Recent Progress of Liquid Metal-Based Electromagnetic Shielding Materials
Abstract
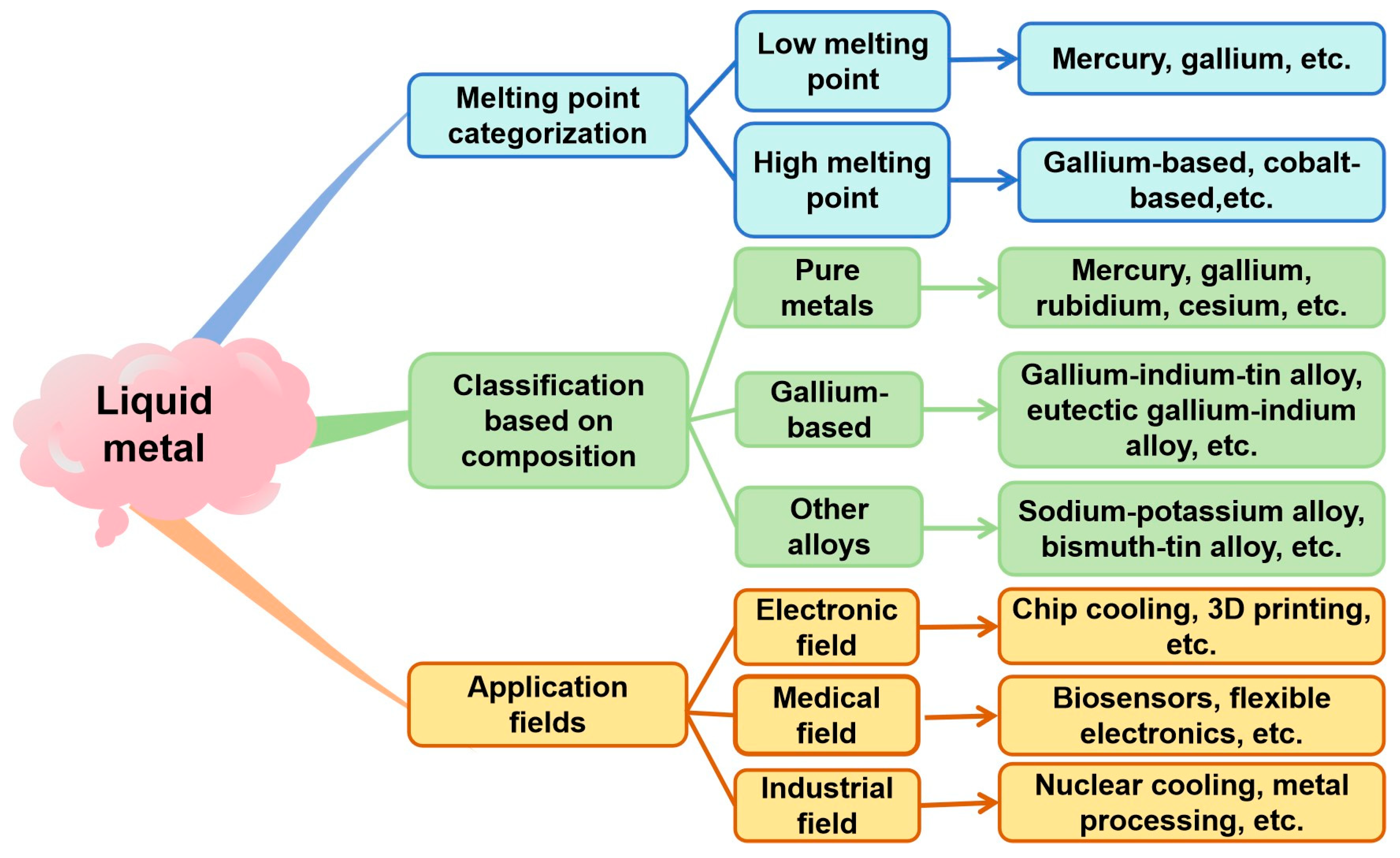
1. Introduction
1.1. Pure Metal Liquid Metals
1.2. Gallium-Based Liquid Metals
1.3. Other Alloy-Based Liquid Metals
2. Preparation Methods of Liquid Metal-Based Materials
2.1. Direct Molding Method
2.2. Composite Preparation Method
2.3. 3D Printing Technology
2.4. Other Preparation Methods
3. Electromagnetic Shielding Principles and Methods
3.1. The Basic Principles of Electromagnetic Shielding
- 1.
- Reflection Loss: Reflection is one of the principal mechanisms of electromagnetic interference (EMI) shielding. Upon encountering a shielding layer, an electromagnetic wave undergoes partial reflection at the interface due to the impedance mismatch between the shielding material and the surrounding medium. The extent of reflection loss is contingent upon the material properties of the shielding layer, its thickness, and the frequency of the incident electromagnetic wave, as depicted in Equation (3) [88]:
- 2.
- Absorption Loss: After entering the shielding layer, the electromagnetic wave propagates within the material, where it is absorbed, leading to energy attenuation. The absorption loss primarily depends on the magnetic permeability, electrical conductivity, and thickness of the shielding material, as shown in Equation (4) [89]:
- 3.
- Multiple Reflection Loss: Electromagnetic waves undergo multiple reflections within the interfaces of the shielding layer. Each reflection results in a portion of the energy being absorbed or reflected back into the original medium, thereby further increasing the propagation path and loss of the electromagnetic waves, enhancing the shielding effect. This process can be repeated until the energy of the electromagnetic waves is completely dissipated, as shown in Equation (5) [90]:
3.2. Methods for Electromagnetic Shielding Testing
- Shielded Room Method: By constructing an enclosed shielding space to simulate real-world complex electromagnetic environments, this method allows for a systematic evaluation of the shielding effectiveness of large-scale equipment or entire buildings. The material to be tested is placed inside the shielded room, where electromagnetic waves of known intensity are emitted. The intensity of the electromagnetic waves that penetrate through the shielding room is measured outside. This measurement is used to assess the shielding effectiveness of the room, thereby inferring the electromagnetic shielding performance of the tested device or material.
