Effect of Ultra-Small Platinum Single-Atom Additives on Photocatalytic Activity of the CuOx-Dark TiO2 System in HER
Abstract
1. Introduction
2. Experimental Part
2.1. Sample Preparation
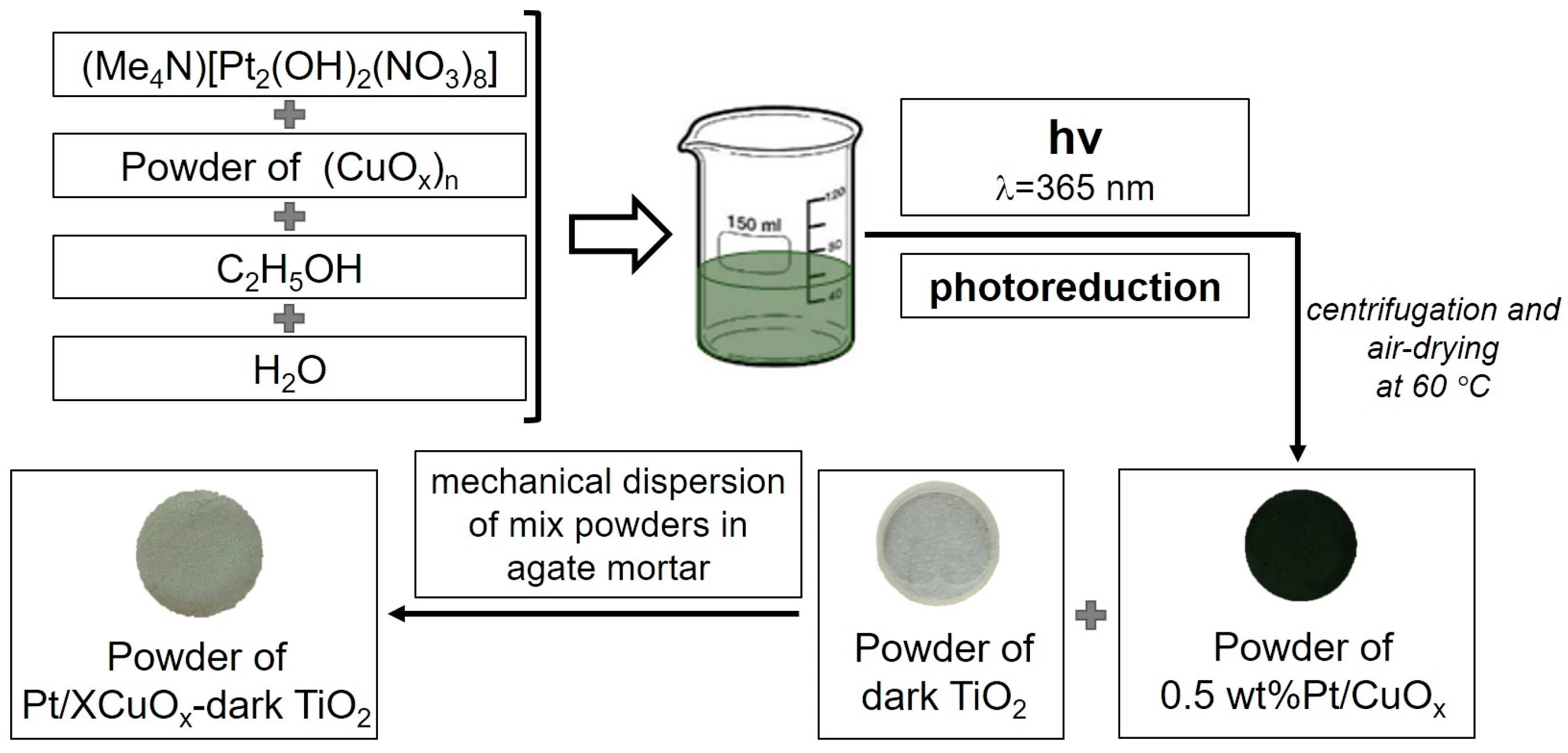
