Solanaceous Crops-Derived Nitrogen-Doped Biomass Carbon Material as Anode for Lithium-Ion Battery
Abstract
1. Introduction
2. Experimental
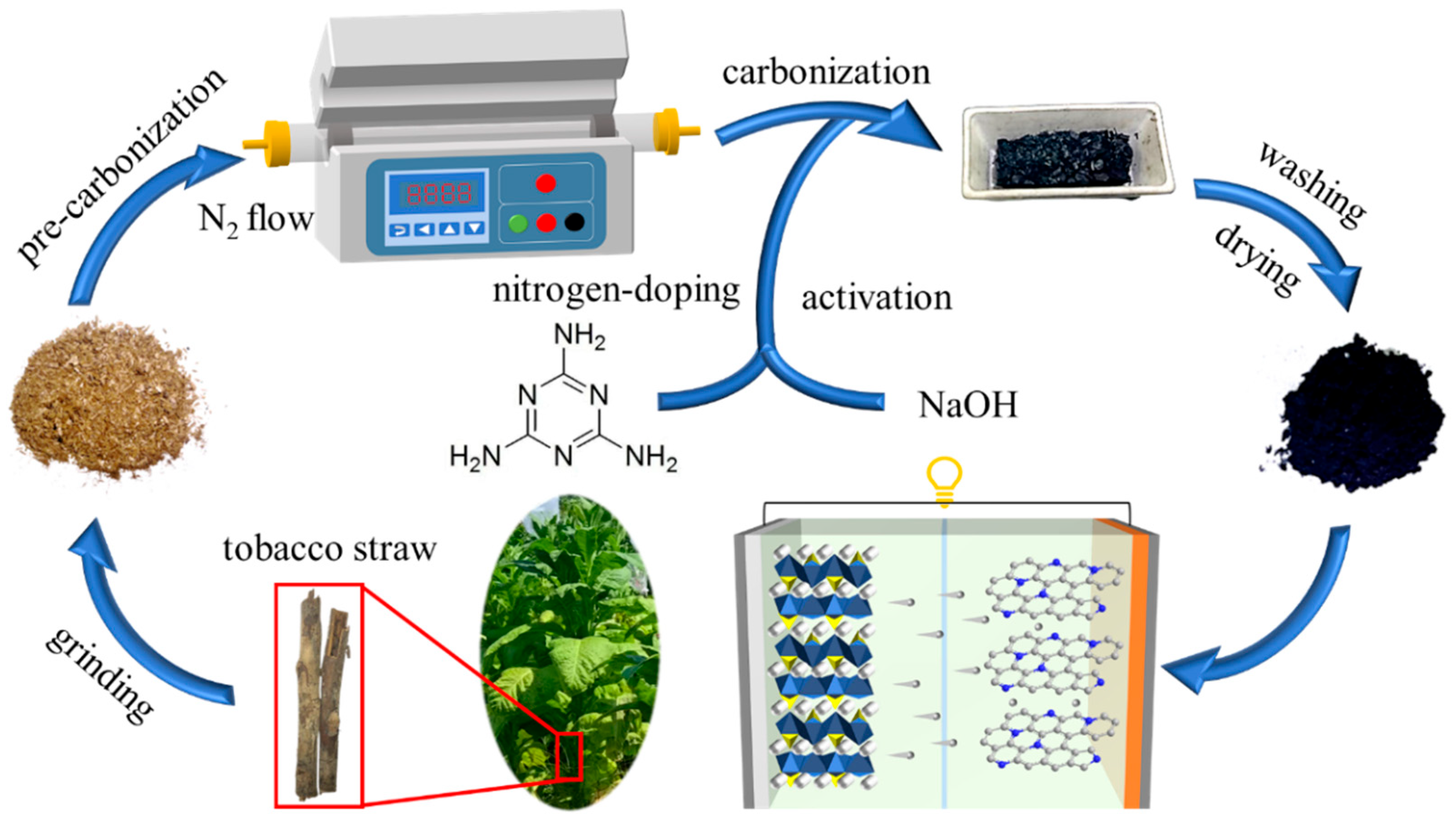
2.1. Preparation of TsC and TsNC
2.2. Materials Characterization
2.3. Electrochemical Measurement
3. Results and Discussion
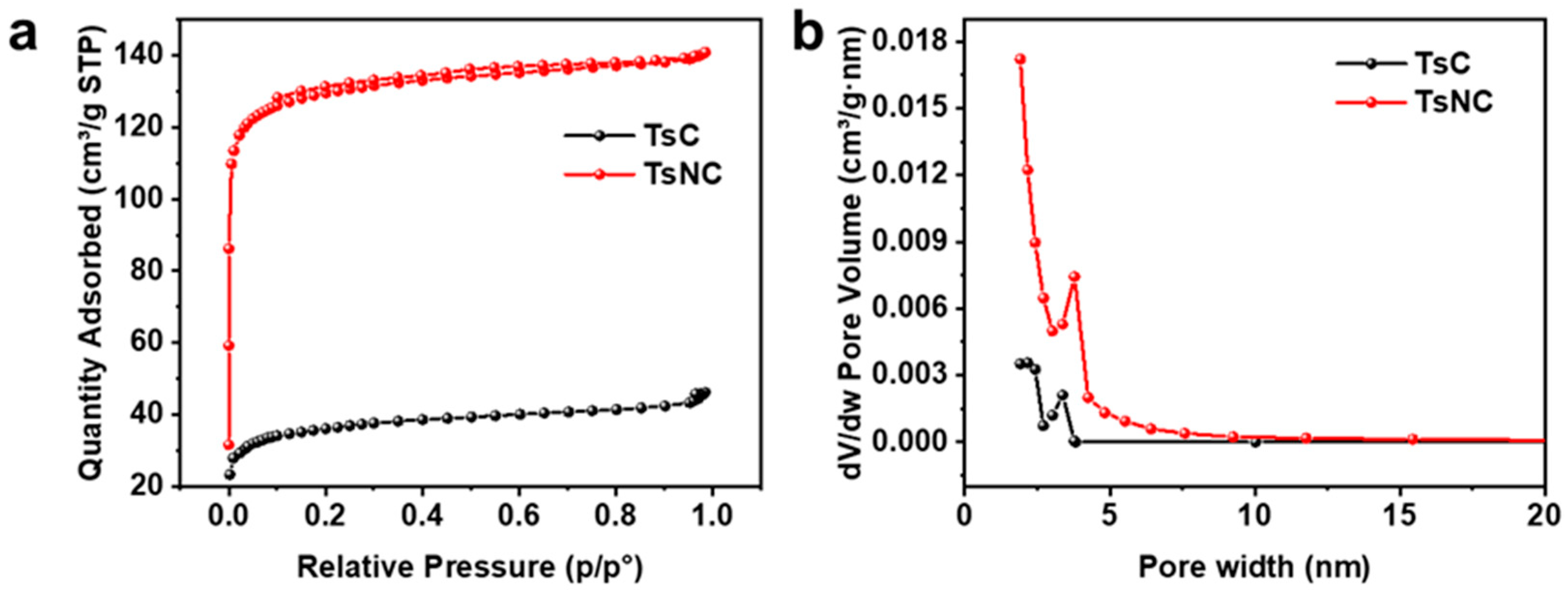
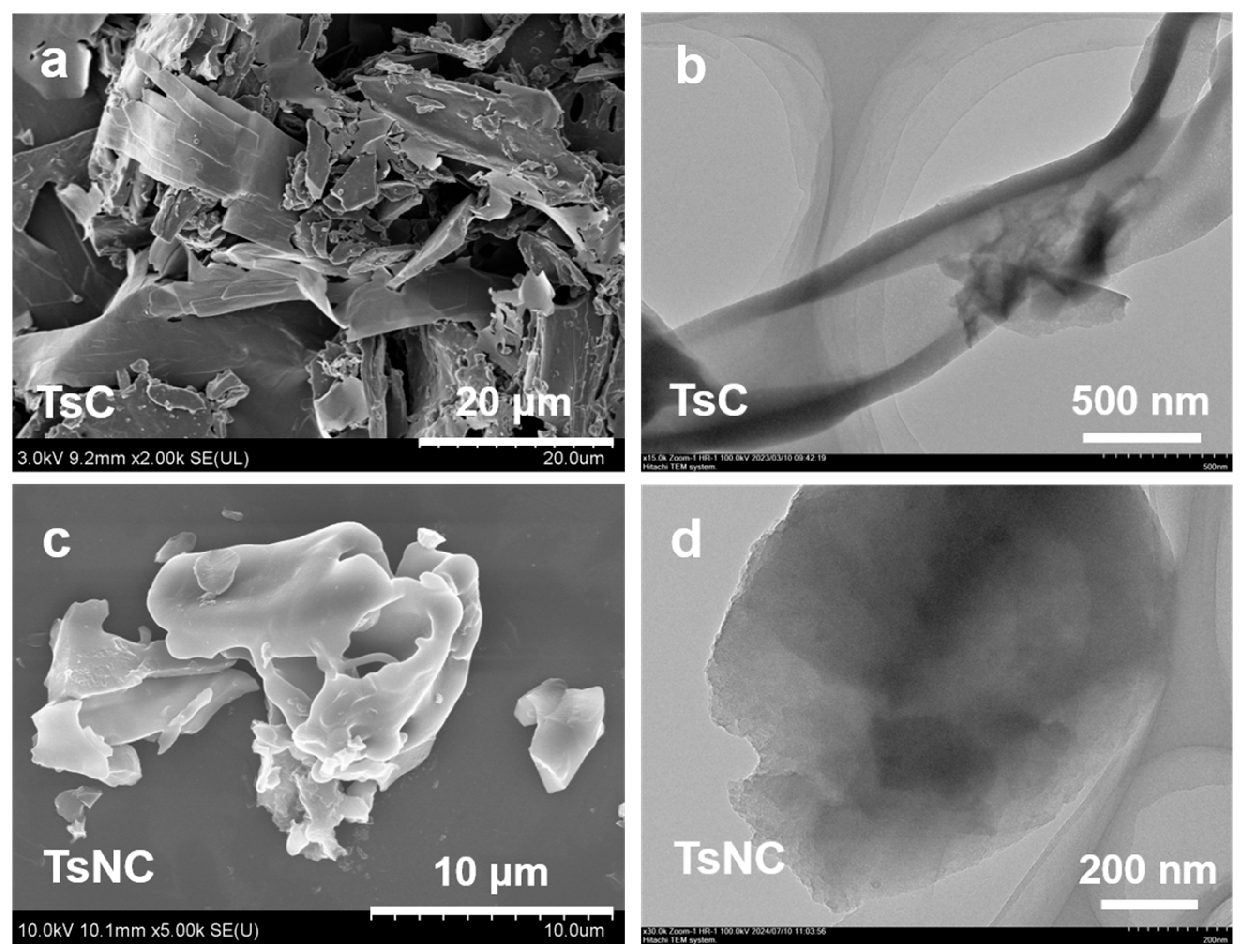
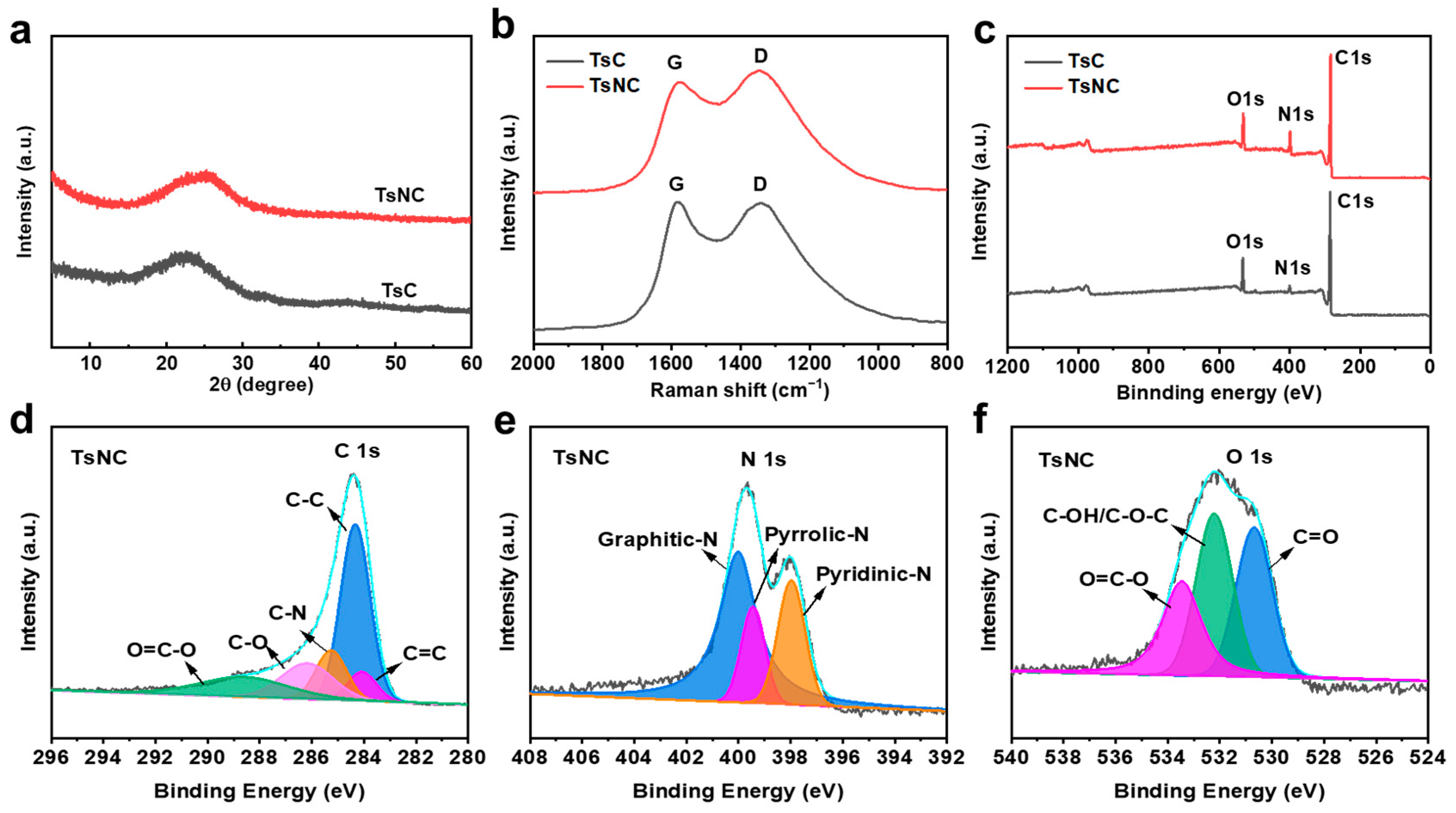
3.1. Morphology and Structure Characterization
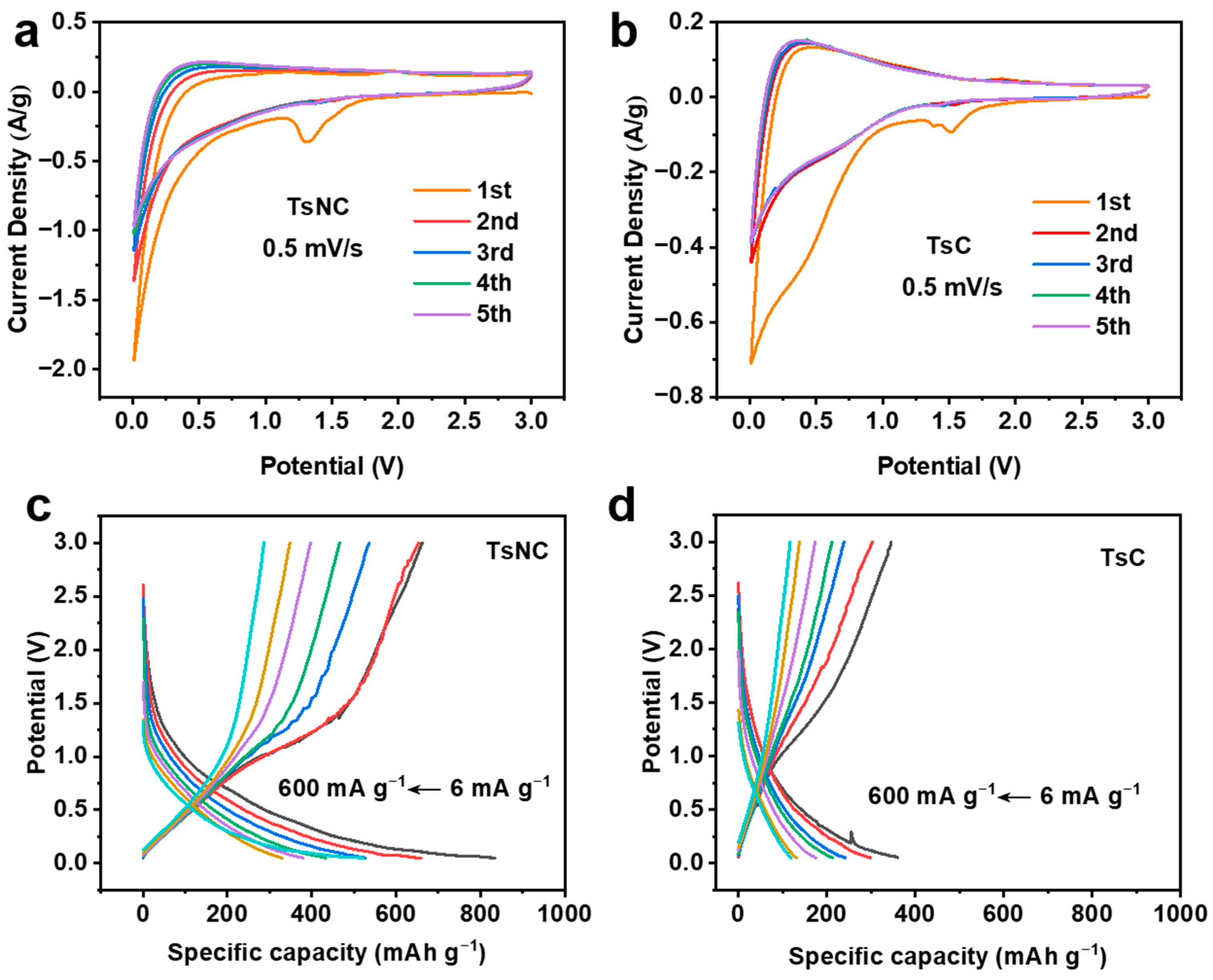
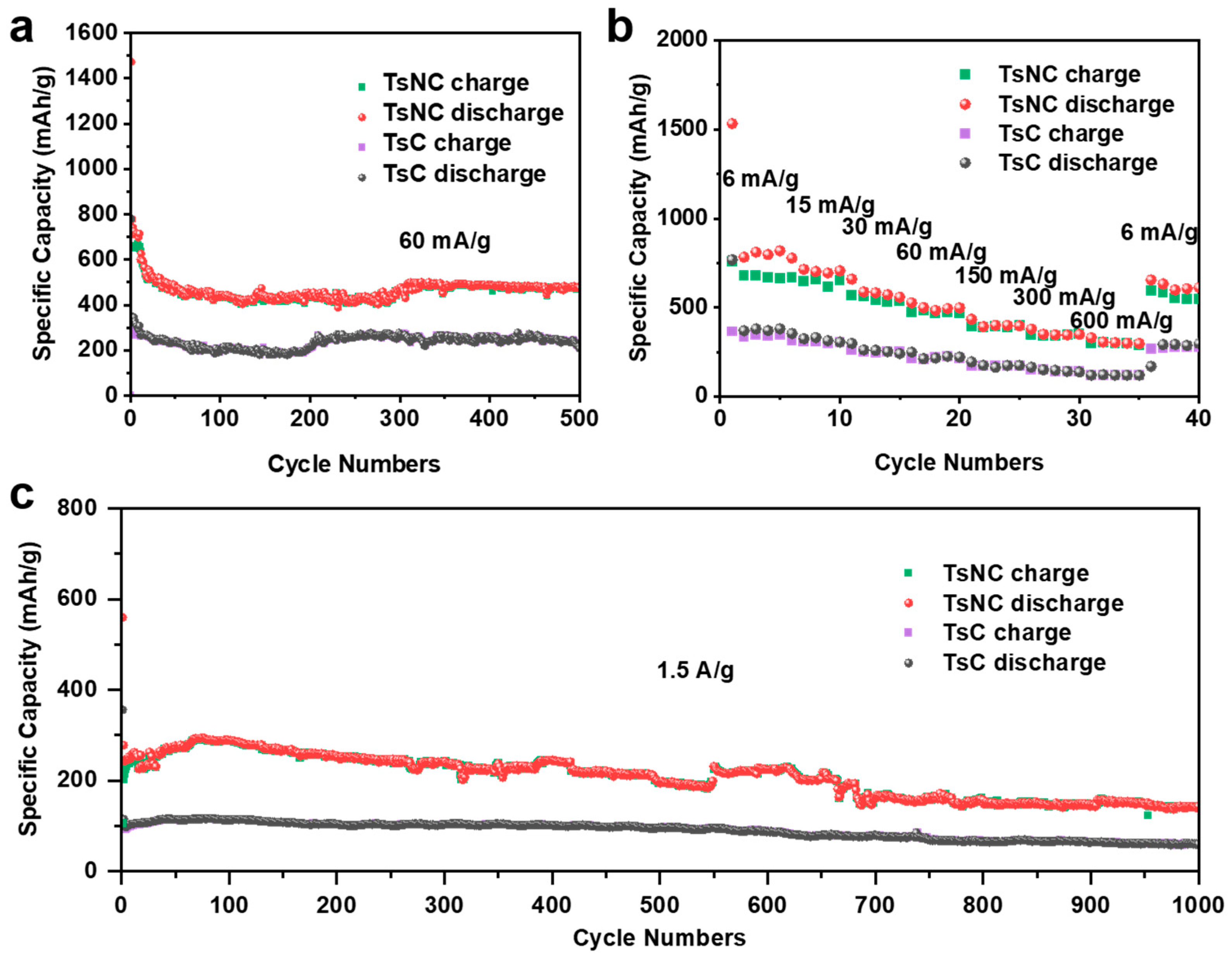
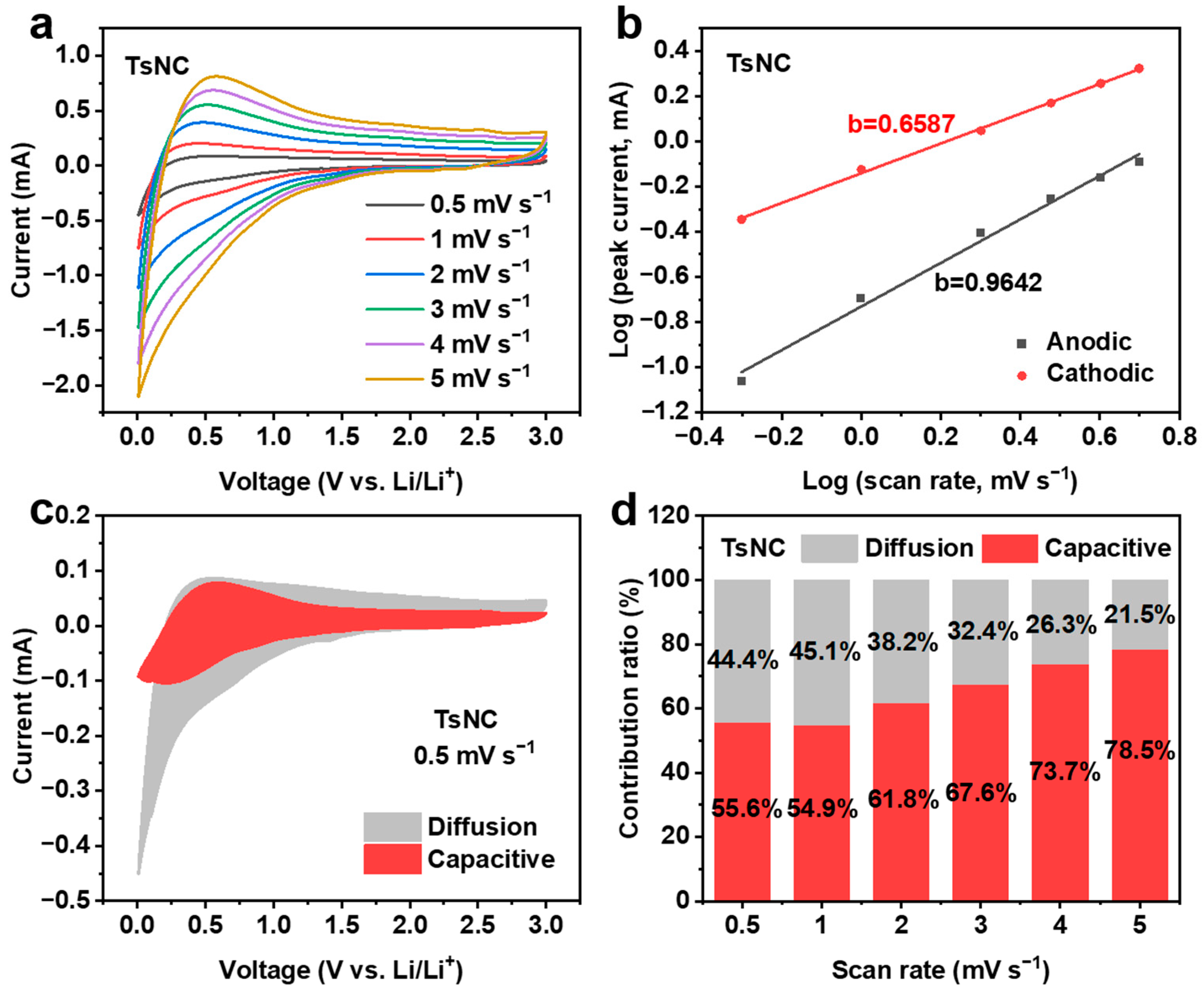
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.X.; Xu, T.; Liu, K.; Zhang, M.; Cai, X.M.; Si, C.L. Biomass-based materials for advanced supercapacitor: Principles, progress, and perspectives. Aggregate 2023, 5, e428. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Kaur, P.; Park, J.S. From coconut shell biomass to oxygen reduction reaction catalyst: Tuning porosity and nitrogen doping. Renew. Sustain. Energy Rev. 2021, 147, 111173. [Google Scholar] [CrossRef]
- Baskar, A.V.; Singh, G.; Ruban, A.M.; Davidraj, J.M.; Bahadur, R.; Sooriyakumar, P.; Kumar, P.; Karakoti, A.; Yi, J.B.; Vinu, A. Recent progress in synthesis and application of biomass-based hybrid electrodes for rechargeable batteries. Adv. Funct. Mater. 2022, 33, 2208349. [Google Scholar] [CrossRef]
- Liedel, C. Sustainable battery materials from biomass. ChemSusChem 2020, 13, 2110–2141. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Shi, H.D.; Wu, Z.S. Recent status, key strategies and challenging perspectives of fast-charging graphite anodes for lithium-ion batteries. Energy Environ. Sci. 2023, 16, 4834–4871. [Google Scholar] [CrossRef]
