Synthesis of RuO2-Co3O4 Composite for Efficient Electrocatalytic Oxygen Evolution Reaction
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of Samples
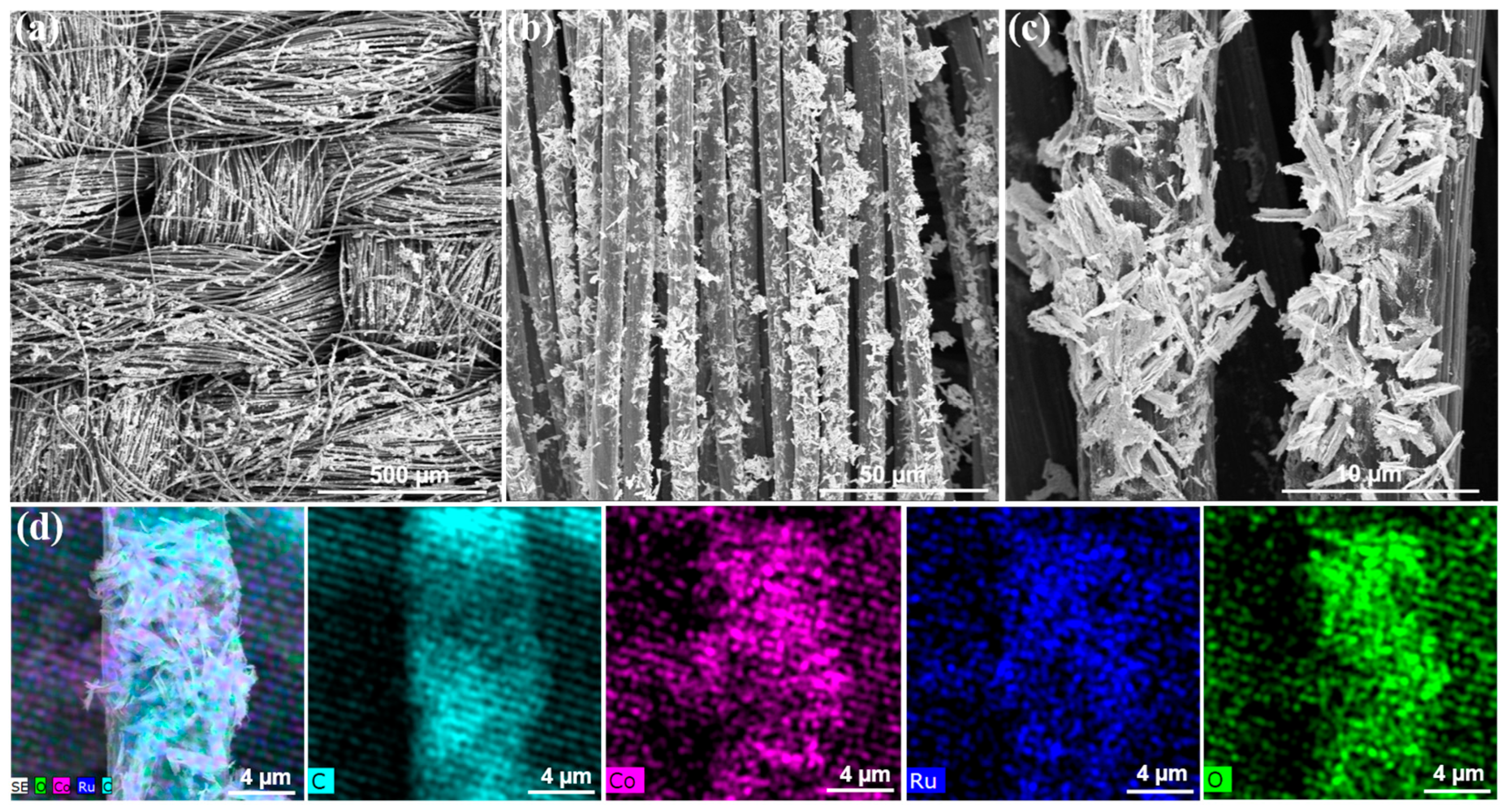
2.3. Material Characterization
2.4. Electrode Preparation and Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, H.; Liu, H.; Chu, W.; Wu, C.; Xie, Y. Structural Transformation of Heterogeneous Materials for Electrocatalytic Oxygen Evolution Reaction. Chem. Rev. 2021, 121, 13174–13212. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.; Jiang, H.; Mei, G.L.; Sun, Y.J.; You, B. Bifunctional Electrocatalysts for Overall and Hybrid Water Splitting. Chem. Rev. 2024, 124, 3694–3812. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.T.; Zhang, S.; Wang, P.T.; Jaroniec, M.; Zheng, Y.; Qiao, S.Z. Urea catalytic oxidation for energy and environmental applications. Chem. Soc. Rev. 2024, 53, 1552–1591. [Google Scholar] [CrossRef]
- Yan, D.F.; Mebrahtu, C.; Wang, S.Y.; Palkovits, R. Innovative Electrochemical Strategies for Hydrogen Production: From Electricity Input to Electricity Output. Angew. Chem. Int. Ed. 2023, 62, e202214333. [Google Scholar] [CrossRef]
- Chen, L.; Yu, C.; Dong, J.T.; Han, Y.N.; Huang, H.L.; Li, W.B.; Zhang, Y.F.; Tan, X.Y.; Qiu, J.S. Seawater electrolysis for fuels and chemicals production: Fundamentals, achievements, and perspectives. Chem. Soc. Rev. 2024, 53, 7455–7488. [Google Scholar] [CrossRef]
- Li, Y.; Wei, X.F.; Chen, L.S.; Shi, J.L. Electrocatalytic Hydrogen Production Trilogy. Angew. Chem. Int. Ed. 2021, 60, 19550–19571. [Google Scholar] [CrossRef]
- Yu, Z.P.; Liu, L.F. Recent Advances in Hybrid Seawater Electrolysis for Hydrogen Production. Adv. Mater. 2024, 36, 2308647. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.Q.; Cao, Z.Y.; Yin, Z.H.; Ma, T.L.; Chen, S.R. Current progress of metal sulfides derived from metal–organic frameworks for advanced electrocatalysis: Potential electrocatalysts with diverse applications. J. Mater. Chem. A 2022, 10, 1617–1641. [Google Scholar] [CrossRef]
