From Spirulina platensis to Nanomaterials: A Comparative Study of AgNPs Obtained from Two Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extract Preparation
2.3. Analytical Techniques for Characterization of the AgNPs
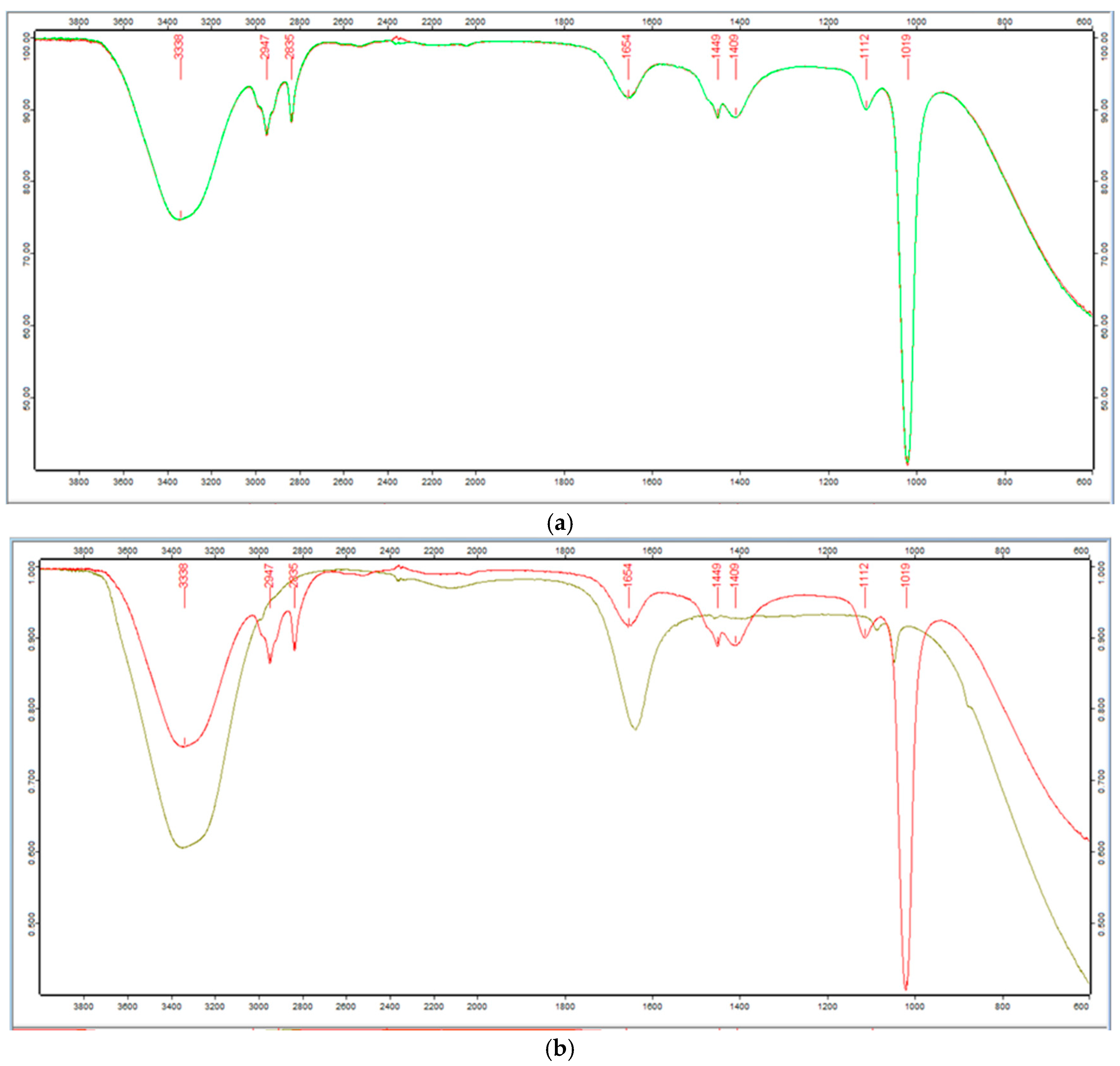
2.3.1. FTIR Spectra
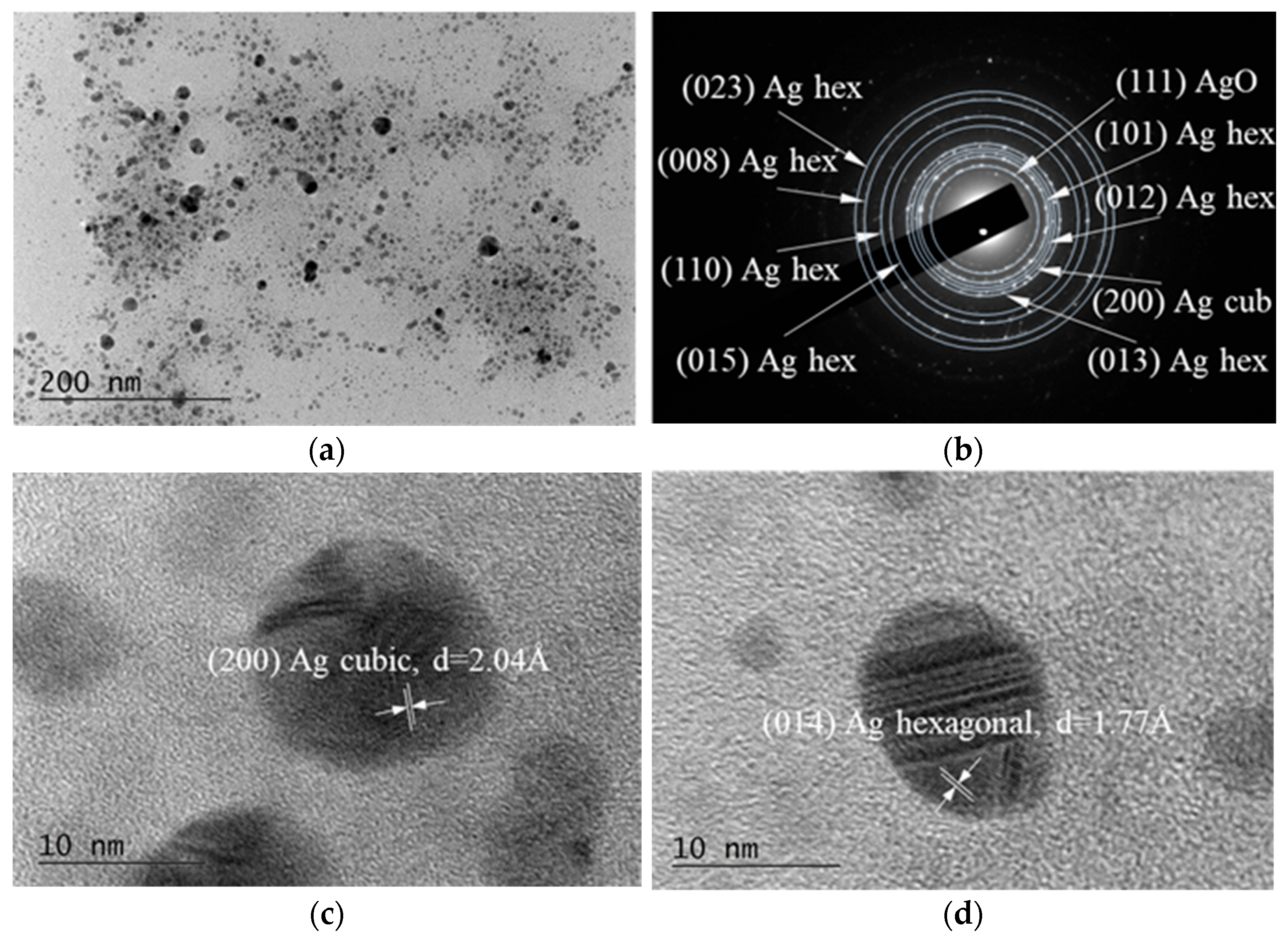
2.3.2. TEM (Transmission Electron Microscopy)
2.3.3. Zeta Potential
2.4. Chemical Composition
2.4.1. Fatty Acid Composition
2.4.2. Tocopherol Composition