- Li, X.; Deng, C.; Liu, M.; Xiong, J.; Zhang, X.; Yan, Q.; Lin, J.; Chen, C.; Wu, F.; Zhao, Y.; et al. Reutilization and upcycling of spent graphite for sustainable lithium-ion batteries: Progress and perspectives. eScience 2025, 5, 100394. [Google Scholar] [CrossRef]
- Zhu, Z.; Men, Y.; Zhang, W.; Yang, W.; Wang, F.; Zhang, Y.; Zhang, Y.; Zeng, X.; Xiao, J.; Tang, C.; et al. Versatile carbon-based materials from biomass for advanced electrochemical energy storage systems. eScience 2024, 4, 100249. [Google Scholar] [CrossRef]
- Mao, H.; Yang, S.; Yang, Y.; Yang, J.; Yuan, G.; Zheng, M.; Hu, H.; Liang, Y.; Yu, X. Hybrid catalyst-assisted synthesis of multifunctional carbon derived from Camellia shell for high-performance sodium-ion batteries and sodium-ion hybrid capacitors. Carbon Neutraliz. 2024, 3, 673–688. [Google Scholar] [CrossRef]
- Luo, F.Q.; Lyu, T.Y.; Wang, D.C.; Zheng, Z.F. A review on green and sustainable carbon anodes for lithium ion batteries: Utilization of green carbon resources and recycling waste graphite. Green Chem. 2023, 25, 8950–8969. [Google Scholar] [CrossRef]
- Li, L.F.; Fan, C.L.; Zeng, B.; Tan, M.C. Effect of pyrolysis temperature on lithium storage performance of pyrolitic-PVDF coated hard carbon derived from cellulose. Mater. Chem. Phys. 2020, 242, 122380. [Google Scholar] [CrossRef]
- Yu, K.F.; Wang, J.J.; Wang, X.F.; Liang, J.C.; Liang, C. Sustainable application of biomass by-products: Corn straw-derived porous carbon nanospheres using as anode materials for lithium ion batteries. Mater. Chem. Phys. 2020, 243, 122644. [Google Scholar] [CrossRef]
- Xi, R.F.; Li, Y.Y.; Zhang, Y.; Wang, P.; Hu, D.M. Effects of activation method on biomass carbon-based materials used for electrochemical hydrogen evolution reaction catalyst. Int. J. Hydrogen Energy 2024, 51, 1–19. [Google Scholar] [CrossRef]
