Preparation of Silk Fibroin–Carboxymethyl Cellulose Composite Binder and Its Application in Silicon-Based Anode for Lithium-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Silk Fibroin (SF) [25]
2.2. Preparation of CMC-SF Composite Binders
2.3. Structure Characterization of CMC-SF Composite Binders
2.4. Electrochemical Performance Characterization
3. Results and Discussion
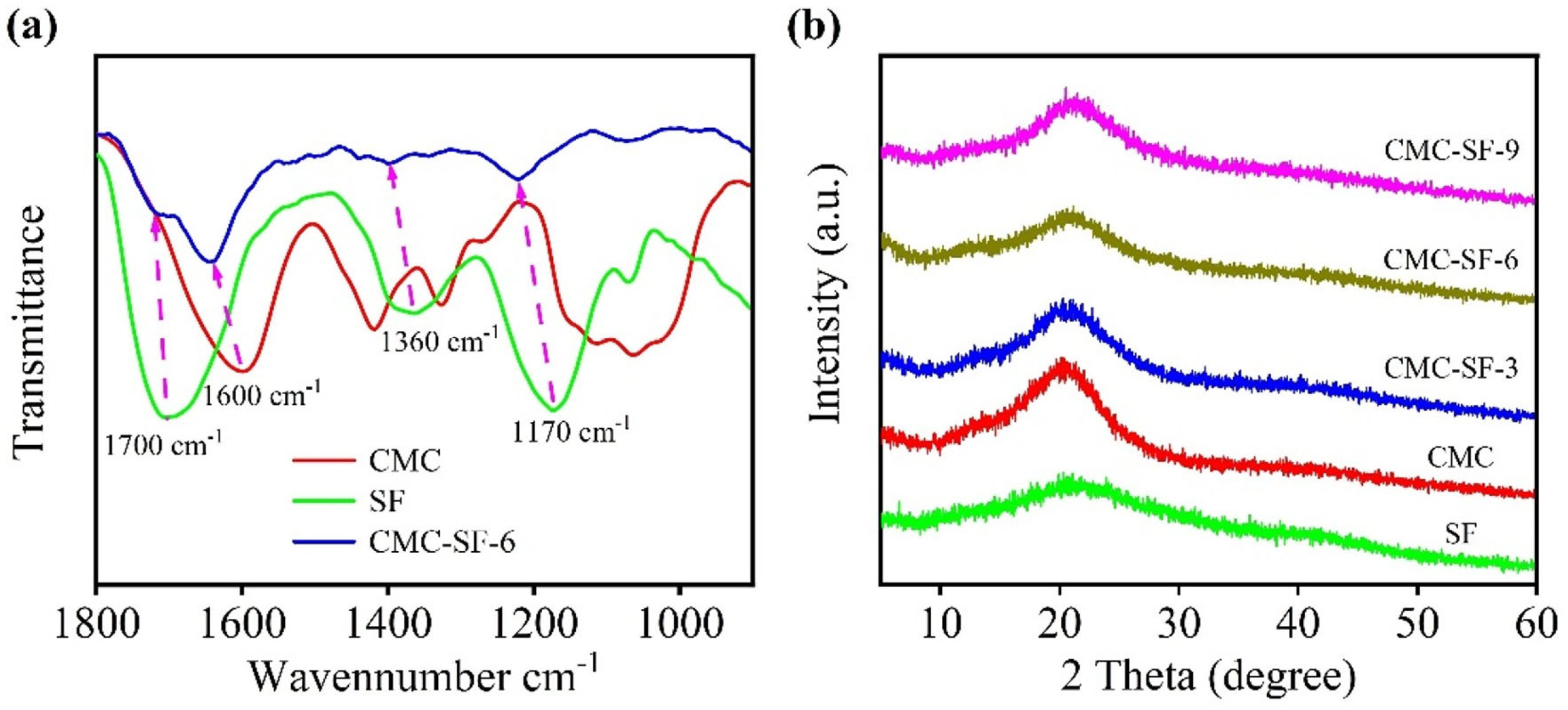
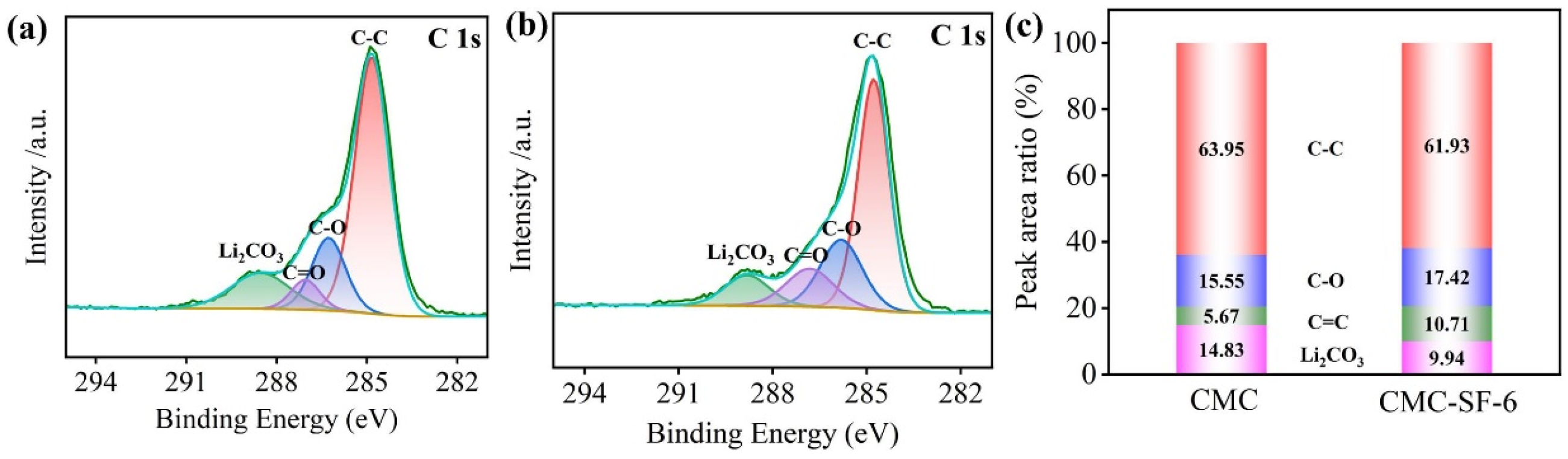
3.1. Subsection Chemical Interactions Between CMC and SF
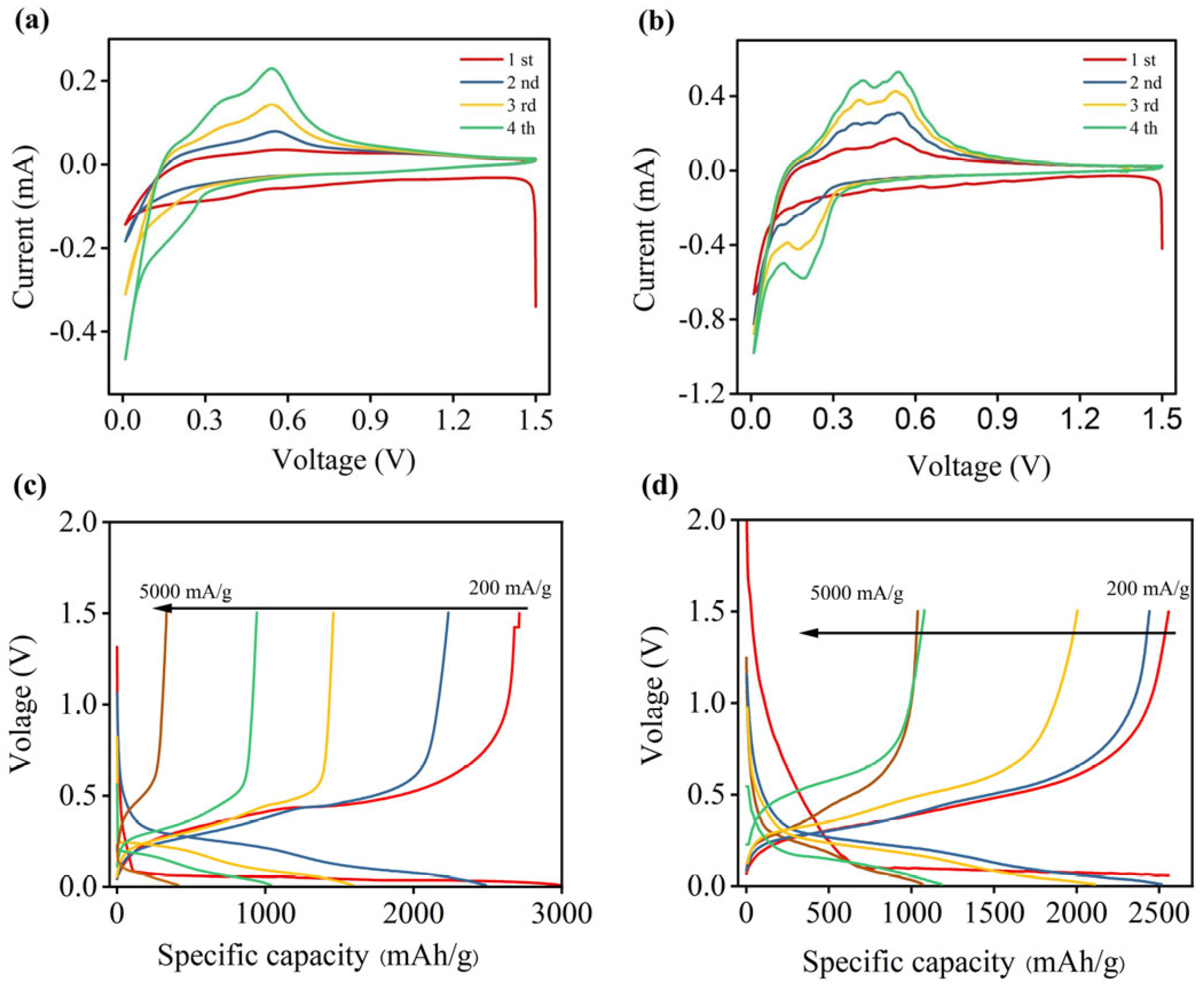
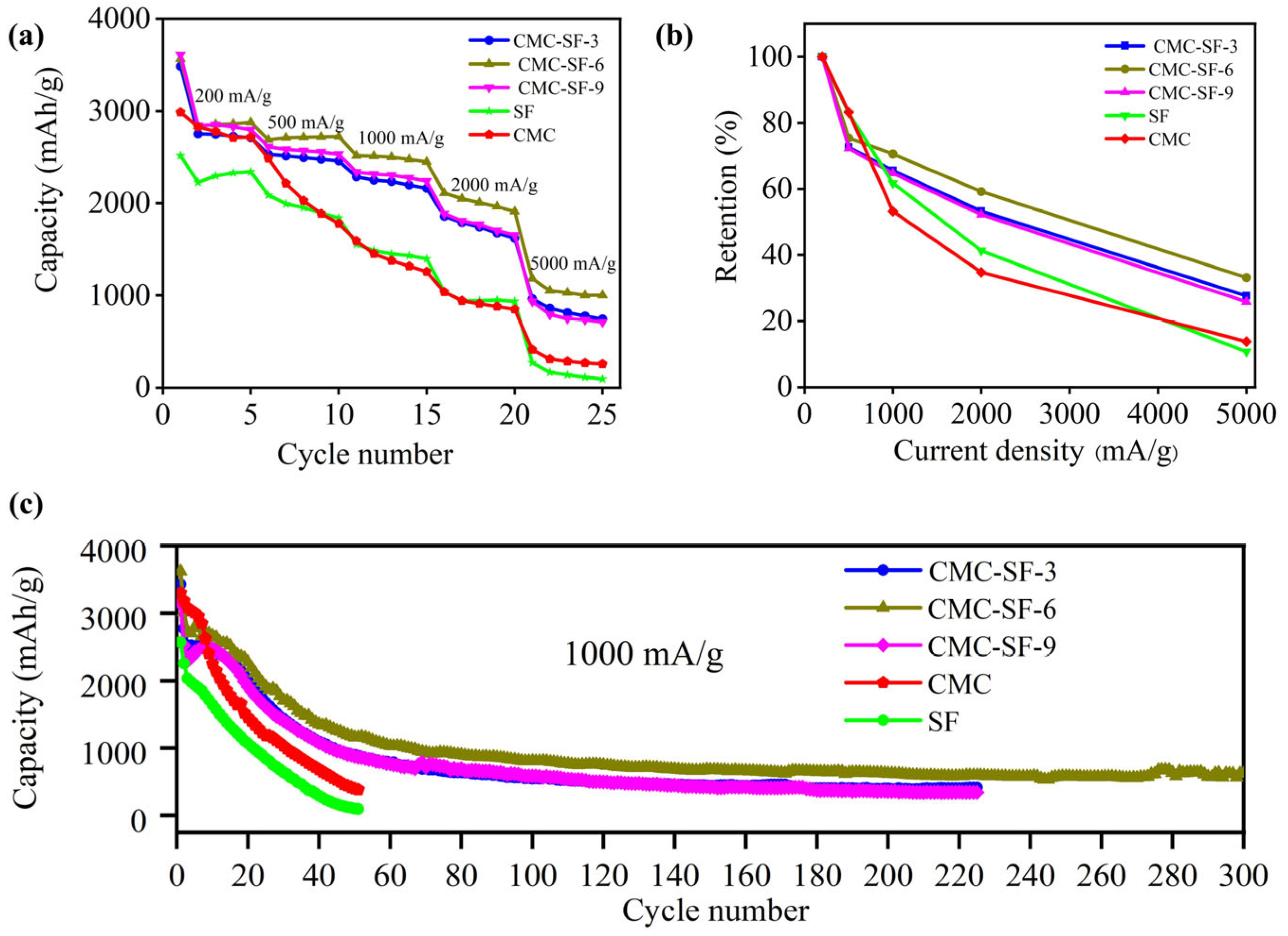
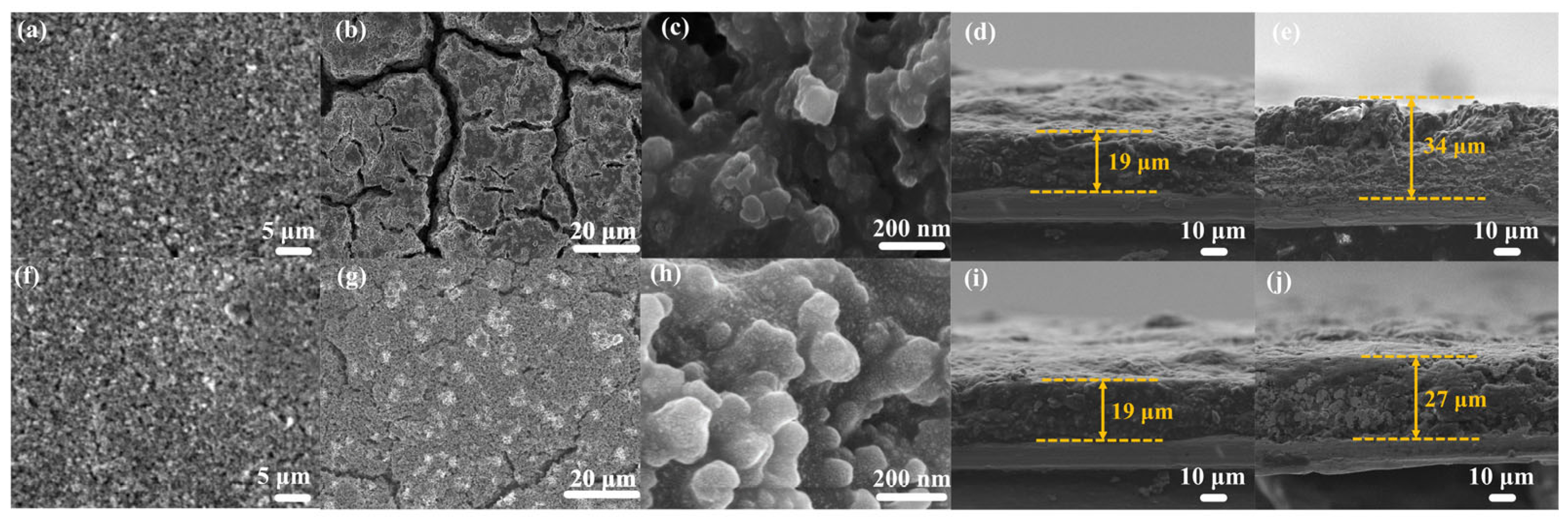
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, Y.; Yang, C.; Jiang, Y.; Shang, Z.; Zhu, J.; Zhang, J.; Jiang, M. Robust Nitrogen/Sulfur Co-Doped Carbon Frameworks as Multifunctional Coating Layer on Si Anodes Toward Superior Lithium Storage. Adv. Energy Mater. 2025, 15, 2403086. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, H.P.; Zhou, H.; Yang, B.; Li, Z.K.; Zhang, S.; Feng, T.T.; Xu, Z.Q.; Fang, Z.X.; Wu, M.Q. Highly Si loading on three-dimensional carbon skeleton via CVD method for a stable SiC composite anode. J. Energy Storage 2025, 116, 116083. [Google Scholar] [CrossRef]
- Fu, S.; Wang, X.; Yao, F.; He, Q.; Xie, F.; Wu, X.; Tong, S.; Wu, M. A simple and effective approach to relieve stress and enhance cyclability of Si-based materials toward high-energy lithium-ion batteries. Chem. Eng. J. 2024, 489, 151151. [Google Scholar] [CrossRef]
