Structure and Magnetic Properties of Vanadium-Doped Heusler Ni-Mn-In Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
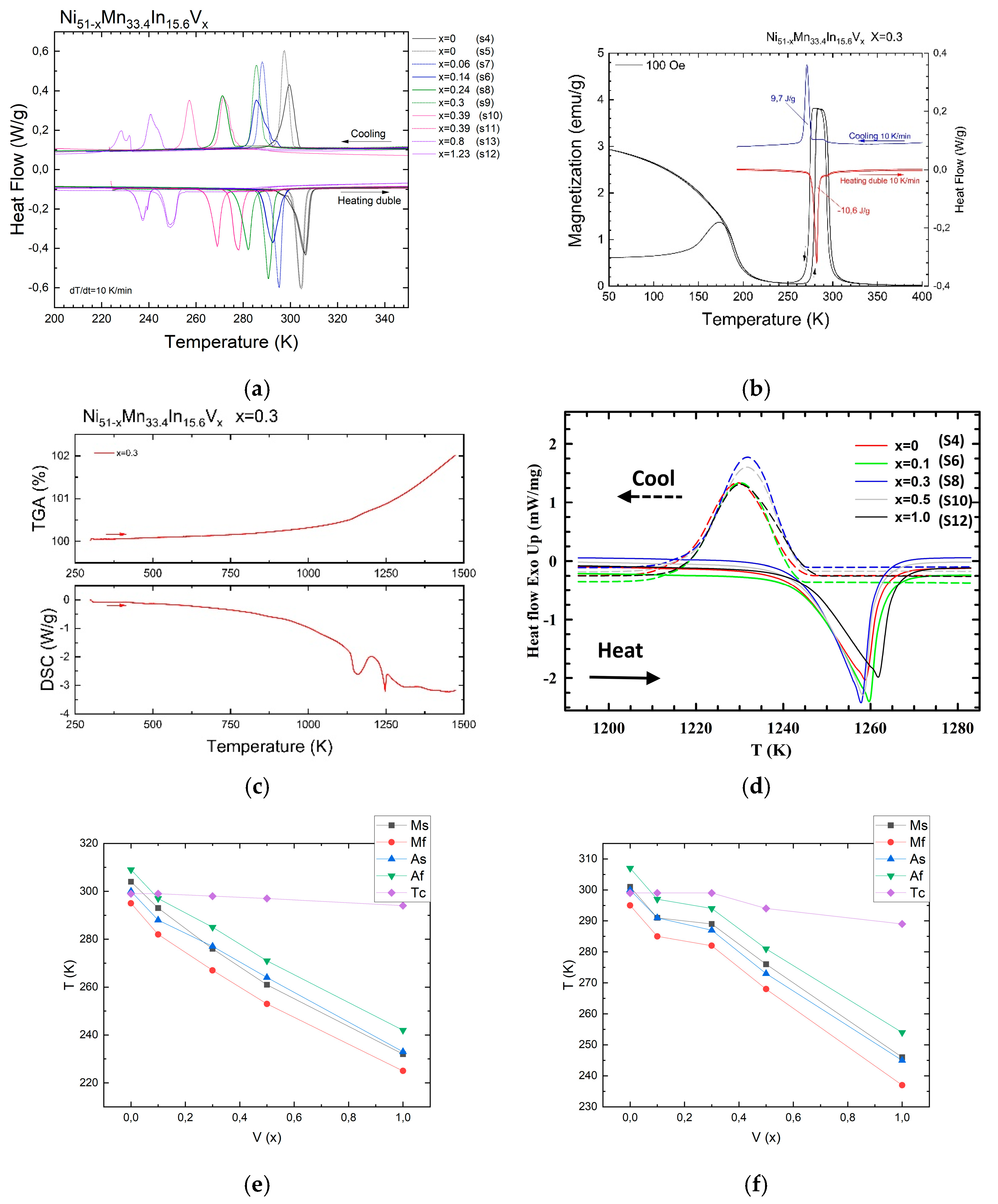
3.1. Thermal Measurements
