Chrysin-Loaded Micelles Regulate Cell Cycle and Induce Intrinsic and Extrinsic Apoptosis in Ovarian Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
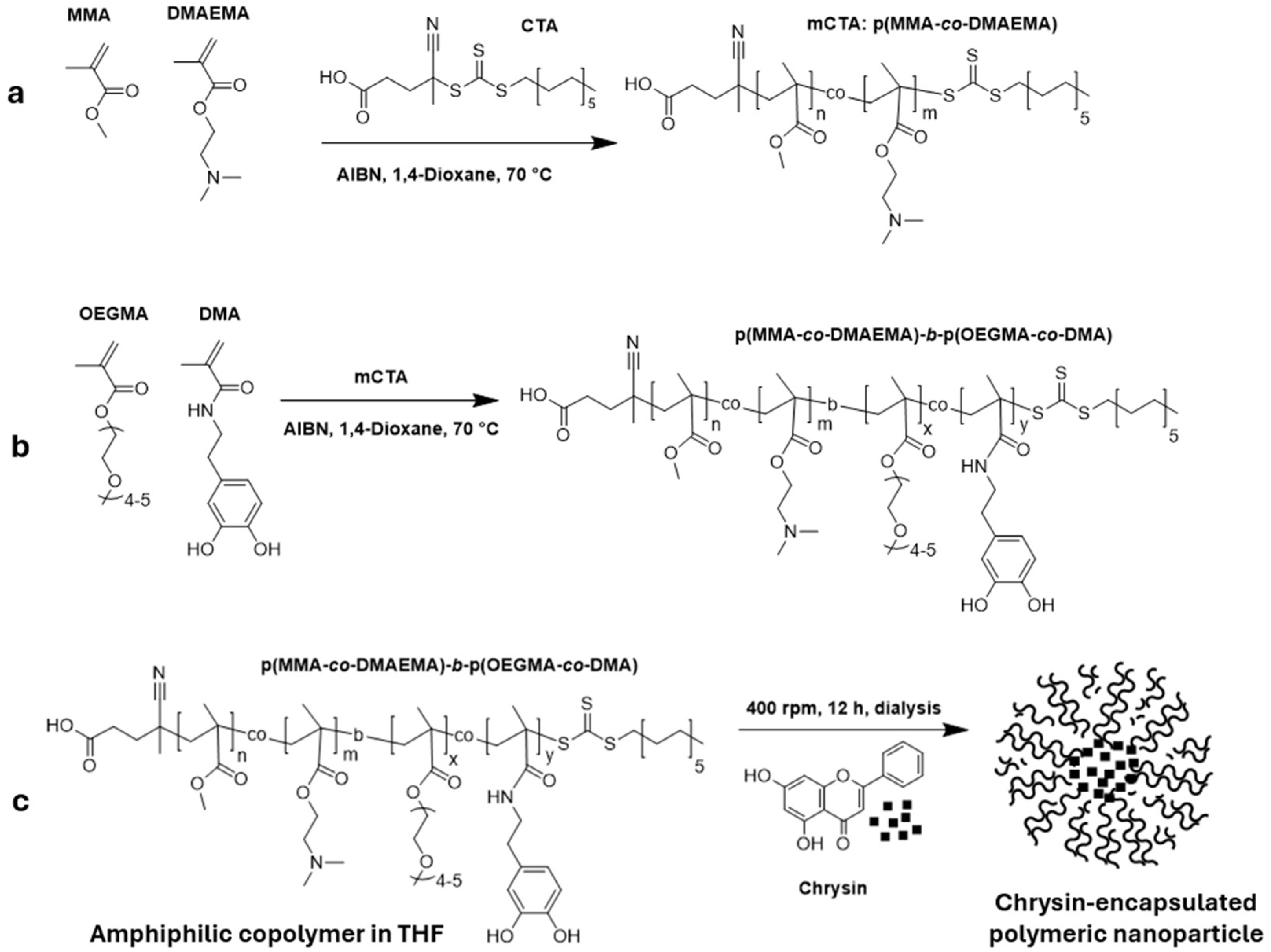
2.1. Synthesis
2.1.1. RAFT Polymerization
2.1.2. Synthesis of DMA Monomer
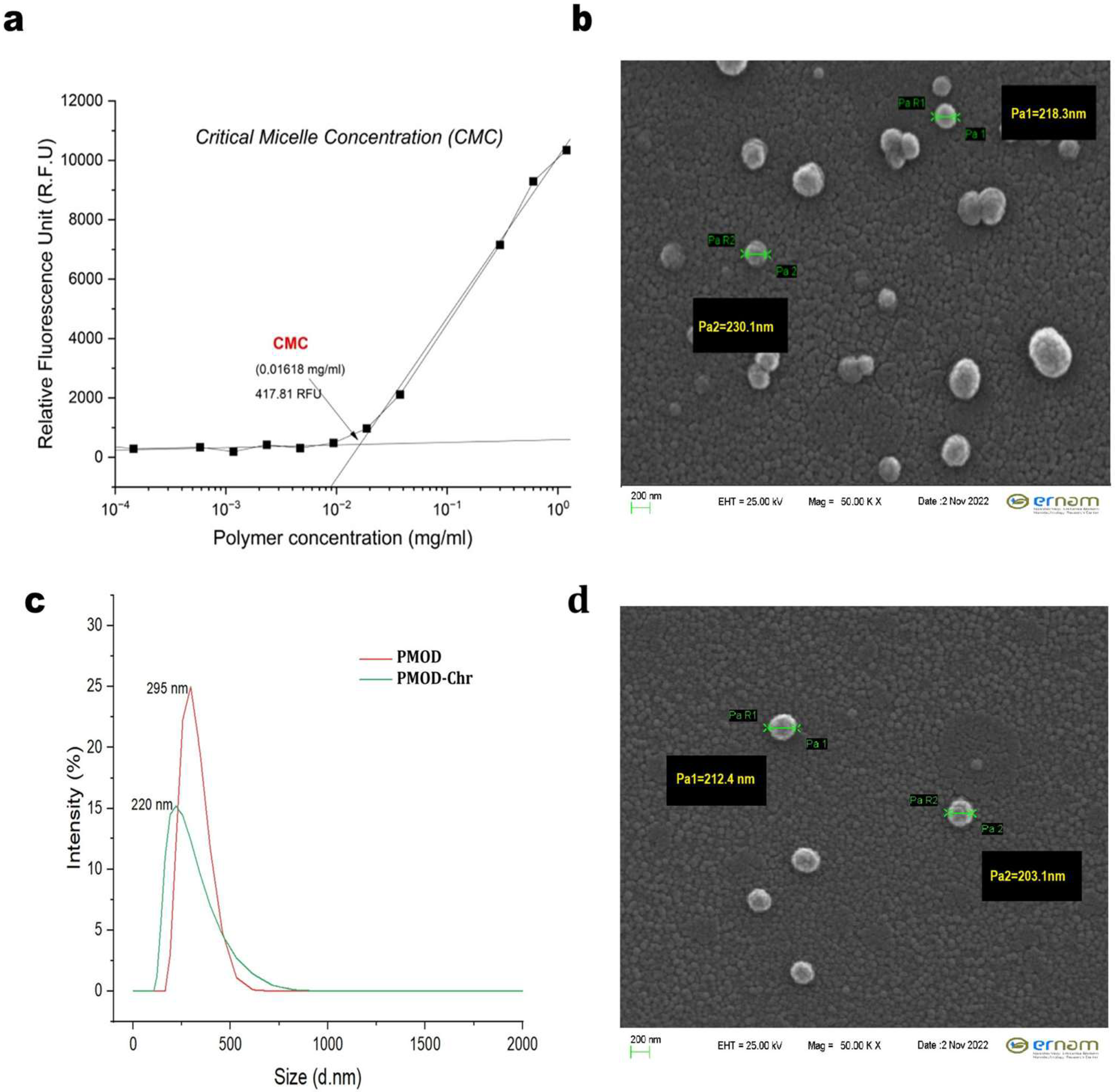
2.2. CMC Evaluation
2.3. Nanoprecipitation of PMOD-Chr
2.4. Cell Culture
2.5. Cell Proliferation Assay
2.6. Colony Formation Assay
2.7. Apoptosis and Cell Cycle Assay
2.8. Gene Expression Analysis
2.9. Statistical Analysis
3. Results
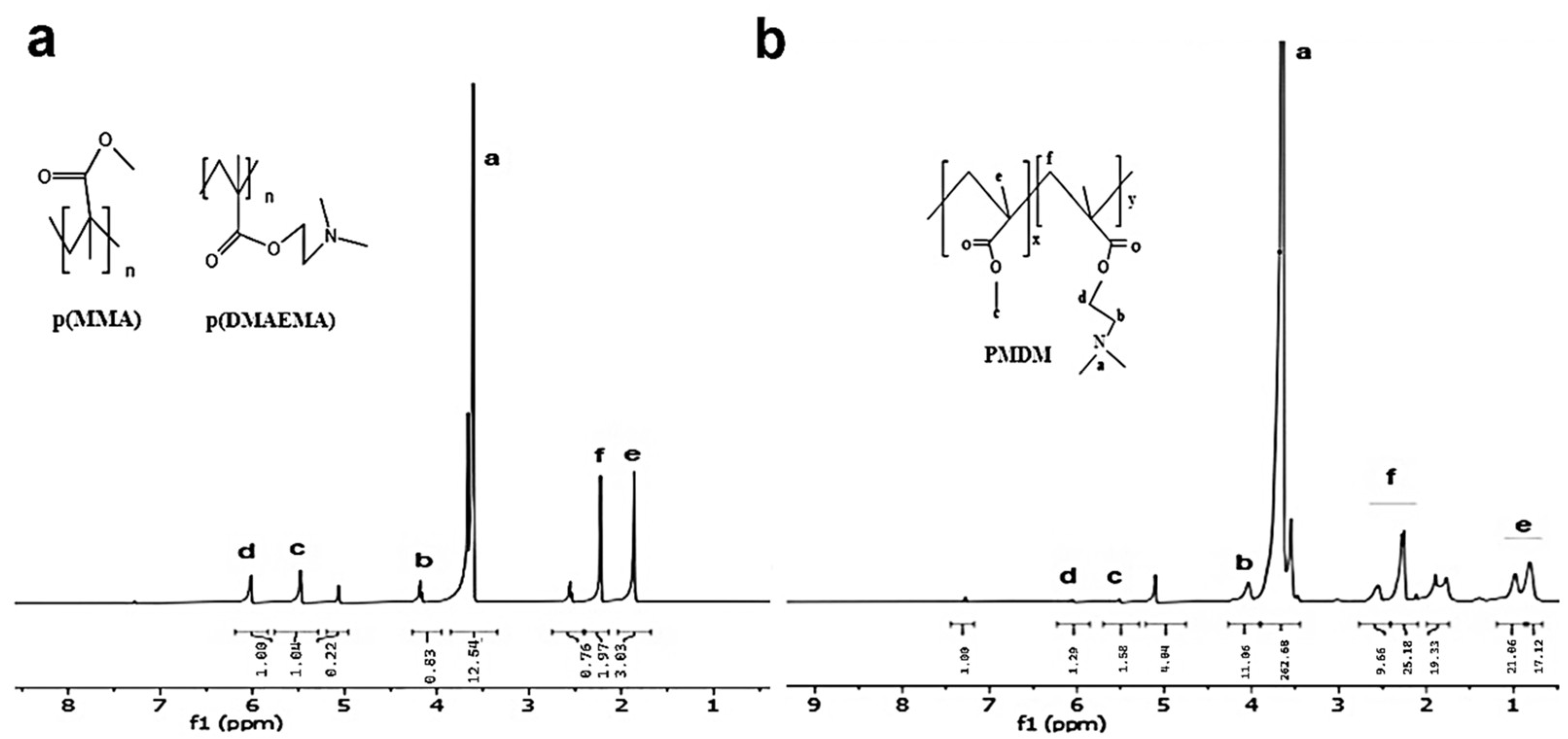
3.1. Characterizations of First Block Copolymers (Ps) as macroCTA Agent (mCTA)
3.2. Characterizations of Second Block Copolymers (BCPs)
3.3. Physicochemical Characterization of PMOD-Chr Nanoparticles
3.4. Determination of EE (%) and DLC (%) of PMOD-Chr
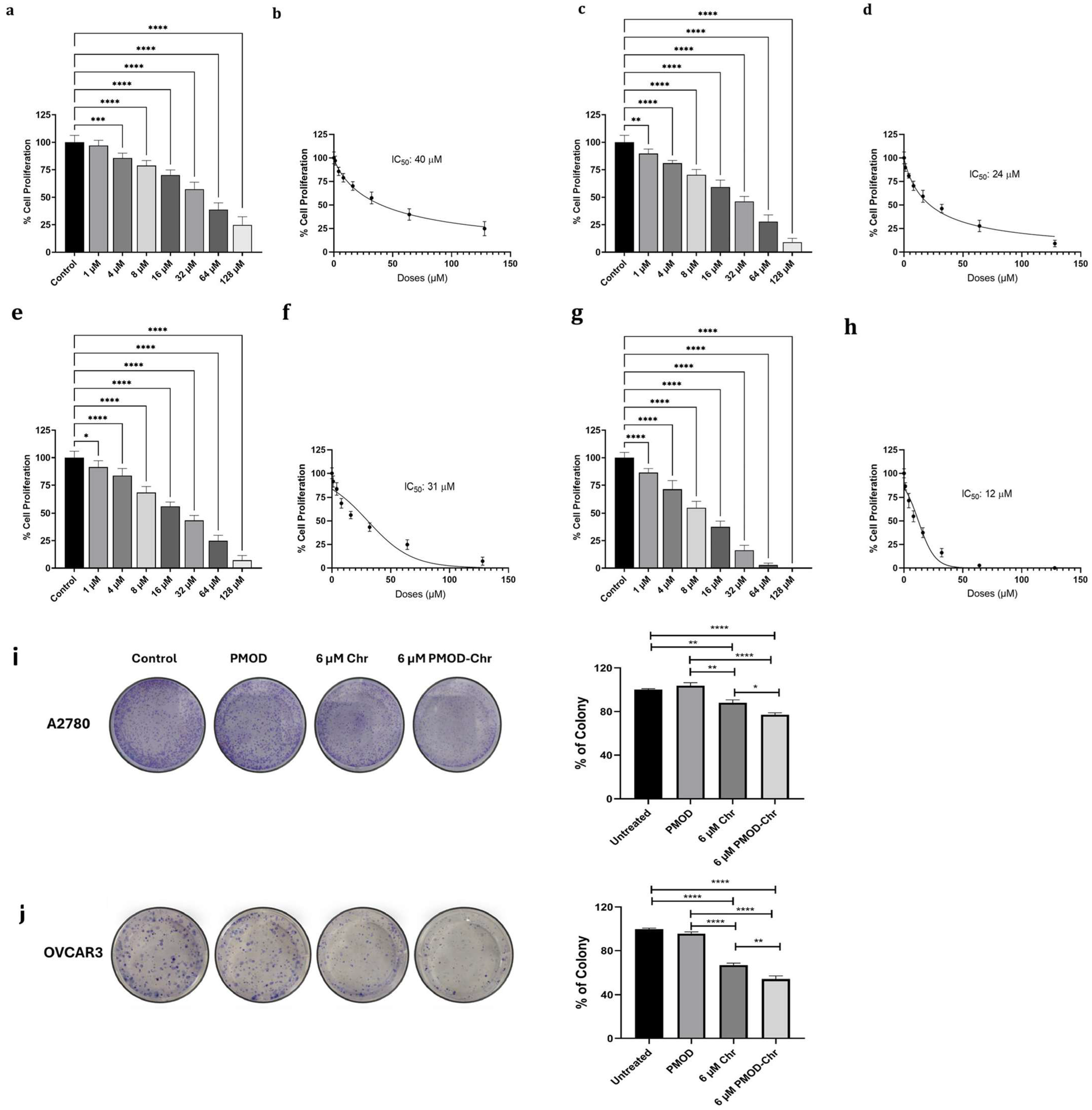
3.5. PMOD-Chr Inhibits Cell Proliferation and Clonogenic Survival in Human Ovarian Cancer Cells
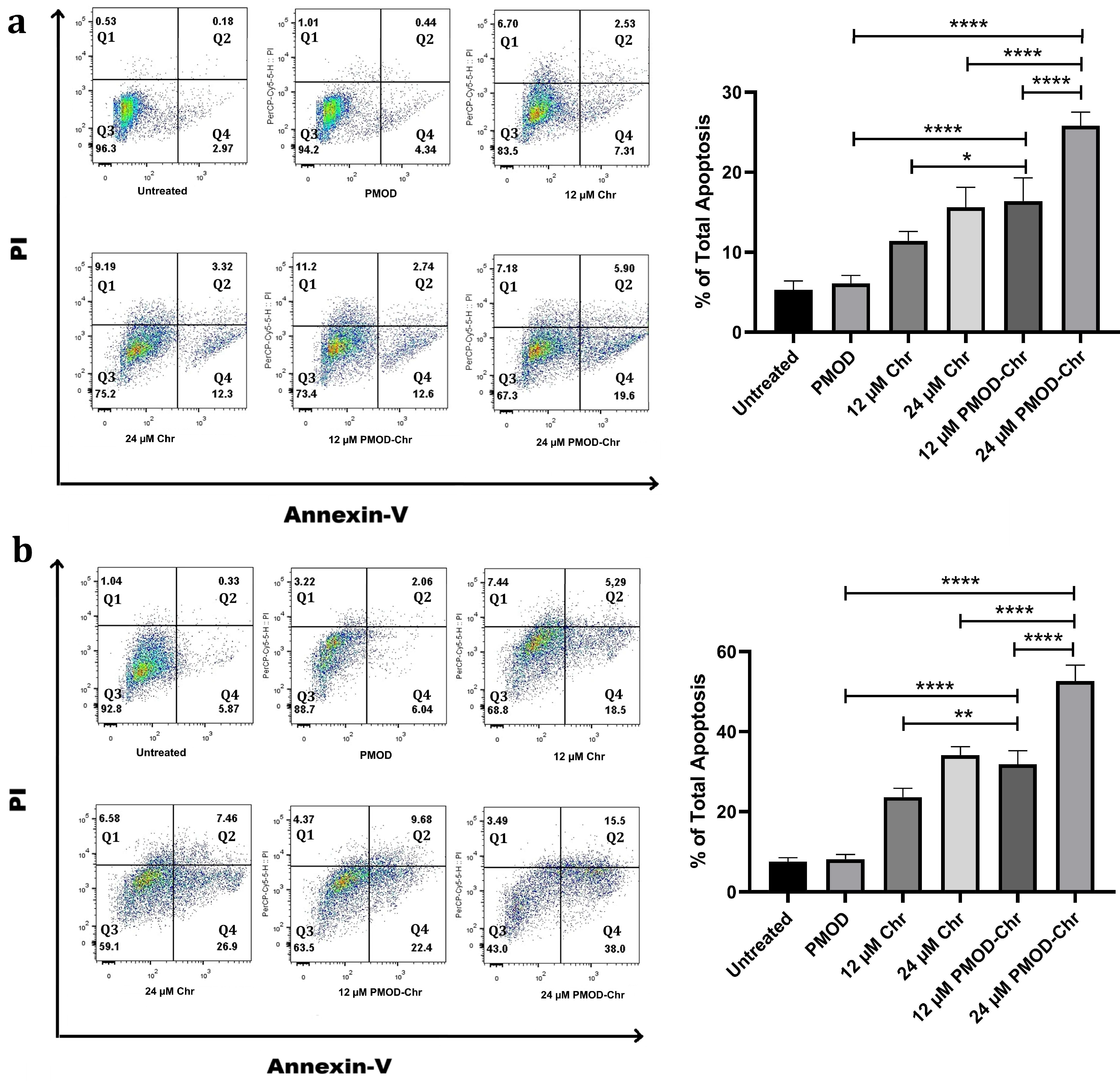
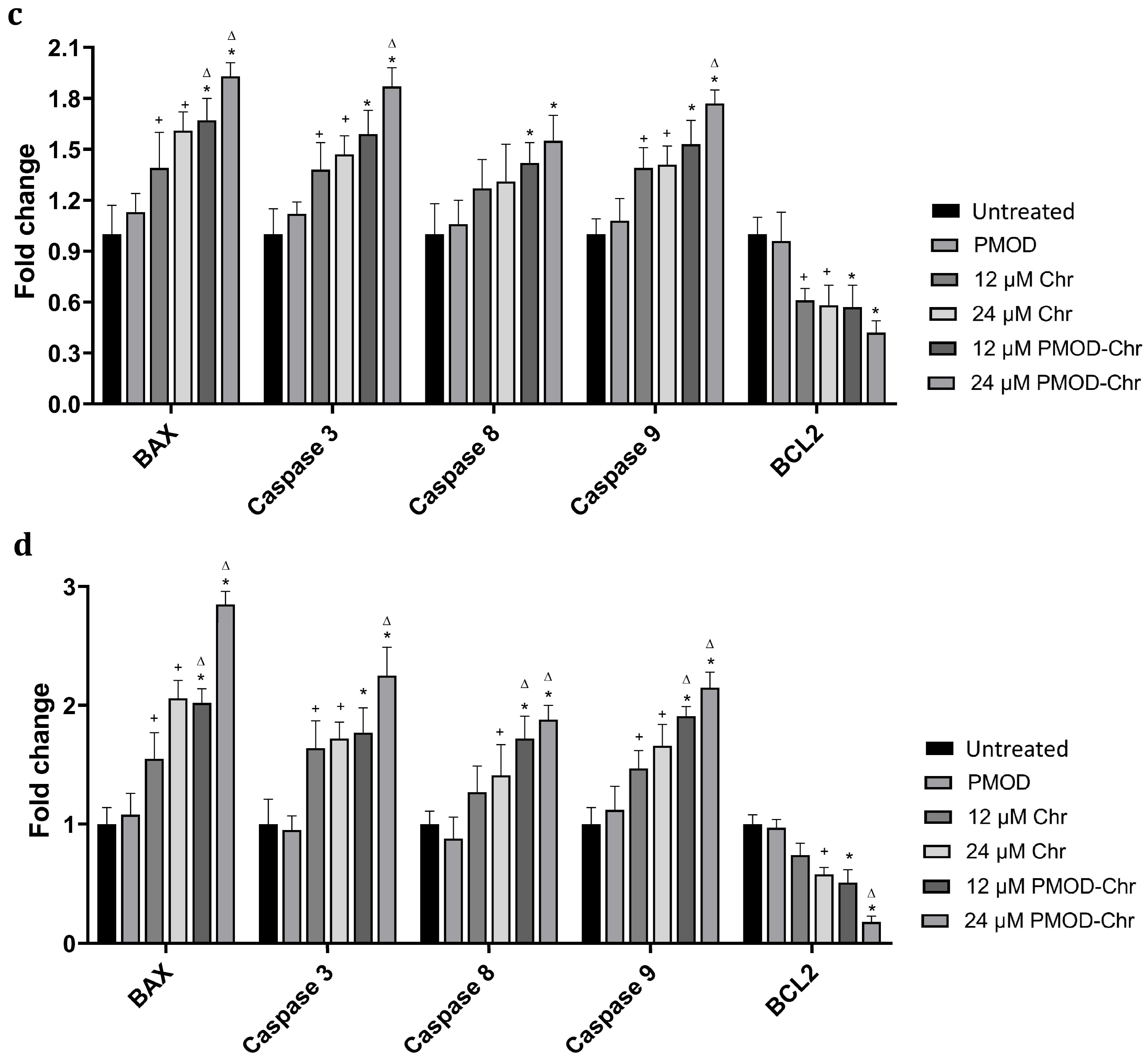
3.6. PMOD-Chr Increased the Rate of Apoptosis Compared to Free Chr
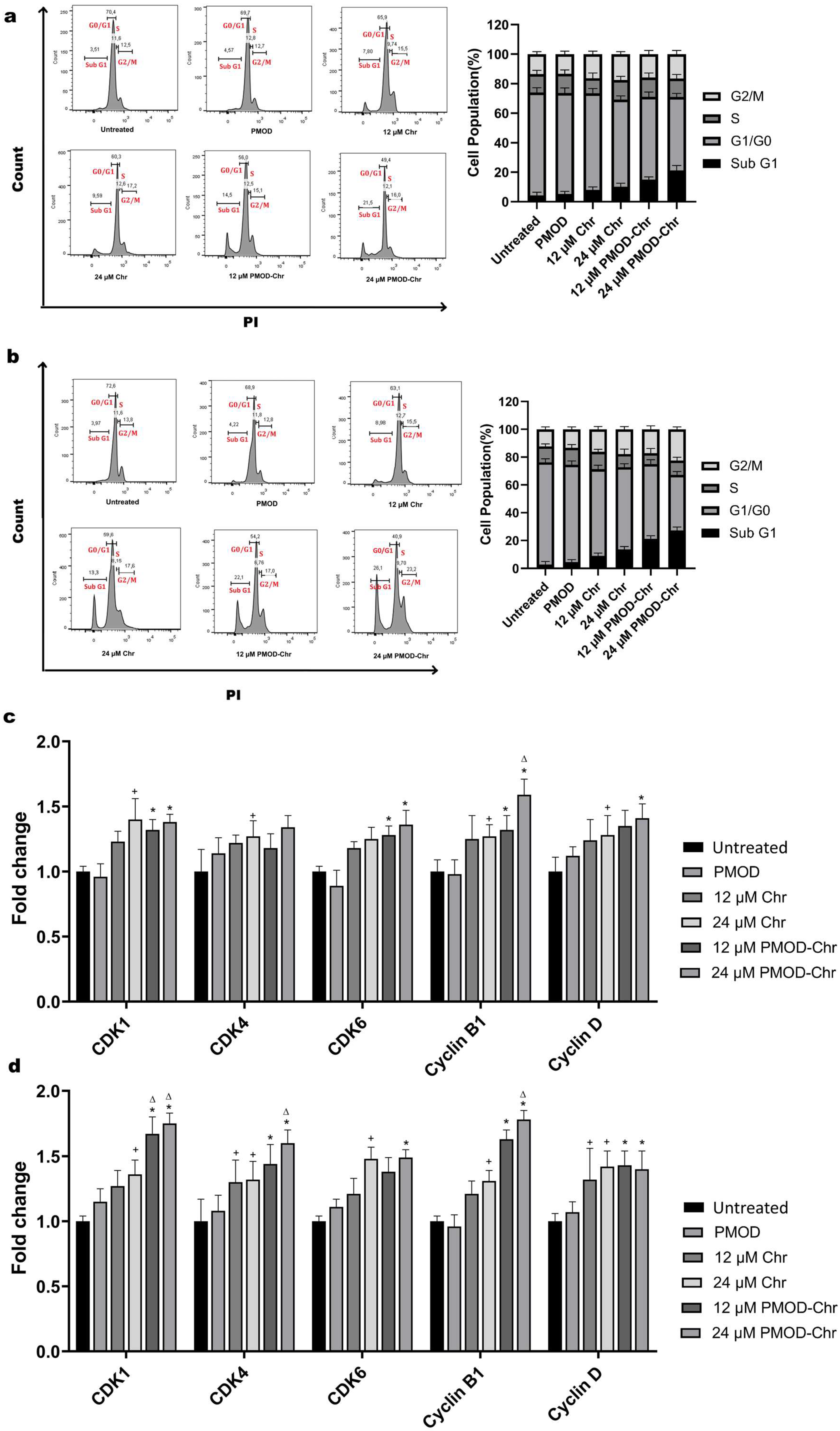
3.7. Cell Cycle Is Arrested by PMOD-Chr in the Sub-G1 and G2/M Phase
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Chr | Chrysin |
| PMMA | Poly (Methyl Methacrylate) |
| DMAEMA | 2-(Dimethyl Amino) Ethyl Methacrylate |
| OEGMA | Oligo (Ethylene Glycol) Methacrylate |
| PMDM | p(MMA-co-DMAEMA) |
| DMA | Dopamine Metacrylamide |
| PMOD | p(MMA-co-DMAEMA)-b-(OEGMA-co-DMA) |
| BCP | Block Copolymer |
| RAFT | Reversible Addition–Fragmentation Chain Transfer |
| EPR | Enhanced Permeability and Retention Effect |
References
- Maleki, Z.; Vali, M.; Nikbakht, H.A.; Hassanipour, S.; Kouhi, A.; Sedighi, S.; Farokhi, R.; Ghaem, H. Survival Rate of Ovarian Cancer in Asian Countries: A Systematic Review and Meta-Analysis. BMC Cancer 2023, 23, 558. [Google Scholar] [CrossRef]
- González-Martín, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.E.; Pothuri, B.; Ray-Coquard, I.; Tan, D.S.P.; Bellet, E.; Oaknin, A.; et al. Newly Diagnosed and Relapsed Epithelial Ovarian Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2023, 34, 833–848. [Google Scholar] [CrossRef]
