Silicon Photocatalytic Water-Treatment: Synthesis, Modifications, and Machine Learning Insights
Abstract
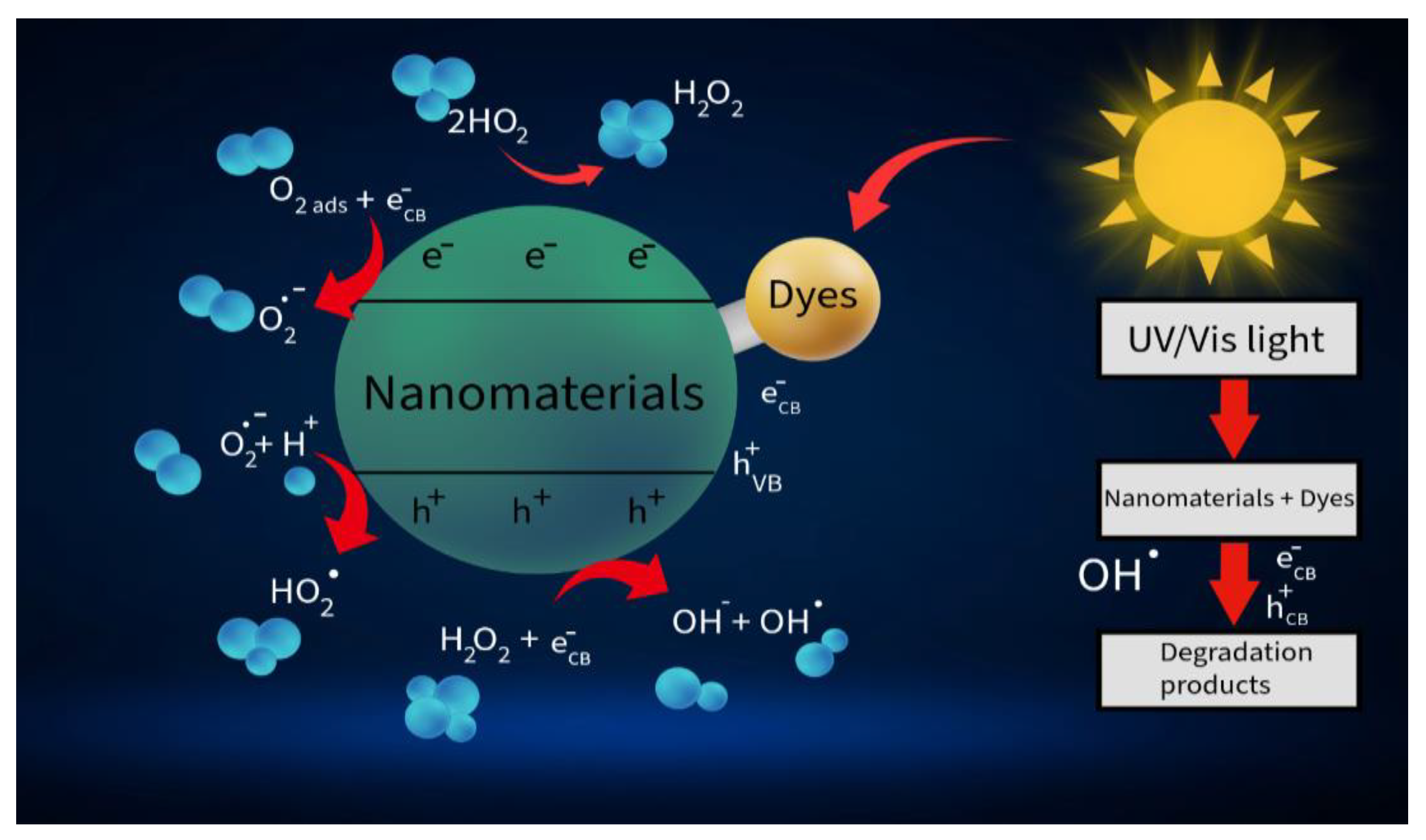
1. Introduction
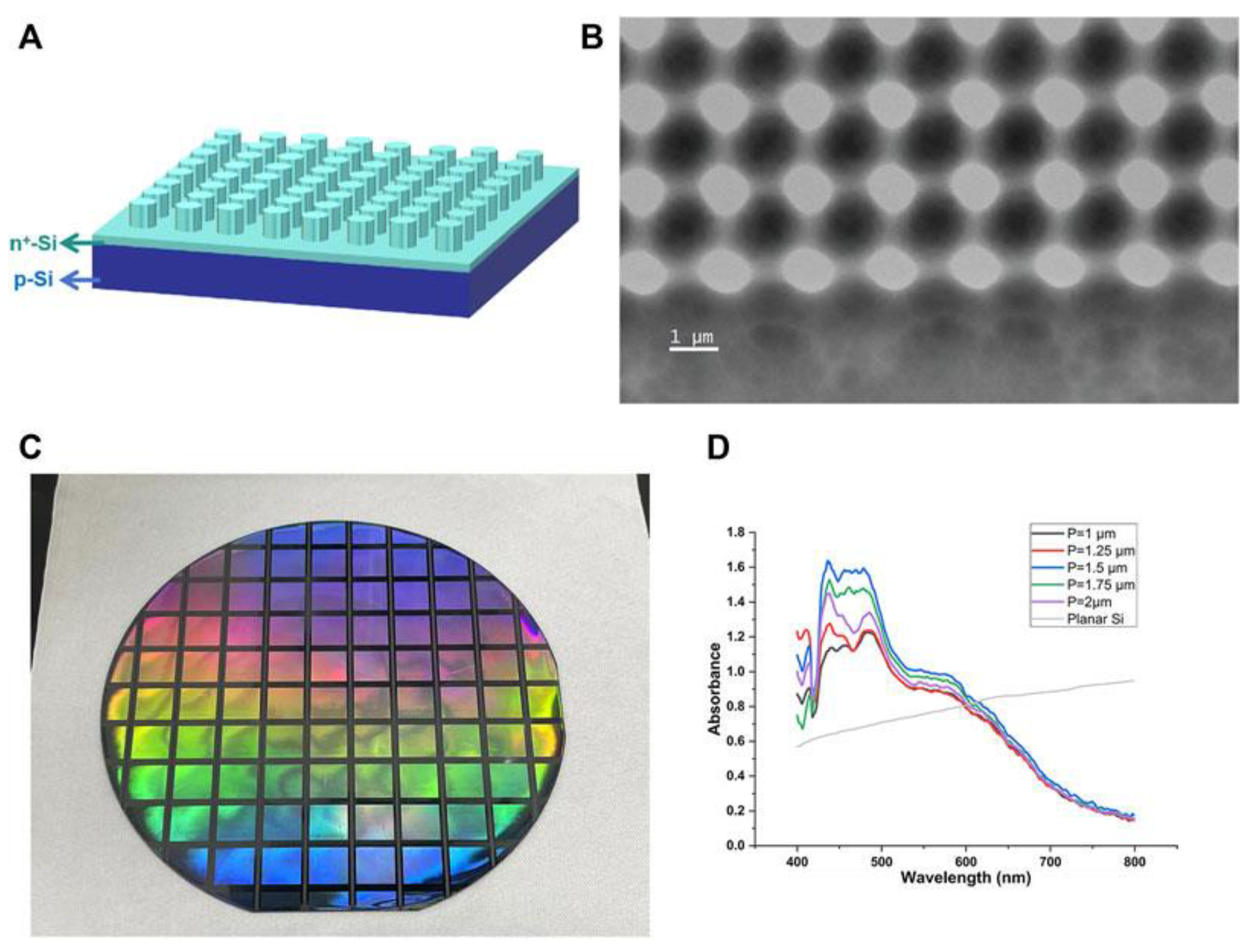
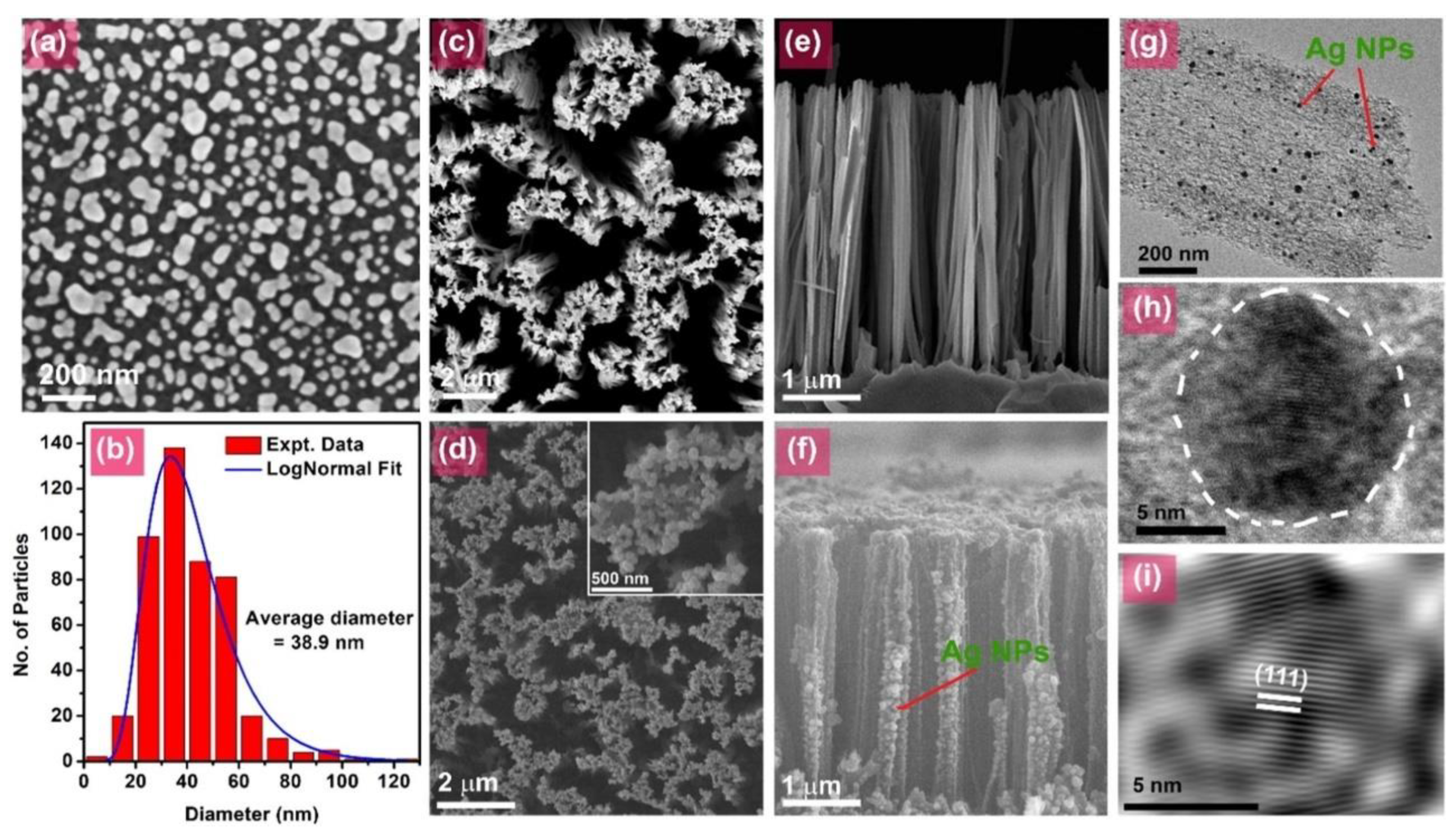
2. Synthesis and Modification of Si-Based Nanostructures
3. Protective Coatings, Nanostructures, and Specialized Applications of Silicon in Photocatalysis
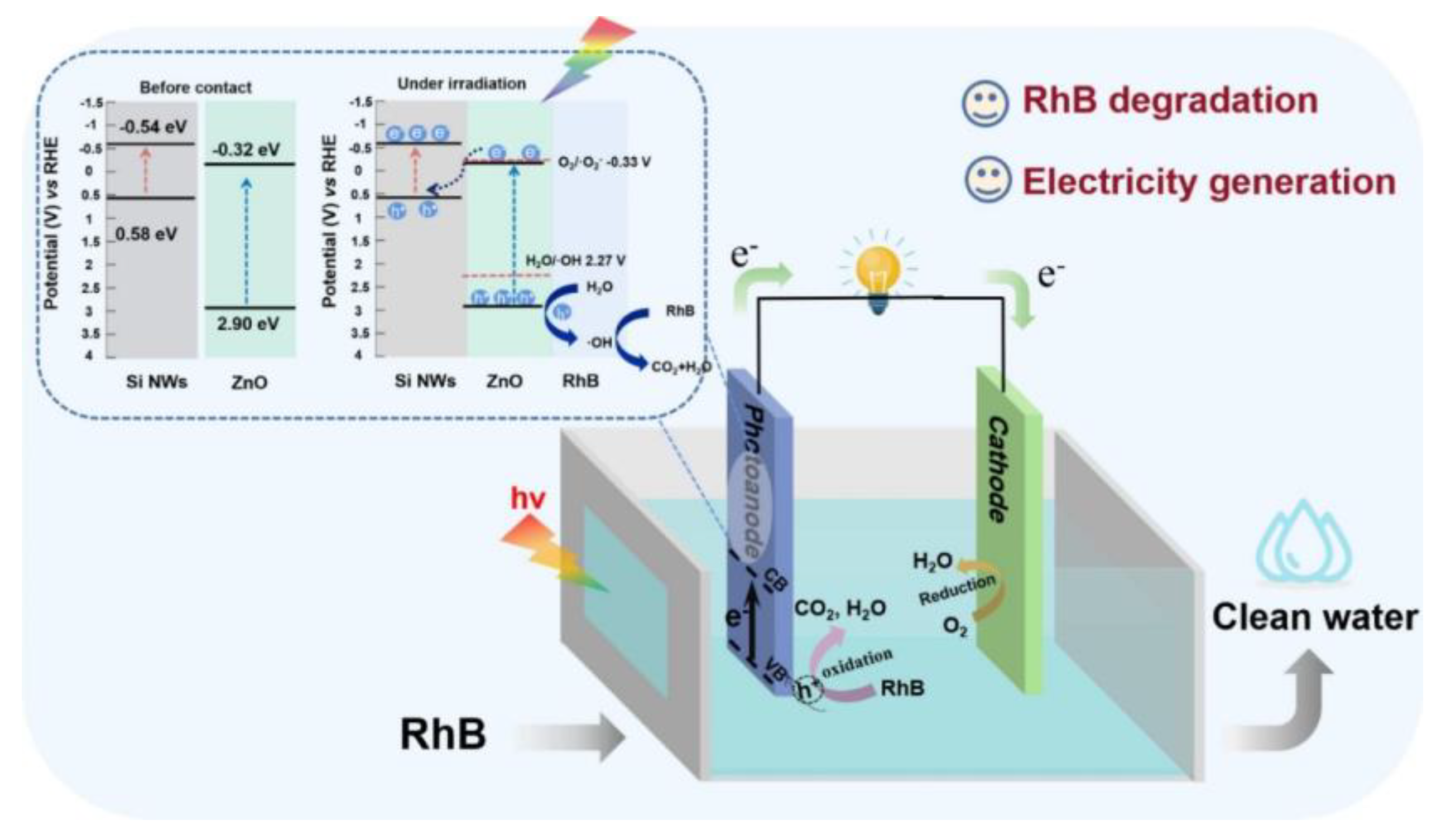
4. Silicon-Based Heterostructures for Photocatalysis
| Photocatalysts | Object of Decomposition | Photocatalytic Parameters | Light Source | Source |
|---|---|---|---|---|
| Si/TiO2 nanotubes (500 nm length, 80 nm diameter, 16 nm wall thickness) | Rhodamine B | ~1.78 times higher kinetic constants compared to TiO2 nanotubes | UV | [82] |
| Si substrates with Ag nanostructures | Methyl Orange | Rate constant 33.5 × 10−3 min−1 | sunlight | [83] |
| Si/SiC@C@TiO2 | Methylene Blue | Rate constants 4.5 (UV) and 7.9 (visible) times higher than TiO2 | UV, simulated solar | [84] |
| Sulfonated mesoporous silica/ZnO | Methylene Blue | 97.419% efficiency | simulated solar | [85] |
| SBA-16/TiO2 | Paraquat herbicide | Si/Ti ratios 5.6, 1.4, 0.7, complete decolorization under UV, 70% PQ reduction in 24 h by 1.4 ratio | UV | [86] |
| Fe3O4/PDA/Si-Ca-Mg (FPS) | Methylene Blue | Adsorption capacity 100.23 mg/g, recyclable adsorbent | sunlight | [87] |
| Cu-Si Nanoparticles | Methylene Green | Photocatalyst doses: 10 mg (46.4%), 20 mg (81.7%), 30 mg (95.7%) degradation efficiency | sunlight | [88] |
| TiO2/RH-SBA-15 | Methyl Orange | 30% TiO2 ratio, 50 ppm initial dye concentration, 200 mg catalyst, 63% higher efficiency than bare TiO2 | simulated solar | [89] |
| V(0.005)-NSiT, V(0.02)-NSiT | DMSO | 40% (V(0.005)) and 29% (V(0.02)) DMSO decomposition in 10 h under visible light | simulated solar | [90] |
- (1)
- SiNWs + hν→SiNWs (e−, h+)
- (2)
- ZnO + hν→ZnO (e−, h+)
- (3)
- SiNWs (e−, h+) + ZnO (e−, h+)→SiNWs (e−) + ZnO (h+)
- (4)
- H2O + h+→⋅OH + H+
- (5)
- RhB + h+→CO2 + H2O
- (6)
- O2 + 4e− + 4H+→2H2O
| Si Nanowires Description | Dye Degraded | Photocatalytic Parameters | Light Source | Source |
|---|---|---|---|---|
| Si nanowires combined with ZnFe2O4/Ag. | Methyl Orange | Degradation rate: 19% in 90 min. | UV | [104] |
| Si nanowires coated with TiO2 and MoS2 nanosheets. | Rhodamine B | Degradation efficiency ~ 90% within 180 min. | UV | [105] |
| Si nanowires mixed with TiO2 microparticles. | Remoazol Black 5 | Degradation efficiency 50.9% after 150 min of continuous 580 nm. | simulated solar | [95] |
| Si nanowires modified with Au, Pt, Pd nanoparticles | Methylene Blue | Pd-modified SiNWs: degradation rate of 97% after UV irradiation 200 min. | UV | [106] |
| Si nanowires (23–30 µm length) decorated with CoO, Cu, Ag nanoparticles. | Methyl Orange | Degradation: Si-NWs-Cu-NPs (88.9%), Si-NWs (85.3%), Si-NWs-CoONPs (49.3%). | simulated solar | [107] |
| Si nanowires decorated with Ni-doped ZnO | Methylene Blue | 97% degradation efficiency with 5% Ni-ZnO/SiNWs | UV | [108] |
| Si nanowires (2.5–13.5 µm) coated with TiO2 nanoparticles | Methylene Blue | 96% degradation efficiency with 3.5 µm nanowire length; stable after 190 days. | UV | [96] |
| Si nanowires (80–100 nm diameter) | Rose Bengal | ~96% degradation in ~90 min under light illumination. | sunlight | [109] |
| Si nanowires decorated with Bi nanoparticles | Methylene Blue | 44% degradation under UV and 89% under solar irradiation in 120 min. | UV, sunlight | [110] |
| Si nanowire arrays prepared by metal-assisted chemical etching with varying H2O2 concentrations. | Rhodamine B | 35% degradation after 5 h of irradiation for 20% H2O2 | simulated solar | [111] |
| Si nanowires (1–42 µm length) modified with graphene oxide. | Methylene Blue | 92% degradation at 10 min etching, 4:1:8 etchant ratio; 96% with H2O2; bare Si (16%), GO/bare Si (31%). | UV | [112] |
| Si nanowires coated with CeO2 nanoparticles | Rhodamine B | Quasi-total discoloration in 75 min; 67% for bare Si-NWs; involves e−, •OH, O2•−, h+ species. | sunlight | [113] |
5. Machine Learning in the Optimization of Photocatalysts
6. Silicon Doping to Enhance Photocatalytic Activity
7. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Li, X.; Zhou, C.; Xiong, W.; Zeng, G.; Huang, D.; Zhang, C.; Wang, W.; Song, B.; Tang, X.; et al. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review. Water Res. 2020, 184, 116200. [Google Scholar] [CrossRef]
- He, S.; Xie, D.; Wang, B.; Zhu, M.; Hu, S. Photocatalytic fuel cell based on integrated silicon nanowire arrays/zinc oxide heterojunction anode for simultaneous wastewater treatment and electricity production. J. Colloid Interface Sci. 2023, 650, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, M.; Gao, H.; Kong, L.; Fan, S.; Wang, L.; Shao, H.; Long, M.; Guo, X. Cyclic coupling of photocatalysis and adsorption for completely safe removal of N-nitrosamines in water. Water Res. 2022, 209, 117904. [Google Scholar] [CrossRef]
- Rani, E.; Talebi, P.; Cao, W.; Huttula, M.; Singh, H. Harnessing photo/electro-catalytic activity via nano-junctions in ternary nanocomposites for clean energy. Nanoscale 2020, 12, 23461–23479. [Google Scholar] [CrossRef]
- Li, Y.; Dong, H.; Li, L.; Tang, L.; Tian, R.; Li, R.; Chen, J.; Xie, Q.; Jin, Z.; Xiao, J.; et al. Recent advances in waste water treatment through transition metal sulfides-based advanced oxidation processes. Water Res. 2021, 192, 116850. [Google Scholar] [CrossRef]
- Claro, E.M.T.; Bidoia, E.D.; de Moraes, P.B. A high-performance doped photocatalysts for inactivation of total coliforms in superficial waters using different sources of radiation. J. Environ. Manag. 2016, 177, 264–270. [Google Scholar] [CrossRef]
