An Approach to the Optimization of Ba-Mn-Cu Perovskites as Catalysts for CO Oxidation: The Role of Cerium
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
2.2. Activity Tests
3. Results and Discussion
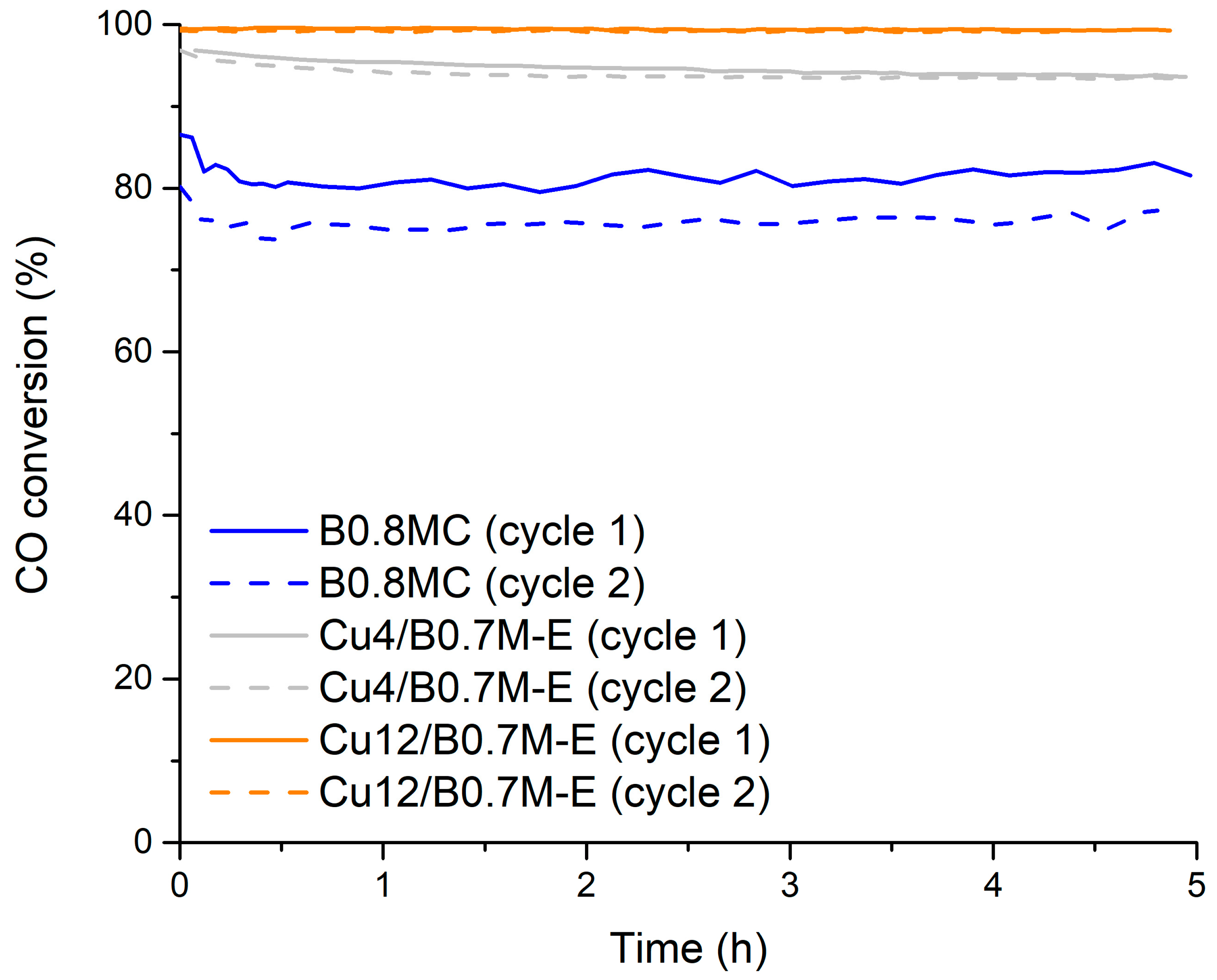
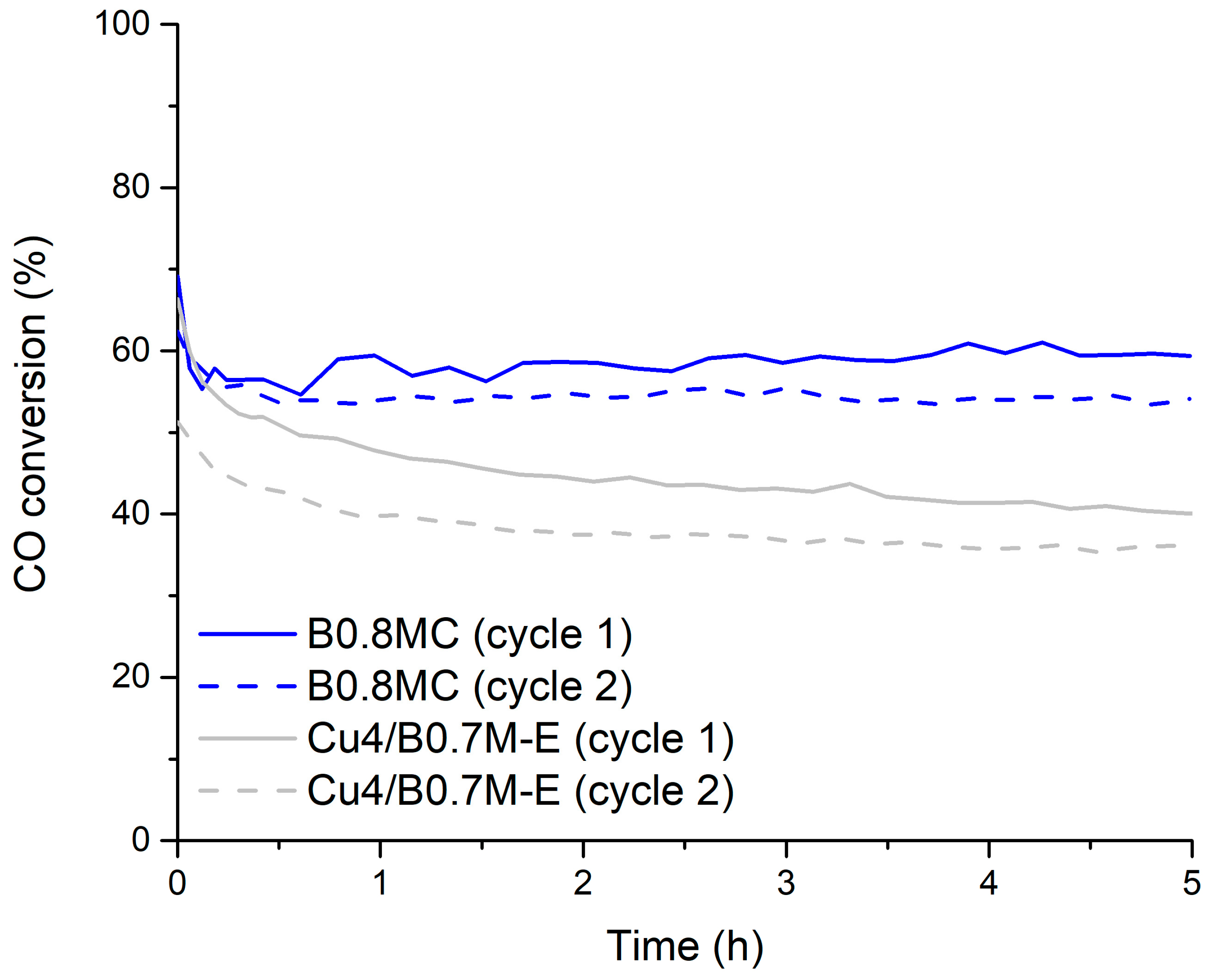
3.1. Selection of the Perovskites
- *
- From a Cu/Ba0.7MnO3 series of samples, with nominal Cu contents of 4, 8 and 12 wt% (denoted as Cux/B0.7M-E, x = 4, 8 and 12), the Cu4/B0.7M-E and Cu12/B0.7M-E formulations feature the best catalytic performance as they present a high proportion of Cu species with a strong interaction with the perovskite support [34].
- *
- From the BaxMn0.7Cu0.3O3 series of samples (with x = 1, 0.9, 0.8 and 0.7), the Ba0.8Mn0.7Cu0.3O3 composition (denoted as B0.8MC) shows the best catalytic behavior due to its high amount of Mn (IV) ions, oxygen vacancies and reduced copper species (Cu (I)), that are more active than oxidized Cu species (Cu (II)) [33].
- (i)
- for testing the stability of the samples at a temperature lower than 300 °C, two reaction cycles at 250 °C under the 1% CO, 1% O2 in He reactant mixture.
- (ii)
- in order to determine the tolerance of the catalysts to CO2 [25], a CO oxidation reaction at 300 °C using the 1% CO, 1% O2 in He reactant mixture, in which a 15% of CO2 was added to simulate the average composition typically found in the actual gasoline exhaust.