- Xu, P.; Guo, D.; Lin, X.; Wang, X.; Zhang, Z.; Zeng, C.; Liao, M.; Su, Z.; Huang, Q.; Zhang, M. Non-toxic synthesis of sandwich-like graphite sheet@Si@C anode material with strong face-to-face bonding by Si-C bonds for lithium-ion batteries. J. Energy Storage 2024, 98, 113225. [Google Scholar] [CrossRef]
- Huang, X.; Guo, R.; Lin, Y.; Cao, Y.; Wu, J. Compact homogeneous Si/SiC/C in-situ nanocomposite microspheres as anode materials for lithium-ion batteries. J. Energy Storage 2024, 87, 111374. [Google Scholar] [CrossRef]
- Li, M.; Li, S.; Yan, D.; Ma, Y.; Niu, X.; Wang, L. Electrolyte design weakens lithium-ion solvation for a fast-charging and long-cycling Si anode. Chem. Sci. 2025, 16, 2609–2618. [Google Scholar] [CrossRef]
- Cheng, K.; Tu, S.; Zhang, B.; Wang, W.; Wang, X.; Tan, Y.; Chen, X.; Li, C.; Li, C.; Wang, L.; et al. Material–electrolyte interfacial interaction enabling the formation of an inorganic-rich solid electrolyte interphase for fast-charging Si-based lithium-ion batteries. Energy Environ. Sci. 2024, 17, 2631–2641. [Google Scholar] [CrossRef]
- Lv, M.; Zhao, R.; Hu, Z.; Yang, J.; Han, X.; Wang, Y.; Wu, C.; Bai, Y. Binder design strategies for cathode materials in advanced secondary batteries. Energy Environ. Sci. 2024, 17, 4871–4906. [Google Scholar] [CrossRef]