- Coaxial Transmission Line Method: Based on the transmission characteristics of electromagnetic waves in coaxial structures, this method utilizes coaxial transmission lines to carry electromagnetic waves. The shielding material to be tested is either fabricated into a coaxial structure or placed around a coaxial line. By measuring the power changes of the electromagnetic waves in the transmission line before and after passing through the shielding material, the shielding effectiveness can be calculated. This method focuses on the precise measurement of shielding performance for sheet-like and strip-like materials.
- Free-Space Method: In free space, electromagnetic waves are transmitted towards the material under test, and the waves that have passed through the shielding material are received at a certain distance. By comparing the intensities of the transmitted and received signals, the shielding effectiveness is determined. This method is suitable for testing the shielding effectiveness of objects of various shapes under conditions that approximate real-world applications.
- Near-Field Probe Method: This method employs a near-field probe positioned close to the surface of the object under test to measure the distribution of the electromagnetic field on the object’s surface. The electromagnetic shielding performance of the object is assessed by analyzing the attenuation of the electromagnetic field. With its high-resolution detection capability, this method can rapidly identify localized electromagnetic leakage points in electronic devices.
4. Research Progress on Liquid Metal-Based Electromagnetic Shielding Materials
4.1. Electromagnetic Functional Materials Based on Pure Liquid Metal as a Single Filler
4.2. Electromagnetic Functional Materials Combining Liquid Metal with Conductive Fillers
4.3. Electromagnetic Functional Materials Based on the Combination of Liquid Metal and Magnetic Substances
4.4. Effects of Different Liquid Metal Forming Structures on Electromagnetic Waves
4.5. Research on Electromagnetic Shielding Performance of Liquid Metal-Based Multifunctional Materials
5. Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Choi, C.; Liu, L.; Hwang, B. Liquid metal composites: Recent advances and applications. Int. J. Miner. Metall. Mater. 2025, 32, 1008–1024. [Google Scholar] [CrossRef]
- Fakharian, M.M.; Sharbati, V.; Bao, X. Dynamic reconfigurable MIMO antenna based on tunable liquid metal. Phys. Scr. 2025, 100, 055553. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, L.; Guan, L.; Zhu, Y.; Lin, M.; Li, Q.; Guo, X.; Wang, Z.; Lian, Y.; Chen, P. Elastic liquid metal composite with strain-independent EMI shielding properties. J. Mater. Sci. Technol. 2025, 235, 146–155. [Google Scholar] [CrossRef]
- Li, D.; Dai, Y.; Yang, B.; Liu, D.; Deng, Y. Purification of indium by vacuum distillation and its analysis. J. Cent. South Univ. 2013, 20, 337–341. [Google Scholar] [CrossRef]
- Feng, J.; Gao, Z.; Wang, Z.; Yang, H.; Niu, J. Extraction and application of gallium in industrial production. Bull. Chin. Ceram. Soc. 2018, 37, 2852–2856+2861. [Google Scholar]
- Huang, J.; Chen, Y.; Wen, B.; Hua, Y.; Zhang, Z.; Li, Z.; Han, B.; Zhao, Y. Softly encapsulated liquid metal grease as high-performance thermal interface material with corrosion resistance to Aluminium. Surf. Interfaces 2025, 68, 106665. [Google Scholar] [CrossRef]
- Ikeda, Y.; Han, S.; Wallis, T.; Kamachali, R.D.; Maaß, R. On the preference of liquid-metal embrittlement along high-angle grain-boundaries in galvanized steels. Scr. Mater. 2025, 265, 116723. [Google Scholar] [CrossRef]
- Belashchenko, D.K. Application of the Embedded Atom Model to Liquid Metals: Liquid Mercury. High Temp. 2006, 44, 675–686. [Google Scholar] [CrossRef]
- Stankus, S.V.; Abdullaev, R.N.; Khairulin, R.A. Density changes of bismuth and alkaline metals at the transition to the liquid state. Thermophys. Aeromech. 2016, 23, 913–918. [Google Scholar] [CrossRef]
- Mori, H.; Hoshino, K.; Watabe, M. Molecular Dynamics Study of the Structure of Expanded Liquid Caesium. J. Phys. Soc. Jpn. 1990, 59, 3254–3259. [Google Scholar] [CrossRef]
- Zeng, J.; Li, X.; Liang, T.; Rao, T.; Zheng, Z.; Yao, Y.; Xu, J.B.; Sun, R.; Li, L. Thermally conductive and self-healable liquid metal elastomer composites based on poly(ionic liquid)s. Compos. Part A 2025, 194, 108922. [Google Scholar] [CrossRef]
- Guan, T.; Guo, M.; Wang, L.; Liu, J. Production and recycling of the cutting edge material of gallium: A review. Sci. Total Environ. 2025, 971, 179046. [Google Scholar] [CrossRef]
- Zhao, K.; Sun, X.; Wang, C.; Song, X.; Wang, F.; Li, K.; Ning, P. Supported catalysts for simultaneous removal of SO2, NOx, and Hg0 from industrial exhaust gases: A review. Chin. Chem. Lett. 2021, 32, 2963–2974. [Google Scholar] [CrossRef]
- Qiao, M.; Xing, Z.; Jun-Heng, F.; Jing, L. Multiphase flow physics of room temperature liquid metals and its applications. Sci. China (Technol. Sci.) 2023, 66, 1483–1510. [Google Scholar] [CrossRef]
- Xiang, S.; Yang, L.; Xu, Y.; Cao, G.; Zhou, H. Selective electrosorption of Cs(I) from high-salinity radioactive wastewater using CNT-interspersed potassium zinc ferrocyanide electrodes. Acta Phys.-Chim. Sin. 2025, 41, 100097. [Google Scholar] [CrossRef]