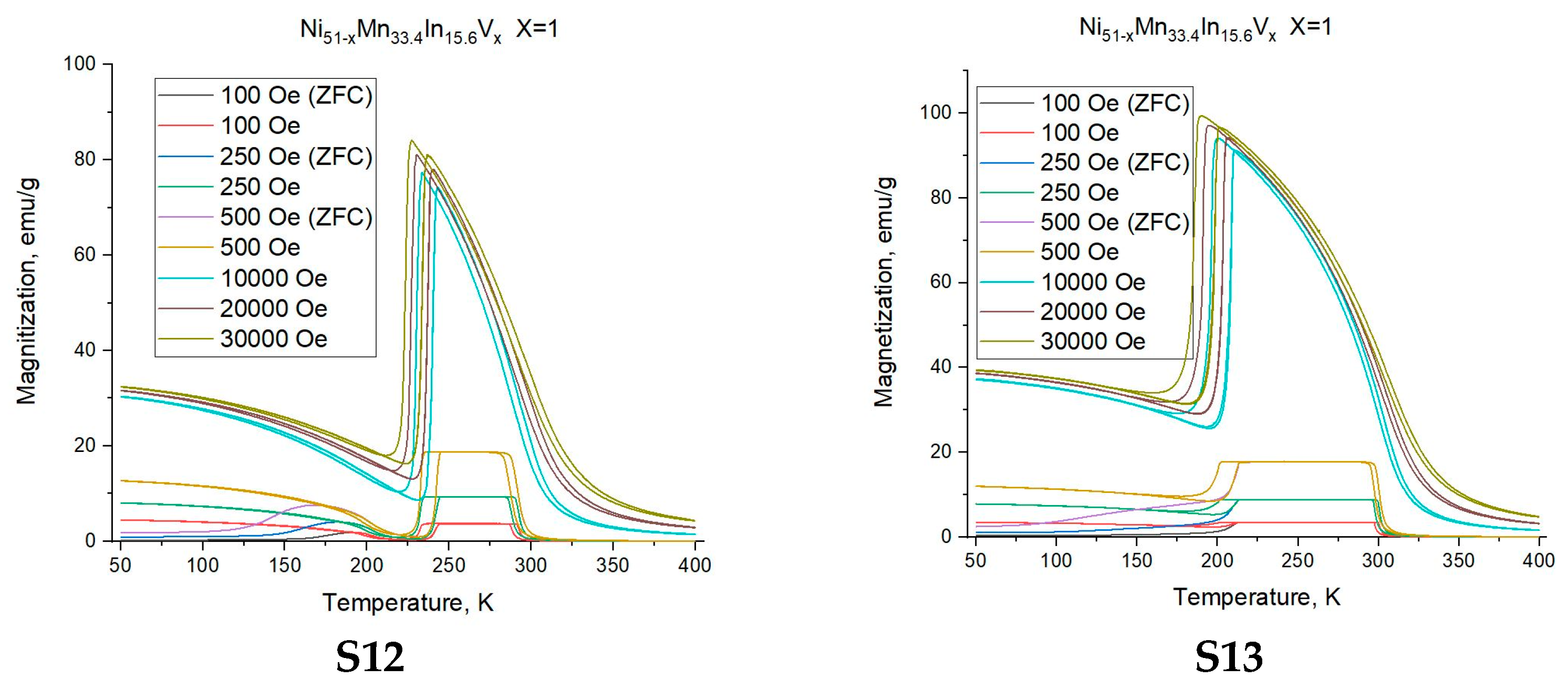
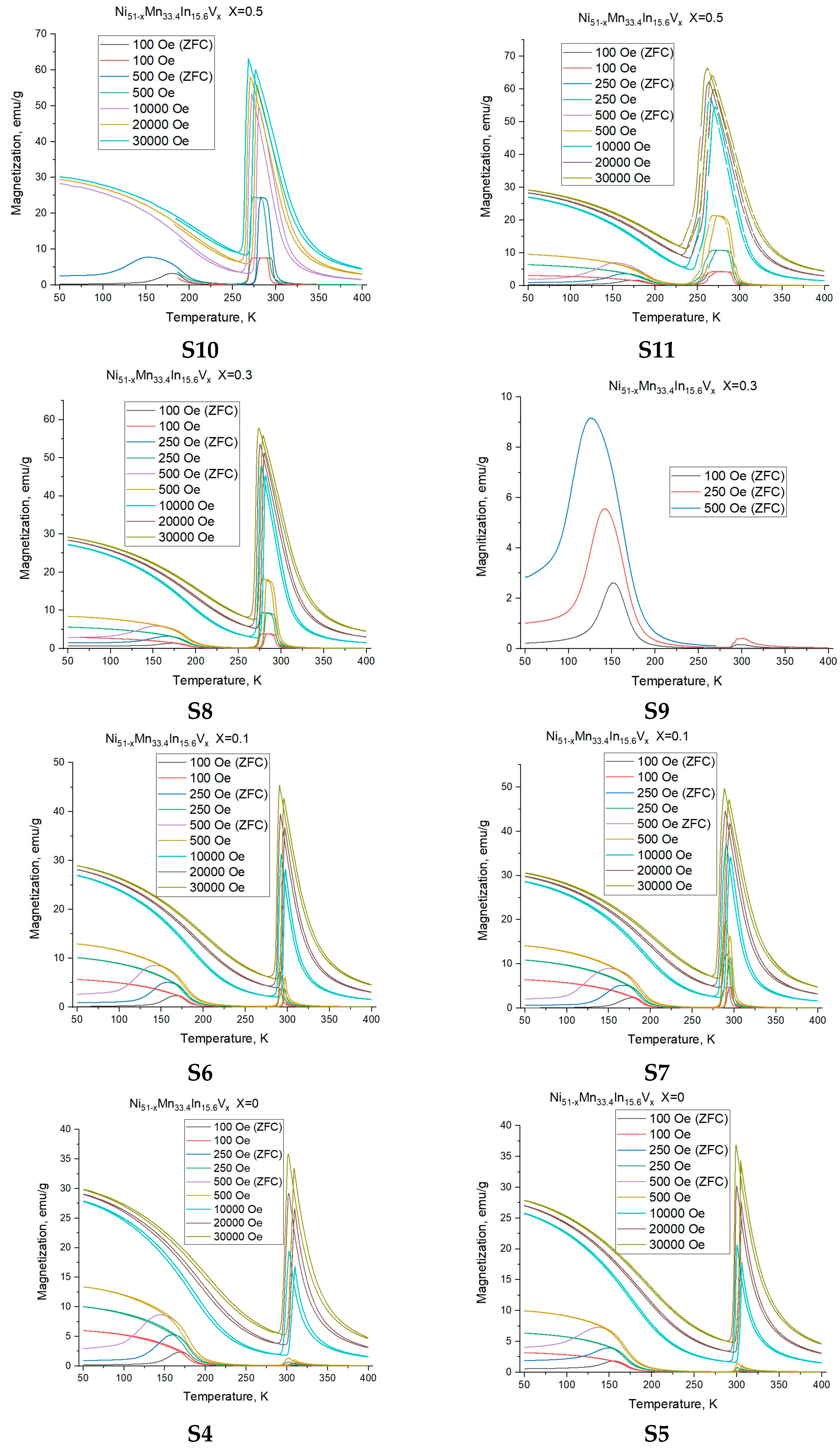
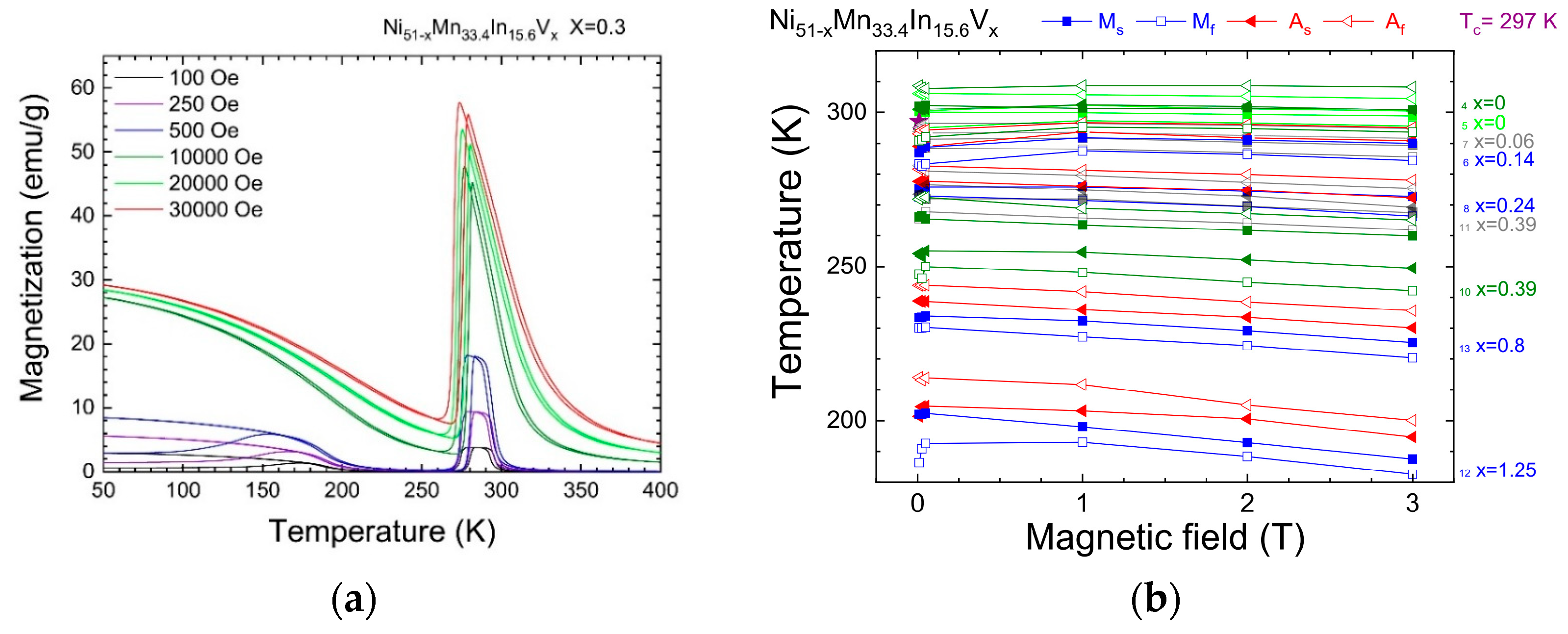
3.2. Magnetic Measurements
3.3. Structural Analysis
3.3.1. X-Ray Diffraction Analysis