- Li, X.; Yuan, S.; Li, M.; Zhu, P.; Duan, H.; Chen, Y. Designing multifunctional, 3D cross-linked network binder for actual use in high performance lithium ion batteries silicon based anodes. J. Power Sources 2024, 623, 235490. [Google Scholar] [CrossRef]
- Reddy, B.S.; Ahn, H.; Ahn, J.; Cho, G.; Cho, K. Cost-effective water-soluble three-dimensional cross-linked polymeric binder for high-performance lithium-sulfur batteries. J. Energy Storage 2023, 66, 107400. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, C.; Zhu, J.; Xue, B.; Zhang, J.; Jiang, M. An Advanced 3D Crosslinked Conductive Binder for Silicon Anodes: Leveraging Glycerol Chemistry for Superior Lithium-Ion Battery Performance. Angew. Chem. Int. Ed. 2025, 64, e202418794. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Ning, J.; Chen, H.; Jiang, Z.; Wang, J.; Chen, D.; Zhao, C.; Liu, Z.; Perepichka, I.F.; Meng, H.; et al. Achievements, challenges, and perspectives in the design of polymer binders for advanced lithium-ion batteries. Chem. Soc. Rev. 2024, 53, 7091–7157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tai, Z.; Zhou, T.; Sencadas, V.; Zhang, J.; Zhang, L.; Konstantinov, K.; Guo, Z.; Liu, H.K. An All-Integrated Anode via Interlinked Chemical Bonding between Double-Shelled–Yolk-Structured Silicon and Binder for Lithium-Ion Batteries. Adv. Mater. 2017, 29, 1703028. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; He, L.; Lu, Y.; Zheng, J.; Li, L.; Geng, X.; Sun, C.; Zhao, H.; Jiang, G.; Di, F.; et al. Volumetric Stress Managements on Silicon Anode of Lithium-Ion Batteries by a Self-Adaptable Binder. Energy Environ. Sci. 2025, 8, e12859. [Google Scholar] [CrossRef]