2.1.1. Preparation of Dark TiO2, CuOx, and Pt/CuOx Nanopowders
2.1.2. Preparation of XCuOx-Dark TiO2 and Pt/XCuOx-Dark TiO2 Catalysts
2.2. Sample Characterization Methods
2.3. Photocatalytic Activity Studies
3. Results and Discussions
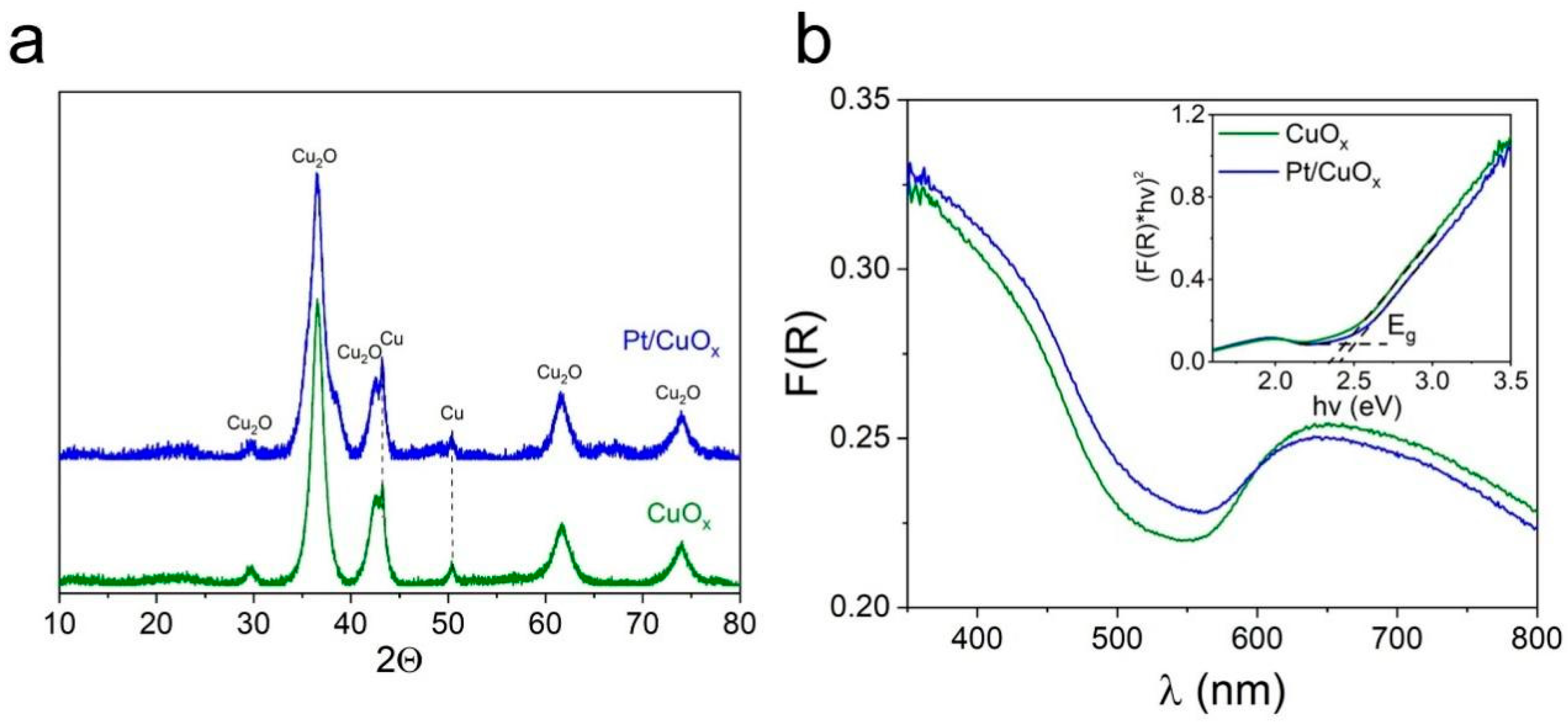
