN 2018, 14, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Justin, C.; Samrot, A.V.; P, D.S.; Sahithya, C.S.; Bhavya, K.S.; Saipriya, C. Preparation, Characterization and Utilization of Coreshell Super Paramagnetic Iron Oxide Nanoparticles for Curcumin Delivery. PLoS ONE 2018, 13, e0200440. [Google Scholar] [CrossRef] [PubMed]
- Situ, J.-Q.; Wang, X.-J.; Zhu, X.-L.; Xu, X.-L.; Kang, X.-Q.; Hu, J.-B.; Lu, C.-Y.; Ying, X.-Y.; Yu, R.-S.; You, J.; et al. Multifunctional SPIO/DOX-Loaded A54 Homing Peptide Functionalized Dextran-g-PLGA Micelles for Tumor Therapy and MR Imaging. Sci. Rep. 2016, 6, 35910. [Google Scholar] [CrossRef]
- Farshchi, H.K.; Azizi, M.; Jaafari, M.R.; Nemati, S.H.; Fotovat, A. Green Synthesis of Iron Nanoparticles by Rosemary Extract and Cytotoxicity Effect Evaluation on Cancer Cell Lines. Biocatal. Agric. Biotechnol. 2018, 16, 54–62. [Google Scholar] [CrossRef]
- Al-Obaidy, R.; Haider, A.J.; Al-Musawi, S.; Arsad, N. Targeted Delivery of Paclitaxel Drug Using Polymer-Coated Magnetic Nanoparticles for Fibrosarcoma Therapy: In Vitro and in Vivo Studies. Sci. Rep. 2023, 13, 3180. [Google Scholar] [CrossRef]
- Serio, F.; Silvestri, N.; Kumar Avugadda, S.; Nucci, G.E.P.; Nitti, S.; Onesto, V.; Catalano, F.; D’Amone, E.; Gigli, G.; Del Mercato, L.L.; et al. Co-Loading of Doxorubicin and Iron Oxide Nanocubes in Polycaprolactone Fibers for Combining Magneto-Thermal and Chemotherapeutic Effects on Cancer Cells. J. Colloid. Interface Sci. 2022, 607, 34–44. [Google Scholar] [CrossRef]
- Buyukhatipoglu, K.; Clyne, A.M. Superparamagnetic Iron Oxide Nanoparticles Change Endothelial Cell Morphology and Mechanics via Reactive Oxygen Species Formation. J. Biomed. Mater. Res. 2011, 96A, 186–195. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy: A Combinational Approach for Enhanced Delivery of Nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Herranz-López, M.; Losada-Echeberría, M.; Barrajón-Catalán, E. The Multitarget Activity of Natural Extracts on Cancer: Synergy and Xenohormesis. Medicines 2018, 6, 6. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic Interactions Between Anticancer Chemotherapeutics and Phenolic Compounds and Anticancer Synergy Between Polyphenols. Postepy. Hig. Med. Dosw. 2014, 68, 528–540. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Agulló-Chazarra, L.; Herranz-López, M.; Valdés, A.; Cifuentes, A.; Micol, V. Rosemary (Rosmarinus Officinalis) Extract Causes ROS-Induced Necrotic Cell Death and Inhibits Tumor Growth in Vivo. Sci. Rep. 2019, 9, 808. [Google Scholar] [CrossRef]
- Einbond, L.S.; Wu, H.; Kashiwazaki, R.; He, K.; Roller, M.; Su, T.; Wang, X.; Goldsberry, S. Carnosic Acid Inhibits the Growth of ER-Negative Human Breast Cancer Cells and Synergizes with Curcumin. Fitoterapia 2012, 83, 1160–1168. [Google Scholar] [CrossRef]
- Mangaiyarkarasi, R.; Chinnathambi, S.; Karthikeyan, S.; Aruna, P.; Ganesan, S. Paclitaxel Conjugated Fe3O4@LaF3:Ce3+,Tb3+ Nanoparticles as Bifunctional Targeting Carriers for Cancer Theranostics Application. J. Magn. Magn. Mater. 2016, 399, 207–215. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Tian, C.; Guo, H.; Shen, Y.; Zhu, M. Fe3O4@MnO2@PPy Nanocomposites Overcome Hypoxia: Magnetic-Targeting-Assisted Controlled Chemotherapy and Enhanced Photodynamic/Photothermal Therapy. J. Mater. Chem. B 2018, 6, 6848–6857. [Google Scholar] [CrossRef]
- Menon, J.U.; Kuriakose, A.; Iyer, R.; Hernandez, E.; Gandee, L.; Zhang, S.; Takahashi, M.; Zhang, Z.; Saha, D.; Nguyen, K.T. Dual-Drug Containing Core-Shell Nanoparticles for Lung Cancer Therapy. Sci. Rep. 2017, 7, 13249. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Bullo, S.; Buskara, K.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Release of a Liver Anticancer Drug, Sorafenib from Its PVA/LDH- and PEG/LDH-Coated Iron Oxide Nanoparticles for Drug Delivery Applications. Sci. Rep. 2020, 10, 21521. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Low, P.S. Folate-Mediated Targeting of Antineoplastic Drugs, Imaging Agents, and Nucleic Acids to Cancer Cells. J. Control. Release 1998, 53, 39–48. [Google Scholar] [CrossRef]
- Tran, P.A.; Nguyen, H.T.; Fox, K.; Tran, N. In Vitro Cytotoxicity of Iron Oxide Nanoparticles: Effects of Chitosan and Polyvinyl Alcohol as Stabilizing Agents. Mater. Res. Express 2018, 5, 035051. [Google Scholar] [CrossRef]
- Popescu, R.C.; Savu, D.; Dorobantu, I.; Vasile, B.S.; Hosser, H.; Boldeiu, A.; Temelie, M.; Straticiuc, M.; Iancu, D.A.; Andronescu, E.; et al. Efficient Uptake and Retention of Iron Oxide-Based Nanoparticles in HeLa Cells Leads to an Effective Intracellular Delivery of Doxorubicin. Sci. Rep. 2020, 10, 10530. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Moosavi, M.A.; Tavakol, S.; Vardar, D.Ö.; Hosseini, A.; Rahmati, M.; Dini, L.; Hussain, S.; Mandegary, A.; Klionsky, D.J. Necrotic, Apoptotic and Autophagic Cell Fates Triggered by Nanoparticles. Autophagy 2019, 15, 4–33. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Lockshin, R.A.; Zakeri, Z. Programmed Cell Death and Apoptosis: Origins of the Theory. Nat. Rev. Mol. Cell Biol. 2001, 2, 545–550. [Google Scholar] [CrossRef]
- Fink, S.L.; Cookson, B.T. Apoptosis, Pyroptosis, and Necrosis: Mechanistic Description of Dead and Dying Eukaryotic Cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef]
