Biochar-Based Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Synergies, and Sustainable Prospects
Abstract
1. Introduction
1.1. Background and Scope
1.2. Methodology of the Literature Review
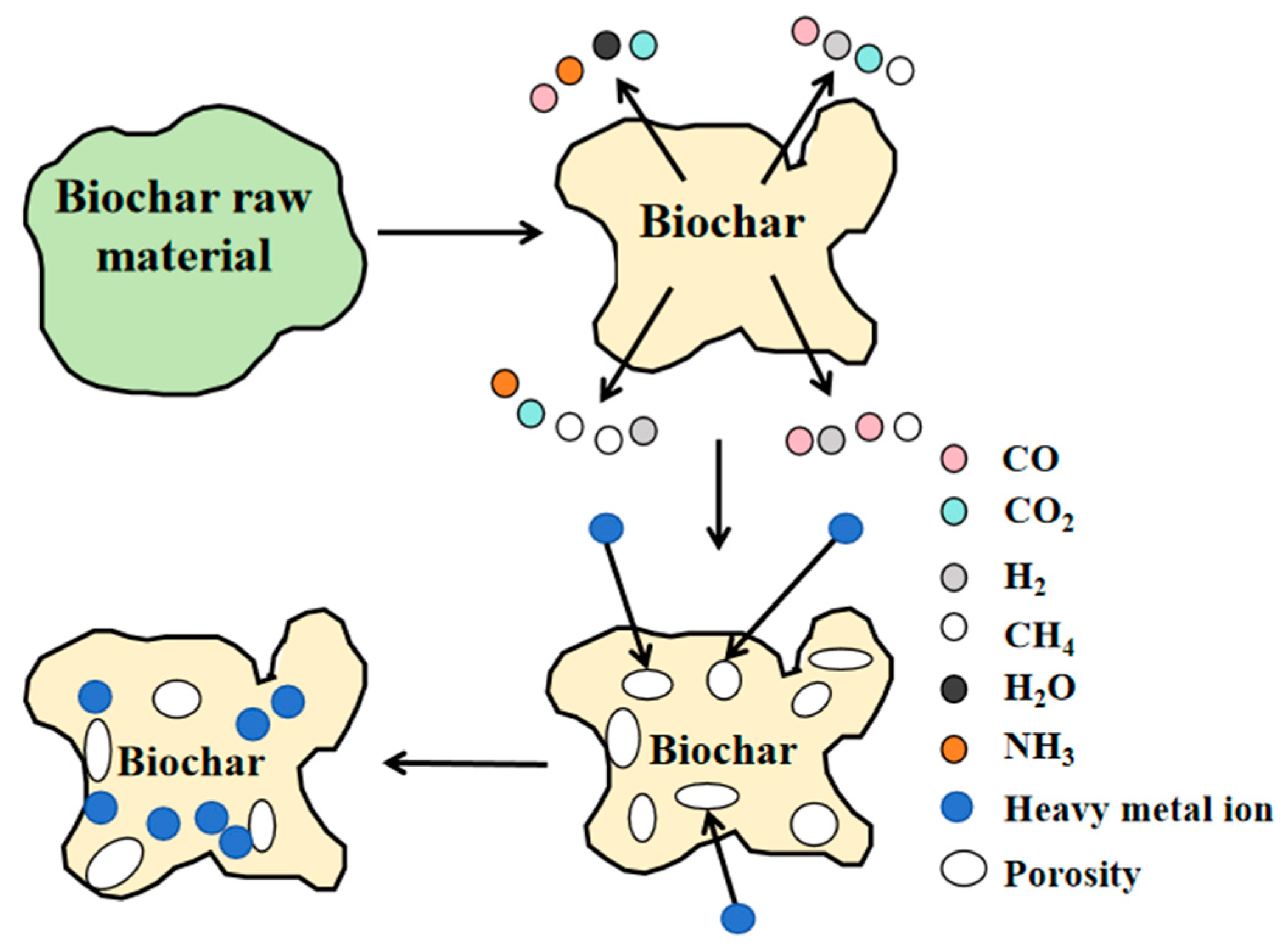
2. Preparation of Biochar
2.1. Preparation Methods
2.2. Effects of Raw Materials and Pyrolysis Temperature on the Physicochemical Properties of Biochar
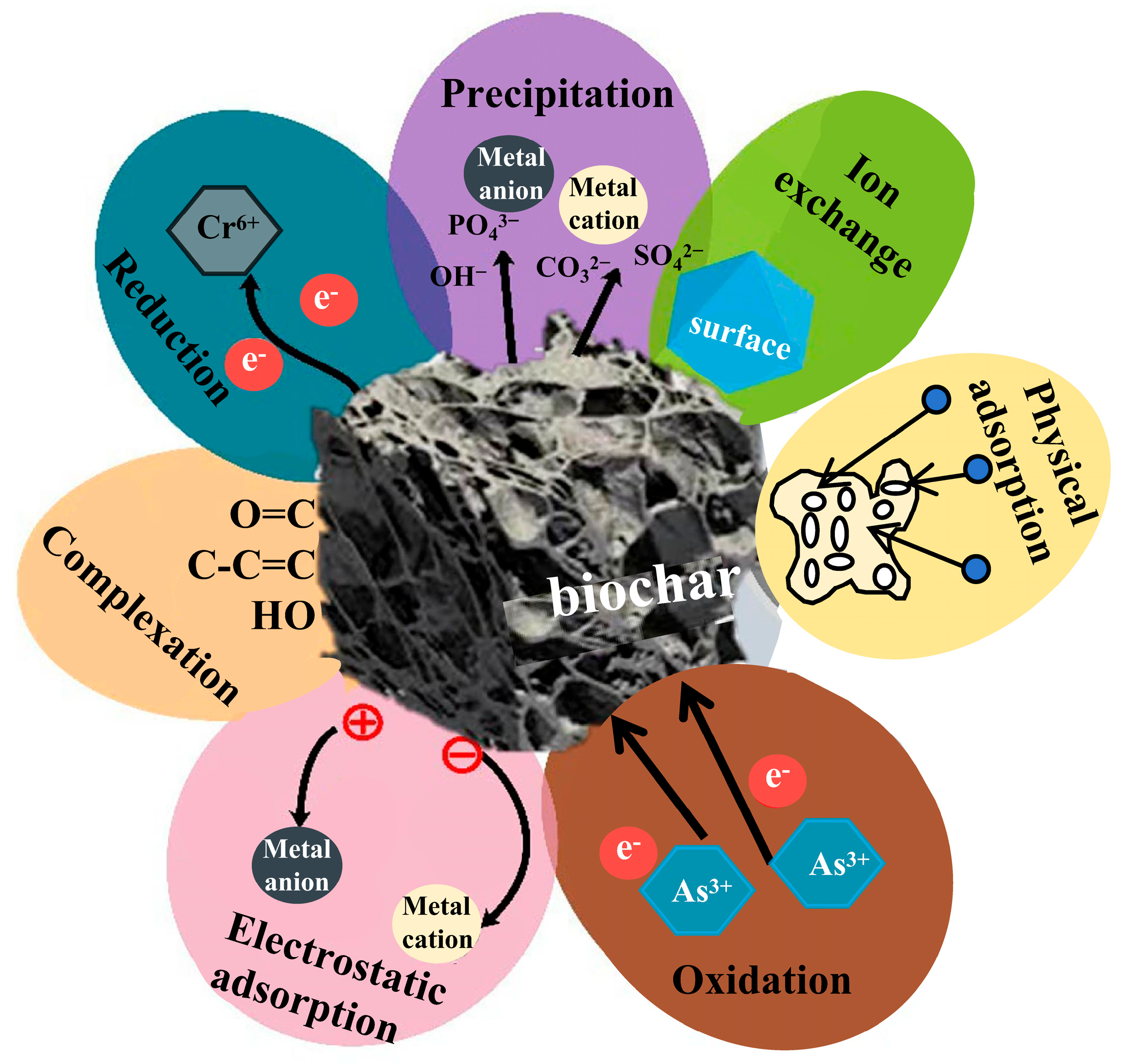
3. Mechanisms of HM Remediation by Biochar
3.1. Physical Adsorption
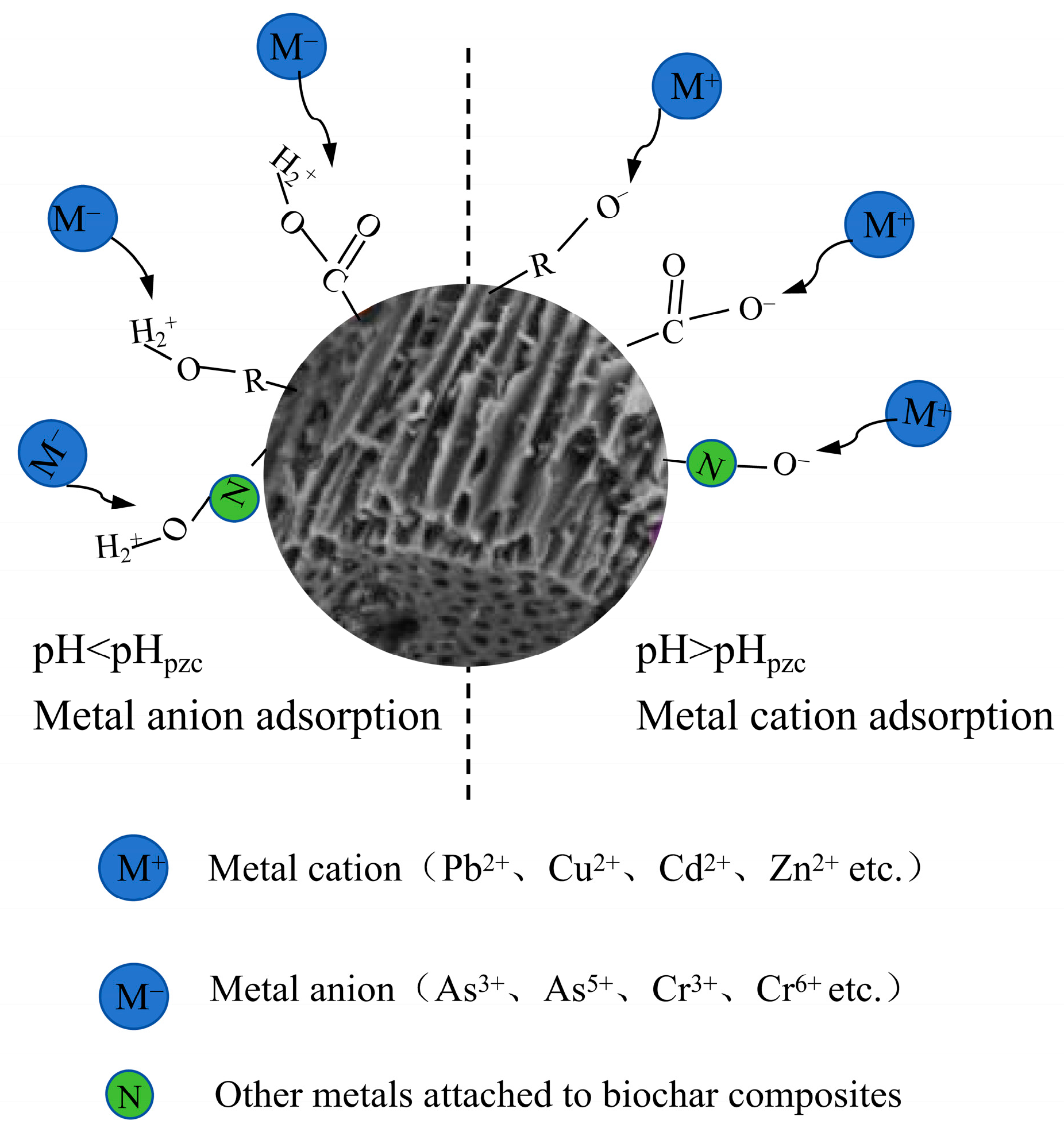
3.2. Electrostatic Adsorption
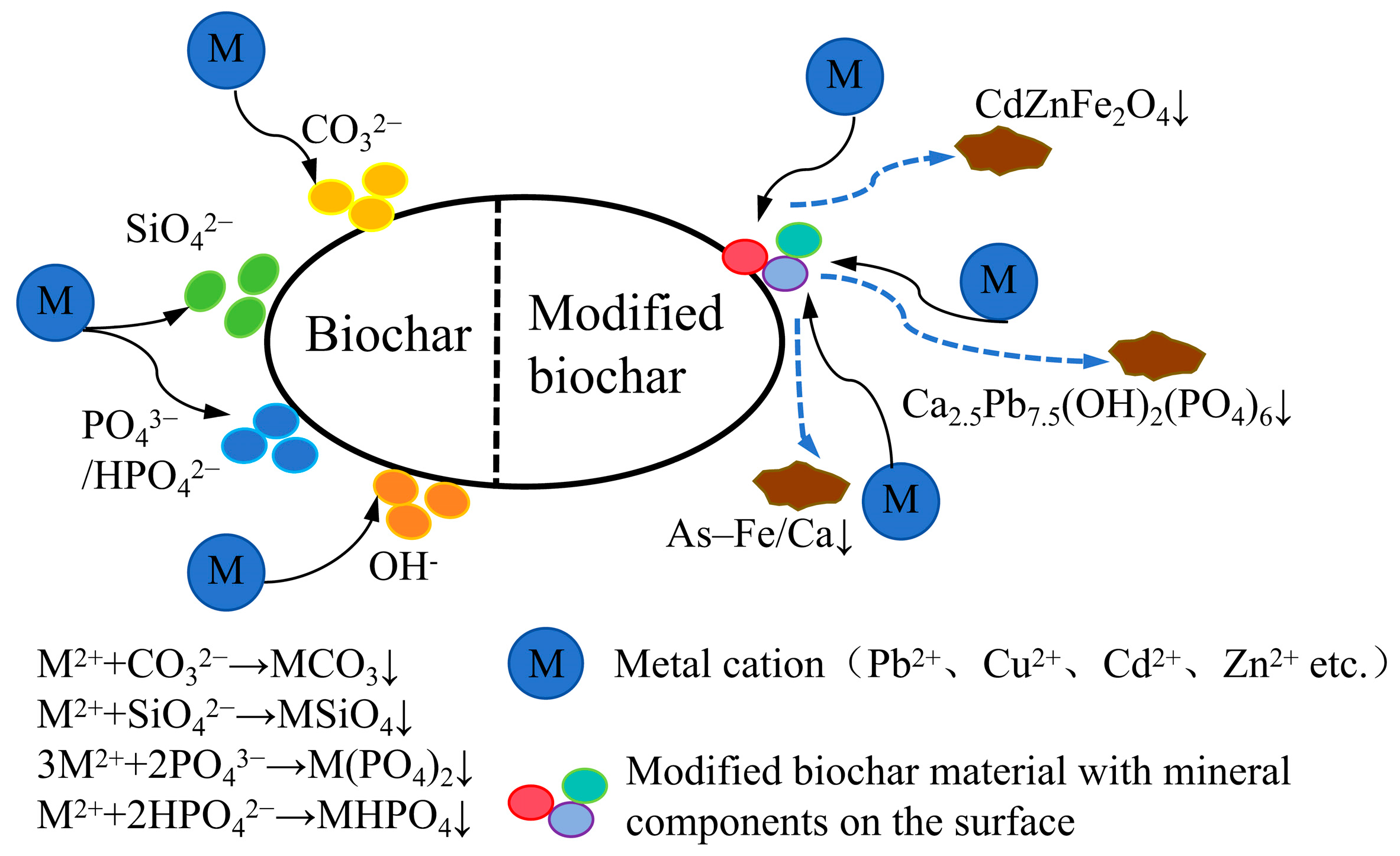
3.3. Precipitation Reaction
3.4. Ion Exchange
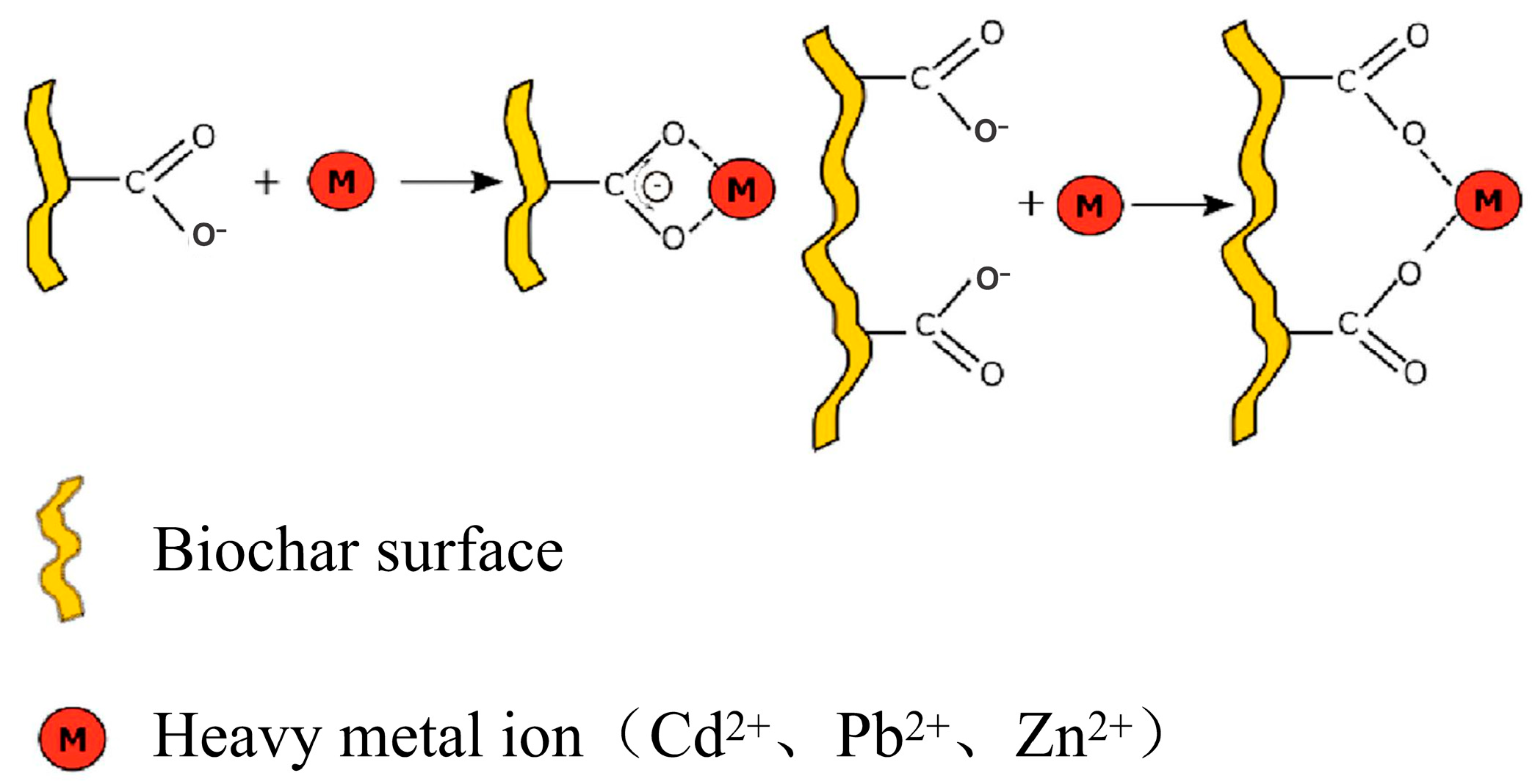
3.5. Organic Functional Group Complexation
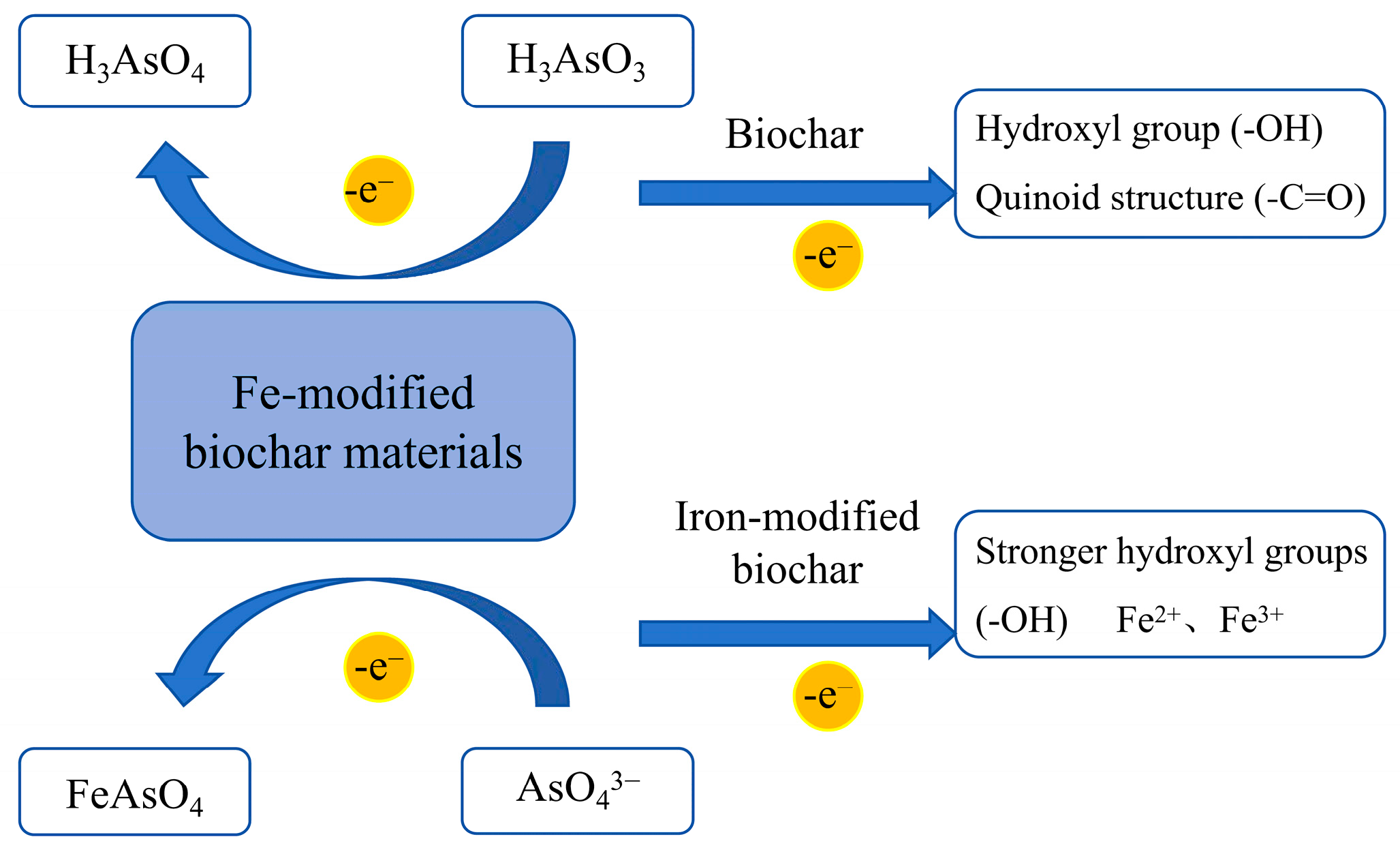
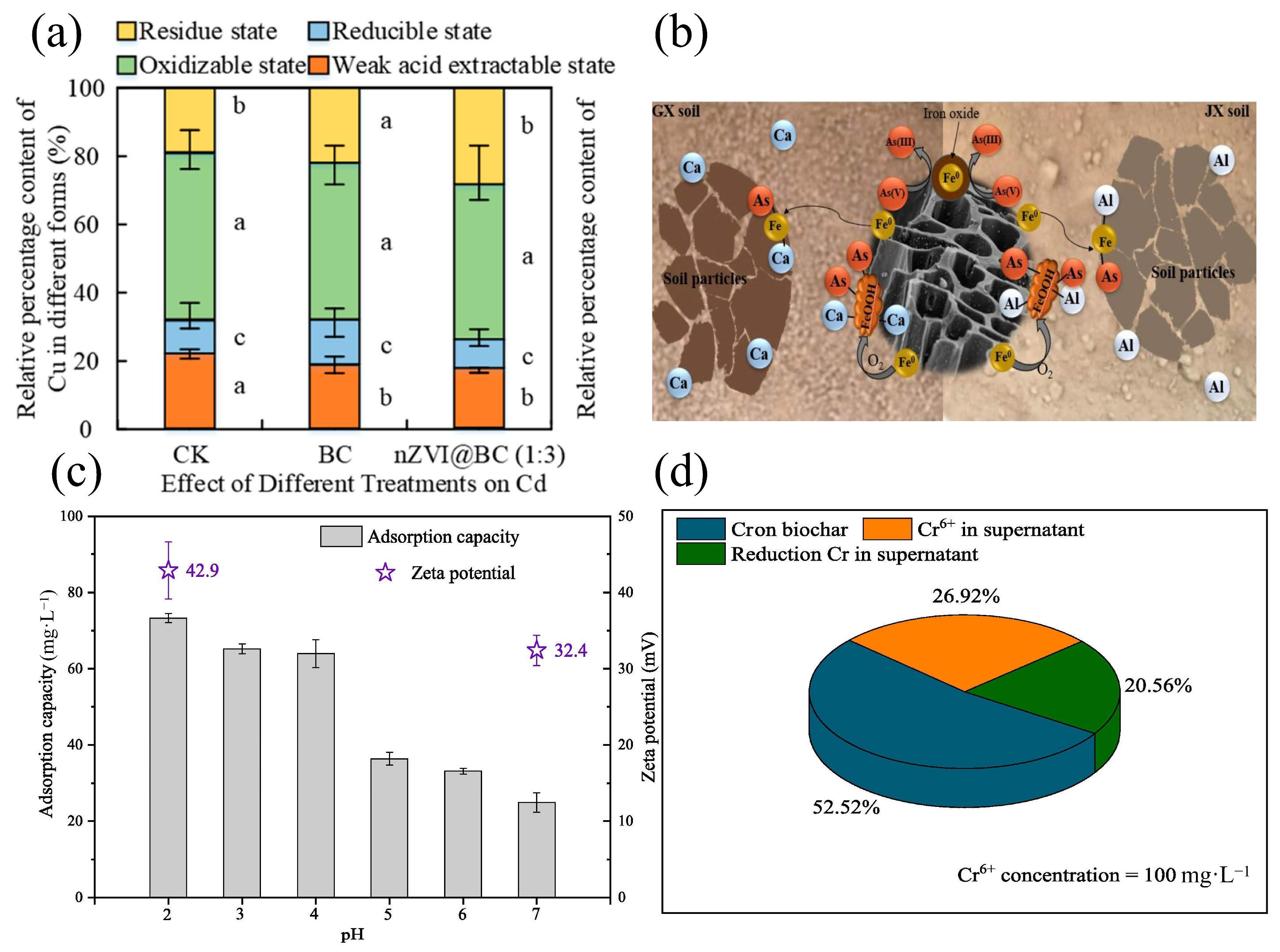
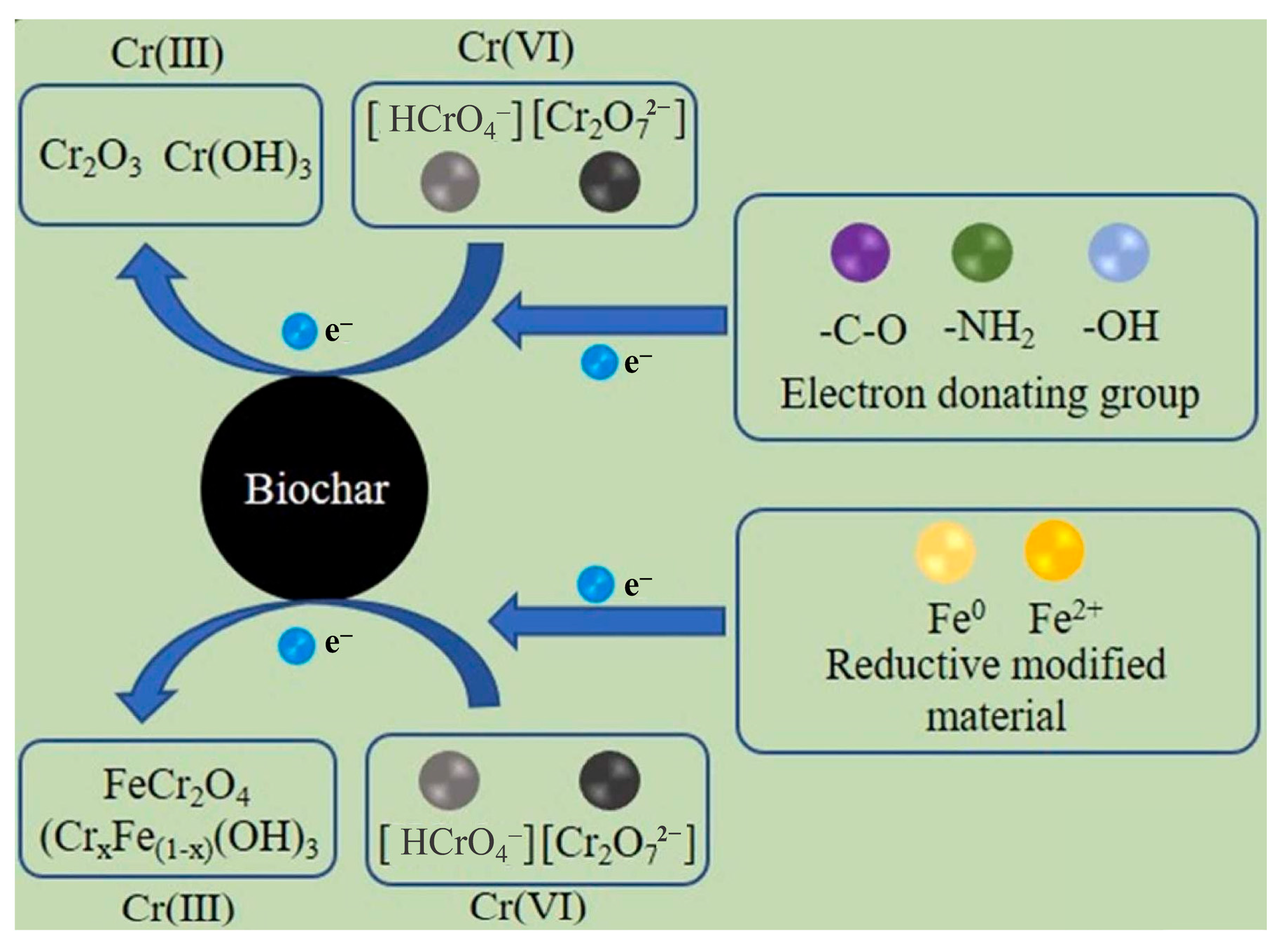
3.6. Reduction-Oxidation Reaction
3.6.1. Oxidation Reaction
| Raw Materials | Pyrolysis Temperature (°C) | Biochar pH Value | Modification Method | Soil pH Value | Biochar Addition | HM Immobilization Effect | Ref |
|---|---|---|---|---|---|---|---|
| Eggshell–corncob | 450 | 9.66 | / | 5.56 | 2% | −53.4% | [84] |
| Eggshell | 450 | 9.53 | / | 5.56 | 2% | −40.1% | |
| Corncob | 450 | 9.83 | / | 5.56 | 2% | −67.8% | |
| Rice straw | 400 | 8.32 | / | 4.65 | 2% | −26% | [83] |
| Peanut shells | 300 | 7.6 | / | 8.00 | 10% | −112% | [88] |
| Pine needles | 700 | 9.7 | / | 8.00 | 10% | −32% | |
| Rice straw | 300 | / | FeCl3 | 7.50 | 1% | 25% | [89] |
| Rice straw | 300 | / | Fe0 | 7.50 | 1% | 35% | [89] |
| Eggshell–corncob | 450 | 8.7 | FeSO4·7H2O | 5.56 | 2% | 56% | [90] |
| Red cedar sawdust | 300 | 9.7–10.3 | Fe3O4 | 5.7 | 9% | 20–30% | [91] |
| Sawdust | 800 | 8.3 | Fe2O3 | 8 | 2–5% | 93.7–97.7% | [52] |
| Corn stalks | 600 | 10.8 | KMnSO4 | 7.21 | 1% | 58% | [32] |
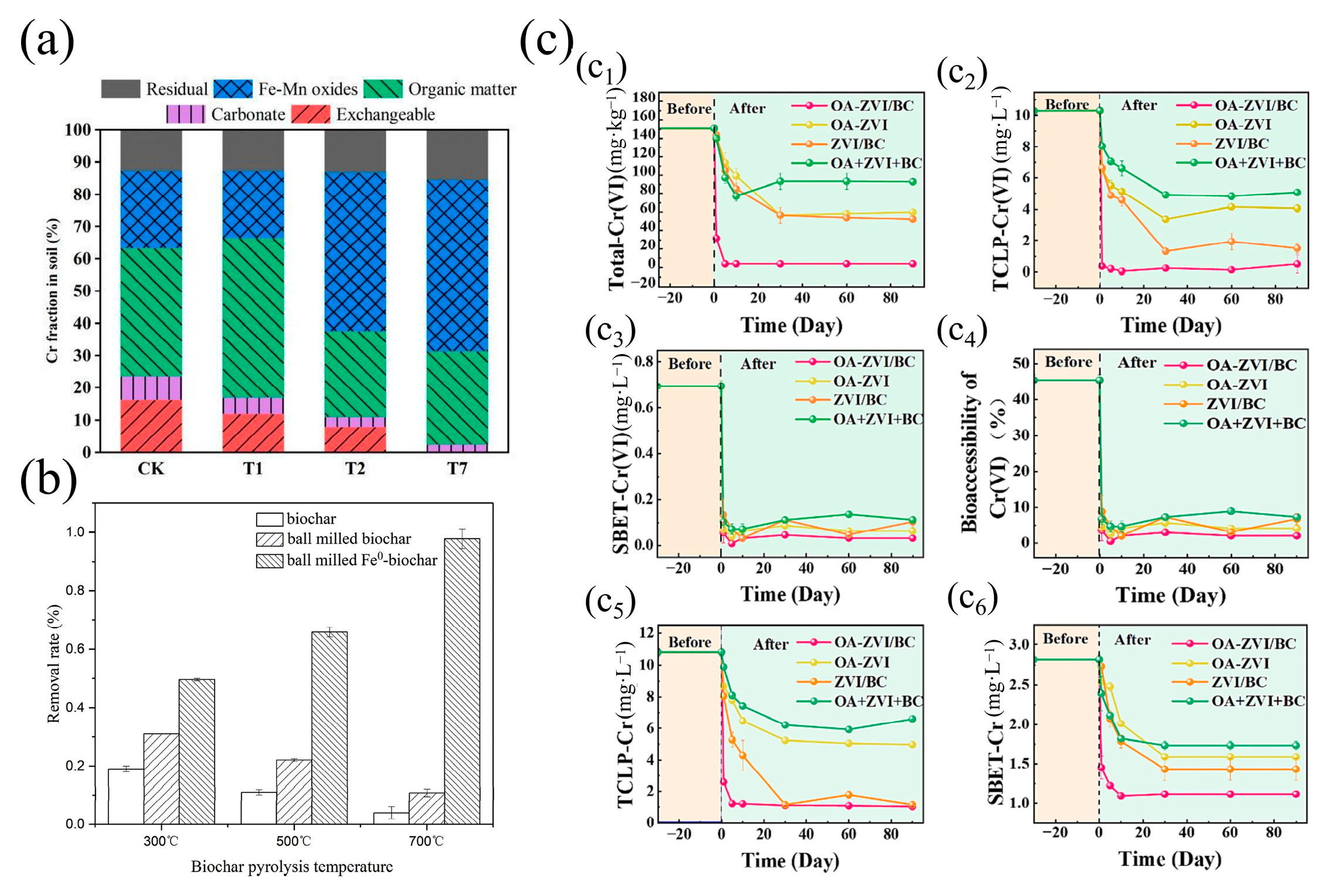
3.6.2. Reduction Reaction
3.7. Biochar in Synergistic Bioremediation
3.7.1. Biochar in Combination with Plants
3.7.2. Biochar in Combination with Microorganisms
4. Advantages of Biochar in Remediating HM-Contaminated Soil
4.1. Carbon Sequestration and Emission Reduction
4.2. Enhancing Soil Properties and Mitigating Erosion
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.L.; Song, S.; Wang, R.S.; Liu, Z.Y.; Meng, J.; Sweetman, A.J.; Jenkins, A.; Ferrier, R.C.; Li, H.; Luo, W.; et al. Impacts of soil and water pollution on food safety and health risks in China. Environ. Int. 2015, 77, 5–15. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef]
- Xiang, M.T.; Li, Y.; Yang, J.Y.; Lei, K.G.; Li, Y.; Li, F.; Zheng, D.F.; Fang, X.Q.; Cao, Y. Heavy metal contamination risk assessment and correlation analysis of heavy metal contents in soil and crops. Environ. Pollut. 2021, 278, 116911. [Google Scholar] [CrossRef]
- Mununga Katebe, F.; Raulier, P.; Colinet, G.; Ngoy Shutcha, M.; Mpundu Mubemba, M.; Jijakli, M.H. Assessment of Heavy Metal Pollution of Agricultural Soil, Irrigation Water, and Vegetables in and Nearby the Cupriferous City of Lubumbashi, (Democratic Republic of the Congo). Agronomy 2023, 13, 357. [Google Scholar] [CrossRef]
- Ngure, V.; Kinuthia, G. Health risk implications of lead, cadmium, zinc, and nickel for consumers of food items in Migori Gold mines, Kenya. J. Geochem. Explor. 2020, 209, 106430. [Google Scholar] [CrossRef]
- Kumar, R.; Ivy, N.; Bhattacharya, S.; Dey, A.; Sharma, P. Coupled effects of microplastics and heavy metals on plants: Uptake, bioaccumulation, and environmental health perspectives. Sci. Total Environ. 2022, 836, 155619. [Google Scholar] [CrossRef]
- Liu, B.L.; Ai, S.W.; Zhang, W.Y.; Huang, D.J.; Zhang, Y.M. Assessment of the bioavailability, bioaccessibility and transfer of heavy metals in the soil-grain-human systems near a mining and smelting area in NW China. Sci. Total Environ. 2017, 609, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.J.; Cai, C.; Chi, H.F.; Reid, B.J.; Coulon, F.; Zhang, Y.; Hou, Y. Remediation of cadmium and lead polluted soil using thiol-modified biochar. J. Hazard. Mater. 2020, 388, 122037. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Liu, J.; Xiao, T.; Lin, K.; Huang, Y.; Deng, P.; Hu, H.; Wang, J. Remediation of uranium-contaminated water and soil by biochar-based materials: A review. Biochar 2025, 7, 41. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of heavy metals in contaminated soil by phytoremediation mechanism: A review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Sui, X.; Wang, X.M.; Li, Y.H.; Ji, H.B. Remediation of petroleum-contaminated soils with microbial and microbial combined methods: Advances, mechanisms, and challenges. Sustainability 2021, 13, 9267. [Google Scholar] [CrossRef]