- Yue, Y.; Zhong, X.Y.; Sun, M.Z.; Du, J.; Gao, W.S.; Hu, W.; Zhao, C.Y.; Li, J.; Huang, B.L.; Li, Z.L.; et al. Fluorine Engineering Induces Phase Transformation in NiCo2O4 for Enhanced Active Motifs Formation in Oxygen Evolution Reaction. Adv. Mater. 2025, 37, 2418058. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, T.; Jiang, J.; Feng, Y.; Xu, G.; Zhang, L. Engineering Ru-Complementary Catalytic Centers on Co2P/CoP Heterojunction for Industrial Alkaline Water Electrolysis. Adv. Funct. Mater. 2025, 35, e07040. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Mao, Y.; Ye, C.Z.; Wang, Z.Y.; Wei, S.H.; Kennedy, J.V.; Zhao, Y.F.; Yang, H.; Cowie, B.C.C.; Waterhouse, G.I.N. Ru Single Atoms Anchored on Co3O4 Nanorods for Efficient Overall Water Splitting under pH- Universal Conditions. Adv. Energy Mater. 2025, 15, 2500700. [Google Scholar] [CrossRef]
- Wang, X.; Pi, W.; Li, Z.B.; Hu, S.; Bao, H.F.; Xu, W.L.; Yao, N. Orbital-level band gap engineering of RuO2 for enhanced acidic water oxidation. Nat. Commun. 2025, 16, 4845. [Google Scholar] [CrossRef]
- Jiao, J.X.; Chen, D.; Zhao, H.Y.; Dong, Y.; Mu, S.C. Durable ruthenium oxide catalysts for water oxidation reaction. Sci. China Chem. 2025, 68, 2217–2233. [Google Scholar] [CrossRef]
- Kandel, M.R.; Pan, U.N.; Dhakal, P.P.; Ghising, R.B.; Sidra, S.; Kim, D.H.; Kim, N.H.; Lee, J.H. Manganese-Doped Bimetallic (Co,Ni)2P Integrated CoP in N,S Co−Doped Carbon: Unveiling a Compatible Hybrid Electrocatalyst for Overall Water Splitting. Small 2024, 20, 2307241. [Google Scholar] [CrossRef]
- Wang, Z.P.; Huang, J.H.; Wang, L.; Liu, Y.Y.; Liu, W.H.; Zhao, S.L.; Liu, Z.Q. Cation-Tuning Induced d-Band Center Modulation on Co-Based Spinel Oxide for Oxygen Reduction/Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202114696. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.Z.; Ge, L.; Yang, Y.S.; Li, M.R.; Jia, Y.; Yao, X.D.; Zhu, Z.H. Ultrathin Iron-Cobalt Oxide Nanosheets with Abundant Oxygen Vacancies for the Oxygen Evolution Reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Budiyanto, E.; Tüysüz, H. Principles of Water Electrolysis and Recent Progress in Cobalt-, Nickel-, and Iron-Based Oxides for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2022, 61, e202103824. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, H.; Xi, S.; Lee, W.S.V.; Xue, J. Understanding of Oxygen Redox in the Oxygen Evolution Reaction. Adv. Mater. 2022, 34, 2107956. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Fu, Q.; Song, B.; Xu, P. Regulation Strategy of Transition Metal Oxide-Based Electrocatalysts for Enhanced Oxygen Evolution Reaction. Acc. Mater. Res. 2022, 3, 1088–1100. [Google Scholar] [CrossRef]
- Sun, X.; Yuan, Y.; Liu, S.Z.; Zhao, H.Q.; Yao, S.Q.; Sun, Y.Y.; Zhang, M.Y.; Liu, Y.J.; Lin, Z.Q. Recent Advances in Perovskite Oxides for Oxygen Evolution Reaction: Structures, Mechanisms, and Strategies for Performance Enhancement. Adv. Funct. Mater. 2025, 35, 2416705. [Google Scholar] [CrossRef]
- Over, H. Fundamental Studies of Planar Single-Crystalline Oxide Model Electrodes (RuO2, IrO2) for Acidic Water Splitting. ACS Catal. 2021, 11, 8848–8871. [Google Scholar] [CrossRef]
- Yang, J.; Huang, L.; Chen, Y.; Li, D.; Sun, J.; Jiang, R.B.; Kang, J.H.; Fang, Y.P. Recent Development of Ir- and Ru-Based Electrocatalysts for Acidic Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2025, 17, 20519–20559. [Google Scholar] [CrossRef]
- Ke, J.; Zhu, W.X.; Ji, Y.J.; Chen, J.X.; Li, C.C.; Wang, Y.; Wang, Q.; Huang, W.-H.; Hu, Z.W.; Li, Y.Y.; et al. Optimizing Acidic Oxygen Evolution Reaction via Modulation Doping in Van der Waals Layered Iridium Oxide. Angew. Chem. Int. Ed. 2025, 64, e202422740. [Google Scholar] [CrossRef]
- Ikram, F.; Cheong, S.; Persson, I.; Ramadhan, Z.R.; Poerwoprajitno, A.R.; Gooding, J.J.; Tilley, R.D. Iridium Nanocrystals Enriched with Defects and Atomic Steps to Enhance Oxygen Evolution Reaction Performance. J. Am. Chem. Soc. 2025, 147, 10784–10790. [Google Scholar] [CrossRef]
- Bertelsen, A.D.; Kløve, M.; Broge, N.L.N.; Bondesgaard, M.; Stubkjær, R.B.; Dippel, A.-C.; Li, Q.Y.; Tilley, R.; Jørgensen, M.R.V.; Iversen, B.B. Formation Mechanism and Hydrothermal Synthesis of Highly Active Ir1−xRuxO2 Nanoparticles for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2024, 146, 23729–23740. [Google Scholar] [CrossRef]
