Combating Healthcare-Associated Infections in Modern Hospitals: Nanotechnology-Based Approaches in the Era of Antimicrobial Resistance
Abstract
1. The Problem of Hospital-Acquired Infections
1.1. Definition and Epidemiological Impact
1.2. Risk Factors and Transmission Mechanisms
1.3. Global Burden and Mortality Statistics
1.4. Pathogen Reservoirs and Transmission Vehicles
1.5. Device-Associated Infections
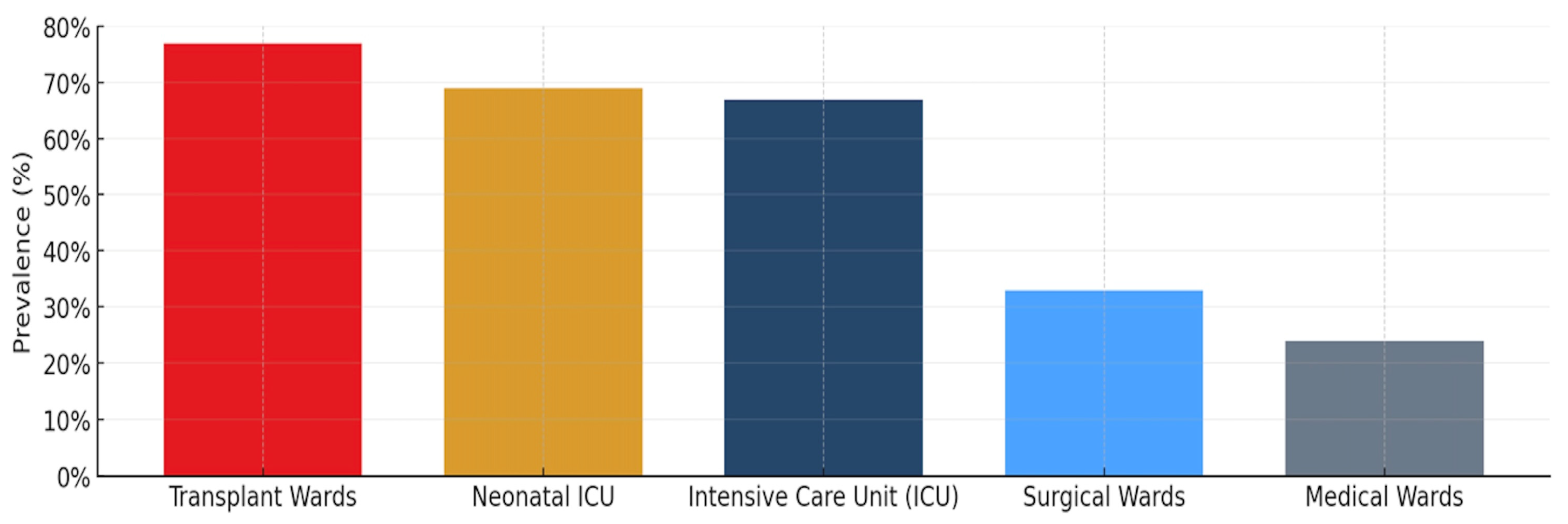
1.6. Ward-Specific Infection Prevalence
1.7. Biofilm-Associated Infections and Resistant Pathogens
1.8. COVID-19 Pandemic Impact
2. Antimicrobial Resistance
2.1. Historical Perspective and Emergence
2.2. Current Mortality and Morbidity Burden
2.3. One Health Approach and Resistance Mechanisms
2.4. ESKAPE Pathogens
3. Strategies Adopted in Hospital Settings to Combat Antimicrobial Resistance
3.1. International Collaboration and Policy Frameworks
3.2. Targets and Classification Systems
3.3. Environmental Control and Cleaning Protocols
3.4. Hand Hygiene Protocols and Compliance
3.5. Textile Management and Antimicrobial Surfaces
3.6. Antimicrobial Stewardship Programs
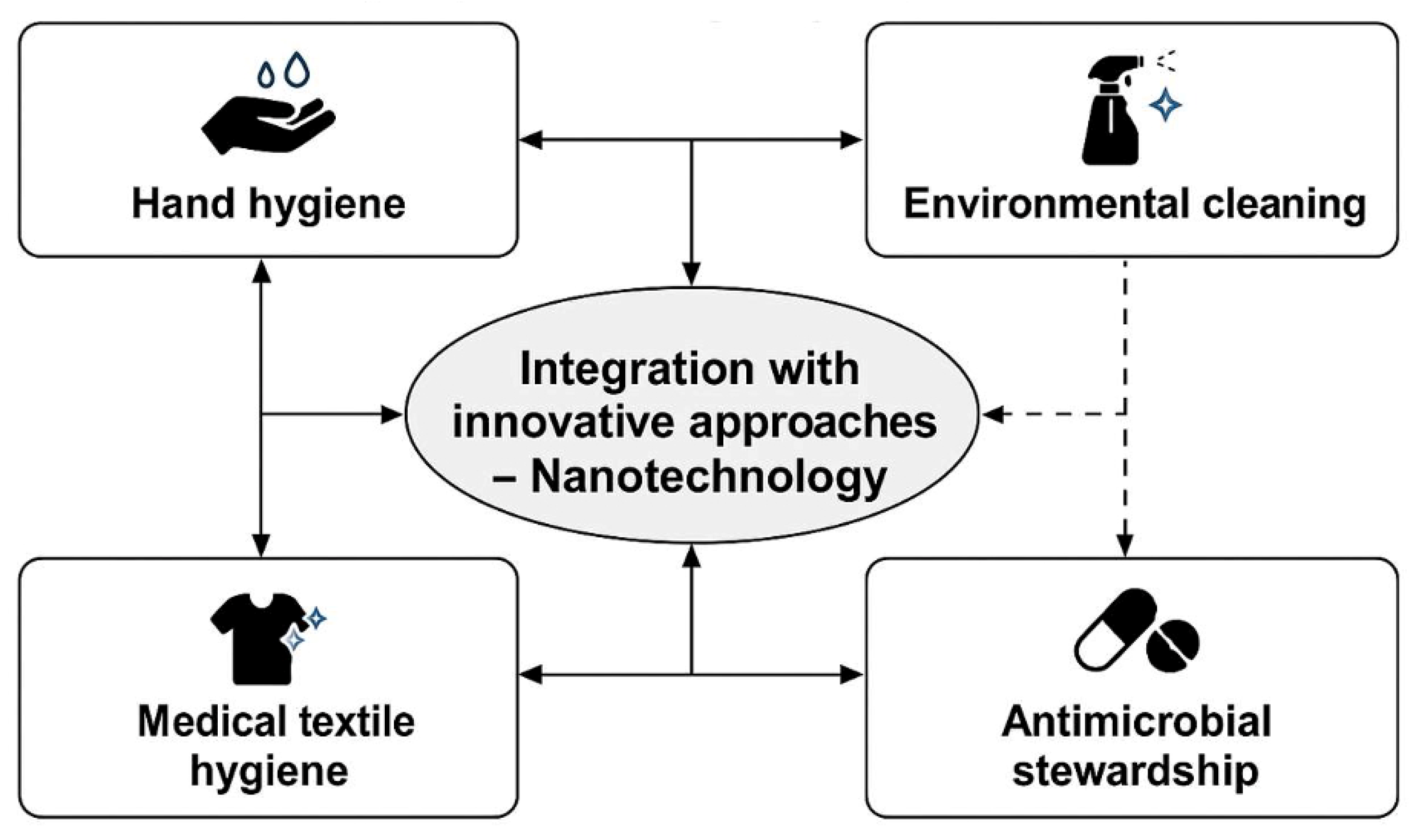
3.7. Emerging Technologies and Innovation
4. New Antimicrobial Approaches Based on Nanotechnology
4.1. Introduction to Nanotechnology in Healthcare
4.2. Nanotechnology as a Solution to Antimicrobial Resistance
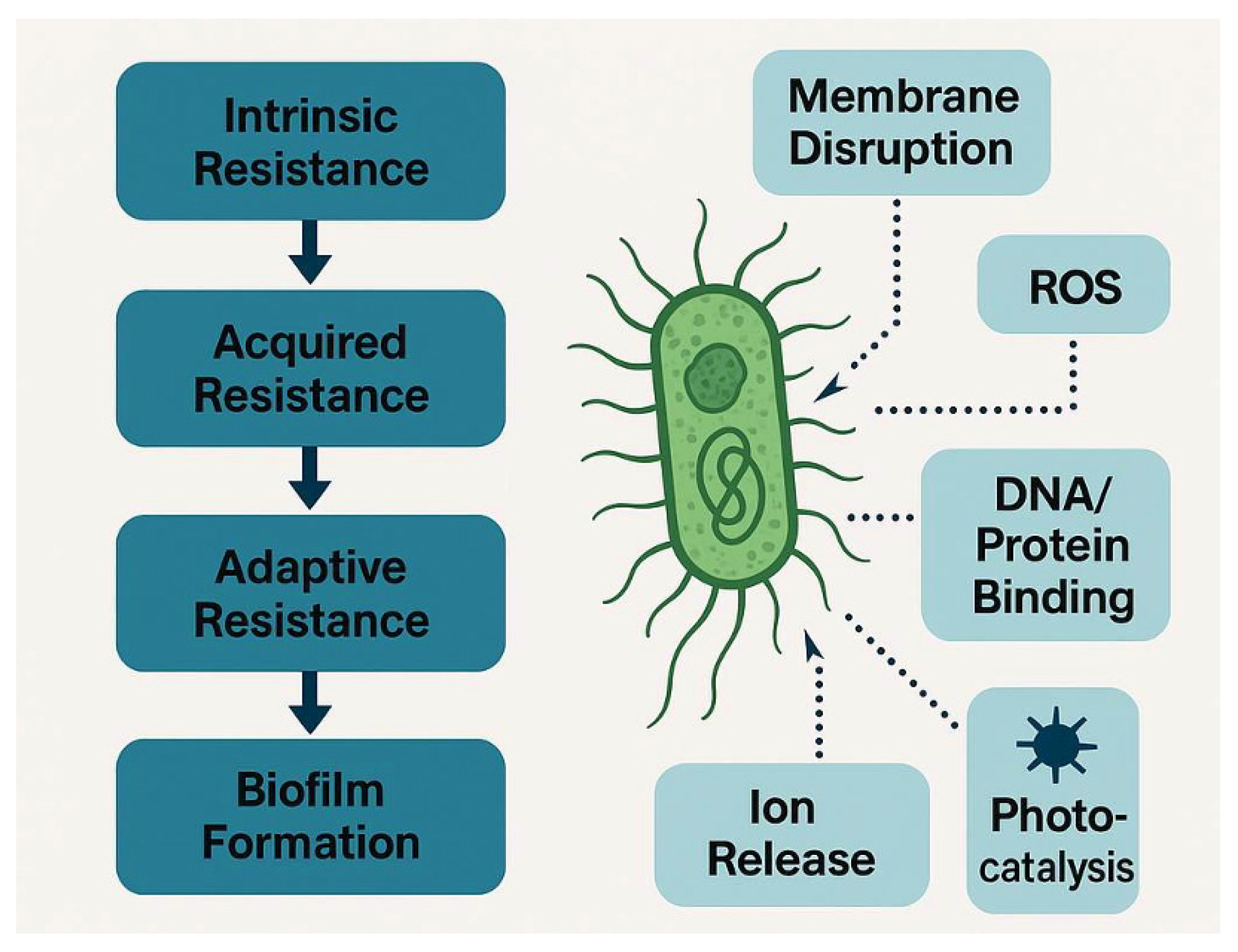
4.3. Mechanisms of Action and Biofilm Penetration
4.4. Metal-Based Nanoparticles and Their Efficacy in Resistance Prevention
4.5. Historical Context, Modern Applications and Advanced Nanomaterial Categories
4.6. Surface Modification and Medical Device Applications
4.7. Safety Considerations and Regulatory Aspects
5. Silver Nanoparticles as Innovative Solutions for HAI Control in Clinical Settings
5.1. Historical Foundation and Antimicrobial Mechanisms
5.2. Broad-Spectrum Antimicrobial Activity
5.3. Recent Advances in Clinical Applications
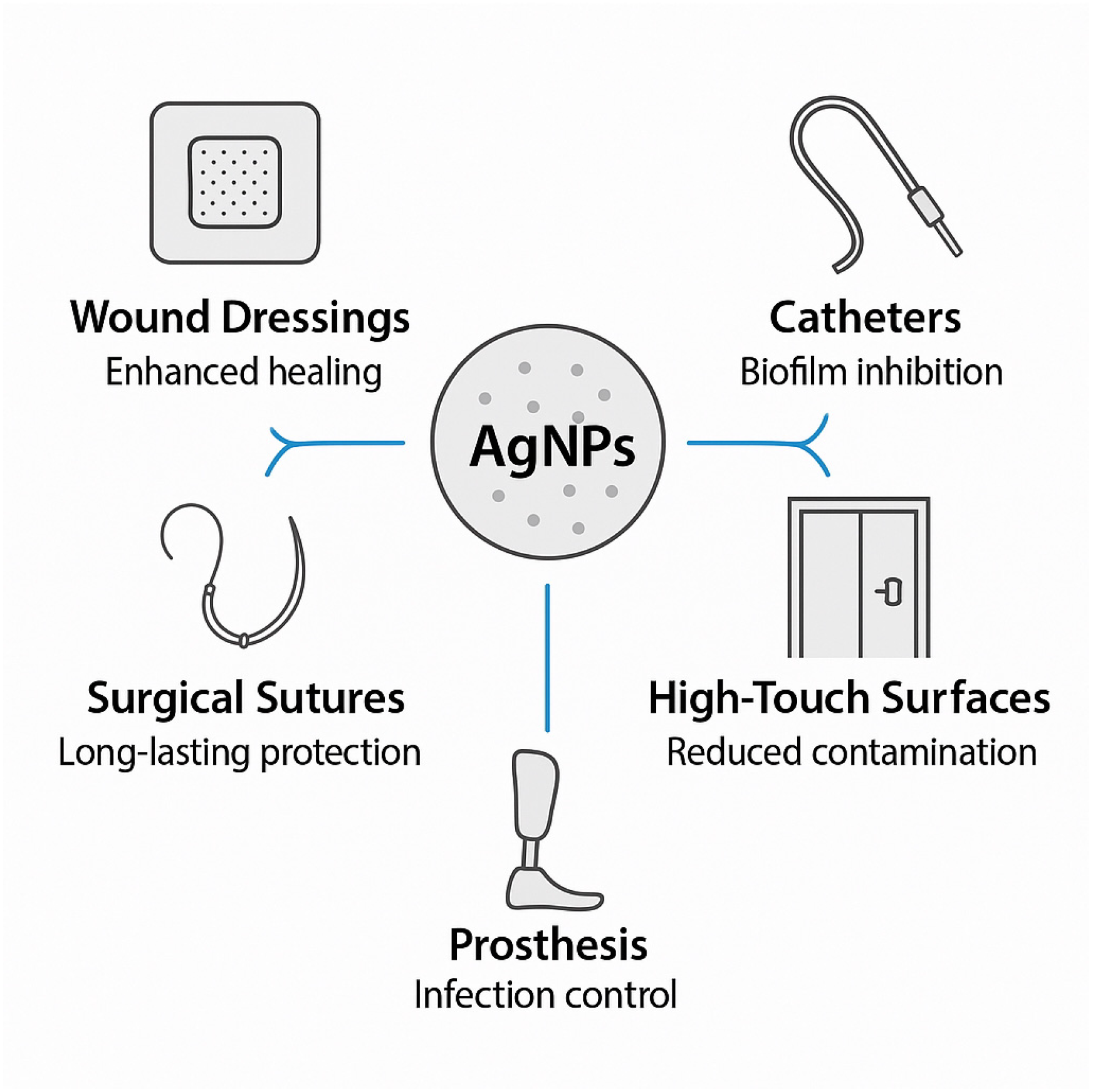
5.3.1. Wound Care Applications
5.3.2. Medical Device Applications
5.3.3. Hospital Surface Applications
5.3.4. Clinical Trials and Ongoing Research
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HAIs | Healthcare-associated infections |
| AMR | Antimicrobial resistance |
| CLABSIs | Central line-associated bloodstream infections |
| CAUTIs | Catheter-associated urinary tract infections |
| SSIs | Surgical site infections |
| VAP | Ventilator-associated pneumonia |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| HIV | Human Immunodeficiency Virus |
| GLASS | Global Antimicrobial Resistance and Use Surveillance System |
| HGT | Horizontal Gene Transfer |
| GAP | Global Action Plan |
| NAP | National Action Plans |
| AHC | Alcohol-Based Handrub Consumption |
| MDROs | Multidrug-Resistant Organisms |
| EPA | Environmental Protection Agency |
References
- Lowe, H.; Woodd, S.; Lange, I.L.; Ahmed, B.; Thwaites, C.L.; Kesteman, T.; Weerakoon, A.; Nygaard-Hansen, O.; Azza Agha, S.; Maes, P.; et al. Challenges and opportunities for infection prevention and control in hospitals in conflict-affected settings: A qualitative study. Confl. Health 2021, 15, 94. [Google Scholar] [CrossRef]
- Ananda, T.; Modi, A.; Chakraborty, I.; Managuli, V.; Mukhopadhyay, C.; Mazumder, N. Nosocomial Infections and Role of Nanotechnology. Bioengineering 2022, 9, 51. [Google Scholar] [CrossRef]
- Murphy, F.; Tchetchik, A.; Furxhi, I. Reduction of Health Care-Associated Infections (HAIs) with Antimicrobial Inorganic Nanoparticles Incorporated in Medical Textiles: An Economic Assessment. Nanomaterials 2020, 10, 999. [Google Scholar] [CrossRef]
- Bennett, N.; Maskell, V.; Beech, E.F.; Silvester, A.; Lal, S.; Wilcock, M. The impact of medicines reconciliation by pharmacy teams in secondary care: A rapid systematic review. BMJ Public Health 2024, 2, e000504. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Gizdavic-Nikolaidis, M.; Swift, S. Antimicrobial Coatings: Reviewing Options for Healthcare Applications. Appl. Microbiol. 2023, 3, 145–174. [Google Scholar] [CrossRef]
- El Guerche-Séblain, C.; Amour, S.; Bénet, T.; Hénaff, L.; Escuret, V.; Schellevis, F.; Vanhems, P. Incidence of hospital-acquired influenza in adults: A prospective surveillance study from 2004 to 2017 in a French tertiary care hospital. Am. J. Infect. Control 2021, 49, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Report on Infection Prevention and Control 2024; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/i/item/9789240103986 (accessed on 28 August 2025).
- European Centre for Disease Prevention and Control (ECDC). Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals, 2022–2023; ECDC: Stockholm, Sweden, 2024; Available online: https://www.ecdc.europa.eu/en/publications-data/PPS-HAI-AMR-acute-care-europe-2022-2023 (accessed on 28 August 2025).
- Bernatchez, S.F. Reducing antimicrobial resistance by practicing better infection prevention and control. Am. J. Infect. Control 2023, 51, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Bouhrour, N.; Nibbering, P.H.; Bendali, F. Medical Device-Associated Biofilm Infections and Multidrug-Resistant Pathogens. Pathogens 2024, 13, 393. [Google Scholar] [CrossRef]
- Murphy, D.J.; Needham, D.M.; Goeschel, C.; Fan, E.; Cosgrove, S.E.; Pronovost, P.J. Monitoring and Reducing Central Line-Associated Bloodstream Infections: A National Survey of State Hospital Associations. Am. J. Med. Qual. 2010, 25, 255–260. [Google Scholar] [CrossRef]