- Baradács, I.; Teutsch, B.; Vincze, Á.; Hegyi, P.; Szabó, B.; Nyirády, P.; Ács, N.; Melczer, Z.; Bánhidy, F.; Lintner, B. Efficacy and Safety of Combination Therapy with PARP Inhibitors and Anti-Angiogenic Agents in Ovarian Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2025, 14, 1776. [Google Scholar] [CrossRef]
- Satora, M.; Kułak, K.; Zaremba, B.; Grunwald, A.; Świechowska-Starek, P.; Tarkowski, R. New Hopes and Promises in the Treatment of Ovarian Cancer Focusing on Targeted Treatment—A Narrative Review. Front. Pharmacol. 2024, 15, 1416555. [Google Scholar] [CrossRef]
- Meneses Do Rêgo, A.C.; Araújo-Filho, I. Decoding PARP Inhibitor Resistance in Ovarian Cancer: Molecular Insights and Emerging Therapeutic Strategies. Int. J. Innov. Res. Med. Sci. 2024, 9, 652–664. [Google Scholar] [CrossRef]
- McMullen, M.; Karakasis, K.; Madariaga, A.; Oza, A.M. Overcoming Platinum and Parp-Inhibitor Resistance in Ovarian Cancer. Cancers 2020, 12, 1607. [Google Scholar] [CrossRef]
- Colombo, I.; Karakasis, K.; Suku, S.; Oza, A.M. Chasing Immune Checkpoint Inhibitors in Ovarian Cancer: Novel Combinations and Biomarker Discovery. Cancers 2023, 15, 3220. [Google Scholar] [CrossRef]
- Dumitru, A.; Dobrica, E.C.; Croitoru, A.; Cretoiu, S.M.; Gaspar, B.S. Focus on PD-1/PD-L1 as a Therapeutic Target in Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12067. [Google Scholar] [CrossRef]
- Sun, S.; Fang, H. Curcumin Inhibits Ovarian Cancer Progression by Regulating Circ-PLEKHM3/MiR-320a/SMG1 Axis. J. Ovarian Res. 2021, 14, 158. [Google Scholar] [CrossRef]
- Ribeiro, E.; Vale, N. The Role of Resveratrol in Cancer Management: From Monotherapy to Combination Regimens. Targets 2024, 2, 307–326. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Jahanshahi, M.; Badehnoosh, B.; Shafabakhsh, R.; Asemi, Z.; Hallajzadeh, J. Inhibitory Effects of Berberine on Ovarian Cancer: Beyond Apoptosis. Med. Chem. Res. 2021, 30, 1605–1613. [Google Scholar] [CrossRef]