- Pestana, C.J.; Santos, A.A.; Capelo-Neto, J.; Melo, V.M.; Reis, K.C.; Oliveira, S.; Rogers, R.; Pacheco, A.B.; Hui, J.; Skillen, N.C.; et al. Suppressing cyanobacterial dominance by UV-LED TiO2-photocatalysis in a drinking water reservoir: A mesocosm study. Water Res. 2022, 226, 119299. [Google Scholar] [CrossRef]
- Yang, D.; Youden, B.; Yu, N.; Carrier, A.J.; Jiang, R.; Servos, M.R.; Oakes, K.D.; Zhang, X. Surface-Enhanced Raman Spectroscopy for the Detection of Reactive Oxygen Species. ACS Nano 2025, 19, 2013–2028. [Google Scholar] [CrossRef] [PubMed]
- Jammaer, J.; Aprile, C.; Verbruggen, S.W.; Lenaerts, S.; Pescarmona, P.P.; Martens, J.A. A Non-Aqueous Synthesis of TiO2/SiO2 Composites in Supercritical CO2 for the Photodegradation of Pollutants. ChemSusChem 2011, 4, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Huang, A.; Su, H.; Tan, T. The activity of acrylic-silicon/nano-TiO2 films for the visible-light degradation of formaldehyde and NO2. Build. Environ. 2013, 65, 215–221. [Google Scholar] [CrossRef]
- Lanjwani, M.F.; Tuzen, M.; Khuhawar, M.Y.; Saleh, T.A. Trends in photocatalytic degradation of organic dye pollutants using nanoparticles: A review. Inorg. Chem. Commun. 2023, 159, 111613. [Google Scholar] [CrossRef]
- Jin, R.; Wu, Z.; Liu, Y.; Jiang, B.; Wang, H. Photocatalytic reduction of NO with NH3 using Si-doped TiO2 prepared by hydrothermal method. J. Hazard. Mater. 2009, 161, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hao, X.; Mu, L.; Shi, W.; She, G. Photoelectrocatalytic Utilization of CO2: A Big Show of Si-based Photoelectrodes. Chem. A Eur. J. 2024, 30, e202303552. [Google Scholar] [CrossRef]
- Tuan, N.; Thu, V.; Trung, D.; Tu, N.; Tran, M.; Duong, P.; Anh, T.; Hong, N.; Loan, P.; Tam, T.; et al. On the origin of photoluminescence enhancement of Si nanocrystals on silica glass template and Si/SiO2 superlattice. Phys. B Condens. Matter 2023, 662, 414970. [Google Scholar] [CrossRef]
- Putwa, S.; Curtis, I.S.; Dasog, M. Nanostructured silicon photocatalysts for solar-driven fuel production. iScience 2023, 26, 106317. [Google Scholar] [CrossRef]
- Wang, K.; He, T. Plasmon photocatalytic CO2 reduction reactions over Au particles on various substrates. Nanoscale 2023, 15, 12398–12405. [Google Scholar] [CrossRef]
- Mendoza-Diaz, M.-I.; Lecestre, A.; Salvagnac, L.; Bounor, B.; Pech, D.; Djafari-Rouhani, M.; Esteve, A.; Rossi, C. High surface area TiO2 photocatalyst for H2 production through silicon micromachining. Appl. Surf. Sci. 2022, 588, 152919. [Google Scholar] [CrossRef]
- Jeon, E.H.; Yang, S.; Kim, Y.; Kim, N.; Shin, H.-J.; Baik, J.; Kim, H.S.; Lee, H. Comparative study of photocatalytic activities of hydrothermally grown ZnO nanorod on Si(001) wafer and FTO glass substrates. Nanoscale Res. Lett. 2015, 10, 361. [Google Scholar] [CrossRef]
- Karpyna, V.; Myroniuk, L.; Bykov, O.; Myroniuk, D.; Kolomys, O.; Strelchuk, V.; Petrosian, L.; Ievtushenko, A. Structure, Optical and Photocatalytic Properties of ZnO Nanostructures Grown on Ag-Coated Si Substrates. Acta Phys. Pol. A 2022, 142, 644–650. [Google Scholar] [CrossRef]
- Kumari, S.; Raturi, S.; Kulshrestha, S.; Chauhan, K.; Dhingra, S.; András, K.; Thu, K.; Khargotra, R.; Singh, T. A comprehensive review on various techniques used for synthesizing nanoparticles. J. Mater. Res. Technol. 2023, 27, 1739–1763. [Google Scholar] [CrossRef]
- Allad, L.; Kaci, S.; Benfadel, K.; Allam, D.; Ouerk, A.; Boukezzata, A.; Torki, C.; Anas, S.; Talbi, L.; Ouadah, Y.; et al. Optical and Photoelectrocatalytic Properties of PbS Loaded Si Based Photocathode. Silicon 2022, 14, 7991–8000. [Google Scholar] [CrossRef]
- Lee, M.; Kim, T.; Jang, W.; Lee, S.; So, J.-P.; Jang, G.; Choi, S.; Kim, S.; Bae, J.; Kim, T.; et al. Nontypical Wulff-Shape Silicon Nanosheets with High Catalytic Activity. J. Am. Chem. Soc. 2023, 145, 22620–22632. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Giri, P.K. Efficient visible light photocatalysis and tunable photoluminescence from orientation controlled mesoporous Si nanowires. RSC Adv. 2016, 6, 35365–35377. [Google Scholar] [CrossRef]
- Liu, D.; Ma, J.; Long, R.; Gao, C.; Xiong, Y. Silicon nanostructures for solar-driven catalytic applications. Nano Today 2017, 17, 96–116. [Google Scholar] [CrossRef]
- Barrera, C.; Ajay, P.; Mallavarapu, A.; Hrdy, M.; Sreenivasan, S.V. Metal Assisted Chemical Etch of Polycrystalline Silicon. J. Micro Nano-Manuf. 2022, 10, 021002. [Google Scholar] [CrossRef]
- Xi, Y.Y.; Hsu, Y.F.; Chan, W.K. Hydrothermal Synthesis of Nanostructures. Recent Patents Nanotechnol. 2007, 1, 121–128. [Google Scholar] [CrossRef]
- Ghosh, R.; Giri, P.K. Silicon nanowire heterostructures for advanced energy and environmental applications: A review. Nanotechnology 2016, 28, 012001. [Google Scholar] [CrossRef]
- Barghout, N.; Kashyout, A.E.-H.B.; Ibrahim, M.A.M.; El Nemr, A. Novel Synthesis of SiC-SiO2 Nanotubes from Cinachyrella sp. and Its Improvement of the Corrosion Resistance of Low Carbon Steel in 3.5% NaCl Water Solution. J. Mater. Eng. Perform. 2023, 32, 10857–10876. [Google Scholar] [CrossRef]
- Wan, H.; Ma, W.; Zhou, K.; Cao, Y.; Liu, X.; Ma, R. Advanced silicon nanostructures derived from natural silicate minerals for energy storage and conversion. Green Energy Environ. 2022, 7, 205–220. [Google Scholar] [CrossRef]
- Dong, Z.; Cai, Y.; Zhang, K.; Chu, Z.; Han, S.; Li, Z. Electrochemical reduction induced self-doping of oxygen vacancies into Ti–Si–O nanotubes as efficient photoanode for boosted photoelectrochemical water splitting. Int. J. Hydrogen Energy 2021, 46, 3554–3564. [Google Scholar] [CrossRef]