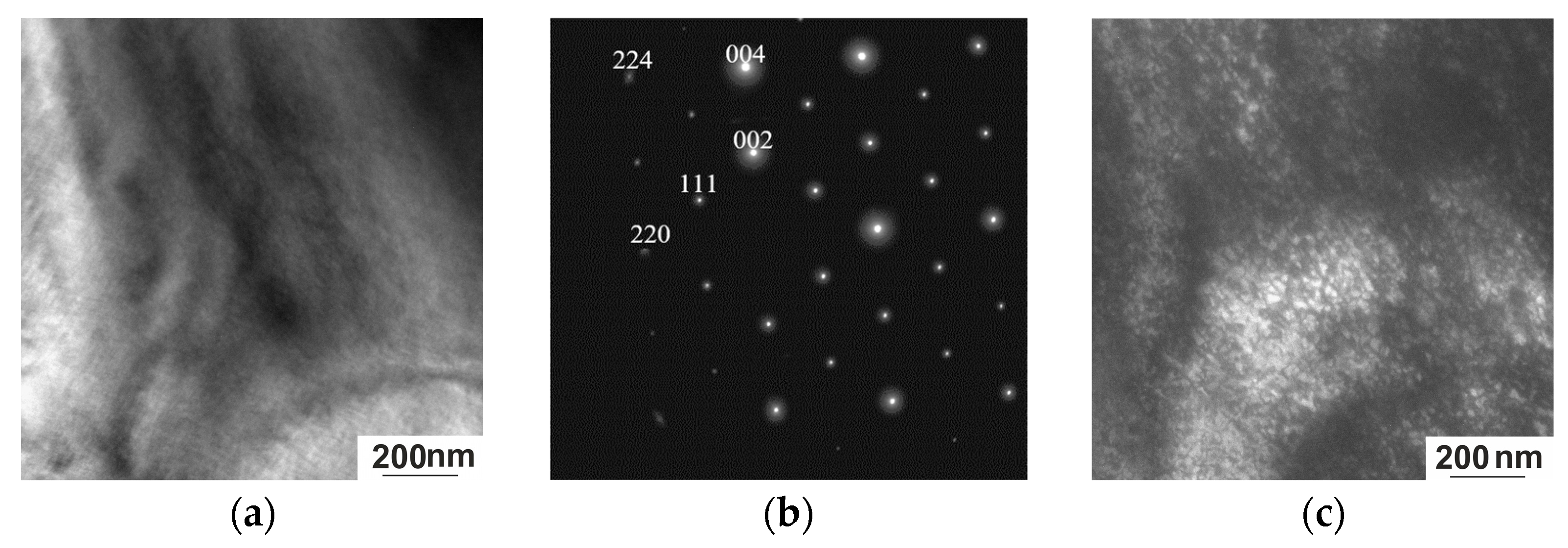
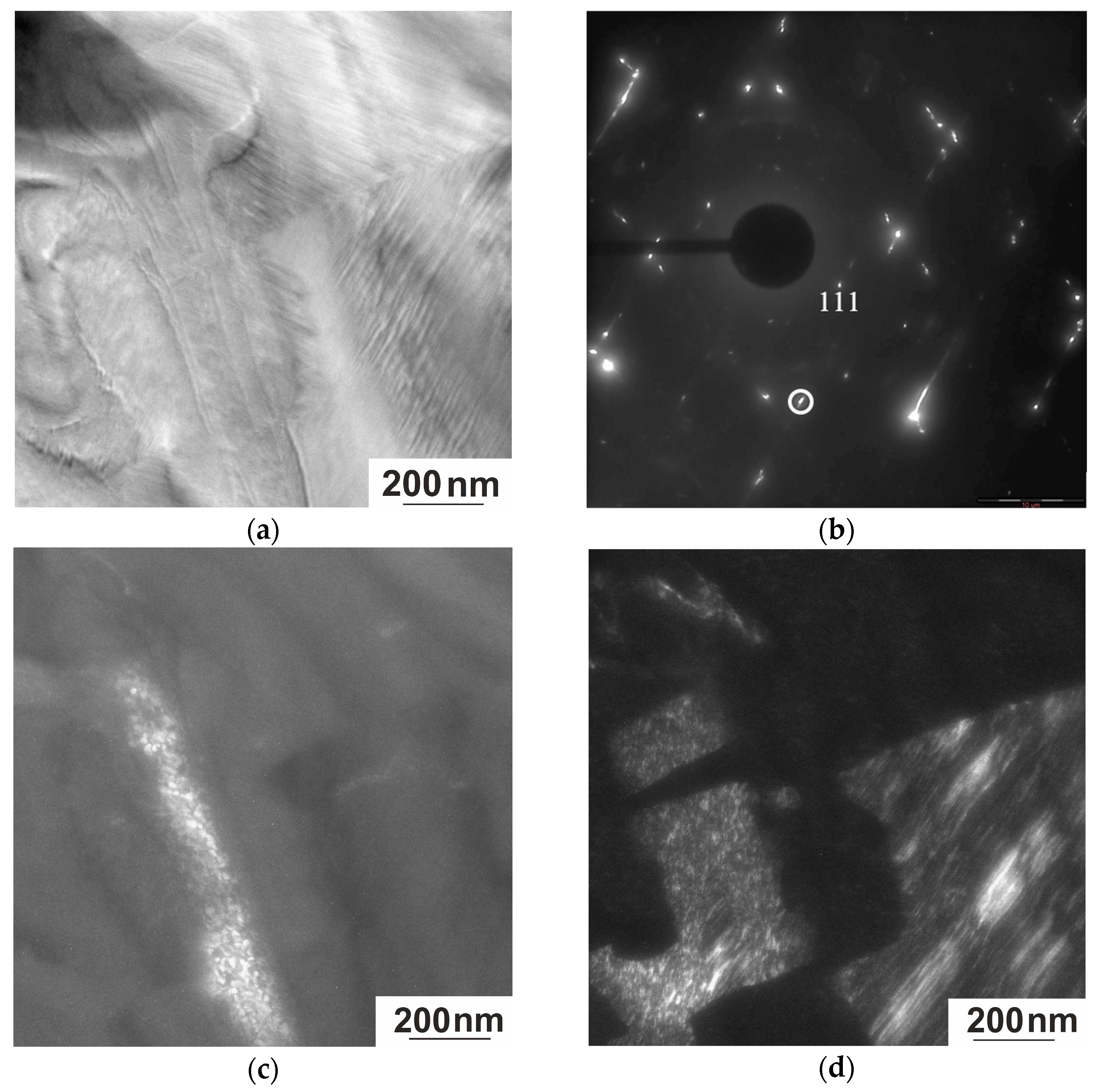
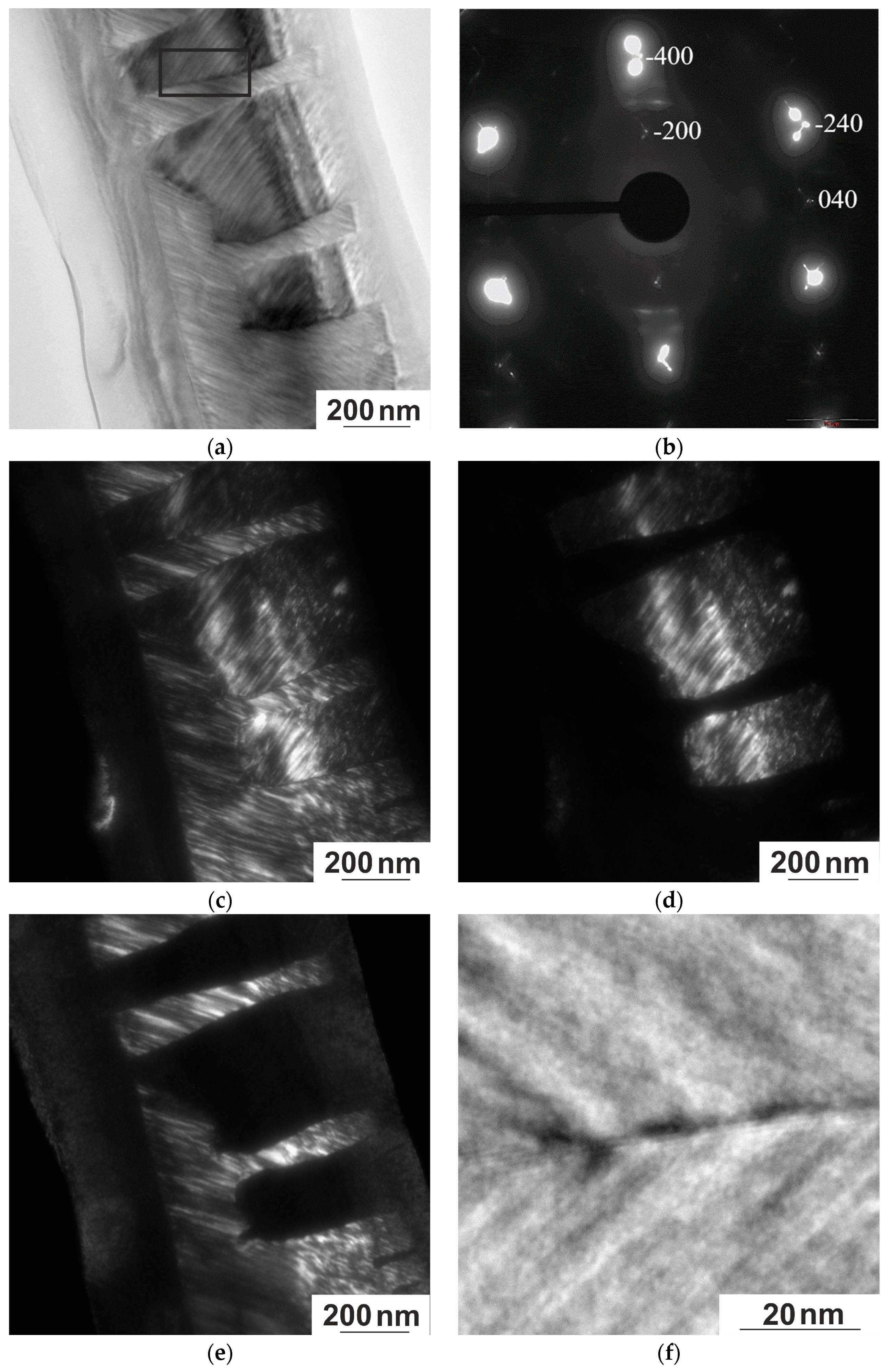
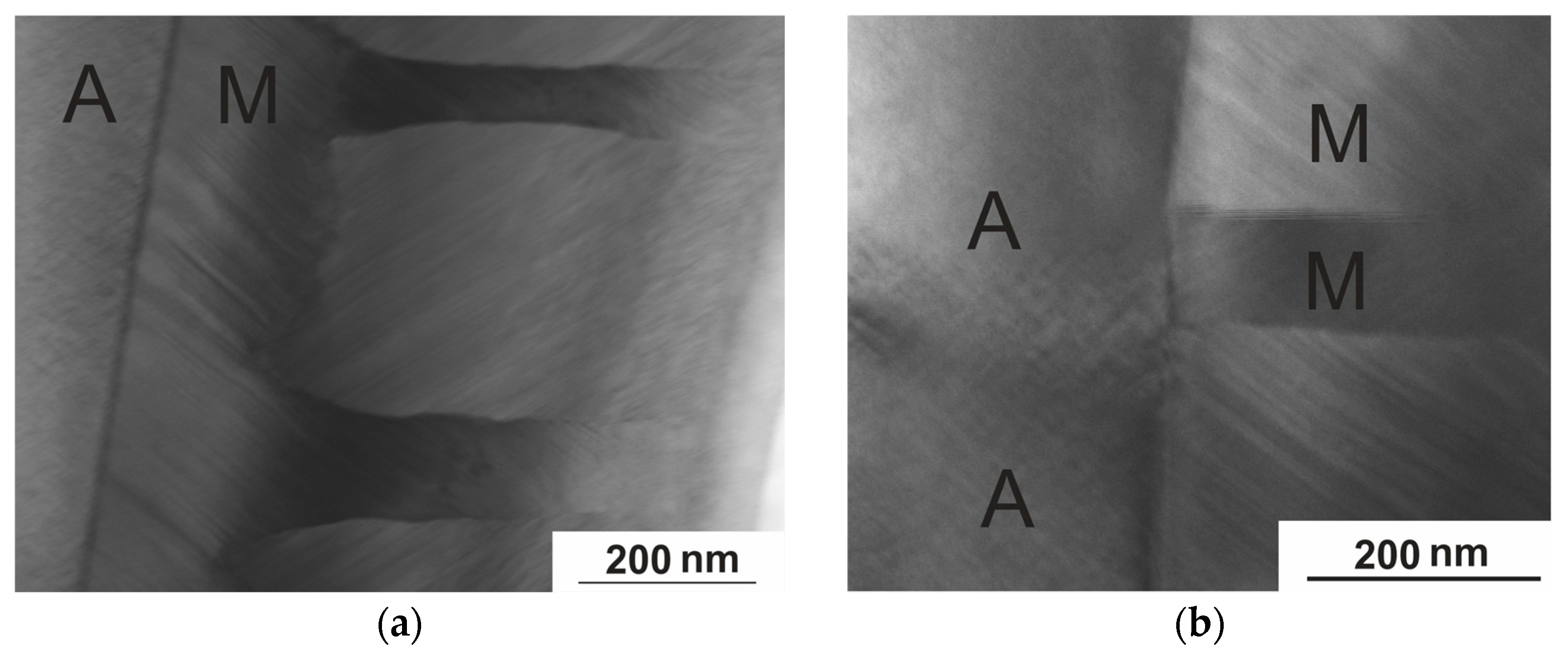
3.3.2. Electron Microscopy
4. Conclusions
- The microstructure of the austenite of the Heusler alloy studied is formed by the spinodal decomposition mechanism, and not through nucleation and growth, since, in essence, the system is structurally divided into regions that differ primarily in the degree of ordering and, accordingly, chemical composition without low- or high-angle boundaries—an antiphase domain structure.