- Gong, M.; Yang, H.; Zhang, S.; Yang, Y.; Zhang, D.; Qi, Y.; Zou, L. Superparamagnetic core/shell GoldMag nanoparticles: Size-, concentration- and time-dependent cellular nanotoxicity on human umbilical vein endothelial cells and the suitable conditions for magnetic resonance imaging. J. Nanobiotechnol. 2015, 13, 24. [Google Scholar] [CrossRef]
- Nosrati, H.; Salehiabar, M.; Davaran, S.; Danafar, H.; Manjili, H.K. Methotrexate-Conjugated L-Lysine Coated Iron Oxide Magnetic Nanoparticles for Inhibition of MCF-7 Breast Cancer Cells. Drug Dev. Ind. Pharm. 2018, 44, 886–894. [Google Scholar] [CrossRef]
- Tousi, M.S.; Sepehri, H.; Khoee, S.; Farimani, M.M.; Delphi, L.; Mansourizadeh, F. Evaluation of Apoptotic Effects of mPEG-b-PLGA Coated Iron Oxide Nanoparticles as a Eupatorin Carrier on DU-145 and LNCaP Human Prostate Cancer Cell Lines. J. Pharm. Anal. 2021, 11, 108–121. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Symonds, P.; Murray, J.C.; Hunter, A.C.; Debska, G.; Szewczyk, A. A Two-Stage Poly(Ethylenimine)-Mediated Cytotoxicity: Implications for Gene Transfer/Therapy. Mol. Ther. 2005, 11, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.S.; Adireddy, S.; Jaiswal, A.; Mandava, S.; Lee, B.R.; Chrisey, D.B. In Vitro/In Vivo Toxicity Evaluation and Quantification of Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2015, 16, 24417–24450. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhao, B.; Zhou, L.; Zhang, Z.; Shen, Y.; Lv, H.; AlQudsy, L.H.H.; Shang, P. Ferroptosis, a Novel Pharmacological Mechanism of Anti-Cancer Drugs. Cancer Lett. 2020, 483, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, S.; Wu, L.; Yang, L.; Yang, L.; Wang, J. The Diversified Role of Mitochondria in Ferroptosis in Cancer. Cell Death Dis. 2023, 14, 519. [Google Scholar] [CrossRef]
- Johnson, J.; Mercado-Ayon, E.; Mercado-Ayon, Y.; Dong, Y.N.; Halawani, S.; Ngaba, L.; Lynch, D.R. Mitochondrial Dysfunction in the Development and Progression of Neurodegenerative Diseases. Arch. Biochem. Biophys. 2021, 702, 108698. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, Z.; Jin, T.; Xu, K.; Liu, M.; Xu, H. Ferroptosis in Low-Grade Glioma: A New Marker for Diagnosis and Prognosis. Med. Sci. Monit. 2020, 26, e921947. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Olzmann, J.A. The Cell Biology of Ferroptosis. Nat. Rev. Mol. Cell Biol. 2024, 25, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Li, X.; Yan, H.; Guo, Q.; Liu, J.; Li, Y. Iron Oxide Nanoparticles Induce Ferroptosis under Mild Oxidative Stress in Vitro. Sci. Rep. 2024, 14, 31383. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Q.; Zhou, L.; Xia, X. Ferroptosis: A Double-Edged Sword. Cell Death Discov. 2024, 10, 265. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. IJMS 2022, 24, 449. [Google Scholar] [CrossRef]
- Cui, K.; Wang, K.; Huang, Z. Ferroptosis and the Tumor Microenvironment. J. Exp. Clin. Cancer Res. 2024, 43, 315. [Google Scholar] [CrossRef]
- Nie, Q.; Chen, W.; Zhang, T.; Ye, S.; Ren, Z.; Zhang, P.; Wen, J. Iron Oxide Nanoparticles Induce Ferroptosis via the Autophagic Pathway by Synergistic Bundling with Paclitaxel. Mol. Med. Rep. 2023, 28, 198. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Liu, X.; Tuerxun, H.; Zhao, Y.; Li, Y.; Wen, S.; Li, X.; Zhao, Y. Crosstalk between Ferroptosis and Autophagy: Broaden Horizons of Cancer Therapy. J. Transl. Med. 2025, 23, 18. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. The Roles of Autophagy in Cancer. IJMS 2018, 19, 3466. [Google Scholar] [CrossRef]
- Adiseshaiah, P.P.; Hall, J.B.; McNeil, S.E. Nanomaterial Standards for Efficacy and Toxicity Assessment. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol 2010, 2, 99–112. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Chen, B.; Ding, J.; Xia, G.; Gao, C.; Cheng, J.; Jin, N.; Zhou, Y.; Li, X.; et al. Pharmacokinetic Parameters and Tissue Distribution of Magnetic Fe3O4 Nanoparticles in Mice. Int. J. Nanomed. 2010, 5, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical Characteristics of Nanoparticles Affect Circulation, Biodistribution, Cellular Internalization, and Trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic Iron Oxide Nanoparticles for Imaging, Targeting and Treatment of Primary and Metastatic Tumors of the Brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef]
- Apopa, P.L.; Qian, Y.; Shao, R.; Guo, N.L.; Schwegler-Berry, D.; Pacurari, M.; Porter, D.; Shi, X.; Vallyathan, V.; Castranova, V.; et al. Iron Oxide Nanoparticles Induce Human Microvascular Endothelial Cell Permeability through Reactive Oxygen Species Production and Microtubule Remodeling. Part. Fibre Toxicol. 2009, 6, 1. [Google Scholar] [CrossRef]
- Larsen, E.K.U.; Nielsen, T.; Wittenborn, T.; Birkedal, H.; Vorup-Jensen, T.; Jakobsen, M.H.; Østergaard, L.; Horsman, M.R.; Besenbacher, F.; Howard, K.A.; et al. Size-Dependent Accumulation of PEGylated Silane-Coated Magnetic Iron Oxide Nanoparticles in Murine Tumors. ACS Nano 2009, 3, 1947–1951. [Google Scholar] [CrossRef]
