Viscous Rheological Behavior of Nanosuspensions of Fumed Silica Nanoparticles and Cellulose Nanocrystals
Abstract
1. Introduction
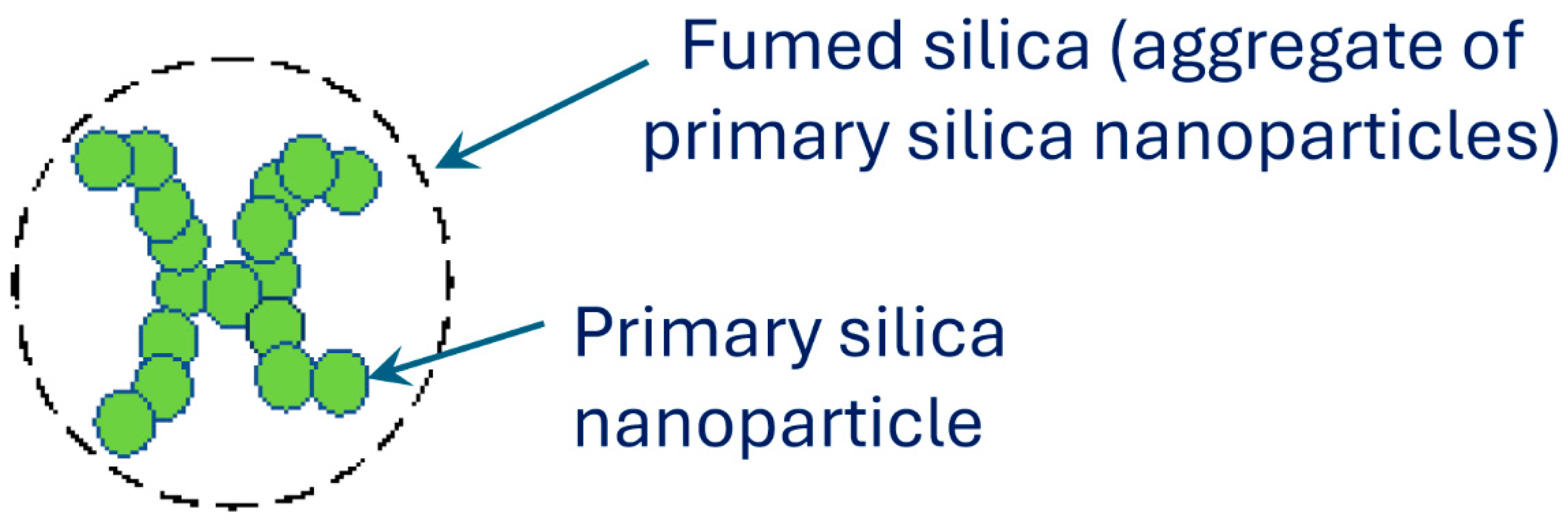
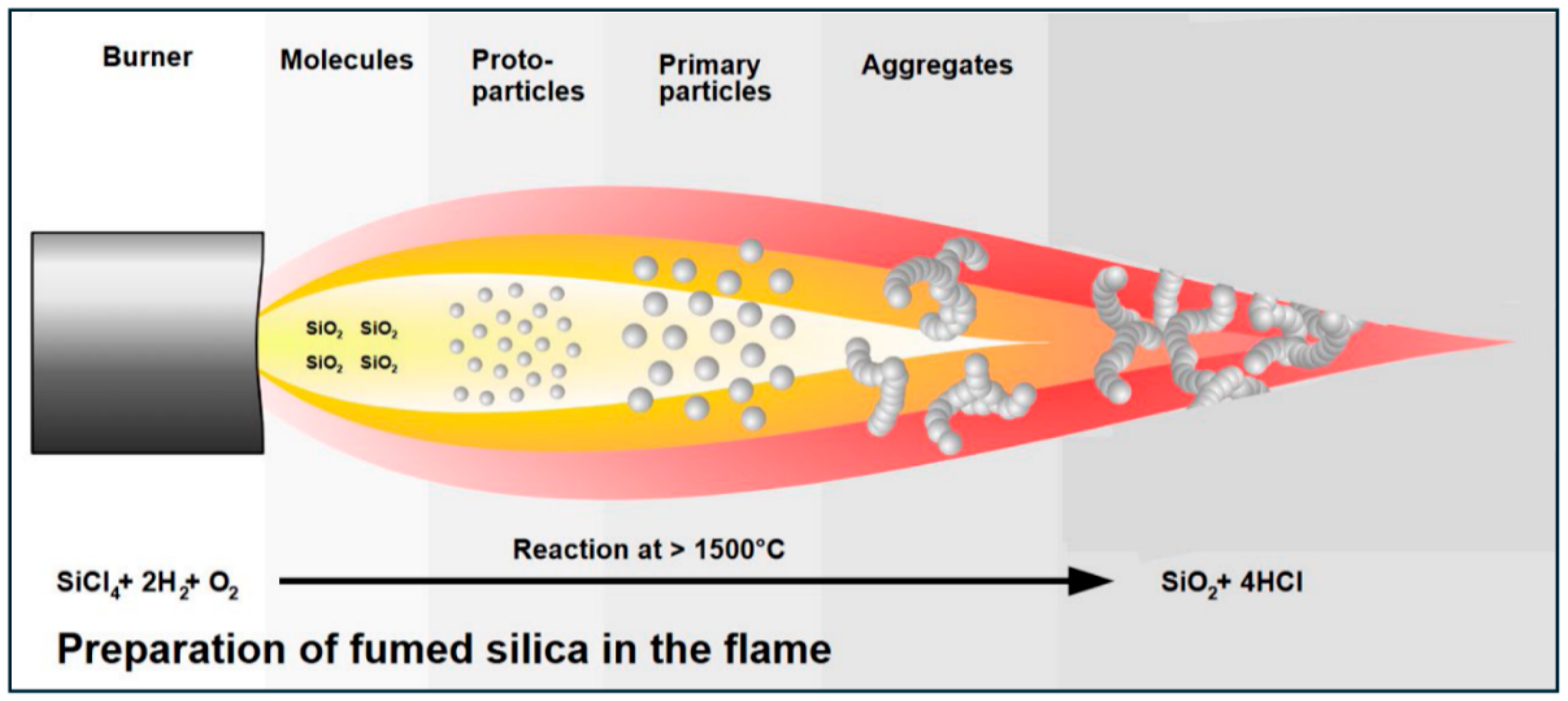
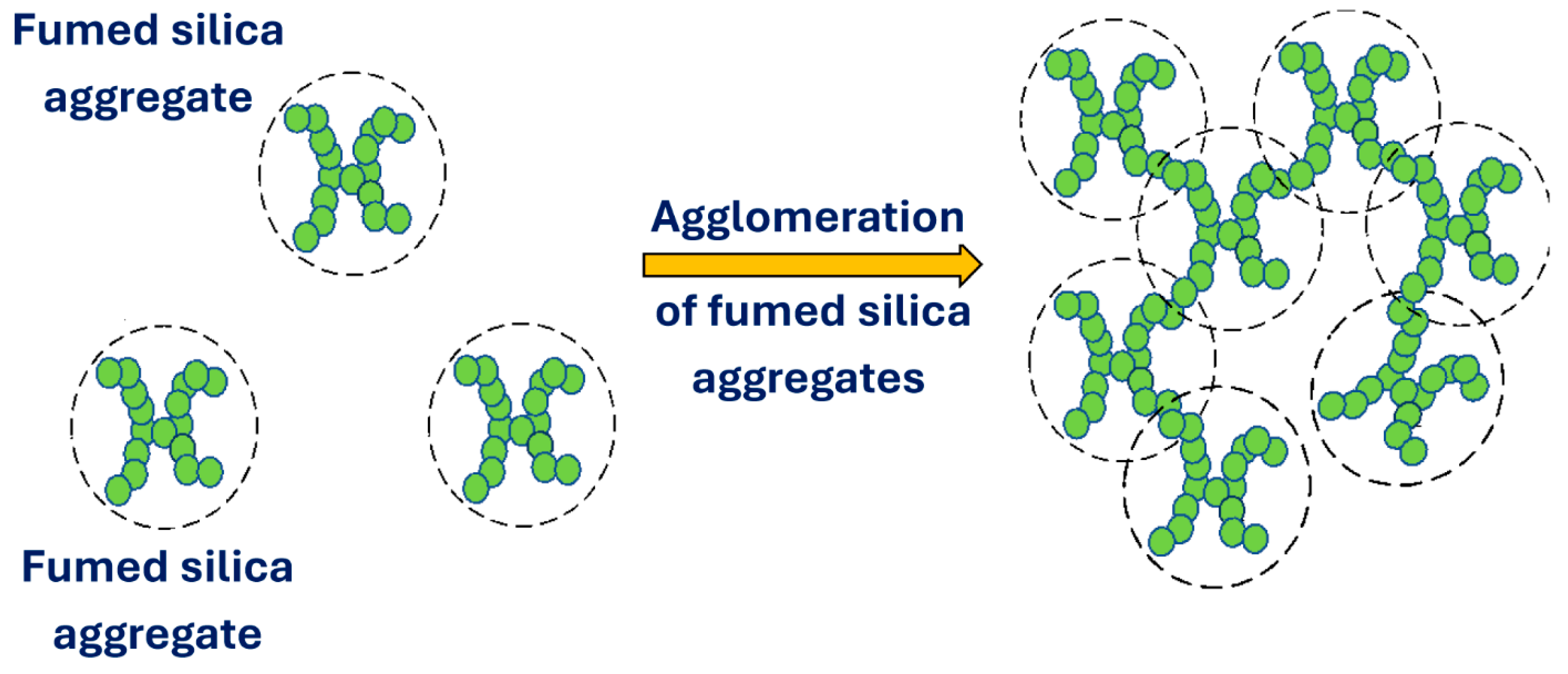
1.1. Fumed Silica
1.2. Nanocrystalline Cellulose
2. Materials and Methods
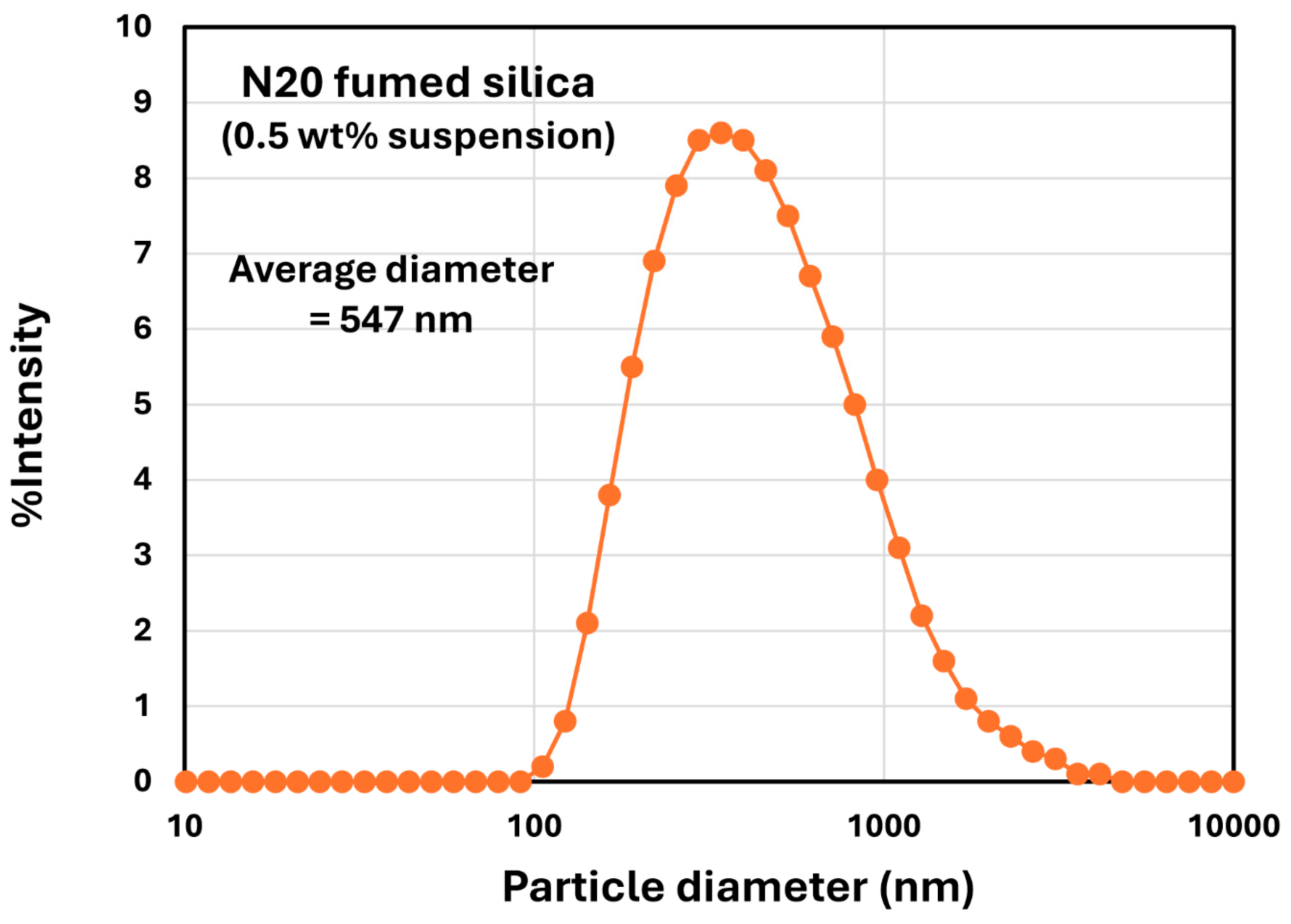
2.1. Materials
2.2. Preparation of Suspensions of Fumed Silica (N20) and Nanocrystalline Cellulose (NCC)
2.3. Preparation of Suspensions of Mixed Fumed Silica (N20) and Nanocrystalline Cellulose (NCC)
2.4. Measurement of Viscous Rheological Behavior of Suspensions
3. Results and Discussion
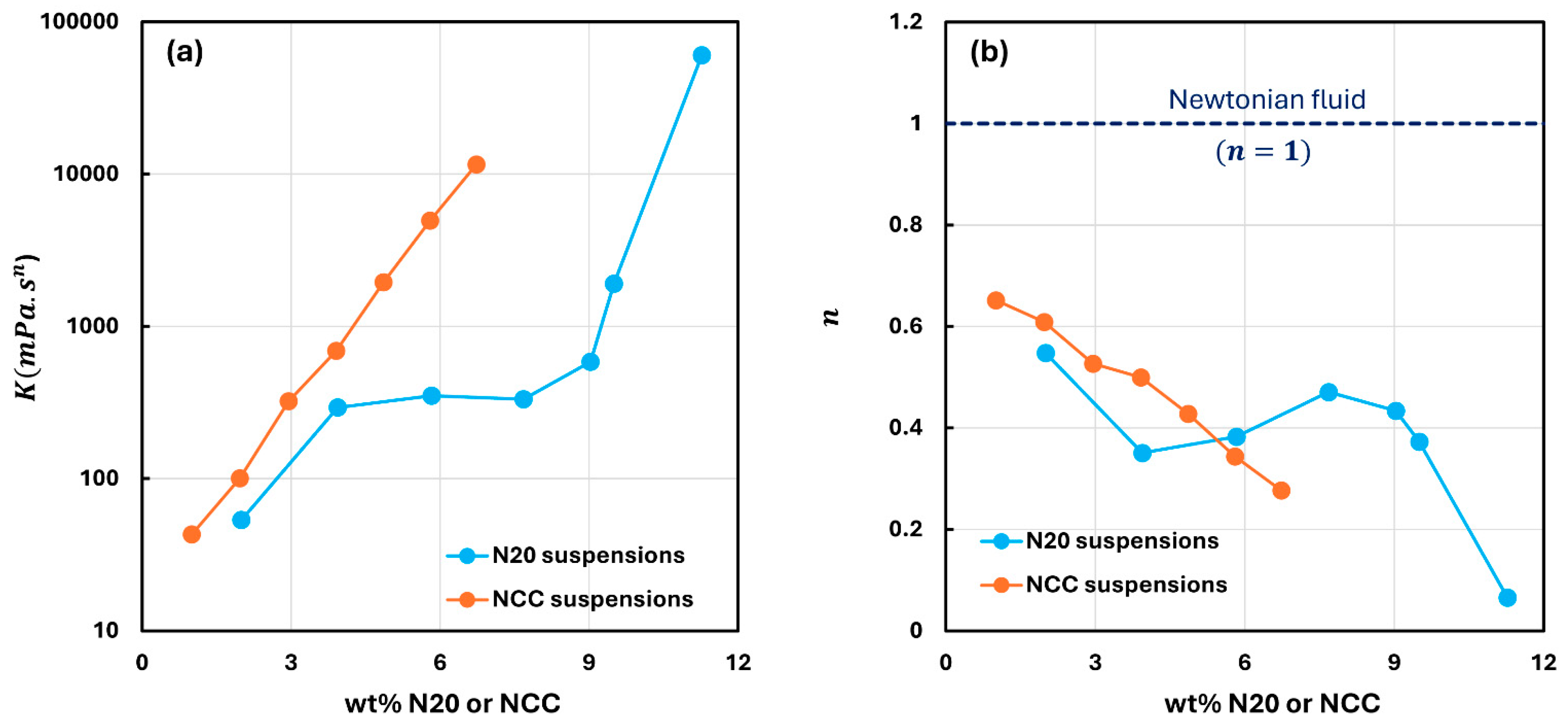
