Abstract
This study demonstrates a novel route to synthesize gold-decorated exfoliated graphite (EG) through graphite intercalation compounds (GICs) with tetrachloroauric acid (HAuCl4). We aimed to develop a scalable method for producing EG/Au composites with controlled nanoparticle morphology by investigating the effects of precursor chemistry and thermal expansion conditions. II-stage GIC–HAuCl4 (average gross-composition: C23HAuCl4; intercalate layer thickness di = 6.85 Å) was prepared via an exchange reaction of HAuCl4 with graphite nitrate. Interaction of this GIC with liquid methylamine yielded an occlusive complex, where methylamine-bound HAuCl4 occupies both interlayer and intercrystalline spaces in the graphite matrix. Methylamine treatment of GIC reduces the onset temperature of exfoliation by ≈100 °C and enhances the expansion efficiency, yielding EG with a low bulk density range of 4–6 g/L when processed at 900 °C in air or nitrogen. Thermal exfoliation of these GICs yielded EG decorated with gold nanoparticles, exhibiting a broad size distribution from a few nanometers to several hundred nanometers, as confirmed by electron microscopy. An X-ray diffraction analysis identified the coexistence of crystalline gold and hexagonal graphite phases, with no detectable impurity phases.
1. Introduction
Exfoliated graphite (EG) is a unique material produced by intercalation and subsequent thermal expansion of natural graphite [1]. This process results in the formation of thin, flake-like structures with a significantly increased surface area and porosity [2,3]. The crystal structure of EG is close to the structure of the original graphite, due to which composites based on it have high thermal stability, chemical inertness, and thermal and electrical conductivity along the graphene layers, together with high mechanical strength, elasticity, and flexibility [4,5]. Exfoliated graphite is widely used in the production of gaskets, seals [6,7,8], thermal management [9,10,11], and energy storage [12,13,14,15] systems due to its unique properties.
To impart new functional properties to exfoliated graphite, it can be doped with various metal-containing phases. The specific nature of these phases plays a critical role in determining the material’s performance for different applications. For instance, when developing magnetic sorbents for oil and hydrocarbon, EG is functionalized with ferro- or ferrimagnetic phases, which facilitate the collection and transportation of sorbate-saturated material [16,17,18,19]. For catalytic applications, EG/metal (EG/M) composites require not only a metal-containing phase of precise composition but also stringent control over the particle size, often necessitating dimensions below several tens of nanometers [20,21].
The following composites have already proven their effectiveness: EG/Fe in the decomposition of heavy metal salts [22], EG/Co in the Fischer–Tropsch processes [20], and EG/Pt for the formation of gas diffusion layers of fuel cells in chemical power sources [21]. The number of studies in which EG/Au or EG/Pt composites are used as catalysts is relatively small, since many researchers are focused on studying catalytic systems based on Au and Pt on more traditional supports, including activated carbon [23,24], carbon black [25,26], and “sibunit” [27]. This may be partly due to the current lack of scalable technology for producing such composites.
A common method for synthesizing EG/M composites involves depositing a metal-containing precursor onto expandable graphite, followed by thermal decomposition in an inert or reducing atmosphere to yield the desired metallic phase [28,29]. This approach enables precise control over the composition, size, morphology, and even shape of the resulting metal particles [30]. However, it is extremely low-tech, since the small mass of EG takes up a large volume. However, in our view, this method suffers from poor scalability due to the high volume-to-mass ratio of EG. EG exhibits an exceptionally low bulk density (≈2–4 g/L) [1], meaning any post-processing inevitably becomes a low-throughput operation.
An alternative approach enabling concurrent formation of the metal-containing phase during thermal expansion was previously developed. In this method, a mixture of expandable graphite, metal nitrates, and melamine undergoes thermal shock, producing EG decorated with iron, cobalt, and nickel particles of micron or submicron dimensions [31,32]. However, this system is unsuitable for investigating the factors controlling metal particle size. During air-based thermal treatment, the reduced metals rapidly oxidize to form oxides, while under inert atmospheres, they react with carbon to yield carbides.
Various ions, atoms, or molecules can be placed between the graphite layers. The compounds formed are called graphite intercalation compounds. The ions or atoms themselves, located between the graphite layers, are called intercalates. The amount of intercalate in graphite is determined by the stage number, which is numerically equal to the number of graphene layers between two adjacent intercalate layers [33,34].
GICs with gold chloride can be synthesized as either I-stage C12.5AuCl3 or II-stage C25AuCl3 compounds, characterized by an intercalated layer thickness (di) range of 6.80–6.86 Å These compounds are prepared through a gas-phase synthesis approach using either single-zone or two-zone methods in sealed tubes. The synthesis requires elevated temperatures (200–300 °C) and prolonged reaction times ranging from 48 to 96 h [35,36,37]. The liquid-phase method enables synthesis of GICs with HAuCl4 under milder reaction conditions and with significantly reduced processing time compared to conventional approaches [38]. The synthesis is achieved by passing chlorine (acting as an oxidizer) through a molten mixture of tetrachloroauric acid hydrate and graphite, resulting in acid intercalation into the graphite matrix. A potentially more efficient approach involves an exchange reaction between HAuCl4 and pre-formed graphite nitrate. In this case, the graphite matrix already possesses a positive charge, potentially eliminating the need for additional oxidizers [21]. While previous work [21] confirms the successful preparation of GIC–H2PtCl6 via this exchange method, no published studies have documented its application for GIC–HAuCl4 synthesis.
This study investigates how synthesis conditions affect the structure of gold-containing thermally expanded graphite and the size distribution of gold particles derived from graphite intercalation compounds with HAuCl4.
2. Materials and Methods
2.1. Sample Preparation
To obtain GIC with gold chloride, the following reagents were used: natural flake graphite (particle size 250–300 µm, purity 99.7%), concentrated (98%) nitric acid, gold (purity 99.9%), nitric acid (solution 68–70%), hydrochloric acid (solution 35–38%), and isopropyl alcohol.