- Liu, S.M.; Yang, B.; Liang, Y.S.; Xiao, Y.H.; Fang, J. Prospect of phytoremediation combined with other approaches for remediation of heavy metal-polluted soils. Environ. Sci. Pollut. Res. 2020, 27, 16069–16085. [Google Scholar] [CrossRef]
- Xu, D.M.; Fu, R.B.; Wang, J.X.; Shi, Y.X.; Guo, X.P. Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods-a critical review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Song, P.P.; Xu, D.; Yue, J.Y.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Chandra, S.; Medha, I.; Tiwari, A.K. The role of modified biochar for the remediation of coal mining-impacted contaminated soil: A Review. Sustainability 2023, 15, 3973. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.Q.; Ju, M.T.; Liu, L. Preparation and modification of biochar materials and their application in soil remediation. Appl. Sci. 2019, 9, 1365. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Improving electrochemical characteristics of plant roots by biochar is an efficient mechanism in increasing cations uptake by plants. Chemosphere 2023, 313, 137365. [Google Scholar] [CrossRef]
- Liu, J.J.; Hou, Q.D.; Ju, M.T.; Ji, P.; Sun, Q.; Li, W. Biomass pyrolysis technology by catalytic fast pyrolysis, catalytic co-pyrolysis and microwave-assisted pyrolysis: A review. Catalysts 2020, 10, 742. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, C.; Guo, S.; Xu, L.; Xiao, S.; Shen, Z. Microwave treatment of pre-oxidized fibers for improving their structure and mechanical properties. Ceram. Int. 2019, 45, 1379–1384. [Google Scholar] [CrossRef]
- Zhang, P.Z.; Zhang, X.X.; Yuan, X.R.; Xie, R.; Han, L. Characteristics, adsorption behaviors, Cu(II) adsorption mechanisms by cow manure biochar derived at various pyrolysis temperatures. Bioresour. Technol. 2021, 331, 125013. [Google Scholar] [CrossRef]
- Abdelhafez, A.A.; Li, J.H.; Abbas, M.H.H. Feasibility of biochar manufactured from organic wastes on the stabilization of heavy metals in a metal smelter contaminated soil. Chemosphere 2014, 117, 66–71. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Gao, B.; Zimmerman, A.R.; Fang, J.; Sun, Y.; Cao, X. Sorption of heavy metals on chitosan-modified biochars and its biological effects. Chem. Eng. J. 2013, 231, 512–518. [Google Scholar] [CrossRef]
- Zhang, G.X.; Guo, X.F.; Zhao, Z.H.; He, Q.S.; Wang, S.F.; Zhu, Y.; Yan, Y.L.; Liu, X.T.; Sun, K.; Zhao, Y.; et al. Effects of biochars on the availability of heavy metals to ryegrass in an alkaline contaminated soil. Environ. Pollut. 2016, 218, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Usman, A.R.A.; Al-Faraj, A.S.; Ahmad, M.; Sallam, A.; Al-Wabel, M.I. Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants. Chemosphere 2018, 194, 327–339. [Google Scholar] [CrossRef]
- Karimi, A.; Moezzi, A.; Chorom, M.; Enayatizamir, N. Chemical fractions and availability of Zn in a calcareous soil in response to biochar amendments. J. Soil Sci. Plant Nutr. 2019, 19, 851–864. [Google Scholar] [CrossRef]
- Ahmad, J.; Patuzzi, F.; Rashid, U.; Shahabz, M.; Ngamcharussrivichai, C.; Baratieri, M. Exploring untapped effect of process conditions on biochar characteristics and applications. Environ. Technol. Innov. 2021, 21, 101310. [Google Scholar] [CrossRef]
- Rees, F.; Simonnot, M.O.; Morel, J.L. Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Bio/Technol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Trakal, L.; Bingöl, D.; Pohorely, M.; Hruska, M.; Komárek, M. Geochemical and spectroscopic investigations of Cd and Pb sorption mechanisms on contrasting biochars: Engineering implications. Bioresour. Technol. 2014, 171, 442–451. [Google Scholar] [CrossRef]