- Curry, F.E.; Adamson, R.H. Endothelial Glycocalyx: Permeability Barrier and Mechanosensor. Ann. Biomed. Eng. 2012, 40, 828–839. [Google Scholar] [CrossRef]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of Transport Pathways in Tumor Vessels: Role of Tumor Type and Microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Malik, A.B. Signaling Mechanisms Regulating Endothelial Permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Jiang, K.; Chung, E.J. Improving Kidney Targeting: The Influence of Nanoparticle Physicochemical Properties on Kidney Interactions. J. Control. Release 2021, 334, 127–137. [Google Scholar] [CrossRef]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.; Maaza, M.; Ezema, F.I. Bio-Inspired Encapsulation and Functionalization of Iron Oxide Nanoparticles for Biomedical Applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Sionkowska, A. Current Research on the Blends of Natural and Synthetic Polymers as New Biomaterials: Review. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Veronese, F.M. Peptide and Protein PEGylation. Biomaterials 2001, 22, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Peñate Medina, T.; Gerle, M.; Humbert, J.; Chu, H.; Köpnick, A.-L.; Barkmann, R.; Garamus, V.M.; Sanz, B.; Purcz, N.; Will, O.; et al. Lipid-Iron Nanoparticle with a Cell Stress Release Mechanism Combined with a Local Alternating Magnetic Field Enables Site-Activated Drug Release. Cancers 2020, 12, 3767. [Google Scholar] [CrossRef]
- Ogden, S.G.; Lewis, D.; Shapter, J.G. Silane Functionalisation of Iron Oxide Nanoparticles. In Proceedings of the SPIE Smart Materials, Nano- and Micro-Smart Systems, Melbourne, Australia, 9–12 December 2008; p. 72670A. [Google Scholar]
- Janjua, T.I.; Cao, Y.; Kleitz, F.; Linden, M.; Yu, C.; Popat, A. Silica Nanoparticles: A Review of Their Safety and Current Strategies to Overcome Biological Barriers. Adv. Drug Deliv. Rev. 2023, 203, 115115. [Google Scholar] [CrossRef]
- Jain, N.K.; Prabhuraj, R.S.; Bavya, M.C.; Prasad, R.; Bandyopadhyaya, R.; Naidu, V.G.M.; Srivastava, R. Niclosamide Encapsulated Polymeric Nanocarriers for Targeted Cancer Therapy. RSC Adv. 2019, 9, 26572–26581. [Google Scholar] [CrossRef]
- Kim, J.-E.; Shin, J.-Y.; Cho, M.-H. Magnetic Nanoparticles: An Update of Application for Drug Delivery and Possible Toxic Effects. Arch. Toxicol. 2012, 86, 685–700. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Tarasevych, A.V.; Kukhar, V.P.; Boukherroub, R.; Szunerits, S. Recent Advances in Surface Chemistry Strategies for the Fabrication of Functional Iron Oxide Based Magnetic Nanoparticles. Nanoscale 2013, 5, 10729. [Google Scholar] [CrossRef]
- Wagstaff, A.J.; Brown, S.D.; Holden, M.R.; Craig, G.E.; Plumb, J.A.; Brown, R.E.; Schreiter, N.; Chrzanowski, W.; Wheate, N.J. Cisplatin Drug Delivery Using Gold-Coated Iron Oxide Nanoparticles for Enhanced Tumour Targeting with External Magnetic Fields. Inorganica Chimica Acta 2012, 393, 328–333. [Google Scholar] [CrossRef]
- Jeong, Y.; Kim, S.; Jin, S.; Ryu, H.; Jin, Y.; Jung, T.; Kim, I.; Jung, S. Cisplatin-incorporated Hyaluronic Acid Nanoparticles Based on Ion-complex Formation. J. Pharm. Sci. 2008, 97, 1268–1276. [Google Scholar] [CrossRef]
- Mohamadkazem, M.; Neshastehriz, A.; Amini, S.M.; Moshiri, A.; Janzadeh, A. Radiosensitising Effect of Iron Oxide-gold Nanocomplex for Electron Beam Therapy of Melanoma in Vivo by Magnetic Targeting. IET Nanobiotechnol. 2023, 17, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Guo, Z.; Gao, F.; Gao, Q.; Wang, D.; Liaw, B.; Cai, Q.; Sun, X.; Wang, X.; Zhao, L. Shape-, Size- and Structure-Controlled Synthesis and Biocompatibility of Iron Oxide Nanoparticles for Magnetic Theranostics. Theranostics 2018, 8, 3284–3307. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.; Chiappini, C.; Fine, D.; Bhavane, R.C.; Peluccio, M.S.; Cheng, M.M.-C.; Liu, X.; Ferrari, M.; Decuzzi, P. The Effect of Shape on the Margination Dynamics of Non-Neutrally Buoyant Particles in Two-Dimensional Shear Flows. J. Biomech. 2008, 41, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Doshi, N.; Prabhakarpandian, B.; Rea-Ramsey, A.; Pant, K.; Sundaram, S.; Mitragotri, S. Flow and Adhesion of Drug Carriers in Blood Vessels Depend on Their Shape: A Study Using Model Synthetic Microvascular Networks. J. Control. Release 2010, 146, 196–200. [Google Scholar] [CrossRef]
- Öztürk, K.; Kaplan, M.; Çalış, S. Effects of Nanoparticle Size, Shape, and Zeta Potential on Drug Delivery. Int. J. Pharm. 2024, 666, 124799. [Google Scholar] [CrossRef]
- Di Bona, K.R.; Xu, Y.; Ramirez, P.A.; DeLaine, J.; Parker, C.; Bao, Y.; Rasco, J.F. Surface Charge and Dosage Dependent Potential Developmental Toxicity and Biodistribution of Iron Oxide Nanoparticles in Pregnant CD-1 Mice. Reprod. Toxicol. 2014, 50, 36–42. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Shokrgozar, M.A.; Sardari, S.; Moghadam, M.K.; Vali, H.; Laurent, S.; Stroeve, P. Irreversible Changes in Protein Conformation Due to Interaction with Superparamagnetic Iron Oxide Nanoparticles. Nanoscale 2011, 3, 1127–1138. [Google Scholar] [CrossRef]
- Xie, D.; Sun, L.; Wu, M.; Li, Q. From Detection to Elimination: Iron-Based Nanomaterials Driving Tumor Imaging and Advanced Therapies. Front. Oncol. 2025, 15, 1536779. [Google Scholar] [CrossRef]
- Ju, H.; Liu, Y.; Wang, Y.; Lu, R.; Yang, B.; Wang, D.; Wang, J. The Cellular Response and Molecular Mechanism of Superoxide Dismutase Interacting with Superparamagnetic Iron Oxide Nanoparticles. NanoImpact 2024, 35, 100515. [Google Scholar] [CrossRef]