- There is a microstructural connection between austenite and martensite. The type of modulated twin structure of martensite is determined by the high density of AFD and AFB in the initial austenite phase. The microtwin structure of martensite corresponds to the size of the domains of the tweed structure of austenite, and a defect-free boundary with good coherence of the A–M phases is observed.
- The alloy microstructure consists predominantly of six-layer modulated martensite up to 113 K. Modulated structures of 10 M and 14 M martensite are also observed. An HRTEM study revealed a thin layer of 4–10 nm thick with a banded contrast of 2 to 5 atomic layers at the boundary of martensite packets.
- The formation of multiple martensite variants undoubtedly affects the conditions for the formation of the MT. The shift in the temperature of the onset of martensitic transformation kMs above 1 T is −0.5 K/T, −1.6 K/T, −3.5 K/T for alloys with a nominal vanadium doping level of x = 0 (S4), 0.3 (S8), and 1 (S12) at.%, respectively, and the temperature hystereses (ΔThys) are 5.6 K, 5.0 K, and 9.1 K, with an increase in width of about 0 K/T, 0.33 K/T, and 0.45 K/T.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Yan, H.L.; Huang, X.M.; Esling, C. Recent Progress in Crystallographic Characterization, Magnetoresponsive and Elastocaloric Effects of Ni-Mn-In-Based Heusler Alloys—A Review. Front. Mater. 2022, 9, 85. [Google Scholar] [CrossRef]
- Bachaga, T.; Zhang, J.; Khitouni, M.; Sunol, J.J. NiMn-based Heusler magnetic shape memory alloys: A review. Int. J. Adv. Manuf. Technol. 2019, 103, 2761–2772. [Google Scholar] [CrossRef]
- Elphick, K.; Frost, W.; Samiepour, M.; Kubota, T.; Takanashi, K.; Sukegawa, H.; Mitani, S.; Hirohata, A. Heusler alloys for spintronic devices: Review on recent development and future perspectives. Sci. Technol. Adv. Mater. 2021, 22, 235–271. [Google Scholar] [CrossRef] [PubMed]
- Bekhouche, A.; Alleg, S.; Dadda, K.; Daoudi, M.I.; Saurina, J.; Sunol, J.J. Microstructure, martensitic transformation kinetics, and magnetic properties of (Ni50Mn40In10)100−xCox melt-spun ribbons. J. Therm. Anal. Calorim. 2025, 150, 953–964. [Google Scholar] [CrossRef]
- Tavares, S.; Yang, K.; Meyers, M.A. Heusler alloys: Past, properties, new alloys and prospects. Prog. Mater. Sci. 2023, 132, 101017. [Google Scholar] [CrossRef]
- Krenke, T.; Acet, M.; Wassermann, E.F.; Moya, X.; Mañosa, L.; Planes, A. Ferromagnetism in the austenitic and martensitic states of Ni−Mn−In alloys. Phys. Rev. B 2006, 73, 174413. [Google Scholar] [CrossRef]
- Liu, Z.H.; Li, G.T.; Wu, Z.G.; Ma, X.Q.; Liu, Y.; Wu, G.H. Tailoring martensitic transformation and martensite structure of NiMnIn alloy by Ga doping. J. Alloys Compd. 2012, 535, 120–123. [Google Scholar] [CrossRef]
- Rama Rao, N.V.; Chandrasekaran, V.; Suresh, K.G. Effect of Ni/Mn ratio on phase transformation and magnetic properties in Ni–Mn–In alloys. J. Appl. Phys. 2010, 108, 043913. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Sharma, V.K.; Chouhan, A.; Arora, P.; Roy, S.B. Combined effect of hydrostatic pressure and magnetic field on the martensitic transition in the Ni49CuMn34In16 alloy. Phys. Rev. B 2011, 8, 064417. [Google Scholar] [CrossRef]
- Erager, K.R.; Sokolovskiy, V.V.; Buchelnikov, V.D. First-principle studies of the tendency towards segregation in Heusler alloys Ni2Mn1+xSb1-x with different atomic ordering. Phys. Solid State 2022, 63, 2028–2033. [Google Scholar] [CrossRef]
- Li, X.-Z.; Zhang, W.-Y.; Sellmyer, D.J. Structural investigation of phase segregation in Mn2CrGa-based alloys. Acta Mater. 2017, 140, 188–195. [Google Scholar] [CrossRef]
- Załęski, K.; Ekholm, M.; Alling, B.; Abrikosov, I.A.; Dubowik, J. Local atomic configuration approach to the nonmonotonic concentration dependence of magnetic properties of Ni2Mn1+xZ1−x (Z = In, Sn, Sb) Heusler alloys. Scr. Mater. 2021, 194, 113646. [Google Scholar] [CrossRef]
- Tian, H.F.; Lu, J.B.; Ma, L.; Shi, H.L.; Yang, H.X.; Wu, G.H.; Li, J.Q. Martensitic transformation and magnetic domains in Mn50Ni40Sn10 studied by in-situ transmission electron microscopy. J. Appl. Phys. 2012, 112, 033904. [Google Scholar] [CrossRef]