- Lee, S.-C.; Hur, J.-M.; Seo, C.-S. Silicon powder production by electrochemical reduction of SiO2 in molten LiCl–Li2O. J. Ind. Eng. Chem. 2008, 14, 651–654. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, R.; Yu, M.; Tian, Y.; Lin, Y.; Pan, F. Controllable Growth of Silver Crystallites on the Rear Ag–Si Contact Interface of TOPCon Solar Cells Through an Electrochemical Reduction Reaction Triggered by Laser-Enhanced Contact Optimization. Sol. RRL 2025, 9, 2500233. [Google Scholar] [CrossRef]
- Ohshita, J.; Hino, K.; Iwawaki, T.; Kunai, A. Electrochemical reduction of alkoxychlorosilanes for Si–Si bond formation. J. Electroanal. Chem. 2009, 625, 138–143. [Google Scholar] [CrossRef]
- Coy, E.; Siuzdak, K.; Pavlenko, M.; Załęski, K.; Graniel, O.; Ziółek, M.; Balme, S.; Miele, P.; Weber, M.; Bechelany, M.; et al. Enhancing photocatalytic performance and solar absorption by schottky nanodiodes heterojunctions in mechanically resilient palladium coated TiO2/Si nanopillars by atomic layer deposition. Chem. Eng. J. 2020, 392, 123702. [Google Scholar] [CrossRef]
- Blagoev, B.S.; Georgieva, B.; Starbova, K.; Starbov, N.; Avramova, I.; Buchkov, K.; Tzvetkov, P.; Stoykov, R.; Terziyska, P.; Delibaltov, D.; et al. A Novel Approach to Obtaining Metal Oxide HAR Nanostructures by Electrospinning and ALD. Materials 2023, 16, 7489. [Google Scholar] [CrossRef] [PubMed]
- Gudovskikh, A.; Uvarov, A.; Morozov, I.; Baranov, A.; Kudryashov, D.; Nikitina, E.; Kleider, J. n-GaP/p-Si Heterojunction Solar Cells Fabricated by PE-ALD. In Physica Status Solidi C: Current Topics in Solid State Physics; Wiley: Weinheim, Germany, 2017; Volume 14. [Google Scholar]
- Maina, J.W.; Merenda, A.; Weber, M.; Pringle, J.M.; Bechelany, M.; Hyde, L.; Dumée, L.F. Atomic layer deposition of transition metal films and nanostructures for electronic and catalytic applications. Crit. Rev. Solid State Mater. Sci. 2020, 46, 468–489. [Google Scholar] [CrossRef]
- Ziegler, M.; Yüksel, S.; Goerke, S.; Weber, K.; Cialla-May, D.; Popp, J.; Pollok, K.; Wang, D.; Langenhorst, F.; Hübner, U.; et al. Growth of Hierarchically 3D Silver–Silica Hybrid Nanostructures by Metastable State Assisted Atomic Layer Deposition (MS-ALD). Adv. Mater. Technol. 2017, 2, 1700015. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, R.; Oh, J.; Yoo, B.; Lee, H. Novel synthesis of dual-suspended architectures between Si-pillars for enhanced photocatalytic performance. RSC Adv. 2017, 7, 2880–2883. [Google Scholar] [CrossRef]
- Liu, J.; Bai, Y.; Chen, P.; Cui, N.; Yin, H. Reaction synthesis of TiSi2 and Ti5Si3 by ball-milling and shock loading and their photocatalytic activities. J. Alloys Compd. 2013, 555, 375–380. [Google Scholar] [CrossRef]
- Abdallah, B.; Kakhia, M.; Zetoun, W. HRTEM study of ZnS Nanowires Films Deposited by Thermal Evaporation. J. Nanostruct. 2020, 10, 713–722. [Google Scholar] [CrossRef]
- Deng, T.; Li, M.; Wang, Y.; Liu, Z. Development of solid-state nanopore fabrication technologies. Sci. Bull. 2015, 60, 304–319. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Curtis, I.S.; Wills, R.J.; Dasog, M. Photocatalytic hydrogen generation using mesoporous silicon nanoparticles: Influence of magnesiothermic reduction conditions and nanoparticle aging on the catalytic activity. Nanoscale 2021, 13, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Wang, Y.; Wang, J.; Zhang, J.; Yu, C.; Qin, Y.; Cui, J.; Zhang, Y.; Tiwary, C.S.; Wu, Y. Accelerated hydrogen production on atomically thin silicon nanosheets photocatalyst with unique surface adsorption chemistry. Int. J. Hydrogen Energy 2023, 51, 929–935. [Google Scholar] [CrossRef]
- Feng, Y.; Zhao, S.; Liang, P.; Xia, Z.; Peng, H. Application of Silicon Nanowires. Curr. Nanosci. 2025, 21, 373–384. [Google Scholar] [CrossRef]
- Lv, P.; Xu, C.; Peng, B. Design of a Silicon Photocatalyst for High-Efficiency Photocatalytic Water Splitting. ACS Omega 2020, 5, 6358–6365. [Google Scholar] [CrossRef]
- Brik, A.; Naama, S.; Hadjersi, T.; Benamar, M.E.A.; Bouanik, S.; Manseri, A. Photodegradation of methylene blue under UV and visible light irradiation by Er2O3-coated silicon nanowires as photocatalyst. React. Kinet. Catal. Lett. 2020, 131, 525–536. [Google Scholar] [CrossRef]
- Islam, A.; Teo, S.H.; Awual, R.; Taufiq-Yap, Y.H. Assessment of clean H2 energy production from water using novel silicon photocatalyst. J. Clean. Prod. 2020, 244, 118805. [Google Scholar] [CrossRef]
- Tong, W.; Yin, Q.; Fang, D.; Zeng, T.; Yi, J. Silicon Quantum Dots: Chemical, Physical Synthesis, and Applications in Fluorescence Detection, Solar Cell, Photocatalyst, and Composite. Curr. Nanosci. 2022, 18, 182–202. [Google Scholar] [CrossRef]
- Qu, Y.; Xue, T.; Zhong, X.; Lin, Y.; Liao, L.; Choi, J.; Duan, X. Heterointegration of Pt/Si/Ag Nanowire Photodiodes and Their Photocatalytic Properties. Adv. Funct. Mater. 2010, 20, 3005–3011. [Google Scholar] [CrossRef]
- Cheng, C.; Zhang, W.; Chen, X.; Peng, S.; Li, Y. Strategies for improving photoelectrochemical water splitting performance of Si-based electrodes. Energy Sci. Eng. 2022, 10, 1526–1543. [Google Scholar] [CrossRef]
- Amdouni, S.; Cherifi, Y.; Coffinier, Y.; Addad, A.; Zaïbi, M.A.; Oueslati, M.; Boukherroub, R. Gold nanoparticles coated silicon nanowires for efficient catalytic and photocatalytic applications. Mater. Sci. Semicond. Process. 2018, 75, 206–213. [Google Scholar] [CrossRef]
- Kamel, L.; Anbia, M. Preparation and evaluation of nanoporous-pyramids structured silicon powder as an effective photocatalyst for degradation of methyl red. Int. J. Environ. Sci. Technol. 2017, 16, 2101–2108. [Google Scholar] [CrossRef]