- Ye, P.; Ye, Y.; Chen, X.; Zou, H.; Zhou, Y.; Zhao, X.; Chang, Z.; Han, B.; Kong, X. Ultrasmall Fe3O4 Nanoparticles Induce S-Phase Arrest and Inhibit Cancer Cells Proliferation. Nanotechnol. Rev. 2020, 9, 61–69. [Google Scholar] [CrossRef]
- Nemmar, A.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Tariq, S.; Attoub, S.; Ali, B.H. Ultrasmall Superparamagnetic Iron Oxide Nanoparticles Acutely Promote Thrombosis and Cardiac Oxidative Stress and DNA Damage in Mice. Part. Fibre Toxicol. 2015, 13, 22. [Google Scholar] [CrossRef]
- Hsiao, Y.-P.; Shen, C.-C.; Huang, C.-H.; Lin, Y.-C.; Jan, T.-R. Iron Oxide Nanoparticles Attenuate T Helper 17 Cell Responses in Vitro and in Vivo. Int. Immunopharmacol. 2018, 58, 32–39. [Google Scholar] [CrossRef]
- Shestovskaya, M.V.; Luss, A.L.; Bezborodova, O.A.; Makarov, V.V.; Keskinov, A.A. Iron Oxide Nanoparticles in Cancer Treatment: Cell Responses and the Potency to Improve Radiosensitivity. Pharmaceutics 2023, 15, 2406. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic Nanoparticles for Precision Oncology: Theranostic Magnetic Iron Oxide Nanoparticles for Image-Guided and Targeted Cancer Therapy. Nanomedicine 2017, 12, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Thiele, L.; Merkle, H.P.; Walter, E. Phagocytosis and Phagosomal Fate of Surface-Modified Microparticles in Dendritic Cells and Macrophages. Pharm. Res. 2003, 20, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Role of Target Geometry in Phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef]
- Doshi, N.; Mitragotri, S. Designer Biomaterials for Nanomedicine. Adv. Funct. Mater. 2009, 19, 3843–3854. [Google Scholar] [CrossRef]
- Champion, J.A.; Mitragotri, S. Shape Induced Inhibition of Phagocytosis of Polymer Particles. Pharm. Res. 2009, 26, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Von Maltzahn, G.; Zhang, L.; Schwartz, M.P.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Magnetic Iron Oxide Nanoworms for Tumor Targeting and Imaging. Adv. Mater. 2008, 20, 1630–1635. [Google Scholar] [CrossRef]
- Wojtera, K.; Pietrzak, L.; Szymanski, L.; Wiak, S. Investigation of Ferromagnetic Nanoparticles’ Behavior in a Radio Frequency Electromagnetic Field for Medical Applications. Electronics 2024, 13, 2287. [Google Scholar] [CrossRef]
- Baniasadi, F.; Hajiaghalou, S.; Shahverdi, A.; Ghalamboran, M.R.; Pirhajati, V.; Fathi, R. The Beneficial Effects of Static Magnetic Field and Iron Oxide Nanoparticles on the Vitrification of Mature Mice Oocytes. Reprod. Sci. 2023, 30, 2122–2136. [Google Scholar] [CrossRef]
- Semeano, A.T.; Tofoli, F.A.; Corrêa-Velloso, J.C.; De Jesus Santos, A.P.; Oliveira-Giacomelli, Á.; Cardoso, R.R.; Pessoa, M.A.; Da Rocha, E.L.; Ribeiro, G.; Ferrari, M.F.R.; et al. Effects of Magnetite Nanoparticles and Static Magnetic Field on Neural Differentiation of Pluripotent Stem Cells. Stem Cell Rev. Rep. 2022, 18, 1337–1354. [Google Scholar] [CrossRef]
- Nandakumaran, N.; Barnsley, L.; Feoktystov, A.; Ivanov, S.A.; Huber, D.L.; Fruhner, L.S.; Leffler, V.; Ehlert, S.; Kentzinger, E.; Qdemat, A.; et al. Unravelling Magnetic Nanochain Formation in Dispersion for In Vivo Applications. Adv. Mater. 2021, 33, 2008683. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. JPM 2021, 11, 771. [Google Scholar] [CrossRef]
- Johannsen, M.; Jordan, A.; Scholz, R.; Koch, M.; Lein, M.; Deger, S.; Roigas, J.; Jung, K.; Loening, S. Evaluation of Magnetic Fluid Hyperthermia in a Standard Rat Model of Prostate Cancer. J. Endourol. 2004, 18, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Talesh, S.; Koohi, M.; Zayerzadeh, E.; Hasan, J.; Shabanian, M. Acute Toxicity Investigation Regarding Clinical and Pathological Aspects Following Repeated Oral Administration of Iron Oxide Nanoparticles in Rats. Nanomed. Res. J. 2019, 4, 228–233. [Google Scholar] [CrossRef]
- Parivar, K.; Malekvand Fard, F.; Bayat, M.; Alavian, S.M.; Motavaf, M. Evaluation of Iron Oxide Nanoparticles Toxicity on Liver Cells of BALB/c Rats. Iran. Red. Crescent Med. J. 2016, 18, e28939. [Google Scholar] [CrossRef]
- Manescu, V.; Paltanea, G.; Antoniac, I.; Vasilescu, M. Magnetic Nanoparticles Used in Oncology. Materials 2021, 14, 5948. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, J.; Gu, G.; Guo, X.; He, C.; Sun, J.; Zou, H.; Wang, H.; Liu, S.; Li, X.; et al. Pulmonary Delivery of Theranostic Nanoclusters for Lung Cancer Ferroptosis with Enhanced Chemodynamic/Radiation Synergistic Therapy. Nano Lett. 2022, 22, 963–972. [Google Scholar] [CrossRef]
- Zhu, M.-T.; Feng, W.-Y.; Wang, Y.; Wang, B.; Wang, M.; Ouyang, H.; Zhao, Y.-L.; Chai, Z.-F. Particokinetics and Extrapulmonary Translocation of Intratracheally Instilled Ferric Oxide Nanoparticles in Rats and the Potential Health Risk Assessment. Toxicol. Sci. 2009, 107, 342–351. [Google Scholar] [CrossRef]
- Bae, C.; Hernández Millares, R.; Ryu, S.; Moon, H.; Kim, D.; Lee, G.; Jiang, Z.; Park, M.H.; Kim, K.H.; Koom, W.S.; et al. Synergistic Effect of Ferroptosis-Inducing Nanoparticles and X-Ray Irradiation Combination Therapy. Small 2024, 20, 2310873. [Google Scholar] [CrossRef]
- Carbon Nanoparticle-Loaded Iron [CNSI-Fe(II)] in the Treatment of Advanced Solid Tumor (CNSI-Fe(II)). Available online: https://clinicaltrials.gov/study/NCT06048367?term=NCT06048367%20&rank=1 (accessed on 9 August 2025).