The synthesis of GIC with gold chloride was carried out by conducting an exchange interaction between IV-stage GIC–HNO3 (graphite nitrate) and HAuCl4. Graphite nitrate was obtained using a standard method during the interaction of graphite with concentrated 98% (fuming) HNO3 in the ratio for graphite/HNO3 of 1:0.5.
Gold was completely dissolved in aqua regia (3:1 mass ratio of 35–38% HCl to 68–70% HNO3) with gentle heating to 50–60 °C. The solution was then concentrated by evaporating excess solvent at 85–90 °C. The endpoint was determined by two visual indicators: near-complete cessation of vapor emission and a color evolution from initial yellow-orange to final dark red.
The resulting HAuCl4∙xH2O melt (in its residual crystallization water) was combined with freshly prepared graphite nitrate in a 1:5 mass ratio of graphite nitrate-to-HAuCl4∙xH2O. The reaction proceeded for 2 h at 85–90 °C with periodic stirring until constant mass was achieved. During this process, gradual mixture thickening and evolution of nitric acid vapor from the graphite intercalation compound as HAuCl4 replacing HNO3 were observed. After cooling to room temperature, the product was washed with excess isopropanol using a glass filter to remove residual acids, followed by drying in a furnace until constant mass was achieved.
GIC–HAuCl4 was saturated with ammonia and methylamine. For saturation, the following reagents were used: 25% aqueous ammonia solution, 38% aqueous methylamine solution, granulated potassium hydroxide, acetone, and liquid nitrogen.
The treatment of GIC–HAuCl4 with liquid ammonia (Tbp = −33 °C) and methylamine (Tbp = −6 °C) was carried out. Granulated KOH was added to a Wurtz flask with an aqueous solution of ammonia or amine. At the same time, gas began to release and passed through a column of alkali to dry out the water vapor captured from the solution. After passing through the column, the gas was condensed in a test tube placed in a thermos filled with acetone, which was pre-cooled with liquid nitrogen to a temperature of −50 ± 5 °C. After condensation of 3–4 mL amine (for 1 g of GIC), the gas supply to the test tube was stopped, and the resulting liquid phase was transferred to test tubes with GIC–HAuCl4.
After adding excess amine, the tube was sealed and kept at room temperature until the GIC was completely saturated with amine. After the holding period for at least 240 h, the tube was opened, and the excess amine evaporated at room temperature until the sample stopped losing weight. Next, the amine weight uptake was estimated, expressed as a mass percentage of the mass of the initially taken GIC.
The exfoliated graphite (EG) samples were prepared by thermal shock treatment of the GIC–HAuCl4 at temperatures of 700 °C or 900 °C, conducted either in ambient air using a muffle furnace or under nitrogen atmosphere in a custom quartz reactor.
2.2. Investigation Techniques
Phase compositions of all specimens were characterized by powder X-ray (XRD) diffraction using a Rigaku Ultima IV diffractometer (Tokyo, Japan), with CuKα radiation (λKα1 = 1.5405 Å, λKα2 = 1.5443 Å).
Thermal decomposition behavior was investigated using a NETZSCH STA 449C Jupiter thermal analyzer (Selb, Germany). Samples (2–3 mg) were heated from 40 up to 400 °C at a rate of 5 °C/min under a nitrogen purge (100 mL/min).
The expansion of graphite particles was performed on an OLYMPUS BX51 optical microscope (Tokyo, Japan) with an OLYMPUS U-MSSP heated objective table (Tokyo, Japan) (temperature change rate of 50°/min). Filming was performed using an E3ISPM06300KPB color USB camera (Saint Petersburg, Russia). During editing, the video was sped up 5 times.
The bulk density (dEG, g/L) of gold-containing EG was calculated from the mass (mEG) and volume (VEG) of the powder:
dEG = mEG/VEG
The error in determining the bulk density of EG was ±1 g/L.
The metal content was determined via a gravimetric analysis. The gold-containing EG samples (mEG ~ 0.3 g) were heated in a ceramic crucible at 900 °C for 3 h in a muffle furnace to ensure complete combustion of the carbon matrix. The residual gold mass (mAu) was then measured, enabling calculation of the metal content in the expanded graphite:
ωAu = mAu/mEG × 100%
The bulk density of pure EG (d′EG, g/L) was recalculated using the Au content (ωAu) in the sample:
d′EG = (1 − ωAu/100) × dEG
The morphology and elemental composition of the synthesized metal-containing EG samples were characterized by scanning electron microscopy (SEM) with energy-dispersive X-ray spectroscopy (EDX) using a TESCAN VEGA3 LMU instrument (Brno, Czech Republic).
The average metal particle size was determined using two complementary methods: a statistical analysis involving size measurements of 50 randomly selected particles across five representative micrographs per sample, and automated image processing, where the mean particle diameter was calculated from the total projected area of all particles in selected micrographs and manual particle counts, assuming spherical morphology.
Samples of EG with Au were examined by transmission electron microscopy (TEM) using a JEM-2010 transmission electron microscope (Tokyo, Japan).
EG was studied using the Raman scattering method using a Renishaw spectrometer (Wotton-under-Edge, the UK) with excitation by a laser, with a wavelength of 532 nm and laser power of 5% (0.8 mW, a spot diameter of 1 μm for objective ×50).
3. Results and Discussion
3.1. Synthesis of Graphite Intercalation Compounds with HAuCl4
GIC–HNO3 is a charged matrix [39] and is capable of entering into exchange interactions, such as with sulfuric acid and phosphoric acids, which are not capable of spontaneous intercalation without an external oxidizer [40], or hexachloroplatinic acid [21]. HAuCl4 is also a strong acid, so a similar reaction can be expected.