3.1. Rheology of Suspensions of Pure Fumed Silica and Pure Nanocrystalline Cellulose
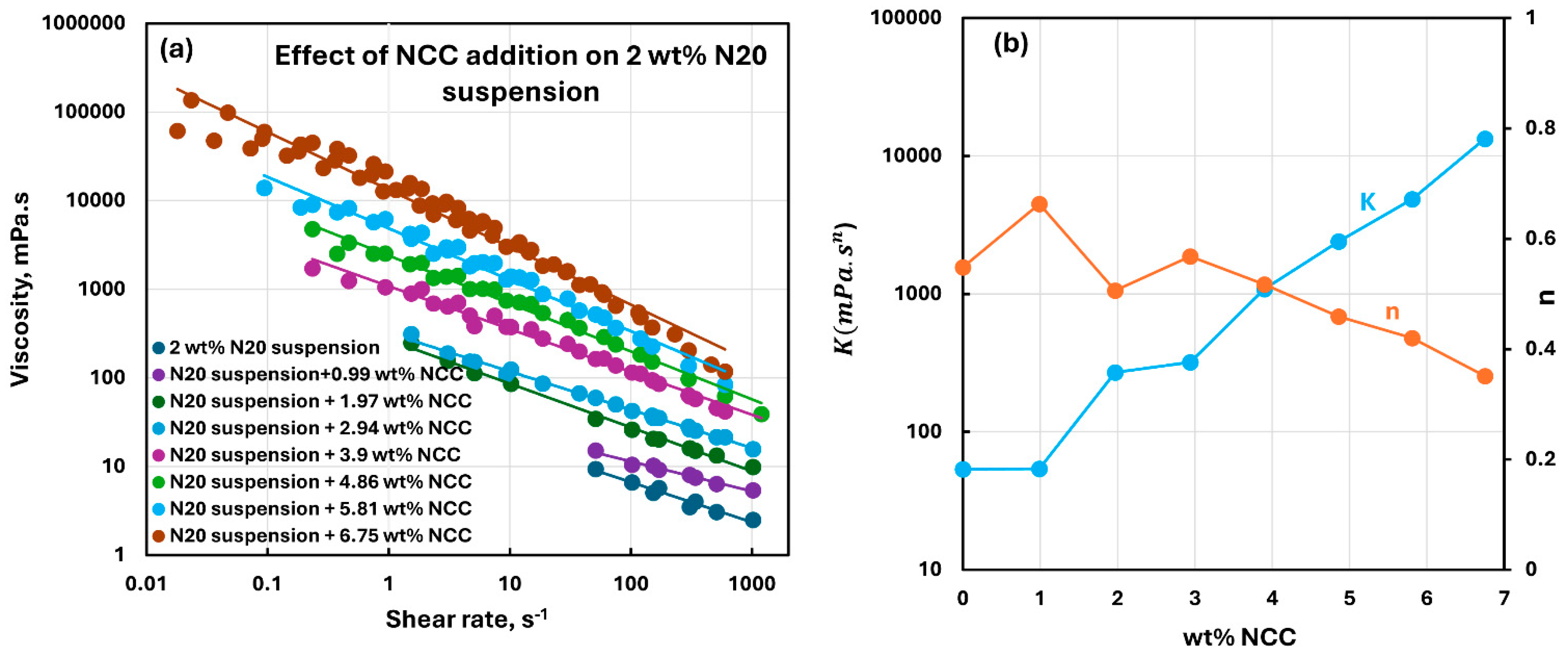
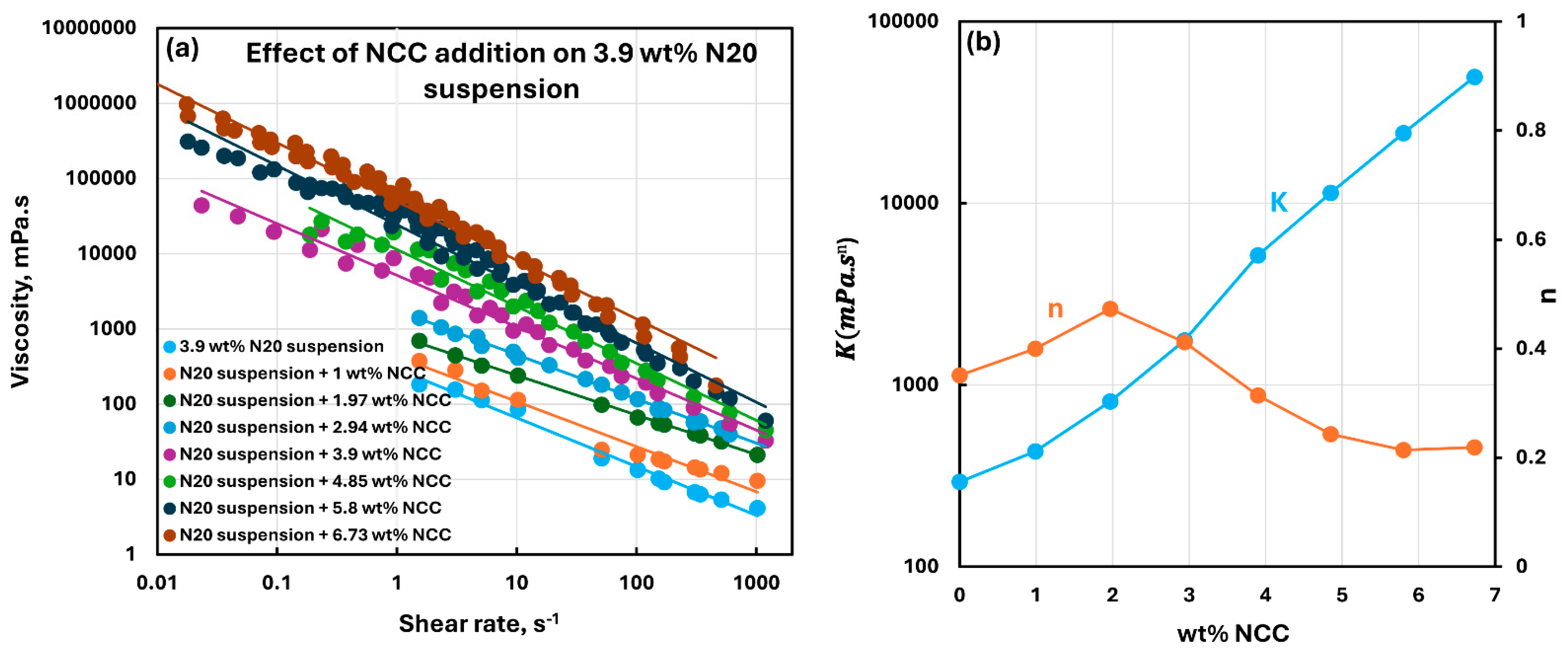
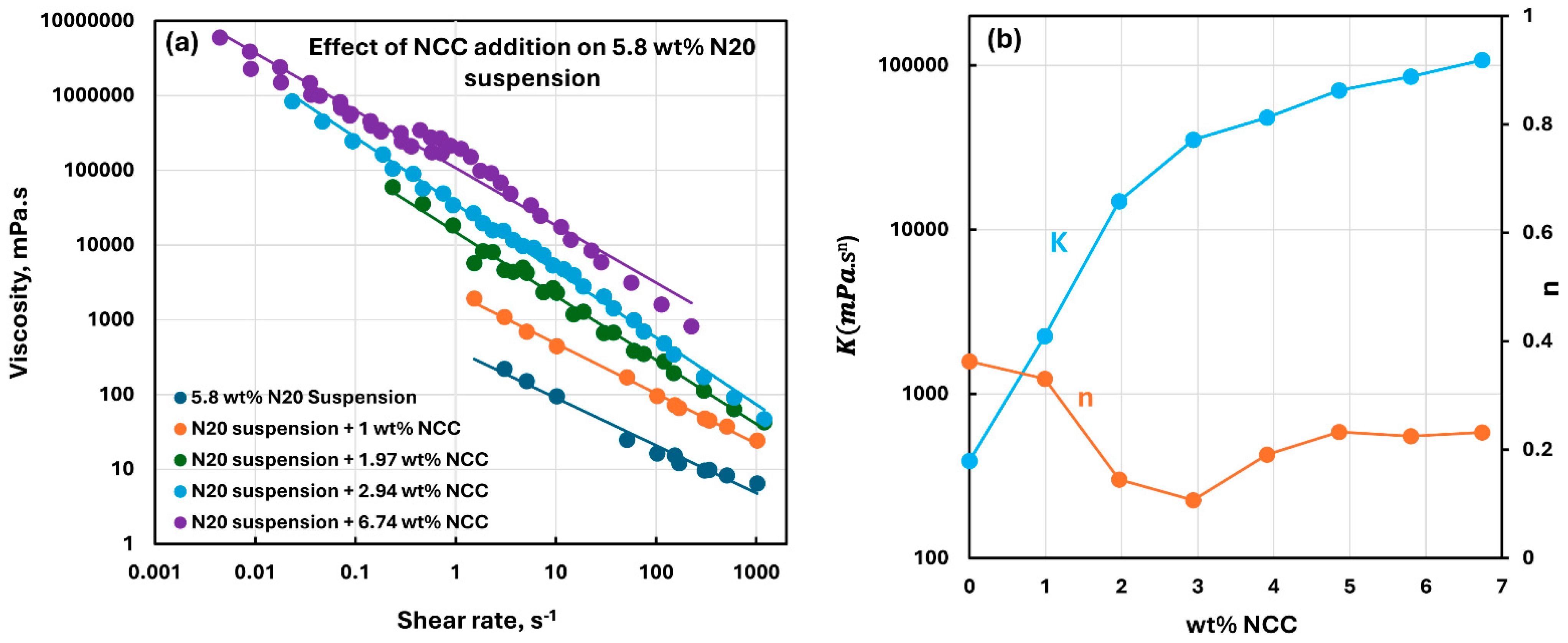
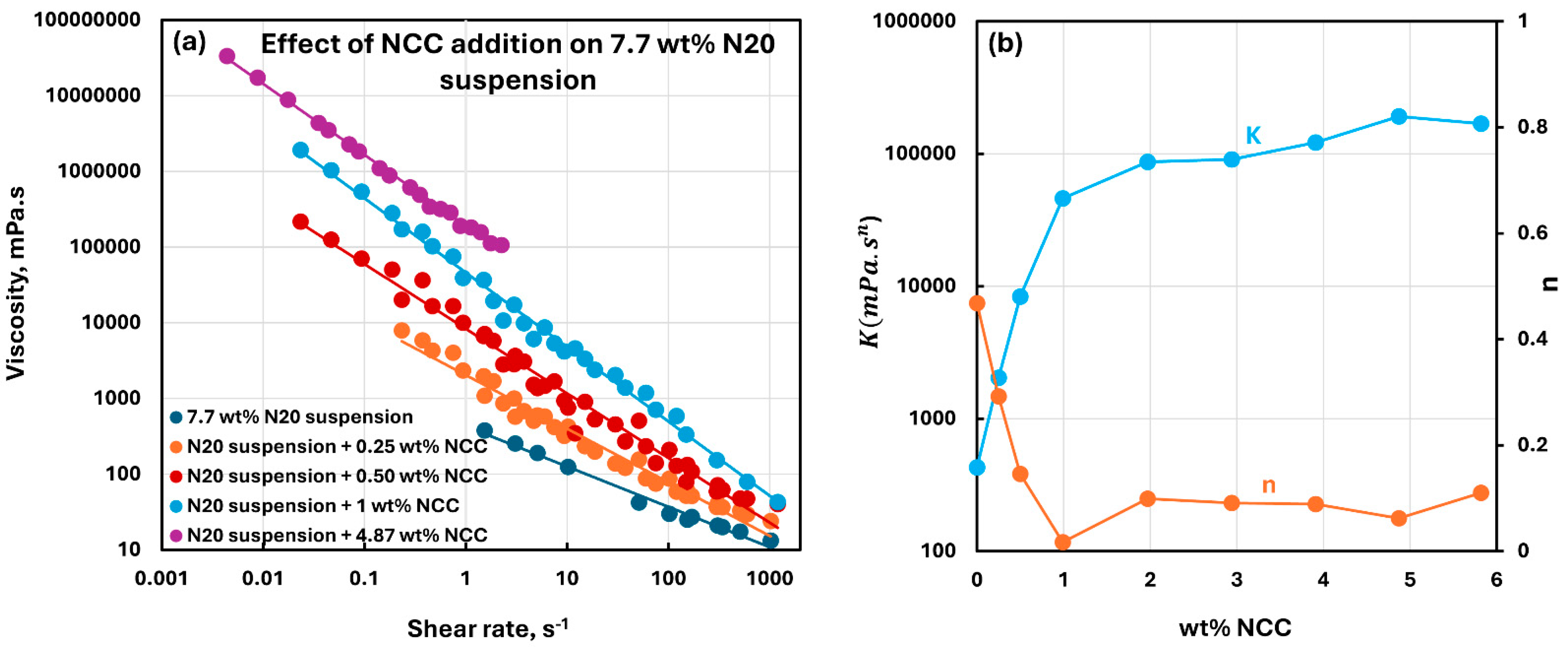
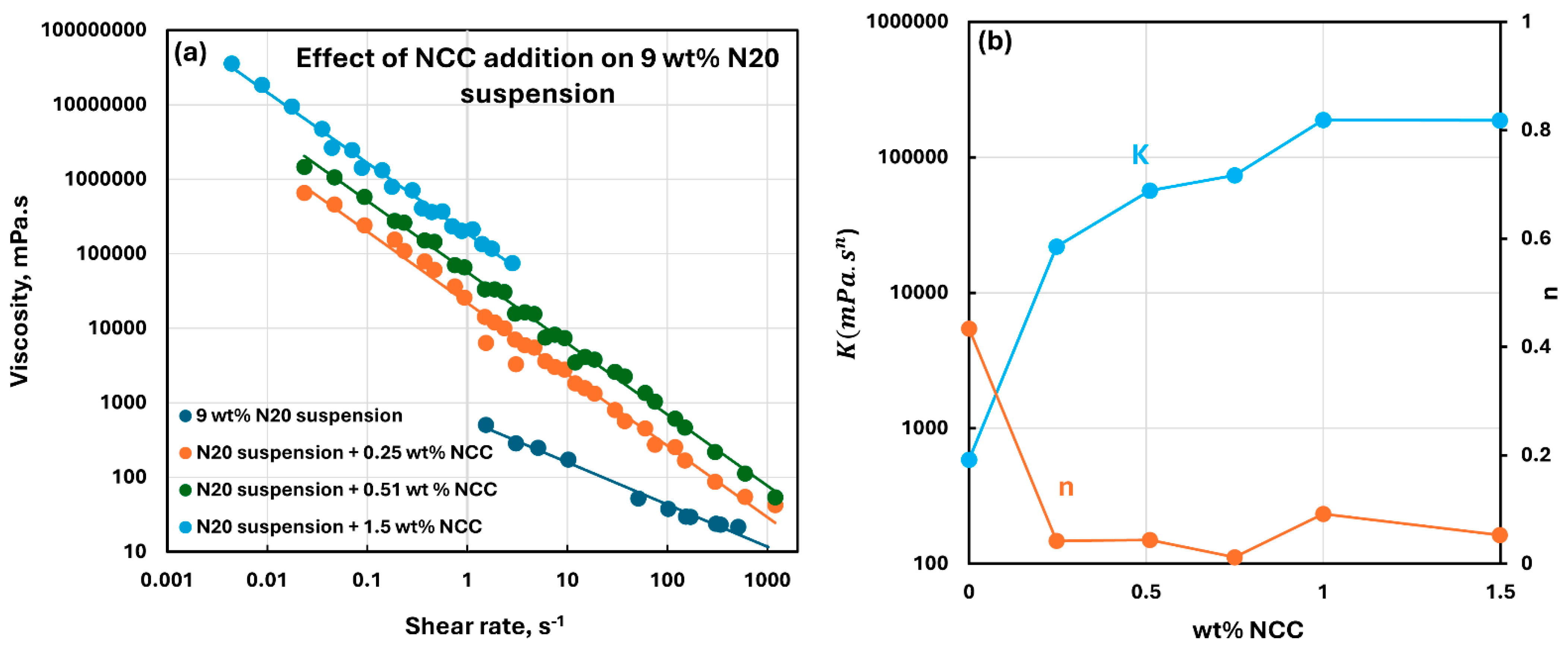
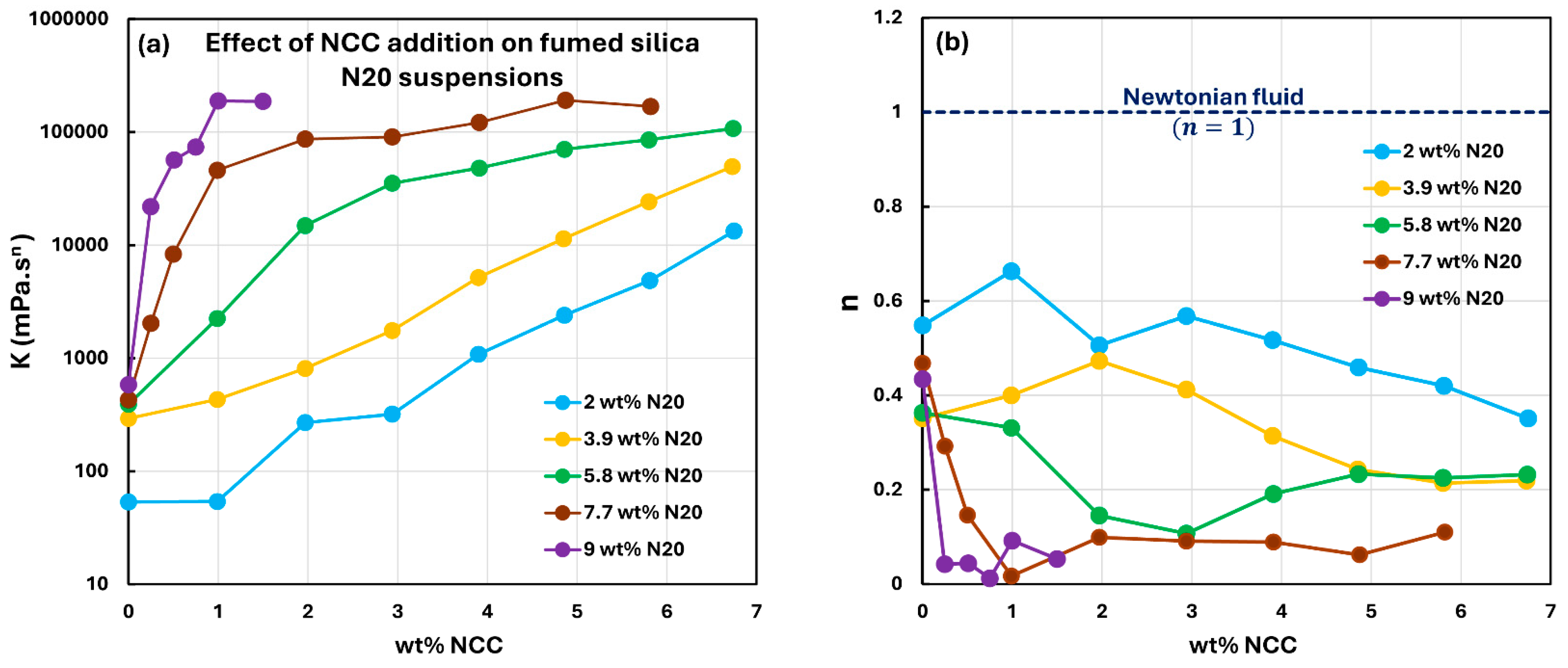
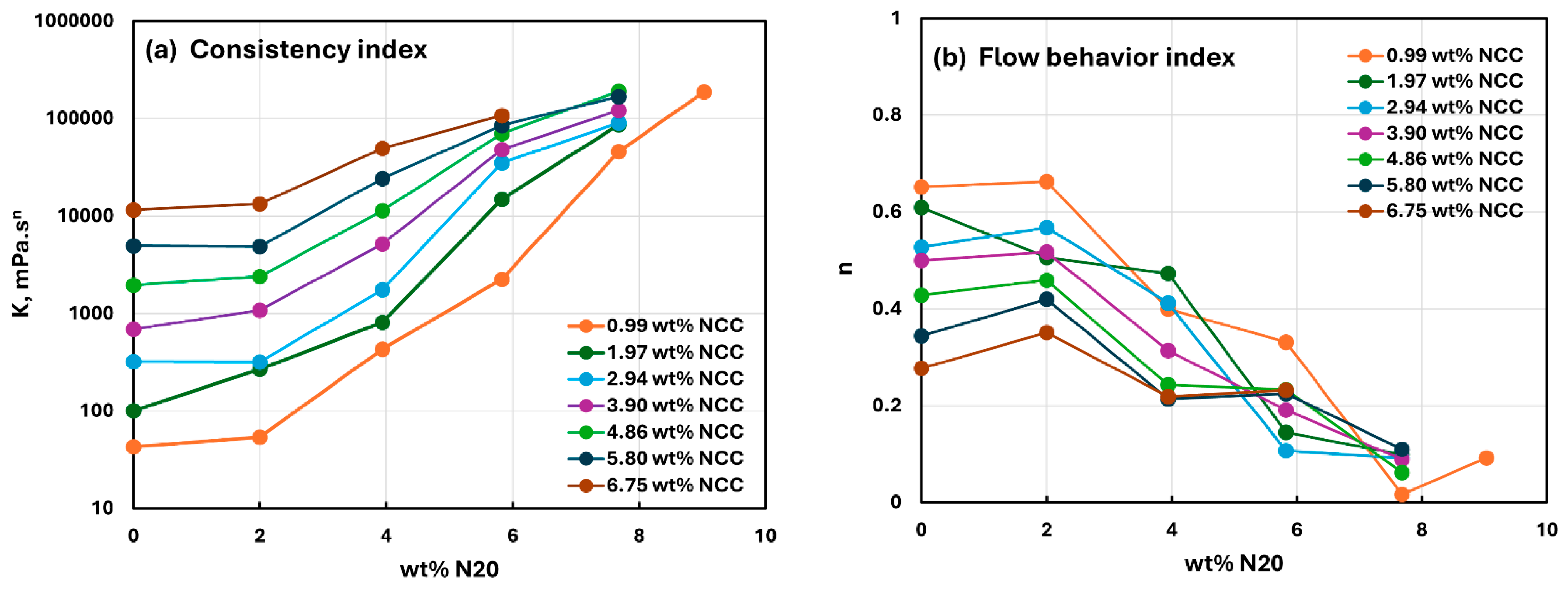
3.2. Rheology of Suspensions of Mixtures of Fumed Silica and Nanocrystalline Cellulose
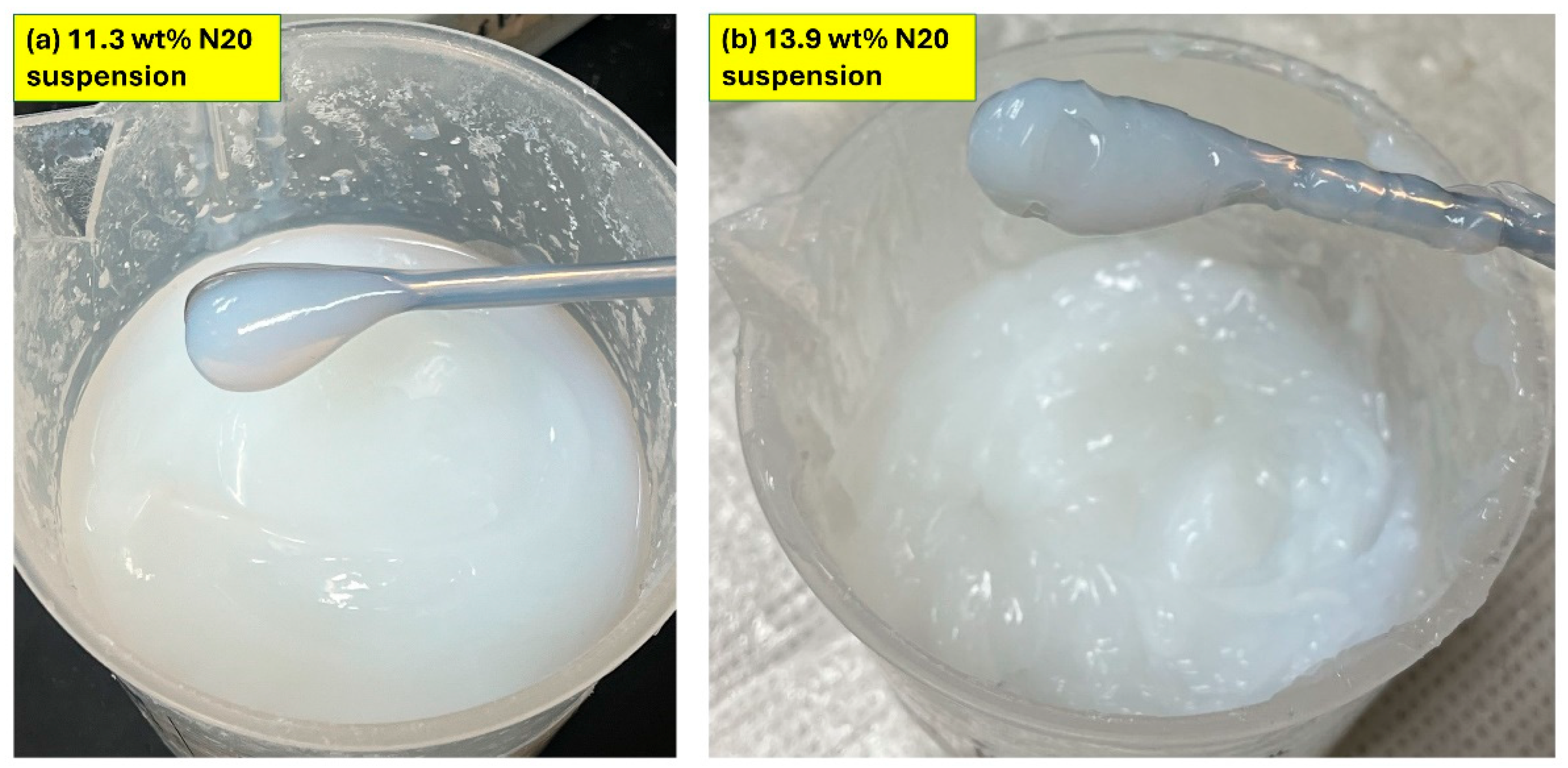
3.3. Visual Inspection of Suspensions
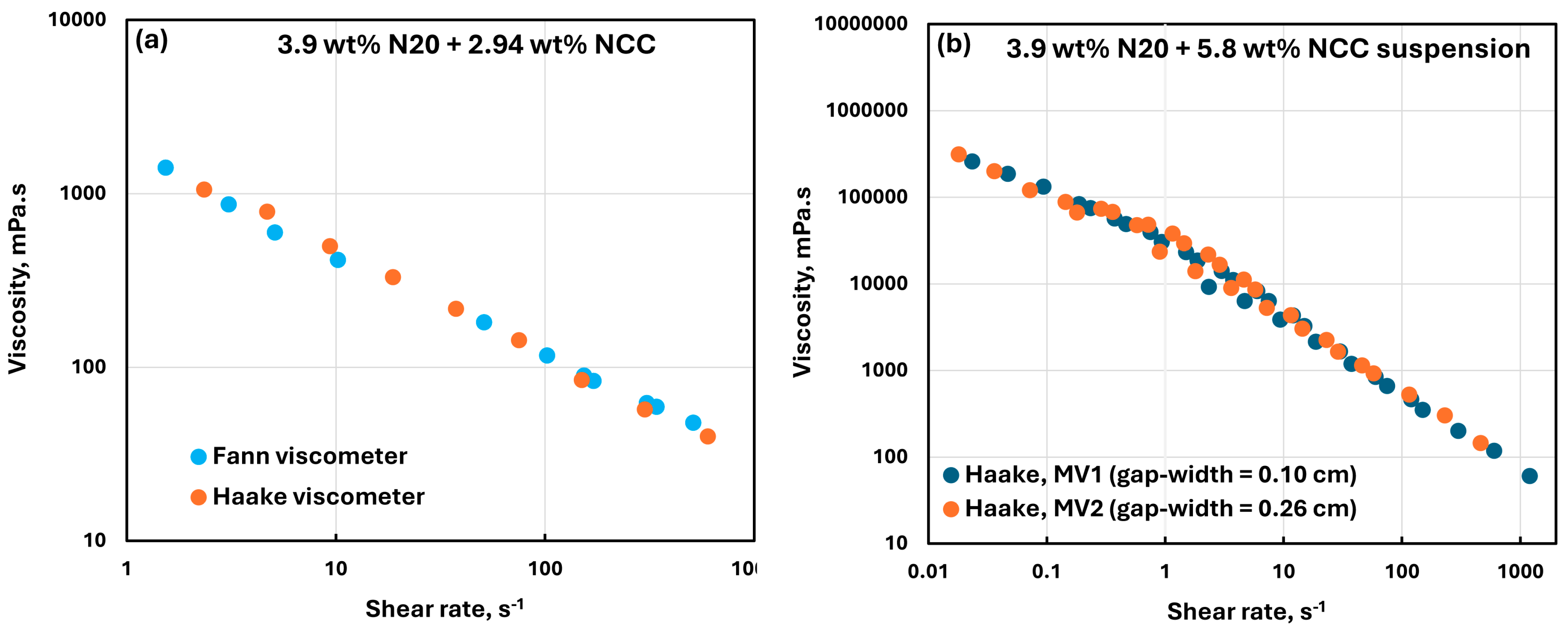
3.4. Reliability and Error Analysis of Rheological Measurements
4. Conclusions
- •