- Mirzaei, N.; Wärnberg, F.; Zaar, P.; Leonhardt, H.; Olofsson Bagge, R. Ultra-Low Dose of Superparamagnetic Iron Oxide Nanoparticles for Sentinel Lymph Node Detection in Patients with Breast Cancer. Ann. Surg. Oncol. 2023, 30, 5685–5689. [Google Scholar] [CrossRef]
- Pantiora, E.; Eriksson, S.; Wärnberg, F.; Karakatsanis, A. Magnetically Guided Surgery after Primary Systemic Therapy for Breast Cancer: Implications for Enhanced Axillary Mapping. Br. J. Surg. 2024, 111, znae008. [Google Scholar] [CrossRef] [PubMed]
- Thoeny, H.C.; Triantafyllou, M.; Birkhaeuser, F.D.; Froehlich, J.M.; Tshering, D.W.; Binser, T.; Fleischmann, A.; Vermathen, P.; Studer, U.E. Combined Ultrasmall Superparamagnetic Particles of Iron Oxide–Enhanced and Diffusion-Weighted Magnetic Resonance Imaging Reliably Detect Pelvic Lymph Node Metastases in Normal-Sized Nodes of Bladder and Prostate Cancer Patients. Eur. Urol. 2009, 55, 761–769. [Google Scholar] [CrossRef]
- Michalak, S. NanoTherm in Adjuvant Therapy of Glioblastoma Multiforme (ANCHIALE). Available online: https://clinicaltrials.gov/study/NCT06271421 (accessed on 7 February 2025).
- Lee, D.; Sohn, J.; Kirichenko, A. Quantifying Liver Heterogeneity via R2*-MRI with Super-Paramagnetic Iron Oxide Nanoparticles (SPION) to Characterize Liver Function and Tumor. Cancers 2022, 14, 5269. [Google Scholar] [CrossRef] [PubMed]
- To Evaluate Dose and Safety of NanoEcho Particle-1 Using NanoEcho Imaging Device Examinations of Rectal Lymph Nodes in Healthy Volunteers and Rectal Cancer Patients. Available online: https://clinicaltrials.gov/study/NCT06693375 (accessed on 9 August 2025).
- Wang, Y.X. Superparamagnetic iron oxide based MRI contrast agents: Current status of clinical application. Quant. Imaging Med. Surg. 2011, 1, 35–40. [Google Scholar] [CrossRef]
- Shah, A.; Dobrovolskaia, M.A. Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: Therapeutic benefits, toxicity, mechanistic insights, and translational considerations. Nanomedicine 2018, 14, 977–990. [Google Scholar] [CrossRef]