A graphite intercalation compound with HAuCl4 (GIC–HAuCl4) was prepared through an exchange reaction between graphite nitrate (GIC–HNO3) and tetrachloroauric acid. The GIC–HNO3 precursor was synthesized by treating natural graphite (Figure 1a) with fuming nitric acid. The identity period of GIC–HNO3 (Ic = 17.80 Å) was calculated based on an XRD analysis (Figure 1b), which confirmed the formation of stage IV. Metallic gold was first dissolved in aqua regia under gentle heating, with the solution subsequently concentrated through controlled evaporation until characteristic color changes were observed. The resulting HAuCl4∙xH2O melt was combined with GIC–HNO3, yielding a progressively thickening mixture as nitric acid was displaced from the graphite matrix.
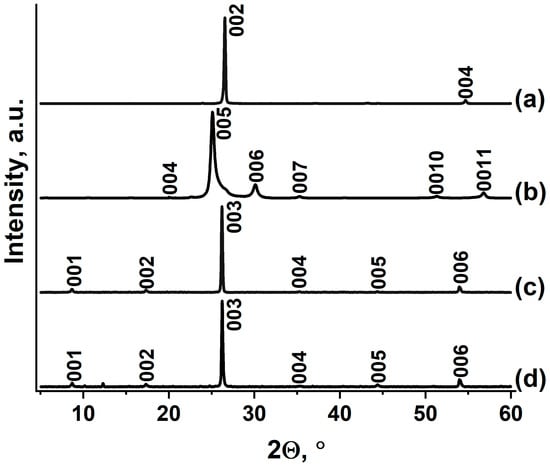
Figure 1.
XRD patterns of (a) natural graphite flake, (b) GIC–HNO3, (c) GIC–HAuCl4, and (d) GIC–HAuCl4 treated by methylamine.
During the reaction between GIC–HNO3 and HAuCl4∙xH2O, a known excess of melt HAuCl4∙xH2O is taken, i.e., theoretically, HAuCl4 should be sufficient to form a I-stage compound with the maximum degree of filling of the interlayer space of graphite, but this does not happen, apparently because the charge on the graphite matrix is insufficient for the intercalation process to lead to the formation of the I-stage, but is sufficient for the II-stage to form. In the paper devoted to the intercalation of H2PtCl6 during the exchange reaction with GIC–HNO3, it was also shown that only the II-stage GIC with H2PtCl6 is formed [21].
The final purification involved thorough washing with organic solvent to remove residual acids, followed by thermal drying to a constant mass. The average composition of the synthesized graphite intercalation compound was determined to be C23HAuCl4. In various experiments, the gross compositions of the obtained GICs varied from C22HAuCl4 to C26HAuCl4. This may be due to the existence of a fairly wide homogeneity region for this compound, which in principle is characteristic of acceptor-type GIC with strong acids.
The synthesized GIC–HAuCl4 exhibits an identity period of 10.20 Å, with an intercalated layer thickness (di) of 6.85 Å, consistent with previously reported values [38]. These values correspond to the formation of II-stage GIC–HAuCl4. The XRD pattern (Figure 1c) demonstrates complete phase purity, showing no detectable reflections corresponding to either the initial graphite nitrate precursor, unreacted graphite, or non-intercalated gold species. These observations confirm the successful preparation of a homogeneous GIC through the exchange reaction between IV-stage GIC–HNO3 and HAuCl4.
It was found that GIC–HAuCl4 does not interact with liquid ammonia, unlike pure HAuCl4 [41]. While GIC–HAuCl4 remains inert toward liquid ammonia, it undergoes a selective reaction with liquid methylamine to form an occlusive complex with the stoichiometry {23C}HAuCl4‧2.7CH3NH2, corresponding to a 13.5 wt% methylamine mass gain. In order to distinguish “true” intercalated compounds from inclusion compounds in which the intercalate is located both in the interlayer and in the intercrystalline space of graphite obtained by processing the GIC with methylamine, we chose the following form {23C}HAuCl4‧2.7CH3NH2 to record the gross composition. This form of recording was proposed to be used by the authors of [42] when they studied the interaction of GIC–CuCl2 with ammonia.
Methylamine treatment of GIC–HAuCl4 does not result in the disappearance of the initial GIC diffraction peaks (Figure 1d), although their intensity decreases substantially, suggesting structural disordering. The appearance of two new unidentified reflections at 2Θ = 10.2 and 12.3° indicates the formation of either reaction products between deintercalated HAuCl4 and methylamine, or an intercalated complex within the graphite matrix. The reaction with methylamine results in partial extraction of HAuCl4 from the graphite matrix, while also forming a stable complex within the interlayer spaces near particle peripheries. Steric or kinetic limitations appear to restrict deeper intercalation, leaving some HAuCl4 unreacted in the bulk graphite, as evidenced by residual GIC reflections in the diffraction pattern. The room-temperature stability of the methylamine complex (despite methylamine’s low boiling point, −6 °C) suggests strong chemical interactions, likely coordination bonding between methylamine and the intercalated gold chloride species.
3.2. Thermal Decomposition of GIC–HAuCl4
The comparative analysis of thermogravimetric curves of GIC–HAuCl4 and GIC treated by methylamine reveals significantly lower thermal stability of the latter, which initiates decomposition at ≈50 °C, compared to 150 °C for the pristine GIC–HAuCl4 (Figure 2). The thermal decomposition profile of GIC–HAuCl4 exhibits a single distinct mass loss step, while GIC treated by methylamine demonstrates more complex degradation behavior.
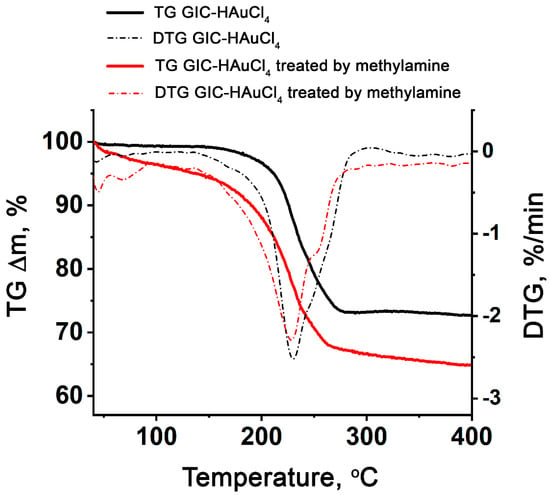
Figure 2.