- Eksi, O.B.; Guler, A.; Akdeniz, M.; Atalay, P.; Hamurcu, Z.; Aydin, O. Development of Silver-Based Hybrid Nanoparticles Loaded with EEF2 K-SiRNA and Quercetin against Triple-Negative Breast Cancer. Drug Deliv. Transl. Res. 2025, 1–23. [Google Scholar] [CrossRef]
- Fu, Y.; Ge, Y.; Yi, S.; Peng, Q.; Jiang, H.; Zhou, J. A Review of Synergistic Strategies in Cancer Therapy: Resveratrol-Loaded Hydrogels for Targeted and Multimodal Treatment. Discov. Oncol. 2025, 16, 1382. [Google Scholar] [CrossRef]
- Garg, A.; Chaturvedi, S. A Comprehensive Review on Chrysin: Emphasis on Molecular Targets, Pharmacological Actions and Bio-Pharmaceutical Aspects. Curr. Drug Targets 2021, 23, 420–436. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, Beneficial Pharmacological Activities, and Molecular Mechanism of Action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Mehdi, S.H.; Nafees, S.; Zafaryab, M.; Khan, M.A.; Alam Rizvi, M.M. Chrysin: A Promising Anticancer Agent Its Current Trends and Future Perspectives. Eur. J. Exp. Biol. 2018, 8, 16. [Google Scholar] [CrossRef]
- Li, H.; Chen, A.; Yuan, Q.; Chen, W.; Zhong, H.; Teng, M.; Xu, C.; Qiu, Y.; Cao, J. NF-ΚB/Twist Axis Is Involved in Chysin Inhibition of Ovarian Cancer Stem Cell Features Induced by Co-Treatment of TNF-α and TGF-β. Int. J. Clin. Exp. Pathol. 2019, 12, 101–112. [Google Scholar] [PubMed]
- Jung, G.H.; Lee, J.H.; Han, S.H.; Woo, J.S.; Choi, E.Y.; Jeon, S.J.; Han, E.J.; Jung, S.H.; Park, Y.S.; Park, B.K.; et al. Chrysin Induces Apoptosis via the MAPK Pathway and Regulates ERK/MTOR-Mediated Autophagy in MC-3 Cells. Int. J. Mol. Sci. 2022, 23, 15747. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.J.; Jeong, Y.J.; Park, J.W.; Kwon, T.K. Chrysin-Induced Apoptosis Is Mediated through Caspase Activation and Akt Inactivation in U937 Leukemia Cells. Biochem. Biophys. Res. Commun. 2004, 325, 1215–1222. [Google Scholar] [CrossRef]
- Cai, S.; Li, Q.; Zhou, H.; Xu, Y.; Song, J.; Gan, C.; Qi, Z.; Qi, S. Mechanism of PI3K/AKT/MTOR Signaling Pathway for Mediating Anti-Inflammatory and Anti-Oxidant Effects of Chrysin: A Protein Microarray-Based Study. J. South. Med. Univ. 2021, 41, 1554–1561. [Google Scholar]