- Huang, H.; Huang, L.; Yan, S.; Li, H.; Qiao, L.; Tian, S.; Zhang, Y.; Liu, K.; Feng, X.; Wang, Y.; et al. A bundle-based approach on catheter-associated urinary tract infection: A multi-center study in Chinese tertiary hospitals. BMC Infect. Dis. 2025, 25, 248. [Google Scholar] [CrossRef]
- Raoofi, S.; Pashazadeh Kan, F.; Rafiei, S.; Hosseinipalangi, Z.; Noorani Mejareh, Z.; Khani, S.; Abdollahi, B.; Seyghalani Talab, F.; Sanaei, M.; Zarabi, F.; et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS ONE 2023, 18, e0274248. [Google Scholar] [CrossRef]
- Bucataru, A.; Balasoiu, M.; Ghenea, A.E.; Zlatian, O.M.; Vulcanescu, D.D.; Horhat, F.G.; Bagiu, I.C.; Sorop, V.B.; Sorop, M.I.; Oprisoni, A.; et al. Factors Contributing to Surgical Site Infections: A Comprehensive Systematic Review of Etiology and Risk Factors. Clin. Pract. 2024, 14, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Tejada, G.G.; Chico-Sánchez, P.; Gras-Valentí, P.; Jaime-Sánchez, F.A.; Galiana-Ivars, M.; Balboa-Esteve, S.; Gómez-Sotero, I.L.; Sánchez-Payá, J.; Ronda-Pérez, E. Estimation of Additional Costs in Patients with Ventilator-Associated Pneumonia. Antibiotics 2024, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Breslawec, A.P.; Liang, T.; Deng, Z.; Kuperman, L.L.; Yu, Q. Strategy to combat biofilms: A focus on biofilm dispersal enzymes. npj Biofilms Microbiomes 2023, 9, 63. [Google Scholar] [CrossRef]
- Zafer, M.M.; Mohamed, G.A.; Ibrahim, S.R.M.; Ghosh, S.; Bornman, C.; Elfaky, M.A. Biofilm-mediated infections by multidrug-resistant microbes: A comprehensive exploration and forward perspectives. Arch. Microbiol. 2024, 206, 101. [Google Scholar] [CrossRef]
- Perez, S.; Innes, G.K.; Walters, M.S.; Mehr, J.; Arias, J.; Greeley, R.; Chew, D. Carbapenem-Resistant Acinetobacter baumannii Infection and Colonization in an Acute Care Hospital During a Surge in COVID-19 Admissions-New Jersey, February–July 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1827–1831. [Google Scholar] [CrossRef]
- Tham, N.; Fazio, T.; Johnson, D.; Skandarajah, A.; Hayes, I.P. Hospital Acquired Infections in Surgical Patients: Impact of COVID-19-Related Infection Prevention Measures. World J. Surg. 2022, 46, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, C.; Russotto, A.; Bussolino, R.; Castagnotto, M.; Gastaldo, C.; Bresciano, L.; Bazzolo, S.; Gamba, D.; Corcione, S.; De Rosa, F.G.; et al. Impact of COVID-19 on healthcare-associated infections and antimicrobial use in Italy, 2022. J. Hosp. Infect. 2024, 149, 14–21. [Google Scholar] [CrossRef]
- Petrakis, V.; Panopoulou, M.; Rafailidis, P.; Lemonakis, N.; Lazaridis, G.; Terzi, I.; Papazoglou, D.; Panagopoulos, P. The Impact of the COVID-19 Pandemic on Antimicrobial Resistance and Management of Bloodstream Infections. Pathogens 2023, 12, 780. [Google Scholar] [CrossRef]
- Lessa, F.C.; Sievert, D.M. Antibiotic Resistance: A Global Problem and the Need to Do More. Clin. Infect. Dis. 2023, 77, S1–S3. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients With COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Okeke, I.N.; de Kraker, M.E.A.; Van Boeckel, T.P.; Kumar, C.K.; Schmitt, H.; Gales, A.C.; Bertagnolio, S.; Sharland, M.; Laxminarayan, R. The scope of the antimicrobial resistance challenge. Lancet 2024, 403, 2426–2438. [Google Scholar] [CrossRef]
- Kariuki, S. Global burden of antimicrobial resistance and forecasts to 2050. Lancet 2024, 404, 1172–1173. [Google Scholar] [CrossRef]
- Galgano, M.; Pellegrini, F.; Catalano, E.; Capozzi, L.; Del Sambro, L.; Sposato, A.; Lucente, M.S.; Vasinioti, V.I.; Catella, C.; Odigie, A.E.; et al. Acquired Bacterial Resistance to Antibiotics and Resistance Genes: From Past to Future. Antibiotics 2025, 14, 222. [Google Scholar] [CrossRef]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2023; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- Ntziora, F.; Giannitsioti, E. Bloodstream infections in the era of the COVID-19 pandemic: Changing epidemiology of antimicrobial resistance in the intensive care unit. J. Intensive Med. 2024, 4, 269–280. [Google Scholar] [CrossRef]
- Velazquez-Meza, M.E.; Galarde-López, M.; Carrillo-Quiróz, B.; Alpuche-Aranda, C.M. Antimicrobial resistance: One Health approach. Vet. World 2022, 15, 743–749. [Google Scholar] [CrossRef]
- Despotovic, M.; de Nies, L.; Busi, S.B.; Wilmes, P. Reservoirs of antimicrobial resistance in the context of One Health. Curr. Opin. Microbiol. 2023, 73, 102291. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Bisen, M.; Harjai, K.; Chhibber, S.; Azizov, S.; Lalhlenmawia, H.; Kumar, D. Advances in Nanotechnology for Biofilm Inhibition. ACS Omega 2023, 8, 21391–21409. [Google Scholar] [CrossRef]
- Sathe, N.; Beech, P.; Croft, L.; Suphioglu, C.; Kapat, A.; Athan, E. Pseudomonas aeruginosa: Infections and novel approaches to treatment “Knowing the enemy” the threat of Pseudomonas aeruginosa and exploring novel approaches to treatment. Infect. Med. 2023, 2, 178–194. [Google Scholar] [CrossRef]
- Carelli, D.E.; Mitsouli, E.T.; Ogne, J.B.; Pierre, J. The best laid plans?: International governance perspectives in AMR national action plans in Europe. Eur. J. Public Health 2023, 33, 682–686. [Google Scholar] [CrossRef]
- Anderson, M.; Kluge, H.H.P.; Lo Fo Wong, D.; Butler, R.; Mossialos, E. Promoting sustainable national action to tackle antimicrobial resistance: A proposal to develop an antimicrobial resistance accountability index. Lancet Microbe 2024, 5, 100997. [Google Scholar] [CrossRef] [PubMed]