- Chen, X.; Lee, C.-S.; Meng, X.; Zhang, W. Diameter- and Shape-Controlled ZnS/Si Nanocables and Si Nanotubes for SERS and Photocatalytic Applications. J. Nanomater. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, P.; Wang, C.; Zhang, X.; Zhang, C. Silicon Nanowires/Reduced Graphene Oxide Composites for Enhanced Photoelectrochemical Properties. ACS Appl. Mater. Interfaces 2013, 5, 1961–1966. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, G.; Papandrea, B.; Li, M.; Xu, Y.; Chen, Y.; Chen, C.-Y.; Zhou, H.; Xue, T.; Li, Y.; et al. Reduced graphene oxide/silicon nanowire heterostructures with enhanced photoactivity and superior photoelectrochemical stability. Nano Res. 2015, 8, 2850–2858. [Google Scholar] [CrossRef]
- Han, T.; Privitera, S.; Milazzo, R.; Bongiorno, C.; Di Franco, S.; La Via, F.; Song, X.; Shi, Y.; Lanza, M.; Lombardo, S. Photo-electrochemical water splitting in silicon based photocathodes enhanced by plasmonic/catalytic nanostructures. Mater. Sci. Eng. B 2017, 225, 128–133. [Google Scholar] [CrossRef]
- Chen, Y.; Teng, C.; Li, F.; Tang, Z.; Xu, L.; Zhang, L.; Huang, Q.; Zhu, J.; Yuan, W. Mechanochemical synthesis of self-circulating Z-scheme AgCl/SiC photocatalyst from retired PV silicon: pH stability, reusability and broad applicability. Appl. Surf. Sci. 2024, 672, 160733. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Zeng, H.; Zhong, L.; Liu, K.; Cao, H.; Li, W.; Yan, H. Silicon carbide recovered from photovoltaic industry waste as photocatalysts for hydrogen production. J. Hazard. Mater. 2017, 329, 22–29. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.; Zeng, X.; Yuan, W. Mechanism of Water Splitting to Hydrogen by Silicon Carbide Nanoparticles. Sci. Adv. Mater. 2013, 5, 155–159. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, G.; Guo, P.; Ye, C.; Chen, Z.; Ma, Z.; Zhang, M.; Li, J. Enhancing photoelectrochemical CO2 reduction with silicon photonic crystals. Front. Chem. 2023, 11, 1326349. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-L.; Yuan, J.-H.; Song, Y.-Q.; Wang, S.; Xue, K.-H.; Xu, M.; Cheng, X.-M.; Miao, X.-S. Two-dimensional silicon chalcogenides with high carrier mobility for photocatalytic water splitting. J. Mater. Sci. 2019, 54, 11485–11496. [Google Scholar] [CrossRef]
- Mahmood, I.; Ahmad, I.; Zhao, T.-K. Photodegradation of Melamine Using Magnetic Silicon Quantum Dots. Mater. Sci. 2021, 27, 127–132. [Google Scholar] [CrossRef]
- Peng, K.-Q.; Wang, X.; Li, L.; Hu, Y.; Lee, S.-T. Silicon nanowires for advanced energy conversion and storage. Nano Today 2013, 8, 75–97. [Google Scholar] [CrossRef]
- Jia, L.; Wang, C.; Liu, H.; Wu, K.; Chen, R. Fabrication and visible-light photocatalytic activity of Si-α-Fe2O3/In2S3 composites. J. Solid State Chem. 2022, 314, 123410. [Google Scholar] [CrossRef]
- Ghosh, R.; Ghosh, J.; Das, R.; Mawlong, L.P.; Paul, K.K.; Giri, P. Multifunctional Ag nanoparticle decorated Si nanowires for sensing, photocatalysis and light emission applications. J. Colloid Interface Sci. 2018, 532, 464–473. [Google Scholar] [CrossRef]
- Xia, Z.; Zhou, X.; Li, J.; Qu, Y. Protection strategy for improved catalytic stability of silicon photoanodes for water oxidation. Sci. Bull. 2015, 60, 1395–1402. [Google Scholar] [CrossRef]
- Su, R.; Zhu, Y.; Gao, B.; Li, Q. Progress on mechanism and efficacy of heterogeneous photocatalysis coupled oxidant activation as an advanced oxidation process for water decontamination. Water Res. 2024, 251, 121119. [Google Scholar] [CrossRef]
- Li, D.; Yuranova, T.; Albers, P.; Kiwi, J. Accelerated photobleaching of Orange II on novel (H5FeW12O4010H2O)/silica structured fabrics. Water Research. 2004, 38, 3541–3550. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.; Kanojia, R.; Attri, P. Transition Metal Oxides and Their Composites for Photocatalytic Dye Degradation. J. Compos. Sci. 2021, 5, 82. [Google Scholar] [CrossRef]
- Sun, E.; Bai, X.; Chang, Y.; Li, Q.; Hui, X.; Li, Y.; Wang, Y. Preparation of PMMA Electrospun Fibers Bearing Porphyrin Pendants and Photocatalytic Degradation of Organic Dyes. Molecules 2022, 27, 8132. [Google Scholar] [CrossRef]
- Sun, N.; Si, X.; He, L.; Zhang, J.; Sun, Y. Strategies for enhancing the photocatalytic activity of semiconductors. Int. J. Hydrogen Energy 2024, 58, 1249–1265. [Google Scholar] [CrossRef]
- Atyaoui, M.; Ezzaouia, H. Hybrid system of polypyrrole conducting polymer and silicon nanowires for application on azoic dye photodegradation. J. Solid State Electrochem. 2025, 29, 3787–3796. [Google Scholar] [CrossRef]
- Dvorsky, R.; Svoboda, L.; Šollová, K.; Trojková, J.; Bednář, J.; Kostura, B.; Matýsek, D.; Pomiklová, M. Synthesis of Core-shell Nanoparticles Si-ZnS by Reactive Deposition of Photocatalytic ZnS Layer on the Surface of Carrier Si Nanoparticles in Aerosol Microdrops. Procedia Soc. Behav. Sci. 2015, 195, 2122–2129. [Google Scholar] [CrossRef]
- Zhu, W.; Han, D.; Niu, L.; Wu, T.; Guan, H. Z-scheme Si/MgTiO3 porous heterostructures: Noble metal and sacrificial agent free photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2016, 41, 14713–14720. [Google Scholar] [CrossRef]
- Yu, X.; Jiajun, L.; Xianglin, Y.; Feng, Z.; Yongjun, L.; Junbo, L. Preparation of graphdiyne-doped TiO2/SiO2 composite for enhanced photocatalytic activity. J. Nanoparticle Res. 2020, 22, 1–10. [Google Scholar] [CrossRef]
- Yuan, W.; Chen, Y.; Tang, Z.; Teng, C.; Zhang, L.; Huang, Q. Direct recycle waste silicon wafer as the charge transfer bridge to assemble a Z-scheme heterojunction for enhanced photocatalytic performance. J. Photochem. Photobiol. A Chem. 2024, 452, 115582. [Google Scholar] [CrossRef]
- Huang, Q.; Yuan, W.; Ke, Q.; Guo, Y.; Wang, L.; Wang, C. A TiO2/Si carrier derived from photovoltaic solid waste to assemble Ag3PO4/Co3(PO4)2/TiO2/Si heterostructure for enhancing visible-light photocatalytic activity. J. Environ. Chem. Eng. 2023, 11, 109696. [Google Scholar] [CrossRef]