- Suspensions of fumed silica are non-Newtonian shear-thinning. They obey the power-law model over the N20 concentration range of 2 to 11.3 wt%. With the increase in N20 concentration, both the consistency and the degree of shear-thinning of suspension are enhanced.
- •
- The suspensions of cellulose nanocrystals are also non-Newtonian shear-thinning, and they follow the power-law model over the NCC concentration range of 1 to 6.75 wt%. With the increase in NCC concentration, both the consistency and the degree of shear-thinning of suspension are enhanced.
- •
- The suspensions of mixed additives, that is, N20 and NCC, are non-Newtonian shear-thinning. The power-law model describes the rheological behavior of the mixed suspension systems well in most cases. In some cases, especially at high concentrations of NCC in the mixed N20 and NCC suspensions, the power-law model describes the rheological data only approximately. The consistency and level of shear-thinning in suspensions of mixed additives are strongly dependent on the concentrations of both additives. The consistency and the level of shear-thinning increase substantially with the increases in N20 and NCC concentrations.
- •
- The mechanisms of shear-thinning in suspensions are reasoned to be as follows: With the increase in shear rate, the rod-shaped cellulose nanocrystals become aligned in the direction of flow and hence offer less resistance to flow. Furthermore, the large agglomerates of fumed silica aggregates undergo breakup with the increase in shear rate, resulting in a reduction in viscosity.