TG and DTG curves of GIC–HAuCl4 and GIC–HAuCl4 treated by methylamine.
According to [43], the thermal decomposition of the compound HAuCl4 occurs through concurrent dechlorination processes, with the HCl evolution occurring near 150 °C. The decomposition product, AuCl3, forms during the initial decomposition stage at 75–180 °C, consistent with the following reaction:
HAuCl4(int) → AuCl3(s) + HCl(g)
The secondary decomposition stage (180–235 °C) corresponds to decomposition of AuCl3:
AuCl3(s) → AuCl(s) + Cl2(g)
The final decomposition stage occurs between 235 and 320 °C, ultimately yielding elemental gold Au as the stable end product. This transformation is accurately represented by the reaction, consistent with previous observations [43]:
2AuCl(s) → 2Au + Cl2(g)
The thermal analysis reveals concurrent decomposition processes occurring near 230 °C, including the decomposition of deintercalated gold chlorides, yielding gaseous HCl and Cl2 evolution, and breakdown of the GIC matrix. The GIC–HAuCl4–methylamine system displays additional thermal features on its TG/DTG patterns, including two low-temperature minima (<100 °C) corresponding to methylamine releasing from the complex, alongside the primary decomposition reactions (DTG minimum at 228 °C).
The thermogravimetric analysis reveals distinct mass losses of 27.3% for GIC–HAuCl4 and 35.2% for GIC–HAuCl4 treated by methylamine during decomposition. Assuming complete reduction to elemental gold on the graphite surface, the theoretical residuals from C23HAuCl4 and {23C}HAuCl4∙2.7CH3NH2 should both yield 23C + Au. This corresponds to calculated mass losses of GIC and treated GIC of 23.4% and 32.6%, respectively. The close agreement between experimental and theoretical values (with minor discrepancies attributable to methodological variations) confirms the proposed decomposition pathway.
To elucidate the deintercalation mechanism, we conducted in situ optical microscopy studies of GIC particle expansion during thermal treatment. Selected particles of both GIC–HAuCl4 and GIC treated by methylamine were deposited on a heating substrate. The samples were heated at a controlled rate of ≈50 °C/min, with temperature monitoring via a thermocouple integrated into the microscope stage. The entire thermal expansion process was recorded using the microscope’s digital imaging system (Video S1).
Optical microscopy observations (Figure 3) demonstrate distinct thermal expansion behaviors between the two materials. GIC–HAuCl4 treated by methylamine complex initiates expansion at ≈60–100 °C, while the GIC–HAuCl4 remains unchanged until reaching 190–200 °C. Complete expansion occurs at 280 °C, transforming the original flat GIC flakes into elongated, worm-like exfoliated graphite structures with visible metallic particles. These visual findings correlate well with thermogravimetric data. Methylamine treatment facilitates earlier GIC thermal expansion onset and enhances the overall expansion degree compared to the non-treated GIC.
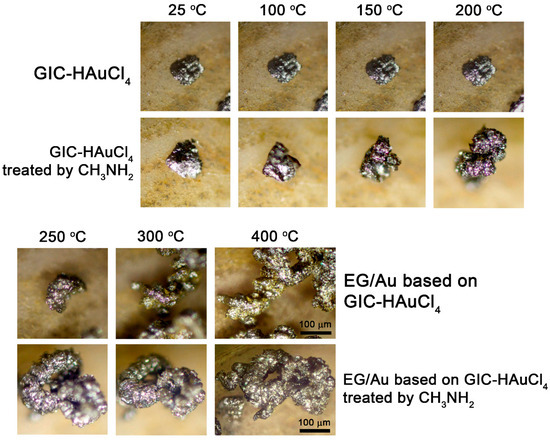
Figure 3.
Optical images of GIC–HAuCl4 and GIC–HAuCl4 treated by methylamine after thermal treatment at different temperatures.
3.3. Preparation of Exfoliated Graphite with Gold Particles
Rapid thermal treatment of acid-intercalated graphite induces violent intercalate evaporation and decomposition, generating substantial pressure within the graphite matrix [44]. During thermal decomposition, GIC–HAuCl4 and GIC–HAuCl4 treated by methylamine release HCl, Cl2, and melamine, which drive pronounced particle expansion and delamination. This process yields low-density powders consisting of elongated, worm-like particles of exfoliated graphite.
The resulting powder, composed of EG particles, exhibits a characteristically low bulk density. The EG samples were prepared through thermal expansion of GIC–HAuCl4 and GIC–HAuCl4 treated by methylamine at 700 °C and 900 °C in either air or nitrogen atmosphere. Three key experimental variables were investigated for their influence on material characteristics, namely the methylamine treatment, atmosphere, and expansion temperature. These parameters were hypothesized to affect both the structural properties of the EG matrix and the morphological distribution of surface gold nanoparticles. Table 1 presents the measured bulk densities of the EG samples and their corresponding gravimetrically determined Au contents.

Table 1.
Characteristics of exfoliated graphite obtained from GIC–HAuCl4 under various processing conditions.
The experimental data demonstrate that lower EG bulk density is achieved under three key conditions: higher processing temperatures, which generate greater dispersive pressure; thermal expansion of GIC treated by methylamine, which enhances the dispersive pressure more significantly than temperature effects alone; and atmospheric processing in air rather than nitrogen, attributable not to the gas composition itself but to the superior thermal shock achievable in a muffle furnace compared to nitrogen-purged reactor systems. While the composition of EG remains largely unaffected by production conditions, oxidative processing in air results in partial carbon oxidation. This leads to a measurable increase in the gold-to-carbon ratio within the final product. The XRD analysis confirms that the phase composition of gold-containing EG remains consistent across all production conditions, exclusively comprising metallic gold with a face-centered cubic crystal structure and graphite (Figure 4).
