Graphene/Zirconia Composites for Components in Solid Oxide Fuel Cells: Microstructure and Electrical Conductivity
Abstract
1. Introduction
2. Materials and Methods
2.1. Processing and Sintering of Composite Powders
2.2. Characterization of Composite Powders and Sintered Specimens
3. Results
3.1. Microstructural Study
3.2. Electrical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chun, O.; Jamshaid, F.; Khan, M.Z.; Gohar, O.; Hussain, I.; Zhang, Y.; Zheng, K.; Saleem, M.; Motola, M.; Hanif, M.B. Advances in Low-Temperature Solid Oxide Fuel Cells: An Explanatory Review. J. Power Sources 2024, 610, 234719. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in Material Selection for Solid Oxide Fuel Cell Technology: A Review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Sengupta, P.; Bhattacharjee, A.; Maiti, H.S. Zirconia: A Unique Multifunctional Ceramic Material. Trans. Indian Inst. Met. 2019, 72, 1981–1998. [Google Scholar] [CrossRef]
- Zakaria, Z.; Abu Hassan, S.H.; Shaari, N.; Yahaya, A.Z.; Boon Kar, Y. A Review on Recent Status and Challenges of Yttria Stabilized Zirconia Modification to Lowering the Temperature of Solid Oxide Fuel Cells Operation. Int. J. Energy Res. 2020, 44, 631–650. [Google Scholar] [CrossRef]
- Shri Prakash, B.; Senthil Kumar, S.; Aruna, S.T. Properties and Development of Ni/YSZ as an Anode Material in Solid Oxide Fuel Cell: A Review. Renew. Sustain. Energy Rev. 2014, 36, 149–179. [Google Scholar] [CrossRef]
- Wu, W.; Wang, G.L.; Guan, W.B.; Zhen, Y.F.; Wang, W.G. Effect of Contact Method between Interconnects and Electrodes on Area Specific Resistance in Planar Solid Oxide Fuel Cells. Fuel Cells 2013, 13, 743–750. [Google Scholar] [CrossRef]
- Morris, T.A.; Barringer, E.A.; Kung, S.C.; McKain, R.W. An All-Ceramic Interconnect for Use in Solid-Oxide Fuel Cell Stacks. MRS Bull. 2005, 30, 596–600. [Google Scholar] [CrossRef]
- Miranzo, P.; Belmonte, M.; Osendi, M.I. From Bulk to Cellular Structures: A Review on Ceramic/Graphene Filler Composites. J. Eur. Ceram. Soc. 2017, 37, 3649–3672. [Google Scholar] [CrossRef]
- Glukharev, A.G.; Konakov, V.G. Synthesis and Properties of Zirconia-Graphene Composite Ceramics: A Brief Review. Rev. Adv. Mater. Sci. 2018, 56, 124–138. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, C.; Ji, H.; Yang, M.; Zhang, H.; Tian, W.; Wu, Y.; Tolochko, O.V.; Wang, Y. A Review of CNTs and Graphene Reinforced YSZ Nanocomposites: Preparation, Mechanical and Anti-Irradiation Properties. J. Mater. Sci. Technol. 2023, 167, 27–49. [Google Scholar] [CrossRef]
- Hudelja, P.; Schmidt, R.; Amorín, H.; Drev, S.; Iveković, A.; Abram, A.; Kocjan, A.; Wicklein, B. Microstructure-Property Relationships in Composites of 8YSZ Ceramics and in Situ Graphitized Nanocellulose. J. Eur. Ceram. Soc. 2022, 42, 4594–4606. [Google Scholar] [CrossRef]
- Ahmad, K.; Ahmad, M.A.; Raza, R.; Khan, M.A.; Rehman, Z.U.; Abbas, G. Graphene Incorporated Nanocomposite Anode for Low Temperature SOFCs. J. Electron. Mater. 2019, 48, 7507–7514. [Google Scholar] [CrossRef]
- Timurkutluk, C.; Zan, R.; Timurkutluk, B.; Toruntay, F.; Onbilgin, S.; Hasret, O.; Altuntepe, A. Effect of Reduced Graphene Oxide Addition on Cathode Functional Layer Performance in Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2023, 48, 23127–23135. [Google Scholar] [CrossRef]
- Kurapova, O.Y.; Glukharev, A.G.; Glumov, O.V.; Kurapov, M.Y.; Boltynjuk, E.V.; Konakov, V.G. Structure and Electrical Properties of YSZ-RGO Composites and YSZ Ceramics, Obtained from Composite Powder. Electrochim. Acta 2019, 320, 134573. [Google Scholar] [CrossRef]
- Marinha, D.; Belmonte, M. Mixed-Ionic and Electronic Conduction and Stability of YSZ-Graphene Composites. J. Eur. Ceram. Soc. 2019, 39, 389–395. [Google Scholar] [CrossRef]
- Glukharev, A.; Glumov, O.; Temnikova, M.; Shamshirgar, A.S.; Kurapova, O.; Hussainova, I.; Konakov, V. YSZ-RGO Composite Ceramics by Spark Plasma Sintering: The Relation between Thermal Evolution of Conductivity, Microstructure and Phase Stability. Electrochim. Acta 2021, 367, 137533. [Google Scholar] [CrossRef]
- Gómez-Gómez, A.; Ramírez, C.; Llorente, J.; Garcia, A.; Moreno, P.; Reveron, H.; Chevalier, J.; Osendi, M.I.; Belmonte, M.; Miranzo, P. Improved Crack Resistance and Thermal Conductivity of Cubic Zirconia Containing Graphene Nanoplatelets. J. Eur. Ceram. Soc. 2020, 40, 1557–1565. [Google Scholar] [CrossRef]