- Oustriere, N.; Marchand, L.; Galland, W.; Gabbon, L.; Lottier, N.; Motelica, M.; Mench, M. Influence of biochars, compost and iron grit, alone and in combination, on copper solubility and phytotoxicity in a Cu-contaminated soil from a wood preservation site. Sci. Total Environ. 2016, 566, 816–825. [Google Scholar] [CrossRef]
- Ahmad, M.; Ok, Y.S.; Kim, B.Y.; Ahn, J.H.; Lee, Y.H.; Zhang, M.; Moon, D.H.; Al-Wabel, M.I.; Lee, S.S. Impact of soybean stover- and pine needle-derived biochars on Pb and As mobility, microbial community, and carbon stability in a contaminated agricultural soil. J. Environ. Manag. 2016, 166, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.H.; Qiu, W.W.; Wang, F.; Lei, M.; Wang, D.; Song, Z. Effects of manganese oxide-modified biochar composites on arsenic speciation and accumulation in an indica rice (Oryza sativa L.) cultivar. Chemosphere 2017, 168, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.K.; Xu, F.; Xie, Y.L.; Wang, C.; Zhang, A.K.; Li, L.L.; Xu, H. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci. Total Environ. 2018, 645, 702–709. [Google Scholar] [CrossRef]
- Freitas, A.M.; Nair, V.D.; Harris, W.G. Biochar as influenced by feedstock variability: Implications and opportunities for phosphorus management. Front. Sustain. Food Syst. 2020, 4, 510982. [Google Scholar] [CrossRef]
- Manikandan, S.K.; Pallavi, P.; Shetty, K.; Bhattacharjee, D.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Nair, V. Effective usage of biochar and microorganisms for the removal of heavy metal ions and pesticides. Molecules 2023, 28, 719. [Google Scholar] [CrossRef]
- Li, G.; Khan, S.; Ibrahim, M.; Sun, T.-R.; Tang, J.-F.; Cotner, J.B.; Xu, Y.-Y. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J. Hazard. Mater. 2018, 348, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.E.; Tsang, D.C.W.; Zhou, F.; Zhang, W.; Qiu, R. Stabilization of cationic and anionic metal species in contaminated soils using sludge-derived biochar. Chemosphere 2016, 149, 263–271. [Google Scholar] [CrossRef]
- Ambika, S.; Kumar, M.; Pisharody, L.; Malhotra, M.; Kumar, G.; Sreedharan, V.; Singh, L.; Nidheesh, P.; Bhatnagar, A. Modified biochar as a green adsorbent for removal of hexavalent chromium from various environmental matrices: Mechanisms, methods, and prospects. Chem. Eng. J. 2022, 439, 135716. [Google Scholar] [CrossRef]
- Lobzenko, I.; Burachevskaya, M.; Zamulina, I.; Barakhov, A.; Bauer, T.; Mandzhieva, S.; Sushkova, S.; Minkina, T.; Tereschenko, A.; Kalinichenko, V.; et al. Development of a unique technology for the pyrolysis of rice husk biochar for promising heavy metal remediation. Agriculture 2022, 12, 1689. [Google Scholar] [CrossRef]
- Awang, N.A.; Salleh, W.N.W.; Aziz, F.; Yusof, N.; Ismail, A.F. A review on preparation, surface enhancement and adsorption mechanism of biochar-supported nano zero-valent iron adsorbent for hazardous heavy metals. J. Chem. Technol. Biotechnol. 2023, 98, 22–44. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Fang, J.; Gao, B.; Chen, J.J.; Zimmerman, A.R. Hydrochars derived from plant biomass under various conditions: Characterization and potential applications and impacts. Chem. Eng. J. 2015, 267, 253–259. [Google Scholar] [CrossRef]
- Shen, Z.T.; Zhang, J.Z.; Hou, D.Y.; Tsang, D.C.W.; Ok, Y.S.; Alessi, D.S. Synthesis of MgO-coated corncob biochar and its application in lead stabilization in a soil washing residue. Environ. Int. 2019, 122, 357–362. [Google Scholar] [CrossRef]
- Song, Z.G.; Lian, F.; Yu, Z.H.; Zhu, L.; Xing, B.; Qiu, W. Synthesis and characterization of a novel MnOx-loaded biochar and its adsorption properties for Cu2+ in aqueous solution. Chem. Eng. J. 2014, 242, 36–42. [Google Scholar] [CrossRef]
- Liu, S.C.; Xie, Z.L.; Zhu, Y.T.; Zhu, Y.; Jiang, Y.; Wang, Y.; Gao, H. Adsorption characteristics of modified rice straw biochar for Zn and in-situ remediation of Zn contaminated soil. Environ. Technol. Innov. 2021, 22, 101388. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Ma, J.; Chen, F.; Yu, R.L.; Hu, G.R.; Zhang, S.L. Remediation of soil polluted with Cd in a postmining area using thiourea-modified biochar. Int. J. Environ. Res. Public Health 2020, 17, 7654. [Google Scholar] [CrossRef]
- Rodriguez, A.; Lemos, D.; Trujillo, Y.T.; Amaya, J.G.; Ramos, L.D. Effectiveness of biochar obtained from corncob for immobilization of lead in contaminated soil. J. Health Pollut. 2019, 9, 190907. [Google Scholar] [CrossRef]