- Vogl, U.S.; Das, P.K.; Weber, A.Z.; Winter, M.; Kostecki, R.; Lux, S.F. Mechanism of Interactions between CMC Binder and Si Single Crystal Facets. Langmuir 2014, 30, 10299–10307. [Google Scholar] [CrossRef]
- Wei, L.M.; Hou, Z.Y. High performance polymer binders inspired by chemical finishing of textiles for silicon anodes in lithium ion batteries. J. Mater. Chem. A 2017, 5, 22156–22162. [Google Scholar] [CrossRef]
- Hamzelui, N.; Eshetu, G.G.; Figgemeier, E. Customizing Active Materials and Polymeric Binders: Stern Requirements to Realize Silicon-Graphite Anode Based Lithium-Ion Batteries. J. Energy Storage 2021, 35, 102098. [Google Scholar] [CrossRef]
- Lee, D.; Park, H.; Goliaszewski, A.; Byeun, Y.; Song, T.; Paik, U. In Situ Cross-linked Carboxymethyl Cellulose-Polyethylene Glycol Binder for Improving the Long-Term Cycle Life of Silicon Anodes in Li Ion Batteries. Ind. Eng. Chem. Res. 2019, 58, 8123–8130. [Google Scholar] [CrossRef]
- Dong, S.; Xie, G.; Xu, S.; Tan, X.; Chaudhary, M.; Zhang, Y.; Wu, R.; Wen, F.; Ayranci, C.; Michaelis, V.K.; et al. Cellulose-Encapsulated Composite Electrolyte Design: Toward Chemically and Mechanically Enhanced Solid-Sodium Batteries. ACS Nano 2024, 18, 16285–16296. [Google Scholar] [CrossRef]