- Liu, L.; Wang, R.; Yu, W.; An, H.; Sun, Q.; Gao, Z. Preliminary exploration of driving factors for the development of radiation protection. Radiat. Prot. 2025, 45, 197–206. [Google Scholar]
- Zhuo, Y. The Magnetic Properties of Pr-Doped YBa2Cu3O7-δ Single Crystals and Grain-Aligned Hg0.8Pb0.2Ba1.5Sr0.5Ca2Cu3Oy. Ph.D. Thesis, Northeast University, Shenyang, China, 1996. [Google Scholar]
- Altaf, A.R. Catalytic Adsorption and Oxidation of Gaseous Hg0 by Magnetic Biochar. Ph.D. Thesis, University of Artois, Arras, France, 2021. [Google Scholar]
- Qiu, H.; Yu, Z.; Zhao, T.; Zhang, Q.; Xu, M.; Li, P.; Li, T.; Bao, W.; Chai, Y.; Chen, S.; et al. Two-dimensional materials for future information technology: Status and prospects. Sci. China (Inf. Sci.) 2024, 67, 160400. [Google Scholar] [CrossRef]
- Jasrotia, R.; Raj, K.; Ramya, M.; Kumar, R.; Pathania, D.; Kumar, Y.; Kandwal, A. Magnesium ferrites and their composites based photocatalysts: Synthesis approaches, effect of doping, and operational parameters on photocatalytic performance for wastewater remediation. J. Magnes. Alloys 2024, 12, 3996–4044. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Wang, M.; Cheng, Q.; Ji, H.; Qian, T.; Yan, C. Advanced In Situ Characterization Techniques for Direct Observation of Gas-Involved Electrochemical Reactions. Energy Environ. Mater. 2023, 6, 77–116. [Google Scholar] [CrossRef]
- Peng, M.; Min, E.D.; Yang, H.Y.; Xue, X.D.; Huan, W.; Feng, G.W.; Lei, H.; Dong, H.Z.; Li, B.Z. Downregulation of Serum PTEN Expression in Mercury-Exposed Population and PI3K/AKT Pathway-Induced Inflammation. Biomed. Environ. Sci. 2024, 37, 354–366. [Google Scholar]
- Bai, Y.; Jie, Z.; Zhang, M.; Wei, R. Flexible liquid metal electromagnetic shielding materials. Sci. China (Technol. Sci.) 2023, 66, 2757–2774. [Google Scholar] [CrossRef]
- Xin, Z.; Deng, Z.-S. Roadmap towards new generation liquid metal thermal interface materials. Sci. China (Technol. Sci.) 2023, 66, 1530–1550. [Google Scholar]
- Peng, C.; Huang, X.; Zhao, M.; Liao, S.; Yang, Q.; Yang, N.; Yu, S. High-temperature-tolerant flexible supercapacitors: Gel polymer electrolytes and electrode materials. J. Energy Chem. 2025, 100, 426–457. [Google Scholar] [CrossRef]
- Sun, M.; Wang, S.; Liang, Y.; Wang, C.; Zhang, Y.; Liu, H.; Zhang, Y.; Han, L. Flexible Graphene Field-Effect Transistors and Their Application in Flexible Biomedical Sensing. Nano-Micro Lett. 2025, 17, 260–321. [Google Scholar] [CrossRef]
- Jiang, C.; Rui, G. Liquid metal-based paper electronics: Materials, methods, and applications. Sci. China (Technol. Sci.) 2023, 66, 1595–1616. [Google Scholar] [CrossRef]
- Deng, Y.; E, E.; Jing, L.; Yi, J.; Mei, S.; Yang, Y. Materials, fundamentals, and technologies of liquid metals toward carbon neutrality. Sci. China (Technol. Sci.) 2023, 66, 1576–1594. [Google Scholar] [CrossRef]
- Fang, T.; Sun, Y.; Kong, D. Liquid Metal-Enabled Epidermal Interfaces. ACS Appl. Biol. Mater. 2024, 7, 7791–7798. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, C.; Zeng, M.; Fu, L. Gallium-Based Liquid Metals in Rechargeable Batteries: From Properties to Applications. Small 2024, 20, e2311099. [Google Scholar] [CrossRef]
- Agarwal, R.; Mohamad, A. Gallium-based liquid metals as smart responsive materials: Morphological forms and stimuli characterization. Adv. Colloid Interface Sci. 2024, 329, 103183. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Shen, Y.; Hao, Z.; Liu, X.; Chen, W.; Ma, X. Gallium-based liquid metal micro/nanoparticles for photothermal cancer therapy. J. Mater. Sci. Technol. 2023, 142, 22–33. [Google Scholar] [CrossRef]
- Zhang, P.; Han, R.; Wang, D.; Yan, B. Design of Heat Sink in Power Electronic Device Using Liquid Metal. In Proceedings of the 2019 5th International Conference on Green Materials and Environmental Engineering (GMEE 2019), Guangzhou, China, 27–29 December 2019; p. 7. [Google Scholar]
- Liang, J.; Cao, G.; Zhang, Y.; Li, Z.; Wan, X.; Jiao, J.; Yang, K.; Xiao, L.; Guo, Y.; Zeng, M.; et al. Synthesis of High-Entropy Alloy Polyhedra Using Liquid Metal Dewetting. J. Am. Chem. Soc. 2025, 147, 16742–16746. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, W.; Yang, M.; Tian, Z.; Guo, W.; Sun, J.; Zhou, X.; Fei, B.; An, B.; Sun, R.; et al. High Linearity, Low Hysteresis Ti3C2Tx MXene/AgNW/Liquid Metal Self-Healing Strain Sensor Modulated by Dynamic Disulfide and Hydrogen Bonds. Adv. Funct. Mater. 2023, 33, 2301587. [Google Scholar] [CrossRef]
- Tu, H.-L.; Zhao, H.-B.; Fan, Y.-Y.; Zhang, Q.-Z. Recent developments in nonferrous metals and related materials for biomedical applications in China: A review. Rare Met. 2022, 41, 1410–1433. [Google Scholar] [CrossRef]
- Wang, X.; Guo, J.; Hu, L. Preparation and application of gallium-based conductive materials in the very recent years. Sci. China (Technol. Sci.) 2021, 64, 681–695. [Google Scholar] [CrossRef]
- Liu, Q. Lubrication State and Performance Regulation of Gallium-Based Liquid Metal. Master’s Thesis, Yantai University, Yantai, China, 2024. [Google Scholar]
- Huang, H.; Ding, X.; Mao, X.; Yan, Y.; Lv, F.; Pan, B.; Huang, W.; Wang, L.; Han, N.; Li, Y. Liquid Metal Alloys Enable Efficient Formate Electrosynthesis. Adv. Funct. Mater. 2024, 34, 2408966. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, J.; Mou, P.; Li, L.; Chen, Y.; Yan, L.; Yu, H.; Shu, J.; Zhang, L. Cobalt/Nitrogen Co-Doped Carbon Materials Enhance the Reaction Rate of Sodium-Potassium Alloy Electrodes. Small 2023, 20, e2304981. [Google Scholar] [CrossRef]