2.5. Microbiological Tests
Test-Microorganisms
2.6. Albumin DenaturationInhibition Method
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization
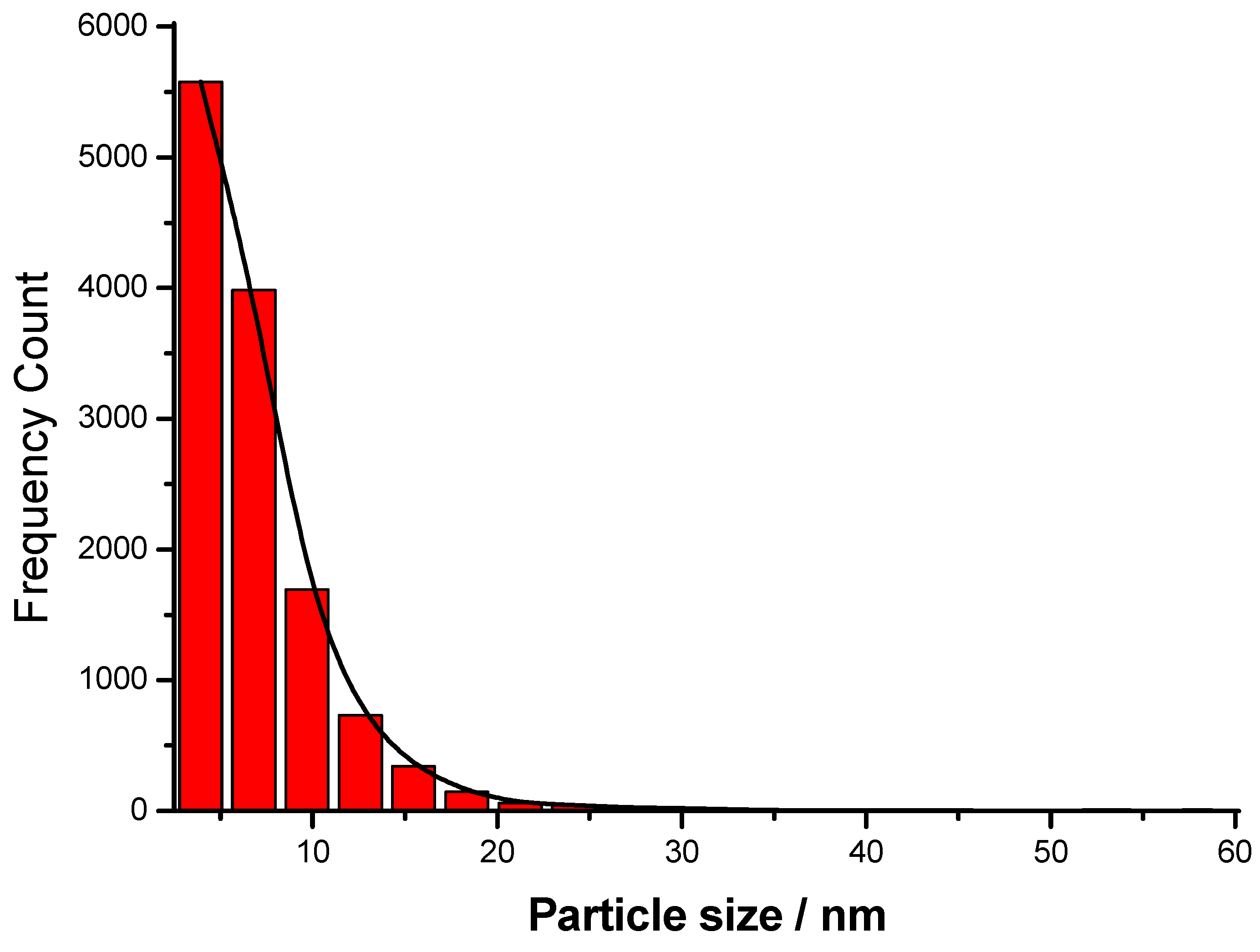
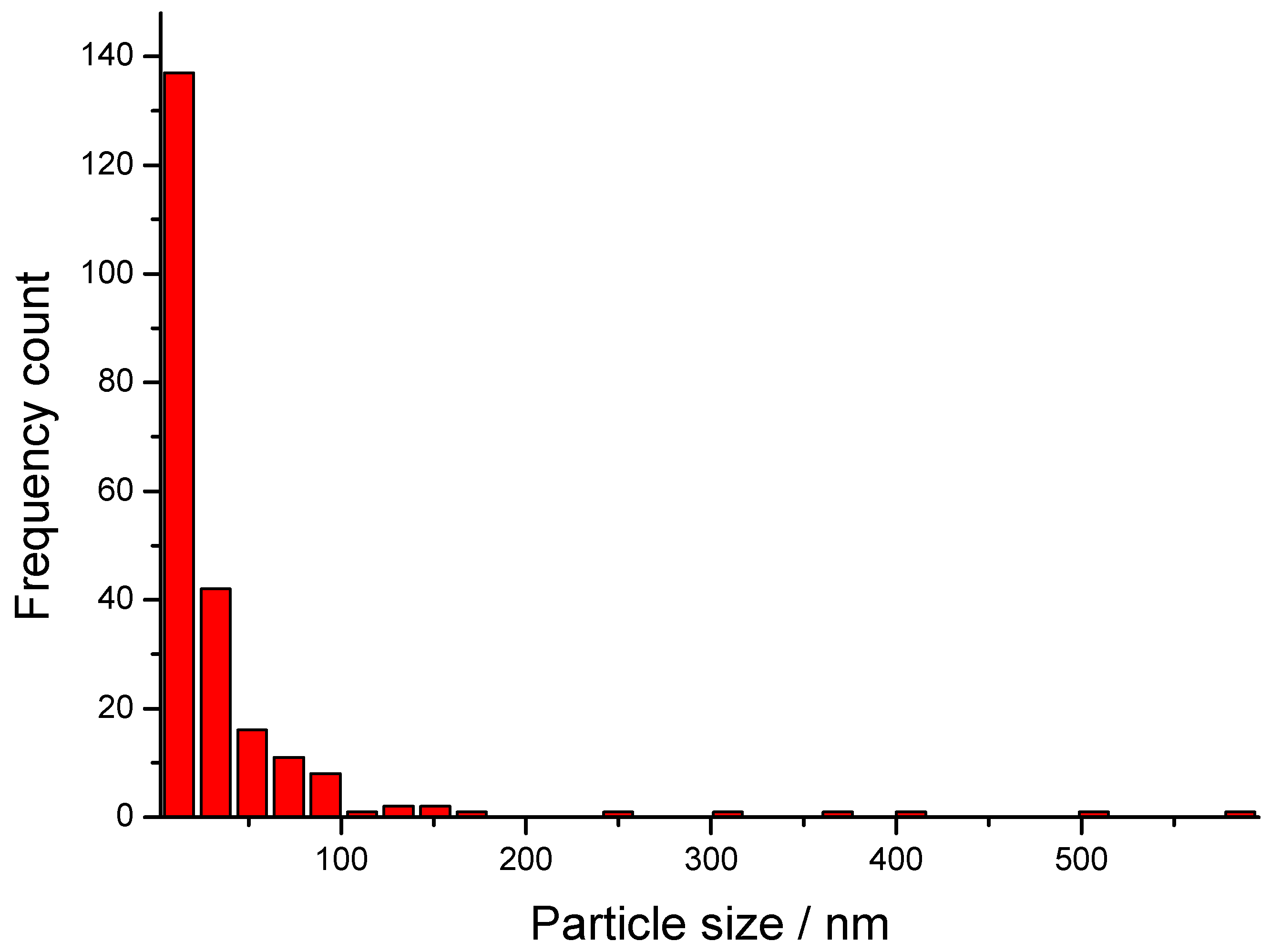
3.2. TEM Micrographs
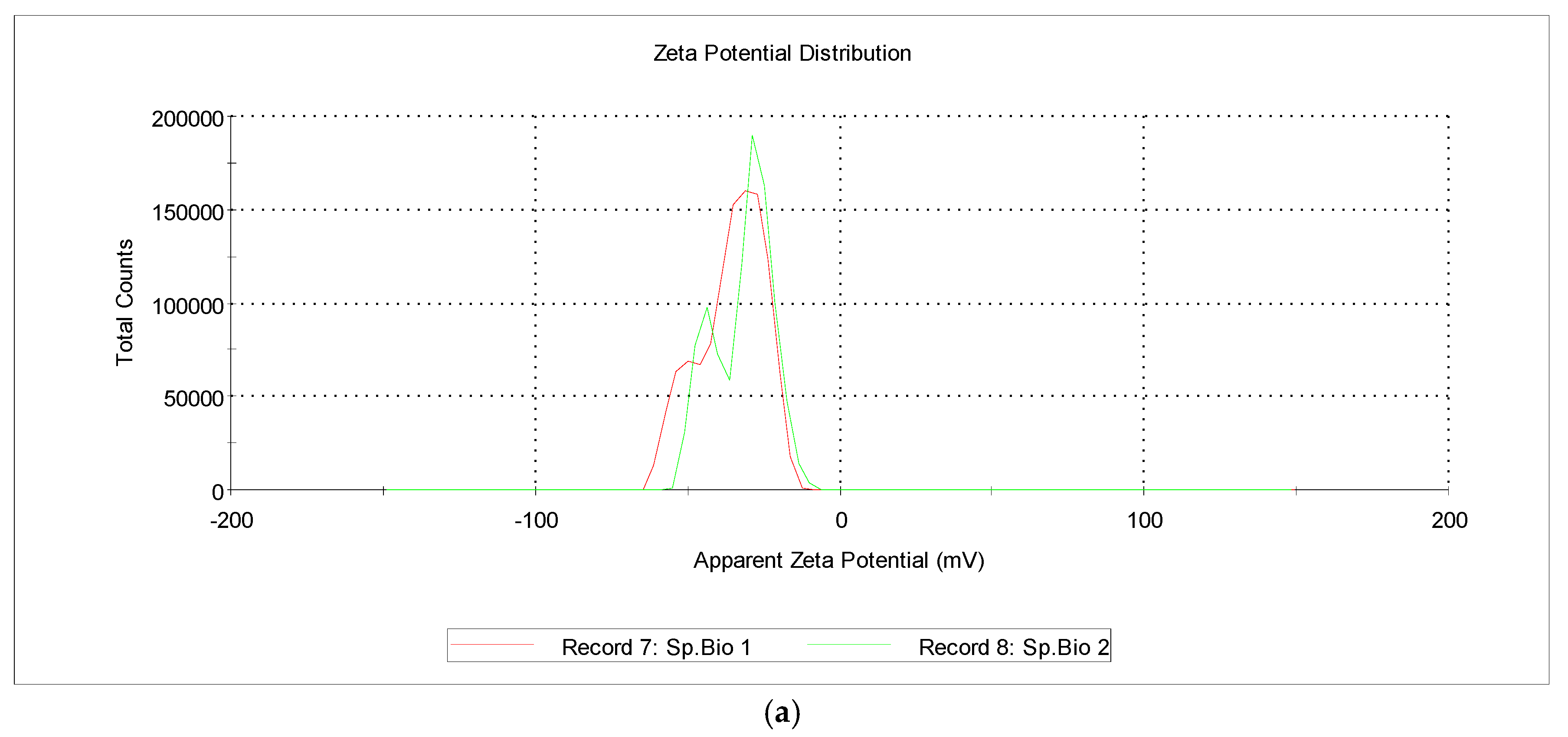
3.3. Zeta-Potential
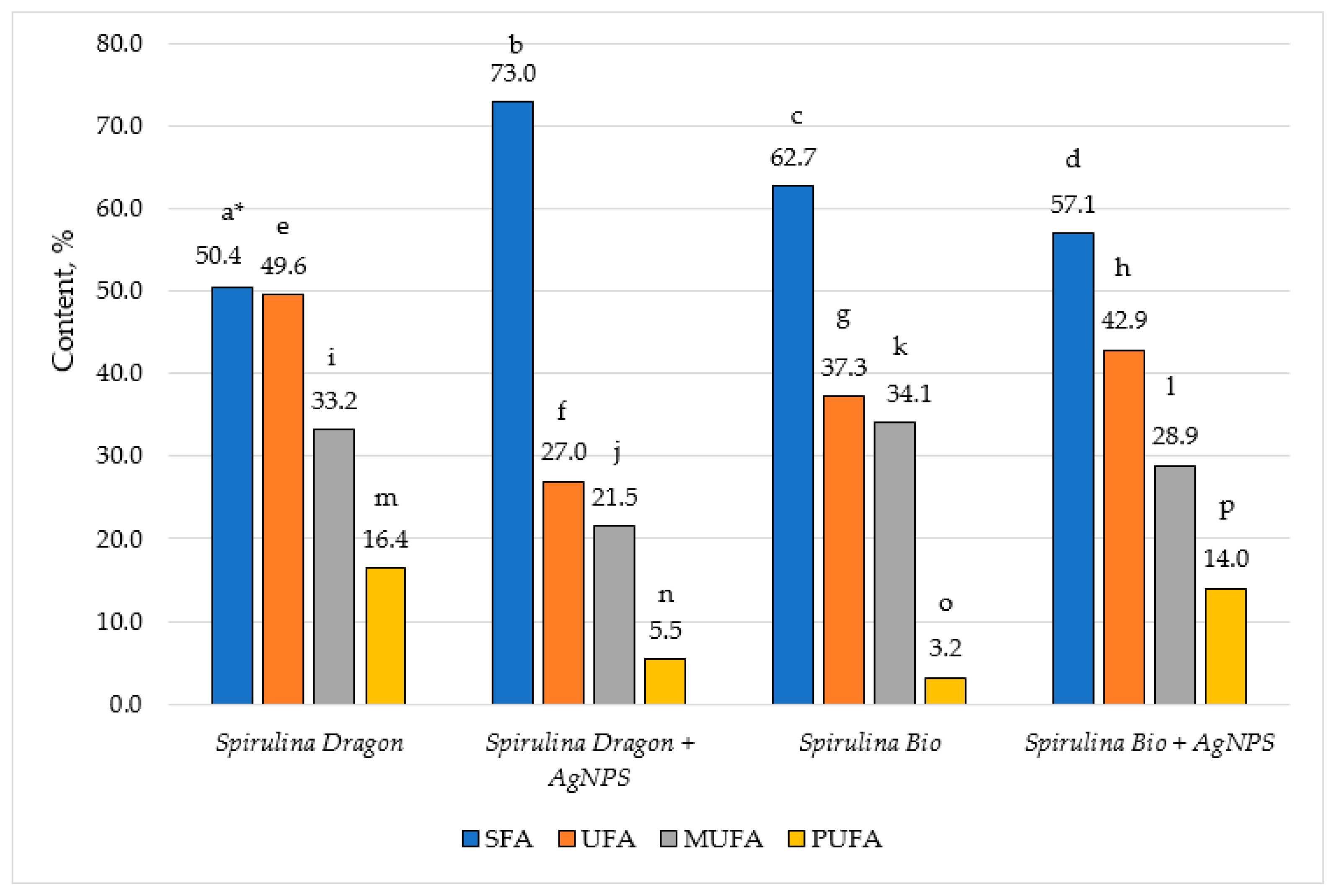
3.4. Chemical Composition Changes
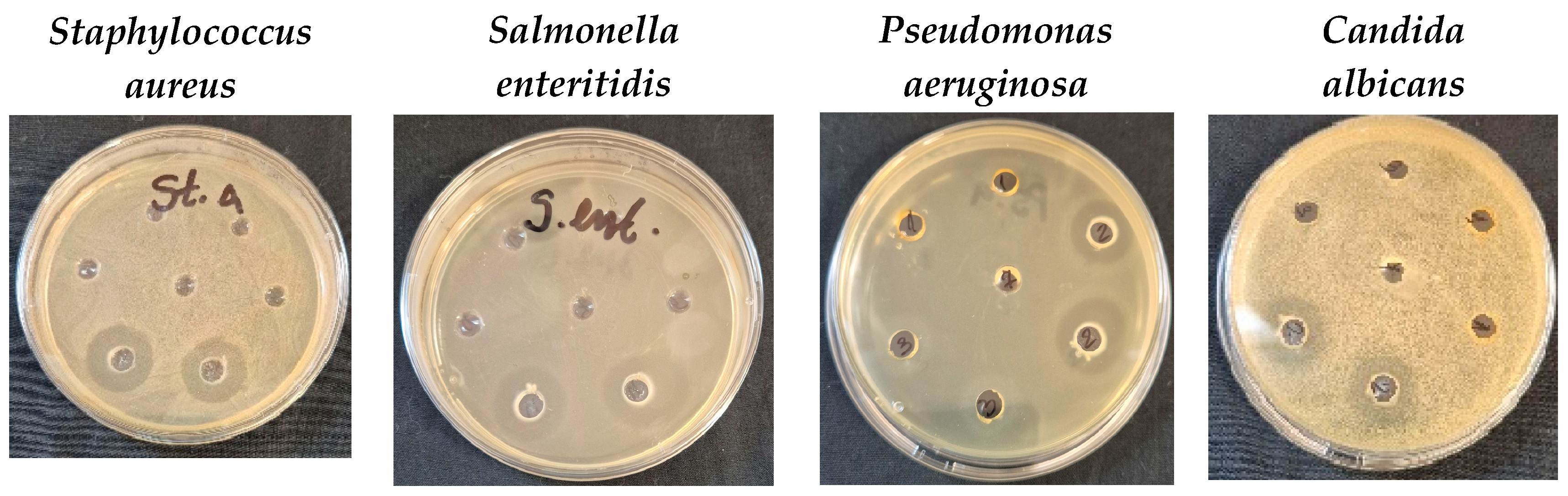
3.5. Antimicrobial Activity
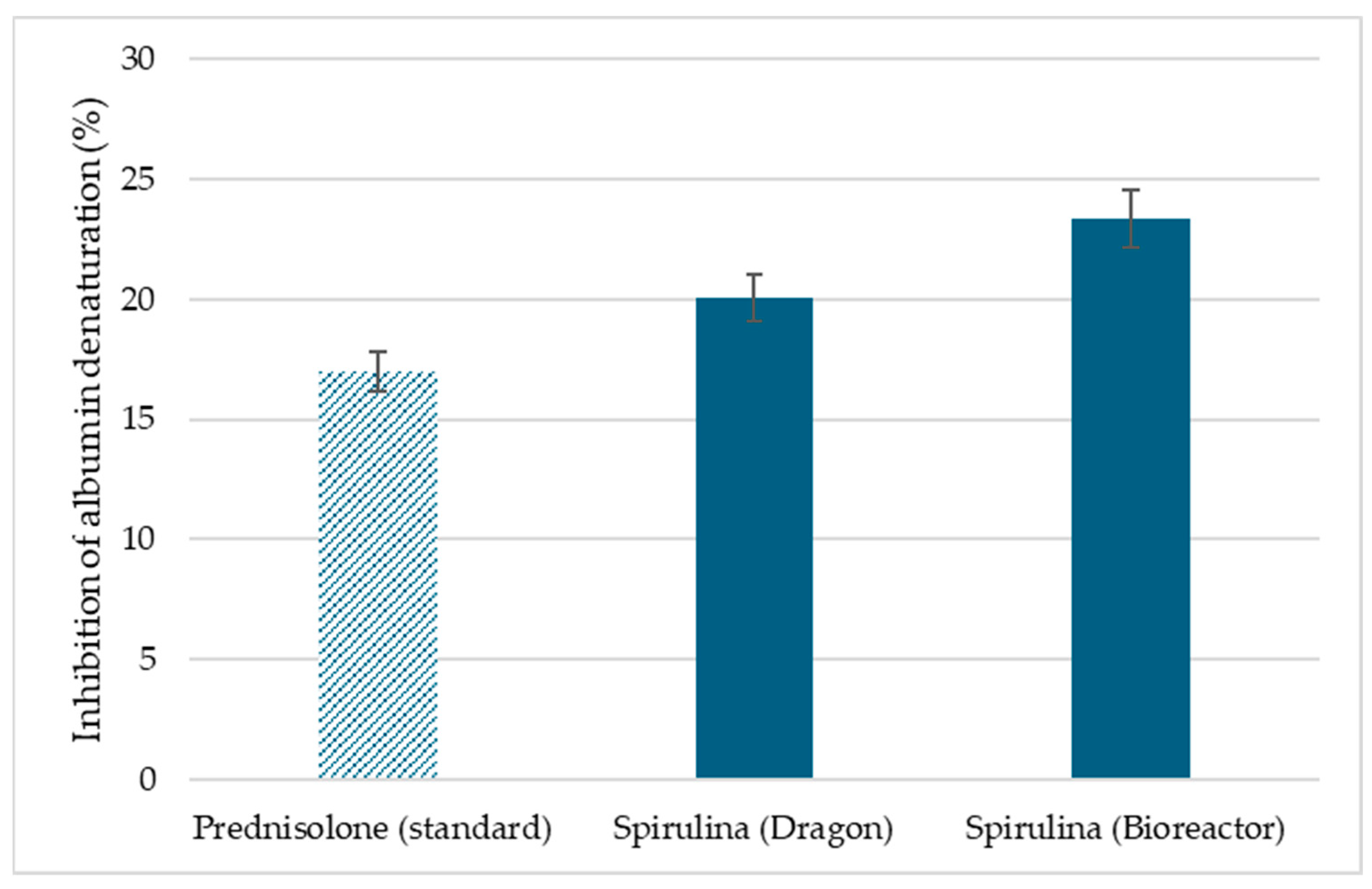
3.6. Inhibition of Albumin Denaturation Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of Fermentation on Enhancing the Nutraceutical Properties of Arthrospira platensis (Spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef]
- Wan, D.; Wu, Q.; Kuča, K. Spirulina. In Nutraceuticals Efficacy, Safety and Toxicity; Gupta, R.C., Ed.; Elsevier Academic Press, Cop: Amsterdam, The Netherlands; London, UK, 2016; ISBN 9780128021477. [Google Scholar]
- Soni, R.A.; Sudhakar, K.; Rana, R.S. Spirulina—From Growth to Nutritional Product: A Review. Trends Food Sci. Technol. 2017, 69, 157–171. [Google Scholar] [CrossRef]
- Ainas, M.; Hasnaoui, S.; Bouarab, R.; Abdi, N.; Drouiche, N.; Mameri, N. Hydrogen Production with the Cyanobacterium Spirulina platensis. Int. J. Hydrogen Energy 2017, 42, 4902–4907. [Google Scholar] [CrossRef]
- GBIF.org. GBIF Backbone Taxonomy: Arthrospira platensis Gomont. Available online: https://www.gbif.org/species/3218108 (accessed on 7 September 2025).