- Dabiri, S.; Jafari, S.; Molavi, O. Advances in Nanocarrier-Mediated Delivery of Chrysin: Enhancing Solubility, Bioavailability, and Anticancer Efficacy. Bioimpacts 2024, 15, 30269. [Google Scholar] [CrossRef]
- Lim, W.; Ryu, S.; Bazer, F.W.; Kim, S.; Song, G. Chrysin Attenuates Progression of Ovarian Cancer Cells by Regulating Signaling Cascades and Mitochondrial Dysfunction. J. Cell Physiol. 2018, 233, 3129–3140. [Google Scholar] [CrossRef]
- Dong, X.; Cao, Y.; Wang, N.; Wang, P.; Li, M. Systematic Study on Solubility of Chrysin in Different Organic Solvents: The Synergistic Effect of Multiple Intermolecular Interactions on the Dissolution Process. J. Mol. Liq. 2021, 325, 115180. [Google Scholar] [CrossRef]
- Jasim, A.J.; Sulaiman, G.M.; Ay, H.; Mohammed, S.A.A.; Mohammed, H.A.; Jabir, M.S.; Khan, R.A. Preliminary Trials of the Gold Nanoparticles Conjugated Chrysin: An Assessment of Anti-Oxidant, Anti-Microbial, and in Vitro Cytotoxic Activities of a Nanoformulated Flavonoid. Nanotechnol. Rev. 2022, 11, 2726–2741. [Google Scholar] [CrossRef]
- Nosrati, H.; Rakhshbahar, A.; Salehiabar, M.; Afroogh, S.; Kheiri Manjili, H.; Danafar, H.; Davaran, S. Bovine Serum Albumin: An Efficient Biomacromolecule Nanocarrier for Improving the Therapeutic Efficacy of Chrysin. J. Mol. Liq. 2018, 271, 639–646. [Google Scholar] [CrossRef]
- Farhadi, A.; Homayouni Tabrizi, M.; Sadeghi, S.; Vala, D.; Khosravi, T. Targeted Delivery and Anticancer Effects of Chrysin-Loaded Chitosan-Folic Acid Coated Solid Lipid Nanoparticles in Pancreatic Malignant Cells. J. Biomater. Sci. Polym. Ed. 2023, 34, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Jangid, A.K.; Solanki, R.; Patel, S.; Medicherla, K.; Pooja, D.; Kulhari, H. Improving Anticancer Activity of Chrysin Using Tumor Microenvironment PH-Responsive and Self-Assembled Nanoparticles. ACS Omega 2022, 7, 15919–15928. [Google Scholar] [CrossRef]
- Ragab, E.M.; El Gamal, D.M.; Mohamed, T.M.; Khamis, A.A. Impairment of Electron Transport Chain and Induction of Apoptosis by Chrysin Nanoparticles Targeting Succinate-Ubiquinone Oxidoreductase in Pancreatic and Lung Cancer Cells. Genes Nutr. 2023, 18, 4. [Google Scholar] [CrossRef]