- Avello, P.; Collins, L.M.; Gómez, S.A.; Luna, F.; Fernández Miyakawa, M.E.; West, H.M.; Iossa, G. National action plans on antimicrobial resistance in Latin America: An analysis via a governance framework. Health Policy Plan. 2024, 39, 188–197. [Google Scholar] [CrossRef]
- Patel, J.; Harant, A.; Fernandes, G.; Mwamelo, A.J.; Hein, W.; Dekker, D.; Sridhar, D. Measuring the global response to antimicrobial resistance, 2020–2021: A systematic governance analysis of 114 countries. Lancet Infect. Dis. 2023, 23, 706–718. [Google Scholar] [CrossRef]
- Bankar, N.J.; Ugemuge, S.; Ambad, R.S.; Hawale, D.V.; Timilsina, D.R. Implementation of Antimicrobial Stewardship in the Healthcare Setting. Cureus 2022, 14, e26664. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, A.; Reid, S.; Assefa, Y. A review of national action plans on antimicrobial resistance: Strengths and weaknesses. Antimicrob. Resist. Infect. Control 2022, 11, 90. [Google Scholar]
- Mendelson, M.; Lewnard, J.A.; Sharland, M.; Cook, A.; Pouwels, K.B.; Alimi, Y.; Mpundu, M.; Wesangula, E.; Weese, J.S.; Røttingen, J.A.; et al. Ensuring progress on sustainable access to effective antibiotics at the 2024 UN General Assembly: A target-based approach. Lancet 2024, 403, 2551–2564. [Google Scholar] [CrossRef]
- Goto, M.; Hasegawa, S.; Balkenende, E.C.; Clore, G.S.; Safdar, N.; Perencevich, E.N.; VA-CDC Practice-Based Research Network. Effectiveness of Ultraviolet-C Disinfection on Hospital-Onset Gram-Negative Rod Bloodstream Infection: A Nationwide Stepped-Wedge Time-Series Analysis. Clin. Infect. Dis. 2023, 76, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.; Stefani, S.; Migliorisi, G. Bacterial Infections in Intensive Care Units: Epidemiological and Microbiological Aspects. Antibiotics 2024, 13, 238. [Google Scholar] [CrossRef]
- van Roekel, H.; Reinhard, J.; Grimmelikhuijsen, S. Improving hand hygiene in hospitals: Comparing the effect of a nudge and a boost on protocol compliance. Behav. Public Policy 2022, 6, 52–74. [Google Scholar] [CrossRef]
- Boyce, J.M. Current issues in hand hygiene. Am. J. Infect. Control 2023, 51, A35–A43. [Google Scholar] [CrossRef]
- Russotto, A.; Rolfini, E.; Paladini, G.; Gastaldo, C.; Vicentini, C.; Zotti, C.M. Hand Hygiene and Antimicrobial Resistance in the COVID-19 Era: An Observational Study. Antibiotics 2023, 12, 583. [Google Scholar] [CrossRef]
- Chakma, S.K.; Hossen, S.; Rakib, T.M.; Hoque, S.; Islam, R.; Biswas, T.; Islam, Z.; Islam, M.M. Effectiveness of a hand hygiene training intervention in improving knowledge and compliance rate among healthcare workers in a respiratory disease hospital. Heliyon 2024, 10, e27286. [Google Scholar] [CrossRef]
- Donskey, C.J. Continuous surface and air decontamination technologies: Current concepts and controversies. Am. J. Infect. Control 2023, 51, A144–A150. [Google Scholar] [CrossRef] [PubMed]
- Khadse, S.N.; Ugemuge, S.; Singh, C. Impact of Antimicrobial Stewardship on Reducing Antimicrobial Resistance. Cureus 2023, 15, e49935. [Google Scholar] [CrossRef]
- Gavi, F.; Fiori, B.; Gandi, C.; Campetella, M.; Bientinesi, R.; Marino, F.; Fettucciari, D.; Rossi, F.; Moretto, S.; Murri, R.; et al. Prevalence and Antimicrobial Resistance Patterns of Hospital Acquired Infections through the COVID-19 Pandemic: A Systematic Review and Meta-Analysis. Antibiotics 2024, 13, 421. [Google Scholar]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Shanay Rab, S.R.; Suman, R. Applications of nanotechnology in medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Keskin, C.; Aslan, S.; Baran, M.F.; Baran, A.; Eftekhari, A.; Adıcan, M.T.; Ahmadian, E.; Arslan, S.; Mohamed, A.J. Green synthesis and characterization of silver nanoparticles using Anchusa officinalis: Antimicrobial and cytotoxic potential. Int. J. Nanomed. 2025, 20, 4481–4502. [Google Scholar] [CrossRef] [PubMed]
- Holubnycha, V.; Husak, Y.; Korniienko, V.; Bolshanina, S.; Tveresovska, O.; Myronov, P.; Holubnycha, M.; Butsyk, A.; Borén, T.; Banasiuk, R.; et al. Antimicrobial Activity of Two Different Types of Silver Nanoparticles against Wide Range of Pathogenic Bacteria. Nanomaterials 2024, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future perspectives. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Medic, B.S.; Tomic, N.; Lagopati, N.; Gazouli, M.; Pojskic, L. Advances in metal and metal oxide nanomaterials for topical antimicrobial applications: Insights and future perspectives. Molecules 2024, 29, 5551. [Google Scholar] [CrossRef]
- de Oliveira, K.B.S.; Leite, M.L.; Melo, N.T.M.; Lima, L.F.; Barbosa, T.C.Q.; Carmo, N.L.; Melo, D.A.B.; Paes, H.C.; Franco, O.L. Antimicrobial peptide delivery systems as promising tools against resistant bacterial infections. Antibiotics 2024, 13, 1042. [Google Scholar] [CrossRef]
- Chicea, D.; Nicolae-Maranciuc, A. A review of chitosan-based materials for biomedical, food, and water treatment applications. Materials 2024, 17, 5770. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Yu, D.-G.; Bligh, S.-W.A. Alginate-based electrospun nanofibers and the enabled drug controlled release profiles: A review. Biomolecules 2024, 14, 789. [Google Scholar] [CrossRef]