- Sun, S.G.; Wan, Z.Q.; Xu, Y.Y.; Zhou, X.M.; Gao, W.; Qian, J.J.; Gao, J.; Cai, D.; Ge, Y.J.; Nie, H.G.; et al. Phase Engineering Modulates the Electronic Structure of the IrO2/MoS2 Heterojunction for Efffcient and Stable Water Splitting. ACS Nano 2025, 19, 12090–12101. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Y.; Cui, X.H.; Xiao, F.L.; Cui, D.L.; Wang, Q.L.; Dang, F.; Yu, H.H.; Lian, G. Modulating the d-Band Center of RuO2 via Ni Incorporation for Efficient and Durable Li–O2 batteries. Small 2024, 20, 2400010. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shi, X.Z.; Wang, Y.Y.; Jin, J.; Liu, H.W.; Yin, J.; Zhao, Y.-Q.; Xi, P.X. Ruthenium-modified porous NiCo2O4 nanosheets boost overall water splitting in alkaline solution. Chin. Chem. Lett. 2022, 33, 4930–4935. [Google Scholar] [CrossRef]
- Du, K.; Zhang, L.F.; Shan, J.Q.; Guo, J.X.; Mao, J.; Yang, C.-C.; Wang, C.-H.; Hu, Z.P.; Ling, T. Interface engineering breaks both stability and activity limits of RuO2 for sustainable water oxidation. Nat. Commun. 2022, 13, 5448. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Xi, M.H.; Qi, Y.B.; Sheng, X.D.; Tian, P.F.; Zhu, Y.H.; Yang, X.L.; Li, C.Z.; Jiang, H.L. Redirecting dynamic structural evolution of nickel- contained RuO2 catalyst during electrochemical oxygen evolution reaction. J. Energy Chem. 2022, 69, 330–337. [Google Scholar] [CrossRef]
- Yang, M.; Lu, W.; Jin, R.X.; Liu, X.-C.; Song, S.Y.; Xing, Y. Superior Oxygen Evolution Reaction Performance of Co3O4/NiCo2O4/Ni Foam Composite with Hierarchical Structure. ACS Sustain. Chem. Eng. 2019, 7, 12214–12221. [Google Scholar] [CrossRef]
- Gao, X.H.; Zhang, H.X.; Li, Q.G.; Yu, X.G.; Hong, Z.L.; Zhang, X.W.; Liang, C.D.; Lin, Z. Hierarchical NiCo2O4 Hollow Microcuboids as Bifunctional Electrocatalysts for Overall Water-Splitting. Angew. Chem. Int. Ed. 2016, 55, 6290–6294. [Google Scholar] [CrossRef]