- Minakshi, M.; Samayamanthry, A.; Whale, J.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Shrestha, L.K. Phosphorous containing activated carbon derived from natural honeydew peel powers aqueous supercapacitors. Chem. Asian J. 2024, 19, e202400622. [Google Scholar] [CrossRef]
- Saikia, D.; Deka, J.R.; Lu, B.J.; Chen, Y.C.; Lian, J.W.; Kao, H.M.; Yang, Y.C. Pinecone-derived biomass carbons as anodes for lithium and sodium-ion batteries by template-assisted and chemically activated approaches. J. Power Sources 2023, 580, 233329. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Qi, H.; Yu, K.F.; Li, X.J. Formation mechanism and characterization of porous biomass carbon for excellent performance lithium-ion batteries. RSC Adv. 2018, 8, 12666–12671. [Google Scholar] [CrossRef]
- Shen, Y.F.; Zhu, Y.P. One-pot synthesis of biomass-derived porous carbons for multipurpose energy applications. J. Mater. Chem. A 2024, 12, 6211–6242. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Martinez-Fernandez, J.; Ruttert, M.; Winter, M.; Placke, T.; Ramirez-Rico, J. An electrochemical evaluation of nitrogen-doped carbons as anodes for lithium ion batteries. Carbon 2020, 164, 261–271. [Google Scholar] [CrossRef]
- Wang, P.F.; Bai, W.D.; Gong, Z.; Shahzad, M.K.; Shi, F.; Cao, D.X.; Zhu, K. Regulating surface condition of cotton-derived carbon towards enhanced lithium ion storage behavior. Electrochim. Acta 2023, 455, 142431. [Google Scholar] [CrossRef]
- Wan, H.R.; Hu, X.F. Nitrogen doped biomass-derived porous carbon as anode materials of lithium ion batteries. Solid State Ion. 2019, 341, 115030. [Google Scholar] [CrossRef]