- Gong, J.; Hou, L.; Ching, Y.C.; Ching, K.Y.; Hai, N.D.; Chuah, C.H. A review of recent advances of cellulose-based intelligent-responsive hydrogels as vehicles for controllable drug delivery system. Int. J. Biol. Macromol. 2024, 264 Pt 2, 130525. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, H.; Luo, Y.; Chen, Y.; Wang, M.; Wu, C.; Hu, P. Recent applications of PLGA in drug delivery systems. Polymers 2024, 16, 2606. [Google Scholar] [CrossRef]
- Bento, C.; Katz, M.; Santos, M.M.M.; Afonso, C.A.M. Striving for uniformity: A review on advances and challenges to achieve uniform polyethylene glycol. Org. Process Res. Dev. 2024, 28, 860–890. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Román-García, M.F.; Ramírez-Díaz, C.A.; Ulloa-Ramírez, M.; Morones-Ramírez, J.R. Nanomaterials-Based Combinatorial Therapy as a Strategy to Combat Antibiotic Resistance. Antibiotics 2022, 11, 794. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.M.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Hetta, H.F.; Ramadan, Y.N.; Al-Harbi, A.I.; Ahmed, E.A.; Battah, B.; Abd Ellah, N.H.; Zanetti, S.; Donadu, M.G. Nanotechnology as a Promising Approach to Combat Multidrug Resistant Bacteria: A Comprehensive Review and Future Perspectives. Biomedicines 2023, 11, 413. [Google Scholar] [CrossRef]
- He, J.; Hong, M.; Xie, W.; Chen, Z.; Chen, D.; Xie, S. Progress and prospects of nanomaterials against resistant bacteria. J. Control. Release 2022, 351, 301–323. [Google Scholar] [CrossRef]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Sannino, A.; Pollini, M. In vivo testing of silver treated fibers for the evaluation of skin irritation effect and hypoallergenicity. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 031–037. [Google Scholar] [CrossRef] [PubMed]
- Chapa González, C.; González García, L.I.; Burciaga Jurado, L.G.; Carrillo Castillo, A. Bactericidal activity of silver nanoparticles in drug-resistant bacteria. Braz. J. Microbiol. 2023, 54, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.S.; Batista, J.G.S.; Rodrigues, M.Á.V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in silver nanoparticles: A comprehensive review on their potential as antimicrobial agents and their mechanisms of action elucidated by proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver nanoparticles: A comprehensive review of synthesis methods and chemical and physical properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef]
- Caron, A.J.; Ali, I.J.; Delgado, M.J.; Johnson, D.; Reeks, J.M.; Strzhemechny, Y.M.; McGillivray, S.M. Zinc oxide nanoparticles mediate bacterial toxicity in Mueller-Hinton Broth via Zn2+. Front. Microbiol. 2024, 15, 1394078. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; Sapata, V.d.M.R.; Lopes, P.R.M.; Moraes, P.B.d.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- Irede, E.L.; Awoyemi, R.F.; Owolabi, B.; Aworinde, O.R.; Kajola, R.O.; Hazeez, A.; Raji, A.A.; Ganiyu, L.O.; Onukwuli, C.O.; Onivefuj, A.P.; et al. Cutting-edge developments in zinc oxide nanoparticles: Synthesis and applications for enhanced antimicrobial and UV protection in healthcare solutions. RSC Adv. 2024, 14, 20992–21034. [Google Scholar] [CrossRef] [PubMed]
- Flores-Rábago, K.M.; Rivera-Mendoza, D.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Castro-Longoria, E. Antibacterial Activity of Biosynthesized Copper Oxide Nanoparticles (CuONPs) Using Ganoderma sessile. Antibiotics 2023, 12, 1251. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Burmistrov, D.E.; Shtarkman, I.N.; Chernikov, A.V.; Bruskov, V.I. Antibacterial Properties of Copper Oxide Nanoparticles (Review). Int. J. Mol. Sci. 2024, 25, 11563. [Google Scholar] [CrossRef]
- Khairy, T.; Amin, D.H.; Salama, H.M.; Elkholy, I.M.A.; Elnakib, M.; Gebreel, H.M.; Sayed, H.A.E. Antibacterial activity of green synthesized copper oxide nanoparticles against multidrug-resistant bacteria. Sci. Rep 2024, 14, 25020. [Google Scholar] [CrossRef]
- He, H.; Zou, Z.; Wang, B.; Xu, G.; Chen, C.; Qin, X.; Yu, C.; Zhang, J. Copper Oxide Nanoparticles Induce Oxidative DNA Damage and Cell Death via Copper Ion-Mediated P38 MAPK Activation in Vascular Endothelial Cells. Int. J. Nanomed. 2020, 15, 3291–3302. [Google Scholar] [CrossRef]
- Moschini, E.; Colombo, G.; Chirico, G.; Capitani, G.; Dalle-Donne, I.; Mantecca, P. Biological mechanism of cell oxidative stress and death during short-term exposure to nano CuO. Sci. Rep. 2023, 13, 2326. [Google Scholar] [CrossRef]
- Saed, M.; Ayivi, R.D.; Wei, J.; Obare, S.O. Gold nanoparticles antibacterial activity: Does the surface matter? Colloid Interface Sci. Commun. 2024, 62, 100804. [Google Scholar] [CrossRef]
- Mutalik, C.; Saukani, M.; Khafid, M.; Krisnawati, D.I.; Widodo; Darmayanti, R.; Puspitasari, B.; Cheng, T.M.; Kuo, T.R. Gold-based nanostructures for antibacterial application. Int. J. Mol. Sci. 2023, 24, 10006. [Google Scholar] [CrossRef] [PubMed]
- Sarma, P.P.; Rai, A.; Baruah, P.K. Recent advances in the development of antibiotics-coated gold nanoparticles to combat antimicrobial resistance. Antibiotics 2024, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.; Sarkhel, S.; Mukherjee, N.; Jaiswal, A. Nanomaterial-Based Antimicrobial Coating for Biomedical Implants: New Age Solution for Biofilm-Associated Infections. ACS Omega 2022, 7, 45962–45980. [Google Scholar] [CrossRef]