- Alanazi, S.T.; Salama, S.A.; El-ebiary, A.M.; Altowairqi, A.K.; Alharthi, A.T.; Alzahrani, S.M.; Althagafi, S.H.; Alotaibi, R.A.; Tammam, A.A.E. Targeting SIRT1, NLRP3 Inflammasome, and Nrf2 Signaling with Chrysin Alleviates the Iron-Triggered Hepatotoxicity in Rats. Toxicology 2024, 504, 153766. [Google Scholar] [CrossRef]
- Kim, K.M.; Jung, J. Upregulation of G Protein-Coupled Estrogen Receptor by Chrysin-Nanoparticles Inhibits Tumor Proliferation and Metastasis in Triple Negative Breast Cancer Xenograft Model. Front. Endocrinol. 2020, 11, 560605. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian Cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Guan, L.; Lu, Y. New Developments in Molecular Targeted Therapy of Ovarian Cancer. Discov. Med. 2018, 26, 219–229. [Google Scholar]
- Radu, M.R.; Prădatu, A.; Duică, F.; Micu, R.; Creţoiu, S.M.; Suciu, N.; Creţoiu, D.; Varlas, V.N.; Rădoi, V.E. Ovarian Cancer: Biomarkers and Targeted Therapy. Biomedicines 2021, 9, 693. [Google Scholar] [CrossRef]
- Raj, S.; Khurana, S.; Choudhari, R.; Kesari, K.K.; Kamal, M.A.; Garg, N.; Ruokolainen, J.; Das, B.C.; Kumar, D. Specific Targeting Cancer Cells with Nanoparticles and Drug Delivery in Cancer Therapy. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 69, pp. 166–177. [Google Scholar]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Gong, L.; Peng, T.; Yao, J.; Lin, Z. Advances in PH-Responsive Drug Delivery Systems. OpenNano 2021, 5, 100031. [Google Scholar] [CrossRef]
- Ramezanian, S.; Moghaddas, J.; Roghani-Mamaqani, H.; Rezamand, A. Dual PH- and Temperature-Responsive Poly(Dimethylaminoethyl Methacrylate)-Coated Mesoporous Silica Nanoparticles as a Smart Drug Delivery System. Sci. Rep. 2023, 13, 20194. [Google Scholar] [CrossRef]
- Singh, J.; Nayak, P. PH-Responsive Polymers for Drug Delivery: Trends and Opportunities. J. Polym. Sci. 2023, 61, 2828–2850. [Google Scholar] [CrossRef]
- Ahmed, A.; Sarwar, S.; Hu, Y.; Munir, M.U.; Nisar, M.F.; Ikram, F.; Asif, A.; Rahman, S.U.; Chaudhry, A.A.; Rehman, I.U. Surface-Modified Polymeric Nanoparticles for Drug Delivery to Cancer Cells. Expert. Opin. Drug Deliv. 2021, 18, 1–24. [Google Scholar] [CrossRef]