- Li, J.C.; Deng, W.F.; Li, H.; Chen, L.; Zhang, Y.P.; Li, J.; Song, Y.Z.; Duan, H. Biomass-derived N–P double-doped porous carbon spheres and their lithium storage mechanism. Int. J. Hydrogen Energy 2024, 56, 828–836. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Gawdzik, B.; Tascón, J.M.D. Phosphorus-containing carbons: Preparation, properties and utilization. Carbon 2019, 157, 796–846. [Google Scholar] [CrossRef]
- Liu, Y.C.; Shi, M.J.; Han, M.N.; Yang, J.; Yu, J.H.; Narayanasamy, M.; Dai, K.; Angaiah, S.; Yan, C. Spontaneous exfoliation and tailoring derived oxygen-riched porous carbon nanosheets for superior Li+ storage performance. Chem. Eng. J. 2020, 387, 124104. [Google Scholar] [CrossRef]
- Sun, X.L.; Chen, Y.; Li, Y.; Luo, F. Biomass alginate derived oxygen-enriched carbonaceous materials with partially graphitic nanolayers for high performance anodes in lithium-ion batteries. Nanomaterials 2023, 13, 82. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Yang, R.X.; Dutta, S.; Ok, Y.S.; Wu, K.C.W. Recent progress in the development of biomass-derived nitrogen-doped porous carbon. J. Mater. Chem. A 2021, 9, 3703–3728. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, X.R.; Chen, X.; Cheng, X.B.; Zhang, X.Q.; Yan, C.; Zhang, Q. Lithiophilic sites in doped graphene guide uniform lithium nucleation for dendrite-free lithium metal anodes. Angew. Chem. Int. Ed. Engl. 2017, 56, 7764–7768. [Google Scholar] [CrossRef]
- Wang, L.; Bai, H.Y.; Zheng, X.C.; Yin, C.F.; Qin, F.; Geng, S.R.; Dong, B.B. Adsorption and migration of lithium ions on the surface of C3N under electric field. Ionics 2024, 30, 779–785. [Google Scholar] [CrossRef]
- Sang, J.J.; Sun, C.X.; Pan, J.H.; Gao, C.; Zhang, R.S.; Jia, F.D.; Wang, F.F.; Wang, Q. Seaweed-Modification of Si by Natural Nitrogen-Doped Porous Biochar for High-Efficiency Lithium Batteries. ACS Appl. Mater. Interfaces 2024, 16, 11389–11399. [Google Scholar] [CrossRef]