- •
- In future work, dynamic rheology of suspensions of mixed nanoparticle/nanocrystal additives will be explored.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pal, R.; Pattath, K. Rheology of suspensions thickened by cellulose nanocrystals. Nanomaterials 2024, 14, 1122. [Google Scholar] [CrossRef] [PubMed]
- Kinra, S.; Pal, R. Rheology of Pickering emulsions stabilized and thickened by cellulose nanocrystals over broad ranges of oil and nanocrystals concentrations. Colloids Interfaces 2023, 7, 36. [Google Scholar] [CrossRef]
- Himashree, P.; Sengar, A.S.; Sunil, C.K. Food thickening agents: Sources, chemistry, properties and applications—A review. Int. J. Gastron. Food Sci. 2022, 27, 100468. [Google Scholar] [CrossRef]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–597. [Google Scholar] [CrossRef]
- Hydrocolloids Market Size, Share & Growth Report, 2030. Available online: https://www.grandviewresearch.com/industry-analysis/hydrocolloids-market (accessed on 3 September 2025).
- How to Thicken Liquids for a Medical Diet. Available online: https://www.verywellhealth.com/what-are-thickened-liquids-1192165 (accessed on 2 September 2025).
- Pal, R. Modeling of sedimentation and creaming in suspensions and Pickering emulsions. Fluids 2019, 4, 186. [Google Scholar] [CrossRef]
- Fumed_Silica_Process. Available online: https://www.hninnotech.com/technology-service/fumed_silica_process/ (accessed on 19 September 2025).