- Wang, W.H.; Kuai, L.; Cao, W.; Huttula, M.; Ollikkala, S.; Ahopelto, T.; Honkanen, A.-P.; Huotari, S.; Yu, M.K.; Geng, B.Y. Mass-Production of Mesoporous MnCo2O4 Spinels with Manganese(IV)-and Cobalt(II)-Rich Surfaces for Superior Bifunctional Oxygen Electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 14977–14981. [Google Scholar] [CrossRef]
- Xiao, K.; Wang, Y.F.; Wu, P.Y.; Hou, L.P.; Liu, Z.-Q. Activating Lattice Oxygen in Spinel ZnCo2O4 through Filling Oxygen Vacancies with Fluorine for Electrocatalytic Oxygen Evolution. Angew. Chem. Int. Ed. 2023, 62, e202301408. [Google Scholar] [CrossRef]
- Xu, K.B.; Ma, S.; Shen, Y.N.; Ren, Q.L.; Yang, J.M.; Chen, X.; Hu, J.Q. CuCo2O4 nanowire arrays wrapped in metal oxide nanosheets as hierarchical multicomponent electrodes for supercapacitors. Chem. Eng. J. 2019, 369, 363–369. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Liu, X.H.; Wang, D.; Wan, H.; Zhang, Y.; Chen, G.; Zhang, N.; Ma, R.Z. Ruthenium composited NiCo2O4 spinel nanocones with oxygen vacancies as a high-efficient bifunctional catalyst for overall water splitting. Chem. Eng. J. 2022, 446, 137037. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, F.C.; Sheng, T.; Ye, T.; Yi, D.; Yang, Y.J.; Liu, S.J.; Wang, X.; Yao, J.N. Clarifying the controversial catalytic active sites of Co3O4 for the oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 23191–23198. [Google Scholar] [CrossRef]
- Rong, C.L.; Sun, Q.; Zhu, J.X.; Arandiyan, H.; Shao, Z.P.; Wang, Y.; Chen, Y. Advances in Stabilizing Spinel Cobalt Oxide-Based Catalysts for Acidic Oxygen Evolution Reaction. Adv. Sci. 2025, 12, e09415. [Google Scholar] [CrossRef]
- Li, C.; Ye, B.R.; Ouyang, B.; Zhang, T.F.; Tang, T.; Qiu, Z.; Li, S.P.; Li, Y.Q.; Chen, R.H.; Wen, W.; et al. Dual Doping of N and F on Co3O4 to Activate the Lattice Oxygen for Efficient and Robust Oxygen Evolution Reaction. Adv. Mater. 2025, 37, 2501381. [Google Scholar] [CrossRef]
- Fan, R.Y.; Liu, H.J.; Ren, J.K.; Li, Y.C.; Nan, J.; Zhou, Y.L.; Liu, C.Y.; Chai, Y.M.; Dong, B. Ligand-Confinement-Induced Catalyst−Support Interface Interactions in Co3O4-Supported RuO2 for Long-Term Stable Acidic Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2024, 12, 2313–2323. [Google Scholar] [CrossRef]