- He, L.Z.; Zhong, H.; Liu, G.X.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Gao, Y.N.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.; Gao, B.; Wang, S.; Wang, B. Biochar as a potential strategy for remediation of contaminated mining soils: Mechanisms, applications, and future perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, Y.G.; Zeng, G.M.; Wang, X.; Hu, X.; Gu, Y.; Yang, Z. Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 2015, 125, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.Q.; Ren, J.; Tao, L.; Ren, H.R.; Wang, M.; Wang, B.Q. Study of the remediation effect and mechanism of biochar-loaded nZVI on heavy metal contaminated soil. Sustainability 2023, 15, 16753. [Google Scholar] [CrossRef]
- Fan, J.; Chen, X.; Xu, Z.B.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. One-pot synthesis of nZVI-embedded biochar for remediation of two mining arsenic-contaminated soils: Arsenic immobilization associated with iron transformation. J. Hazard. Mater. 2020, 398, 122901. [Google Scholar] [CrossRef]
- Yu, Y.; An, Q.; Jin, L.; Luo, N.; Li, Z.; Jiang, J.N. Unraveling sorption of Cr (VI) from aqueous solution by FeCl3 and ZnCl2-modified corn stalks biochar: Implicit mechanism and application. Bioresour. Technol. 2020, 297, 122466. [Google Scholar] [CrossRef]
- Li, A.Y.; Ge, W.Z.; Liu, L.H.; Zhang, Y.T.; Qiu, G.H. Synthesis and application of amine-functionalized MgFe2O4-biochar for the adsorption and immobilization of Cd(II) and Pb(II). Chem. Eng. J. 2022, 439, 135785. [Google Scholar] [CrossRef]
- Song, P.P.; Ma, W.J.; Gao, X.Y.; Ai, S.Y.; Wang, J.; Liu, W.R. Remediation mechanism of Cu, Zn, As, Cd, and Pb contaminated soil by biochar-supported nanoscale zero-valent iron and its impact on soil enzyme activity. J. Clean. Prod. 2022, 378, 134510. [Google Scholar] [CrossRef]
- Wang, Y.F.; Li, J.N.; Li, Q.N.; Xu, L.; Ai, Y.H.; Liu, W.; Zhou, Y.T.; Zhang, B.Y.; Guo, N.; Cao, B.; et al. Effective amendment of cadmium in water and soil before and after aging of nitrogen-doped biochar: Preparation optimization, removal efficiency and mechanism. J. Hazard. Mater. 2024, 477, 135356. [Google Scholar] [CrossRef]
- Yang, T.T.; Xu, Y.M.; Huang, Q.Q.; Sun, Y.B.; Liang, X.F.; Wang, L.; Qin, X.; Zhao, L.J. An efficient biochar synthesized by iron-zinc modified corn straw for simultaneously immobilization Cd in acidic and alkaline soils. Environ. Pollut. 2021, 291, 118129. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Liu, J.; Li, Q.B.; Liu, Z.C.; Xia, K.; Hu, L.; Wu, X.X.; Yan, Q. Highly effective adsorption and passivation of Cd from wastewater and soil by MgO- and Fe3O4-loaded biochar nanocomposites. Front. Environ. Sci. 2023, 11, 1239842. [Google Scholar] [CrossRef]
- Cai, T.; Liu, X.L.; Zhang, J.C.; Tie, B.Q.; Lei, M.; Wei, X.D.; Peng, O.; Du, H.H. Silicate-modified oiltea camellia shell-derived biochar: A novel and cost-effective sorbent for cadmium removal. J. Clean. Prod. 2021, 281, 125390. [Google Scholar] [CrossRef]
- Ji, Y.N.; Zheng, N.; An, Q.R.; Wang, S.; Sun, S.; Li, X.; Chen, C.; Sun, S.; Jiang, Y. Enhanced immobilization of cadmium and lead in contaminated soil using calcium alginate-modified HAP biochar: Improvements in soil health and microbial diversity. Environ. Pollut. 2024, 357, 124445. [Google Scholar] [CrossRef]
- Lyu, P.; Li, L.F.; Huang, J.L.; Ye, J.; Zhu, C.X. Magnetic biochar-supported layered double hydroxide for simultaneous remediation of As and Cd in soil: Effectiveness, retention durability, and insight into a new immobilization mechanism. J. Clean. Prod. 2024, 434, 140136. [Google Scholar] [CrossRef]
- Huang, D.L.; Deng, R.; Wan, J.; Zeng, G.M.; Xue, W.J.; Wen, X.F.; Zhou, C.Y.; Hu, L.; Liu, X.G.; Xu, P.; et al. Remediation of lead-contaminated sediment by biochar-supported nano-chlorapatite: Accompanied with the change of available phosphorus and organic matters. J. Hazard. Mater. 2018, 348, 109–116. [Google Scholar] [CrossRef]