- Deng, R.; Dias, P.R.; Lunardi, M.M.; Ji, J. A sustainable chemical process to recycle end-of-life silicon solar cells. Green Chem. 2021, 23, 10157–10167. [Google Scholar] [CrossRef]
- Wolf, A.T.; Stammer, A. Chemical Recycling of Silicones—Current State of Play (Building and Construction Focus). Polymers 2024, 16, 2220. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Quan, B.; Ji, G.; Zhang, H. Preparation of Si/TiO2 Heterojunction Nanotube Arrays via Electrodeposition and Their Enhanced Photocatalytic Activity. Nanosci. Nanotechnol. Lett. 2015, 7, 840–845. [Google Scholar] [CrossRef]
- Sakir, M.; Onses, M.S. Solid substrates decorated with Ag nanostructures for the catalytic degradation of methyl orange. Results Phys. 2019, 12, 1133–1141. [Google Scholar] [CrossRef]
- Liu, M.-P.; Su, T.; Sun, L.; Du, H.-B. Facile preparation of yolk–shell structured Si/SiC@C@TiO2 nanocomposites as highly efficient photocatalysts for degrading organic dye in wastewater. RSC Adv. 2015, 6, 4063–4069. [Google Scholar] [CrossRef]
- Ulfa, M.; Aziza, H.; Sholeha, N.A. In-situ sulfonated mesoporous silica as ZnO nanomaterial support for enhanced dyes photodegradation. Results Eng. 2025, 26, 104381. [Google Scholar] [CrossRef]
- Vanichvattanadecha, C.; Jaroenworaluck, A.; Henpraserttae, P.; Wimuktiwan, P.; Manpetch, P.; Singhapong, W. Ordered mesoporous silica (SBA-16) supporting titania (TiO2) nanoparticles for photodegradation of paraquat (PQ) herbicide. J. Porous Mater. 2021, 28, 1137–1153. [Google Scholar] [CrossRef]
- Chen, P.; Cao, Z.-F.; Wang, S.; Zhong, H. In situ nano-silicate functionalized magnetic composites by (poly)dopamine to improve MB removal. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 552, 89–97. [Google Scholar] [CrossRef]
- Fazal, Y.; Ali, I.; Chen, J.; Pan, Y.; Bukhari, S.N.U.S.; Liu, X.; Shah, A.A.; Shen, Z. Photothermal Hyperthermia and Photodegradation Studies of Cu-Si Nanoparticles Synthesized by Laser Ablation in Liquid Media. Nano 2024, 2450089. [Google Scholar] [CrossRef]
- Liou, T.-H.; Wang, S.-Y. Utilizing rice husk for sustainable production of mesoporous titania nanocomposites with highly adsorption and photocatalysis. Biomass Bioenergy 2025, 199, 107950. [Google Scholar] [CrossRef]
- Mase, A.; Sugita, T.; Mori, M.; Iwamoto, S.; Tokutome, T.; Katayama, K.; Itabashi, H. Study of vanadium-modified N/Si co-doped TiO2 in aqueous solution and its photocatalytic activity. Chem. Eng. J. 2013, 225, 440–446. [Google Scholar] [CrossRef]
- Zhang, Y.; Ratchford, D.C.; Anthony, R.J.; Yeom, J. ZnO nanowire and silicon nanocrystal heterostructures for photocatalytic applications. In Proceedings of the 2017 IEEE 17th International Conference on Nanotechnology (IEEE-NANO), Pittsburgh, PA, USA, 25–28 July 2017; pp. 873–876. [Google Scholar]
- Zhang, Y.; Mandal, R.; Ratchford, D.C.; Anthony, R.; Yeom, J. Si Nanocrystals/ZnO Nanowires Hybrid Structures as Immobilized Photocatalysts for Photodegradation. Nanomaterials 2020, 10, 491. [Google Scholar] [CrossRef]
- Hoa, N.T.; Van Cuong, V.; Lam, N.D. Mechanism of the photocatalytic activity of p-Si(100)/n-ZnO nanorods heterojunction. Mater. Chem. Phys. 2018, 204, 397–402. [Google Scholar] [CrossRef]
- Hsiao, P.-H.; Li, T.-C.; Chen, C.-Y. ZnO/Cu2O/Si Nanowire Arrays as Ternary Heterostructure-Based Photocatalysts with Enhanced Photodegradation Performances. Nanoscale Res. Lett. 2019, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Chen, A.-H. Photocatalytic Si nanowires/TiO2 microparticles with extended absorption edge up to 700 nm. J. Phys. D Appl. Phys. 2012, 45, 365304. [Google Scholar] [CrossRef]
- Ramachandran, K.; Ziad, R.; Columbus, S.; Daoudi, K.; Hammouche, J.; El Khakani, M.A.; Chidambaram, S.; Gaidi, M. TiO2/Si nanowires hybrid system for efficient photocatalytic degradation of organic dye. J. Mater. Sci. Mater. Electron. 2021, 33, 9194–9203. [Google Scholar] [CrossRef]
- Yao, X.; Chen, L.; Liu, M.; Feng, D.; Wang, C.; Lu, F.; Wang, W.; Wang, X.; Cheng, Y.; Liu, H.; et al. Rational design of Si/TiO2 heterojunction photocatalysts: Transfer matrix method. Appl. Catal. B Environ. 2018, 221, 70–76. [Google Scholar] [CrossRef]
- Hong, T.; Hui-Xiang, W.; Wei-Mei, S.; Yao, X. Microwave-Assisted Solvothermal Synthesis of In-Si Co-Modified TiO2 Photocatalysts with Enhanced Photocatalytic Activity. Acta Phys. Chim. Sin. 2014, 30, 1543–1549. [Google Scholar] [CrossRef]
- Bui, D.-N.; Kang, S.-Z.; Li, X.; Mu, J. Effect of Si doping on the photocatalytic activity and photoelectrochemical property of TiO2 nanoparticles. Catal. Commun. 2011, 13, 14–17. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Li, Z.; Jiang, M.; Yu, X.; Xu, L. “Two-in-one” sulfur and nitrogen co-doped fluorescent silicon nanoparticles: Simultaneous as the fluorescent probe and photocatalyst for in-situ real time visual monitoring and degradation of tetracycline antibiotics. Sci. Total. Environ. 2022, 846, 157470. [Google Scholar] [CrossRef]
- Gusmão, C.d.A.; Palharim, P.H.; Ramos, B.; Teixeira, A.C.S.C. Enhancing the visible-light photoactivity of silica-supported TiO2 for the photocatalytic treatment of pharmaceuticals in water. Environ. Sci. Pollut. Res. 2021, 29, 42215–42230. [Google Scholar] [CrossRef]
- Kalidhasan, S.; Lee, H.-Y. Preparation of TiO2-deposited silica-based catalysts for photocatalytic decomposition of chloro-pesticide to environmentally less toxic species. Chemosphere 2022, 290, 133300. [Google Scholar] [CrossRef]
- AbuKhadra, M.R.; Mohamed, A.S.; El-Sherbeeny, A.M.; Elmeligy, M.A. Enhanced photocatalytic degradation of acephate pesticide over MCM-41/Co3O4 nanocomposite synthesized from rice husk silica gel and Peach leaves. J. Hazard. Mater. 2020, 389, 122129. [Google Scholar] [CrossRef]