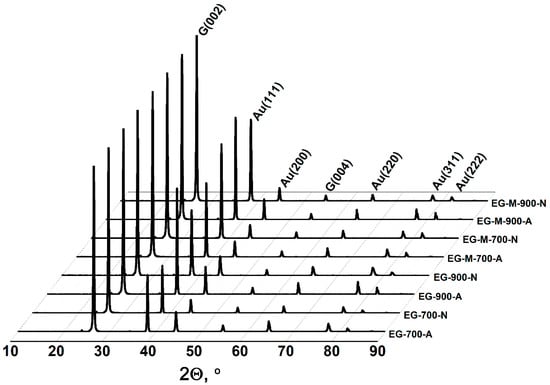
Figure 4.
XRD patterns of gold-containing exfoliated graphite obtained under various processing conditions.
The Raman spectra of EG based on GIC–HAuCl4 and GIC treated by methylamine (Figure 5) show a prominent G-band, confirming high graphitic crystallinity in the EG-900-N and EG-M-900-N samples. EG based on GIC–HAuCl4 treated by methylamine additionally exhibits a distinct D-band, indicative of amorphous carbon formation [45,46]. This structural disorder likely results from thermal decomposition of methylamine’s hydrocarbon moieties under inert atmosphere conditions.
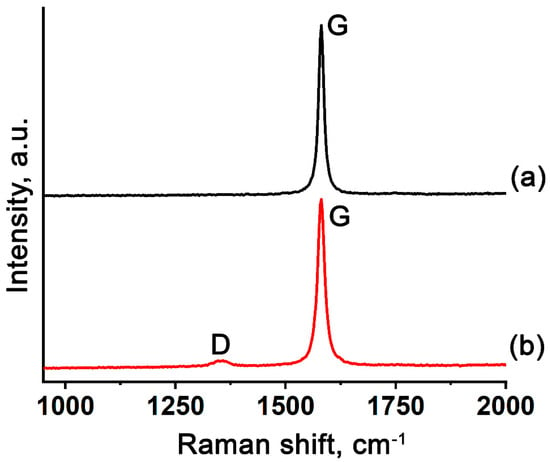
Figure 5.
Raman spectra of gold-containing EG based on (a) GIC–HAuCl4 and (b) GIC–HAuCl4 treated by methylamine.
The SEM analysis reveals the characteristic worm-like morphology of EG particles (Figure 6a). Backscattered electron imaging demonstrates uniform metal distribution across the entire particle surface (Figure 6a,b). The gold particles are less than one micrometer in size (Figure 6c–f). The EDX analysis confirms the surface elemental composition consists exclusively of carbon, oxygen, and gold (Figure 6a). The observed oxygen signal originates from surface oxygen-containing functional groups, which is consistent with previously IR spectroscopy data and characteristic of all exfoliated graphite materials [47].
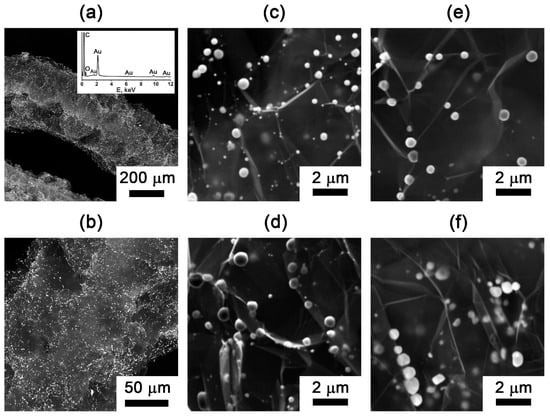
Figure 6.
SEM images with an EDX analysis of the (a,b) gold-containing EG particles (EG-M-900-Air). SEM images of the gold particles on the surface of exfoliated graphite: (c) EG-M-900-Air; (d) EG-M-900-N; (e) EG-900-Air; (f) EG-900-N.
The average metal particle size was determined through a statistical analysis of representative SEM images, collected across all the EG samples. For quantitative assessment, one image from each set was subjected to the detailed image analysis, involving measurement of the total gold particle area, particle counting, and calculation of the mean spherical-equivalent diameter based on circular projection assumptions. The results for the two methods of determining the diameter are shown in Table 2. The two particle sizing methods demonstrate good correlation, with observed variations primarily attributable to the inherent polydispersity of the system. The broader size distribution obtained from the averaging method reflects this significant particle size dispersion.

Table 2.
Results of determination of the average diameters of gold particles on EG.
Gold nanoparticles supported on exfoliated graphite exhibit submicrometer dimensions, with average particle sizes ranging from 300 to 500 nm (Figure 6c–f, Table 2). Our analysis reveals that the preparation atmosphere serves as the primary determinant of particle size, where nitrogen environments consistently yield larger Au particles compared to air. In contrast, both the methylamine treatment of the graphite intercalation compound and processing temperature range (700–900 °C) demonstrate comparatively minor influence on the final gold particle dimensions.
Based on the results of the conducted research and calculations, it turned out that the parameters we considered (methylamine treatment, atmosphere, and expansion temperature) have practically no effect on the size of the metal particles, but they do affect the degree of expansion of graphite and the bulk density of EG.
Transmission electron microscopy was employed to characterize the gold nanoparticles dispersed within the graphite matrix of sample EG-M-900-A, which exhibited the lowest bulk density among all prepared samples (Figure 7). The TEM images reveal a size distribution of Au nanoparticles, ranging from tens of nanometers (Figure 7a) to several nanometers in diameter (Figure 7b–d).