- Gnanasekaran, M.; Satheesh, A. Influence of Joule heating and magneto-hydrodynamic on double diffusion mixed convection in a cavity with liquid sodium-potassium alloy and discrete thermosolutal source. Numer. Heat Transf. Part A Appl. 2025, 86, 3687–3710. [Google Scholar] [CrossRef]
- Dhand, V.; Han, G.; Han, K.; Cho, H.; Jo, H.; Rhee, K.; Lee, K. Investigation on the novel tribological property of the bismuth–tin alloy coated basalt fiber epoxy composite. In Proceedings of the Korean Society of Tribologists & Lubrication Engineers Conference, Republic of Korea; 2022. [Google Scholar]
- Zhang, C.; Hou, H.; Wang, Y.; Dai, C.; Li, J.; Liu, W. Effect of bismuth–tin alloy quenching medium on microstructure and properties of boron-containing steel. Iron Steel Vanadium Titan. 2024, 45, 156–161. [Google Scholar]
- Hmadeh, L.; Jaculli, M.A.; Vedvik, N.; Elahifar, B.; Sangesland, S. The effect of temperature on the sealability of bismuth–tin alloy plugs. Geoenergy Sci. Eng. 2024, 241, 213107. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, G.; Tong, Y.; Chen, L.; Chen, P. Vanadium Oxide Clusters Mediated Bismuth-Tin Alloy for Accelerated Dynamics of Electrocatalytic CO2 Conversion. Adv. Funct. Mater. 2024, 35, 2420177. [Google Scholar] [CrossRef]
- Fang, Y. Preparation of ZIF-8 Derived Nanocomposites and Their Performance in Alkali-Metal-Ion Batteries. Master’s Thesis, Fujian Normal University, Fuzhou, China, 2019. [Google Scholar]
- Xiao, Y.; Guo, J.; Xiong, J.; Jiang, X. Temperature- and pressure-controlled insulator-conductor transition performance of flexible fluoroelastomer, tin bismuth alloy and carbon nanotube composites with a sandwich structure. J. Alloys Compd. 2023, 942, 169113. [Google Scholar]
- Liu, S.y.; Zhou, Y.; Miao, X.c.; Xiao, Q.h.; Guan, R.; Ai, X.g.; Li, S.l. Refining mechanism of tin–bismuth alloy solidified structure upon applying direct current attached mold. J. Iron Steel Res. Int. 2024, 32, 619–628. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Xie, S.; Liu, Y.; Liang, P.; Hu, X.; Wen, Z. 3D printing technology for rechargeable Li/Na-ion batteries. J. Energy Chem. 2025, 103, 237–263. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, H.; Cao, Z.; Wang, S.; Zhu, M. Research progress of lignin-derived materials in lithium/sodium ion batteries. Green Energy Environ. 2025, 10, 322–344. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, X.; Liu, M.; Yang, H.; Zou, D. Review on high-temperature macroencapsulated phase change materials: Encapsulation strategy, thermal storage system, and optimization. J. Energy Chem. 2025, 104, 324–359. [Google Scholar] [CrossRef]
- Tian, C. Application of metal-based nanomaterials in lithium batteries. In Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2024), London, UK, 13–19 January 2024; p. 8. [Google Scholar]
- Hu, S.; Wang, D.; Yang, K.; Wang, Y.-F.; Yang, T.; Peng, Q.-Y.; Tan, X.-D.; Kremenakova, D.; Militky, J.; Venkataraman, M.; et al. Copper Coated Textiles for Inhibition of Virus Spread. In Proceedings of the 13th Textile Bioengineering and Informatics Symposium, Online, 7–10 July 2020; p. 8. [Google Scholar]
- Toshikazu, S.; Toshitaka, I.; Masanori, U. Effect of bismuth–tin alloy particle diameter on bonding strength of copper nanoparticles/bismuth–tin solder hybrid joints. J. Mater. Sci. Mater. Electron. 2018, 29, 7161–7176. [Google Scholar]
- Gicha, B.B.; Tufa, L.T.; Nwaji, N.; Hu, X.; Lee, J. Advances in All-Solid-State Lithium–Sulfur Batteries for Commercialization. Nano-Micro Lett. 2024, 16, 217–254. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Dai, Z.; Gao, D.; Zhao, X. Electrochemical reduction of carbon dioxide to produce formic acid coupled with oxidative conversion of biomass. J. Energy Chem. 2024, 92, 705–729. [Google Scholar] [CrossRef]
- Zhang, W.-T.; Huang, W.; Yu, F.-J.; Zhao, Q.; Zhu, X.-D.; Feng, T.; Jiang, Y.; Li, X.-M.; Miao, X.-F.; Zhu, H.; et al. Frontiers in high entropy alloys and high entropy functional materials. Rare Met. 2024, 43, 4639–4776. [Google Scholar] [CrossRef]
- Yao, H.C.; Chieh, C.C.; Yu, C.H.; Long, P.W.; Wei, L.Y. Fabrication of a Micro Lens Array with a High Filling Factor Using a Direct Molding Method. Mater. Sci. Forum 2013, 773–774, 712–721. [Google Scholar] [CrossRef]
- Dedov, S.; Lehmann, G.; Kawalla, R. Application of Combined Casting-Forging Process for Production of Durable Lightweight Aluminum Parts. Key Eng. Mater. 2013, 2443, 264–273. [Google Scholar] [CrossRef]
- Lai, H.-W.; Ouchi, M. Backbone Degradable Polymers via Chain-growth Radical Polymerization. Chin. J. Polym. Sci. 2025, 43, 887–907. [Google Scholar] [CrossRef]
- Yan, L.; Liu, H.; Yang, Y.; Dai, L.; Si, C. Lignin-derived carbon fibers: A green path from biomass to advanced materials. Carbon Energy 2025, 7, 16–38. [Google Scholar] [CrossRef]
- Gong, F.; Yuan, Y.; Zhang, X.; Cheng, X.; Huang, Y.; Wu, C. Potential application of jet 3D printing technology for crack repair on asphalt pavement. J. Traffic Transp. Eng. 2025, 1–49. Available online: https://kns.cnki.net/kcms/detail/61.1494.u.20250422.1013.002.html (accessed on 27 July 2025).