- Wang, C.; Qi, L.M. Heterostructured Inter-Doped Ruthenium-Cobalt Oxide Hollow Nanosheet Arrays for Highly Efficient Overall Water Splitting. Angew. Chem. Int. Ed. 2020, 59, 17219–17224. [Google Scholar] [CrossRef]
- Pan, S.C.; Zhang, L.L.; Liu, M.L.; Pan, X.C.; Bi, M.; Guo, T.; Zhang, Y.; Sun, J.W.; Vasiliev, A.; Ouyang, X.P.; et al. Neighboring Site Synergies in Co-Defective Ru−Co Spinel Oxide toward Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2023, 11, 290–299. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.; Guo, R.K.; Xiao, J.F. Ru doping boosts electrocatalytic water splitting. Dalton Trans. 2022, 51, 11208–11225. [Google Scholar] [CrossRef]
- Li, W.M.; Liu, R.; Yu, G.T.; Chen, X.J.; Yan, S.; Ren, S.Y.; Chen, J.J.; Chen, W.; Wang, C.; Lu, X.F. Rationally Construction of Mn-Doped RuO2 Nanofibers for High-Activity and Stable Alkaline Ampere-Level Current Density Overall Water Splitting. Small 2024, 20, 2307164. [Google Scholar] [CrossRef]
- Wang, H.; Abruña, H.D. Comparative Study of Ru-Transition Metal Alloys and Oxides as Oxygen Evolution Reaction Electrocatalysts in Alkaline Media. ACS Appl. Energy Mater. 2022, 5, 11241–11253. [Google Scholar] [CrossRef]
- Zhang, F.F.; Hong, S.H.; Qiao, R.X.; Huang, W.-H.; Tang, Z.; Tang, J.Y.; Pao, C.-W.; Yeh, M.-H.; Dai, J.; Chen, Y.; et al. Boosting Alkaline Hydrogen Evolution by Creating Atomic-Scale Pair Cocatalytic Sites in Single-Phase Single-Atom-Ruthenium-Incorporated Cobalt Oxide. ACS Nano 2025, 19, 11176–11186. [Google Scholar] [CrossRef]
- Wu, D.L.; Chen, D.; Zhu, J.W.; Mu, S.C. Ultralow Ru Incorporated Amorphous Cobalt-Based Oxides for High-Current-Density Overall Water Splitting in Alkaline and Seawater Media. Small 2021, 17, 2102777. [Google Scholar] [CrossRef]
- Wu, Q.X.; Dong, A.Q.; Yang, C.C.; Ye, L.; Zhao, L.J.; Jiang, Q. Metal- organic framework derived Co3O4@Mo-Co3S4-Ni3S2 heterostructure supported on Ni foam for overall water splitting. Chem. Eng. J. 2021, 413, 127482. [Google Scholar] [CrossRef]