- Sekhon, S.S.; Park, J.S. Biomass-derived N-doped porous carbon nanosheets for energy technologies. Chem. Eng. J. 2024, 425, 129017. [Google Scholar] [CrossRef]
- Wang, P.F.; Sui, B.B.; Sha, L.; Gong, Z.; Zhang, Y.H.; Wu, Y.H.; Zhao, L.N.; Shi, F.N. Nitrogen-rich Graphite Flake from Hemp as Anode Material for High Performance Lithium-ion Batteries. Chem. Asian J. 2023, 18, e202300279. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Zheng, X.C.; Yan, Z.H.; Huang, Z.Y.; Yao, Y.; Li, H.X.; Kuang, Y.F.; Zhou, H.H. Construction and preparation of nitrogen-doped porous carbon material based on waste biomass for lithium-ion batteries. Int. J. Hydrogen Energy 2021, 46, 17267–17281. [Google Scholar] [CrossRef]
- Chen, X.L.; Li, F.; Su, S.B.; Chen, H.Y.; Zhang, J.H.; Cai, D.D. Efficient honeycomb–shaped biochar anodes for lithium-ion batteries from Eichhornia crassipes biomass. Environ. Chem. Lett. 2021, 19, 3505–3510. [Google Scholar] [CrossRef]
- Wei, X.X.; Deng, Y.R.; Hu, X.M.; Zhao, J.K.; Wei, H.B.; Yang, Z.Y.; Han, G.Y. Biomass derived fibrous porous carbon loaded zinc oxide nanoparticles as high-performance anode materials for lithium ion batteries. J. Energy Storage 2023, 70, 107854. [Google Scholar] [CrossRef]
- Chang, H.Y.; Deng, H.; Wang, Y.; Wang, S.; Cao, L.L.; Dong, Z.F.; Tan, T.L. Synthesis of large mesoporous carbon from cotton stalk for use as an anode for lithium-ion batteries. Biomass Bioenergy 2022, 167, 106641. [Google Scholar] [CrossRef]
- Fromm, O.; Heckmann, A.; Rodehorst, U.C.; Frerichs, J.; Becker, D.; Winter, M.; Placke, T. Carbons from biomass precursors as anode materials for lithium ion batteries: New insights into carbonization and graphitization behavior and into their correlation to electrochemical performance. Carbon 2018, 128, 147–163. [Google Scholar] [CrossRef]