- Han, P.; Li, J.; Zhong, H.; Xie, J.; Zhang, P.; Lu, Q.; Li, J.; Xu, P.; Chen, P.; Leng, L.; et al. Anti-Oxidation Properties and Therapeutic Potentials of Spirulina. Algal Res. 2021, 55, 102240. [Google Scholar] [CrossRef]
- Novoveská, L.; Zapata, A.K.M.; Zabolotney, J.B.; Atwood, M.C.; Sundstrom, E.R. Optimizing Microalgae Cultivation and Wastewater Treatment in Large-Scale Offshore Photobioreactors. Algal Res. 2016, 18, 86–94. [Google Scholar] [CrossRef]
- El-Sayed, H.S.; Hassan, A.; Barakat, K.M.; Ghonam, H.E.-B. Improvement of Growth and Biochemical Constituents of Rosmarinus officinalis by Fermented Spirulina maxima Biofertilizer. Plant Physiol. Biochem. 2024, 208, 108452. [Google Scholar] [CrossRef]
- Saraswathi, K.; Naga Kavitha, C. Spirulina: Pharmacological Activities and Health Benefits. J. Young Pharm. 2023, 15, 441–447. [Google Scholar] [CrossRef]
- Boukid, F.; Castellari, M. Food and Beverages Containing Algae and Derived Ingredients Launched in the Market from 2015 to 2019: A Front-of-Pack Labeling Perspective with a Special Focus on Spain. Foods 2021, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Sommano, S.R.; Bhat, F.M.; Wongkeaw, M.; Sriwichai, T.; Sunanta, P.; Chuttong, B.; Burgett, M. Amino Acid Profiling and Chemometric Relations of Black Dwarf Honey and Bee Pollen. Front. Nutr. 2020, 7, 558579. [Google Scholar] [CrossRef] [PubMed]
- Özyurt, G.; Uslu, L.; Durmuş, M.; Sakarya, Y.; Uzlaşir, T.; Küley, E. Chemical and Physical Characterization of Microencapsulated Spirulina Fermented with Lactobacillus Plantarum. Algal Res. 2023, 73, 103149. [Google Scholar] [CrossRef]
- Mohammed, D.M.; El-Messery, T.M.; Baranenko, D.A.; Hashim, M.A.; Tyutkov, N.; Marrez, D.A.; Elmessery, W.M.; El-Said, M.M. Effect of Spirulina maxima Microcapsules to Mitigate Testicular Toxicity Induced by Cadmium in Rats: Optimization of in Vitro Release Behavior in the Milk Beverage. J. Funct. Foods 2023, 112, 105938. [Google Scholar] [CrossRef]
- Tavakoli, Z.; Kavoosi, G.; Siahbalaei, R.; Karimi, J. Spirulina maxima as a Valuable Ingredient: Determination of Broad Fatty Acid and Amino Acid Profiles and Nutritional Quality and Anti-Amylase Capacity. Appl. Food Res. 2025, 5, 100741. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.; Bandarra, N.M.; Afonso, C. Microalgae as Healthy Ingredients for Functional Food: A Review. Food Funct. 2017, 8, 2672–2685. [Google Scholar] [CrossRef]
- Sharma, R.; Rana, A. Algal Elixirs: Unraveling the Multifaceted Impact of Spirulina in Human Health. Food Biosci. 2024, 62, 105365. [Google Scholar] [CrossRef]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the Food and Functional Food Industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Bhat, A.G.; OKeefe, J. Effects of Spirulina on Weight Loss and Blood Lipids: A Review. Open Heart 2020, 7, e001003. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Petros, R.A.; DeSimone, J.M. Strategies in the Design of Nanoparticles for Therapeutic Applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Virmani, I.; Sasi, C.; Priyadarshini, E.; Kumar, R.; Sharma, S.K.; Singh, G.P.; Pachwarya, R.B.; Paulraj, R.; Barabadi, H.; Saravanan, M.; et al. Comparative Anticancer Potential of Biologically and Chemically Synthesized Gold Nanoparticles. J. Clust. Sci. 2019, 31, 867–876. [Google Scholar] [CrossRef]
- Jain, A.S.; Pawar, P.S.; Sarkar, A.; Junnuthula, V.; Dyawanapelly, S. Bionanofactories for Green Synthesis of Silver Nanoparticles: Toward Antimicrobial Applications. Int. J. Mol. Sci. 2021, 22, 11993. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Moradi, F.; Sedaghat, S.; Moradi, O.; Arab Salmanabadi, S. Review on Green Nano-Biosynthesis of Silver Nanoparticles and Their Biological Activities: With an Emphasis on Medicinal Plants. Inorg. Nano-Met. Chem. 2020, 51, 133–142. [Google Scholar] [CrossRef]
- Appel, A.-K.; Thomann, R.; Mülhaupt, R. Polyurethane Nanocomposites Prepared from Solvent-Free Stable Dispersions of Functionalized Graphene Nanosheets in Polyols. Polymer 2012, 53, 4931–4939. [Google Scholar] [CrossRef]
- Chahardoli, A.; Karimi, N.; Fattahi, A. Biosynthesis, Characterization, Antimicrobial and Cytotoxic Effects of Silver Nanoparticles Using Nigella arvensis Seed Extract. Iran. J. Pharm. Res. 2017, 16, 1167–1175. [Google Scholar]
- Amin, Z.R.; Khashyarmanesh, Z.; Sedigheh, B.; Noghabi, Z.S. Does Biosynthetic Silver Nanoparticles Are More Stable with Lower Toxicity than Their Synthetic Counterparts? Iran. J. Pharm. Res. 2019, 18, 210. [Google Scholar]
- Bhanu Prakash. Functional and Preservative Properties of Phytochemicals; Academic Press: London, UK; San Diego, CA, USA; Cambridge, MA, USA; Oxford, UK, 2020; ISBN 9780128185933. [Google Scholar]
- Khan, M.; Shaik, M.R.; Adil, S.F.; Khan, S.T.; Al-Warthan, A.; Siddiqui, M.R.H.; Tahir, M.N.; Tremel, W. Plant Extracts as Green Reductants for the Synthesis of Silver Nanoparticles: Lessons from Chemical Synthesis. Dalton Trans. 2018, 47, 11988–12010. [Google Scholar] [CrossRef]
- Muthusamy, G.; Thangasamy, S.; Raja, M.; Chinnappan, S.; Kandasamy, S. Biosynthesis of Silver Nanoparticles from Spirulina Microalgae and Its Antibacterial Activity. Environ. Sci. Pollut. Res. Int. 2017, 24, 19459–19464. [Google Scholar] [CrossRef] [PubMed]
- Fahim, M.; Shahzaib, A.; Nishat, N.; Jahan, A.; Bhat, T.A.; Inam, A. Green Synthesis of Silver Nanoparticles: A Comprehensive Review of Methods, Influencing Factors, and Applications. JCIS Open 2024, 16, 100125. [Google Scholar] [CrossRef]
- Rodrigues, A.S.; Batista, J.; Rodrigues, V.; Thipe, V.C.; Minarini, L.A.R.; Lopes, P.S.; Lugão, A.B. Advances in Silver Nanoparticles: A Comprehensive Review on Their Potential as Antimicrobial Agents and Their Mechanisms of Action Elucidated by Proteomics. Front. Microbiol. 2024, 15, 1440065. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Ali, S.A.; Osman, M.E.; Mohamed, E.T. Eco-Friendly Biosynthesis of Silver Nanoparticles Using Marine-Derived Fusarium Exquisite: Optimization, Characterization, and Evaluation of Antimicrobial, Antioxidant, and Cytotoxic Activities. World J. Microbiol. Biotechnol. 2025, 41, 165. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Genova, J.; Chamati, H. Green Synthesis of Gold Nanoparticles: An Eco-Friendly Approach. Chemistry 2022, 4, 345–369. [Google Scholar] [CrossRef]
- Sangeetha, G.; Vidhya, R. In Vitro Anti-Inflammatory Activity of Different Parts of Pedalium murex (L.). Int. J. Herb. Med. 2016, 4, 31–36. [Google Scholar]
- Soror, A.-F.S.; Ahmed, M.W.; Hassan, A.E.A.; Alharbi, M.; Alsubhi, N.H.; Al-Quwaie, D.A.; Alrefaei, G.I.; Binothman, N.; Aljadani, M.; Qahl, S.H.; et al. Evaluation of Green Silver Nanoparticles Fabricated by Spirulina platensis Phycocyanin as Anticancer and Antimicrobial Agents. Life 2022, 12, 1493. [Google Scholar] [CrossRef] [PubMed]
- Gul, A.; Baig, M.N.; Ahmed, D.; Najam, Z.; Aslam, T.; Ali, S. Green Synthesis of Silver Nanoparticles from Spirulina platensis Extract: Antibacterial and Antioxidant Potential. BioNanoScience 2024, 14, 2327–2336. [Google Scholar] [CrossRef]
- Harutyunyan, A.; Gabrielyan, L.; Aghajanyan, A.; Gevorgyan, S.; Schubert, R.; Betzel, C.; Kujawski, W.; Gabrielyan, L. Comparative Study of Physicochemical Properties and Antibacterial Potential of Cyanobacteria Spirulina platensis-Derived and Chemically Synthesized Silver Nanoparticles. ACS Omega 2024, 9, 29410–29421. [Google Scholar] [CrossRef] [PubMed]
- Bej, S.; Swain, S.; Bishoyi, A.K.; Sahoo, C.R.; Jali, B.R.; Khan, M.S.; Padhy, R.N. Biosynthesis, Characterization and Monitoring In Vitro Antibacterial Efficiencies of AgNPs with Filamentous Cyanobacteria Spirulina sp. and S. subsalsa against Pathogenic Bacteria. J. King Saud. Univ. Sci. 2024, 36, 103336. [Google Scholar] [CrossRef]
- Rudi, L.; Zinicovscaia, I.; Cepoi, L.; Chiriac, T.; Grozdov, D.; Kravtsova, A. The Impact of Silver Nanoparticles Functionalized with Spirulina Protein Extract on Rats. Pharmaceuticals 2024, 17, 1247. [Google Scholar] [CrossRef]
- Nikolova, K.; Gentscheva, G.; Gyurova, D.; Pavlova, V.; Dincheva, I.; Velikova, M.; Gerasimova, A.; Makedonski, L.; Gergov, G. Metabolomic Profile of Arthrospira platensis from a Bulgarian Bioreactor—A Potential Opportunity for Inclusion in Dietary Supplements. Life 2024, 14, 174. [Google Scholar] [CrossRef]
- Gentscheva, G.; Milkova-Tomova, I.; Pehlivanov, I.; Gugleva, V.; Nikolova, K.; Petkova, N.; Andonova, V.; Buhalova, D.; Pisanova, E. Chemical Characterization of Selected Algae and Cyanobacteria from Bulgaria as Sources of Compounds with Antioxidant Activity. Appl. Sci. 2022, 12, 9935. [Google Scholar] [CrossRef]
- ISO 659:2014; Oilseeds–Determination of Oil Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2014.