- Park, J.H.; Choppala, G.K.; Bolan, N.S.; Chung, J.W.; Chuasavathi, T. Biochar reduces the bioavailability and phytotoxicity of heavy metals. Plant Soil 2011, 348, 439–451. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Kwon, E.E.; Vithanage, M.; Rinklebe, J.; Moon, D.H.; Meers, E.; Tsang, D.C.W.; Ok, Y.S. Soil lead immobilization by biochars in short-term laboratory incubation studies. Environ. Int. 2019, 127, 190–198. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Wang, S.L.; Lehmann, J.; Rennhofer, H.; Ottner, F.; Wriessnig, K.; Daudin, G.; Lichtenegger, H.; Soja, G.; et al. Enhanced Cu and Cd sorption after soil aging of woodchip-derived biochar: What were the driving factors? Chemosphere 2019, 216, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.Q.; Fang, S.Y.; Yao, Y.Q.; Li, T.Q.; Ni, Q.J.; Yang, X.E.; He, Z.L. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci. Total Environ. 2016, 562, 517–525. [Google Scholar] [CrossRef]
- Ullah, M.H.; Rahman, M.J. Adsorptive removal of toxic heavy metals from wastewater using water hyacinth and its biochar: A review. Heliyon 2024, 10, e36869. [Google Scholar] [CrossRef]
- Jiang, J.H.; Li, R.Y.; Yang, K.X.; Li, Y.; Deng, L.; Che, D. Investigation on Pb2+ adsorption characteristics by AAEMs-rich biochar in aqueous solution: Performance and mechanism. Environ. Res. 2023, 236, 116731. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Yuan, X.Z.; Xiong, T.; Jiang, L.; Wang, H.; Wu, Z. Biochar facilitated hydroxyapatite/calcium silicate hydrate for remediation of heavy metals contaminated soils. Water Air Soil Pollut. 2020, 231, 66. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Hussain, B.; Usman, M.; Gurajala, H.K.; Rashid, M.S.; He, Z.; Yang, X. Efficiency of lime, biochar, Fe containing biochar and composite amendments for Cd and Pb immobilization in a co-contaminated alluvial soil. Environ. Pollut. 2020, 257, 113609. [Google Scholar] [CrossRef]
- Soria, R.I.; Rolfe, S.A.; Betancourth, M.P.; Thornton, S.F. The relationship between properties of plant-based biochars and sorption of Cd(II), Pb(II) and Zn(II) in soil model systems. Heliyon 2020, 6, e05388. [Google Scholar] [CrossRef]
- Diao, Y.Z.; Zhou, L.; Ji, M.Y.; Wang, X.X.; Dan, Y.T.; Sang, W.J. Immobilization of Cd and Pb in soil facilitated by magnetic biochar: Metal speciation and microbial community evolution. Environ. Sci. Pollut. Res. 2022, 29, 71871–71881. [Google Scholar] [CrossRef]
- Sun, T.; Xu, Y.M.; Sun, Y.B.; Wang, L.; Liang, X.F.; Jia, H.T. Crayfish shell biochar for the mitigation of Pb contaminated water and soil: Characteristics, mechanisms, and applications. Environ. Pollut. 2021, 271, 116308. [Google Scholar] [CrossRef]
- Lu, H.P.; Li, Z.A.; Gascó, G.; Méndez, A.; Shen, Y.; Paz-Ferreiro, J. Use of magnetic biochars for the immobilization of heavy metals in a multi-contaminated soil. Sci. Total Environ. 2018, 622, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.C.; Ma, S.L.; Xu, S.J.; Duan, R.; Cheng, G.; Zhao, P. Hierarchically porous magnetic biochar as an efficient amendment for cadmium in water and soil: Performance and mechanism. Chemosphere 2021, 281, 130990. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.X.; Wang, Y.H.; Zhang, L.M.; Huang, Q.Y. Study of the mechanism of remediation of Cd-contaminated soil by novel biochars. Environ. Sci. Pollut. Res. 2017, 24, 24844–24855. [Google Scholar] [CrossRef]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen containing functional groups of biochar: An overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, A.; Ahmed, N.; Mosa, A.; Niazi, N.K.; Yousaf, B.; Sharma, A.; Sarkar, B.; Cai, Y.; Chang, S.X. Nickel in soil and water: Sources, biogeochemistry, and remediation using biochar. J. Hazard. Mater. 2021, 419, 126421. [Google Scholar] [CrossRef]
- Zhou, Q.W.; Liao, B.H.; Lin, L.N.; Qiu, W.; Song, Z. Adsorption of Cu(II) and Cd(II) from aqueous solutions by ferromanganese binary oxide-biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Gong, H.F.; Chi, J.; Ding, Z.; Zhang, F.; Huang, J.J. Removal of lead from two polluted soils by magnetic wheat straw biochars. Ecotoxicol. Environ. Saf. 2020, 205, 111132. [Google Scholar] [CrossRef]
- Xu, X.; Huang, H.; Zhang, Y.; Xu, Z.; Cao, X. Biochar as both electron donor and electron shuttle for the reduction transformation of Cr(VI) during its sorption. Environ. Pollut. 2019, 244, 423–430. [Google Scholar] [CrossRef]