- Hussain, Z.; Khan, S.; Imran, M.; Sohail, M.; Shah, S.W.A.; de Matas, M. PEGylation: A Promising Strategy to Overcome Challenges to Cancer-Targeted Nanomedicines: A Review of Challenges to Clinical Transition and Promising Resolution. Drug Deliv. Transl. Res. 2019, 9, 721–734. [Google Scholar] [CrossRef]
- Qiu, C.; Xia, F.; Zhang, J.; Shi, Q.; Meng, Y.; Wang, C.; Pang, H.; Gu, L.; Xu, C.; Guo, Q.; et al. Advanced Strategies for Overcoming Endosomal/Lysosomal Barrier in Nanodrug Delivery. Research 2023, 6, 0148. [Google Scholar] [CrossRef]
- Aydin, O.; Youssef, I.; Yuksel Durmaz, Y.; Tiruchinapally, G.; Elsayed, M.E.H. Formulation of Acid-Sensitive Micelles for Delivery of Cabazitaxel into Prostate Cancer Cells. Mol. Pharm. 2016, 13, 1413–1429. [Google Scholar] [CrossRef]
- Memet, B.C.; Yildiz, U.; Eksi, O.B.; Aydin, O. DOX-Couped Polymeric Micelles as a State-of-the-Art Strategy Against Triple Negative Breast Cancer. In Proceedings of the 9th World Congress on Recent Advances in Nanotechnology, RAN 2024, London, UK, 8–10 April 2024. [Google Scholar] [CrossRef]
- Shinde, V.R.; Revi, N.; Murugappan, S.; Singh, S.P.; Rengan, A.K. Enhanced Permeability and Retention Effect: A Key Facilitator for Solid Tumor Targeting by Nanoparticles. Photodiagnosis Photodyn. Ther. 2022, 39, 102915. [Google Scholar] [CrossRef]
- Bose, S.; Saha, P.; Alam, M.T.; Chatterjee, B.; Sarkar, M.; Dixit, A.K.; Kumar, D.; Pathak, R.K.; Tripathi, P.P.; Srivastava, A.K. Inhibition of DNA Polymerase Eta-mediated Translesion DNA Synthesis with Small Molecule Sensitises Ovarian Cancer Stem-like Cells to Chemotherapy. Br. J. Pharmacol. 2025, 182, 3594–3611. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.; Ramos, C.; Mendes, C.; Sequeira, C.O.; Tomé, C.S.; Fernandes, D.G.H.; Mota, P.; Pires, R.F.; Urso, D.; Hipólito, A. Targeting Glutathione and Cystathionine β-Synthase in Ovarian Cancer Treatment by Selenium–Chrysin Polyurea Dendrimer Nanoformulation. Nutrients 2019, 11, 2523. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Chen, Y.; Zhang, Q.; Guo, Y.; Huang, Z.; Dai, L.; Cao, S. 8-Bromo-7-Methoxychrysin Induces Apoptosis by Regulating Akt/FOXO3a Pathway in Cisplatin-Sensitive and Resistant Ovarian Cancer Cells. Mol. Med. Rep. 2015, 12, 5100–5108. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef]