- Rajan, A.; Laha, S.S.; Sahu, N.K.; Thorat, N.D.; Shankar, B. Recent advancements and clinical aspects of engineered iron oxide nanoplatforms for magnetic hyperthermia-induced cancer therapy. Mater. Today Bio. 2024, 29, 101348. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.; Devi, S.; Kaur, G.; Singh, S.K.; Mittal, N. Magnetic hyperthermia-based therapies for cancer targeting: Current progress and future perspectives. Med. Oncol. 2025, 42, 453. [Google Scholar] [CrossRef]
- Yan, X.; Li, S.; Yan, H.; Yu, C.; Liu, F. IONPs-Based Medical Imaging in Cancer Care: Moving Beyond Traditional Diagnosis and Therapeutic Assessment. Int. J. Nanomed. 2023, 18, 1741–1763. [Google Scholar] [CrossRef]
- Sato, K.; Ogawa, K.; Nabeshima, S.; Suwabe, S.; Ozeki, T. Fabrication and application of iron oxide-encapsulated PLGA nanoparticles with dual responsiveness to magnetic fields and light for nose-to-brain drug delivery. J. Drug Deliv. Sci. Technol. 2025, 114, 107535. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef] [PubMed]
- Cortajarena, A.L.; Ortega, D.; Ocampo, S.M.; Gonzalez-García, A.; Couleaud, P.; Miranda, R.; Belda-Iniesta, C.; Ayuso-Sacido, A. Engineering Iron Oxide Nanoparticles for Clinical Settings. Nanobiomedicine 2014, 1, 1–20. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron Oxide Nanoparticles Inhibit Tumour Growth by Inducing Pro-Inflammatory Macrophage Polari-zation in Tumour Tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Munawwar, A.; Sajjad, A.; Rasul, A.; Sattar, M.; Jabeen, F. Dissecting the Role of SMYD2 and Its Inhibitor (LLY-507) in the Treatment of Chemically Induced Non-Small Cell Lung Cancer (NSCLC) by Using Fe3O4 Nanoparticles Drug Delivery System. Pharmaceuticals 2023, 16, 986. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Liu, C.; Zhang, X.; Wang, W.; Li, B.; Liu, J.; Liu, Y.; Sun, H.; Zhang, D.; Hou, Y.; et al. Photothermal-Activatable Fe3O4 Superparticle Nanodrug Carriers with PD-L1 Immune Checkpoint Blockade for Anti-Metastatic Cancer Immuno-therapy. ACS Appl. Mater. Interfaces 2018, 10, 20342–20355. [Google Scholar] [CrossRef]