- Gu, R.X.; Fu, D.H.; Jin, Y.H.; Jia, M.Q.; Nie, K.L. Aspergillus niger fermentation residues application to produce biochar for the anode of lithium-ion batteries. J. Environ. Manag. 2023, 346, 118985. [Google Scholar] [CrossRef]
- Luo, W.B.; He, Q.; Zhang, C.Q.; Jiang, Z.Y.; Cheng, Y.; Wang, H.S. Lignin-based polymer networks enabled N, S Co-doped defect-rich hierarchically porous carbon anode for long-cycle Li-ion batteries. ACS Sustain. Chem. Eng. 2024, 12, 2881–2892. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Jin, Y.H.; Han, T.; Yang, H.M.; Gond, R.; Subasi, Y.; Asfaw, H.D.; Younesi, R.; Jönsson, P.G.; Yang, W.H. Bio-based anode material production for lithium–ion batteries through catalytic graphitization of biochar: The deployment of hybrid catalysts. Sci. Rep. 2024, 14, 3966. [Google Scholar] [CrossRef]
- He, W.; Luo, H.; Jing, P.; Wang, H.M.; Xu, C.H.Y.; Wu, H.; Wang, Q.; Zhang, Y. Embedding silicon in biomass-derived porous carbon framework as high-performance anode of lithium-ion batteries. J. Alloys Compd. 2022, 918, 165364. [Google Scholar] [CrossRef]
- Song, Y.J.; Kim, K.H.; Ahn, H.J. Co/CoO particle within F, N-codoped mesoporous carbon framework for anode of lithium-ion batteries. J. Alloys Compd. 2023, 969, 172365. [Google Scholar] [CrossRef]
- Xu, X.J.; Wu, F.Z.; Yang, W.L.; Dai, X.Y.; Wang, T.H.; Zhou, J.W.; Wang, J.; Guo, D. Silicon@ natural nitrogen-doped biomass carbon composites derived from “silicon tofu” as green and efficient anode materials for lithium-ion batteries. ACS Sustain. Chem. Eng. 2021, 9, 13215–13224. [Google Scholar] [CrossRef]
- Belmesov, A.A.; Glukhov, A.A.; Kayumov, R.R.; Podlesniy, D.N.; Latkovskaya, E.M.; Repina, M.A.; Ivanov, N.P.; Tsvetkov, M.V.; Shichalin, O.O. Using aquatic plant-derived biochars as carbon materials for the negative electrodes of Li-ion batteries. Coatings 2023, 13, 2075. [Google Scholar] [CrossRef]