- Ye, L.; Cao, Z.; Liu, X.; Cui, Z.; Li, Z.; Liang, Y.; Zhu, S.; Wu, S. Noble metal-based nanomaterials as antibacterial agents. J. Alloys Compd. 2022, 904, 164091. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver nanoparticles: Bactericidal and mechanistic approach against drug resistant pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Zuo, C.; Liu, M.; Tai, X. A review on Cu2O-based composites in photocatalysis: Synthesis, modification, and applications. Molecules 2023, 28, 5576. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Song, Z.; Du, T.; Du, X. Antimicrobial materials based on photothermal action and their application in wound treatment. Burns Trauma 2024, 12, tkae046. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic effects between metal nanoparticles and commercial antimicrobial agents: A review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Mutalik, C.; Lin, I.H.; Krisnawati, D.I.; Khaerunnisa, S.; Khafid, M.; Widodo; Hsiao, Y.C.; Kuo, T.R. Antibacterial pathways in transition metal-based nanocomposites: A mechanistic overview. Int. J. Nanomed. 2022, 17, 6821–6842. [Google Scholar] [CrossRef]
- Jiang, H.; Li, L.; Li, Z.; Su, Y.; Liu, Y.; Wang, S.; Shang, L. Metal-based nanoparticles in antibacterial application in biomedical field: Current development and potential mechanisms. Biomed. Microdevices 2024, 26, 12. [Google Scholar] [CrossRef]
- Hung, Y.-P.; Chen, Y.-F.; Tsai, P.-J.; Huang, I.-H.; Ko, W.-C.; Jan, J.-S. Advances in the Application of Nanomaterials as Treatments for Bacterial Infectious Diseases. Pharmaceutics 2021, 13, 1913. [Google Scholar] [CrossRef]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, H.; Teng, Z.; Ma, J.; Liu, G. Nanomaterial-enabled anti-biofilm strategies: New opportunities for treatment of bacterial infections. Nanoscale 2025, 17, 5605–5628. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan-based scaffolds incorporated with silver nanoparticles for the treatment of infected wounds. Pharmaceutics 2024, 16, 327. [Google Scholar] [CrossRef] [PubMed]
- Shehabeldine, A.M.; Salem, S.S.; Ali, O.M.; Abd-Elsalam, K.A.; Elkady, F.M.; Hashem, A.H. Multifunctional silver nanoparticles based on chitosan: Antibacterial, antibiofilm, antifungal, antioxidant, and wound-healing activities. J. Fungi 2022, 8, 612. [Google Scholar] [CrossRef]
- Haugen, H.J.; Makhtari, S.; Ahmadi, S.; Hussain, B. The antibacterial and cytotoxic effects of silver nanoparticles coated titanium implants: A narrative review. Materials 2022, 15, 5025. [Google Scholar] [CrossRef]
- Ansari, M.; Shahlaei, M.; Hosseinzadeh, S.; Moradi, S. Recent advances in nanostructured delivery systems for vancomycin. Nanomedicine 2024, 19, 1931–1951. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric nanoparticles for drug delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Sanchez, J.A.; Materon, L.; Parsons, J.G.; Alcoutlabi, M. Synthesis, characterization, and antibacterial activity of graphene oxide/zinc hydroxide nanocomposites. Appl. Sci. 2024, 14, 6274. [Google Scholar] [CrossRef]
- Ye, S.; Zhang, H.; Lai, H.; Xu, J.; Yu, L.; Ye, Z.; Yang, L. MXene: A wonderful nanomaterial in antibacterial. Front. Bioeng. Biotechnol. 2024, 12, 1338539. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Su, L.; Poelstra, K.; Grainger, D.W.; van der Mei, H.C.; Shi, L.; Busscher, H.J. Beyond surface modification strategies to control infections associated with implanted biomaterials and devices-Addressing the opportunities offered by nanotechnology. Biomaterials 2024, 308, 122576. [Google Scholar] [CrossRef]
- Mishra, A.; Aggarwal, A.; Khan, F. Medical Device-Associated Infections Caused by Biofilm-Forming Microbial Pathogens and Controlling Strategies. Antibiotics 2024, 13, 623. [Google Scholar] [CrossRef]
- Wu, J.; Gupta, G.; Buerki-Thurnherr, T.; Nowack, B.; Wick, P. Bridging the gap: Innovative human-based in vitro approaches for nanomaterials hazard assessment and their role in safe and sustainable by design, risk assessment, and life cycle assessment. NanoImpact 2024, 36, 100533. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Romero, J.; Mentxaka, G.; García-Salvador, A.; Katsumiti, A.; Carregal-Romero, S.; Goñi-de-Cerio, F. Assessing the toxicity of metal- and carbon-based nanomaterials in vitro: Impact on respiratory, intestinal, skin, and immune cell lines. Int. J. Mol. Sci. 2024, 25, 10910. [Google Scholar] [CrossRef] [PubMed]
- Kedves, A.; Kónya, Z. Effects of nanoparticles on anaerobic, anammox, aerobic, and algal-bacterial granular sludge: A comprehensive review. Biofilm 2024, 8, 100234. [Google Scholar] [CrossRef]
- Bento de Carvalho, T.; Barbosa, J.B.; Teixeira, P. Assessing antimicrobial efficacy on plastics and other non-porous surfaces: A closer look at studies using the ISO 22196:2011 standard. Biology 2024, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation (EU) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and Repealing Council Directives 90/385/EEC and 93/42/EEC (Text with EEA Relevance). OJ L 117, 5 May 2017, pp. 1–175. Available online: https://eur-lex.europa.eu/eli/reg/2017/745 (accessed on 24 August 2025).
- Ellis Tobin, S.; Brenner, S. Nanotechnology Fundamentals Applied to Clinical Infectious Diseases and Public Health. Open Forum Infect. Dis. 2021, 8, ofab583. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Silver Nanoparticle-Based Antimicrobial Coatings: Sustainable Strategies for Microbial Contamination Control. Microbiol. Res. 2025, 16, 110. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Dove, A.S.; Dzurny, D.I.; Dees, W.R.; Qin, N.; Nunez Rodriguez, C.C.; Alt, L.A.; Ellward, G.L.; Best, J.A.; Rudawski, N.G.; Fujii, K.; et al. Silver nanoparticles enhance the efficacy of aminoglycosides against antibiotic-resistant bacteria. Front. Microbiol. 2023, 13, 1064095. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, E.D.; de Figueiredo, L.F.; Otoch, J.P.; Seckler, M.M.; de Oliveira, R.A.; Franco, F.F.; Marangoni, V.S.; Zucolotto, V.; Levin, A.S.; Costa, S.F. Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. J. Nanobiotechnol. 2015, 13, 64. [Google Scholar] [CrossRef]