- AOAC. Official Methods of Analysis, 20th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2016. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Food Energy–Methods of Analysis and Conversion Factors; FAO Food and Nutrition Paper No. 77; Report of a Technical Workshop; FAO: Rome, Italy, 2003. [Google Scholar]
- ISO 12966-1:2014; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 1: Guidelines on Modern Gas Chromatography of Fatty Acid Methyl Esters. International Organization for Standardization: Geneva, Switzerland, 2014.
- ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 9936:2016; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Contents by High-Performance Liquid Chromatography. International Organization for Standardization: Geneva, Switzerland, 2016.
- Goranov, B.; Gaytanska, Y.; Denkova-Kostova, R.; Ivanova, P.; Denkova, Z.; Kostov, G. Examination of the Bactericidal and Fungicidal Activity of Bacillus amyloliquefaciens M Isolated from Spring Waters in Bulgaria. Appl. Sci. 2024, 14, 3612. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Petkova, N.; Ivanov, I.; Todorova, M.; Ivanova, P.; Nikolov, L.; Grozeva, N.; Nikolova, K. Design of Functional Yogurts with Microencapsulated Biologically Active Compounds. Nat. Life Sci. Commun. 2024, 23, e2024011. [Google Scholar] [CrossRef]
- Milusheva, M.; Todorova, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Pencheva, M.; Nedialkov, P.; Tumbarski, Y.; Yanakieva, V.; Tsoneva, S.; et al. Novel Anthranilic Acid Hybrids—An Alternative Weapon against Inflammatory Diseases. Pharmaceuticals 2023, 16, 1660. [Google Scholar] [CrossRef]
- Yanakieva, V.; Parzhanova, A.; Dimitrov, D.; Vasileva, I.; Raeva, P.; Ivanova, S. Study on the Antimicrobial Activity of Medicinal Plant Extracts and Emulsion Products with Integrated Herbal Extracts. Agric. For. 2023, 69, 71–89. [Google Scholar] [CrossRef]
- Milusheva, M.; Gledacheva, V.; Stefanova, I.; Feizi-Dehnayebi, M.; Mihaylova, R.; Nedialkov, P.; Cherneva, E.; Tumbarski, Y.; Tsoneva, S.; Todorova, M.; et al. Synthesis, Molecular Docking, and Biological Evaluation of Novel Anthranilic Acid Hybrid and Its Diamides as Antispasmodics. Int. J. Mol. Sci. 2023, 24, 13855. [Google Scholar] [CrossRef]
- Kavitha, E.; Stephen, L.D.; Brishti, F.H.; Karthikeyan, S. Two-Trace Two-Dimensional (2T2D) Correlation Infrared Spectral Analysis of Spirulina platensis and Its Commercial Food Products Coupled with Chemometric Analysis. J. Mol. Str. 2021, 1244, 130964. [Google Scholar] [CrossRef]
- Nikolova, K.; Eftimov, T.; Pashev, A.; Karadjov, M.; Tzvetkova, C.; Gentscheva, G.; Brabant, D.; Samia, F. Correlation between Chemical Characteristics and Optical Spectra of Spirulina Commercially Available on the Bulgarian Market. J. Turk. Chem. Soc. A 2023, 10, 465–474. [Google Scholar] [CrossRef]
- Tong, X.; Djearamane, S.; Tanislaus, C.; Wong, L.S. Impact of Silver Nanoparticles on the Nutritional Properties of Arthrospira platensis. PeerJ 2022, 10, e13972. [Google Scholar] [CrossRef]
- Filippo, E.; Serra, A.; Buccolieri, A.; Manno, D. Green Synthesis of Silver Nanoparticles with Sucrose and Maltose: Morphological and Structural Characterization. J. Non-Cryst. Solids 2010, 356, 344–350. [Google Scholar] [CrossRef]
- Ivask, A.; ElBadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity Mechanisms in Escherichia Coli Vary for Silver Nanoparticles and Differ from Ionic Silver. ACS Nano 2013, 8, 374–386. [Google Scholar] [CrossRef]
- Stoehr, L.C.; Gonzalez, E.; Stampfl, A.; Casals, E.; Duschl, A.; Puntes, V.; Oostingh, G.J. Shape Matters: Effects of Silver Nanospheres and Wires on Human Alveolar Epithelial Cells. Part. Fibre Toxicol. 2011, 8, 36. [Google Scholar] [CrossRef]
- Huk, A.; Izak-Nau, E.; Reidy, B.; Boyles, M.; Duschl, A.; Lynch, I.; Dušinska, M. Is the Toxic Potential of Nanosilver Dependent on Its Size? Part. Fibre Toxicol. 2014, 11, 65. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic Potential of Silver Nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef]
- León-Silva, S.; Fernández-Luqueño, F.; López-Valdez, F. Silver Nanoparticles (AgNP) in the Environment: A Review of Potential Risks on Human and Environmental Health. Water Air Soil. Pollut. 2016, 227, 306. [Google Scholar] [CrossRef]
- Khoshnamvand, M.; Huo, C.; Liu, J. Silver Nanoparticles Synthesized Using Allium Ampeloprasum, L. Leaf Extract: Characterization and Performance in Catalytic Reduction of 4-Nitrophenol and Antioxidant Activity. J. Mol. Struct. 2019, 1175, 90–96. [Google Scholar] [CrossRef]
- Alzubaidi, A.K.; Al-Kaabi, W.J.; Ali, A.A.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Asiri, M.; Khane, Y. Green Synthesis and Characterization of Silver Nanoparticles Using Flaxseed Extract and Evaluation of Their Antibacterial and Antioxidant Activities. Appl. Sci. 2023, 13, 2182. [Google Scholar] [CrossRef]
- Liaqat, N.; Jahan, N.; Rahman, K.; Anwar, T.; Qureshi, H. Green Synthesized Silver Nanoparticles: Optimization, Characterization, Antimicrobial Activity, and Cytotoxicity Study by Hemolysis Assay. Front. Chem. 2022, 10, 982006. [Google Scholar] [CrossRef] [PubMed]