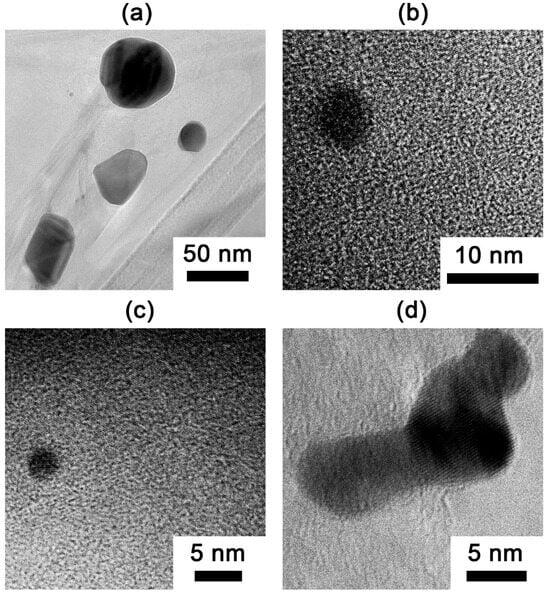
Figure 7.
TEM images of gold nanoparticles: (a) nanoparticles with sizes in the tens of nanometers; (b–d) smaller nanoparticles (several nanometers in size).
Thus, this study demonstrates a versatile route for preparing gold-decorated exfoliated graphite with tunable nanoparticle sizes and distributions through optimization of the thermal conditions, reaction environment, and chemical precursors.
4. Conclusions
This work presents a robust synthetic strategy for fabricating exfoliated graphite decorated with gold nanoparticles via thermal treatment of a graphite intercalation compound with tetrachloroauric acid in air and nitrogen atmospheres. This work establishes, for the first time, a facile synthetic route to HAuCl4-intercalated graphite via exchange reaction between graphite nitrate and tetrachloroauric acid, bypassing the need for chlorine gas oxidation required by conventional gas-phase or liquid-phase methods. The resulting GIC–HAuCl4 can react with methylamine to form an intercalation complex, where coordinated amine–chloride species within the graphite’s matrix lower the onset temperature of exfoliation by ≈100 °C and enhance the expansion efficiency compared to untreated GIC.
Thermally exfoliated graphite composites exhibit gold nanoparticles with sizes ranging from a few nanometers to several hundred nanometers uniformly dispersed on low-density graphite matrices (6–19 g/L), with particle sizes governed primarily by the atmosphere of treatment rather than the processing temperature. The gold nanoparticles exhibit a polydisperse size distribution ranging from a few nanometers (<10 nm) to submicron dimensions (≈300–500 nm).
These fundamental insights into processing–structure relationships provide a rational design framework for engineering graphite-supported functional composites with tailored properties required in catalysis, energy storage, or functional coatings.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15171363/s1, Video S1: Thermal expansion of intercalated graphite particles (GIC–HAuCl4 and GIC–HAuCl4 treated by methylamine).
Author Contributions
A.D.M.: Writing—original draft, investigation, formal analysis. A.V.I.: Writing—review and editing, supervision, formal analysis. V.A.M.: Methodology, investigation. B.A.K.: Investigation, formal analysis. N.V.M.: Validation. V.V.A.: Supervision, resources. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data will be made available on request.
Acknowledgments
This research was performed according to the Development program of the Interdisciplinary Scientific and Educational School of Lomonosov Moscow State University «The future of the planet and global environmental change and State Program» of TIPS RAS and was supported by the Ministry of Education and Science of the Russian Federation and carried out as part of the work on the topic “Substances and materials for safety, reliability and energy efficiency” on assignment by project No. AAAA-A21-121011590086-0.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GIC | Graphite intercalation compound |
| EG | Exfoliated graphite |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
References
- Saikam, L.; Arthi, P.; Senthil, B.; Shanmugam, M. A Review on Exfoliated Graphite: Synthesis and Applications. Inorg. Chem. Commun. 2023, 152, 110685. [Google Scholar] [CrossRef]
- Çalın, Ö.; Kurt, A.; Çelik, Y. Influence of Expansion Conditions and Precursor Flake Size on Porous Structure of Expanded Graphite. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 611–620. [Google Scholar] [CrossRef]
- Goudarzi, R.; Motlagh, G.H. The Effect of Graphite Intercalated Compound Particle Size and Exfoliation Temperature on Porosity and Macromolecular Diffusion in Expanded Graphite. Heliyon 2019, 5, e02595. [Google Scholar] [CrossRef]
- Solfiti, E.; Berto, F. Mechanical Properties of Flexible Graphite: Review. Procedia Struct. Integr. 2020, 25, 420–429. [Google Scholar] [CrossRef]
- Wei, X.H.; Liu, L.; Zhang, J.X.; Shi, J.L.; Guo, Q.G. Mechanical, Electrical, Thermal Performances and Structure Characteristics of Flexible Graphite Sheets. J. Mater. Sci. 2010, 45, 2449–2455. [Google Scholar] [CrossRef]
- Bouzid, A.-H.; Das, S.K. High Temperature Aged Leakage Relaxation Screening Tests on Confined Flexible Graphite Gaskets. In Proceedings of the ASME 2021 Pressure Vessels & Piping Conference, Virtual, 13–15 July 2021; American Society of Mechanical Engineers Digital Collection. ASME: New York, NY, USA, 2021. [Google Scholar]
- Khovavko, A.; Strativnov, E.; Nebesnyi, A.; Filonenko, D.; Sviatenko, O.; Piatova, A.; Barabash, M. Production Technology and Application of Materials Based on Thermally Expanded Graphite. In Carbon Nanostructured Materials: Synthesis, Characterization, and Industrial Applications; Khovavko, A., Strativnov, E., Nebesnyi, A., Filonenko, D., Sviatenko, O., Piatova, A., Barabash, M., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 97–151. ISBN 978-3-031-64121-3. [Google Scholar]