- Kakian, F.; Mirzaei, E.; Moattari, A.; Takallu, S.; Bazargani, A. Determining the cytotoxicity of the Minimum Inhibitory Concentration (MIC) of silver and zinc oxide nanoparticles in ESBL and carbapenemase producing Proteus mirabilis isolated from clinical samples in Shiraz, Southwest Iran. BMC Res. Notes 2024, 17, 40. [Google Scholar] [CrossRef]
- Wypij, M.; Czarnecka, J.; Świecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J. Microbiol. Biotechnol. 2018, 34, 23. [Google Scholar] [CrossRef]
- Yakoup, A.Y.; Kamel, A.G.; Elbermawy, Y.; Abdelsattar, A.S.; El-Shibiny, A. Characterization, antibacterial, and cytotoxic activities of silver nanoparticles using the whole biofilm layer as a macromolecule in biosynthesis. Sci. Rep. 2024, 14, 364. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Alsaleh, N.B.; Aljasham, A.T.; Tawfik, E.A.; Almutairi, M.M.; Assiri, M.A.; Alkholief, M.; Almutairi, M.M. Silver Nanoparticle-Based Combinations with Antimicrobial Agents against Antimicrobial-Resistant Clinical Isolates. Antibiotics 2022, 11, 1219. [Google Scholar] [CrossRef]
- Shrestha, S.; Wang, B.; Dutta, P.K. Commercial Silver-Based Dressings: In Vitro and Clinical Studies in Treatment of Chronic and Burn Wounds. Antibiotics 2024, 13, 910. [Google Scholar] [CrossRef]
- Vijayakumar, V.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent advancements in biopolymer and metal nanoparticle-based materials in diabetic wound healing management. Int. J. Biol. Macromol. 2019, 122, 137–148. [Google Scholar] [CrossRef]
- Paladini, F.; Russo, F.; Masi, A.; Lanzillotti, C.; Sannino, A.; Pollini, M. Silver-Treated Silk Fibroin Scaffolds for Prevention of Critical Wound Infections. Biomimetics 2024, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Rybka, M.; Mazurek, Ł.; Konop, M. Beneficial Effect of Wound Dressings Containing Silver and Silver Nanoparticles in Wound Healing-From Experimental Studies to Clinical Practice. Life 2022, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Michaels, J.A.; Campbell, B.; King, B.; Palfreyman, S.J.; Shackley, P.; Stevenson, M. Randomized controlled trial and cost-effectiveness analysis of silver-donating antimicrobial dressings for venous leg ulcers (VULCAN trial). Br. J. Surg. 2009, 96, 1147–1156. [Google Scholar] [CrossRef]
- Cooper, I.R.; Pollini, M.; Paladini, F. The potential of photo-deposited silver coatings on Foley catheters to prevent urinary tract infections. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Goda, R.M.; El-Baz, A.M.; Khalaf, E.M.; Alharbi, N.K.; Elkhooly, T.A.; Shohayeb, M.M. Combating Bacterial Biofilm Formation in Urinary Catheter by Green Silver Nanoparticle. Antibiotics 2022, 11, 495. [Google Scholar] [CrossRef]
- Gallo, A.L.; Pollini, M.; Paladini, F. A combined approach for the development of novel sutures with antibacterial and regenerative properties: The role of silver and silk sericin functionalization. J. Mater. Sci. Mater. Med. 2018, 29, 133. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, G.D.; Umapathy, V.R.; SumathiJones, C.; Cheema, M.S.; Jayamani, D.R.; Dharani, R.; Sneha, S.; Yamuna, M.; Gayathiri, E.; Yadav, S. Fabrication and characterization of surgical sutures with propolis silver nano particles and analysis of its antimicrobial properties. J. King Saud Univ.-Sci. 2022, 34, 102082. [Google Scholar] [CrossRef]
- Panico, A.; Paladini, F.; Sannino, A.; Pollini, M. Antibacterial silver treatments on polymeric membranes for fouling control and disinfection in water filtration. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Picca, R.A.; Paladini, F.; Mangone, A.; Giannossa, L.C.; Franco, C.D.; Gallo, A.L.; Valentini, A.; Sannino, A.; Pollini, M.; et al. Spectroscopic Characterization and Nanosafety of Ag-Modified Antibacterial Leather and Leatherette. Nanomaterials 2017, 7, 203. [Google Scholar] [CrossRef]
- Gallo, N.; Iaconisi, G.N.; Pollini, M.; Paladini, F.; Pal, S.; Nobile, C.; Capobianco, L.; Licciulli, A.; Buonocore, G.G.; Mansi, A.; et al. Efficacy Evaluation of Cu- and Ag-Based Antibacterial Treatments on Polypropylene Fabric and Comparison with Commercial Products. Coatings 2023, 13, 919. [Google Scholar] [CrossRef]
- Pollini, M.; Paladini, F.; Sannino, A.; Picca, R.A.; Sportelli, M.C.; Cioffi, N.; Nitti, M.A.; Valentini, M.; Valentini, A. Nonconventional Routes to Silver Nanoantimicrobials: Technological Issues, Bioactivity and Applications. In Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Rai, M., Kon, K., Eds.; Elsevier: London, UK; San Diego, CA, USA; Waltham, MA, USA; Oxford, UK, 2015; pp. 87–105. [Google Scholar]
- Ellingson, K.D.; Pogreba-Brown, K.; Gerba, C.P.; Elliott, S.P. Impact of a Novel Antimicrobial Surface Coating on Health Care-Associated Infections and Environmental Bioburden at 2 Urban Hospitals. Clin. Infect. Dis. 2020, 71, 1807–1813. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Reina, G.; Lehner, S.; Jovic, M.; Kovacova, M.; Spitalsky, Z.; Schulte, S.; Fischer, S.; Kock, P.; Neubauer, P.; et al. The release and cytotoxicity of novel light inducible antimicrobial coatings after incineration. Ecotoxicol. Environ. Saf. 2025, 300, 118421. [Google Scholar] [CrossRef] [PubMed]