3.2. Effect of the Reaction Temperature for the CO Oxidation
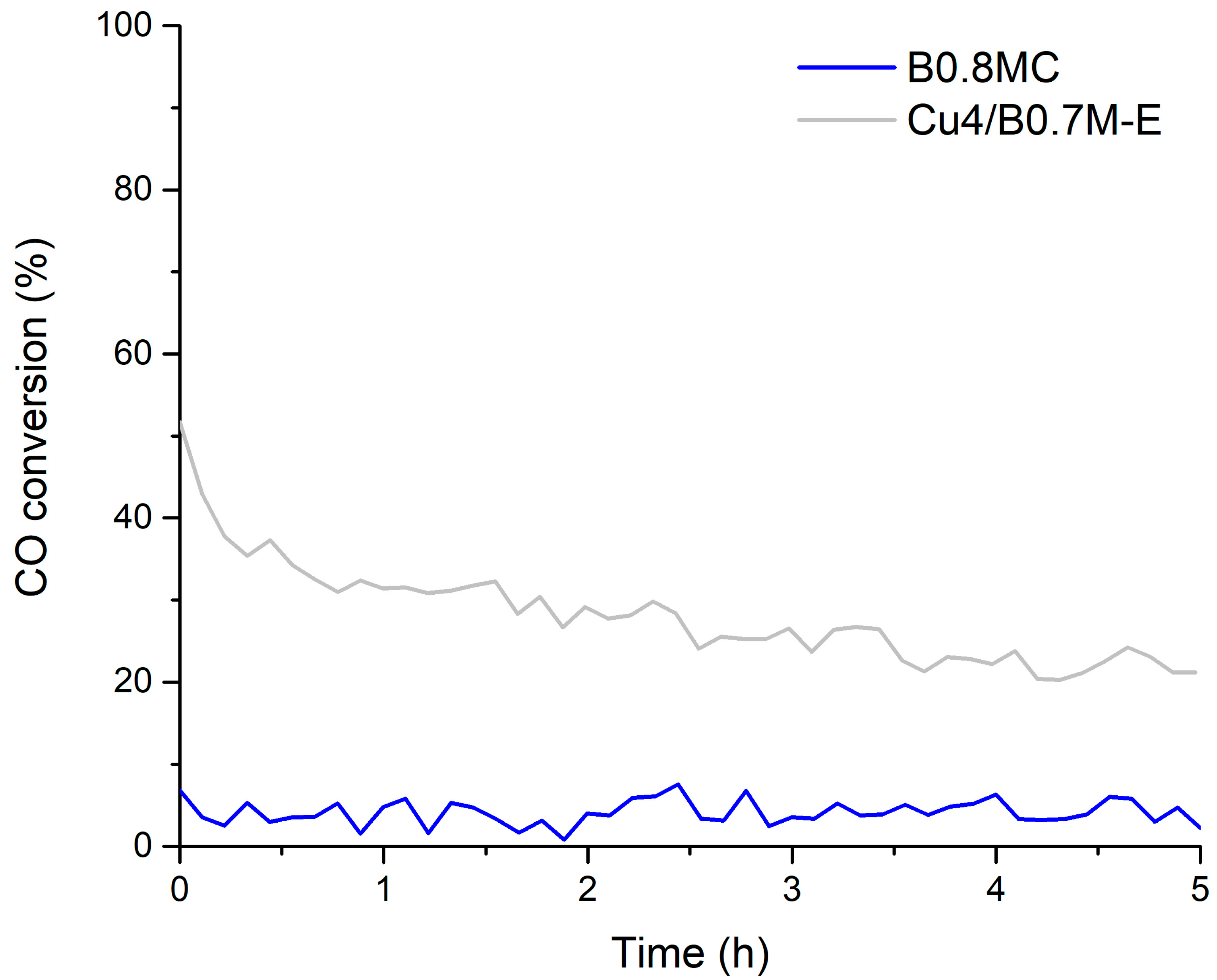
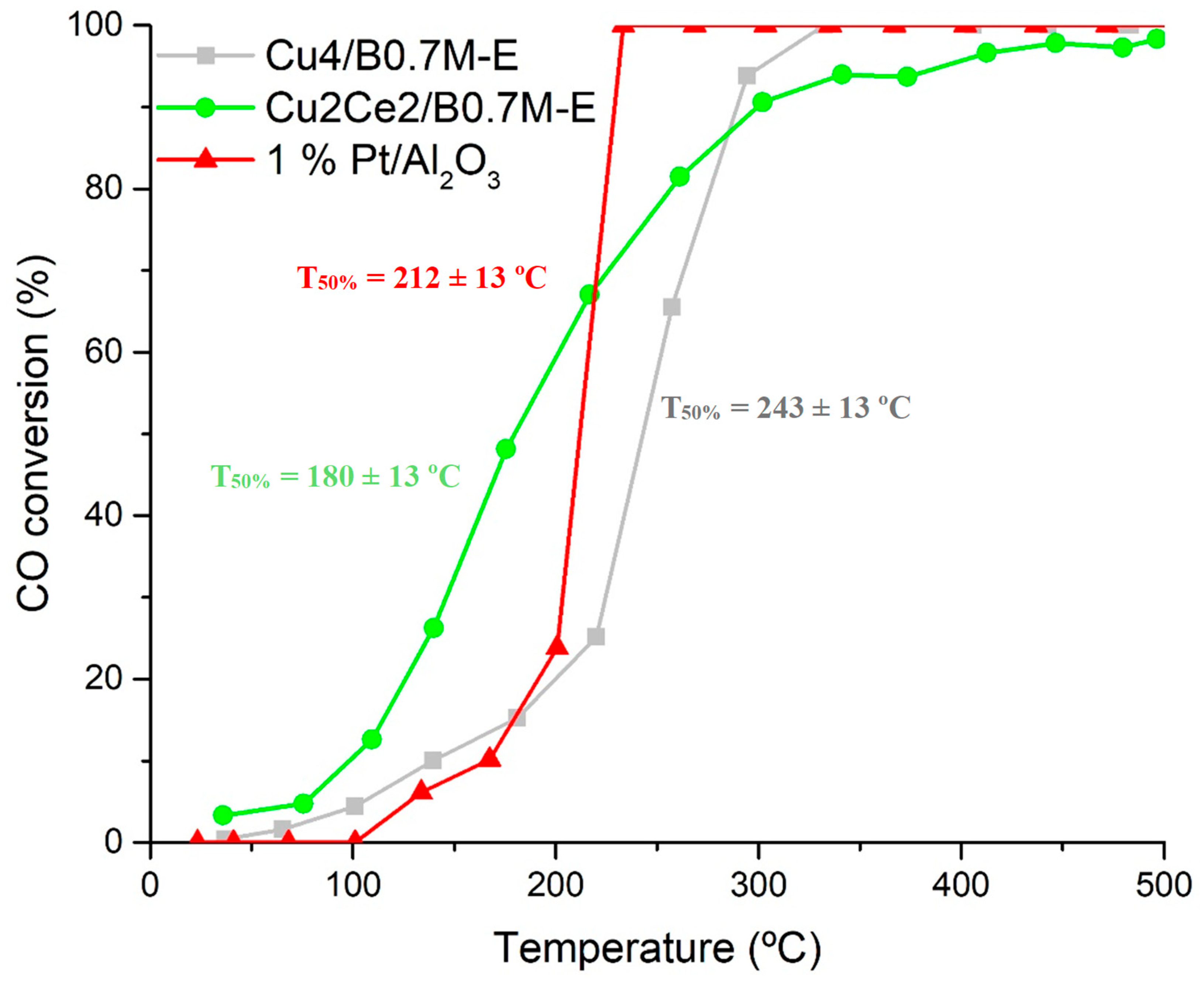
3.3. Tolerance to CO2
3.4. Optimization of the Cu4/B0.7M-E Formulation
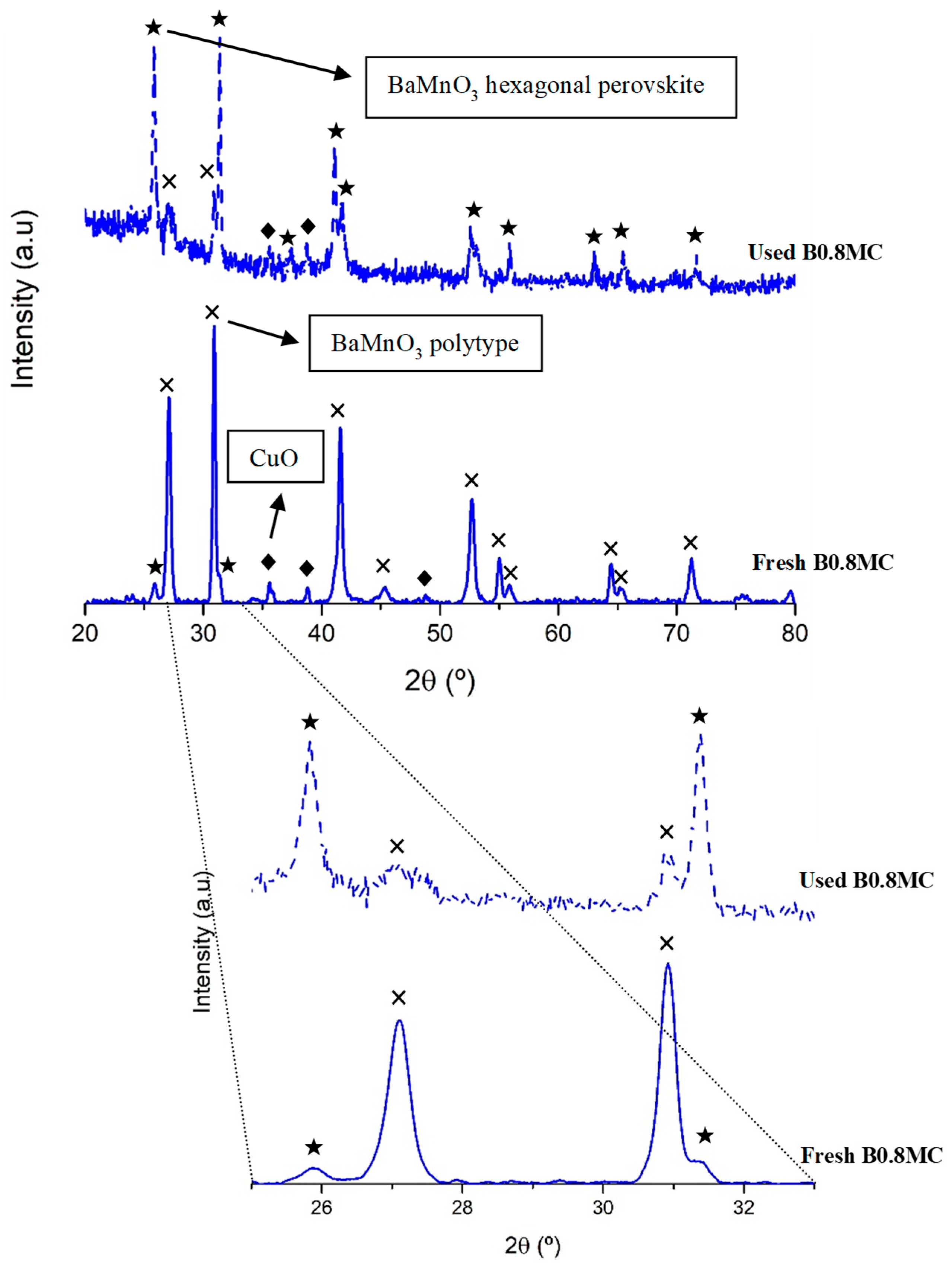
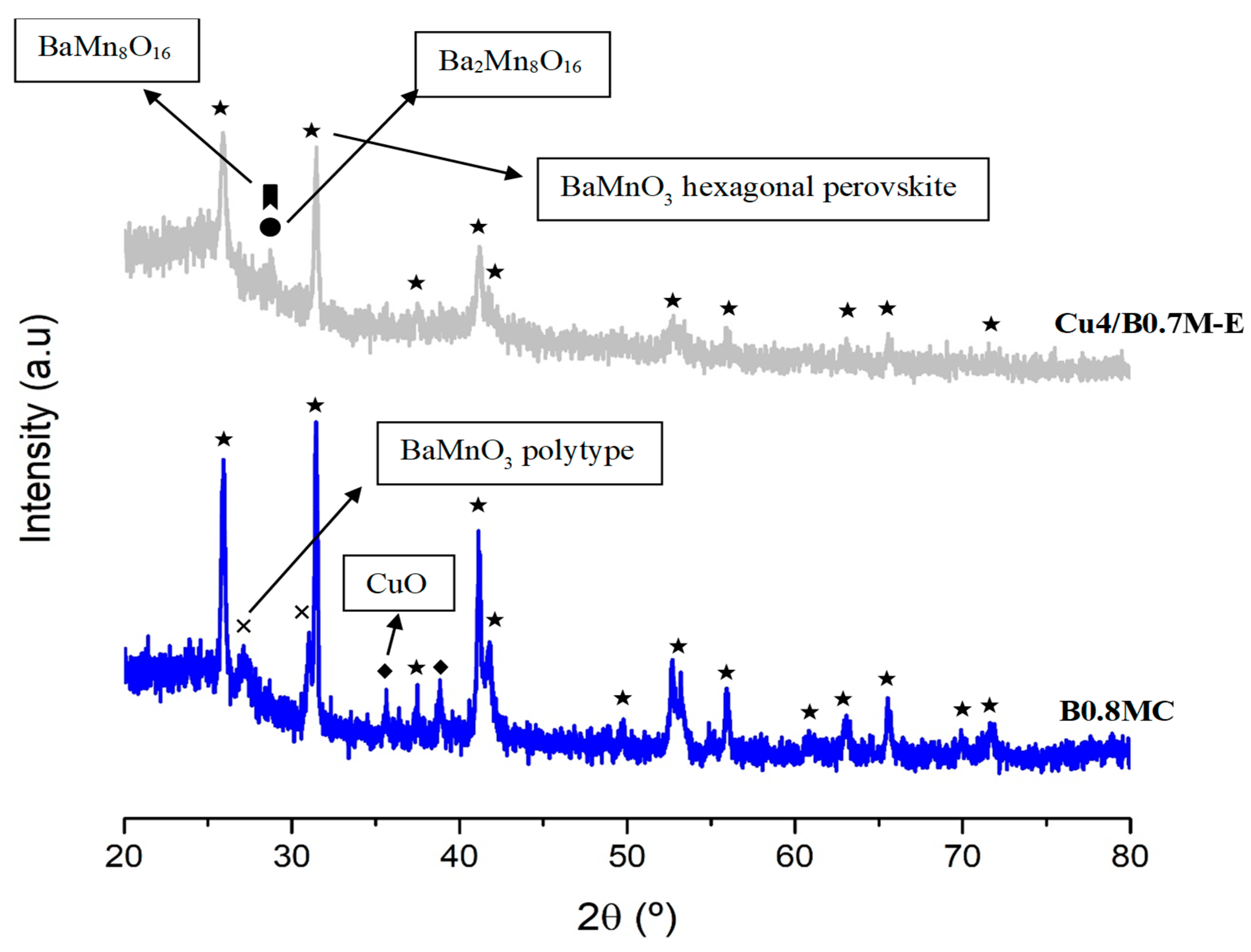
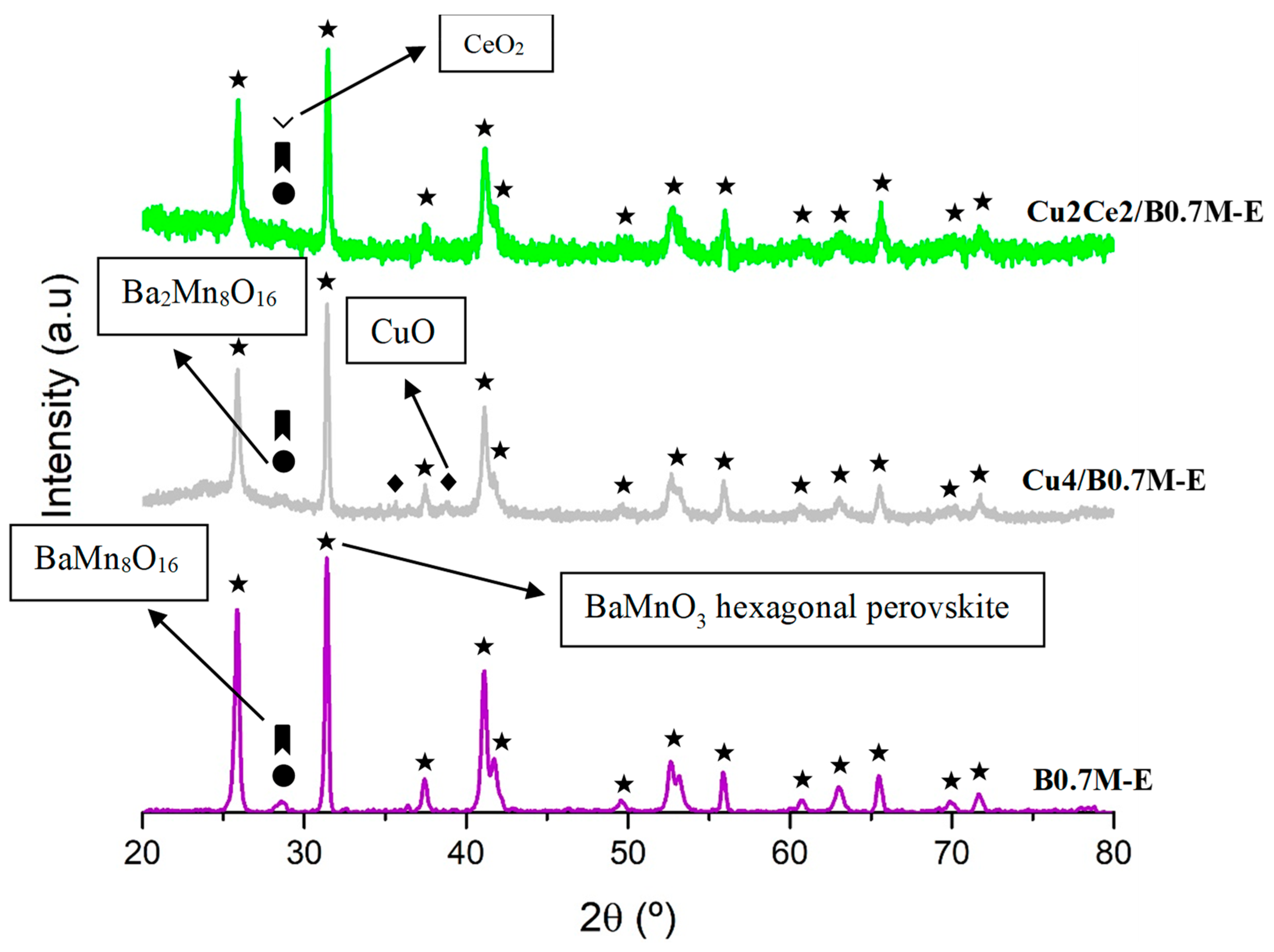
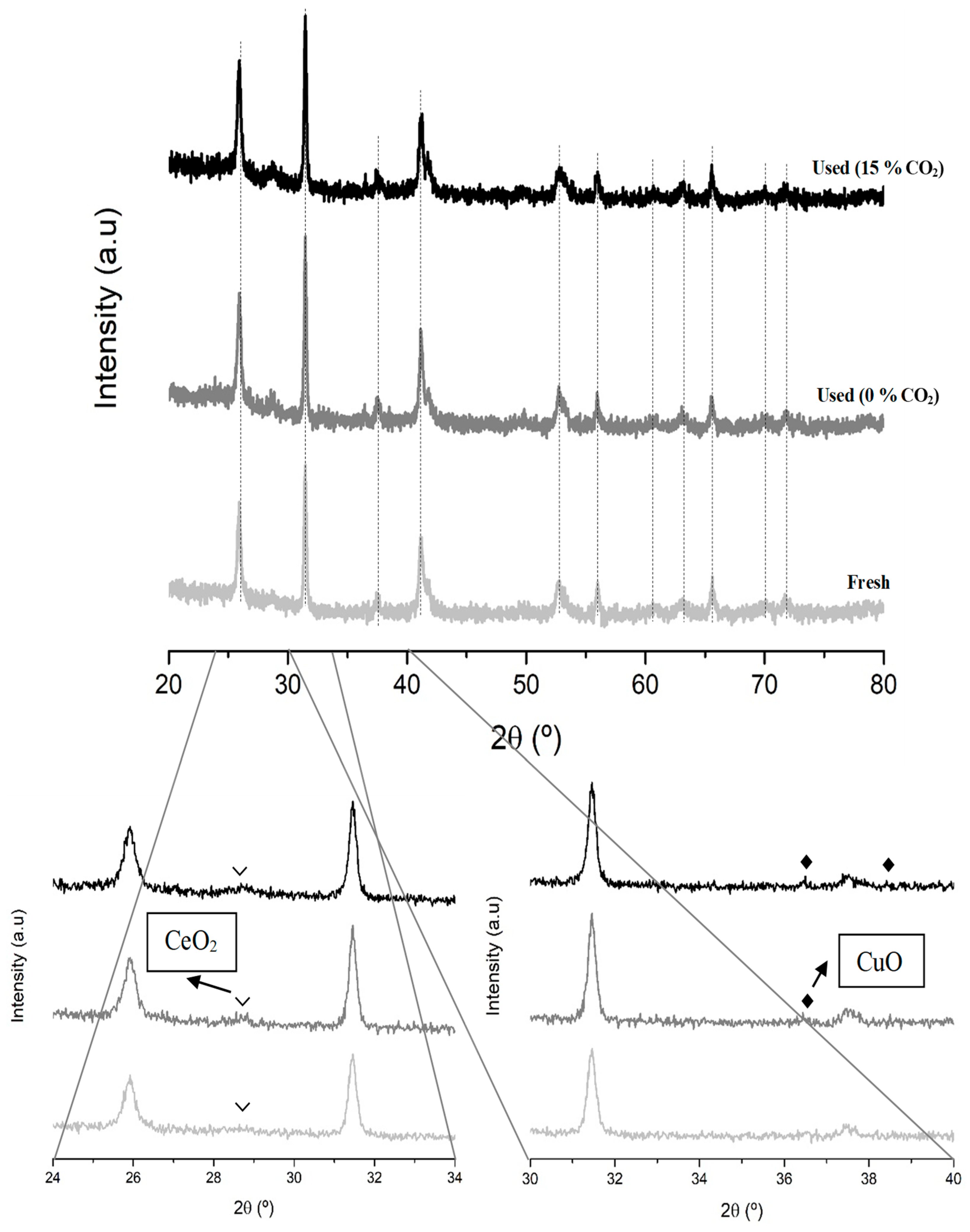
3.4.1. Chemical, Morphological and Structural Characterization
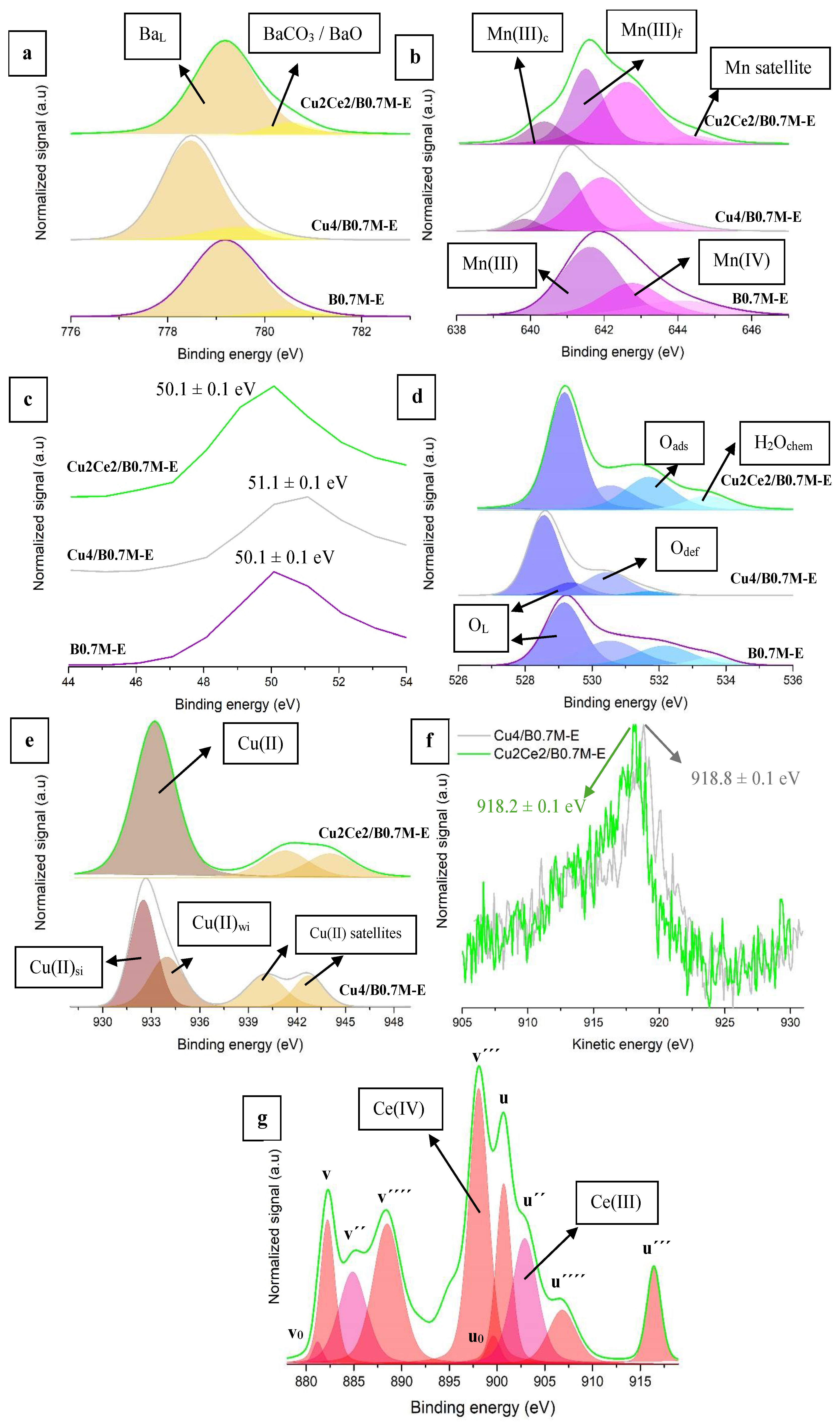
3.4.2. Surface Properties
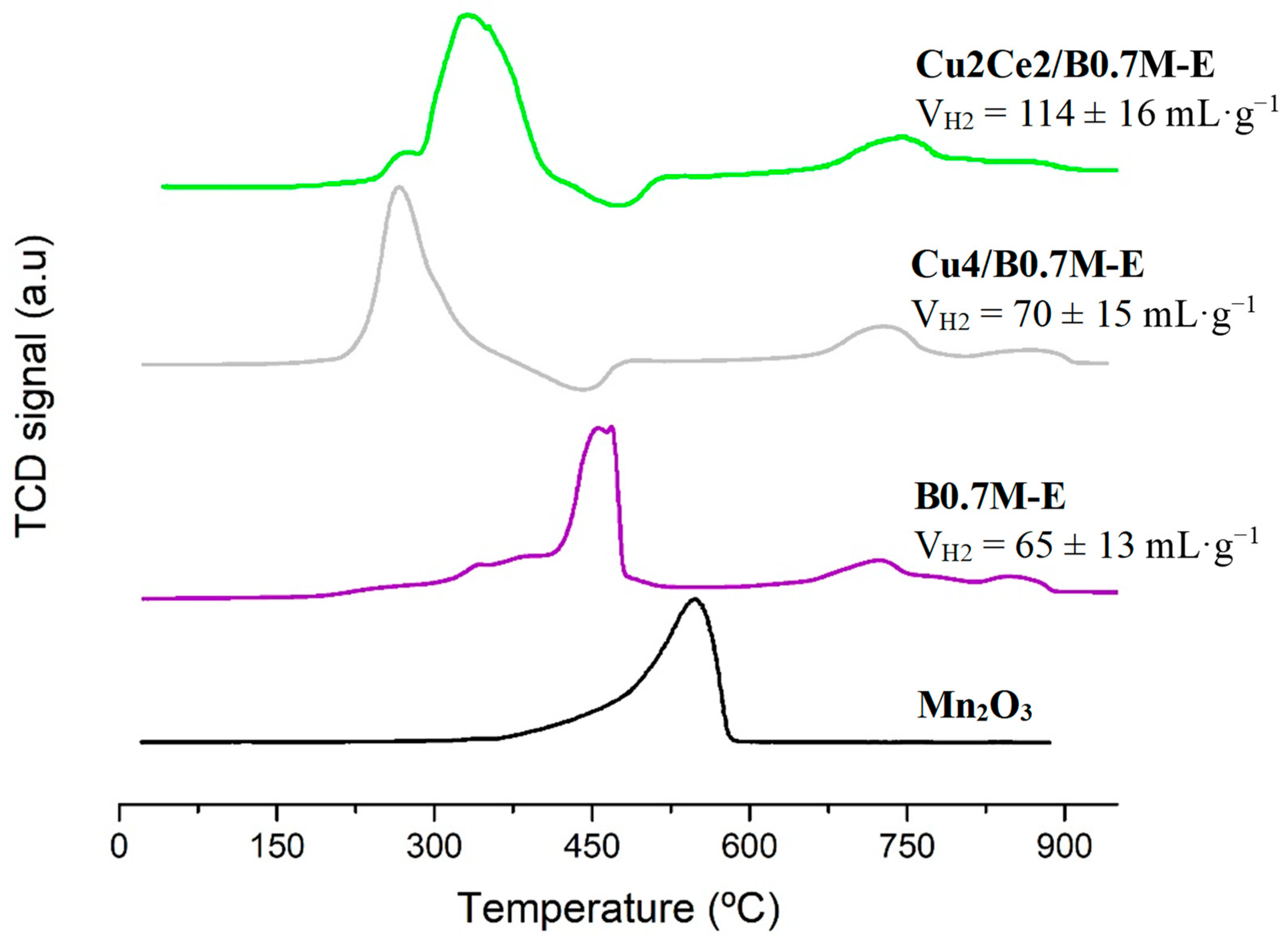
3.4.3. Redox Properties
3.4.4. Catalytic Activity
4. Conclusions
- (i)
- The B0.8MC sample showed the best catalytic performance during an isothermal reaction at 250 °C, but it featured a lower tolerance to CO2 than the Cu4/B0.7M-E sample, which was selected to be optimized by the addition of cerium.
- (ii)
- The impregnation of the B0.7M-E support with cerium and copper did not cause significant structural changes in the perovskite structure.
- (iii)
- The presence of cerium increased the fraction of the reduced copper species on the surface, improving the redox properties of the raw B0.7M-E support due to Mn-Ce synergistic effect.
- (iv)
- The Cu2Ce2/B0.7M-E bimetallic formulation exhibits a better catalytic performance for the CO oxidation reaction than the monometallic one, including a notably higher tolerance to CO2.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GHGs | Greenhouse Gases |
| ICEs | Internal Combustion Engines |
| HCs | Hydrocarbons |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectroscopy |
| XPS | X-ray Photoelectron Spectroscopy |
| H2-TPR | Temperature-Programmed Reduction with H2 |
| XRD | X-Ray Diffraction |
| JCPDS | Joint Committee on Powder Diffraction Standards |
| ICDD | International Centre for Diffraction Data |
| KE | Kinetic Energy |
| O2-TPD | Temperature-Programmed Desorption of O2 |
| TPRe | Temperature-Programmed Reaction |
| TCD | Thermal Conductivity Detector |
| TG-MS | Thermo Gravimetric Mass Spectrometer |
| TWCs | Three-Way Catalysts |
| GHSV | Gas Hourly Space Velocity |
| FE-SEM | Field Emission Scanning Electron Microscopy |
| EDX | Energy Dispersive X-ray spectroscopy |
References
- Sokal, K.; Kachel, M. Impact of agriculture on greenhouse gas emissions—A review. Energies 2025, 18, 2272. [Google Scholar] [CrossRef]
- Yao, S.; Wang, G.; Batista, E.R.; Yang, P. Defect-promoted catalytic conversion of carbon monoxide to methanol on ThO2 surfaces. ACS Appl. Mater. Interfaces 2025, 17, 28864–28872. [Google Scholar] [CrossRef]
- Rakha, H.A.; Farag, M.; Foroutan, H. Electric versus gasoline vehicle particulate matter and greenhouse gas emissions: Large-scale analysis. Transp. Res. D Transp. Environ. 2025, 140, 104622. [Google Scholar] [CrossRef]
- Folęga, P.; Burchart, D. Study of the greenhouse gas emissions from electric buses powered by renewable energy sources in Poland. Energies 2025, 18, 1807. [Google Scholar] [CrossRef]
- Filho, G.C.K.; Silva, F.M.F.; Fagundez, J.L.S.; da Silva, C.D.R.; Lanzanova, T.D.M.; Martins, M.E.S. Design and validation of a combustión chamber for internal combustion engines operated with methane based fuels. Appl. Therm. Eng. 2025, 271, 126007. [Google Scholar] [CrossRef]
- Towoju, O.A.; Ishola, F.A. A case for the internal combustion engine powered vehicle. Energy Rep. 2020, 6, 315–321. [Google Scholar] [CrossRef]
- Martins, J.; Brito, F.P. Alternative fuels for internal combustion engines. Energies 2020, 13, 4086. [Google Scholar] [CrossRef]
- Díaz-Verde, A.; Sanchis, E.J.; Martínez-Munuera, J.C.; García-García, A.; Piqueras, P. Exploring the potential of copper-based catalysts for use in CO oxidation reactions in oxy-fuel combustion engines. Fuel 2025, 391, 134807. [Google Scholar] [CrossRef]