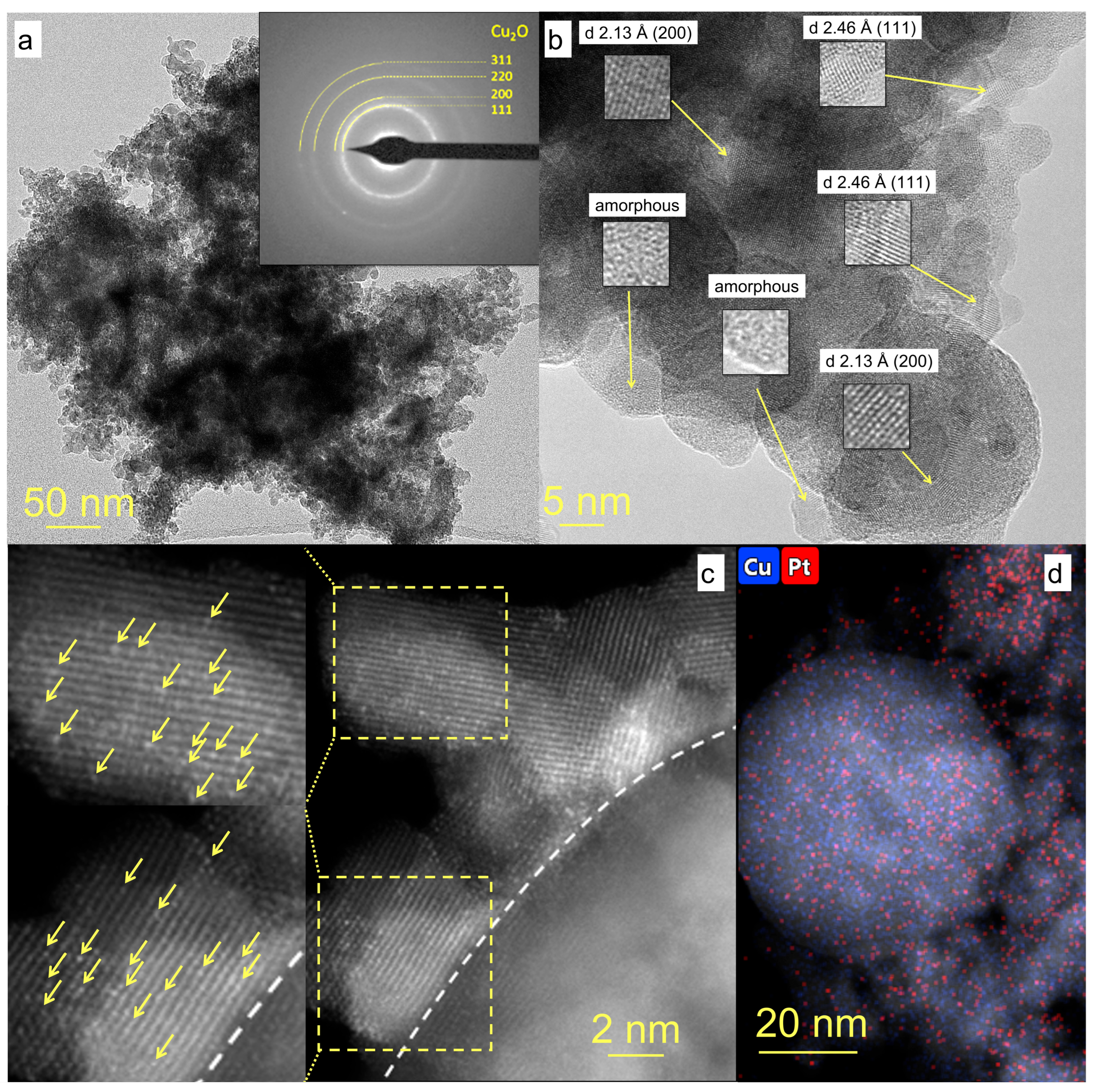
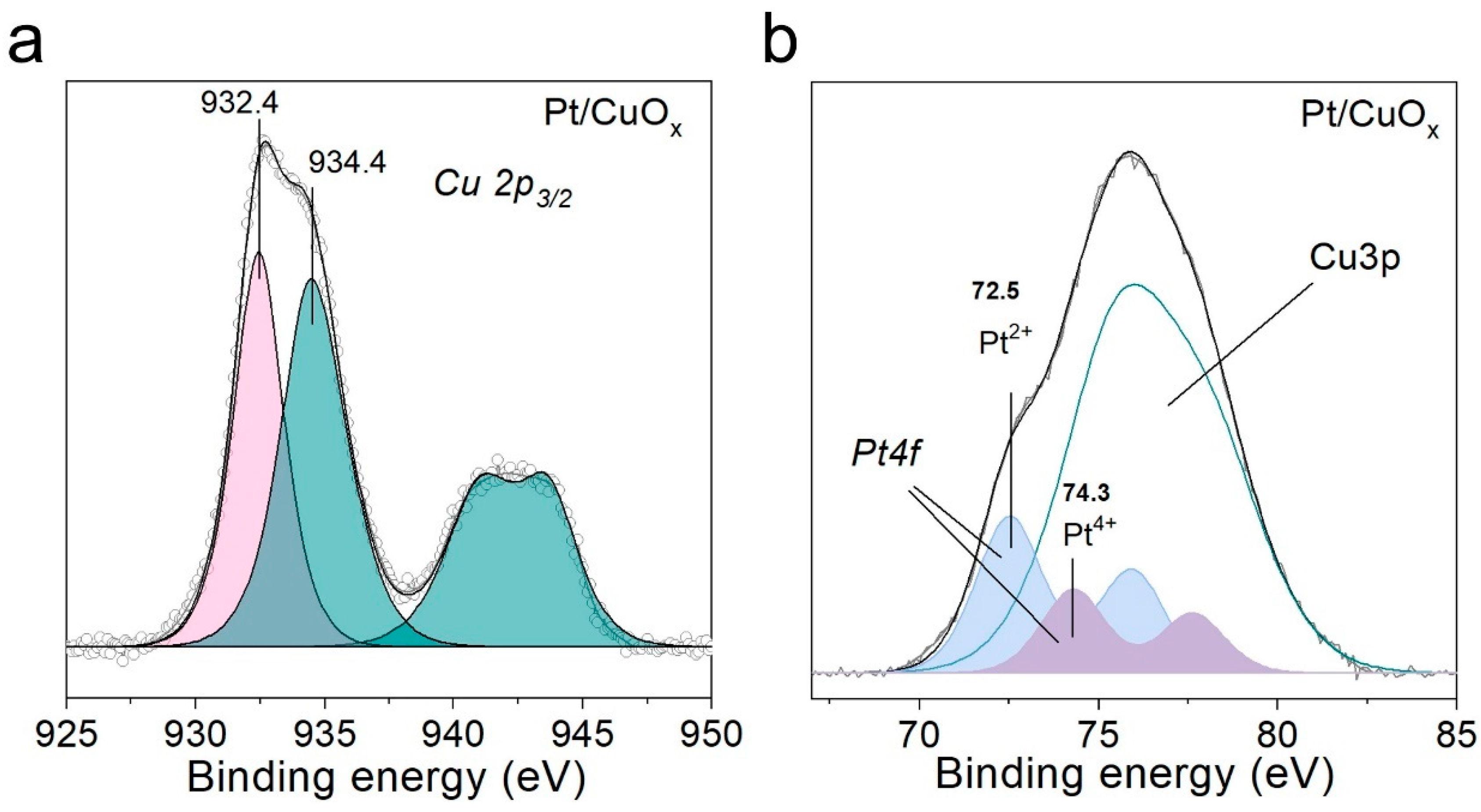
3.1. Pt/CuOx Characterization
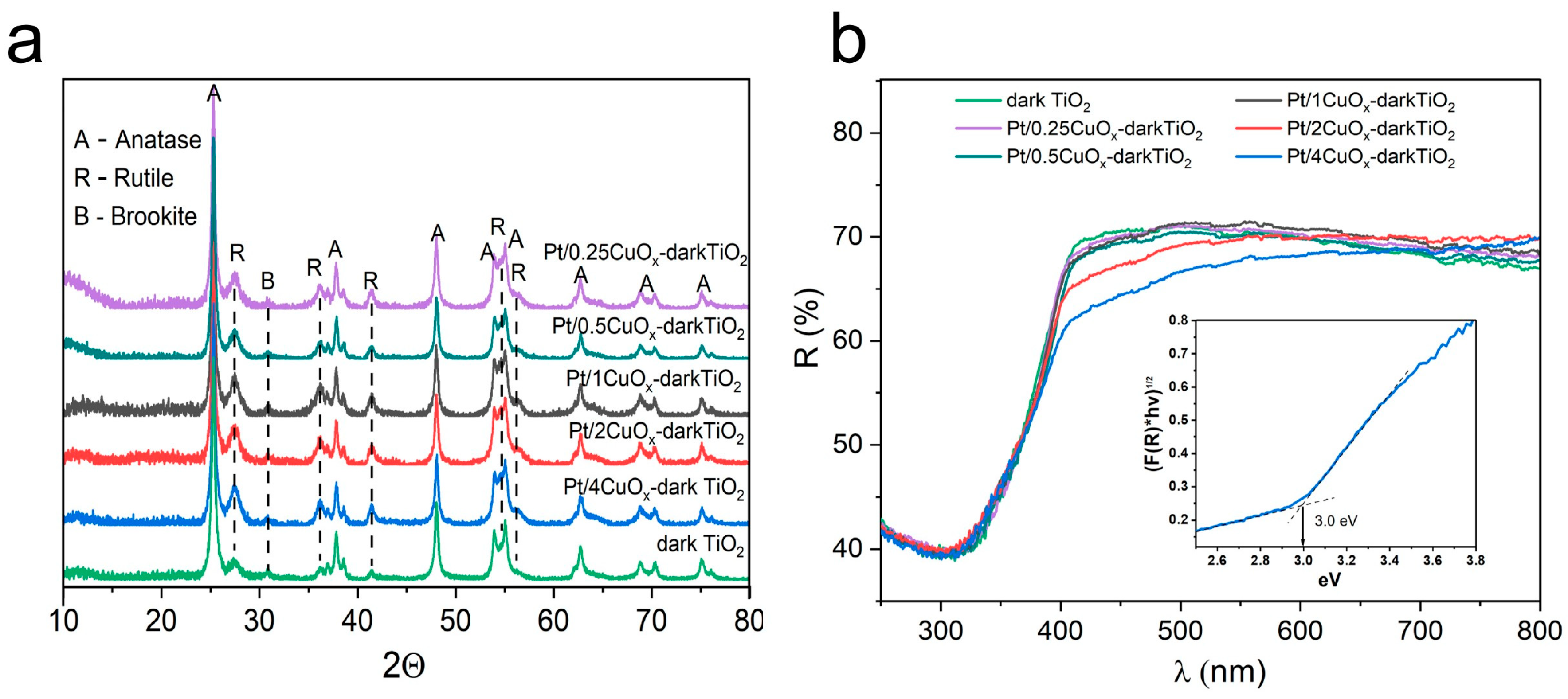
3.2. Pt/XCuOx-Dark TiO2 Characterization
3.3. Studies of Photocatalyst Activity in HER
- (i).