- Li, Z.; Wan, Z.; Zeng, X.; Zhang, S.; Yan, L.; Ji, J.; Wang, H.; Ma, Q.; Liu, T.; Lin, Z.; et al. A robust network binder via localized linking by small molecules for high-areal-capacity silicon anodes in lithium-ion batteries. Nano Energy 2021, 79, 105430. [Google Scholar] [CrossRef]
- Jiao, X.; Yuan, X.; Yin, J.; Boorboor Ajdari, F.; Feng, Y.; Gao, G.; Song, J. Multiple Network Binders via Dual Cross-Linking for Silicon Anodes of Lithium-Ion Batteries. ACS Appl. Energy Mater. 2021, 4, 10306–10313. [Google Scholar] [CrossRef]
- Xue, S.; Fu, Y.; Song, Z.; Chen, S.; Ji, Y.; Zhao, Y.; Wang, H.; Qian, G.; Yang, L.; Pan, F. Coil-to-Stretch Transition of Binder Chains Enabled by “Nano-Combs” to Facilitate Highly Stable SiOx Anode. Energy Environ. Mater. 2022, 5, 1310–1316. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, X.; Zamora, J.; McCloy, J.; Song, M. Silk fibroin-based biopolymer composite binders with gradient binding energy and strong adhesion force for high-performance micro-sized silicon anodes. J. Energy Chem. 2023, 80, 442–451. [Google Scholar] [CrossRef]
- Lin, X.; Li, J.; Dai, L.; Pei, K.; Huang, Z.; Huang, S.; Li, M.; Liang, Y.; Chen, H.; Zhang, S. Silk fibroin-induced conformational change for robust silicon anodes. Nano Energy 2025, 139, 110957. [Google Scholar] [CrossRef]