- Johnsson, F.; Kjärstad, J.; Rootzén, J. The threat to climate change mitigation posed by the abundance of fossil fuels. Clim. Policy 2019, 19, 258–274. [Google Scholar] [CrossRef]
- Ajanovic, A. The future of electric vehicles: Prospects and impediments. WIREs Energy Environ. 2015, 4, 521–536. [Google Scholar] [CrossRef]
- Arif, S.M.; Lie, T.T.; Seet, B.C.; Ayyadi, S.; Jensen, K. Review of electric vehicle technologies, charging methods, standards and optimization techniques. Electronics 2021, 10, 1910. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Deeba, M.; Alerasool, S. Gasoline automobile catalysis and its historical journey to cleaner air. Nat. Catal. 2019, 2, 603–613. [Google Scholar] [CrossRef]
- Resitoglu, I.A.; Ozarslan, H.; Sugozu, B.; Kaya, H.S. The structural properties of an aged catalytic converter and determining the effects on engine characteristics. Int. J. Automot. Technol. 2025, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, J.; Shan, Y.; Wang, F.; Liu, J.; Wang, M.; Liu, Z.; Yan, Y.; Xu, G.; He, G.; et al. Toward synergetic reduction of pollutant and greenhouse gas emissions from vehicles: A catalysis perspective. Chem. Soc. Rev. 2025, 54, 1151–1215. [Google Scholar] [CrossRef]
- Afzal, M.; Agarwal, S.; Elshaikh, R.H.; Babker, A.M.A.; Choudhary, R.K.; Prabhakar, P.K.; Zahir, F.; Sah, A.K. Carbon monoxide poisoning: Diagnosis, prognostic factors, treatment strategies, and future perspectives. Diagnostics 2025, 15, 581. [Google Scholar] [CrossRef]
- Ahn, G.J.; Lee, S.; Lee, S.J.; Cha, Y.S. Internal malignancy risk after carbon monoxide poisoning: A nationwide population-based cohort study. J. Clin. Med. 2025, 14, 937. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, Y.; Zhao, Y.; Liu, X.; Zhu, T. Density functional theory studies of Pt-based catalysts for CO oxidation. Sci. China Technol. Sci. 2025, 68, 1310501. [Google Scholar] [CrossRef]
- Xu, L.; Zheng, P.; Tian, J.; Zhang, T.; Jiang, R.; Gao, J.; Zhang, L.; Xu, G.; Zhong, Z.; Su, F. Understanding the synergistic catalytic functions of Cu-Pt active sites in the bimetallic CuPt/ZrO2 catalyst in CO oxidation. J. Catal. 2025, 444, 115995. [Google Scholar] [CrossRef]
- Ghafoor, A.; Lotfi, M.; Gomaa, A.A.; Goldbach, A.; Shen, W. CO oxidation over nanostructured Pt/ZnO catalysts. ACS Appl. Nano Mater. 2025, 8, 3887–3898. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Xu, L.; Wang, S.; Yao, S. Revealing the positive and negative effects of strong metal support interactions on CO oxidation for Pt-supported different crystals (β- and γ-) MnO2 catalysts: Pt oxides and carbonate species. Appl. Catal. A Gen. 2025, 691, 120075. [Google Scholar] [CrossRef]
- Kautsar, D.B.; Le, P.H.; Ando, A.; Tanabe, E.; Cao, K.L.A.; Septiani, E.L.; Hirano, T.; Ogi, T. Enhancing CO oxidation performance by controlling the interconnected pore structure in porous three-way catalyst particles. Nanoscale 2025, 17, 2841–2851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, D.; Li, D.; He, L.; Guo, Z.; Yeli, G.; Zhang, X.; Liu, B.; Tan, H.; Zhang, F.; et al. Oxygen vacancies promote the activation of O2 in transition metal oxide doped ε-MnO2 for low-temperature CO oxidation. Sep. Purif. Technol. 2025, 352, 128109. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, X.; Zhang, T.; Wu, Z.; Li, J.; Wang, W.; Gao, E.; Zhu, J.; Yao, S. In-situ synthesized Co3O4/Mn3O4 on monolithic nickel foam: Enhanced CO oxidation via MOF-74 derivation. Fuel 2025, 388, 134504. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, S.; Zhang, T.; Wu, Z.; Li, J.; Wang, W.; Zhu, J.; Wu, J.; Ye, D.; Han, R.; et al. Unveiling the mechanistic synergy in Mn-doped NiO catalysts with atomic-burry structure: Enhanced CO oxidation via Ni-OH and Mn bifunctionality. Sep. Purif. Technol. 2025, 354, 129330. [Google Scholar] [CrossRef]