- The distribution of low Pt concentrations in the form of SAs on the CuOx surface, achieved during photoreduction, does not interfere with the self-dispersion of copper oxide NPs on the dark TiO2 surface and their uniform distribution in the form of subnanometer Pt/CuOx clusters during the subsequent synthesis of Pt/XCuOx-dark TiO2 photocatalysts.
- (ii).
- A small amount of SA Pt on the CuOx surface does not significantly affect the interaction of CuOx clusters with oxygen vacancies/Ti3+ ions in titania and does not hinder the reduction of surface Cu(II) to Cu(I). Therefore, the SA Pt also does not hinder the efficient transfer of electrons between CuOx and dark TiO2 in Pt/XCuOx-dark TiO2 samples.
- (iii).
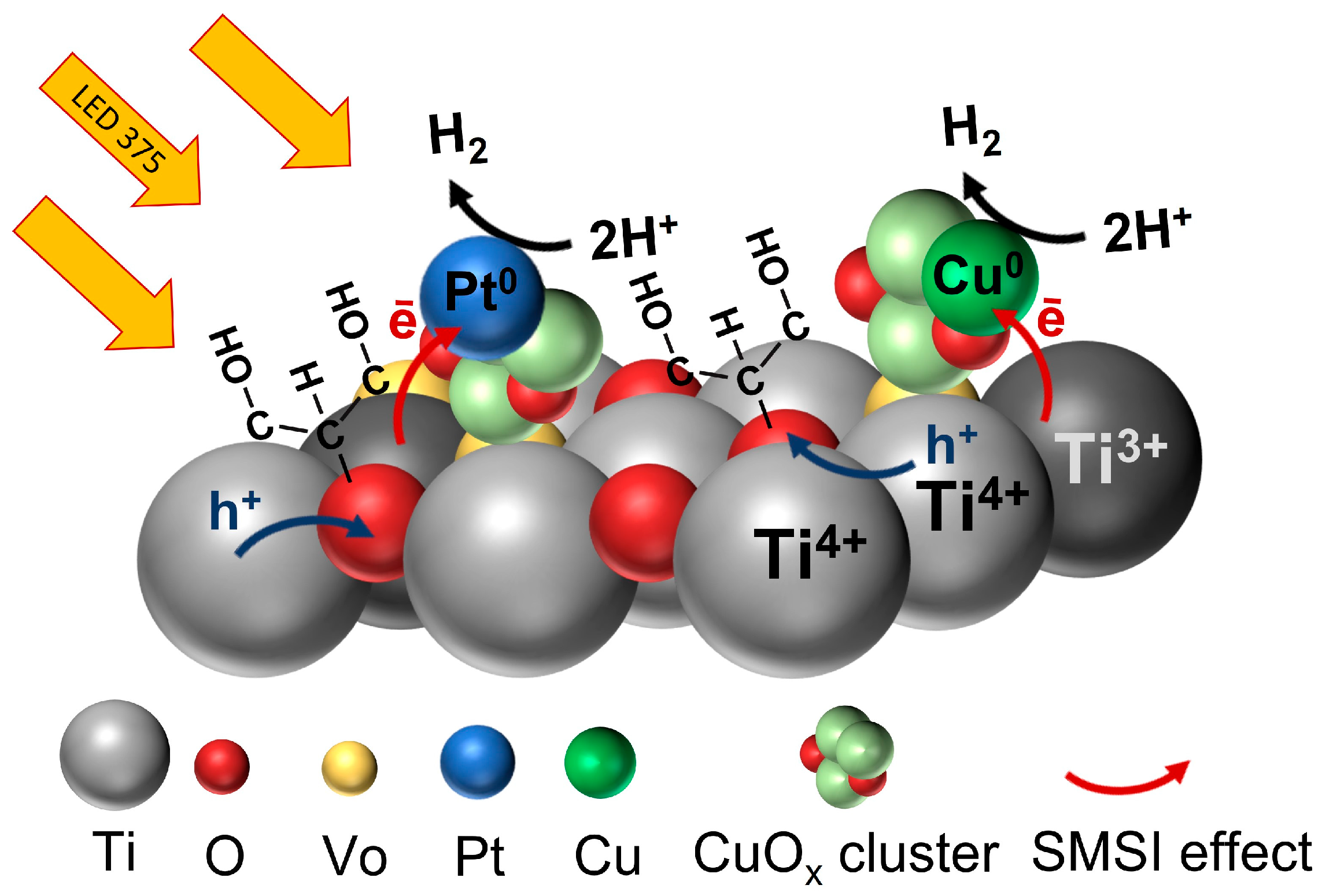
- Since, in this work, platinum was pre-deposited onto CuOx particles, in our Pt/XCuOx-dark TiO2 samples, in addition to short-lived Cu0-CuOx-Ov-dark TiO2 active centers, short-lived Pt0-CuOx-Ov-dark TiO2 active centers were also formed as a result of photoirradiation. In such centers, due to the SMSI effect, efficient electron transfer to Pt or Cu occurs, which leads to better spatial separation of charges. This is confirmed by photocurrent studies, which showed that the number of photo-generated charges increases in the series dark TiO2–CuOx-dark-TiO2–Pt/CuOx-dark-TiO2 (see Figure S4). The method for determining photocurrent is described in detail in our previously published works [42,58]. It can be assumed that during irradiation, the photogenerated electron e− is transferred from dark TiO2 to a CuOx cluster and/or SA Pt with the formation of short-lived states Cu0-CuOx-Ov-dark TiO2 and Pt0-CuOx-Ov-dark TiO2. Then the e− is transferred to a proton H+ with the formation of a H2 molecule. After irradiation termination, electron transfer and formation of short-lived states Cu0 and Pt0 cease (see Figure 7).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Tian, Y.; Gu, H.; Jiang, B. Photocatalytic hydrogen evolution: Recent advances in materials, modifications, and photothermal synergy. Int. J. Hydrogen Energy 2025, 115, 113–130. [Google Scholar] [CrossRef]
- Gupta, A.; Likozar, B.; Jana, R.; Chanu, W.C.; Singh, M.K. A review of hydrogen production processes by photocatalytic water splitting—From atomistic catalysis design to optimal reactor engineering. Int. J. Hydrogen Energy 2022, 47, 33282–33307. [Google Scholar] [CrossRef]
- Xu, F.; Weng, B. Photocatalytic hydrogen production: An overview of new advances in structural tuning strategies. J. Mater. Chem. A 2023, 11, 4473–4486. [Google Scholar] [CrossRef]
- Davis, K.A.; Yoo, S.; Shuler, E.W.; Sherman, B.D.; Lee, S.; Leem, G. Photocatalytic hydrogen evolution from biomass conversion. Nano Converg. 2021, 8, 6. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Sain, M.; Chen, Z. Dissimilar dimensional materials based tailored heterostructures for photocatalytic hydrogen production. Renew. Sustain. Energy Rev. 2023, 181, 113348. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Alevato, V.; Streater, D.; Premtaj, C.; Huang, J.; Brock, S.L. CdS quantum dot aerogels for photocatalytic hydrogen evolution. Nano Res. 2024, 17, 10292–10301. [Google Scholar] [CrossRef]
- Wang, S.; Liao, W.; Su, H.; Pang, S.; Yang, C.; Fu, Y.; Zhang, Y. Review on the Application of Semiconductor Heterostructures in Photocatalytic Hydrogen Evolution: State-of-the-Art and Outlook. Energy Fuels 2023, 37, 1633–1656. [Google Scholar] [CrossRef]
- Bilal, M.; Wang, L.; Rehman, Z.U.; Zheng, K.; Hou, J.; Butt, F.K.; Hussain, A.; Ahmad, J.; Ullah, S.; Jrar, J.A.; et al. Hydrogen production using g-C3N4 based photocatalysts: A review Hydrogen production using g-C3N4 based photocatalysts: A review. Int. J. Hydrogen Energy 2024, 96, 456–473. [Google Scholar] [CrossRef]