- Ge, R.; Li, X.; Lin, M.; Wang, D.; Li, S.; Liu, S.; Tang, Q.; Liu, Y.; Jiang, J.; Liu, L.; et al. Fe3O4@polydopamine Composite Theranostic Superparticles Employing Preassembled Fe3O4 Nanoparticles as the Core. ACS Appl. Mater. Interfaces 2016, 8, 22942–22952. [Google Scholar] [CrossRef]
- Khalkhali, M.; Sadighian, S.; Rostamizadeh, K.; Khoeini, F.; Naghibi, M.; Bayat, N.; Habibizadeh, M.; Hamidi, M. Synthesis and Characterization of Dextran Coated Magnetite Nanoparticles for Diagnostics and Therapy. Bioimpacts 2017, 5, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Han, Y.; An, Y.; Ding, Y.; He, C.; Wang, X.; Tang, Q. NRP-1 Targeted and Cargo-Loaded Exosomes Facilitate Simultaneous Imaging and Therapy of Glioma in Vitro and in Vivo. Biomaterials 2018, 178, 302–316. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, W.; Dong, L.; Hong, C.; Luo, Z.; Hu, Y.; Cai, K. Redox-Responsive Magnetic Nanovectors Self-Assembled from Amphiphilic Polymer and Iron Oxide Nanoparticles for a Remotely Targeted Delivery of Paclitaxel. J. Mater. Chem. B 2021, 9, 6037–6043. [Google Scholar] [CrossRef]
- Srivastava, N.; Chudasama, B.; Baranwal, M. In Vitro Assessment of Polyethylene Glycol-Coated Iron Oxide Nano-particles Integrating Luteinizing Hormone Releasing-Hormone Targeted Magnetic Hyperthermia and Doxorubicin for Lung and Breast Cancer Cells. Biointerphases 2025, 20, 031001. [Google Scholar] [CrossRef]
- Thomas, C.R.; Ferris, D.P.; Lee, J.-H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.-S.; Cheon, J.; Zink, J.I. Noninvasive Remote-Controlled Release of Drug Molecules in Vitro Using Magnetic Actuation of Mechanized Nanopar-ticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef]
- Ma, P.; Xiao, H.; Yu, C.; Liu, J.; Cheng, Z.; Song, H.; Zhang, X.; Li, C.; Wang, J.; Gu, Z.; et al. Enhanced Cisplatin Chemotherapy by Iron Oxide Nanocarrier-Mediated Generation of Highly Toxic Reactive Oxygen Species. Nano Lett. 2017, 17, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Bilbao-Asensio, M.; Ruiz-de-Angulo, A.; Arguinzoniz, A.G.; Cronin, J.; Llop, J.; Zabaleta, A.; Michue-Seijas, S.; Sos-nowska, D.; Arnold, J.N.; Mareque-Rivas, J.C. Redox-Triggered Nanomedicine via Lymphatic Delivery: Inhibition of Melanoma Growth by Ferroptosis Enhancement and a Pt(IV)-Prodrug Chemoimmunotherapy Approach. Adv. Ther. 2023, 6, 2200179. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Chen, G.; Liu, B.; Deng, X.; Wei, Y.; Xia, J.; Xing, B.; Ma, P.; Lin, J. Synthesis and Optimization of MoS2@Fe3O4-ICG/Pt(IV) Nanoflowers for MR/IR/PA Bioimaging and Combined PTT/PDT/Chemotherapy Triggered by 808 Nm Laser. Adv. Sci. 2017, 4, 1600540. [Google Scholar] [CrossRef]
- Sato, I.; Umemura, M.; Mitsudo, K.; Fukumura, H.; Kim, J.-H.; Hoshino, Y.; Nakashima, H.; Kioi, M.; Nakakaji, R.; Sato, M.; et al. Simultaneous Hyperthermia-Chemotherapy with Controlled Drug Delivery Using Single-Drug Nanoparticles. Sci. Rep. 2016, 6, 24629. [Google Scholar] [CrossRef]

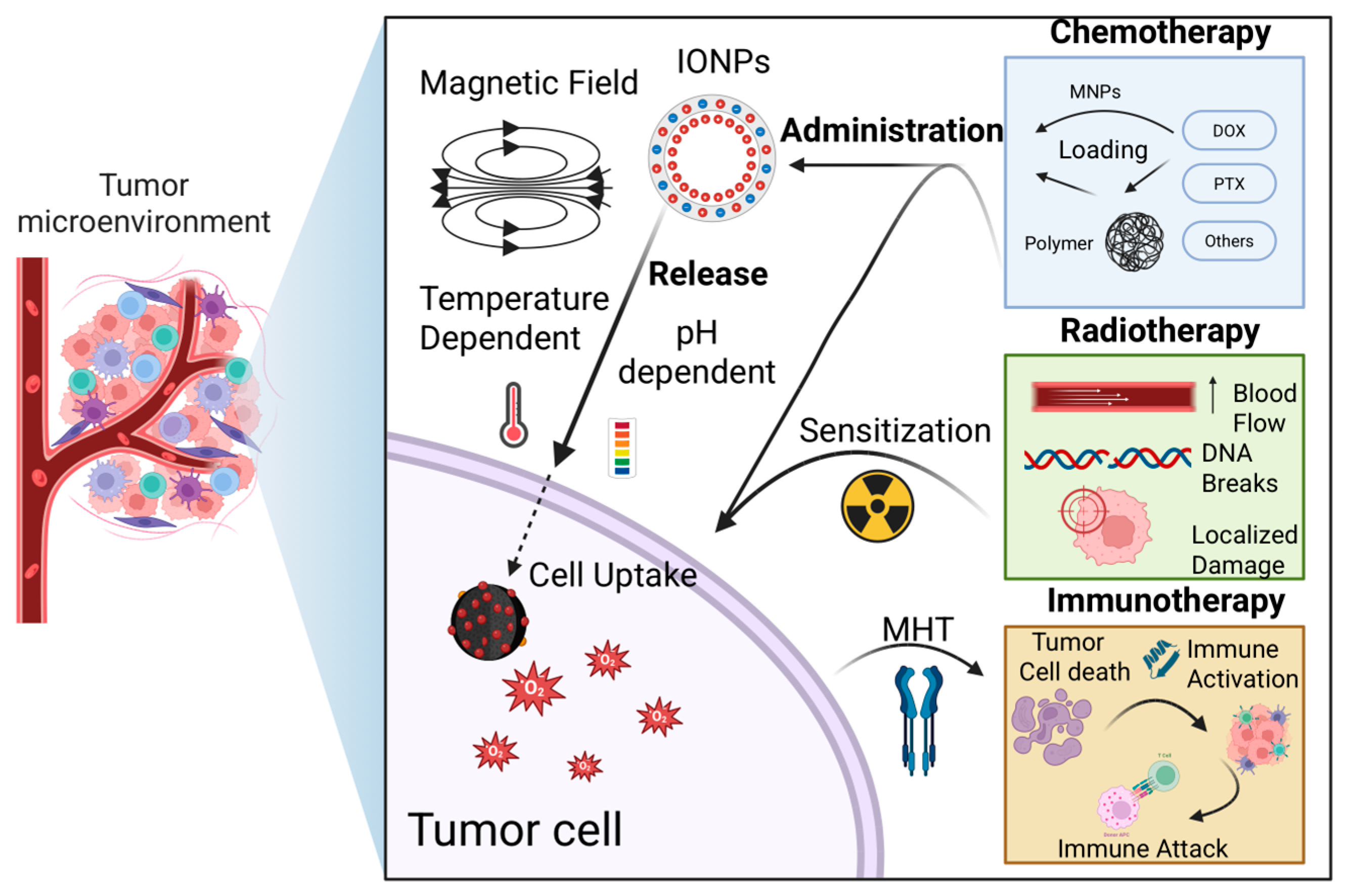
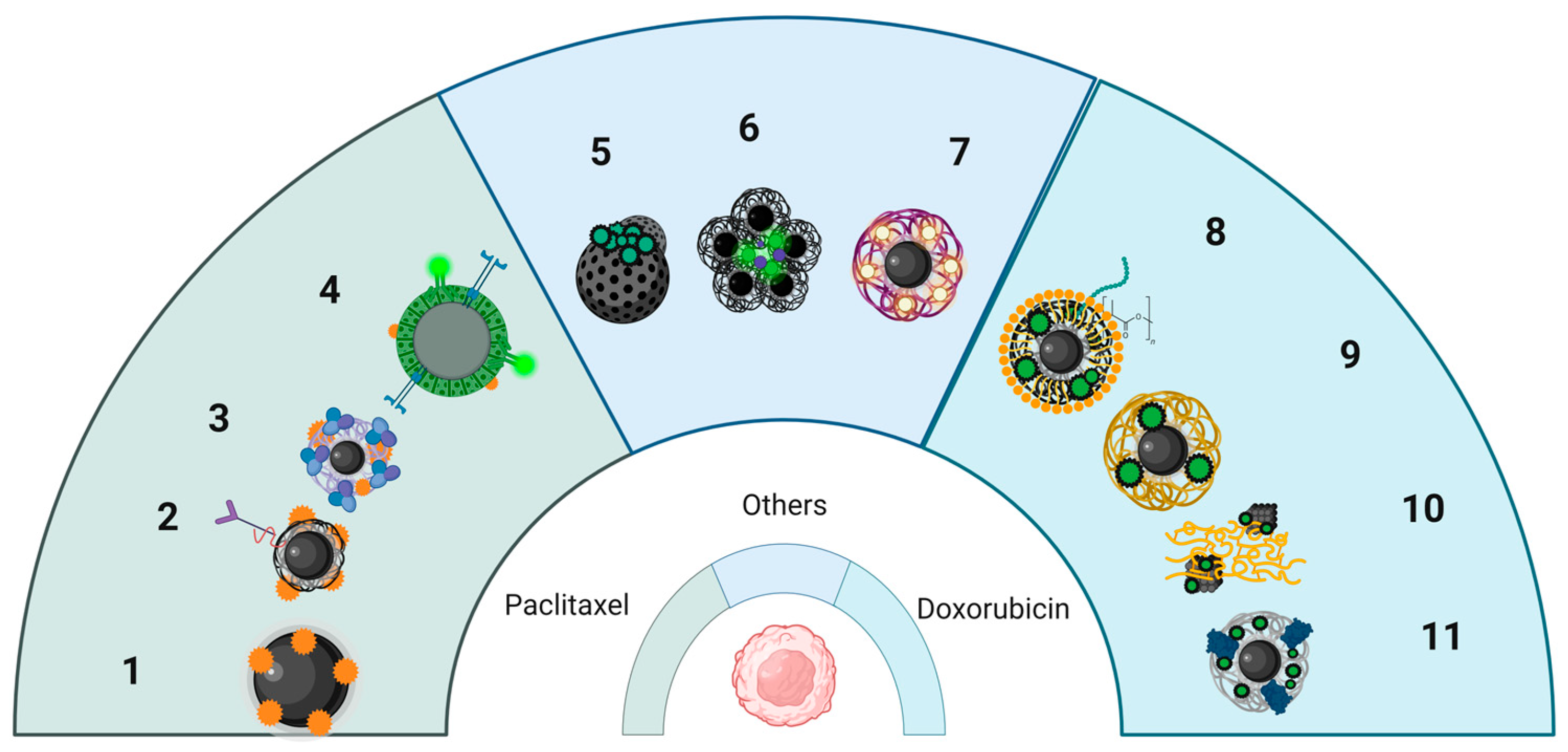
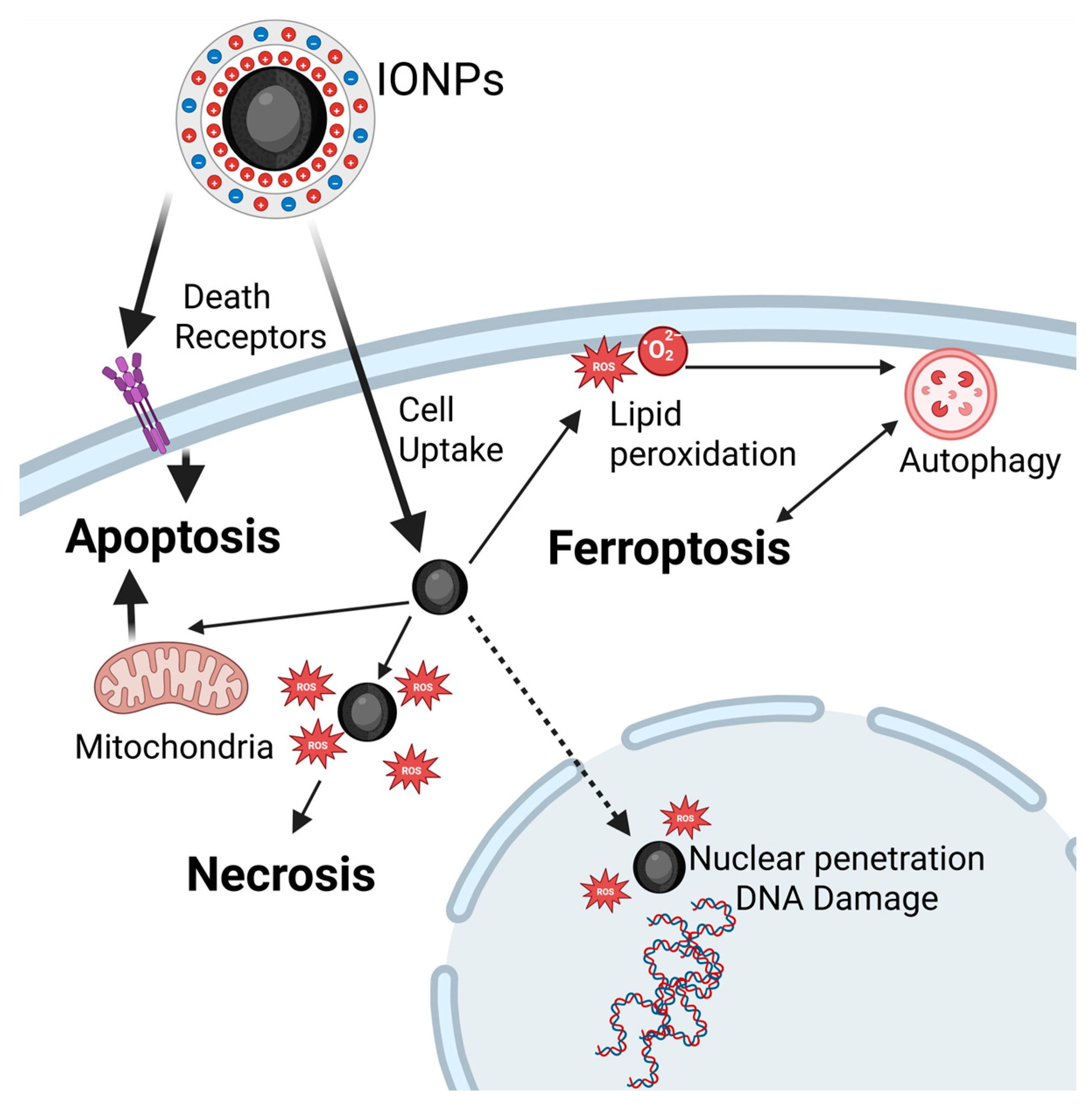
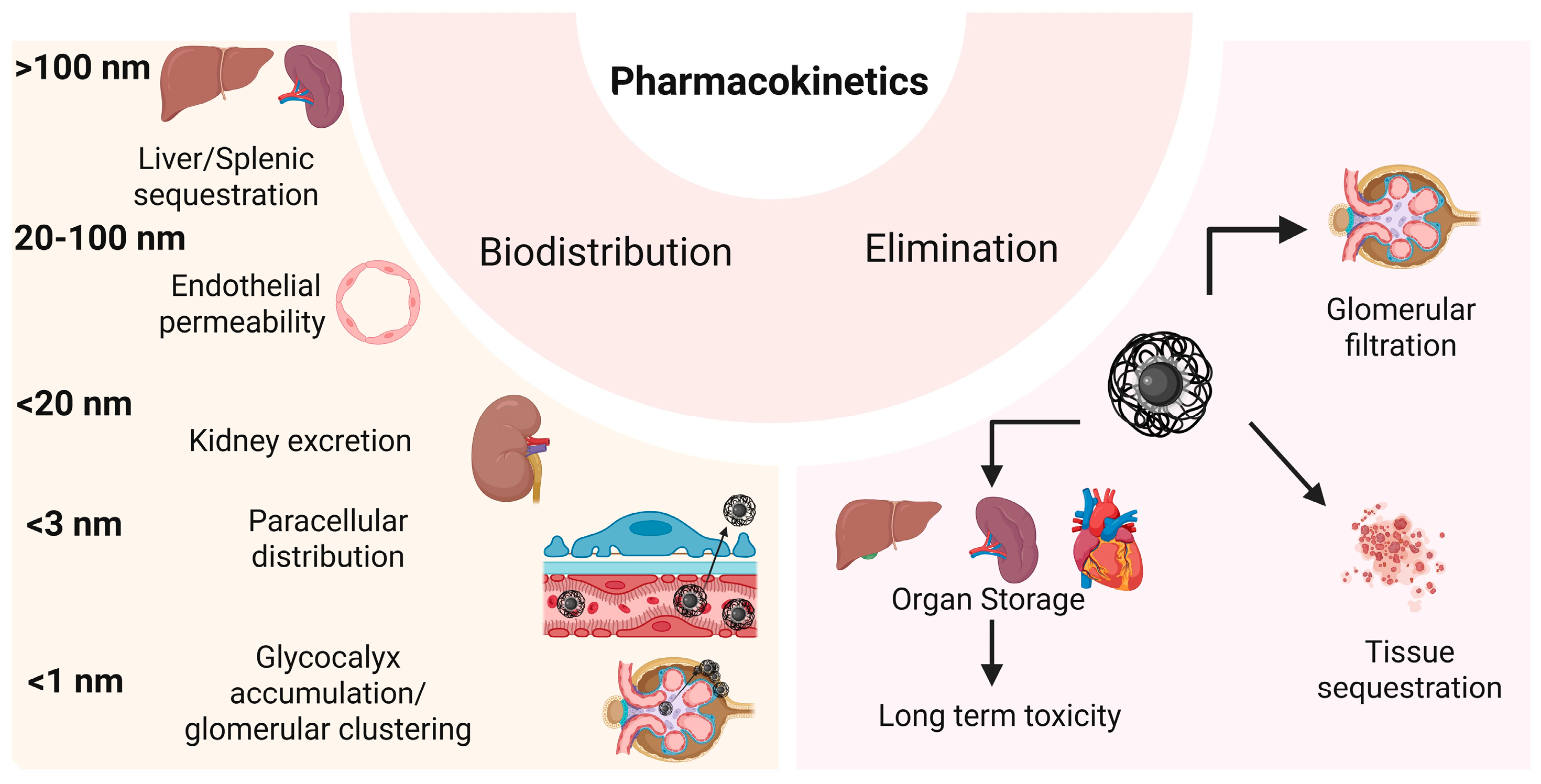
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldea, I.; Iacoviță, C.; Gurgu, R.A.; Vizitiu, A.S.; Râzniceanu, V.; Mitrea, D.R. Magnetic Hyperthermia with Iron Oxide Nanoparticles: From Toxicity Challenges to Cancer Applications. Nanomaterials 2025, 15, 1519. https://doi.org/10.3390/nano15191519
Baldea I, Iacoviță C, Gurgu RA, Vizitiu AS, Râzniceanu V, Mitrea DR. Magnetic Hyperthermia with Iron Oxide Nanoparticles: From Toxicity Challenges to Cancer Applications. Nanomaterials. 2025; 15(19):1519. https://doi.org/10.3390/nano15191519
Chicago/Turabian StyleBaldea, Ioana, Cristian Iacoviță, Raul Andrei Gurgu, Alin Stefan Vizitiu, Vlad Râzniceanu, and Daniela Rodica Mitrea. 2025. "Magnetic Hyperthermia with Iron Oxide Nanoparticles: From Toxicity Challenges to Cancer Applications" Nanomaterials 15, no. 19: 1519. https://doi.org/10.3390/nano15191519
APA StyleBaldea, I., Iacoviță, C., Gurgu, R. A., Vizitiu, A. S., Râzniceanu, V., & Mitrea, D. R. (2025). Magnetic Hyperthermia with Iron Oxide Nanoparticles: From Toxicity Challenges to Cancer Applications. Nanomaterials, 15(19), 1519. https://doi.org/10.3390/nano15191519