- Glisenti, A.; Pacella, M.; Guiotto, M.; Natile, M.M.; Canu, P. Largely Cu-doped LaCo1-xCuxO3 perovskites for TWC: Toward new PGM-free catalysts. Appl. Catal. B Environ. 2016, 180, 94–105. [Google Scholar] [CrossRef]
- Gan, L.; Ye, P.; Tian, X.; Wu, Q.; Shi, H.; Xiong, S.; Chen, J.; Li, J. Mechanism of B-site modulation in LaBO3 perovskites (B=Fe, Co, Ni) promoting CO catalytic oxidation. Appl. Surf. Sci. 2025, 686, 162215. [Google Scholar] [CrossRef]
- Felli, A.; Toso, A.; Braga, A.; Colussi, S.; Boaro, M.; Llorca, J.; Truscott, B.; Artner-Wallner, C.; Trovarelli, A. Perovskite-derived MnOx/LaMnO3 nanocomposites to boost CO oxidation activity. Catal. Sci. Technol. 2025, 15, 1882–1893. [Google Scholar] [CrossRef]
- Rizzato, L.; Cavazzani, J.; Osti, A.; Scavini, M.; Glisenti, A. Cu-doped SrTiO3 nanostructured catalysts for CO2 conversion into solar fuels using localized surface plasmon resonance. Catalysts 2023, 13, 1377. [Google Scholar] [CrossRef]
- Senoner, B.; Osti, A.; Glisenti, A. Enhancing coking resistance of nickel-based catalysts for dry reforming of methane via nitric oxide abatement: A support study. Catal. Sci. Technol. 2024, 14, 6678–6691. [Google Scholar] [CrossRef]
- Osti, A.; Rizzato, L.; Cavazzani, J.; Glisenti, A. Optimizing citrate combustion synthesis of A-site-deficient La,Mn-based perovskites: Application for catalytic CH4 combustion in stoichiometric conditions. Catalysts 2023, 13, 1177. [Google Scholar] [CrossRef]
- Ofoegbuna, T.; Darapaneni, P.; Sahu, S.; Plaisance, C.; Dorman, J.A. Stabilizing the B-site oxidation state in ABO3 perovskite nanoparticles. Nanoscale 2019, 11, 14303–14311. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Verde, Á.; Illán-Gómez, M.J. Enhancing the performance of BaxMnO3 (x = 1, 0.9, 0.8 and 0.7) perovskites as catalysts for CO oxidation by decreasing the Ba content. Nanomaterials 2024, 14, 1334. [Google Scholar] [CrossRef]
- Díaz-Verde, A.; Torregrosa-Rivero, V.; Illán-Gómez, M.J. Copper catalysts supported on barium deficient perovskites for CO oxidation reaction. Top. Catal. 2023, 66, 695–907. [Google Scholar] [CrossRef]
- Díaz-Verde, Á.; dos Santos Veiga, E.L.; Beltrán-Mir, H.; Illán-Gómez, M.J.; Cordoncillo-Cordoncillo, E. Non-stoichiometric BaxMn0.7Cu0.3O3 perovskites as catalysts for CO oxidation: Optimizing the Ba content. Nanomaterials 2025, 15, 103. [Google Scholar] [CrossRef]
- Yin, C.; Lei, Y.; Wu, H.; Xu, Y.; Luo, H.; Wang, H. Synergistic effect of Cu and Ag on porous (Cu, Ag) co-doped CeO2 nanosheets for low-temperature CO oxidation. Inorg. Chem. Commun. 2025, 172, 113729. [Google Scholar] [CrossRef]
- Martínez-Munuera, J.C.; Serrano-Martínez, V.M.; Giménez-Mañogil, J.; Yeste, M.P.; García-García, A. Unraveling the nature of active sites onto copper/ceria-zirconia catalysts for low temperature CO oxidation. Catal. Today 2022, 384–386, 246–256. [Google Scholar] [CrossRef]
- Martínez-Munuera, J.C.; Yeste, M.P.; García-García, A. CO oxidation under lean and stoichiometric conditions over ceria-zirconia with very low metal contents (Cu, Co, Ag and Pt). J. Rare Earths 2024, 42, 1669–1681. [Google Scholar] [CrossRef]
- Pakharukova, V.P.; Kriventsov, V.V.; Ishchenko, A.V.; Potemkin, D.I.; Snytnikov, P.V. Structural studies of copper species in supported CuO/CeO2 catalyst by X-ray absorption spectroscopy coupled with pair distribution function analysis. J. Clust. Sci. 2025, 36, 18. [Google Scholar] [CrossRef]
- Gu, X.; Zhu, S.; Liu, S.; Chong, C.H. Preparation of perovskite catalysts and their mechanism of catalytic degradation and regeneration of waste polyisocyanurate. Polym. Degrad. Stab. 2025, 232, 111138. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Wei, J. High performance Mn/Mg co-modified calcium-based material via EDTA chelating agent for effective solar energy storage. Chem. Eng. J. 2024, 480, 147892. [Google Scholar] [CrossRef]