- Yan, Y.; Meng, Q.; Tian, L.; Cai, Y.; Zhang, Y.; Chen, Y. Engineering of g-C3N4 for Photocatalytic Hydrogen Production: A Review. Int. J. Mol. Sci. 2024, 25, 8842. [Google Scholar] [CrossRef] [PubMed]
- Kharina, S.; Kurenkova, A.; Aydakov, E.; Mishchenko, D.; Gerasimov, E.; Saraev, A.; Zhurenok, A.; Lomakina, V.; Kozlova, E. Activation of g-C3N4 by oxidative treatment for enhanced photocatalytic H2 evolution. Appl. Surf. Sci. 2025, 698, 163074. [Google Scholar] [CrossRef]
- Zhurenok, A.V.; Vasichenko, D.B.; Berdyugin, S.N.; Gerasimov, E.Y.; Saraev, A.A.; Cherepanova, S.V.; Kozlova, E.A. Photocatalysts Based on Graphite-like Carbon Nitride with a Low Content of Rhodium and Palladium for Hydrogen Production under Visible Light. Nanomaterials 2023, 13, 2176. [Google Scholar] [CrossRef]
- Do, H.H.; Nguyen, H.C.; Nguyen, T.V.; Xia, C.; Nguyen, D.L.T.; Raizada, P.; Singh, P.; Nguyen, V.-H.; Ahn, S.H.; Kim, S.Y.; et al. Metal-organic-framework based catalyst for hydrogen production: Progress and perspectives. Int. J. Hydrogen Energy 2022, 47, 37552–37568. [Google Scholar] [CrossRef]
- Musa, E.N.; Yadav, A.K.; Smith, K.T.; Jung, M.S.; Stickle, W.F.; Eschbach, P.; Ji, X.; Stylianou, K.C. Boosting Photocatalytic Hydrogen Production by MOF-Derived Metal Oxide Heterojunctions with a 10.0% Apparent Quantum Yield. Angew. Chem. Int. Ed. 2024, 63, 202405681. [Google Scholar] [CrossRef]
- Tian, L.; Guan, X.; Zong, S.; Dai, A.; Qu, J. Cocatalysts for Photocatalytic Overall Water Splitting: A Mini Review. Catalysts 2023, 13, 355. [Google Scholar] [CrossRef]
- Tuntithavornwat, S.; Saisawang, C.; Ratvijitvech, T.; Watthanaphanit, A.; Hunsom, M.; Kannan, A.M. Recent development of black TiO2 nanoparticles for photocatalytic H2 production: An extensive review. Int. J. Hydrogen Energy 2024, 55, 1559–1593. [Google Scholar] [CrossRef]
- Khan, A.; Le Pivert, M.; Ranjbari, A.; Dragoe, D.; Bahena-Uribe, D.; Colbeau-Justin, C.; Herrero, C.; Rutkowska-Zbik, D.; Deschamps, J.; Remita, H. Cu-Based MOF/TiO2 composite Nanomaterials for Photocatalytic Hydrogen Generation and the Role of Copper. Adv. Funct. Mater. 2025, 2501736. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, X.; Zhang, Y.; Gao, J.; Chen, Z.; Lu, Z. Synergism of oxygen vacancies, Ti3+ and N dopants on the visible-light photocatalytic activity of N-doped TiO2. J. Photochem. Photobiol. A 2019, 382, 111928. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.-T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The roles of metal co-catalysts and reaction media in photocatalytic hydrogen production: Performance evaluation of M/TiO2 photocatalysts (M = Pd, Pt, Au) in different alcohol–water mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Dessal, C.; Martínez, L.; Maheu, C.; Len, T.; Morfin, F.; Rousset, J.L.; Puzenat, E.; Afanasiev, P.; Aouine, M.; Soler, L.; et al. Influence of Pt particle size and reaction phase on the photocatalytic performances of ultradispersed Pt/TiO2 catalysts for hydrogen evolution. J. Catal. 2019, 375, 155–163. [Google Scholar] [CrossRef]
- Chen, Y.; Soler, L.; Armengol-Profitos, M.; Xie, C.; Crespo, D.; Llorca, J. Enhanced photoproduction of hydrogen on Pd/TiO2 prepared by mechanochemistry. Appl. Catal. B Environ. 2022, 309, 121275. [Google Scholar] [CrossRef]
- Kerketta, U.; Tesler, A.B.; Schmuki, P. Single-Atom Co-Catalysts Employed in Titanium Dioxide Photocatalysis. Catalysts 2022, 12, 1223. [Google Scholar] [CrossRef]