- Stintz, M.; Barthel, H.; Heinemann, M.; Weis, J. Particle Size Distribution of Fumed Silica Agglomerates at Low Shear Stress. In Organosilicon Chemistry V; Auner, N., Weis, J., Eds.; Wiley-VCH Verlag GmbH & Co: Weinheim, Germany, 2003; pp. 741–746. [Google Scholar]
- Davis, B.; Sanchez Garcia, A.M.; Matheu, D.M.; Kutsovsky, Y.E. Fumed Silica of Controlled Aggregate Size and Processes for Manufacturing the Same. U.S. Patent 8,038,971B2, 18 October 2011. [Google Scholar]
- Fumed Silica Size & Property—HIFULL Corporation. Available online: https://en.hifull.com/blog/fumed-silica-size-property/ (accessed on 19 September 2025).
- Fumed Silica—Wikipedia. Available online: https://en.wikipedia.org/wiki/Fumed_silica (accessed on 19 September 2025).
- Heinze, T. Cellulose: Structure and properties. In Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Rojas, O., Ed.; Springer: Cham, Switzerland; Berlin/Heidelberg, Germany, 2016; pp. 1–52. [Google Scholar]
- Yu, H.; Qin, Z.; Liang, B.; Liu, N.; Zhou, Z.; Chen, L. Facile extraction of thermally stable cellulose nanocrystals with a high yield of 93% through hydrochloric acid hydrolysis under hydrothermal conditions. J. Mater. Chem. A 2013, 1, 3938–3944. [Google Scholar] [CrossRef]
- Islam, M.; Chen, L.; Sisler, J.; Tam, K. Cellulose nanocrystal (CNC)–inorganic hybrid systems: Synthesis, properties and applications. J. Mater. Chem. B 2018, 6, 864–883. [Google Scholar] [CrossRef]
- Abitbol, T.; Palermo, A.; Moran-Mirabal, J.M.; Cranston, E.D. Fluorescent labeling and characterization of cellulose nanocrystals with varying charge contents. Biomacromolecules 2013, 14, 3278–3284. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Filpponen, I.; Clarke, S.P.; Brougham, D.F.; Argyropoulos, D.S. Production of cellulose nanocrystals using hydrobromic acid and click reactions on their surface. J. Mater. Sci. 2011, 46, 7344–7355. [Google Scholar] [CrossRef]
- Kaushik, M.; Moores, A. Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Molinari, M.; Aguie-Beghin, V. Langmuir-Blodgett procedure to precisely control the coverage of functionalized AFM cantilevers for SMFS measurements: Application with cellulose nanocrystals. Langmuir 2018, 34, 9376–9386. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Chhajed, M.; Gupta, P.; Roy, S.; Maji, P.K. Isolation of cellulose nanocrystals from different waste biomass collating their liquid crystal ordering with morphological exploration. Int. J. Biol. Macromol. 2021, 175, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Miriam de Souza Lima, M.; Borsali, R. Static and Dynamic Light Scattering from Polyelectrolyte Microcrystal Cellulose. Langmuir 2002, 18, 992–996. [Google Scholar] [CrossRef]
- Darpentigny, C.; Molina-Boisseau, S.; Nonglaton, G.; Bras, J.; Jean, B. Ice-templated freeze-dried cryogels from tunicate cellulose nanocrystals with high specific surface area and anisotropic morphological and mechanical properties. Cellulose 2020, 27, 233–247. [Google Scholar] [CrossRef]
- Orts, W.J.; Godbout, L.; Marchessault, R.H.; Revol, J.-F. Enhanced Ordering of Liquid Crystalline Suspensions of Cellulose Microfibrils: A Small Angle Neutron Scattering Study. Macromolecules 1998, 31, 5717–5725. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Influence of surface charge on viscosity behavior of cellulose microcrystal suspension. J. Wood Sci. 1999, 45, 258–261. [Google Scholar] [CrossRef]
- Fagbemi, O.D.; Andrew, J.E.; Sithole, B. Beneficiation of wood sawdust into cellulose nanocrystals for application as a bio-binder in the manufacture of particleboard. Biomass Convers. Biorefin. 2021, 13, 11645–11656. [Google Scholar] [CrossRef]
- Kumar, P.; Miller, K.; Kermanshahi-pour, A.; Brar, S.K.; Beims, R.F.; Xu, C.C. Nanocrystalline cellulose derived from spruce wood: Influence of process parameters. Int. J. Biol. Macromol. 2022, 221, 426–434. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, M.; Hou, Q.; Liu, R.; Wu, T.; Si, C. Further characterization of cellulose nanocrystal (CNC) preparation from sulfuric acid hydrolysis of cotton fibers. Cellulose 2015, 23, 439–450. [Google Scholar] [CrossRef]