- Oliveira, G.V.; de Macedo, V.; Urquieta-González, E.A.; Magriotis, Z.M.; Pereira, C.A. Fe2O3/γ-Al2O3 and NiO/γ-Al2O3 catalysts for the selective catalytic oxidation of ammonia. Catal. Today 2025, 444, 114991. [Google Scholar] [CrossRef]
- Megía, P.J.; García-Moreno, L.; Vizcaíno, A.J.; Calles, J.A.; Carrero, A. Bioethanol steam reforming for hydrogen production over Ni-Cr/SBA 15: Influence of metal loading and Ni/Cr ratio. Molecules 2025, 30, 1206. [Google Scholar] [CrossRef]
- Alam, M.A.; Ahmed, S.; Bishwas, R.K.; Mostofa, S.; Jahan, S.A. X-ray crystallographic diffraction study by whole powder pattern fitting (WPPF) method: Refinement of crystalline nanostructure polymorphs TiO2. S. Afr. J. Chem. Eng. 2025, 51, 68–77. [Google Scholar] [CrossRef]
- Coskuner, A.B.; Erdil, T.; Ozgur, C.; Geyikci, U.; Toparli, C. Tuning the lattice strain through manipulating crystal structure of high entropy oxides enhances electrocatalytic performance. Mater. Res. Bull. 2025, 186, 113333. [Google Scholar] [CrossRef]
- Peter, R.; Petravic, M. Initial stages of oxide formation on copper surfaces during oxygen bombardment at room temperature. J. Phys. Chem. C 2021, 125, 25290–25297. [Google Scholar] [CrossRef]
- Fandré, J.P.; Pennell, S.; Ramesh, S.L.; Lopez, J.; Spolenak, R.; Dunand, D.C. Hierarchically porous SnO2/Cu composites via freeze casting and selective Cu reduction. Adv. Eng. Mater. 2025, 27, 2402635. [Google Scholar] [CrossRef]
- Duell, B.A.; Ramazani, A.; Natesakhawat, S.; Popczun, E.J.; Lekse, J.W.; Duan, Y. Targeted chemical looping materials discovery by an inverse design. Adv. Intell. Syst. 2025, 7, 2401118. [Google Scholar] [CrossRef]
- Guiotto, M.; Pacella, M.; Perin, G.; Iovino, A.; Michelon, N.; Natile, M.M.; Glisenti, A.; Canu, P. Washcoating vs. direct synthesis of LaCoO3 on monoliths for environmental applications. Appl. Catal. A Gen. 2015, 499, 146–157. [Google Scholar] [CrossRef]
- Park, D.C.; Moon, S.; Song, J.H.; Kim, H.; Lee, E.; Lim, Y.H.; Kim, D.H. Widening the operating window of Pt/ZSM-5 catalysts for efficient NOx removal in H2-SCR: Insights from thermal aging. Catal. Today 2024, 425, 114318. [Google Scholar] [CrossRef]
- Wang, F.; Da, B.; Jin, Y.; Sanwal, P.; Cui, L.; Chen, S.; Xu, J.; Xue, B.; Li, G. Insight into the effect of manganese oxidation state on the synthesis of ethylene urea from CO2 and ethylenediamine. J. Environ. Sci. 2025, 155, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Raciulete, M.; Anastasescu, C.; Papa, F.; Atkinson, I.; Bradu, C.; Negrila, C.; Eftemie, D.I.; Culita, D.C.; Miyazaki, A.; Bratan, V.; et al. Band-gap engineering of layered perovskites by Cu spacer insertion as photocatalysts for depollution reaction. Catalysts 2022, 12, 1529. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Liu, J.; Liu, F.; Li, Y. Reaction mechanism and microkinetics of CO catalytic combustion over Ni-doped LaCoO3 perovskite. Proc. Combust. Inst. 2023, 39, 5621–5635. [Google Scholar] [CrossRef]
- González-Varela, D.; Araiza, D.G.; Díaz, G.; Pfeiffer, H. LaNiO3 perovskite synthesis through the EDTA-citrate complexing method and its application to CO oxidation. Catalysts 2022, 12, 57. [Google Scholar] [CrossRef]
- Popa, I.M.; Pischetola, C.; Krumeich, F.; van Bokhoven, J.A.; Carja, G.; Artiglia, L. Exploiting the LDH memory effect in the carbon dioxide to methanol conversion. Adv. Funct. Mater. 2025, 35, 2502812. [Google Scholar] [CrossRef]
- Belda-Marco, S.; Lillo-Ródenas, M.A.; Román-Martínez, M.C. Optimization of active sites in TiO2-Cu photocatalysts for H2 generation via cellulose photo-reforming. ChemCatChem 2025, 17, e202500250. [Google Scholar] [CrossRef]
- LaSurface Database. Available online: http://www.lasurface.com/accueil/ (accessed on 15 May 2025).