- Tang, P.; Lee, H.J.; Hurlbutt, K.; Huang, P.-Y.; Narayanan, S.; Wang, C.; Gianolio, D.; Arrigo, R.; Chen, J.; Warner, J.H.; et al. Elucidating the Formation and Structural Evolution of Platinum Single-Site Catalysts for the Hydrogen Evolution Reaction. ACS Catal. 2022, 12, 3173–3180. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Zhang, C.; Chen, X.; Liu, C.; Weng, W.; Shan, W.; He, H. A simple strategy to improve Pd dispersion and enhance Pd/TiO2 catalytic activity for formaldehyde oxidation: The roles of surface defects. Appl. Catal. B Environ. 2021, 282, 119540. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, G.; Du, H.; Xiao, X.; Yang, X. Pt single-atom-layer ensembles on TiO2 for direct dehydrogenation of methanol to hydrogen and methyl formate. J. Chem. Eng. 2024, 501, 157836. [Google Scholar] [CrossRef]
- Han, B.; Guo, Y.; Huang, Y.; Xi, W.; Xu, J.; Luo, J.; Qi, H.; Ren, Y.; Liu, X.; Qiao, B.; et al. Strong Metal–Support Interactions between Pt Single Atoms and TiO2. Angew. Chem. Int. Ed. 2020, 59, 11824–11829. [Google Scholar] [CrossRef]
- Wu, X.; Sun, S.; Wang, R.; Xiao, X.; Yang, X. Pt single atoms and defect engineering of TiO2-nanosheet-assembled hierarchical spheres for efficient room-temperature HCHO oxidation. J. Hazard. Mater. 2023, 454, 131434. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Chen, X.; Jin, J.; Yan, X.; Huang, J. Single-Atom Ni Supported on TiO2 for Catalyzing Hydrogen Storagein MgH2. J. Am. Chem. Soc. 2024, 146, 10432–10442. [Google Scholar] [CrossRef]
- Kim, J.; Her, S.-H.; Kim, D.-S.; Kim, B.; Jung, S.-M.; Kim, J.; Hwang, H.; Kim, K.-S.; Kim, Y.-T.; Choi, S.-Y.; et al. Stabilized Co Single-Atom Catalyst via Ion Implantation for Efficient Hydrogen Production. Small 2025, 21, 2505383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Wang, H.; Xiao, B.; Zhang, W.; Zhao, X.; Lv, T.; Thangamuthu, M.; Zhang, J.; Guo, Y.; et al. Single-atom Cu anchored catalysts for photocatalytic renewable H2 production with a quantum efficiency of 56%. Nat. Commun. 2022, 13, 58. [Google Scholar] [CrossRef]
- Hejazi, S.; Mohajernia, S.; Wu, Y.; Andryskova, P.; Zoppellaro, G.; Hwang, I.; Tomanec, O.; Zboril, R.; Schmuki, P. Intrinsic Cu nanoparticle decoration of TiO2 nanotubes: A platform for efficient noble metal free photocatalytic H2 production. Electrochem. Commun. 2019, 98, 82–86. [Google Scholar] [CrossRef]
- Guo, S.; Ji, Y.; Li, Y.; Li, H.; An, P.; Zhang, J.; Yan, J.; Liu, S.F.; Ma, T. Amorphous quantum dots co-catalyst: Defect level induced solar-to-hydrogen production. Appl. Catal. B Environ. 2023, 330, 122583. [Google Scholar] [CrossRef]
- Reutova, O.A.; Fakhrutdinova, E.D.; Goncharova, D.A.; Stadnichenko, A.I.; Stonkus, O.A.; Kharlamova, T.S.; Svetlichnyi, V.A.; Vodyankina, O.V. Strong Metal–Support Interactions in Cu(I)-Dark TiO2 Nanoscale Photocatalysts Prepared by Pulsed Laser Ablation for Hydrogen Evolution Reaction. ACS Appl. Nano Mater. 2024, 7, 17062–17073. [Google Scholar] [CrossRef]
- Reutova, O.A.; Fakhrutdinova, E.D.; Stonkus, O.A.; Kibis, L.S.; Vodyankina, O.V.; Svetlichnyi, V.A. Self-dispersing of (CuOx)n species on dark TiO2 surface as a key to high-performance HER photocatalysts. ChemCatChem 2025, 17, e00594. [Google Scholar] [CrossRef]
- Bernareggi, M.; Dozzi, M.V.; Bettini, L.G.; Ferretti, A.M.; Chiarello, G.L.; Selli, E. Flame-Made Cu/TiO2 and Cu-Pt/TiO2 Photocatalysts for Hydrogen Production. Catalysts 2017, 7, 301. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Chiarello, G.L.; Pedroni, M.; Livraghi, S.; Giamello, E.; Selli, E. High photocatalytic hydrogen production on Cu(II) pre-grafted Pt/TiO2. Appl. Catal. B Environ. 2017, 209, 417–428. [Google Scholar] [CrossRef]
- Wang, H.; Qi, H.; Sun, X.; Jia, S.; Li, X.; Miao, T.J.; Xiong, L.; Wang, S.; Zhang, X.; Liu, X.; et al. High quantum efficiency of hydrogen production from methanol aqueous solution with PtCu–TiO2 photocatalysts. Nat. Mater. 2023, 22, 619–626. [Google Scholar] [CrossRef]