- Bellanato, I.; Benito Clemente, A.; Royo, P. A model for the interaction of a pathogen and an innovative antimicrobial nanocoating. Emergent Mater. 2025, 8, 1251–1266. [Google Scholar] [CrossRef]
- Bezzon, A.; Aurisicchio, L.; Castlunger, E.; Martellini, T.C.; Czerwiński, D.; Favuzzi, I.; Jeremiasz, O.; Meduri, A.; Mosinger, J.; Kurylak, W.; et al. Advancing surface safety: The role of sol-gel nanocoatings in the context of MIRIA European project. J. Sol-Gel Sci. Technol. 2024, 112, 639–647. [Google Scholar] [CrossRef]
- Duarte Bernardino, C.; Lee, M.; Ren, Q.; Ruehle, B. Facile spray-coating of antimicrobial silica nanoparticles for high-touch surface protection. ACS Appl. Mater. Interfaces 2025, 17, 12507–12519. [Google Scholar] [CrossRef]
- Lee, M.; Wiesli, L.; Schreiber, F.; Ivask, A.; Ren, Q. Quantitative assessment of microbial transmission onto environmental surfaces using thermoresponsive gelatin hydrogels as a finger mimetic under in situ-mimicking conditions. Adv. Healthc. Mater. 2025, 14, e2403790. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Low, S.Y.; Boon, Y.; Goh, C.; Ng, A.; Ng, A.J.Y.; Teo, J.; Johari, N.H.; Pua, Y.H.; Chua, M.T.; et al. Antimicrobial surface coating in the emergency department as protective technology for infection control (ASEPTIC): A pilot randomized controlled trial. Antimicrob. Resist. Infect. Control 2024, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Hirata, H.; Eto, S.; Hashimoto, A.; Kii, S.; Kobayashi, T.; Tsukamoto, M.; Yoshihara, T.; Toda, Y.; Mawatari, M. Development of silver-containing hydroxyapatite-coated antimicrobial implants for orthopaedic and spinal surgery. Medicina 2022, 58, 519. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Sambri, A.; Morante, L.; Bortoli, M.; Parisi, S.C.; Panzavolta, F.; Alesi, D.; Neri, E.; Neri, M.P.; Tedeschi, S.; et al. Silver-coated distal femur megaprosthesis in chronic infections with severe bone loss: A multicentre case series. J. Clin. Med. 2023, 12, 6679. [Google Scholar] [CrossRef]
- Schoder, S.; Lafuente, M.; Alt, V. Silver-coated versus uncoated locking plates in subjects with fractures of the distal tibia: A randomized, subject- and observer-blinded, multi-center non-inferiority study. Trials 2022, 23, 968. [Google Scholar] [CrossRef]
- Kosugi, C.; Koda, K.; Shimizu, H.; Yamazaki, M.; Shuto, K.; Mori, M.; Usui, A.; Nojima, H.; Endo, S.; Yanagibashi, H.; et al. A randomized trial of ionic silver dressing to reduce surgical site infection after gastrointestinal surgery. Ann. Surg. Open 2024, 5, e402. [Google Scholar] [CrossRef]
- Yi, Q.; Huang, Z.; Tang, B. Impact of silver dressings on wound healing rate in patients with lower extremity ulcers: A systematic review and meta-analysis of randomized controlled trials. Med. Princ. Pract. 2025, 34, 13–24. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Q.; Wang, H.; Välimäki, M.; Zhou, Q.; Dai, W.; Guo, J. Effectiveness of silver and iodine dressings on wound healing: A systematic review and meta-analysis. BMJ Open 2024, 14, e077902. [Google Scholar] [CrossRef]
- Kai-Larsen, Y.; Grass, S.; Mody, B.; Upadhyay, S.; Trivedi, H.L.; Pal, D.K.; Babu, S.; Bawari, B.; Singh, S.K. Foley catheter with noble metal alloy coating for preventing catheter-associated urinary tract infections: A large, multi-center clinical trial. Antimicrob. Resist. Infect. Control 2021, 10, 40. [Google Scholar] [CrossRef]
- Saint, S.; Elmore, J.G.; Sullivan, S.D.; Emerson, S.S.; Koepsell, T.D. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infection: A meta-analysis. Am. J. Med. 1998, 105, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Geng, S.; Zhang, L.; Fan, X.; Tong, F.; Meng, X.; Wang, T.; Fang, X.; Mei, Q.; Pan, A. Prevention of urinary tract infection using a silver alloy hydrogel-coated catheter in critically ill patients: A single-center prospective randomized controlled study. J. Intensive Med. 2023, 4, 118–124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monroy Caltzonci, D.A.; Rasu Chettiar, A.D.; Ibarra, V.C.; Marasamy, L.; Loredo-Tovías, M.; Acosta-Torres, L.S.; Manisekaran, R. Antimicrobial and cytotoxic effect of positively charged nanosilver-coated silk sutures. ACS Omega 2024, 9, 17636–17645. [Google Scholar] [CrossRef]
- Paladini, F.; Panico, A.; Masi, A.; Russo, F.; Sannino, A.; Pollini, M. Silver-Treated Sutures for the Prevention of Biofilm-Associated Surgical Site Infections. Antibiotics 2025, 14, 49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Butler, J.; Morgan, S.; Jones, L.; Upton, M.; Besinis, A. Evaluating the antibacterial efficacy of a silver nanocomposite surface coating against nosocomial pathogens as an antibiofilm strategy to prevent hospital infections. Nanotoxicology 2024, 18, 410–436. [Google Scholar] [CrossRef]
- Gupta, N.; Haughton, S.; Kemper, S.; Koehler, M.; Antoon, R.; Edwards, C.G.; Bardin, A. The antimicrobial effectiveness of chlorhexidine- and chlorhexidine-silver sulfadiazine-impregnated central venous catheters against the emerging fungal pathogen Candida auris. Am. J. Infect. Control 2024, 52, 1283–1288. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K. Development of a silver-based dual-function antimicrobial laundry additive and textile coating for the decontamination of healthcare laundry. J. Appl. Microbiol. 2021, 130, 1012–1022. [Google Scholar] [CrossRef]