- Chitchak, K.; Jaisabuy, K.; Vanalabhpatana, P. Metal salen electrocatalysts for electro-reduction of carbon dioxide. Catal. Lett. 2025, 155, 173. [Google Scholar] [CrossRef]
- Zhang, E.; Song, X.; Chen, J.; Zhang, B.; Wang, G. Hydrothermal synthesis of magnetic cube-like copper ferrite nanocrystallines for reduction 4-nitrophenol. Part. Part. Syst. Charact. 2025, 42, 2400218. [Google Scholar] [CrossRef]
- Ulyanov, A.N.; Yu, S.C.; Yang, D.S. Mn-site-substituted lanthanum manganites: Destruction of electron pathway and local structure effects on Curie temperature. J. Magn. Magn. Mater. 2004, 282, 303–306. [Google Scholar] [CrossRef]
- Yang, Z.; Sun, Y.; Li, P.; Zhang, S.; Sun, X.; Cheng, S.; Jiang, Y. High-performance MnOx-CuO catalysts for low-temperature CO oxidation: Metal interaction and reaction mechanism. Mol. Catal. 2025, 576, 114945. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Liu, W.; Zhao, Z.; Liu, X.; Xu, Y.; Li, T. Enhancing the CO oxidation performance of copper by alloying with immiscible tantalum. ACS Appl. Mater. Interfaces 2025, 17, 6377–6384. [Google Scholar] [CrossRef]
- Thormählen, P.; Fridell, E.; Cruise, N.; Skoglundh, M.; Palmqvist, A. The influence of CO2, C3H6, NO, H2, H2O or SO2 on the low-temperature oxidation of CO on a cobalt-aluminate spinel catalyst (Co1.66Al1.34O4). Appl. Catal. B Environ. 2001, 31, 1–12. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, T.; Zhu, L.; Jia, A.P.; Lu, J.Q.; Luo, M.F. Enhanced performance of CO oxidation over Pt/CuCrOx catalyst in the presence of CO2 and H2O. Appl. Surf. Sci. 2018, 442, 613–621. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic oxidation of carbon monoxide over transition metal oxides. ChemCatChem 2010, 3, 24–65. [Google Scholar] [CrossRef]
- Maul, J.; dos Santos, I.M.G.; Sabrano, J.R.; Casassa, S.; Erba, A. A quantum-mechanical investigation of oxygen vacancies and copper doping in the orthorrombic CaSnO3 perovskite. Phys. Chem. Chem. Phys. 2018, 20, 20970–20980. [Google Scholar] [CrossRef]
- Liu, X.; Ren, X.; Shao, Y.; Dong, A.; Yang, C.; Li, K.; Zhou, B.; Wu, H.; Li, Z. Unveiling reduction of NH3 production in Pd-based Three-Way Catalyst modified by Ru. Catal. Lett. 2025, 155, 101. [Google Scholar] [CrossRef]
- Balzarotti, R.; Peressut, A.B.; Garbarino, G.; Spennati, E.; Basbus, J.F.; Carpanese, M.P.; Latorrata, S.; Cristiani, C.; Finocchio, E. A study of redox properties of ceria and Fe-ceria solid materials through small molecules catalytic oxidation. Materials 2025, 18, 806. [Google Scholar] [CrossRef] [PubMed]
- Prieur, D.; Bonani, W.; Popa, K.; Walter, O.; Kriegsman, K.W.; Engelhard, M.H.; Guo, X.; Eloirdi, R.; Gouder, T.; Beck, A.; et al. Size dependence of lattice parameter and electronic structure in CeO2 nanoparticles. Inorg. Chem. 2020, 59, 5760–5767. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Ferrer, C.; Parres-Esclapez, S.; Lozano-Castelló, D.; Bueno-López, A. Relationship between surface area and crystal size of pure and doped cerium oxides. J. Rare Earths 2010, 28, 647–653. [Google Scholar] [CrossRef]
- Alshaikh, H.; Mohamed, R.M. Synthesis of barium titanate nanocrystals decorated with PtO for enhanced photooxidative desulfurization of thiophene. Mater. Sci. Eng. B 2025, 314, 118028. [Google Scholar] [CrossRef]
- Zeng, Z.; Wu, J.; Xia, C.; Yuan, S.; Ren, X.; Zhao, L.; Zhuang, L.; He, Y. Boosting photocatalytic efficiency with ohmic contact: A novel Bi/BaTiO3 composite for nitrogen fixation and RhB degradation. J. Alloys Compd. 2025, 1010, 178312. [Google Scholar] [CrossRef]
- Padalia, D.; Kumar, U.; Bhandari, P.; Dalal, J.; Ranakoti, L.; Singh, T. Tuning the structural, optical, and dielectric properties of europium-doped barium titanate ceramics. J. Mater. Sci. Mater. Electron. 2024, 35, 1375. [Google Scholar] [CrossRef]
- Garbujo, A.; Pacella, M.; Natile, M.M.; Guiotto, M.; Fabro, J.; Canu, P.; Glisenti, A. On A-doping strategy for tuning the TWC catalytic performance of perovskite based catalysts. Appl. Catal. A Gen. 2017, 544, 94–107. [Google Scholar] [CrossRef]
- Taucher, T.C.; Hehn, I.; Hofmann, O.T.; Zharnikov, M.; Zojer, E. Understanding chemical versus electrostatic shifts in x-ray photoelectron spectra of organic self-assembled monolayers. J. Phys. Chem. C 2016, 120, 3428–3437. [Google Scholar] [CrossRef]
- Jiang, J.; Yang, H.; Jiang, H.; Hu, Y.; Li, C. Boosting catalytic activity of Cu-Ce solid solution catalysts by flame spray pyrolysis with high Cu+ concentration and oxygen vacancies. Chem. Eng. J. 2023, 471, 144439. [Google Scholar] [CrossRef]
- Sajeev, S.; Vincent, M.; Garbacz, P.; Strawski, M.; Zhu, C.; Aoki, Y.; Kowalski, D. The role of Ni substitution in manganite perovskite Li-O2 battery. RSC Appl. Interfaces 2025, 2, 1051–1058. [Google Scholar] [CrossRef]
- Feng, M.; Tang, Y.; Li, Z.; Zhang, Z.; Li, C.; Bao, R.; Yi, J.; Chen, J.; Wang, J. Differentiated modulating the electronic structure of NiFe@Ni/Fe-MnOx via phase transformation engineering to synergy promote bifunctional water splitting reactions. Small 2025, 21, 2410752. [Google Scholar] [CrossRef]
- Liu, G.; Li, J.; Yang, K.; Tang, W.; Liu, H.; Yang, J.; Yue, R.; Chen, Y. Effects of cerium incorporation on the catalytic oxidation of benzene over flame-made perovskite La1-xCexMnO3 catalysts. Particuology 2015, 19, 60–68. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, W.; Lai, S.; Chen, X. Integral structured Co-Mn composite oxides grown on interconnected Ni foam for catalytic toluene oxidation. RSC Adv. 2019, 9, 6533–6541. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Jung, H. Tailoring oxidation state of manganese enables the direct formation of todorokite. ACS Nano 2025, 19, 12910–12919. [Google Scholar] [CrossRef]
- Zhou, J.; Ouyang, R.; Zhong, X.; Guo, L.; Yu, X.; Xu, J.; Fang, X.; Shen, J.; Wang, X. BaBO3 (B = Ti, Sn, Zr) perovskites for oxidative coupling of methane: Elucidating the effects of intrinsic oxygen vacancies, high-temperature crystal phase transition on reaction performance. Phys. Chem. Chem. Phys. 2025, 27, 8887–8897. [Google Scholar] [CrossRef] [PubMed]
- Islam, Z.U.; Want, B. Structural, optical, and dielectric properties of double perovskite NdBaFeTiO6. Ceram. Int. 2025, 51, 1585–1594. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, X.; Ye, J.; Liu, L. Achieving highly moisture-tolerant ozone decomposition over Pd/MnO2-HAC monolith catalyst. ACS Appl. Eng. Mater. 2025, 3, 968–977. [Google Scholar] [CrossRef]
- Gao, H.; Song, Z.; Mao, Y.; Fan, Y.; Li, R.; Chen, X.; Liu, W.; Zhang, J.; Huang, Z.; Zhang, X. Tight coupling of oxygen vacancies and acidity on α-MnO2 through cerium doping engineering for efficient removal of multi-component VOCs. Appl. Catal. B Environ. Energy 2025, 362, 124745. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, L.; Liu, L.; Li, W.; Zhu, C.; Liu, W. Zr-doped CoZrOx solid solution catalysts with enhanced oxygen vacancy for trace ethylene removal under humid conditions. Appl. Surf. Sci. 2025, 689, 162449. [Google Scholar] [CrossRef]
- Paul, M.J.; Suresh, R.; Gayathri, P.; Balasubramani, V.; Al-Anazi, K.M.; Farah, M.A. CuO-La2O3 composite-enabled MIS Schottky barrier diodes: A novel approach to optoelectronic device diversification. J. Inorg. Organomet. Polym. 2025, 35, 396–408. [Google Scholar] [CrossRef]
- Ren, Y.; Chen, G.; Lu, Y.; Li, K.; Li, Z.; Zhu, Y. One-step synthesis, characterization and ammonia-selective catalytic reduction performance of Cu-SAPO-34 catalyst. Eur. J. Inorg. Chem. 2025, 28, e202400576. [Google Scholar] [CrossRef]
- NIST X-Ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20, National Institute of Standards and Technology, Gaithersburg MD, 2000; 20899. Available online: https://dx.doi.org/10.18434/T4T88K (accessed on 20 May 2025).