- Pozio, A.; Di Carli, M.; Aurora, A.; Falconieri, M.; Della Seta, L.; Prosini, P.P. Hard carbons for use as electrodes in Li-S and Li-ion batteries. Nanomaterials 2022, 12, 1349. [Google Scholar] [CrossRef]
- Long, W.Y.; Fang, B.Z.; Ignaszak, A.; Wu, Z.Z.; Wang, Y.J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef]
- Jiang, Q.W.; Ni, Y.Y.; Zhang, Q.; Gao, J.F.; Wang, Z.Q.; Yin, H.H.; Jing, Y.; Wang, J. Sustainable nitrogen self-doped carbon nanofibers from biomass chitin as anodes for high-performance lithium-ion batteries. Energy Fuels 2022, 36, 4026–4033. [Google Scholar] [CrossRef]
- Yang, L.; Lei, Y.Y.; Liang, X.M.; Qu, L.Y.; Xu, K.; Hua, Y.N.; Feng, J.W. SnO2 nanoparticles composited with biomass N-doped carbon microspheres as low cost, environmentally friendly and high-performance anode material for sodium-ion and lithium-ion batteries. J. Power Sources 2022, 547, 232032. [Google Scholar] [CrossRef]
- Xu, X.; Ying, H.J.; Zhang, S.L.; Meng, Z.; Yan, X.F.; Han, W.Q. Biomass-derived 3D interconnected porous carbon-encapsulated nano-FeS2 for high-performance lithium-ion batteries. ACS Appl. Energy Mater. 2020, 3, 5589–5596. [Google Scholar] [CrossRef]
- Li, S.Y.; Jiang, Z.Y.; Liu, A.M.; Lu, J.; Du, J.; Tao, Y.H.; Cheng, Y.; Wang, H.S. A porous carbon based on the surface and structural regulation of wasted lignin for long-cycle lithium-ion battery. Int. J. Biol. Macromol. 2022, 222, 1414–1422. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.X.; Li, H.X.; Wen, Y.L.; Xu, C.X.; Qin, S.F.; Kuang, Y.F.; Zhou, H.H.; Huang, Z.Y. The in situ formation of ZnS nanodots embedded in honeycomb-like NS co-doped carbon nanosheets derived from waste biomass for use in lithium-ion batteries. New Carbon Mater. 2023, 38, 543–552. [Google Scholar] [CrossRef]