- Lima, A.P.B.; Melo, A.S.; Ferreira, G.M.; da Silva, G.N. Chrysin Inhibits the Cell Viability, Induces Apoptosis and Modulates Expression of Genes Related to Epigenetic Events in Bladder Cancer Cells. Nat. Prod. Res. 2023, 37, 1877–1881. [Google Scholar] [CrossRef]
- Han, H.; Lee, S.-O.; Xu, Y.; Kim, J.-E.; Lee, H.-J. SPHK/HIF-1α Signaling Pathway Has a Critical Role in Chrysin-Induced Anticancer Activity in Hypoxia-Induced PC-3 Cells. Cells 2022, 11, 2787. [Google Scholar] [CrossRef]
- Ramos, P.S.; Ferreira, C.; Passos, C.L.A.; Silva, J.L.; Fialho, E. Effect of Quercetin and Chrysin and Its Association on Viability and Cell Cycle Progression in MDA-MB-231 and MCF-7 Human Breast Cancer Cells. Biomed. Pharmacother. 2024, 179, 117276. [Google Scholar] [CrossRef]
- Jung, Y.; Kraikivski, P.; Shafiekhani, S.; Terhune, S.S.; Dash, R.K. Crosstalk between Plk1, P53, Cell Cycle, and G2/M DNA Damage Checkpoint Regulation in Cancer: Computational Modeling and Analysis. NPJ Syst. Biol. Appl. 2021, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Lim, B. Targeting Apoptosis in Cancer. Curr. Oncol. Rep. 2022, 24, 273–284. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Simal-Gandara, J.; Kopustinskiene, D.M.; Bernatoniene, J.; Samarghandian, S. Emerging Cellular and Molecular Mechanisms Underlying Anticancer Indications of Chrysin. Cancer Cell Int. 2021, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Faraji, F.; Jafarpour, S.; Faraji, F.; Rasoulpoor, S.; Dokaneheifard, S.; Mohammadi, M. Anti-Cancer Activity of Chrysin in Cancer Therapy: A Systematic Review. Indian. J. Surg. Oncol. 2022, 13, 681–690. [Google Scholar] [CrossRef]
- Topacio, B.R.; Zatulovskiy, E.; Cristea, S.; Xie, S.; Tambo, C.S.; Rubin, S.M.; Sage, J.; Kõivomägi, M.; Skotheim, J.M. Cyclin D-Cdk4, 6 Drives Cell-Cycle Progression via the Retinoblastoma Protein’s C-Terminal Helix. Mol. Cell 2019, 74, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.R.; Korolchuk, S.; Martin, M.P.; Stanley, W.A.; Moukhametzianov, R.; Noble, M.E.M.; Endicott, J.A. CDK1 Structures Reveal Conserved and Unique Features of the Essential Cell Cycle CDK. Nat. Commun. 2015, 6, 6769. [Google Scholar] [CrossRef]