- Li, X.; Shi, C.; Feng, Z.; He, J.; Zhang, R.; Yang, Z.; Pan, P.; Li, H.; Feng, D.; Zheng, L. Construction of Si nanowires/ZnFe2O4/Ag photocatalysts with enhanced photocatalytic activity under visible light and magnetic field. J. Alloys Compd. 2023, 946, 169467. [Google Scholar] [CrossRef]
- Hamdi, A.; Boussekey, L.; Roussel, P.; Addad, A.; Ezzaouia, H.; Boukherroub, R.; Coffinier, Y. Hydrothermal preparation of MoS2/TiO2/Si nanowires composite with enhanced photocatalytic performance under visible light. Mater. Des. 2016, 109, 634–643. [Google Scholar] [CrossRef]
- Brahiti, N.; Hadjersi, T.; Menari, H.; Amirouche, S.; El Kechai, O. Enhanced photocatalytic degradation of methylene blue by metal-modified silicon nanowires. Mater. Res. Bull. 2015, 62, 30–36. [Google Scholar] [CrossRef]
- Cortez, O.A.C.; Bueno, J.d.J.P.; Mexicano, Y.C.; López, M.L.M.; Rodríguez, C.H.; Pérez, A.X.M.; Alejandre, D.C.; López, C.M.; Robles, M.R.G.; Oza, G.; et al. CoO, Cu, and Ag Nanoparticles on Silicon Nanowires with Photocatalytic Activity for the Degradation of Dyes. Sustainability 2022, 14, 13361. [Google Scholar] [CrossRef]
- Hammouche, J.; Daoudi, K.; Columbus, S.; Ziad, R.; Ramachandran, K.; Gaidi, M. Structural and morphological optimization of Ni doped ZnO decorated silicon nanowires for photocatalytic degradation of methylene blue. Inorg. Chem. Commun. 2021, 131, 108763. [Google Scholar] [CrossRef]
- Ameen, S.; Park, D.-R.; Shin, H.S. Silicon nanowires arrays for visible light driven photocatalytic degradation of rose bengal dye. J. Mater. Sci. Mater. Electron. 2016, 27, 10460–10467. [Google Scholar] [CrossRef]
- Naffeti, M.; Zaïbi, M.A.; Nefzi, C.; García-Arias, A.V.; Chtourou, R.; Postigo, P.A. Highly efficient photodegradation of methylene blue by a composite photocatalyst of bismuth nanoparticles on silicon nanowires. Environ. Technol. Innov. 2023, 30, 103133. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, G.; Wang, J.; Liang, X.; Zuo, Z.; Shi, Y. Fabrication and photocatalytic properties of silicon nanowires by metal-assisted chemical etching: Effect of H2O2 concentration. Nanoscale Res. Lett. 2012, 7, 663. [Google Scholar] [CrossRef]
- Gaidi, M.; Daoudi, K.; Columbus, S.; Hajjaji, A.; El Khakani, M.A.; Bessais, B. Enhanced photocatalytic activities of silicon nanowires/graphene oxide nanocomposite: Effect of etching parameters. J. Environ. Sci. 2021, 101, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Derkaoui, K.; Hadjersi, T.; Boukhouidem, K.; Bouanik, S.; Naama, S.; Khen, A.; Manseri, A.; Benharrat, L.; Kechouane, M.; Trari, M. Facile CeO2 nanoparticles deposition on Si-nanowires: Application to the rhodamine B photodegradation under visible light. React. Kinet. Catal. Lett. 2023, 136, 1657–1672. [Google Scholar] [CrossRef]
- Teo, S.H.; Islam, A.; Taufiq-Yap, Y.H.; Awual, R. Introducing the novel composite photocatalysts to boost the performance of hydrogen (H2) production. J. Clean. Prod. 2021, 313, 127909. [Google Scholar] [CrossRef]
- Molaei, R.; Bayati, M.R.; Alipour, H.M.; Nori, S.; Narayan, J. Enhanced photocatalytic efficiency in zirconia buffered n-NiO/p-NiO single crystalline heterostructures by nanosecond laser treatment. J. Appl. Phys. 2013, 113, 233708. [Google Scholar] [CrossRef]
- Yao, L.; He, X.; Lv, J.; Xu, G.; Bao, Z.; Cui, J.; Yu, D.; Wu, Y. Efficient degradation of ciprofloxacin by Co3O4/Si nanoarrays heterojunction activated peroxymonosulfate under simulated sunlight: Performance and mechanism. J. Environ. Chem. Eng. 2022, 10, 107397. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Lin, X.; Tang, Z.-R.; Xu, Y.-J. Silicon nanowires@Co3O4 arrays film with Z-scheme band alignment for hydrogen evolution. Catal. Today 2019, 335, 294–299. [Google Scholar] [CrossRef]
- Gu, J.; Yu, H.; Quan, X.; Chen, S.; Niu, J. Utilizing transparent and conductive SnO2 as electron mediator to enhance the photocatalytic performance of Z-scheme Si-SnO2-TiOx. Front. Environ. Sci. Eng. 2020, 14, 72. [Google Scholar] [CrossRef]
- Ali, S.; Razzaq, A.; Kim, H.; In, S.-I. Activity, selectivity, and stability of earth-abundant CuO/Cu2O/Cu0-based photocatalysts toward CO2 reduction. Chem. Eng. J. 2022, 429, 131579. [Google Scholar] [CrossRef]
- Abadi, A.G.; AlSaidi, M.S.; AL Shibli, W.K. DFT-Driven Approaches to Optimizing Small Bandgap Doping Structures: A Brief Review. J. Inorg. Organomet. Polym. Mater. 2025, 35, 6130–6146. [Google Scholar] [CrossRef]
- Abdullah, B.J. Size effect of band gap in semiconductor nanocrystals and nanostructures from density functional theory within HSE06. Mater. Sci. Semicond. Process. 2022, 137, 106214. [Google Scholar] [CrossRef]
- Fehér, P.P.; Madarász, Á.; Stirling, A. Prediction of Redox Power for Photocatalysts: Synergistic Combination of DFT and Machine Learning. J. Chem. Theory Comput. 2023, 19, 4125–4135. [Google Scholar] [CrossRef]
- Deringer, V.L.; Bernstein, N.; Bartók, A.P.; Cliffe, M.J.; Kerber, R.N.; Marbella, L.E.; Grey, C.P.; Elliott, S.R.; Csányi, G. Realistic Atomistic Structure of Amorphous Silicon from Machine-Learning-Driven Molecular Dynamics. J. Phys. Chem. Lett. 2018, 9, 2879–2885. [Google Scholar] [CrossRef]
- Qian, X.; Peng, S.; Li, X.; Wei, Y.; Yang, R. Thermal conductivity modeling using machine learning potentials: Application to crystalline and amorphous silicon. Mater. Today Phys. 2019, 10, 100140. [Google Scholar] [CrossRef]
- Buratti, Y.; Javier, G.M.; Abdullah-Vetter, Z.; Dwivedi, P.; Hameiri, Z. Machine learning for advanced characterisation of silicon photovoltaics: A comprehensive review of techniques and applications. Renew. Sustain. Energy Rev. 2024, 202, 114617. [Google Scholar] [CrossRef]