- Jung, M.; Hart, J.N.; Boensch, D.; Scott, J.; Ng, Y.H.; Amal, R. Hydrogen evolution via glycerol photoreforming over Cu–Ptnanoalloys on TiO2. Appl. Catal. A Gen. 2016, 518, 221–230. [Google Scholar] [CrossRef]
- Bernareggia, M.; Chiarello, G.L.; West, G.; Ratova, M.; Ferretti, A.M.; Kelly, P.; Selli, E. Cu and Pt clusters deposition on TiO2 powders by DC magnetron sputtering for photocatalytic hydrogen production. Cat. Today 2019, 326, 15–21. [Google Scholar] [CrossRef]
- Mao, B.; Zhu, Y.; Huang, D.; Zhang, J.; Liu, Q.; Li, X.; Song, K. Stepwise Reduction and In Situ Loading of Core-Shelled Pt@Cu Nanocrystals on TiO2–NTs for Highly Active Hydrogen Evolution. Adv. Mater. Interfaces 2023, 10, 2201891. [Google Scholar] [CrossRef]
- Fakhrutdinova, E.D.; Shabalina, A.V.; Gerasimova, M.A.; Nemoykina, A.L.; Vodyankina, O.V.; Svetlichnyi, V.A. Highly Defective Dark Nano Titanium Dioxide: Preparation via Pulsed Laser Ablation and Application. Materials 2020, 13, 2054. [Google Scholar] [CrossRef]
- Fakhrutdinova, E.; Reutova, O.; Maliy, L.; Kharlamova, T.; Vodyankina, O.; Svetlichnyi, V. Laser-based Synthesis of TiO2-Pt Photocatalysts for hydrogen generation. Materials 2022, 15, 7413. [Google Scholar] [CrossRef]
- Fakhrutdinova, E.D.; Zinina, E.V.; Bugrova, T.A.; Reutova, O.A.; Svetlichnyi, V.A.; Vodyankina, O.V. Unraveling the mechanism of hydrogen evolution on dark TiO2 obtained by pulsed laser ablation. Kinet. Catal. 2024, 65, 737–744. [Google Scholar] [CrossRef]
- Fakhrutdinova, E.D.; Reutova, O.A.; Bugrova, T.A.; Kibis, L.S.; Stonkus, O.A.; Vasilchenko, D.B.; Vodyankina, O.V.; Svetlychnyi, V.A. Highly Defective Dark TiO2 Modified with Pt: The Effect of Precursor Nature and Preparation Method on Photocatalytic Properties. Trans. Tianjin Univ. 2024, 30, 198–209. [Google Scholar] [CrossRef]
- Goncharova, D.A.; Kharlamova, T.S.; Lapin, I.N.; Svetlichnyi, V.A. Chemical and Morphological Evolution of Copper NPs Obtained by Pulsed Laser Ablation in Liquid. J. Phys. Chem. C 2019, 123, 21731–21742. [Google Scholar] [CrossRef]
- Goncharova, D.A.; Kharlamova, T.S.; Reutova, O.A.; Svetlichnyi, V.A. Water–ethanol CuOx nanoparticle colloids prepared by laser ablation: Colloid stability and catalytic properties in nitrophenol hydrogenation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126115. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Topchiyan, P.; Berdyugin, S.; Filatov, E.; Tkachev, S.; Baidina, I.; Komarov, V.; Slavinskaya, E.; Stadnichenko, A.; Gerasimov, E. Tetraalkylammonium salts of platinum nitrato complexes: Isolation, structure, and relevance to the preparation of PtOx/CeO2 catalysts for low-temperature CO oxidation. Inorg. Chem. 2019, 58, 6075. [Google Scholar] [CrossRef]
- Meyer, B.K.; Polity, A.; Reppin, D.; Becker, M.; Hering, P.; Klar, P.J.; Sander, T.; Reindl, C.; Benz, J.; Eickhoff, M.; et al. Binary copper oxide semiconductors: From materials towards devices. Phys. Status Solidi B 2019, 249, 1487–1509. [Google Scholar] [CrossRef]
- Heinemann, M.; Eifert, B.; Heiliger, C. Band structure and phase stability of the copper oxides Cu2O, CuO, and Cu4O3. Phys. Rev. B 2013, 87, 115111. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, Z.P.; Gerasimova, M.A.; Fakhrutdinova, E.D.; Svetlichnyi, V.A. Effect of laser and temperature treatment on the optical properties of titanium dioxide nanoparticles prepared via pulse laser ablation. Rus. Phys. J. 2022, 64, 2115–2122. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, Z.; Du, L.; Yu, Y.; Huang, W.; Shi, Q. Mesoporous black TiO2 hollow shells with controlled cavity size for enhanced visible light photocatalysis. Opt. Express. 2023, 31, 33883–33897. [Google Scholar] [CrossRef]