- Mantry, S.; Silakabattini, K.; Das, P.K.; Sankaraiah, J.; Barik, C.S.; Panda, S.; Wahab, S.; Khalid, M. Silk fibroin: An innovative protein macromolecule-based hydrogel/scaffold revolutionizing breast cancer treatment and diagnosis-Mechanisms, advancements, and targeting capabilities. Int. J. Biol. Macromol. 2025, 309, 142870. [Google Scholar] [CrossRef] [PubMed]
- Muyonga, H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Karkar, Z.; Guyomard, D.; Roué, L.; Lestriez, B. A comparative study of polyacrylic acid (PAA) and carboxymethyl cellulose (CMC) binders for Si-based electrodes. Electrochim. Acta 2017, 258, 453–466. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Zhu, M.; Chen, G.R.; Dudko, N.; Li, Y.; Liu, H.J.; Shi, L.Y.; Wu, G.; Zhang, D.S. High-Performance Microsized Si Anodes for Lithium-Ion Batteries: Insights into the Polymer Configuration Conversion Mechanism. Adv. Mater. 2022, 34, 2109658. [Google Scholar] [CrossRef]
- Wang, S.F.; He, Y.T.; Zhang, G.; Ma, K.H.; Wang, C.; Zhou, F.S.; Wang, Z.H.; Liu, Z.G.; Lü, Z.; Huang, X.Q.; et al. Multifunctional Silicon-Based Composite Electrolyte Additive Enhances the Stability of the Lithium Metal Anode/Electrolyte Interface. Adv. Energy Mater. 2024, 14, 2401384. [Google Scholar] [CrossRef]




| Sample | Mass Change (%) | Solubility |
|---|---|---|
| CMC-SF-9 | 5.13 | Insoluble |
| CMC-SF-6 | 5.86 | Insoluble |
| CMC-SF-3 | 6.93 | Insoluble |
| CMC | 8.12 | Insoluble |
| Before Cycling | After Cycling | ||||
|---|---|---|---|---|---|
| Rs | Rct | Rs | Rct | RSEI | |