- Fan, L.B.; Meng, T.; Yan, M.X.; Wang, D.W.; Chen, Y.T.; Xing, Z.C.; Wang, E.K.; Yang, X.R. Rational Construction of Ruthenium-Cobalt Oxides Heterostructure in ZIFs-Derived Double-Shelled Hollow Polyhedrons for Efficient Hydrogen Evolution Reaction. Small 2021, 17, 2100998. [Google Scholar] [CrossRef]
- Abitha, M.; Chinnuswamy, V.; Ponpandian, N. Oxide derivatives of metal–organic frameworks for water splitting: A concise review. Sustain. Energy Fuels 2025, 9, 921–941. [Google Scholar] [CrossRef]
- Sun, N.N.; Shah, S.S.A.; Lin, Z.Y.; Zheng, Y.Z.; Jiao, L.; Jiang, H.L. MOF-Based Electrocatalysts: An Overview from the Perspective of Structural Design. Chem. Rev. 2025, 125, 2703–2792. [Google Scholar] [CrossRef]
- Li, Y.J.; Feng, Z.H.; Wang, X.; Han, X.; Li, C.J.; Xia, J.X.; Yin, S.; Li, H.M. MOFs-derived 3D Hierarchical CoFe2O4/RuO2 Hollow Nanosheet Array for Efficient Overall Water Splitting. Electrochim. Acta 2024, 503, 144930. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, Z.Y.; Liu, X.; Ding, F.; Zou, P.; Wang, X.X.; Zhao, Q.B.; Rao, H.B. MOF-Derived NiO/NiCo2O4 and NiO/NiCo2O4-rGO as Highly Efficient and Stable Electrocatalysts for Oxygen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 12511–12521. [Google Scholar] [CrossRef]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Song, X.Z.; Ni, J.C.; Wang, X.B.; Dong, J.H.; Liang, H.J.; Pan, Y.; Dai, Y.; Tan, Z.Q.; Wang, X.F. Hollow Starlike Ag/CoMo-LDH Heterojunction with a Tunable d-Band Center for Boosting Oxygen Evolution Reaction Electrocatalysis. Inorg. Chem. 2023, 62, 13328–13337. [Google Scholar] [CrossRef]
- Zheng, W.R. iR Compensation for Electrocatalysis Studies: Considerations and Recommendations. ACS Energy Lett. 2023, 8, 1952–1958. [Google Scholar] [CrossRef]
- Jin, M.Y.; Han, X.; Yang, A.T.; Chou, T.; Chen, T.T.; Pi, Y.C.; Wang, S.; Yang, Y.; Wang, J.; Jin, H.L. Grain-Boundary-Rich Pt/Co3O4 Nanosheets for Solar-Driven Overall Water Splitting. Inorg. Chem. 2025, 64, 327–334. [Google Scholar] [CrossRef]
- Lazouskaya, M.; Vetik, I.; Tamm, M.; Uppuluri, K.; Scheler, O. Binary RuO2−CuO Electrodes Outperform RuO2 Electrodes in Measuring the pH in Food Samples. ACS Omega 2023, 8, 13275–13284. [Google Scholar] [CrossRef]