- Tarahomi, M.; Firouzi Amandi, A.; Eslami, M.; Yazdani, Y.; Salek Farrokhi, A.; Ghorbani, F.; Taherian, M.; Yousefi, B. Niosomes Nanoparticles as a Novel Approach in Drug Delivery Enhances Anticancer Properties of Chrysin in Human Ovarian Carcinoma Cells (SKOV3): An in Vitro Study. Med. Oncol. 2023, 40, 87. [Google Scholar] [CrossRef]

| MMA | DMAEMA | CTA | AIBN | |
|---|---|---|---|---|
| P1 | 3 g | 471.63 mg | 121.098 mg | 6.84 mg |
| P2 | 3 g | 1.179 g | 151.372 mg | 7.697 mg |
| P3 | 3 g | 2.0122 g | 0.1727 g | 8.796 mg |
| M1/M2/CTA/AIBN | Conv. [%] | Mn (g mol−1), Theo. 1H NMR | Mw | Mn | Mw/Mn (PDI) | |
|---|---|---|---|---|---|---|
| BCP1 | 90/10/1/0.125 | 89.4 | 11,017.67 | 1.324 × 104 | 1.251 × 104 | 1.058 |
| BCP2 | 80/20/1/0.125 | 95.5 | 10,697.76 | 9.901 × 104 | 8.205 × 104 | 1.207 |
| BCP3 | 70/30/1/0.125 | 95.9 | 10,645.75 | 1.636 × 105 | 1.444 × 105 | 1.113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cakir, S.; Yildiz, U.; Yildirim, T.; Aydin, O. Chrysin-Loaded Micelles Regulate Cell Cycle and Induce Intrinsic and Extrinsic Apoptosis in Ovarian Cancer Cells. Nanomaterials 2025, 15, 1362. https://doi.org/10.3390/nano15171362
Cakir S, Yildiz U, Yildirim T, Aydin O. Chrysin-Loaded Micelles Regulate Cell Cycle and Induce Intrinsic and Extrinsic Apoptosis in Ovarian Cancer Cells. Nanomaterials. 2025; 15(17):1362. https://doi.org/10.3390/nano15171362
Chicago/Turabian StyleCakir, Serife, Ummugulsum Yildiz, Turgay Yildirim, and Omer Aydin. 2025. "Chrysin-Loaded Micelles Regulate Cell Cycle and Induce Intrinsic and Extrinsic Apoptosis in Ovarian Cancer Cells" Nanomaterials 15, no. 17: 1362. https://doi.org/10.3390/nano15171362
APA StyleCakir, S., Yildiz, U., Yildirim, T., & Aydin, O. (2025). Chrysin-Loaded Micelles Regulate Cell Cycle and Induce Intrinsic and Extrinsic Apoptosis in Ovarian Cancer Cells. Nanomaterials, 15(17), 1362. https://doi.org/10.3390/nano15171362