- Zhong, D.; Jiang, Y.; Zhao, Z.; Wang, L.; Chen, J.; Ren, S.; Liu, Z.; Zhang, Y.; Tsang, D.C.W.; Crittenden, J.C. pH Dependence of Arsenic Oxidation by Rice-Husk-Derived Biochar: Roles of Redox-Active Moieties. Environ. Sci. Technol. 2019, 53, 9034–9044. [Google Scholar] [CrossRef]
- Wu, J.Z.; Li, Z.T.; Huang, D.; Liu, X.; Tang, C.; Parikh, S.J.; Xu, J. A novel calcium-based magnetic biochar is effective in stabilization of arsenic and cadmium co-contamination in aerobic soils. J. Hazard. Mater. 2020, 387, 122010. [Google Scholar] [CrossRef]
- Islam, M.S.; Magid, A.S.I.A.; Chen, Y.; Weng, L.; Arafat, M.Y.; Khan, Z.H.; Ma, J.; Li, Y. Arsenic and cadmium load in rice tissues cultivated in calcium enriched biochar amended paddy soil. Chemosphere 2021, 283, 131102. [Google Scholar] [CrossRef] [PubMed]
- Arabi, Z.; Rinklebe, J.; El-Naggar, A.; Hou, D.Y.; Sarmah, A.K.; Moreno-Jiménez, E. (Im)mobilization of arsenic, chromium, and nickel in soils via biochar: A meta-analysis. Environ. Pollut. 2021, 286, 117199. [Google Scholar] [CrossRef]
- Zhang, W.; Cho, Y.; Vithanage, M.; Shaheen, S.M.; Rinklebe, J.; Alessi, D.S.; Hou, C.H.; Hashimoto, Y.; Withana, P.A.; Ok, Y.S. Arsenic removal from water and soils using pristine and modified biochars. Biochar 2022, 4, 55. [Google Scholar] [CrossRef]
- Gong, H.B.; Zhao, L.; Rui, X.; Hu, J.; Zhu, N. A review of pristine and modified biochar immobilizing typical heavy metals in soil: Applications and challenges. J. Hazard. Mater. 2022, 432, 128668. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.S.; Lee, S.E.; Al-Wabel, M.I.; Tsang, D.C.W.; Ok, Y.S. Biochar-induced changes in soil properties affected immobilization/mobilization of metals/metalloids in contaminated soils. J. Soils Sediments 2017, 17, 717–730. [Google Scholar] [CrossRef]
- Wu, C.; Cui, M.Q.; Xue, S.G.; Li, W.C.; Huang, L.; Jiang, X.X.; Qian, Z.Y. Remediation of arsenic-contaminated paddy soil by iron-modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 20792–20801. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Magid, A.S.I.A.; Chen, Y.; Weng, L.; Ma, J.; Arafat, M.Y.; Khan, Z.H.; Li, Y. Effect of calcium and iron-enriched biochar on arsenic and cadmium accumulation from soil to rice paddy tissues. Sci. Total Environ. 2021, 785, 147163. [Google Scholar] [CrossRef]
- Wan, X.M.; Li, C.Y.; Parikh, S.J. Simultaneous removal of arsenic, cadmium, and lead from soil by iron-modified magnetic biochar. Environ. Pollut. 2020, 261, 114157. [Google Scholar] [CrossRef]
- Li, M.H.; Guo, X.L.; Wei, Y.; Liu, A.; Lu, J.; Niu, X.; Ma, Y.; Li, S.; Shang, Z.; Liu, X. Adsorption mechanism and structure-performance relationship of chromium ions by biochar. Water Air Soil Pollut. 2020, 231, 517. [Google Scholar] [CrossRef]
- Ding, K.L.; Zhou, X.Y.; Hadiatullah, H.; Lu, Y.; Zhao, G.; Jia, S.; Zhang, R.; Yao, Y. Removal performance and mechanisms of toxic hexavalent chromium (Cr(VI)) with ZnCl2 enhanced acidic vinegar residue biochar. J. Hazard. Mater. 2021, 420, 126551. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; Nguyen, D.T.; Le, G.T.; Tomul, F.; Lima, E.C.; Woo, S.H.; Sarmah, A.K.; Hung Quang, N.; Phuong Tri, N.; Dinh Duc, N.; et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: A systematic in-depth review. J. Hazard. Mater. 2019, 373, 258–270. [Google Scholar] [CrossRef]
- Yang, J.W.; Tan, X.P.; Shaaban, M.; Cai, Y.J.; Wang, B.Y.; Peng, Q.A. Remediation of Cr(VI)-contaminated soil by biochar-supported nanoscale zero-valent iron and the consequences for indigenous microbial communities. Nanomaterials 2022, 12, 3541. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Sun, Y.B.; Tang, J.C.; He, J.; Sun, H. Aqueous Cr(VI) removal by a novel ball milled Fe0-biochar composite: Role of biochar electron transfer capacity under high pyrolysis temperature. Chemosphere 2020, 241, 125044. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Chen, Q.J.; Liu, Y.Y.; Ma, Q.; Zhang, J.; Tang, C.; Duan, G.; Lin, A.; Zhang, T.; Li, S. Enhanced remediation of Cr(VI)-contaminated soil by modified zero-valent iron with oxalic acid on biochar. Sci. Total Environ. 2023, 905, 167399. [Google Scholar] [CrossRef]