| CMC | 3.812 | 134.8 | 8.078 | 233.0 | 352.4 |
| CMC-SF-3 | 6.120 | 148.2 | 6.837 | 142.4 | 263.5 |
| CMC-SF-6 | 4.667 | 175.1 | 8.414 | 139.5 | 241.2 |
| CMC-SF-9 | 4.328 | 189.9 | 7.797 | 157.4 | 323.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Wang, R.; Lei, M.; Geng, Q.; Li, Q.; Zhang, J.; Zhang, J. Preparation of Silk Fibroin–Carboxymethyl Cellulose Composite Binder and Its Application in Silicon-Based Anode for Lithium-Ion Batteries. Nanomaterials 2025, 15, 1509. https://doi.org/10.3390/nano15191509
Huang S, Wang R, Lei M, Geng Q, Li Q, Zhang J, Zhang J. Preparation of Silk Fibroin–Carboxymethyl Cellulose Composite Binder and Its Application in Silicon-Based Anode for Lithium-Ion Batteries. Nanomaterials. 2025; 15(19):1509. https://doi.org/10.3390/nano15191509
Chicago/Turabian StyleHuang, Shuai, Ruyi Wang, Mingke Lei, Qingxuan Geng, Qingwei Li, Jiwei Zhang, and Jingwei Zhang. 2025. "Preparation of Silk Fibroin–Carboxymethyl Cellulose Composite Binder and Its Application in Silicon-Based Anode for Lithium-Ion Batteries" Nanomaterials 15, no. 19: 1509. https://doi.org/10.3390/nano15191509
APA StyleHuang, S., Wang, R., Lei, M., Geng, Q., Li, Q., Zhang, J., & Zhang, J. (2025). Preparation of Silk Fibroin–Carboxymethyl Cellulose Composite Binder and Its Application in Silicon-Based Anode for Lithium-Ion Batteries. Nanomaterials, 15(19), 1509. https://doi.org/10.3390/nano15191509