- Tian, W.Y.; Xie, X.; Zhang, X.G.; Li, J.H.; Waterhouse, G.I.N.; Ding, J.; Liu, Y.S.; Lu, S.Y. Synergistic Interfacial Effect of Ru/Co3O4 Heterojunctions for Boosting Overall Water Splitting. Small 2024, 20, 2309633. [Google Scholar] [CrossRef]
- Shah, K.; Dai, R.; Mateen, M.; Hassan, Z.; Zhuang, Z.; Liu, C.; Israr, M.; Cheong, W.C.; Hu, B.; Tu, R.; et al. Cobalt Single Atom Incorporated in Ruthenium Oxide Sphere: A Robust Bifunctional Electrocatalyst for HER and OER. Angew. Chem. Int. Ed. 2022, 61, e202114951. [Google Scholar] [CrossRef]

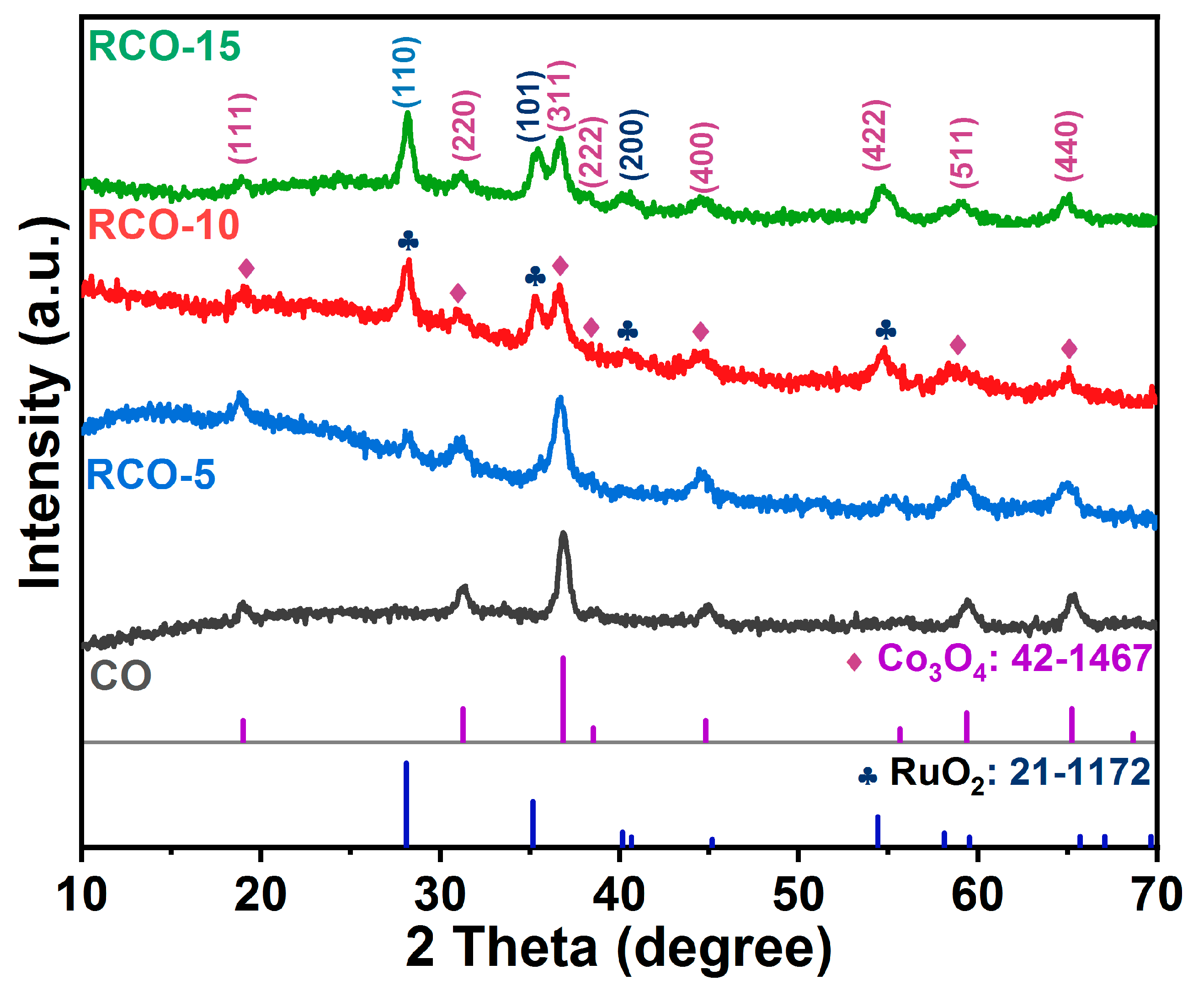
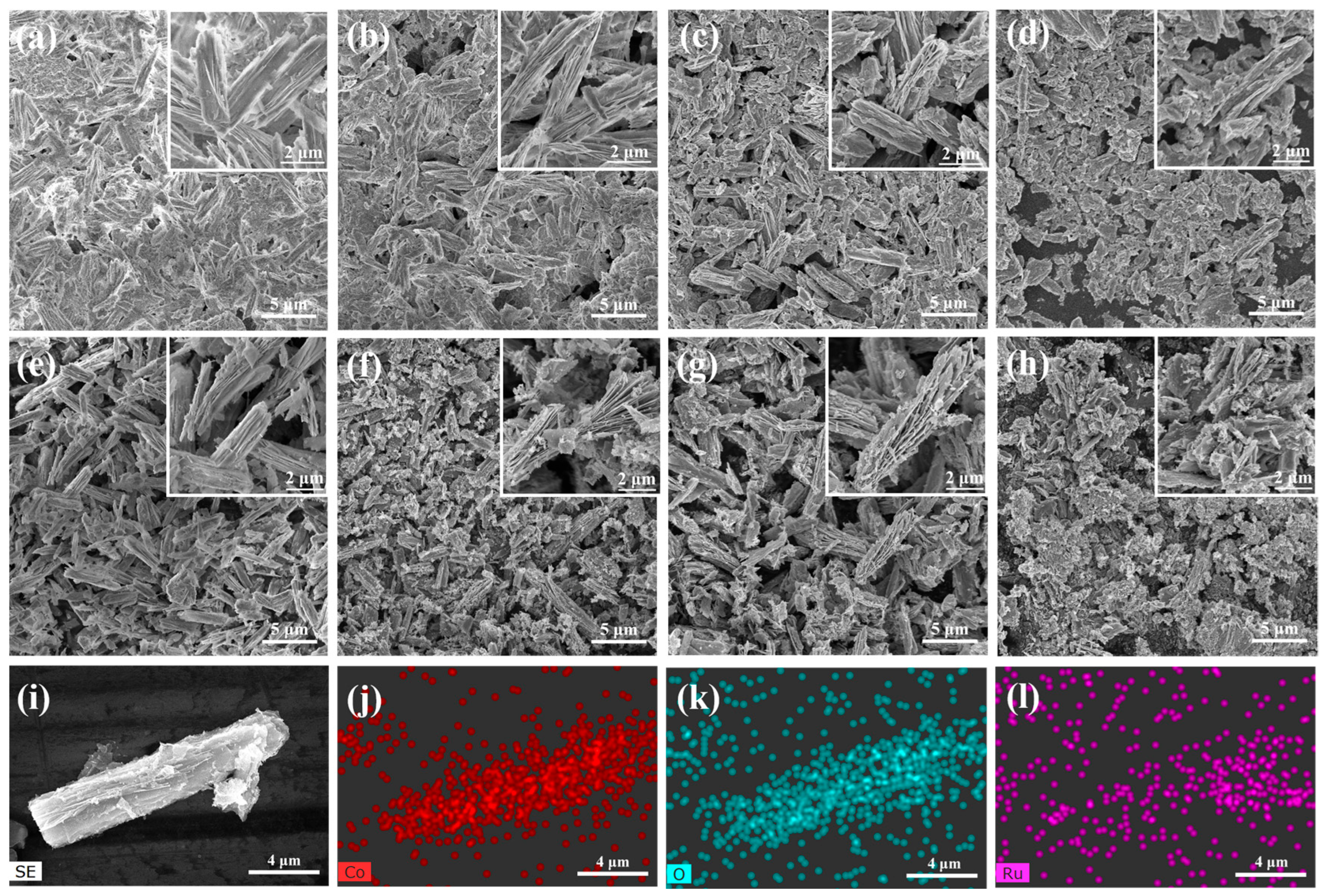

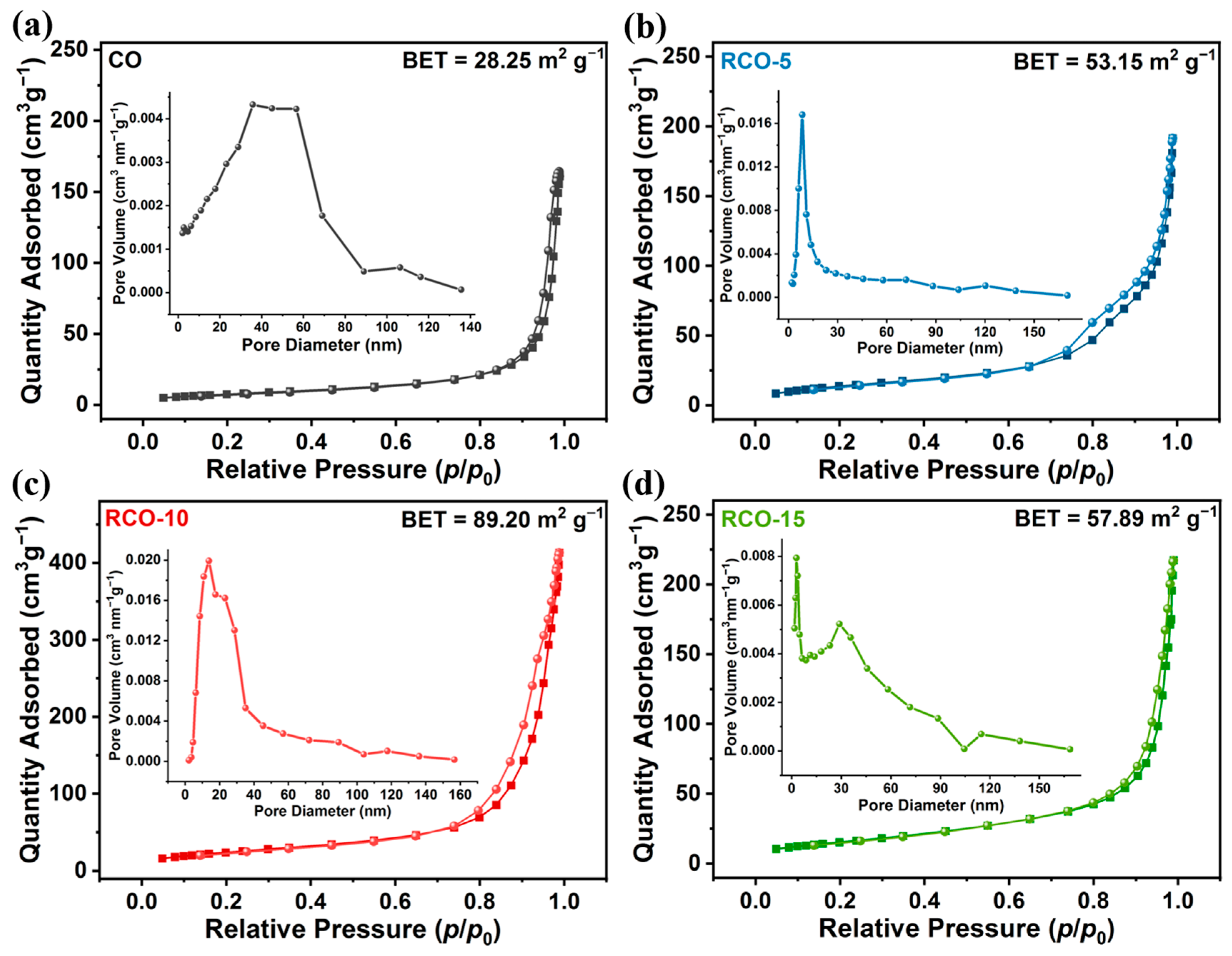
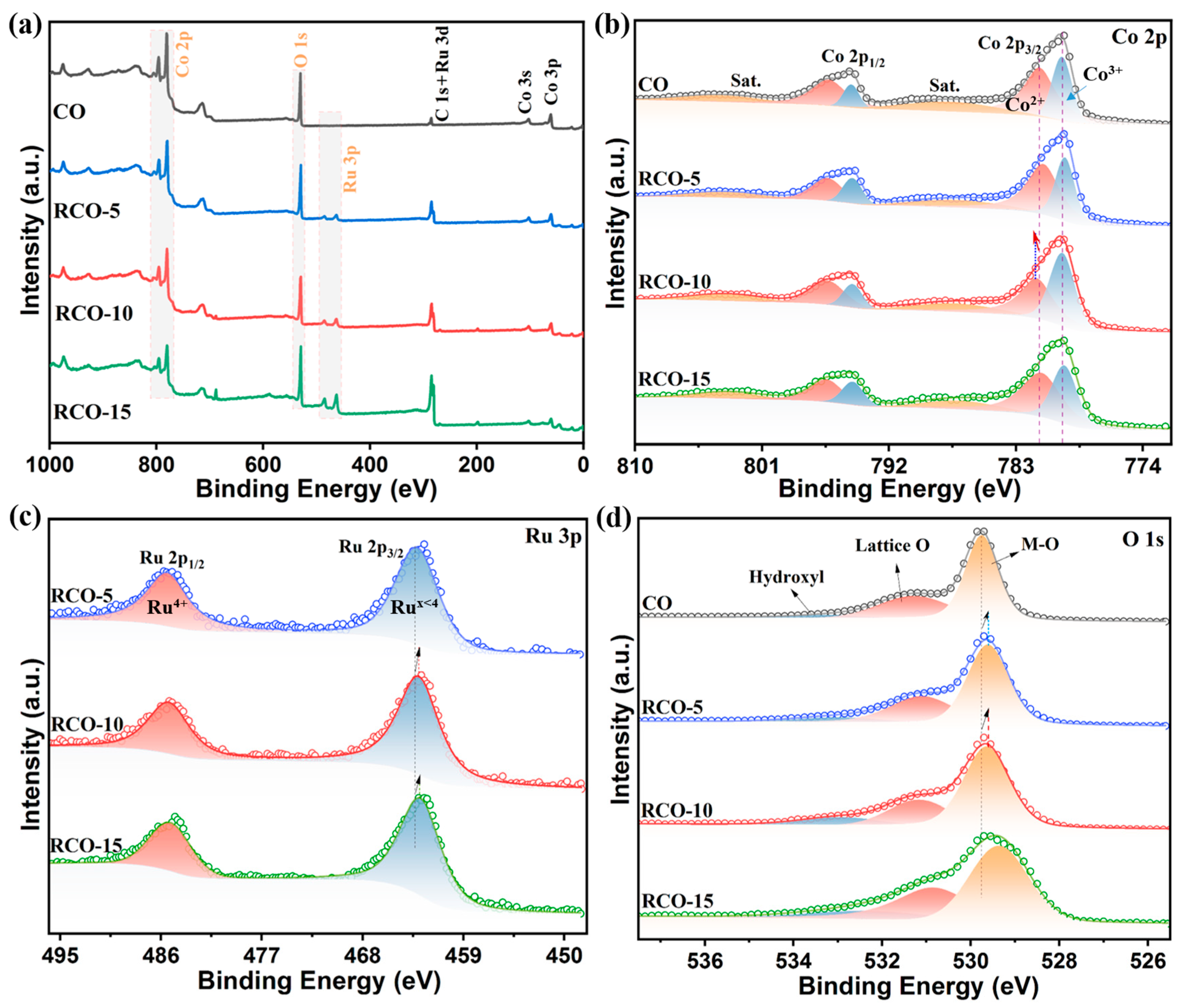
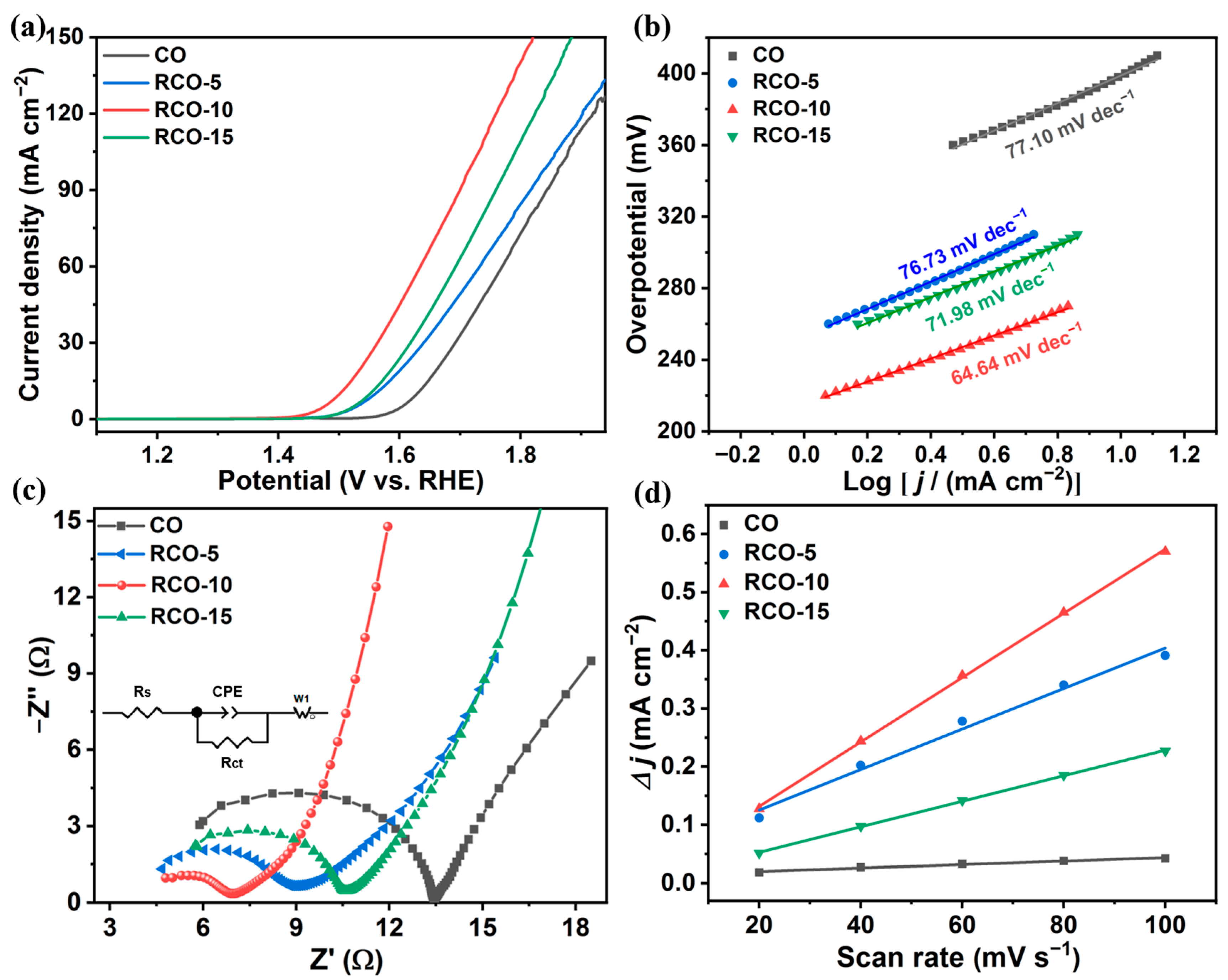

| Catalysts | Overpotential (mV) (j = 10 mA cm−2) | Tafel Slope (mV dec−1) | Rct (Ω) |
|---|---|---|---|
| CO | 399 | 77.10 | 8.70 |
| RCO-5 | 331 | 76.73 | 4.03 |
| RCO-10 | 272 | 66.64 | 2.42 |
| RCO-15 | 322 | 71.98 | 5.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Bu, Y.; Hao, J.; Zhang, W.; Xiao, Y.; Zhao, N.; Zhang, R.; Zhang, D. Synthesis of RuO2-Co3O4 Composite for Efficient Electrocatalytic Oxygen Evolution Reaction. Nanomaterials 2025, 15, 1356. https://doi.org/10.3390/nano15171356
Zhang J, Bu Y, Hao J, Zhang W, Xiao Y, Zhao N, Zhang R, Zhang D. Synthesis of RuO2-Co3O4 Composite for Efficient Electrocatalytic Oxygen Evolution Reaction. Nanomaterials. 2025; 15(17):1356. https://doi.org/10.3390/nano15171356
Chicago/Turabian StyleZhang, Jingchao, Yingping Bu, Jia Hao, Wenjun Zhang, Yao Xiao, Naihui Zhao, Renchun Zhang, and Daojun Zhang. 2025. "Synthesis of RuO2-Co3O4 Composite for Efficient Electrocatalytic Oxygen Evolution Reaction" Nanomaterials 15, no. 17: 1356. https://doi.org/10.3390/nano15171356
APA StyleZhang, J., Bu, Y., Hao, J., Zhang, W., Xiao, Y., Zhao, N., Zhang, R., & Zhang, D. (2025). Synthesis of RuO2-Co3O4 Composite for Efficient Electrocatalytic Oxygen Evolution Reaction. Nanomaterials, 15(17), 1356. https://doi.org/10.3390/nano15171356