- Lau, K.; Niemann, F.; Abdiaziz, K.; Heidelmann, M.; Yang, Y.; Tong, Y.; Fechtelkord, M.; Schmidt, T.C.; Schnegg, A.; Campen, R.K.; et al. Differentiating between Acidic and Basic Surface Hydroxyls on Metal Oxides by Fluoride Substitution: A Case Study on Blue TiO2 from Laser Defect Engineering. Angew. Chem. Int. Ed. 2023, 62, e202213968. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Z.; Curto, A.G.; Zeng, Y.-J.; Van Thourhout, D. Plasmonic semiconductors: Materials, tunability and applications. Prog. Mater. Sci. 2023, 138, 101158. [Google Scholar] [CrossRef]
- Dahlman, C.J.; Agrawal, A.; Staller, C.M.; Adair, J.; Milliron, D.J. Anisotropic Origins of Localized Surface Plasmon Resonance in n-Type Anatase TiO2 Nanocrystals. Chem. Mater. 2019, 31, 502–511. [Google Scholar] [CrossRef]
- Xi, H.; Xu, Y.; Zou, W.; Ji, J.; Cai, Y.; Wan, H.; Dong, L. Enhanced methanol selectivity of CuxO/TiO2 photocatalytic CO2 reduction: Synergistic mechanism of surface hydroxyl and low-valence copper species. J. CO2 Util. 2022, 55, 101825. [Google Scholar] [CrossRef]
- Wei, T.; Zhu, Y.N.; An, X.; Liu, L.M.; Cao, X.; Liu, H.; Qu, J. Defect Modulation of Z Scheme TiO2/Cu2O Photocatalysts for Durable Water Splitting. ACS Catal. 2019, 9, 8346–8354. [Google Scholar] [CrossRef]
- Golubovskaya, A.G.; Kharlamova, T.S.; Gavrilenko, E.A.; Fakhrutdinova, E.D.; Vodyankina, O.V.; Kulinich, S.A.; Svetlichnyi, V.A. Photocatalytic decomposition of rhodamine B and selective oxidation of 5-hydroxymethylfurfural by β-Bi2O3/Bi12SiO20 nanocomposites produced by laser. J. Compos. Sci. 2024, 8, 42. [Google Scholar] [CrossRef]

| Sample | Cu (wt.%) | Pt * (wt.%) | HER (mM/gcat) | AQY | HER (Cu + Pt)/ HER (Cu)/ |
|---|---|---|---|---|---|
| dark TiO2 | – | – | 0.12 | 0.02 | – |
| CuOx | – | – | 0 | 0 | – |
| Pt/CuOx | – | 0.5 | 0 | 0 | 0 |
| 0.25CuOx-dark TiO2 | 0.25 | – | 2.23 | 0.38 | – |
| 0.50CuOx-dark TiO2 | 0.50 | – | 2.62 | 0.45 | – |
| 1.00CuOx-dark TiO2 | 1.00 | – | 2.92 | 0.50 | – |
| 2.00CuOx-dark TiO2 | 2.00 | – | 2.40 | 0.41 | – |
| 4.00CuOx-dark TiO2 | 4.00 | – | 1.85 | 0.31 | – |
| Pt/0.25CuOx-dark TiO2 | 0.25 | 0.00125 | 2.94 | 0.50 | 1.32 |
| Pt/0.50CuOx-dark TiO2 | 0.50 | 0.0025 | 3.51 | 0.60 | 1.34 |
| Pt/1.00CuOx-dark TiO2 | 1.00 | 0.005 | 3.84 | 0.65 | 1.32 |
| Pt/2.00CuOx-dark TiO2 | 2.00 | 0.01 | 2.88 | 0.54 | 1.20 |
| Pt/4.00CuOx-dark TiO2 | 4.00 | 0.02 | 1.87 | 0.31 | 1.01 |
| 0.0025Pt/dark TiO2 | – | 0.0025 | 0.66 | 0.11 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fakhrutdinova, E.D.; Gorbina, O.A.; Vodyankina, O.V.; Kulinich, S.A.; Svetlichnyi, V.A. Effect of Ultra-Small Platinum Single-Atom Additives on Photocatalytic Activity of the CuOx-Dark TiO2 System in HER. Nanomaterials 2025, 15, 1378. https://doi.org/10.3390/nano15171378
Fakhrutdinova ED, Gorbina OA, Vodyankina OV, Kulinich SA, Svetlichnyi VA. Effect of Ultra-Small Platinum Single-Atom Additives on Photocatalytic Activity of the CuOx-Dark TiO2 System in HER. Nanomaterials. 2025; 15(17):1378. https://doi.org/10.3390/nano15171378
Chicago/Turabian StyleFakhrutdinova, Elena D., Olesia A. Gorbina, Olga V. Vodyankina, Sergei A. Kulinich, and Valery A. Svetlichnyi. 2025. "Effect of Ultra-Small Platinum Single-Atom Additives on Photocatalytic Activity of the CuOx-Dark TiO2 System in HER" Nanomaterials 15, no. 17: 1378. https://doi.org/10.3390/nano15171378
APA StyleFakhrutdinova, E. D., Gorbina, O. A., Vodyankina, O. V., Kulinich, S. A., & Svetlichnyi, V. A. (2025). Effect of Ultra-Small Platinum Single-Atom Additives on Photocatalytic Activity of the CuOx-Dark TiO2 System in HER. Nanomaterials, 15(17), 1378. https://doi.org/10.3390/nano15171378