- Wang, L.; Liang, D.; Yu, R.; Wang, M.; Tian, Y.; Ma, T.; Yang, B.; Xu, B.; Jiang, W. Progress and prospects in magnesium alloy scrap recycling. J. Magnes. Alloys 2024, 12, 4828–4867. [Google Scholar] [CrossRef]
- Jiang, C.; Gu, L.; Xiong, H.; Wang, J.; Zhou, L.; Shen, M.; Wang, J.; Ying, T.; Zeng, X. Review of progress on fabrication technology of Mg matrix composites. J. Magnes. Alloys 2024, 12, 4768–4807. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Suttora, N.; Zheng, J.; Wang, Q. Mechanism Design and Drive Control of Bionic Robotic Fish Based on DEAP Material. In Proceedings of the 28th Chinese Control and Decision Conference, Yinchuan, China, 28–30 May 2016; p. 6. [Google Scholar]
- Gawdzińska, K.; Grabian, J.; Nagolska, D.; Kwiecińska, B. Innovative Multilayer Polymer-glass Composites Reinforced with Metal-ceramic Foam. In Proceedings of the 2018 3rd International Conference on Design, Mechanical and Material Engineering (D2ME 2018). Phuket, Thailand, 27–29 September 2018; p. 7. [Google Scholar]
- Camacho, P.A.R. Lithium-ion Storage of Transition Metal Sulfides and Oxides via Different Nanostructure-Enhancing Methods. Master’s Thesis, Dalian University of Technology, Dalian, China, 2021. 004852. [Google Scholar]
- Peng, Y.-C.; Liu, X.; Li, H.-J. A Review on Electrostatic Spinning Functional Nanofiber Air Filtration Membranes. In Proceedings of the 17th Textile Bioengineering and Informatics Symposium, Daegu, Republic of Korea, 20–23 August 2024; p. 8. [Google Scholar]
- Wang, Q. Research on the preparation of colloidal photonic crystals and the anti-counterfeiting applications. In Proceedings of the 3rd International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2023), San Francisco, CA, USA, 18 March 2023; p. 8. [Google Scholar]
- Li, Y. The development of carbon fiber epoxy resin composite material and its applications in aerospace. In Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering (CONF-FMCE 2023), Oxford, UK, 26 August 2023; p. 6. [Google Scholar]
- Liang, Y.; Ran, W.; Kuang, D.; Wang, Z. Design strategies and insights of flexible infrared optoelectronic sensors. J. Semicond. 2025, 46, 24–60. [Google Scholar] [CrossRef]
- Yu, S.; Jiang, Y.; Yu, L.; Wang, H.; Pan, L.; Zhang, J.; Zhang, Y.; Hou, X. Liquid-solid composites with confined interface behaviors. Natl. Sci. Rev. 2025, 12, 405–418. [Google Scholar] [CrossRef]
- Xu, J.; Ma, S.; Gao, P.; Guo, Y.; Sun, J.; Qiao, D.; Ma, B.; Yuan, W.; Ramakrishna, S.; Ye, T. The state-of-the-art fundamentals and applications of micro-energy systems on-chip. Natl. Sci. Open 2025, 4, 114–161. [Google Scholar] [CrossRef]
- Patirupanusara, P.; Rubkumintara, T.; Suwanprateeb, J. Direct Fabrication of Three-Dimensional Printing Parts Made from Polymethyl Methacrylate for Biomedical Applications. In Proceedings of the 2012 International Conference on Recent Trends in Computer & Information Engineering, International Conference on Mechanical, Materials and Automotive Engineering, International Conference on Advances in Electrical and Electronics Engineering, International Conference on Ecological, Environmental and Bio-Sciences, International Conference on Business, Economics, and Behavioral Sciences and International Conference on Chemical Engineering and Pharmaceutical Sciences, Pattaya, Thailand, 13–15 April 2012; p. 3. [Google Scholar]
- Zhou, C. Application of 3D printing technology and related materials in total hip arthroplasty. In Proceedings of the 2nd International Conference on Biological Engineering and Medical Science (ICBioMed 2022), Oxford, UK, 7–13 November 2022; p. 6. [Google Scholar]
- Zhu, C.; Xiang, C.; Wu, M.; Yu, C.; Dai, S.; Sun, Q.; Zhou, T.; Wang, H.; Xu, M. Recent advances in wave-driven triboelectric nanogenerators: From manufacturing to applications. Int. J. Extrem. Manuf. 2024, 6, 267–300. [Google Scholar] [CrossRef]
- Rashid, H.U. Design and Synthesis of Magnetic Resonance Imaging (MRI)Contrast Agents & Catalyst-Enhanced Chemical Vapor Deposition (CECVD) of Palladium, Platinum, Palladium-Platinum Bilayer and Their Bimetal on Polymer Surface. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2012. [Google Scholar]
- Hajalilou, A.; Parvini, E.; Morgado, A.T.; Lopes, A.P.; Jorge, M.E.M.; Freitas, M.; Tavakoli, M. Replacing the Gallium Oxide Shell with Conductive Ag: Toward a Printable and Recyclable Composite for Highly Stretchable Electronics, Electromagnetic Shielding, and Thermal Interfaces. ACS Appl. Mater. Interfaces 2024, 16, 61157–61168. [Google Scholar] [CrossRef]
- Cheng, J.; Li, C.; Xiong, Y.; Zhang, H.; Raza, H.; Ullah, S.; Wu, J.; Zheng, G.; Cao, Q.; Zhang, D.; et al. Recent Advances in Design Strategies and Multifunctionality of Flexible Electromagnetic Interference Shielding Materials. Nano-Micro Lett. 2022, 14, 128–158. [Google Scholar] [CrossRef]
- Shi, Y.; Wu, M.; Ge, S.; Li, J.; Alshammari, A.S.; Luo, J.; Amin, M.A.; Qiu, H.; Jiang, J.; Asiri, Y.M.; et al. Advanced Functional Electromagnetic Shielding Materials: A Review Based on Micro-Nano Structure Interface Control of Biomass Cell Walls. Nano-Micro Lett. 2025, 17, 106–142. [Google Scholar] [CrossRef] [PubMed]
- Haoran, W.; Xiaofei, W.; Hao, M. Study on electromagnetic protective clothing structure for shielding effectiveness. In Proceedings of the 2015 IEEE 12th International Conference on Electronic Measurement & Instruments, Qingdao, China, 16–18 July 2015; p. 5. [Google Scholar]