- Li, X.N.; Li, R.P.; Zhan, M.Q.; Hou, Q.; Zhang, H.Y.; Wu, G.Q.; Ding, L.Q.; Lv, X.F.; Xu, Y. Combined magnetic biochar and ryegrass enhanced the remediation effect of soils contaminated with multiple heavy metals. Environ. Int. 2024, 185, 108498. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Z.W.; Liu, C.J.; Dong, Y. Technologies for removing heavy metal from contaminated soils on farmland: A review. Chemosphere 2022, 305, 135457. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.Y.; Su, A.X.; Lv, X.; Qiu, Y.; Xu, Y. Co-planting improves the phytoremediation efficiency of combined phenanthrene and copper co-contaminated soils. J. Clean. Prod. 2023, 382, 135380. [Google Scholar] [CrossRef]
- Jun, L.; Wei, H.; Aili, M.; Juan, N.; Hongyan, X.; Jingsong, H.; Yunhua, Z.; Cuiying, P. Effect of lychee biochar on the remediation of heavy metal-contaminated soil using sunflower: A field experiment. Environ. Res. 2020, 188, 109886. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Fan, Z.; Zhang, G.L.; Qin, L.; Fang, J. Application progress of microbial immobilization technology based on biomass materials. Bioresources 2021, 16, 8509–8524. [Google Scholar] [CrossRef]
- Liu, Y.L.; Tie, B.Q.; Peng, O.; Luo, H.Y.; Li, D.Y.; Liu, S.T.; Lei, M.; Wei, X.D.; Liu, X.L.; Du, H.H. Inoculation of Cd-contaminated paddy soil with biochar-supported microbial cell composite: A novel approach to reducing cadmium accumulation in rice grains. Chemosphere 2020, 247, 125850. [Google Scholar] [CrossRef]
- Chen, H.M.; Zhang, J.W.; Tang, L.Y.; Su, M.; Tian, D.; Zhang, L.; Li, Z.; Hu, S.J. Enhanced Pb immobilization via the combination of biochar and phosphate solubilizing bacteria. Environ. Int. 2019, 127, 395–401. [Google Scholar] [CrossRef]
- Chen, J.Q.; Dong, J.; Chang, J.J.; Guo, T.; Yang, Q.; Jia, W.; Shen, S. Characterization of an Hg(II)-volatilizing Pseudomonas sp. strain, DC-B1, and its potential for soil remediation when combined with biochar amendment. Ecotoxicol. Environ. Saf. 2018, 163, 172–179. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H.R.; Wu, J.Z.; Huang, L.; Brookes, P.C.; Mazza Rodrigues, J.L.; Xu, J.; Liu, X. Effects of magnetic biochar-microbe composite on Cd remediation and microbial responses in paddy soil. J. Hazard. Mater. 2021, 414, 125494. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of biochar application to the environment and economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Yang, F.; Wang, B.; Shi, Z.M.; Li, L.; Li, Y.; Mao, Z.; Liao, L.; Zhang, H.; Wu, Y. Immobilization of heavy metals (Cd, Zn, and Pb) in different contaminated soils with swine manure biochar. Environ. Pollut. Bioavailab. 2021, 33, 55–65. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Feng, D.; Guo, D.; Zhang, Y.; Sun, S.; Zhao, Y.; Shang, Q.; Sun, H.; Wu, J.; Tan, H. Functionalized construction of biochar with hierarchical pore structures and surface O-/N-containing groups for phenol adsorption. Chem. Eng. J. 2021, 410, 127707. [Google Scholar] [CrossRef]


| Raw Material | Temperature (°C) | Time (h) | Rate (°C·min−1) | N% | C% | pH Value | CEC (cmol·kg−1) | C/H | C/N | Ash (%) | SSA (m2·g−1) | Average Pore Size (nm) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cow manure | 300 | 1 | 20 | 2.72 | 38.97 | 8.62 | 185.89 a | 0.93 | / | 34.54 | 3.42 c | 4.44 | [20] |
| Cow manure | 700 | 1 | 20 | 1.54 | 34.76 | 10.83 | 147.52 a | 3.44 | / | 50.45 | 10.61 c | 16.1 | [20] |
| Orange peel | 500 | / | / | 2.10 | 66 | 8.80 | 68.28 a | 1.52 | 36.66 | 11 | 0.21 c | / | [21] |
| Sugar cane bagasse | 500 | / | / | 1.00 | 74 | 9.60 | 69.60 a | 2.37 | 86.33 | 12 | 92.30 c | 65 | [21] |
| Peanut hull | 600 | 2 | / | 0.94 | 86.39 | 6.90 | / | / | 91.90 | / | 27.10 c | / | [22] |
| Walnut shells | 200 | 4 | 15 | 0.30 | 61.94 | 6.47 | / | 0.95 | 240.8 | 1.41 | 1.73 c | / | [23] |
| Walnut shells | 600 | 4 | 15 | 0.30 | 90.56 | 10.33 | / | 3.57 | 352.2 | 1.57 | 315.1 c | / | [23] |
| Date palm leaf | 600 | 2 | 5 | 0 | 76.23 | 10.23 | 39.86 b | 6.35 | / | 38.68 | 164.7 c | 4.92 | [24] |
| Bamboo | 600 | 2 | / | 0.15 | 80.89 | 7.9 | / | / | 539.3 | / | 470.4 c | / | [22] |
| Corn | 350 | 3 | 5 | 1.64 | 57.01 | 8.17 | 31.95 a | 1.22 | 34.72 | 28.13 | 32.1 c | / | [25] |
| Corn | 500 | 3 | 5 | 1.56 | 59.91 | 9.19 | 23.87 a | 2.32 | 38.49 | 33.00 | 61.83 c | / | [25] |
| Rice straws | 200 | 4 | 15 | 0.98 | 59.99 | 6.81 | / | 0.88 | 71.42 | 13.97 | 2.37 c | / | [23] |
| Rice straws | 600 | 4 | 15 | 1.67 | 90.79 | 12.39 | / | 3.13 | 63.43 | 27.22 | 211.9 c | / | [23] |