- Bouzid, A.-H. A Study on Liquid Leak Rates in Packing Seals. Appl. Sci. 2021, 11, 1936. [Google Scholar] [CrossRef]
- Solfiti, E.; Berto, F. A Review on Thermophysical Properties of Flexible Graphite. Procedia Struct. Integr. 2020, 26, 187–198. [Google Scholar] [CrossRef]
- Chriaa, I.; Karkri, M.; Trigui, A.; Jedidi, I.; Abdelmouleh, M.; Boudaya, C. The Performances of Expanded Graphite on the Phase Change Materials Composites for Thermal Energy Storage. Polymer 2021, 212, 123128. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, J.; Wei, T.; Liu, X. Thermal Conductivity Experiment of Interface Material Based on Graphite Sheet. J. Phys. Conf. Ser. 2024, 2825, 012040. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Pan, W.; Wang, M.; Zhang, H.; Zhang, D.; Zhang, D. Application of Expanded Graphite-Based Materials for Rechargeable Batteries beyond Lithium-Ions. Nanoscale 2021, 13, 19291–19305. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Nagarajan, R.D.; Shetty, B.H.; Govindasamy, M.; Sundramoorthy, A.K. Recent Trends in the Applications of Thermally Expanded Graphite for Energy Storage and Sensors—A Review. Nanoscale Adv. 2021, 3, 6294–6309. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, C.; Zhang, W.; Pan, W.; Wang, Q.; Li, L. Expanded Graphite-Based Materials for Supercapacitors: A Review. Molecules 2022, 27, 716. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Zhang, S.; Ji, X.; Li, L. Synthesis of Expanded Graphite-Based Materials for Application in Lithium-Based Batteries. J. Energy Storage 2023, 60, 106678. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Q.; Zhang, Y.; Fan, J.; Ma, L. Sorption and Regeneration of Magnetic Exfoliated Graphite as a New Sorbent for Oil Pollution. Desalination 2010, 263, 183–188. [Google Scholar] [CrossRef]
- Takeuchi, K.; Fujishige, M.; Kitazawa, H.; Akuzawa, N.; Medina, J.O.; Morelos-Gomez, A.; Cruz-Silva, R.; Araki, T.; Hayashi, T.; Terrones, M.; et al. Oil Sorption by Exfoliated Graphite from Dilute Oil–Water Emulsion for Practical Applications in Produced Water Treatments. J. Water Process Eng. 2015, 8, 91–98. [Google Scholar] [CrossRef]
- Vinh, N.H.; Hieu, N.P.; Van Thinh, P.; Diep, N.T.M.; Thuan, V.N.; Trinh, N.D.; Thuy, N.H.; Long Giang, B.; Quynh, B.T.P. Magnetic NiFe2O4/Exfoliated Graphite as an Efficient Sorbent for Oils and Organic Pollutants. J. Nanosci. Nanotechnol. 2018, 18, 6859–6866. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Volkova, S.I.; Maksimova, N.V.; Pokholok, K.V.; Kravtsov, A.V.; Belik, A.A.; Posokhova, S.M.; Kalachev, I.L.; Avdeev, V.V. Exfoliated Graphite with γ-Fe2O3 for the Removal of Oil and Organic Pollutants from the Water Surface: Synthesis, Mossbauer Study, Sorption and Magnetic Properties. J. Alloys Compd. 2023, 960, 170619. [Google Scholar] [CrossRef]
- Asalieva, E.; Sineva, L.; Sinichkina, S.; Solomonik, I.; Gryaznov, K.; Pushina, E.; Kulchakovskaya, E.; Gorshkov, A.; Kulnitskiy, B.; Ovsyannikov, D.; et al. Exfoliated Graphite as a Heat-Conductive Frame for a New Pelletized Fischer–Tropsch Synthesis Catalyst. Appl. Catal. A Gen. 2020, 601, 117639. [Google Scholar] [CrossRef]
- Dunaev, A.V.; Arkhangelsky, I.V.; Zubavichus, Y.V.; Avdeev, V.V. Preparation, Structure and Reduction of Graphite Intercalation Compounds with Hexachloroplatinic Acid. Carbon 2008, 46, 788–795. [Google Scholar] [CrossRef]
- Cai, X.; Qiu, Y.; Zhou, Y.; Jiao, X. Nanoscale Zero-Valent Iron Loaded Vermiform Expanded Graphite for the Removal of Cr(VI) from Aqueous Solution. R. Soc. Open Sci. 2021, 8, 210801. [Google Scholar] [CrossRef] [PubMed]
- Brandão, R.D.; de Freitas Júnior, A.M.; Linares, J.J.; Suarez, P.A.Z.; Dutra, R.C.; Garnier, J.; Tonhá, M.S.; Ballesteros-Plata, D.; Rodríguez-Castellón, E.; Prauchner, M.J. Activated Carbon-Supported Pt Catalysts Intended for the Hydroprocessing of Lipid Feedstocks: Effects of Support Surface Composition and Impregnation Protocol. Molecules 2025, 30, 2862. [Google Scholar] [CrossRef]
- Paris, C.B.; Howe, A.G.; Lewis, R.J.; Hewes, D.; Morgan, D.J.; He, Q.; Edwards, J.K. Impact of the Experimental Parameters on Catalytic Activity When Preparing Polymer Protected Bimetallic Nanoparticle Catalysts on Activated Carbon. ACS Catal. 2022, 12, 4440–4454. [Google Scholar] [CrossRef]
- Islam, J.; Kim, S.-K.; Kim, K.-H.; Lee, E.; Park, G.-G. Enhanced Durability of Pt/C Catalyst by Coating Carbon Black with Silica for Oxygen Reduction Reaction. Int. J. Hydrogen Energy 2021, 46, 1133–1143. [Google Scholar] [CrossRef]