- Beche, E.; Peraudeau, G.; Flaud, V.; Perarnau, D. An XPS investigation of (La2O3)1-x (CeO2)2x (ZrO2)2 compounds. Surf. Interface Anal. 2012, 44, 1045–1050. [Google Scholar] [CrossRef]
- Larachi, F.; Pierre, J.; Adnot, A.; Bernis, A. Ce 3d XPS study of composite CexMn1-xO2-y wet oxidation catalysts. Appl. Surf. Sci. 2002, 195, 236–250. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.; Hao, Y.; Chen, R.; Chen, T. Porous Mn-based oxides for complete ethanol and toluene catalytic oxidation: The relationship between structure and performance. Catal. Sci. Technol. 2020, 10, 1941–1951. [Google Scholar] [CrossRef]
- Kobayashi, M.; Naniwa, S.; Goto, K.; Matsuo, H.; Iguchi, S.; Tanaka, T.; Teramura, K. Promoting effect of Pd nanoparticles on SrTi0.8Mn0.2O3 in the Reverse Water-Gas Shift reaction via the Mars-van Krevelen mechanism. ChemCatChem 2024, 16, e202400871. [Google Scholar] [CrossRef]
- Santamaria, A.; Méndez, A.C.; Gallego, J. LaMn1−xCoxO3 perovskite-like doped and impregnated with Cu for the enhancement of toluene total oxidation. Appl. Surf. Sci. 2024, 669, 160587. [Google Scholar] [CrossRef]
- Tada, S.; Kondo, M.; Joutsuka, T. Oxide solid-solution catalysts with good redox properties for liquid-phase aerobic additive-free oxidation: A review. ChemCatChem 2024, 16, e202301367. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, P.; Yang, C.; Zhang, T.; Zhang, X.; Xiong, J.; Sun, X.; Wei, Y.; Li, Z. Alkaline earth metal enhancement of cerium-manganese-based three-dimensional ordered microporous catalysts for NO oxidation. ChemCatChem 2025, 17, e202401984. [Google Scholar] [CrossRef]
- Lee, J.; Christopher, P. Does H2 Temperature-Programmed Reduction always probe solid-state redox chemistry? The case of Pt/CeO2. Angew. Chem. Ind. Ed. 2024, 64, e202414388. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Sun, Y.; Li, P.; Jiang, Y.; Sun, X.; Yang, Z. Effect of Mn/Cu molar ratios on CO oxidation activity of Mn-Cu bimetallic catalysts. Catalysts 2025, 15, 353. [Google Scholar] [CrossRef]
- Martín-Martín, J.A.; González-Marcos, M.P.; Aranzabal, A.; González-Velasco, J.R.; Finocchio, E. Promotion of different active phases in MnOx-CeO2 catalysts for simulataneous NO reduction and o-DCB oxidation. Top. Catal. 2025, 68, 13–32. [Google Scholar] [CrossRef]
- Li, H.X.; Jin, L.Y.; Zhang, A.C.; Sun, Z.J.; Zhang, X.M.; Zhu, Q.F.; Yang, C.Z.; Zhang, S.B. Investigation of Co-doped Mn oxide catalyst for NH3-SCR activity and SO2/H2O resistance. J. Fuel Chem. Technol. 2022, 50, 1404–1416. [Google Scholar] [CrossRef]
- Kim, M.H.; Cho, K.H.; Shin, C.H.; Kang, S.E.; Ham, S.W. Total oxidation of propane over Cu-Mn mixed oxide catalysts prepared by co-precipitation method. Korean J. Chem. Eng. 2011, 28, 1139–1143. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, Y.; Gao, Y.; Ding, N.; Wang, D.; Long, L.; Wang, B.; Lang, J.; Vovk, E.I.; Yang, Y. Mechanistic understanding of dissociated hydrogen in Cu/CeO2-catalyzed methanol synthesis. ACS Appl. Mater. Interfaces 2025, 17, 7151–7163. [Google Scholar] [CrossRef]
- Fulham, G.J.; Wu, X.; Liu, W.; Marek, E.J. Mechanistic insights into the role of zinc oxide, zirconia and ceria supports in Cu-based catalysts for CO2 hydrogenation to methanol. Chem. Eng. J. 2024, 480, 147732. [Google Scholar] [CrossRef]
- Kumar, S.; Teraoka, Y.; Joshi, A.G.; Rayalu, S.; Labhsetwar, N. Ag promoted La0.8Ba0.2MnO3 type perovskite catalyst for N2O decomposition in the presence of O2, NO and H2O. J. Mol. Catal. A Chem. 2011, 348, 42–54. [Google Scholar] [CrossRef]
- Moradkhani, F.; Kootenaei, A.S.; Maghsoodi, S.; Mirzaei, M.; Azimi, A. A double perovskite oxide with A-site deficiency: A facile way to yield a boosted propane combustion catalyst. Pet. Chem. 2023, 63, 1322–1334. [Google Scholar] [CrossRef]
- Harada, K.; Oishi, T.; Hamamoto, S.; Ishihara, T. Lattice oxygen activity in Pr- and La-doped CeO2 for low-temperature soot oxidation. J. Phys. Chem. C 2014, 118, 559–568. [Google Scholar] [CrossRef]
- Tan, X.; Lan, H.; Xie, H.; Zhou, G.; Jiang, Y. Role of surface oxygen species of mesoporous CeCu oxide catalyst in OVOCs catalytic combustion. J. Environ. Chem. Eng. 2017, 5, 2068–2076. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Kappis, K.; Papavasiliou, J.; Vakros, J.; Kuśmierz, M.; Gac, W.; Georgiou, Y.; Deligiannakis, Y.; Avgouropoulos, G. Copper-promoted ceria catalysts for CO oxidation reaction. Catal. Today 2020, 355, 647–653. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, K.; Guo, Z.; Guo, Y.; Jiang, Y.; Wu, X.; Fu, Z.; Zhang, H.; Li, P.; Zeng, S. Abundant surface adsorbed oxygen species originated from defect/vacancy sites during preferential CO oxidation. Chem. Eng. J. 2025, 504, 158672. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, Z.; Li, Z.; Gao, G.; Ji, L.; Xu, H.; Huang, W.; Qu, Z.; Yan, N. The unique CO activation effects for boosting NH3 selective catalytic oxidation over CuOx-CeO2. Environ. Sci. Technol. 2022, 56, 10402–10411. [Google Scholar] [CrossRef]
- Porta, A.; Visconti, C.G.; Castoldi, L.; Matarrese, R.; Jeong-Potter, C.; Farrauto, R.; Lietti, L. Ru-Ba synergistic effect in dual functioning materials for cyclic CO2 capture and methanation. Appl. Catal. B Environ. 2021, 283, 119654. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Papavasiliou, J.; Tabakova, T.; Idakiev, V.; Ioannides, T. A comparative study of ceria-supported gold and copper oxide catalysts for preferential CO oxidation reaction. Chem. Eng. J. 2006, 124, 41–45. [Google Scholar] [CrossRef]
- Landi, G.; Benedetto, A.D.; Colussi, S.; Barbato, P.S.; Lisi, L. Effect of carbon dioxide and water on the performances of an iron-promoted copper/ceria catalyst for CO preferential oxidation in H2-rich streams. Int. J. Hydrogen Energy 2016, 41, 7332–7341. [Google Scholar] [CrossRef]