- Chen, J. Research on the Basic Electromagnetic Compatibility of Locomotive Based on Electromagnetic Simulation. In Proceedings of the 2020 4th International Conference on Electrical, Mechanical and Computer Engineering (ICEMCE 2020), Jinan, China, 19–21 June 2020; p. 6. [Google Scholar]
- Tao, Y.; Liang, H.; Wu, J.; Huang, J.; Pang, W.; Wu, X. Experimental Research of Electromagnetic Interference Caused by Disconnector Operation in Gas Insulated Switchgear. In Proceedings of the 2019 4th Asia Conference on Power and Electrical Engineering (ACPEE 2019), Hangzhou, China, 28–31 March 2019; p. 7. [Google Scholar]
- Yang, Z.K.; Xiong, L.; Luo, Y.; Chen, J.P.; Miao, X.F.; Wu, Y.M. Experiment Research on the Frequency Effects of Pulsed Electromagnetic Field on Industrial Circulating Water. In Proceedings of the 2015 International Conference on Computer Science and Environmental Engineering (CSEE 2015), Beijing, China, 17–18 May 2015; p. 10. [Google Scholar]
- Zhang, G.; Ma, X.; Zang, Y.; Xie, L.; Zhang, Y. Analysis of Electromagnetic Compatibility and Its Testing Technology Characteristics. In Proceedings of the 2018 5th International Conference on Electrical & Electronics Engineering and Computer Science (ICEEECS 2018), Beijing, China, 29–30 June 2018; p. 4. [Google Scholar]
- Sambyal, P.; Noh, S.J.; Hong, J.P.; Kim, W.N.; Iqbal, A.; Hwang, S.S.; Hong, S.M.; Koo, C.M. FeSiAl/metal core shell hybrid composite with high-performance electromagnetic interference shielding. Compos. Sci. Technol. 2019, 172, 66–73. [Google Scholar] [CrossRef]
- Jagdees, P.; Kumar, S.A.; Monika, T.; Vinay, G.; Kedar, S. Hydrothermal synthesis of micro-flower like morphology aluminum-doped MoS2/rGO nanohybrids for high efficient electromagnetic wave shielding materials. Ceram. Int. 2021, 47, 15648–15660. [Google Scholar]
- Naqvi, S.T.A.; Godara, S.K.; Ray, B.; Datar, S.; Singh, C. Tunable structural, magnetic, complex permittivity/permeability, and reflection loss parameters of Co2+ and Ni2+-Co-doped M-type strontium ferrites for microwave absorber applications. J. Mater. Sci. Mater. Electron. 2025, 36, 213. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, H.; Lv, H.; Yang, L.; Liang, G.; Zhang, J.; Lai, Y.; Cheng, H.W.; Che, R. Recent progress in carbon-based materials and loss mechanisms for electromagnetic wave absorption. Carbon 2024, 219, 118834. [Google Scholar] [CrossRef]
- Nikhil, S.; Jaya, M.; Rahul, P. Advancing Accuracy in Perovskite Tandem Solar Cell Efficiency via Transfer Matrix-Based Realistic Device Simulations. J. Electron. Mater. 2024, 53, 4214–4223. [Google Scholar] [CrossRef]
- Vacuumschmelze GmbH & Co., KG. Multi-Shelled Shielded Room and Method for the Production of a Multi-Shelled Shielded Room. USPTO 20200037475, 27 August 2024. [Google Scholar]
- Li, B.; Hu, X.; Wang, M.; Dou, R.; Liu, X.; Ma, Y.; Ma, S. Research on the electromagnetic shielding properties and absorbing properties of carbon fiber reinforced cement-based composite. In Proceedings of the Global Intelligent Industry Conference 2020, Guangzhou, China, 20–21 November 2020; p. 11780. [Google Scholar]
- Nuvotronics Inc. Coaxial Transmission Line Microstructures and Methods of Formation Thereof. USPTO 20190067790, 24 September 2013. [Google Scholar]
- Zhu, Y.; Li, X.; Mao, J. An Ultra-Wideband Substrate Integrated Coaxial Line Array Based on Silicon-Based MEMS Process. J. Circuits Syst. Comput. 2024, 33, 2550016. [Google Scholar] [CrossRef]
- Sheng, M.; Zhou, Z.; Ren, J.; Gu, Y.; Zhou, Q.; Xing, B. Study of the method of electromagnetic parameters test of conductive rubber. Measurement 2025, 244, 116520. [Google Scholar] [CrossRef]
- Elnail, K.E.I.; Huang, X.; Tan, L. Optimal Resonance Reactive Current Shielding Design In Wireless Power Transfer Systems. In Proceedings of the 2017 International Electrical and Energy Conference (CIEEC 2017), Beijing, China, 25–27 October 2017; p. 4. [Google Scholar]
- Akhmetkhanov, R.S. Analysis of the Effect of the Direction of Reinforcement of a Fibrous Composite Material on the Inhomogeneity of Localization of Deformations and Stresses Using the Method of Thermoelastic Response. Inorg. Mater. 2025, 60, 502–509. [Google Scholar] [CrossRef]
- Balashov, A.A. Regression Analysis Using Neutral Networks for Nondestructive Control of the Thermal Characteristics of Polymers. Dokl. Phys. 2025, 69, 35–39. [Google Scholar] [CrossRef]
- Yue, Z.; Wei, Z. Research on the Functional Reconstruction of Industrial Tourism under Supply Power Obstacles. In Proceedings of the 2025 (23th) Euro-Asia Conference on CSR and Environmental Management, Tianjin, China, 13 May 2025; p. 16. [Google Scholar]
- Xia, Q.; Chen, Z.; Yang, L.; Hou, J.; Zhang, J.B.; Wang, Z.X.; Wu, W.L.; Ye, Z.W.; Tan, H.S. Structural vibration control of the curved bridge based on the combined effects of TLMD and LMD. Adv. Struct. Eng. 2025, 28, 1910–1922. [Google Scholar] [CrossRef]
- Jiang, H.; Yuan, B.; Guo, H.; Pan, F.; Meng, F.; Wu, Y.; Wang, X.; Ruan, L.; Zheng, S.; Yang, Y. Malleable, printable, bondable, and highly conductive MXene/liquid metal plasticine with improved wettability. Nat. Commun. 2024, 15, 6138. [Google Scholar] [CrossRef]