- Minakshi, M.; Aughterson, R.; Sharma, P.; Sunda, A.P.; Ariga, K.; Shrestha, L.K. Micelle-Assisted Electrodeposition of γ-MnO2 on Lead Anodes: Structural and Electrochemical Insights. ChemNanoMat 2025, e202500270. [Google Scholar] [CrossRef]
- Sui, D.; Yao, M.; Si, L.Q.; Yan, K.; Shi, J.G.; Wang, J.S.; Xu, C.C.; Zhang, Y.S. Biomass-derived carbon coated SiO2 nanotubes as superior anode for lithium-ion batteries. Carbon 2023, 205, 510–518. [Google Scholar] [CrossRef]
- Shang, H.; Hao, X.; Zhou, Y.; Peng, J.; Guo, L.; Li, H.; Sun, B. Activated Carbon from Spartina alterniflora and Its N-Doped Material for Li-Ion Battery Anode. Nanomaterials 2025, 15, 658. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, H.; Zhou, Y.; Li, H.; Peng, J.; Hao, X.; Guo, L.; Sun, B. Solanaceous Crops-Derived Nitrogen-Doped Biomass Carbon Material as Anode for Lithium-Ion Battery. Nanomaterials 2025, 15, 1357. https://doi.org/10.3390/nano15171357
Shang H, Zhou Y, Li H, Peng J, Hao X, Guo L, Sun B. Solanaceous Crops-Derived Nitrogen-Doped Biomass Carbon Material as Anode for Lithium-Ion Battery. Nanomaterials. 2025; 15(17):1357. https://doi.org/10.3390/nano15171357
Chicago/Turabian StyleShang, Hong, Yougui Zhou, Huipeng Li, Jia Peng, Xinmeng Hao, Lihua Guo, and Bing Sun. 2025. "Solanaceous Crops-Derived Nitrogen-Doped Biomass Carbon Material as Anode for Lithium-Ion Battery" Nanomaterials 15, no. 17: 1357. https://doi.org/10.3390/nano15171357
APA StyleShang, H., Zhou, Y., Li, H., Peng, J., Hao, X., Guo, L., & Sun, B. (2025). Solanaceous Crops-Derived Nitrogen-Doped Biomass Carbon Material as Anode for Lithium-Ion Battery. Nanomaterials, 15(17), 1357. https://doi.org/10.3390/nano15171357