- Das, R.; Sarma, S.; Perumal, A.; Srinivasan, A. Effect of Co and Cu substitution on the magnetic entropy change in Ni46Mn43Sn11 alloy. J Appl. Phys. 2011, 109, 07A901. [Google Scholar] [CrossRef]
- Zhang, K.; Tian, X.; Tan, C.; Guo, E.; Zhao, W.; Cai, W. Designing a New Ni-Mn-Sn Ferromagnetic Shape Memory Alloy with Excellent Performance by Cu Addition. Metals 2018, 8, 152. [Google Scholar] [CrossRef]
- Liu, J.; You, X.; Huang, B.; Batashev, I.; Maschek, M.; Gong, Y.; Miao, X.; Xu, F.; van Dijk, N.; Brück, E. Reversible low-field magnetocaloric effect in Ni-Mn-In-based Heusler alloys. Phys. Rev. Mater. 2019, 3, 084409. [Google Scholar] [CrossRef]
- Skripov, V.P.; Skripov, A.V. Spinodal decomposition (phase transition involving unstable states). Usp. Fiz. Nauk. 1979, 128, 193–231. [Google Scholar] [CrossRef]
- Allen, S.M. Spinodal Decomposition. Encycl. Mater. Sci. Technol. 2001, 8761–8764. [Google Scholar] [CrossRef]
- Boytsova, O.V.; Makarevich, O.N.; Sharovarov, D.I.; Makarevich, A.M. Spinodal Decomposition in the Chemistry and Technology of Inorganic Materials. Inorg. Mater. 2022, 58, 673–686. [Google Scholar] [CrossRef]
- Khachaturian, A.G. Theory of Phase Transitions and Structure of Solid Solutions; Nauka: Moscow, Russia, 1979. (In Russian) [Google Scholar]
- Kuznetsov, D.; Kuznetsova, E.; Mashirov, A.; Danilov, D.; Shandryuk, G.; Musabirov, I.; Shchetinin, I.; Prokunin, A.; von Gratowski, S.; Shavrov, V. Influence of the Cooling Rate on Austenite Ordering and Martensite Transformation in a Non-Stoichiometric Alloy Based on Ni-Mn-In. J. Compos. Sci. 2023, 7, 514. [Google Scholar] [CrossRef]
- Kuznetsov, D.D.; Mashirov, A.V.; Kuznetsova, E.I.; Prokunin, A.V.; Danilov, D.V.; Musabirov, I.I.; Koledov, V.V.; Shavrov, V.G. Structural domains of austenite of non-stochiometric heusler alloys based on Ni-Mn-In. J. Radio Electron. 2024, 5, 1684–1719. (In Russian) [Google Scholar] [CrossRef]
- Kuznetsov, D.D.; Kuznetsova, E.I.; Mashirov, A.V.; Loshachenko, A.S.; Danilov, D.V.; Mitsiuk, V.I.; Kuznetsov, A.S.; Shavrov, V.G.; Koledov, V.V.; Ari-Gur, P. Magnetocaloric Effect, Structure, Spinodal Decomposition and Phase Transformations Heusler Alloy Ni-Mn-In. Nanomaterials 2023, 13, 1385. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, D.D.; Kuznetsova, E.I.; Mashirov, A.V.; Loshachenko, A.S.; Danilov, D.V.; Shandryuk, G.A.; Shavrov, V.G.; Koledov, V.V. In Situ TEM Study of Phase Transformations in Nonstoichiometric Ni46Mn41In13 Heusler Alloy. Phys. Solid State 2022, 64, 15–21. [Google Scholar] [CrossRef]
- Sagaradze, V.V.; Belozerov, Y.V.; Pecherkina, N.L.; Mukhin, M.L.; Zaynutdinov, Y.R. The shape memory effect in high-strength precipitation-hardening austenitic steels. Mater. Sci. Eng. A 2006, 438–440, 812–815. [Google Scholar] [CrossRef]
- Sagaradze, V.V.; Belozerov, Y.V.; Mukhin, M.L.; Zaynutdinov, Y.R.; Pecherkina, N.L.; Zavalishin, V.A. A New Approach to Creation of High-Strength Austenitic Steels with a Controlled Shape-Memory Effect. Phys. Met. Metallogr. 2006, 101, 506–512. [Google Scholar] [CrossRef]
- Konovalova, E.V.; Perevalova, O.B.; Koneva, N.A.; Smirnov, A.I.; Kozlov, E.V. Influence of the antiphase domain structure on the long-range atomic order parameter in the Ni3Mn alloy with superstructure L 12. Phys. Solid State 2013, 55, 2116–2119. [Google Scholar] [CrossRef]
- Pushin, V.; Kuranova, N.; Marchenkova, E.; Pushin, A. Design and development of Ti–Ni, Ni–Mn–Ga and Cu–Al–Ni-based alloys with high and low temperature shape memory effects. Materials 2019, 12, 2616. [Google Scholar] [CrossRef]
- Sudareva, S.V.; Romanov, E.P.; Krinitsina, T.P.; Blinova, Y.u.V.; Kuznetsova, E.I. Mechanism of phase transformations and fine structure of a nonstoichiometric compound YBa2Cu3O7−δ at temperatures of 200 and 300 °C. Phys. Met. Metallogr. 2006, 102, 205–212. [Google Scholar] [CrossRef]
- Bobylev, I.B.; Kuznetsova, E.I.; Krinitsina, T.P.; Zyuzeva, N.A.; Sudareva, S.V.; Romanov, E.P. Restoration of the microstructure of Ba2YCu3O7−δ at T > 900 °C after low-temperature decomposition. Phys. Met. Metallogr. 2011, 112, 165–169. [Google Scholar] [CrossRef]