- Haouache, S.; Jimenez-Saelices, C.; Cousin, F.; Falourd, X.; Pontoire, B.; Cahier, K.; Jérome, F.; Capron, I. Cellulose nanocrystals from native and mercerized cotton. Cellulose 2022, 29, 1567–1581. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Q.; Chang, C.; Zhang, L. Phase transition identification of cellulose nanocrystal suspensions derived from various raw materials. J. Appl. Polym. Sci. 2017, 135, 45702. [Google Scholar] [CrossRef]
- Mondal, K.; Sakurai, S.; Okahisa, Y.; Goud, V.V.; Katiyar, V. Effect of cellulose nanocrystals derived from Dunaliella tertiolecta marine green algae residue on crystallization behaviour of poly(lactic acid). Carbohydr. Polym. 2021, 261, 117881. [Google Scholar] [CrossRef]
- Araki, J.; Kuga, S. Effect of Trace Electrolyte on Liquid Crystal Type of Cellulose Microcrystals. Langmuir 2001, 17, 4493–4496. [Google Scholar] [CrossRef]
- Sommer, A.; Staroszczyk, H. Bacterial cellulose vs. bacterial cellulose nanocrystals as stabilizer agents for O/W pickering emulsions. Food Hydrocoll. 2023, 145, 109080. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Grachev, V.; Deschaume, O.; Lang, P.R.; Lettinga, M.P.; Bartic, C.; Thielemans, W. Dimensions of Cellulose Nanocrystals from Cotton and Bacterial Cellulose: Comparison of Microscopy and Scattering Techniques. Nanomaterials 2024, 14, 455. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose nanocrystal-based composites: A review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Xu, J.; Wang, P.; Yuan, B.; Zhang, H. Rheology of cellulose nanocrystal and nanofibril suspensions. Carbohydr. Polym. 2024, 324, 121527. [Google Scholar] [CrossRef]
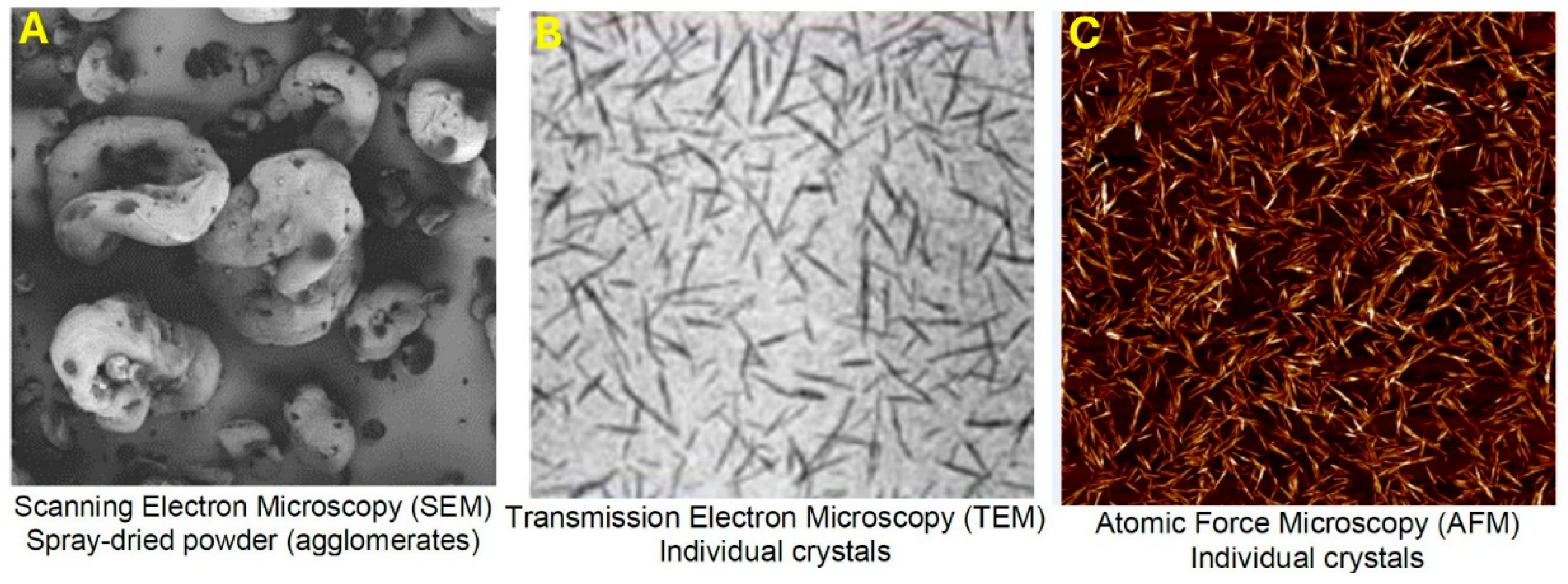
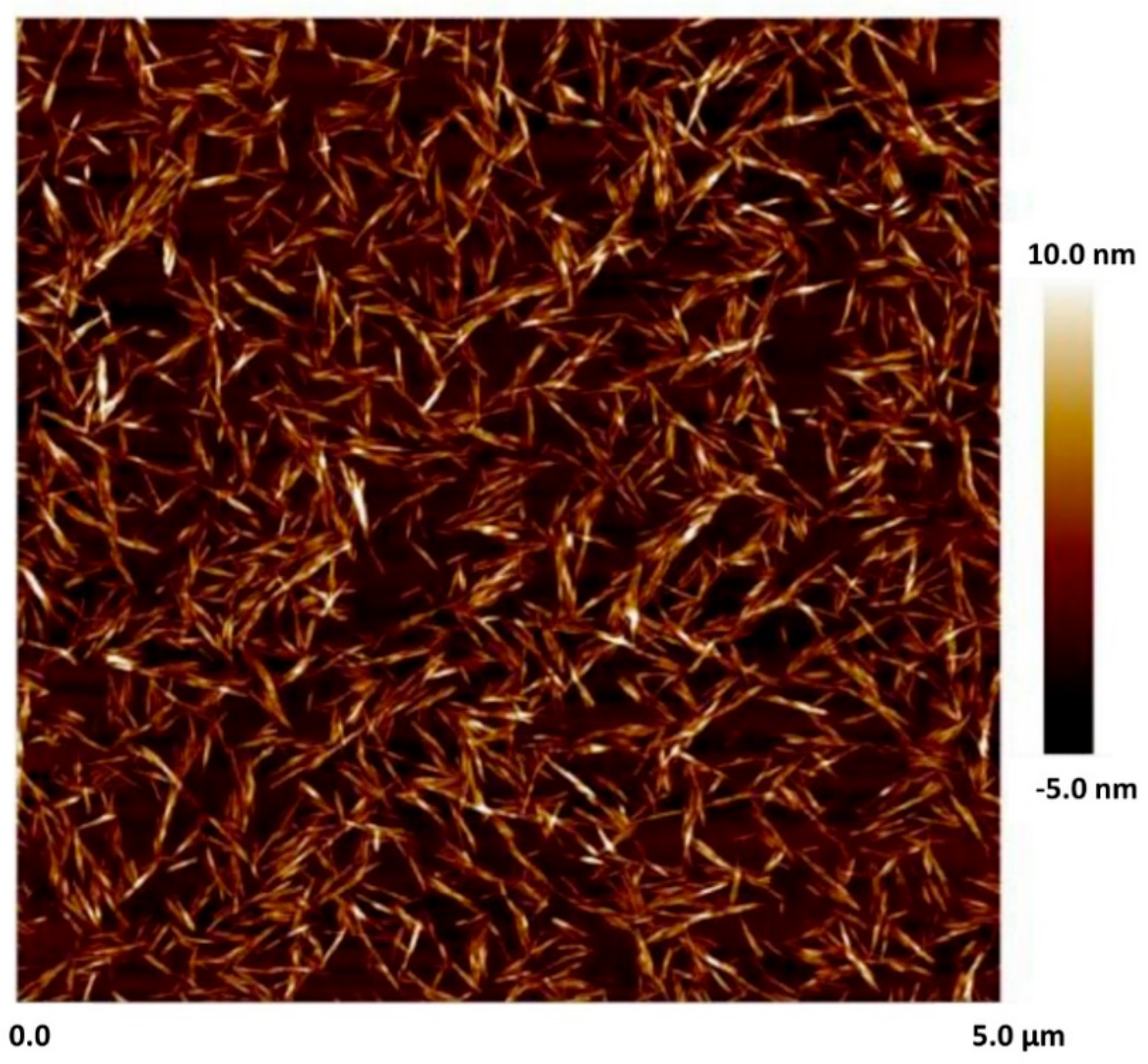



| Fumed Silica (N20) Concentration in N20–Water Suspension (wt%) | Concentration of Cellulose Nanocrystals (NCC) in Mixed N20–NCC–Water Suspension (wt%) |
|---|---|
| 2.0 | Seven concentrations: 0.99, 1.97, 2.94, 3.9, 4.86, 5.81, 6.75 |
| 3.9 | Seven concentrations: 0.99, 1.97, 2.94, 3.9, 4.85, 5.8, 6.73 |
| 5.8 | Seven concentrations: 0.99, 1.97, 2.94, 3.91, 4.86, 5.80, 6.74 |
| 7.7 | Eight concentrations: 0.25, 0.50, 0.99, 1.97, 2.94, 3.91, 4.87, 5.82 |
| 9.0 | Five concentrations: 0.247, 0.51, 0.75, 1.0, 1.498 |
| 9.5 | No NCC added |
| 11.3 | No NCC added |
| Viscometer | (cm) | Length of Inner Cylinder (cm) | Gap-Width Between Cylinders (cm) | |
|---|---|---|---|---|
| Fann viscometer | 1.72 | 1.84 | 3.8 | 0.12 |
| Haake viscometer with MV I bob | 2.00 | 2.1 | 6.0 | 0.10 |
| Haake viscometer with MV II bob | 1.84 | 2.1 | 6.0 | 0.26 |
| Haake viscometer with MV III bob | 1.52 | 2.1 | 6.0 | 0.58 |
| Viscometer | Shear Rate, s−1 | Shear Rate Range of Device, s−1 | Shear Stress, mPa |
|---|---|---|---|
| Fann viscometer with R1 bob | |||
| Haake viscometer with MV I bob | |||
| Haake viscometer with MV II bob | |||
| Haake viscometer with MV III bob |
| NCC Concentration (wt%) | |||
|---|---|---|---|
| 0 | 53.48 | 0.548 | 0.9769 |
| 0.99 | 54 | 0.663 | 0.9738 |
| 1.97 | 269.24 | 0.506 | 0.9911 |
| 2.94 | 319.76 | 0.568 | 0.9791 |
| 3.90 | 1083.5 | 0.517 | 0.9582 |
| 4.86 | 2400 | 0.459 | 0.9488 |
| 5.81 | 4859.2 | 0.42 | 0.945 |
| 6.75 | 13,331 | 0.351 | 0.7631 |
| NCC Concentration (wt%) | RMSE (mPa.s) | APRE (%) | |
|---|---|---|---|
| 0 | 0.33 | 0.38 | 6.43 |
| 0.99 | 0.48 | 0.22 | 3.86 |
| 1.97 | 9.17 | 0.34 | 5.87 |
| 2.94 | 11.55 | 0.12 | 3.96 |
| 3.90 | 114.97 | 0.94 | 9.87 |
| 4.86 | 345.22 | 1.20 | 12.59 |
| 5.81 | 1354.89 | 1.74 | 15.34 |
| 6.75 | 19,259.76 | 6.08 | 27.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, R.; Alizadeh, H. Viscous Rheological Behavior of Nanosuspensions of Fumed Silica Nanoparticles and Cellulose Nanocrystals. Nanomaterials 2025, 15, 1468. https://doi.org/10.3390/nano15191468
Pal R, Alizadeh H. Viscous Rheological Behavior of Nanosuspensions of Fumed Silica Nanoparticles and Cellulose Nanocrystals. Nanomaterials. 2025; 15(19):1468. https://doi.org/10.3390/nano15191468
Chicago/Turabian StylePal, Rajinder, and Hanie Alizadeh. 2025. "Viscous Rheological Behavior of Nanosuspensions of Fumed Silica Nanoparticles and Cellulose Nanocrystals" Nanomaterials 15, no. 19: 1468. https://doi.org/10.3390/nano15191468
APA StylePal, R., & Alizadeh, H. (2025). Viscous Rheological Behavior of Nanosuspensions of Fumed Silica Nanoparticles and Cellulose Nanocrystals. Nanomaterials, 15(19), 1468. https://doi.org/10.3390/nano15191468