- Mai, H.; Le, T.C.; Chen, D.; Winkler, D.A.; Caruso, R.A. Machine Learning for Electrocatalyst and Photocatalyst Design and Discovery. Chem. Rev. 2022, 122, 13478–13515. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Ke, Y.; Li, X. Machine learning integrated photocatalysis: Progress and challenges. Chem. Commun. 2023, 59, 5795–5806. [Google Scholar] [CrossRef] [PubMed]
- Masood, H.; Toe, C.Y.; Teoh, W.Y.; Sethu, V.; Amal, R. Machine Learning for Accelerated Discovery of Solar Photocatalysts. ACS Catal. 2019, 9, 11774–11787. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, M. Accelerated Design for Perovskite-Oxide-Based Photocatalysts Using Machine Learning Techniques. Materials 2024, 17, 3026. [Google Scholar] [CrossRef]
- Mai, H.; Li, X.; Le, T.C.; Russo, S.P.; Winkler, D.A.; Chen, D.; Caruso, R.A. Rapid Design of Efficient Mn3O4-Based Photocatalysts by Machine Learning and Density Functional Theory Calculations. Adv. Energy Sustain. Res. 2025, 6, 2400397. [Google Scholar] [CrossRef]
- Soltani, A.; Djani, F.; Abdesslam, Y. Machine learning predictive model to estimate the photo-degradation performance of stannates and hydroxystannates photocatalysts on a variety of waterborne contaminants. Comput. Theor. Chem. 2024, 1244, 115003. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, J.; Tong, M.; Samia, A.C.; Zhang, H.; Yu, X. A Novel Machine Learning Model to Predict the Photo-Degradation Performance of Different Photocatalysts on a Variety of Water Contaminants. Catalysts 2021, 11, 1107. [Google Scholar] [CrossRef]
- Ali, M.; Hossen, A.; Aziz, A.A. Progress in prediction of photocatalytic CO2 reduction using machine learning approach: A mini review. Next Mater. 2025, 8, 100522. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, M.; Tang, F.; Ling, L.; Yu, H.; Chen, X. Machine learning accelerates the screening of efficient metal-oxide catalysts for photocatalytic water splitting. Mater. Res. Bull. 2024, 179, 112956. [Google Scholar] [CrossRef]
- Tranter, A.D.; Kranz, L.; Sutherland, S.; Keizer, J.G.; Gorman, S.K.; Buchler, B.C.; Simmons, M.Y. Machine Learning-Assisted Precision Manufacturing of Atom Qubits in Silicon. ACS Nano 2024, 18, 19489–19497. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Long, J.; Shi, D.; Chen, X.; Hou, M.; Gao, J.; Liu, H.; He, Y.; Fan, B.; et al. Achieving a sub-10 nm nanopore array in silicon by metal-assisted chemical etching and machine learning. Int. J. Extreme Manuf. 2021, 3, 035104. [Google Scholar] [CrossRef]
- Morrow, J.D.; Ugwumadu, C.; Drabold, D.A.; Elliott, S.R.; Goodwin, A.L.; Deringer, V.L. Understanding Defects in Amorphous Silicon with Million-Atom Simulations and Machine Learning. Angew. Chem. Int. Ed. Engl. 2024, 63, e202403842. [Google Scholar] [CrossRef]
- Erhard, L.C.; Rohrer, J.; Albe, K.; Deringer, V.L. Modelling atomic and nanoscale structure in the silicon–oxygen system through active machine learning. Nat. Commun. 2024, 15, 1927. [Google Scholar] [CrossRef]
- Wang, R.; Yu, H.; Zhong, Y.; Xiang, H. Identifying Direct Bandgap Silicon Structures with High-Throughput Search and Machine Learning Methods. J. Phys. Chem. C 2024, 128, 12677–12685. [Google Scholar] [CrossRef]
- Jenisha, J.; Raj, A. Photocatalytic treatment of N-Nitrosomorpholine by undoped TiO2-ZnO & Si-doped TiO2-ZnO nanocatalyst. Indian J. Chem. Technol. 2024, 31, 240–247. [Google Scholar] [CrossRef]
- Shivaraju, H.; Sparsha, M.; Yashas, S.; Sonu, K.; Harini, R.; Jenkins, D. Preparation of supporting photocatalysts for water treatment using natural sunlight as an alternative driving energy. In Materials Today-Proceedings; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3936–3944. [Google Scholar]
- Dong, Z.; Ding, D.; Li, T.; Ning, C. High-efficiency photoelectrochemical water splitting with heterojunction photoanode of In2O3-x nanorods/black Ti–Si–O nanotubes. Int. J. Hydrogen Energy 2019, 44, 17611–17621. [Google Scholar] [CrossRef]
- Ma, N.; Quan, X.; Zhang, Y.; Chen, S.; Zhao, H. Integration of separation and photocatalysis using an inorganic membrane modified with Si-doped TiO2 for water purification. J. Membr. Sci. 2009, 335, 58–67. [Google Scholar] [CrossRef]
- Vohra, M.S.; Lee, J.; Choi, W. Enhanced photocatalytic degradation of tetramethylammonium on silica-loaded titania. J. Appl. Electrochem. 2005, 35, 757–763. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, H.; Dai, D.; Liu, C. Preparation of silicon-doped TiO2 fiber and supported TiO2 and comparison study of their photocatalytic activity. J. Sol-Gel Sci. Technol. 2017, 83, 243–251. [Google Scholar] [CrossRef]
- Li, T.; Ding, D. Ni/Si-Codoped TiO2 Nanostructure Photoanode for Enhanced Photoelectrochemical Water Splitting. Materials 2019, 12, 4102. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Ding, D. Photoelectrochemical water splitting with black Ni/Si-doped TiO2 nanostructures. Int. J. Hydrogen Energy 2020, 45, 20983–20992. [Google Scholar] [CrossRef]
- Ai, Z.; Gao, Z.; Su, K.; Ho, W.; Zhang, L. Aerosol flow synthesis of N, Si-codoped TiO2 hollow microspheres with enhanced visible-light driven photocatalytic performance. Catal. Commun. 2012, 29, 189–193. [Google Scholar] [CrossRef]
- Chen, C.; Wei, Y.; Yuan, G.; Liu, Q.; Lu, R.; Huang, X.; Cao, Y.; Zhu, P. Synergistic Effect of Si Doping and Heat Treatments Enhances the Photoelectrochemical Water Oxidation Performance of TiO2 Nanorod Arrays. Adv. Funct. Mater. 2017, 27, 1701575. [Google Scholar] [CrossRef]
- Tong, M.; Sun, D.; Zhang, R.; Liu, H.; Chen, R. Preparation of Si–α-Fe2O3/CdS composites with enhanced visible-light photocatalytic activity for p-nitrophenol degradation. J. Alloys Compd. 2021, 862, 158271. [Google Scholar] [CrossRef]