- Liao, S.-Y.; Wang, X.-Y.; Li, X.-M.; Wan, Y.-J.; Zhao, T.; Hu, Y.-G.; Zhu, P.-L.; Sun, R.; Wong, C.-P. Flexible liquid metal/cellulose nanofiber composites film with excellent thermal reliability for highly efficient and broadband EMI shielding. Chem. Eng. J. 2021, 422, 129962. [Google Scholar] [CrossRef]
- Wang, C.; Tang, L.; Zhou, Y.; Zhao, X.; Liu, C.; Yan, L. Transparent and switchable ultra-wideband frequency-selective-surface electromagnetic shielding. Acta Phys. Sin. 2024, 73, 99–108. [Google Scholar]
- Zhang, X.; Deng, Z.; Song, H.; Guo, M.; Li, L. Liquid metal electromagnetic wave shielding and absorbing film for solving electromagnetic interference in flexible sensors. Sci. China Mater. 2024, 67, 3976–3985. [Google Scholar] [CrossRef]
- Nie, X.; Xiong, M.; Zeng, J.; Li, C.; Chen, Y.; Xu, Z.; Fan, W. Surface Nanopatterning and Structural Coloration of Liquid Metal Gallium Through Hypergravity Nanoimprinting. Adv. Funct. Mater. 2025, 35, 2570044. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, Z.; Yang, D. Liquid Metal Hydrothermal Rheological Modification Method for High Performance Gallium-Coated Carbon Microparticle Composites. Adv. Mater. Technol. 2024, 10, 2400737. [Google Scholar] [CrossRef]
- Steenbergen, K.G.; Lambie, S.; Gaston, N. Discerning order from chaos: Characterising the surface structure of liquid gallium. Mater. Horiz. 2024, 12, 1314–1322. [Google Scholar] [CrossRef]
- He, X.; Ni, M.; Wu, J.; Xuan, S.; Gong, X. Hard-magnetic liquid metal droplets with excellent magnetic field dependent mobility and elasticity. J. Mater. Sci. Technol. 2021, 92, 60–68. [Google Scholar] [CrossRef]
- Jiang, J. Curved-Surface Printing of Gallium-Based Liquid Metal and Its Applications in Plant Electronics. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2022. [Google Scholar]
- Yin, F.; Zhao, Y.; Li, Z. Research progress on applications of gallium-based liquid metal. Mod. Chem. Ind. 2022, 42, 24–29. [Google Scholar]
- Lu, B.; Li, A.; Yang, S.; Yuan, Z.; Hui, J. Research progress of magnetic Ga-based liquid metal composites. Mater. Rep. 2024, 38, 170–184. [Google Scholar]
- Zhao, B.; Du, Y.; Lv, H.; Yan, Z.; Jian, H.; Chen, G.; Wu, Y.; Fan, B.; Zhang, J.; Wu, L. Liquid-Metal-Assisted Programmed Galvanic Engineering of Core–shell Nanohybrids for Microwave Absorption. Adv. Funct. Mater. 2023, 33, 2302172. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; Rajavel, K.; Zhao, T.; Zhu, P.; Hu, Y.; Sun, R.; Wong, C.-P. Tailorable, Lightweight and Superelastic Liquid Metal Monoliths for Multifunctional Electromagnetic Interference Shielding. Nano-Micro Lett. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Rui, G.; Sun, X.; Yuan, B.; Wang, H.; Liu, J. Magnetic Liquid Metal (Fe-EGaIn) Based Multifunctional Electronics for Remote Self-Healing Materials, Degradable Electronics, and Thermal Transfer Printing. Adv. Sci. 2019, 6, 1901478. [Google Scholar]
- Xiang, W.; Lu, Y.; Wang, H.; Sun, X.; Sen, C.; He, Z.; Liu, J. Liquid-metal-based magnetic fluids. Nat. Rev. Mater. 2024, 9, 433–449. [Google Scholar] [CrossRef]
- Shen, M.; Xu, X.; Qi, J.; Li, X.; Xue, B.; Zhu, M.; Zhang, Z.; Zheng, X.; Li, B.; Shang, Z. PMDI cross-linked rare earth/liquid metal reinforced ANF/MXene membranes for multifunctional electromagnetic interference shielding. Compos. Part A 2024, 182, 108178. [Google Scholar] [CrossRef]
- Li, N.; Zhan, F.; Su, J.; Li, Y.Q.; Chen, X.Q.; Guo, M.H.; Wang, L.; Liu, J. Antagonistic Effect Between Deformation and Magnetism in Liquid Metal Coils Smart Architecture for Multi-Mode Sensing. Adv. Funct. Mater. 2025, 2507514. [Google Scholar] [CrossRef]
- Wei, Y.; Bhuyan, P.; Kwon, S.J.; Kim, S.; Bae, Y.; Singh, M.; Tran, D.T.; Ha, M.; Jeong, K.-U.; Ma, X.; et al. Liquid Metal Grid Patterned Thin Film Devices Toward Absorption-Dominant and Strain-Tunable Electromagnetic Interference Shielding. Nano-Micro Lett. 2024, 16, 551–563. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, P.; Zhang, C.; Wang, Y.; Chang, H.; Rao, W. Porous and anisotropic liquid metal composites with tunable reflection ratio for low-temperature electromagnetic interference shielding. Appl. Mater. Today 2020, 19, 100612. [Google Scholar] [CrossRef]
















| Preparation Method | Advantages | Disadvantages | Scalability Potential |
|---|---|---|---|
| Direct Molding Method [58,59,60,61,62,63,64] | Elementary process with simple operation and low equipment cost; Leverages low melting point and high fluidity of liquid metals for easy shaping; High production efficiency via rapid raw material conversion. | Limited to simple geometries (cannot fabricate complex structures); Mold-dependent (additional mold design/manufacturing cost); Internal stress-imbalance risk during natural cooling solidification. | Well-suited for industrial mass production via continuous molding and automated cooling systems; ideal for standardized components. |
| Composite Preparation Method [65,66,67,68,69,70] | Integrates liquid metal advantages (high conductivity/fluidity) with other materials to enable new/enhanced properties; Versatile for liquid metal–metal/ceramic/polymer composites; Achieves comprehensive properties unavailable in single materials. | Complex process (requires pretreatment, mixing–dispersion, shaping–curing); Interface compatibility issues between components (affects stability); Difficulty in controlling mixing uniformity (causes performance fluctuations). | Mature for lab-scale production; mass production requires breakthroughs in automated mixing equipment and interface optimization; gradually applied in functional materials. |