- Mirmohammadsadeghi, S.; Juhas, D.; Parker, M.; Peranidze, K.; Horn, D.A.V.; Sharma, A.; Patel, D.; Sysoeva, T.A.; Klepov, V.; Reukov, V. The highly durable antibacterial gel-like coatings for textiles. Gels 2024, 10, 398. [Google Scholar] [CrossRef]
- Wang, F.; Harker, A.; Edirisinghe, M.; Parhizkar, M. Micro- and nanomanufacturing for biomedical applications and nanomedicine: A perspective. Small Sci. 2023, 3, 2300039. [Google Scholar] [CrossRef]
- Tchouaket, E.N.; El-Mousawi, F.; Robins, S.; Kruglova, K.; Séguin, C.; Kilpatrick, K.; Jubinville, M.; Leroux, S.; Beogo, I.; Sia, D. A systematic review of economic evaluation of healthcare associated infection prevention and control interventions in long-term care facilities. Health Econ. Rev. 2024, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.E.; Weyandt, R.; Dewald, W.; Tolksdorf, T.; Müller, L.; Braun, A. A realistic approach for evaluating antimicrobial surfaces for dry surface exposure scenarios. Appl. Environ. Microbiol. 2024, 90, e01150-24. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Ho, M.H.; Yuan, S.H.; Chou, H.W.; Chang, C.C.; Chen, C.H.; Lin, Y.E. The evaluation of antibacterial coatings against commonly found pathogenic bacteria in the environment—Implications for environmental safety and infection prevention. Pathogens 2024, 14, 13. [Google Scholar] [CrossRef]
- Yong, L.X.; Calautit, J.K. A comprehensive review on the integration of antimicrobial technologies onto various surfaces of the built environment. Sustainability 2023, 15, 3394. [Google Scholar] [CrossRef]

| Nanomaterial | Size (nm) | Tested Pathogens | Antimicrobial Efficacy % | Mechanisms of Action | References |
|---|---|---|---|---|---|
| Silver Nanoparticles (AgNPs) | 2–100 | S. aureus, E. coli, P. aeruginosa, A. flavus, C. albicans | 95; >99.9% bacterial reduction and biofilm disruption | Cell membrane disruption, Ag+ release, ROS generation, DNA binding, protein synthesis inhibition, cell division arrest | [34,65,68,69,70,71,72] |
| Zinc Oxide (ZnO) | 21–100 | S. aureus, P. aeruginosa, E. coli, S. epidermidis, B. subtilis | 80–92% bacterial reduction and growth inhibition | Photocatalysis, ROS generation (•OH, O2−, H2O2), Zn2+ release, cell wall disruption, intracellular pH alteration | [73,74,75,76] |
| Copper-based Nanoparticles (CuNPs/CuO) | 4.5–40 | S. aureus, E. coli, P. aeruginosa, B. subtilis | 85–95% bacterial reduction and killing efficacy | Cu2+ ion release, ROS generation, membrane disruption, intracellular oxidative stress, DNA damage, respiratory enzyme inhibition, cytoplasmic protein denaturation | [77,78,79,80,81] |
| Gold Nanoparticles (AuNPs) | 5–15 | S. aureus, E. coli, MRSA | 65–85% bacterial reduction (synergistic with antibiotics) | ATP synthesis inhibition, protein binding, tRNA binding interference, increased membrane permeability to antibiotics, enhanced antibiotic uptake, membrane destabilization | [65,67,82,83,84] |
| Application Area | Specific Use | Key Findings | Clinical Impact | References |
|---|---|---|---|---|
| Wound Dressings | Diabetic foot ulcers; surgical wounds | Improved healing rates and reduced bioburden (clinical studies/meta-analyses) | Shorter time-to-healing in selected indications | [144,145,146] |
| Urinary Catheters | CAUTI prevention | Risk reduction of bacteriuria/CAUTI with silver-alloy or noble-metal-alloy catheters (setting-dependent) | Significant CAUTI reduction vs. standard catheters; effect varies by duration/material | [147,148,149] |
| Surgical Sutures | Post-operative infection prevention | Sustained antimicrobial activity (preclinical evidence) | Clinical benefit shown for silver dressings on surgical incisions | [150,151] |
| Hospital Surfaces | High-touch surface coating | 75–79% reduction in environmental contamination (study-dependent) | Decreased HAI transmission in multi-unit studies | [134,140,152] |
| Central Venous Catheters | CLABSI prevention | Reduced catheter colonization/CRBSI with antimicrobial-impregnated CVCs | Improved outcomes in meta-analyses | [153] |
| Textile Applications | Hospital linens; uniforms | Persistent antimicrobial activity retained after washing (technology-dependent) | Potential IPC benefit via reduced textile contamination | [154,155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paladini, F.; D’Urso, F.; Broccolo, F.; Pollini, M. Combating Healthcare-Associated Infections in Modern Hospitals: Nanotechnology-Based Approaches in the Era of Antimicrobial Resistance. Nanomaterials 2025, 15, 1405. https://doi.org/10.3390/nano15181405
Paladini F, D’Urso F, Broccolo F, Pollini M. Combating Healthcare-Associated Infections in Modern Hospitals: Nanotechnology-Based Approaches in the Era of Antimicrobial Resistance. Nanomaterials. 2025; 15(18):1405. https://doi.org/10.3390/nano15181405
Chicago/Turabian StylePaladini, Federica, Fabiana D’Urso, Francesco Broccolo, and Mauro Pollini. 2025. "Combating Healthcare-Associated Infections in Modern Hospitals: Nanotechnology-Based Approaches in the Era of Antimicrobial Resistance" Nanomaterials 15, no. 18: 1405. https://doi.org/10.3390/nano15181405
APA StylePaladini, F., D’Urso, F., Broccolo, F., & Pollini, M. (2025). Combating Healthcare-Associated Infections in Modern Hospitals: Nanotechnology-Based Approaches in the Era of Antimicrobial Resistance. Nanomaterials, 15(18), 1405. https://doi.org/10.3390/nano15181405