- Khachaturyan, A.G.; Shapiro, S.M.; Semenovskaya, S. Adaptive phase formation in martensitic transformation. Phys. Rev. B 1991, 43, 10832. [Google Scholar] [CrossRef]
- Kaufmann, S.; Niemann, R.; Thersleff, T.; Rößler, U.; Heczko, O.; Buschbeck, J.; Holzapfel, B.; Schultz, L.; Fähler, S. Modulated martensite: Why it forms and why it deforms easily. N. J. Phys. 2011, 13, 053029. [Google Scholar] [CrossRef]
- Chulist, R.; Wójcik, A.; Sozinov, A.; Tokarski, T.; Faryna, M.; Schell, N.; Skrotzki, W.; Li, B.; Sehitoglu, H.; Li, X.; et al. Adaptive Phase or Variant Formation at the Austenite/Twinned Martensite Interface in Modulated Ni–Mn–Ga Martensite. Adv. Funct. Mater. 2024, 34, 2307322. [Google Scholar] [CrossRef]
- Zhou, L.; Schneider, M.M.; Giri, A.; Cho, K.; Sohn, Y. Microstructural and crystallographic characteristics of modulated martensite, non-modulated martensite, and pre-martensitic tweed austenite in Ni-Mn-Ga alloys. Acta Mater. 2017, 134, 93–103. [Google Scholar] [CrossRef]
- Lega, P.; Karstev, A.; Nedospasov, I.; Lv, S.; Lv, S.; Tabachkova, N.; Irzhak, A.; Orlov, A.; Koledov, V. Blocking of martensitic transition at the nanoscale in the Ti2NiCu wedge. Phys. Rev. B 2020, 101, 214111. [Google Scholar] [CrossRef]








| № | Nominal Composition (at.%) | EDX Average Value, at. %. (Ni; Mn; In; V) | EDX Maximum Value, at. % (Ni; Mn; In; V) | EDX Minimum Value, at. % (Ni; Mn; In; V) | EDX Standard Deviation, at. % (Ni; Mn; In; V) |
|---|---|---|---|---|---|
| S4 | Ni51−xMn33.4In15.6Vx (x = 0) | 50.38; 34.02; 15.60; 0 | 50.89; 35.14; 15.83; 0 | 49.51; 33.49; 15.35; 0 | 0.59; 0.50; 0.17; 0 |
| S5 | Ni51−xMn33.4In15.6Vx (x = 0) | 50.36; 34.04; 15.59; 0 | 50.75; 34.36; 15.67; 0 | 50.07; 33.64; 15.52; 0 | 0.28; 0.25; 0.06; 0 |
| S6 | Ni51−xMn33.4In15.6Vx (x = 0.1) | 50.19; 34.04; 15.63; 0.14; | 50.37; 34.24; 15.94; 0.20; | 49.88; 33.87; 15.40; 0.10; | 0.04; 0.16; 0.17; 0.19 |
| S7 | Ni51−xMn33.4In15.6Vx (x = 0.1) | 50.34; 33.92; 15.69;0.06; | 50.51; 34.26; 15.93; 0.15; | 50.11; 33.69; 15.37; 0.00; | 0.06; 0.24; 0.16; 0.22 |
| S8 | Ni51−xMn33.4In15.6Vx (x = 0.3) | 50.24; 33.87; 15.65; 0.24; | 50.47; 34.06; 15.82; 0.31; | 50.00; 33.65; 15.55; 0.18; | 0.05; 0.15; 0.18; 0.11 |
| S9 | Ni51−xMn33.4In15.6Vx (x = 0.3) | 50.31; 33.79; 15.61; 0.30; | 50.62; 34.09; 15.94; 0.48; | 49.99; 33.54; 15.29; 0.22; | 0.09; 0.21; 0.26; 0.23 |
| S10 | Ni51−xMn33.4In15.6Vx (x = 0.5) | 49.85; 34.03; 15.73; 0.39; | 50.02; 34.14; 15.88; 0.50; | 0.33; 33.88; 49.74; 15.57 | 0.07; 0.09; 0.12; 0.10 |
| S11 | Ni51−xMn33.4In15.6Vx (x = 0.5) | 49.95; 33.94; 15.72; 0.39; | 50.24; 34.05; 15.86; 0.50; | 49.73; 33.81; 15.57; 0.31; | 0.08; 0.11; 0.24; 0.11 |
| S12 | Ni51−xMn33.4In15.6Vx (x = 1) | 49.80; 33.54; 15.43; 1.23; | 50.12; 33.66; 15.71; 2.15; | 49.28; 33.29; 15.08; 0.84; | 0.49; 0.14; 0.29; 0.24 |
| S13 | Ni51−xMn33.4In15.6Vx (x = 1) | 49.93; 33.59; 15.68; 0.80; | 50.14; 33.73; 15.94; 0.89; | 49.80; 33.47; 15.55; 0.74; | 0.06; 0.11; 0.12; 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsov, D.; Kuznetsova, E.; Mashirov, A.; Kamantsev, A.; Danilov, D.; Shandryuk, G.; Taskaev, S.; Musabirov, I.; Gaifullin, R.; Kolkov, M.; et al. Structure and Magnetic Properties of Vanadium-Doped Heusler Ni-Mn-In Alloys. Nanomaterials 2025, 15, 1466. https://doi.org/10.3390/nano15191466
Kuznetsov D, Kuznetsova E, Mashirov A, Kamantsev A, Danilov D, Shandryuk G, Taskaev S, Musabirov I, Gaifullin R, Kolkov M, et al. Structure and Magnetic Properties of Vanadium-Doped Heusler Ni-Mn-In Alloys. Nanomaterials. 2025; 15(19):1466. https://doi.org/10.3390/nano15191466
Chicago/Turabian StyleKuznetsov, Dmitry, Elena Kuznetsova, Alexey Mashirov, Alexander Kamantsev, Denis Danilov, Georgy Shandryuk, Sergey Taskaev, Irek Musabirov, Ruslan Gaifullin, Maxim Kolkov, and et al. 2025. "Structure and Magnetic Properties of Vanadium-Doped Heusler Ni-Mn-In Alloys" Nanomaterials 15, no. 19: 1466. https://doi.org/10.3390/nano15191466
APA StyleKuznetsov, D., Kuznetsova, E., Mashirov, A., Kamantsev, A., Danilov, D., Shandryuk, G., Taskaev, S., Musabirov, I., Gaifullin, R., Kolkov, M., Koledov, V., & Ari-Gur, P. (2025). Structure and Magnetic Properties of Vanadium-Doped Heusler Ni-Mn-In Alloys. Nanomaterials, 15(19), 1466. https://doi.org/10.3390/nano15191466