- Meekins, B.H.; Thompson, A.B.; Gopal, V.; Mehrabadi, B.A.T.; Elvington, M.C.; Ganesan, P.; Newhouse-Illige, T.A.; Shepard, A.W.; Scipioni, L.E.; Greer, J.A.; et al. In-Situ and Ex-Situ Comparison of the Electrochemical Oxidation of SO2 on Carbon Supported Pt and Au Catalysts. Int. J. Hydrogen Energy 2020, 45, 1940–1947. [Google Scholar] [CrossRef]
- Borisov, V.A.; Iost, K.N.; Temerev, V.L.; Surovikin, Y.V.; Osipov, A.R.; Trenikhin, M.V.; Smorokov, A.A.; Shlyapin, D.A. Effect of Sibunite Graphitization on the Stability of Ru (Pt, Pd)/Sibunit Catalysts in an Oxidizing Atmosphere at Elevated Temperatures. Catal. Ind. 2021, 13, 252–257. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Shen, J.; Tang, T.; Huang, R. Dispersion of Magnetic Metals on Expanded Graphite for the Shielding of Electromagnetic Radiations. Appl. Phys. Lett. 2007, 90, 133117. [Google Scholar] [CrossRef]
- Yao, Y.; Jin, S.; Sun, J.; Li, L.; Zou, H.; Wen, P.; Lv, G.; Lv, X.; Shu, Q. Sandwich-like Sulfur-Free Expanded Graphite/CoNi Hybrids and Their Synergistic Enhancement of Microwave Absorption. J. Alloys Compd. 2021, 862, 158005. [Google Scholar] [CrossRef]
- Song, N.-N.; Yang, H.-T.; Liu, H.-L.; Ren, X.; Ding, H.-F.; Zhang, X.-Q.; Cheng, Z.-H. Exceeding Natural Resonance Frequency Limit of Monodisperse Fe3O4 Nanoparticles via Superparamagnetic Relaxation. Sci. Rep. 2013, 3, 3161. [Google Scholar] [CrossRef] [PubMed]
- Muravev, A.D.; Ivanov, A.V.; Mukhanov, V.A.; Razuvaeva, V.A.; Vasiliev, A.V.; Kazin, P.E.; Avdeev, V.V. Preparation of Exfoliated Graphite Containing Ferromagnetic Iron, Cobalt, and Nickel Alloys. Inorg. Mater. 2024, 60, 838–845. [Google Scholar] [CrossRef]
- Muravev, A.D.; Ivanov, A.V.; Mukhanov, V.A.; Pokholok, K.V.; Vasiliev, A.V.; Kazin, P.E.; Sividova, V.D.; Maksimova, N.V.; Kalachev, I.L.; Avdeev, V.V. Preparation of Magnetic Composite Sorbent Based on Exfoliated Graphite with Metallic Iron, Cobalt and Nickel Using Melamine as a Reducing Agent. J. Alloys Compd. 2024, 1000, 175125. [Google Scholar] [CrossRef]
- Li, M.; Fan, Q.; Gao, L.; Liang, K.; Huang, Q. Chemical Intercalation of Layered Materials: From Structure Tailoring to Applications. Adv. Mater. 2024, 36, 2312918. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Ren, H.; Duan, X.; Shakir, I.; Huang, Y.; Duan, X. Layered Intercalation Materials. Adv. Mater. 2021, 33, 2004557. [Google Scholar] [CrossRef]
- Stumpp, E. The Intercalation of Metal Chlorides and Bromides into Graphite. Mater. Sci. Eng. 1977, 31, 53–59. [Google Scholar] [CrossRef]
- Ishii, T.; Shibayama, Y.; Enoki, T.; Bandow, S.; Shirotani, I.; Sugihara, K. C-Axis Compressibility and Thermal Expansion of Gold Trichloride-Graphite Intercalation Compounds (AuCl3-GICs). J. Phys. Soc. Jpn. 1995, 64, 4748–4758. [Google Scholar] [CrossRef]
- Vangelisti, R.; Herold, A. Observations macroscopiques et microscopiques des produits d’insertion du graphite avec le trichlorure d’or. Carbon 1977, 15, 327–333. [Google Scholar] [CrossRef]
- Stumpp, E. From Iron Chloride to Metal Nitrate GICs. Recent Synthetic Developments in GIC Chemistry. Mater. Sci. Forum 1992, 91–93, 1–9. [Google Scholar] [CrossRef]
- Forsman, W.C.; Vogel, F.L.; Carl, D.E.; Hoffman, J. Chemistry of Graphite Intercalation by Nitric Acid. Carbon 1978, 16, 269–271. [Google Scholar] [CrossRef]
- Sorokina, N.E.; Maksimova, N.V.; Avdeev, V.V. Intercalation of Graphite in the Ternary Systems C–HNO3–R (R = H2O, CH3COOH, H3PO4, H2SO4). Inorg. Mater. 2002, 38, 564–570. [Google Scholar] [CrossRef]
- Vittal, J.J.; Puddephatt, R.J. Gold: Inorganic & Coordination Chemistry. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; Wiley: Hoboken, NJ, USA, 2005; ISBN 978-1-119-95143-8. [Google Scholar]
- Stumpp, E.; Ehrhardt, C. Reaction of Metal Chloride Graphite Intercalation Compounds with Ammonia. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1994, 245, 237–242. [Google Scholar] [CrossRef]
- Otto, K.; Oja Acik, I.; Krunks, M.; Tõnsuaadu, K.; Mere, A. Thermal Decomposition Study of HAuCl4·3H2O and AgNO3 as Precursors for Plasmonic Metal Nanoparticles. J. Therm. Anal. Calorim. 2014, 118, 1065–1072. [Google Scholar] [CrossRef]
- Chung, D.D.L. A Review of Exfoliated Graphite. J. Mater. Sci. 2016, 51, 554–568. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Le, G.T.T.; Manyam, J.; Opaprakasit, P.; Chanlek, N.; Grisdanurak, N.; Sreearunothai, P. Divergent Mechanisms for Thermal Reduction of Graphene Oxide and Their Highly Different Ion Affinities. Diam. Relat. Mater. 2018, 89, 246–256. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Divitskaya, D.A.; Lavrin, M.A.; Kravtsov, A.V.; Volkova, S.I.; Maksimova, N.V.; Kalachev, I.L.; Kirichenko, A.N.; Rodionov, N.B.; Malakho, A.P.; et al. Exfoliated Graphite for Sorption of Liquid Hydrocarbons from the Water Surface: Effect of Preparation Conditions on Sorption Capacity and Water Wettability. Adsorption 2024, 30, 755–767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).