- Hanisha, R.; Balaganapathy, M.; Eswar, B.; Kathirvelan, P.; Rajabathar, J.R.; Siddiqui, N.; Dinakarkumar, Y. Biogenic Synthesis of Silver Nanoparticles Using Spirulina maxima Extract and Their Bactericidal Activity. J. Umm Al-Qura Univ. Appl. Sci. 2024, 1–11. [Google Scholar] [CrossRef]
- Al-Badwy, A.H.; Khalil, A.M.; Bashal, A.H.; Kebeish, R. Polysaccharides from Spirulina platensis (PSP): Promising Biostimulants for the Green Synthesis of Silver Nanoparticles and Their Potential Application in the Treatment of Cancer Tumors. Microb. Cell Factories 2023, 22, 247. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Tocopherol-Mediated Synthesis of Silver Nanoparticles and Preparation of Antimicrobial PBAT/Silver Nanoparticles Composite Films. LWT–Food Sci. Technol. 2016, 72, 149–156. [Google Scholar] [CrossRef]
- Abbas, R.; Luo, J.; Qi, X.; Naz, A.; Khan, I.A.; Liu, H.; Yu, S.; Wei, J. Silver Nanoparticles: Synthesis, Structure, Properties and Applications. Nanomaterials 2024, 14, 1425. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R. Antioxidant Activity and Total Phenolic Content of Moringa Oleifera Leaves in Two Stages of Maturity. Plant Foods Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef]
- Mohamed, A.; Dayo, M.; Alahmadi, S.; Ali, S. Anti-Inflammatory and Antimicrobial Activity of Silver Nanoparticles Green-Synthesized Using Extracts of Different Plants. Nanomaterials 2024, 14, 1383. [Google Scholar] [CrossRef] [PubMed]
- Arun, N.; Gupta, S.; Singh, D. Antimicrobial and Antioxidant Property of Commonly Found Microalgae Spirulina platensis, Nostoc muscorum and Chlorella pyrenoidosa against Some Pathogenic Bacteria and Fungi. Int. J. Pharm. Sci. Res. 2012, 3, 103. [Google Scholar] [CrossRef]
- Okoli, C.O.; Akah, P.A.; Nwafor, S.V.; Anisiobi, A.I.; Ibegbunam, I.N.; Erojikwe, O. Anti-Inflammatory Activity of Hexane Leaf Extract of Aspilia Africana C.D. Adams. J. Ethnopharmacol. 2007, 109, 219–225. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Jayashree, V.; Bagyalakshmi, S.; Manjula Devi, K.; Richard, D.D. In Vitro Anti-Inflammatory Activity of 4-Benzylpiperidine. Asian J. Pharm. Clin. Res. 2016, 9, 108. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) and Organ Damage: A Current Perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Del Grossi Moura, M.; Cruz Lopes, L.; Silva, M.T.; Barberato-Filho, S.; Motta, R.H.L.; Bergamaschi, C.d.C. Use of Steroid and Nonsteroidal Anti-Inflammatories in the Treatment of Rheumatoid Arthritis: Systematic Review Protocol. Medicine 2018, 97, e12658. [Google Scholar] [CrossRef]
- Madhuranga, H.; Samarakoon, D. Committed to Create Value for Researchers in Vitro Anti-Inflammatory Egg Albumin Denaturation Assay: An Enhanced Approach in Vitro Anti-Inflammatory Egg Albumin Denaturation Assay: An Enhanced Approach. J. Nat. Ayurved. Med. 2023, 7, 000411. [Google Scholar] [CrossRef]
- Manoharan, K.; Chitra, P. In Vitro Antioxidant, Anti-Inflammatory and Antibacterial Activities of Microencapsulated Elaeocarpus tectorius (Lour.) Poir Leaf Extracts. Asian J. Biol. Life Sci. 2022, 10, 597–607. [Google Scholar] [CrossRef]
- Enechi, O.C.; Okeke, E.S.; Nwankwo, N.E.; Nweze, J.E.; Obilor, C.P.; Okoye, C.I.; Awoh, O.E. Membrane Stabilization, Albumin Denaturation, Protease Inhibition, and Antioxidant Activity as Possible Mechanisms for the Anti-Inflammatory Effects of Flavonoid-Rich Extract of Peltophorum pterocarpum (DC.) K.Heyne (FREPP) Stem Bark. Trop. J. Nat. Prod. Res. 2020, 4, 812–816. [Google Scholar] [CrossRef]
- Anokwah, D.; Kwatia, E.A.; Amponsah, I.K.; Jibira, Y.; Harley, B.K.; Ameyaw, E.O.; Obese, E.; Biney, R.P.; Mensah, A.Y. Evaluation of the Anti-Inflammatory and Antioxidant Potential of the Stem Bark Extract and Some Constituents of Aidia genipiflora (DC.) Dandy (Rubiaceae). Heliyon 2022, 8, e10082. [Google Scholar] [CrossRef] [PubMed]
- Kaddour, S.M.; Arrar, L.; Baghiani, A. Anti-Inflammatory Potential Evaluation (In-Vitro and In-Vivo) of Arthrophytum Scoparium Aerial Part. J. Drug Deliv. Ther. 2020, 10, 213–218. [Google Scholar] [CrossRef]


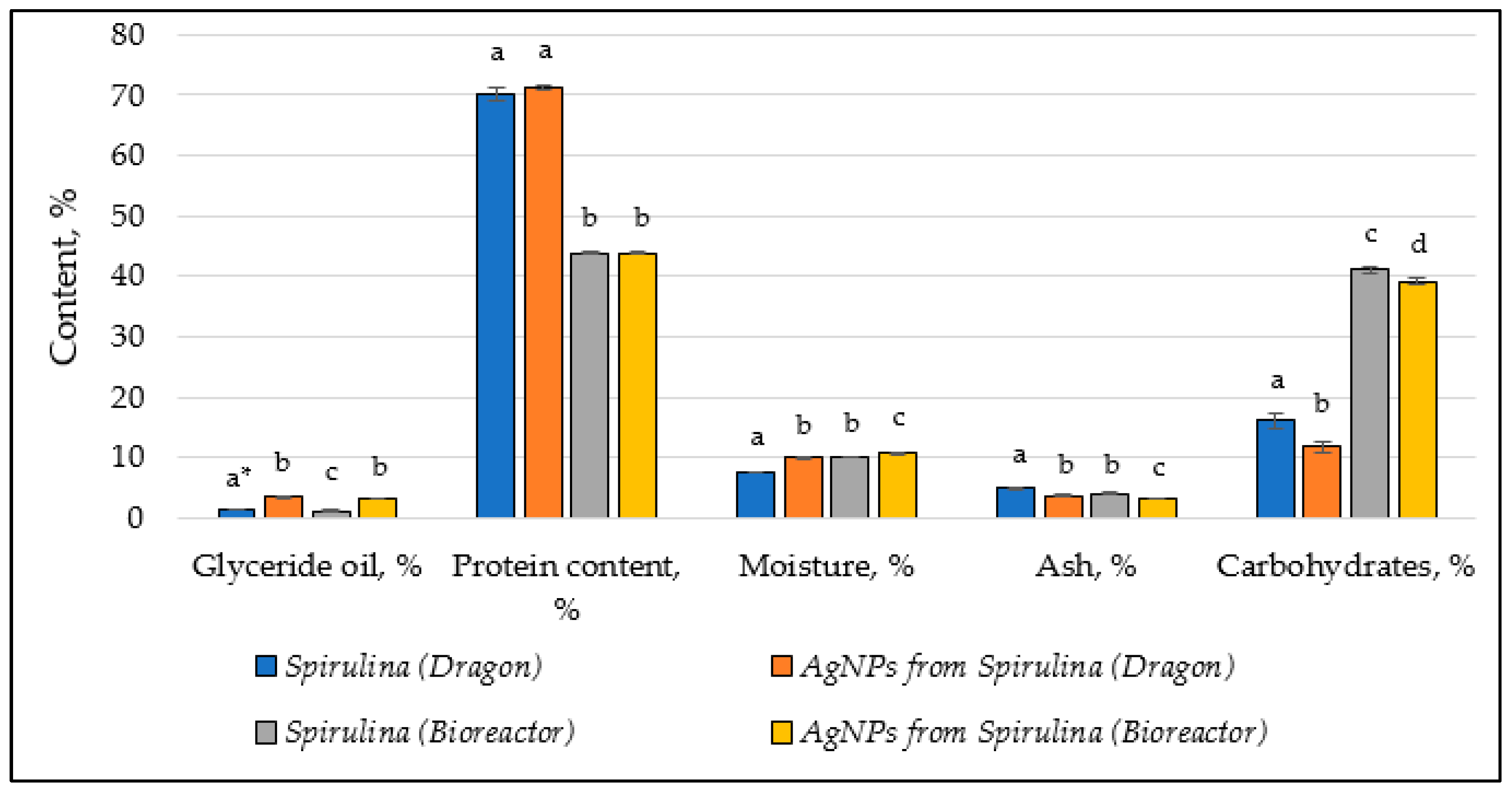
| Chemical Composition | Spirulina (Dragon) | AgNPs from Spirulina (Dragon) | Spirulina (Bioreactor) | AgNPs from Spirulina (Bioreactor) |
|---|---|---|---|---|
| Glyceride oil, % | 1.37 ± 0.05 a * | 3.37 ± 0.17 b | 1.12 ± 0.12 c | 3.10 ± 0.10 b |
| Protein content, % | 70.20 ± 1.00 a | 71.25 ± 0.50 a | 43.80 ± 0.20 b | 43.90 ± 0.10 b |
| Moisture, % | 7.54 ± 0.10 a | 9.95 ± 0.15 b | 10.00 ± 0.10 b | 10.72 ± 0.12 c |
| Ash, % | 4.84 ± 0.25 a | 3.69 ± 0.15 b | 3.97 ± 0.21 b | 3.19 ± 0.13 c |
| Carbohydrates, % | 16.05 ± 1.40 a | 11.69 ± 0.80 b | 41.11 ± 0.58 c | 39.09 ± 0.52 d |
| Fatty Acids, % | Spirulina (Dragon) | AgNPs from Spirulina (Dragon) | Spirulina (Bioreactor) | AgNPs from Spirulina (Bioreactor) | |
|---|---|---|---|---|---|
| C 8:0 | caprylic | 0.6 ± 0.1 a * | 0.4 ± 0.1 b | 0.9 ± 0.2 c | 1.6 ± 0.3 d |