| 3D Printing Technology [71,72,73,74] | Enables precise fabrication of complex structures; Supports personalized customization with flexible design adjustments; Layer-by-layer deposition enhances material performance via structural control. | High equipment cost and maintenance difficulty; Slow printing speed (low throughput, unsuitable for mass production); Strict requirements on fluidity/stability of liquid metal-material mixtures. | Superior for precision components and personalized products; Mass production needs high-speed printing and cost reduction; Currently for small-batch customization. |
| Mechanical Stirring [75] | Low maintenance cost; Realizes liquid metal micronization (microdroplets/lamellae) and homogenization. | Impurity introduction risk; Wide particle size distribution. | Suitable for medium-scale powder/microdroplet production; Needs impurity control for scaling. |
| Microfluidic Control [76] | Micron-level precision; Uniform product size/composition (ideal for micro/nano-composites). | Chip-dependent (high cost); Ultra-low throughput (lab-scale only). | Lab R&D focus; Hard to scale; For micro/nano-device small-batch production. |
| Vapor Deposition [77] | High-purity films/coatings with good compactness. | Slow deposition rate; Complex equipment (limited to films, not bulk materials). | Small-scale film production; Needs deposition rate improvement for scaling. |
| Ultrasonic Electroplating Replacement [78] | Metallurgical bonding between substrate and liquid metal; Uniform interface composition. | Narrow process window (strict temperature/immersion time control); Limited substrate applicability. | Suitable for specific substrate coatings; Needs process stability optimization for scaling. |
| Researchers | Material Systems | Characteristic Advantages | Ref | |
|---|---|---|---|---|
| LM Single filler | Liao et al. | LM/CNF composite shielding film | stable electromagnetic shielding effectiveness of 65 dB | [102] |
| Liu et al. | PDMS/EGaIn composite elastomer | high flexibility, stable conductivity (<10% resistance variation), and consistent EMI shielding under 100% strain | [3] | |
| Zhang et al. | EWSA film | As the effective printing area increases, the shielding performance improves, reaching 53 dB | [104] | |
| LM/ conductive fillers | Zhao et al. | Ga-based liquid metal | core–shell Ga–Ni and Ga–CoNi nanohybrids are 3.92 and 3.8 GHz at a thickness of 1.4 mm, respectively. | [112] |
| Xu et al. | EM/LM foam | ultralight and highly resilient, with a shielding effectiveness of 98.7 dB | [113] | |
| Jiang et al. | MXene/LM | ultra-thin and 3D-printable, with a shielding effectiveness of 105 dB | [101] | |
| LM/ magnetic substances | Guo et al. | Fe-EGaIn magnetic circuit material | magnetic-field-controlled repair, easy-to-shape printing | [114] |
| Xiang et al. | LMMFs magnetically controlled material | magnetic fluid: conductivity > 104 S/m, Young’s modulus increased by ~4 times | [115] | |
| Shen et al. | ANF/MXene@Ga/Gd2O3 membrane | enables rapid heat dissipation, with a shielding efficiency per unit volume reaching 6788 dB·cm−1·g−1 | [116] | |
| LM different forming structures | Liao et al. | Layered hierarchical structure; LM/CNF composite film | broadband coverage, high-temperature stability, shielding effectiveness of 65 dB (4–18 GHz) | [102] |
| Xu et al. | Porous foam structure; EM/LM composite foam | ultrahigh compressive resilience, shielding effectiveness of 98.7 dB (8.2–40 GHz) | [113] | |
| Li et al. | Dynamic tuning structure; ALMA antagonistic coil | increasing temperature leads to decreased magnetic permeability and dynamic narrowband | [117] | |
| LM-based multifunctional materials | Wei et al. | LM/mesh pattern layer | wide frequency range, lightweight and stretchable, excellent electromagnetic shielding effectiveness | [118] |
| Zhang et al. | LM/PDMS novel porous material | high electromagnetic shielding effectiveness under extreme conditions, with tunability | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suo, J.; Guan, L.; Chen, P.; Zhu, Y.; Lin, M.; Hu, Y.; Liu, Z.; Han, S.; Han, S.; Bai, Z.; et al. Recent Progress of Liquid Metal-Based Electromagnetic Shielding Materials. Nanomaterials 2025, 15, 1346. https://doi.org/10.3390/nano15171346
Suo J, Guan L, Chen P, Zhu Y, Lin M, Hu Y, Liu Z, Han S, Han S, Bai Z, et al. Recent Progress of Liquid Metal-Based Electromagnetic Shielding Materials. Nanomaterials. 2025; 15(17):1346. https://doi.org/10.3390/nano15171346
Chicago/Turabian StyleSuo, Jialu, Li Guan, Peng Chen, Yujie Zhu, Mengmeng Lin, Yuanhua Hu, Zhen Liu, Shijie Han, Shixuan Han, Zhongyi Bai, and et al. 2025. "Recent Progress of Liquid Metal-Based Electromagnetic Shielding Materials" Nanomaterials 15, no. 17: 1346. https://doi.org/10.3390/nano15171346
APA StyleSuo, J., Guan, L., Chen, P., Zhu, Y., Lin, M., Hu, Y., Liu, Z., Han, S., Han, S., Bai, Z., Guo, X., Zhao, B., & Zhang, R. (2025). Recent Progress of Liquid Metal-Based Electromagnetic Shielding Materials. Nanomaterials, 15(17), 1346. https://doi.org/10.3390/nano15171346