| Sample | XPS 1 | |||||
| Mn(IV)/Mn(III) 2 | Cu/(Ba+Mn+Cu) (Nominal) | Cusi/Cuwi | OL/(Ba+Mn(+Cu)) (Nominal) 3 | |||
| B0.8MC | 0.96 ± 0.10 | 0.08 ± 0.01 (0.17) | 1.40 ± 0.09 | 0.86 ± 0.03 (1.67) | ||
| Cu4/B0.7M-E | 1.28 ± 0.05 | 0.10 ± 0.01 (0.07) | 1.62 ± 0.07 | 1.41 ± 0.02 (1.76) | ||
| Cu12/B0.7M-E | 1.34 ± 0.10 | 0.18 ± 0.01 (0.15) | 1.30 ± 0.04 | 1.15 ± 0.03 (1.76) | ||
| Sample | H2-TPR | O2-TPD | CO Specific Activity 4 | |||
| mL H2 ·(g sample)−1 | μmol O2 ·(g sample)−1 | |||||
| B0.8MC | 60 ± 15 | 367 ± 35 | 0.424 ± 0.048/0.401 ± 0.048 | |||
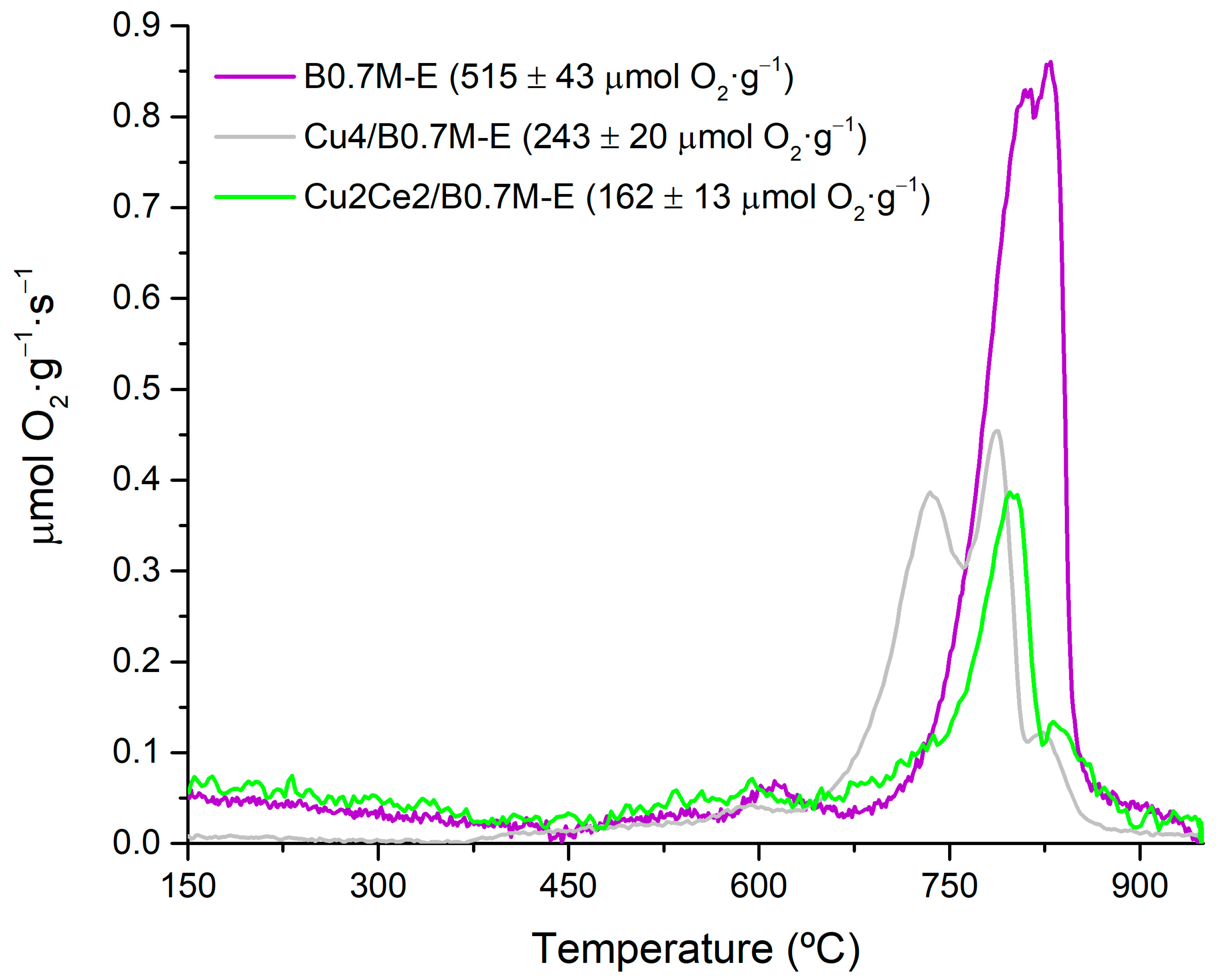
| Cu4/B0.7M-E | 70 ± 15 | 243 ± 20 | 1.233 ± 0.043/1.231 ± 0.043 | |||
| Cu12/B0.7M-E | 90 ± 16 | 100 ± 10 | 0.588 ± 0.042/0.588 ± 0.042 | |||
| Sample | B0.8MC | Cu4/B0.7M-E | ||
|---|---|---|---|---|
| Cycle | 1 | 2 | 1 | 2 |
| ΔCCO (%) 1 | −10 ± 6 | −8 ± 6 | −26 ± 6 | −15 ± 6 |
| aCO (5 h) 2 | 0.309 ± 0.062 | 0.281 ± 0.067 | 0.518 ± 0.087 | 0.469 ± 0.094 |
| Sample | Mn(IV)/Mn(III) 1 | Cu/(Ba+Mn+Cu) | OL/(Ba+Mn(+Cu)) 2 | BaCO3/BaL | ||
| Fresh B0.8MC | 0.96 ± 0.10 | 0.08 ± 0.01 | 0.86 ± 0.03 | 0.15 ± 0.01 | ||
| Used B0.8MC | 0.58 ± 0.01 | 0.09 ± 0.01 | 0.18 ± 0.01 | 0.15 ± 0.01 | ||
| Fresh Cu4/B0.7M-E | 1.28 ± 0.05 | 0.10 ± 0.01 | 1.41 ± 0.02 | 0.16 ± 0.01 | ||
| Used Cu4/B0.7M-E | 0.79 ± 0.01 | 0.10 ± 0.01 | 0.22 ± 0.01 | 0.11 ± 0.01 | ||
| Sample | KE Cu L3M4.5M4.5 (eV) 3 | Cu (II) (%) 4 | Cusi (%) 4,5 | |||
| Fresh B0.8MC | 917.5 ± 0.1 | 22 ± 12 | 78 ± 18 | |||
| Used B0.8MC | 917.2 ± 0.1 | 42 ± 2 | 58 ± 5 | |||
| Fresh Cu4/B0.7M-E | 918.8 ± 0.1 | 55 ± 5 | 45 ± 14 | |||
| Used Cu4/B0.7M-E | 917.5 ± 0.1 | 21 ± 4 | 79 ± 5 | |||
| Sample | B0.8MC | Cu4/B0.7M-E |
|---|---|---|
| ΔCCO (%) 1 | - 3 | −31 ± 6 |
| aCO (5 h) 2 | 0.012 ± 0.001 | 0.457 ± 0.030 |
| Sample | Mn(IV)/Mn(III) 1 | Cu/(Ba+Mn+Cu) | OL/(Ba+Mn(+Cu)) 2 | BaCO3/BaL | |||
| Fresh B0.8MC | 0.96 ± 0.01 | 0.08 ± 0.01 | 0.86 ± 0.03 | 0.15 ± 0.01 | |||
| Used B0.8MC | 0.84 ± 0.03 | 0.04 ± 0.01 | 0.17 ± 0.01 | 0.06 ± 0.01 | |||
| Fresh Cu4/B0.7M-E | 1.28 ± 0.05 | 0.10 ± 0.01 | 1.41 ± 0.02 | 0.16 ± 0.01 | |||
| Used Cu4/B0.7M-E | 0.45 ± 0.02 | 0.05 ± 0.01 | 0.20 ± 0.01 | 0.41 ± 0.01 | |||
| Sample | KE Cu L3M4.5M4.5 (eV) 3 | Cu (II) (%) 4 | Cusi (%) 4,5 | ||||
| Fresh B0.8MC | 917.5 ± 0.1 | 22 ± 12 | 78 ± 18 | ||||
| Used B0.8MC | 917.6 ± 0.1 | 37 ± 8 | 63 ± 16 | ||||
| Fresh Cu4/B0.7M-E | 918.8 ± 0.1 | 55 ± 5 | 45 ± 14 | ||||
| Used Cu4/B0.7M-E | 917.6 ± 0.1 | 61 ± 4 | 39 ± 19 | ||||
| Sample | Cell Parameters 1 | Perovskite Average Crystal Size (nm) | Lattice Strain | |
|---|---|---|---|---|
| a (Å) | c (Å) | |||
| B0.7M-E | 5.68 ± 0.21 | 4.82 ± 0.32 | 26.2 ± 2.1 | 0.0002 ± 2 |
| Cu4/B0.7M-E | 5.69 ± 0.20 | 4.80 ± 0.28 | 15.2 ± 2.3 | 0.0017 ± 0.0004 |
| Cu2Ce2/B0.7M-E | 5.68 ± 0.20 | 4.79 ± 0.27 | 32.3 ± 2.8 | 0.0030 ± 0.0003 |
| Sample | BaCO3/BaL | Mn(IV)/Mn(III) 1 | Mn(III)c/Mn(III)f | OL/(Ba+Mn) (Nominal = 1.76) | ||
| B0.7M-E | 0.08 ± 0.01 | 0.48 ± 0.01 | - | 1.06 ± 0.01 | ||
| Cu4/B0.7M-E | 0.16 ± 0.01 | 1.28 ± 0.05 | 0.18 ± 0.03 | 1.41 ± 0.02 | ||
| Cu2Ce2/B0.7M-E | 0.10 ± 0.01 | 1.05 ± 0.03 | 0.31 ± 0.02 | 0.29 ± 0.01 | ||
| Sample | Cu/(Ba+Mn+Cu(+Ce)) (nominal) | Ce(IV)/Ce(III) | Ce/(Ba+Mn+Cu+Ce) (nominal) | |||
| B0.7M-E | - | - | - | |||
| Cu4/B0.7M-E | 0.10 ± 0.01 (0.07) | - | - | |||
| Cu2Ce2/B0.7M-E | 0.07 ± 0.01 (0.04) | 1.83 ± 0.06 | 0.47 ± 0.01 (0.02) | |||
| Sample | Cu (II) (%) 2 | Cured/Cusi (%) 2,3 | ||||
| B0.7M-E | - | - | ||||
| Cu4/B0.7M-E | 55 ± 5 | 45 ± 14 | ||||
| Cu2Ce2/B0.7M-E | 41 ± 5 | 59 ± 16 | ||||
| Sample | Cu2Ce2/B0.7M-E | Cu4/B0.7M-E | ||
|---|---|---|---|---|
| CO2 Content (%) | 0 | 15 | 0 | 15 |
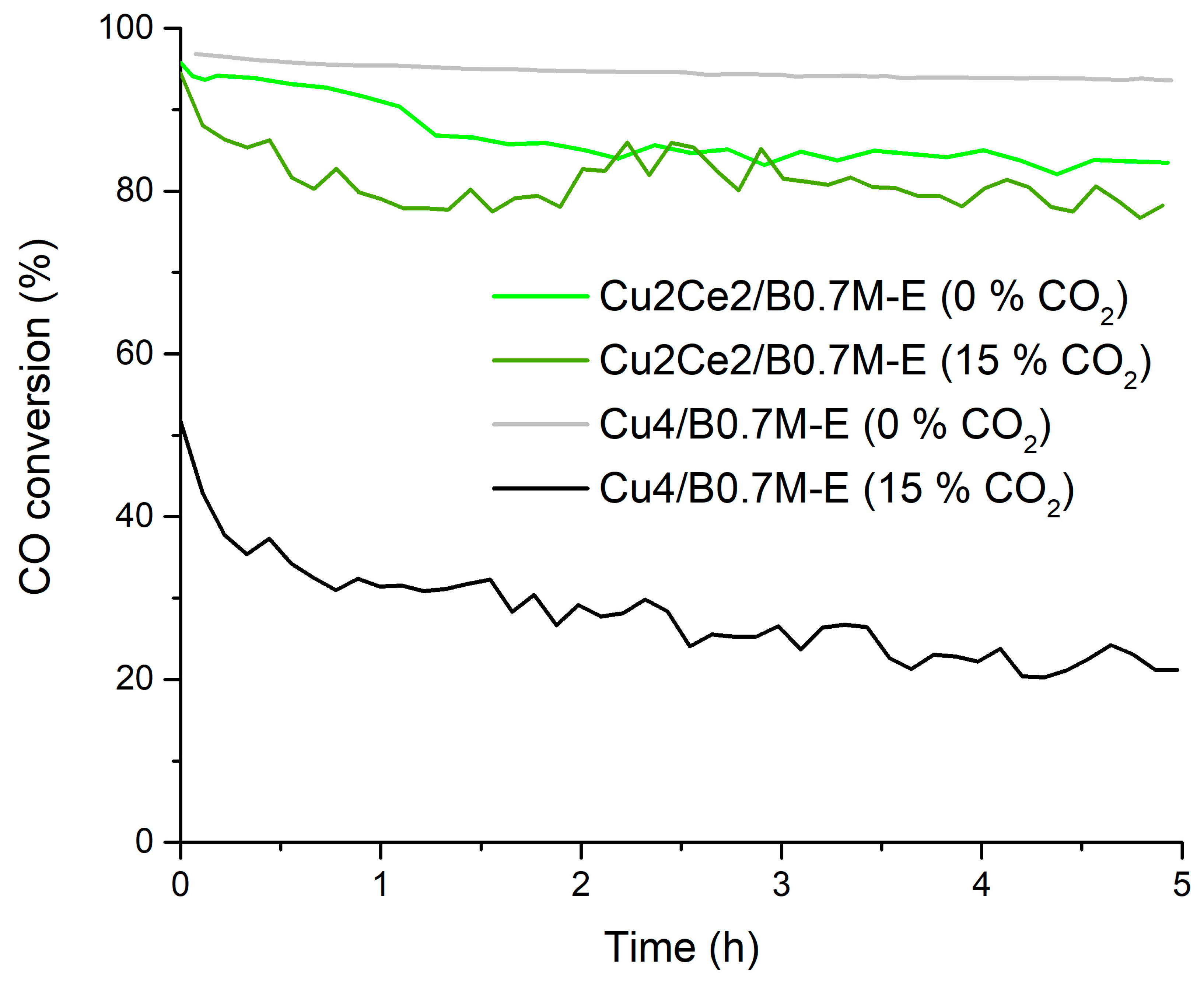
| ΔCCO (%) 1 | −13 ± 6 | −20 ± 6 | −2 ± 6 3 | −31 ± 6 |
| aCO (5 h) 2 | 2.274 ± 0.108 | 1.620 ± 0.097 | 1.231 ± 0.043 | 0.457 ± 0.030 |
| Sample | BaCO3/BaL | Mn(IV)/Mn(III) 1 | Mn(III)c/Mn(III)f | OL/(Ba+Mn) (Nominal = 1.76) |
| Fresh | 0.10 ± 0.01 | 1.05 ± 0.03 | 0.31 ± 0.01 | 0.29 ± 0.01 |
| Used (0% CO2) | 0.09 ± 0.01 | 0.75 ± 0.01 | 0.31 ± 0.01 | 0.29 ± 0.01 |
| Used (15% CO2) | 0.08 ± 0.01 | 0.77 ± 0.02 | 0.35 ± 0.02 | 0.29 ± 0.01 |
| Sample | Cu/(Ba+Mn+Cu+Ce) (Nominal = 0.04) | KE Cu L3M4.5M4.5 (eV) 2 | Ce(IV)/Ce(III) | Ce/(Ba+Mn+Cu+Ce) (Nominal = 0.02) |
| Fresh | 0.07 ± 0.01 | 918.2 ± 0.1 | 1.83 ± 0.06 | 0.47 ± 0.01 |
| Used (0% CO2) | 0.07 ± 0.01 | 917.9 ± 0.1 | 2.37 ± 0.04 | 0.46 ± 0.01 |
| Used (15% CO2) | 0.06 ± 0.01 | 917.4 ± 0.1 | 2.57 ± 0.06 | 0.44 ± 0.01 |
| Sample | Cu (II) (%) 2 | Cured (%) 3,4 | ||
| Fresh | 41 ± 5 | 59 ± 16 | ||
| Used (0% CO2) | 42 ± 5 | 58 ± 14 | ||
| Used (15% CO2) | 41 ± 6 | 59 ± 18 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Verde, Á.; Illán-Gómez, M.J. An Approach to the Optimization of Ba-Mn-Cu Perovskites as Catalysts for CO Oxidation: The Role of Cerium. Nanomaterials 2025, 15, 1467. https://doi.org/10.3390/nano15191467
Díaz-Verde Á, Illán-Gómez MJ. An Approach to the Optimization of Ba-Mn-Cu Perovskites as Catalysts for CO Oxidation: The Role of Cerium. Nanomaterials. 2025; 15(19):1467. https://doi.org/10.3390/nano15191467
Chicago/Turabian StyleDíaz-Verde, Álvaro, and María José Illán-Gómez. 2025. "An Approach to the Optimization of Ba-Mn-Cu Perovskites as Catalysts for CO Oxidation: The Role of Cerium" Nanomaterials 15, no. 19: 1467. https://doi.org/10.3390/nano15191467
APA StyleDíaz-Verde, Á., & Illán-Gómez, M. J. (2025). An Approach to the Optimization of Ba-Mn-Cu Perovskites as Catalysts for CO Oxidation: The Role of Cerium. Nanomaterials, 15(19), 1467. https://doi.org/10.3390/nano15191467