- Liang, Z.; Ba, G.; Li, H.; Du, N.; Hou, W. Facile synthesis of silicon-doped polymeric carbon nitride with enhanced photocatalytic performance. J. Alloys Compd. 2020, 815, 152488. [Google Scholar] [CrossRef]
- Wen, J.; Li, N.; Lin, P.; Han, Y.; Chen, G.; Bai, L.; Guo, S.; Wu, H.; He, W.; Zhang, J. Electronic, magnetic and photocatalytic properties of Si doping in g-ZnO monolayer with point defects. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 134, 114913. [Google Scholar] [CrossRef]
- Mavengere, S.; Kim, J.-S. UV–visible light photocatalytic properties of NaYF4:(Gd, Si)/TiO2 composites. Appl. Surf. Sci. 2018, 444, 491–496. [Google Scholar] [CrossRef]
- Singh, J.; Jadhav, S.; Avasthi, S.; Sen, P. Designing Photocatalytic Nanostructured Antibacterial Surfaces: Why Is Black Silica Better than Black Silicon? ACS Appl. Mater. Interfaces 2020, 12, 20202–20213. [Google Scholar] [CrossRef]
- Convertino, A.; Maiolo, L.; Scuderi, V.; Di Mauro, A.; Scuderi, M.; Nicotra, G.; Impellizzeri, G.; Fortunato, G.; Privitera, V. A forest of SiO2 nanowires covered by a TiO2 thin film for an efficient photocatalytic water treatment. RSC Adv. 2016, 6, 91121–91126. [Google Scholar] [CrossRef]
- Olejnik, T.; Pasieczna-Patkowska, S.; Lesiuk, A.; Ryczkowski, J. Phenol and methylene blue photodegradation over Ti/SBA-15 materials under uv light. PJCT 2016, 18, 30–38. [Google Scholar] [CrossRef]
- Takeuchi, M.; Matsuoka, M.; Yamashita, H.; Anpo, M. Preparation of Ti–Si binary oxide thin film photocatalysts by the application of an ionized cluster beam method. J. Synchrotron Radiat. 2001, 8, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Pan, Z.; Zhang, B.; Liu, G.; Zhang, H.; Song, X.; Hu, G.; Xiao, C.; Wei, Z.; Zheng, Y. The research of photocatalytic activity on Si doped TiO2 nanotubes. Mater. Lett. 2017, 188, 66–68. [Google Scholar] [CrossRef]
- Van Viet, P.; Huy, T.H.; You, S.-J.; Van Hieu, L.; Thi, C.M. Hydrothermal synthesis, characterization, and photocatalytic activity of silicon doped TiO2 nanotubes. Superlattices Microstruct. 2018, 123, 447–455. [Google Scholar] [CrossRef]
- Van Viet, P.; Huy, T.H.; Sang, T.T.; Nguyet, H.M.; Thi, C.M. One-pot hydrothermal synthesis of Si doped TiO2 nanotubes from commercial material sources for visible light-driven photocatalytic activity. Mater. Res. Express 2019, 6, 055006. [Google Scholar] [CrossRef]
- Rafajilović, M.R.; Radulović, K.; Pergal, M.V.; Blanuša, J.; Rajić, V.; Cvjetićanin, N.; Vasiljević-Radović, D. Si/Pyrex glass and poly(dimethylsiloxane)-based microfluidic devices with integrated heating elements for TiO2 nanoparticle synthesis. J. Sci. Adv. Mater. Devices 2025, 10, 100877. [Google Scholar] [CrossRef]
- Ounas, A.; Baha, A.A.; Izghri, Z.; Idouhli, R.; Tabit, K.; Yaacoubi, A.; Abouelfida, A.; Bacaoui, A. Biochar/Zeolite-Supported TiO2 Nanocatalyst for the Photodegradation of Organic Pollutants in Aqueous Media. Chem. Afr. 2025, 8, 3139–3150. [Google Scholar] [CrossRef]
- Ho, T.-N. Enhanced Photocatalytic Activation of Peroxymonosulfate Using Ag-TiO2/SiO2 Monolith for Phenol Degradation. Catal. Surv. Asia 2025, 29, 185–193. [Google Scholar] [CrossRef]
- Yang, R.; Zhu, R.; Fan, Y.; Hu, L.; Chen, B. Construction of an artificial inorganic leaf CdS–BiVO4 Z-scheme and its enhancement activities for pollutant degradation and hydrogen evolution. Catal. Sci. Technol. 2019, 9, 2426–2437. [Google Scholar] [CrossRef]
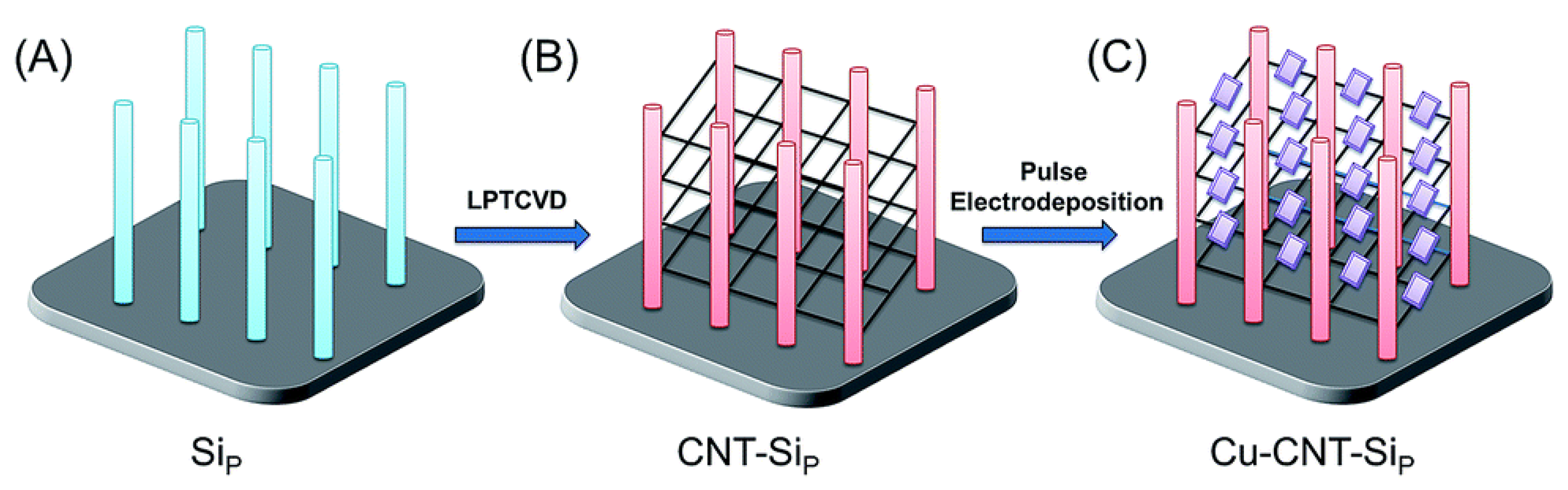
| Structure | Object of Decomposition | Photocatalyst Parameters | Light Source | Source |
|---|---|---|---|---|
| Si-doped TiO2 nanotubes | Methyl Orange | 5% Si-TiO2 NTs showed much higher photocatalytic activity | UV | [158] |
| Si-doped TiO2 nanotubes | Methylene Blue | 10% Si-doped TiO2 nanotubes tripled MB degradation efficiency compared to undoped TiO2 | UV | [159] |
| Si-doped TiO2 nanotubes | Phenol | 10% Si-doped TiO2 nanotubes showed ~9 times higher phenol degradation under visible light compared to undoped TiO2. | simulated solar | [160] |
| TiO2/SiO2 (Si-doped TiO2 in PDMS microreactor) | Methylene Blue | Degradation efficiency: 93.59% after 90 min | UV | [161] |
| TiO2-SiO2 (Si-doped TiO2 hybrid) | Rhodamine B | Degradation efficiency: 95.81% for 60 mg/L RhB over 3 cycles-High surface area from biochar/zeolite support | UV | [162] |
| TiO2-SiO2 (Si-doped TiO2 monolith) | Phenol | Degradation efficiency: 92% after 240 min with PMS | UV | [163] |
| CdS-BiVO4 (Si-doped artificial leaf) | Rhodamine B | Degradation efficiency: 92% after 2 h visible light irradiation (2.1 times higher than no-template BiVO4) | sunlight | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serikkanov, A.S.; Bakranov, N.B.; Idrissova, T.K.; Bakranova, D.I.; Boukhvalov, D.W. Silicon Photocatalytic Water-Treatment: Synthesis, Modifications, and Machine Learning Insights. Nanomaterials 2025, 15, 1514. https://doi.org/10.3390/nano15191514
Serikkanov AS, Bakranov NB, Idrissova TK, Bakranova DI, Boukhvalov DW. Silicon Photocatalytic Water-Treatment: Synthesis, Modifications, and Machine Learning Insights. Nanomaterials. 2025; 15(19):1514. https://doi.org/10.3390/nano15191514
Chicago/Turabian StyleSerikkanov, Abay S., Nurlan B. Bakranov, Tunyk K. Idrissova, Dina I. Bakranova, and Danil W. Boukhvalov. 2025. "Silicon Photocatalytic Water-Treatment: Synthesis, Modifications, and Machine Learning Insights" Nanomaterials 15, no. 19: 1514. https://doi.org/10.3390/nano15191514
APA StyleSerikkanov, A. S., Bakranov, N. B., Idrissova, T. K., Bakranova, D. I., & Boukhvalov, D. W. (2025). Silicon Photocatalytic Water-Treatment: Synthesis, Modifications, and Machine Learning Insights. Nanomaterials, 15(19), 1514. https://doi.org/10.3390/nano15191514