| C 10:0 | capric | - | - | 0.2 ± 0.0 a | 0.5 ± 0.1 b |
| C 12:0 | lauric | - | - | 0.3 ± 0.1 a | 1.0 ± 0.2 b |
| C 14:0 | myristic | 1.3 ± 0.3 a | 0.9 ± 0.2 a | 1.4 ± 0.1 a | 1.5 ± 0.2 a |
| C 14:1 | myristoleic | 3.6 ± 0.4 a | 5.3 ± 0.3 b | 2.7 ± 0.2 c | 1.1 ± 0.1 d |
| C 15:0 | pentadecanoic | - | - | 0.2 ± 0.0 a | 0.6 ± 0.1 b |
| C 15:1 | pentadecenoic | 1.9 ± 0.3 a | 0.2 ± 0.0 b | 0.9 ± 0.2 c | 1.5 ± 0.4 a |
| C 16:0 | palmitic | 30.8 ± 0.5 a | 63.8 ± 0.5 b | 42.5 ± 0.4 c | 37.0 ± 0.3 d |
| C 16:1 | palmitoleic | 4.8 ± 0.3 a | 5.5 ± 0.3 b | 2.3 ± 0.2 c | 1.7 ± 0.2 d |
| C 16:2 | 7,10-hexadecadienoic | 11.0 ± 0.4 a | 2.8 ± 0.2 b | 2.0 ± 0.1 c | 7.1 ± 0.2 d |
| C 17:0 | heptadecanoic | 12.6 ± 0.5 a | 3.9 ± 0.3 b | 2.7 ± 0.2 c | 8.4 ± 0.4 d |
| C 16:3 | 7,10,13- hexadecatrienoic | 3.7 ± 0.2 a | 1.0 ± 0.1 b | 0.7 ± 0.1 c | 2.4 ± 0.2 d |
| C 17:1 | heptadecenoic | 17.2 ± 0.5 a | 5.5 ± 0.3 b | 3.7 ± 0.2 c | 12.1 ± 0.3 d |
| C 18:0 | stearic | 4.7 ± 0.3 a | 3.7 ± 0.2 b | 13.9 ± 0.4 c | 5.6 ± 0.2 d |
| C 18:1 | oleic | 5.7 ± 0.3 a | 5.0 ± 0.4 a | 23.8 ± 0.5 c | 12.5 ± 0.3 d |
| C 18:2 | linoleic | 0.7 ± 0.2 a | 0.6 ± 0.1 a | 0.2 ± 0.0 c | 3.8 ± 0.3 d |
| C 18:3 n-3 | α-linolenic | 0.4 ± 0.1 a | 0.7 ± 0.2 a | - | - |
| C 18:4 | stearidonic | - | - | 0.3 ± 0.1 a | 0.7 ± 0.2 b |
| C 20:0 | arachidic | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.3 ± 0.1 a | - |
| C 20:1 | eicosenoic | - | - | 0.7 ± 0.2 | - |
| C 20:4 | arachidonic | 0.6 ± 0.1 a | 0.4 ± 0.1 a | - | - |
| C 22:0 | behenic | 0.2 ± 0.0 a | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.7 ± 0.2 c |
| C 24:0 | lignoceric | - | - | 0.2 ± 0.0 a | 0.2 ± 0.0 a |
| Tocopherols | Spirulina (Dragon) | AgNPs in Spirulina (Dragon) | Spirulina (Bioreactor) | AgNPs in Spirulina (Bioreactor) |
|---|---|---|---|---|
| α-tocopherol, % | 84.1 ± 0.5 a * | 100 ± 0.0 b | - | - |
| α-tocotrienol, % | 3.8 ± 0.2 a | - | 66.1 ± 0.4 b | 100 ± 0.0 c |
| β-tocopherol, % | 1.5 ± 0.1 | - | - | - |
| γ-tocopherol, % | 7.4 ± 0.4 | - | - | - |
| γ-tocotrienol, % | 3.2 ± 0.2 a | - | 33.9 ± 0.3 b | - |
| Total tocopherol content, mg/kg | 2432 ± 30 a | 92.4 ± 10 b | 102 ± 12 b | 42 ± 6 c |
| Tested Microorganisms | Inhibition Zones, mm | |||
|---|---|---|---|---|
| AgNPs from Spirulina (Dragon) | AgNPs from Spirulina (Bioreactor) | |||
| 24 h | 48 h | 24 h | 48 h | |
| Staphylococcus aureus | 18 ± 0.0 a | 18 ± 0.0 a | 19 ± 1.0 a | 19 ± 1.0 a |
| Listeria monocytogenes | 20 ± 0.0 a | 20 ± 0.0 a | 20 ± 0.0 a | 20 ± 0.0 a |
| Klebsiella sp. | 8.5 ± 0.5 a | 8.5 ± 0.5 a | 10 ± 0.0 a | 10 ± 0.0 a |
| Enterococcus faecalis | 17 ± 0.0 a | 17.5 ± 0.5 a | 16 ± 0.0 a | 17 ± 0.0 b |
| Escherichia coli | 17 ± 0.0 a | 17 ± 0.0 a | 17 ± 1.0 a | 17.5 ± 0.5 b |
| Salmonella enteritidis | 23 ± 1.0 a | 23 ± 1.0 a | 21 ± 1.0 a | 21 ± 1.0 a |
| Proteus vulgaris | 17.5 ± 0.5 a | 17.5 ± 0.5 a | 15 ± 1.0 a | 17 ± 0.0 b |
| Pseudomonas aeruginosa | 19 ± 1.0 a | 23 ± 1.0 a | 17.5 ± 0.5 a | 19 ± 1.0 b |
| Candida albicans | 17.5 ± 0.5 a | 17.5 ± 0.5 a | 16.5 ± 0.5 a | 16.5 ± 0.5 a |
| Bacilluscereus | 14.5 ± 0.5 a | 14.5 ± 0.5 a | 15 ± 1.0 a | 15 ± 1.0 a |
| Bacillussubtilis | 12.5 ± 0.5 a | 12.5 ± 0.5 a | 12 ± 0.0 a | 12 ± 0.0 a |
| Saccharomyces cerevisiae | 9 ± 0.0 a | 9 ± 0.0 a | 8.5 ± 0.5 a | 8.5 ± 0.5 a |
| Aspergillus niger | 8 ± 0.0 a | - | 8 ± 0.0 a | - |
| Aspergillus flavus | 8 ± 0.0 a | 8 ± 0.0 a | 10 ± 0.0 a | 10 ± 0.0 a |
| Penicillium chrysogenum | 10 ± 0.0 a | 10 ± 0.0 a | 11 ± 0.0 a | 10.5 ± 0.5 a |
| Mucor sp. | - | - | - | - |
| Fusarium moniliforme | 8 ± 0.0 a | - | 8 ± 0.0 a | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, A.; Todorova, M.; Petrov, D.; Petkova, Z.; Teneva, O.; Antova, G.; Angelova-Romova, M.; Yanakieva, V.; Tsoneva, S.; Gledacheva, V.; et al. From Spirulina platensis to Nanomaterials: A Comparative Study of AgNPs Obtained from Two Extracts. Nanomaterials 2025, 15, 1392. https://doi.org/10.3390/nano15181392
Ivanova A, Todorova M, Petrov D, Petkova Z, Teneva O, Antova G, Angelova-Romova M, Yanakieva V, Tsoneva S, Gledacheva V, et al. From Spirulina platensis to Nanomaterials: A Comparative Study of AgNPs Obtained from Two Extracts. Nanomaterials. 2025; 15(18):1392. https://doi.org/10.3390/nano15181392
Chicago/Turabian StyleIvanova, Alexandra, Mina Todorova, Dimitar Petrov, Zhana Petkova, Olga Teneva, Ginka Antova, Maria Angelova-Romova, Velichka Yanakieva, Slava Tsoneva, Vera Gledacheva, and et al. 2025. "From Spirulina platensis to Nanomaterials: A Comparative Study of AgNPs Obtained from Two Extracts" Nanomaterials 15, no. 18: 1392. https://doi.org/10.3390/nano15181392
APA StyleIvanova, A., Todorova, M., Petrov, D., Petkova, Z., Teneva, O., Antova, G., Angelova-Romova, M., Yanakieva, V., Tsoneva, S., Gledacheva, V., Nikolova, K., Karashanova, D., & Nikolova, S. (2025). From Spirulina platensis to Nanomaterials: A Comparative Study of AgNPs Obtained from Two Extracts. Nanomaterials, 15(18), 1392. https://doi.org/10.3390/nano15181392