| Raw Material | Pyrolysis Temperature (°C) | Modification Method | SSA Before Modification (m2·g−1) | SSA After Modification (m2·g−1) | Pore Size Before Modification (nm) | Pore Size After Modification (nm) | Fixed HMs | Fixed Effect Before Modification | Fixed Effect After Modification | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Poplar bark | 600 | SC(NH2)2 impregnation | 2.77 c | 5.70 c | 1.94 | 6.42 | Cd2+ | 36.26% | 79.56% | [46] |
| Corncob | 600 | H2O2 modification | 80.14 c | 196.4 c | / | / | Pb2+ | 35.90% | 39.86% | [47] |
| Rice straw | 500 | KOH modification | 19.96 c | 54.66 c | 9.24 | 4.63 | Zn2+ | 22.54 mg·g−1 | 25.37 mg·g−1 | [45] |
| Corn stalks | 600 | KMnO4 modification | 61.0 c | 2.28 c | 23.70 | 92.20 | Cu2+ | / | 160 mg·g−1 | [44] |
| Rice straw | 500 | HCl-HF modification | 19.96 c | 22.45 c | 9.24 | 9.12 | Zn2+ | 22.54 mg·g−1 | 16.53 mg·g−1 | [45] |
| Rice straw | 500 | R-SH modification | 7.82 c | 0.34 c | 20.82 | 16.69 | Cd2+ | 41.20 mg·g−1 | 45.10 mg·g−1 | [8] |
| Raw Material | Temperature (°C) | Modification Method | Soil pH Value | Precipitation | Fixation Effect | Ref |
|---|---|---|---|---|---|---|
| Corn stalks | 900 | Nitrogen doped (urea) | / | CaC2O4, CaCO3 | After modification, the adsorption capacity of Cd2+ by biochar increased from 55.87 mg·g−1 to 74.37 mg·g−1 | [56] |
| Corn stalks | 500 | Zinc iron oxide | 5.69 | CdZnFe2O4 | After modification, the content of extractable Cd in soil decreased by 12.77–57.45% | [57] |
| 7.87 | In alkaline soil, DTPA-Cd content decreased by 23.73–52.50% | [57] | ||||
| Wheat straw | 500 | MgO | 7.4 | Cd(OH)2, CdCO3 | The extraction rate of Cd in soil decreased by 50.85% | [58] |
| Oiltea camellia shells | 500 | Na2SiO3 | / | CdCO3, CdSiO3 and Cd2SiO4 | After modification, the adsorption capacity of biochar for Cd2+ increased from 62.2 mg·g−1 to 120–211 mg·g−1 | [59] |
| Corncob | 600 | MgO | 9.14 | Pb(OH)2, Pb3(CO3)2(OH)2 | The extraction rate of Pb was reduced by 50.71% | [43] |
| Wood ear mushroom sticks | 650 | Calcium alginate | 5.36 | Ca2.5Pb7.5(OH)2(PO4)6 | The effective concentration of Pb was reduced by 31.9–78.6% | [60] |
| 7.46 | The effective concentration of Pb was reduced by 26.4–62.3% | [60] | ||||
| Bamboo | 800 | Iron, aluminum and magnesium metal oxides | 2.88 | As-Fe/Ca precipitate | Fixed more than 85% As | [61] |
| Raw Material | Pyrolysis Temperature (°C) | HM | Complexing Functional Group | Biochar Fixation Effect | Ref |
|---|---|---|---|---|---|
| Rice straw | 500 | Pb | -OH | The fixation rate of Pb in paddy soil was 50.47% | [72] |
| Crayfish shell | 300–700 | Pb | Ester group (-COOR) and -OH | The DTPA-Pb content in acidic soil was reduced by 1.87–16.48% | [73] |
| Poultry litter | 300–500 | Zn | -COOH or -OH | Reduced the content of acid-soluble Cd in soil by 8~10% | [74] |
| Wheat straw | 550 | Zn | -COOH, C=O and Ar-OH | / | [71] |
| Wheat straw | 700 | Cd | -COOH and -OH | Reduced the content of available Cd in soil by 47.97–61.38% | [75] |
| Rice straw | 400 | Cd | -COOR, C=O and -OH | The content of Cd in the roots, stems, and leaves of lettuce was reduced by 62–67% | [76] |
| Poultry litter | 300–500 | Cu | -COOH, Ar-OH, -OH and C=O | Reduced the acid-soluble Cd content in soil by 8–10% | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Ma, J.; Liu, K.; Zhang, S.; Wang, J. Biochar-Based Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Synergies, and Sustainable Prospects. Nanomaterials 2025, 15, 1487. https://doi.org/10.3390/nano15191487
Wei Y, Ma J, Liu K, Zhang S, Wang J. Biochar-Based Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Synergies, and Sustainable Prospects. Nanomaterials. 2025; 15(19):1487. https://doi.org/10.3390/nano15191487
Chicago/Turabian StyleWei, Yuxin, Jingjing Ma, Kuankuan Liu, Shuai Zhang, and Junqi Wang. 2025. "Biochar-Based Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Synergies, and Sustainable Prospects" Nanomaterials 15, no. 19: 1487. https://doi.org/10.3390/nano15191487
APA StyleWei, Y., Ma, J., Liu, K., Zhang, S., & Wang, J. (2025). Biochar-Based Remediation of Heavy Metal-Contaminated Soils: Mechanisms, Synergies, and Sustainable Prospects. Nanomaterials, 15(19), 1487. https://doi.org/10.3390/nano15191487