- Pérez-Coll, D.; Ruiz-Morales, J.C.; Marrero-López, D.; Núñez, P.; Frade, J.R. Effect of Sintering Additive and Low Temperature on the Electrode Polarization of CGO. J. Alloys Compd. 2009, 467, 533–538. [Google Scholar] [CrossRef]
- Moriche, R.; Guisado-Arenas, E.; Muñoz-Ferreiro, C.; López-Pernía, C.; Morales-Rodríguez, A.; Jiménez-Piqué, E.; Gallardo-López, Á.; Poyato, R. Influence of Graphene-Based Nanostructures Processing Routes and Aspect Ratio in Flexural Strength and Fracture Mechanisms of 3Y-TZP-Matrix Composites. Ceram. Int. 2024, 50, 19217–19227. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, Y.; Chen, W.; Li, X.; Zheng, Q.; Li, K.; Guo, R. Fabrication and Properties of in Situ Reduced Graphene Oxide-Toughened Zirconia Composite Ceramics. J. Am. Ceram. Soc. 2018, 101, 3498–3507. [Google Scholar] [CrossRef]
- Ramírez, C.; Wang, Q.; Belmonte, M.; Miranzo, P.; Isabel Osendi, M.; Sheldon, B.W.; Padture, N.P. Direct in Situ Observation of Toughening Mechanisms in Nanocomposites of Silicon Nitride and Reduced Graphene-Oxide. Scr. Mater. 2018, 149, 40–43. [Google Scholar] [CrossRef]
- Muñoz-Ferreiro, C.; López-Pernía, C.; Gallardo-López, Á.; Poyato, R. Unravelling the Optimization of Few-Layer Graphene Crystallinity and Electrical Conductivity in Ceramic Composites by Raman Spectroscopy. J. Eur. Ceram. Soc. 2021, 41, 290–298. [Google Scholar] [CrossRef]
- López-Pernía, C.; Morales-Rodríguez, A.; Gallardo-López, Á.; Poyato, R. Enhancing the Electrical Conductivity of In-Situ Reduced Graphene Oxide-Zirconia Composites through the Control of the Processing Routine. Ceram. Int. 2021, 47, 9382–9391. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Fan, Y.; Kang, L.; Zhou, W.; Jiang, W.; Wang, L.; Kawasaki, A. Control of Doping by Matrix in Few-Layer Graphene/ Metal Oxide Composites with Highly Enhanced Electrical Conductivity. Carbon. N. Y 2015, 81, 83–90. [Google Scholar] [CrossRef]
- Díez-Betriu, X.; Álvarez-García, S.; Botas, C.; Álvarez, P.; Sánchez-Marcos, J.; Prieto, C.; Menéndez, R.; De Andrés, A. Raman Spectroscopy for the Study of Reduction Mechanisms and Optimization of Conductivity in Graphene Oxide Thin Films. J. Mater. Chem. C Mater. 2013, 1, 6905–6912. [Google Scholar] [CrossRef]
- Muñoz-Ferreiro, C.; Morales-Rodríguez, A.; Rojas, T.C.; Jiménez-Piqué, E.; López-Pernía, C.; Poyato, R.; Gallardo-López, A. Microstructure, Interfaces and Properties of 3YTZP Ceramic Composites with 10 and 20 Vol% Different Graphene-Based Nanostructures as Fillers. J. Alloys Compd. 2019, 777, 213–224. [Google Scholar] [CrossRef]
- Flaureau, A.; Weibel, A.; Chevallier, G.; Estournès, C. Study of the Densification and Grain Growth Mechanisms Occurring during Spark Plasma Sintering of Different Submicronic Yttria-Stabilized Zirconia Powders. J. Eur. Ceram. Soc. 2021, 41, 3581–3594. [Google Scholar] [CrossRef]
- Rajeswari, K.; Suresh, M.B.; Hareesh, U.S.; Rao, Y.S.; Das, D.; Johnson, R. Studies on Ionic Conductivity of Stabilized Zirconia Ceramics (8YSZ) Densified through Conventional and Non-Conventional Sintering Methodologies. Ceram. Int. 2011, 37, 3557–3564. [Google Scholar] [CrossRef]
- Panthi, D.; Hedayat, N.; Du, Y. Densification Behavior of Yttria-Stabilized Zirconia Powders for Solid Oxide Fuel Cell Electrolytes. J. Adv. Ceram. 2018, 7, 325–335. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Rafique, M.; Usman, M.; ul Ain, Q.; Ghaffar, A. Evaluation of Densification Effects on the Properties of 8 Mol % Yttria Stabilized Zirconia Electrolyte Synthesized by Cost Effective Coprecipitation Route. Ceram. Int. 2021, 47, 2857–2863. [Google Scholar] [CrossRef]
- Román-Manso, B.; Domingues, E.; Figueiredo, F.M.; Belmonte, M.; Miranzo, P. Enhanced Electrical Conductivity of Silicon Carbide Ceramics by Addition of Graphene Nanoplatelets. J. Eur. Ceram. Soc. 2015, 35, 2723–2731. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.L.; Palermo, V.; Pugno, N.; et al. Science and Technology Roadmap for Graphene, Related Two-Dimensional Crystals, and Hybrid Systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef] [PubMed]
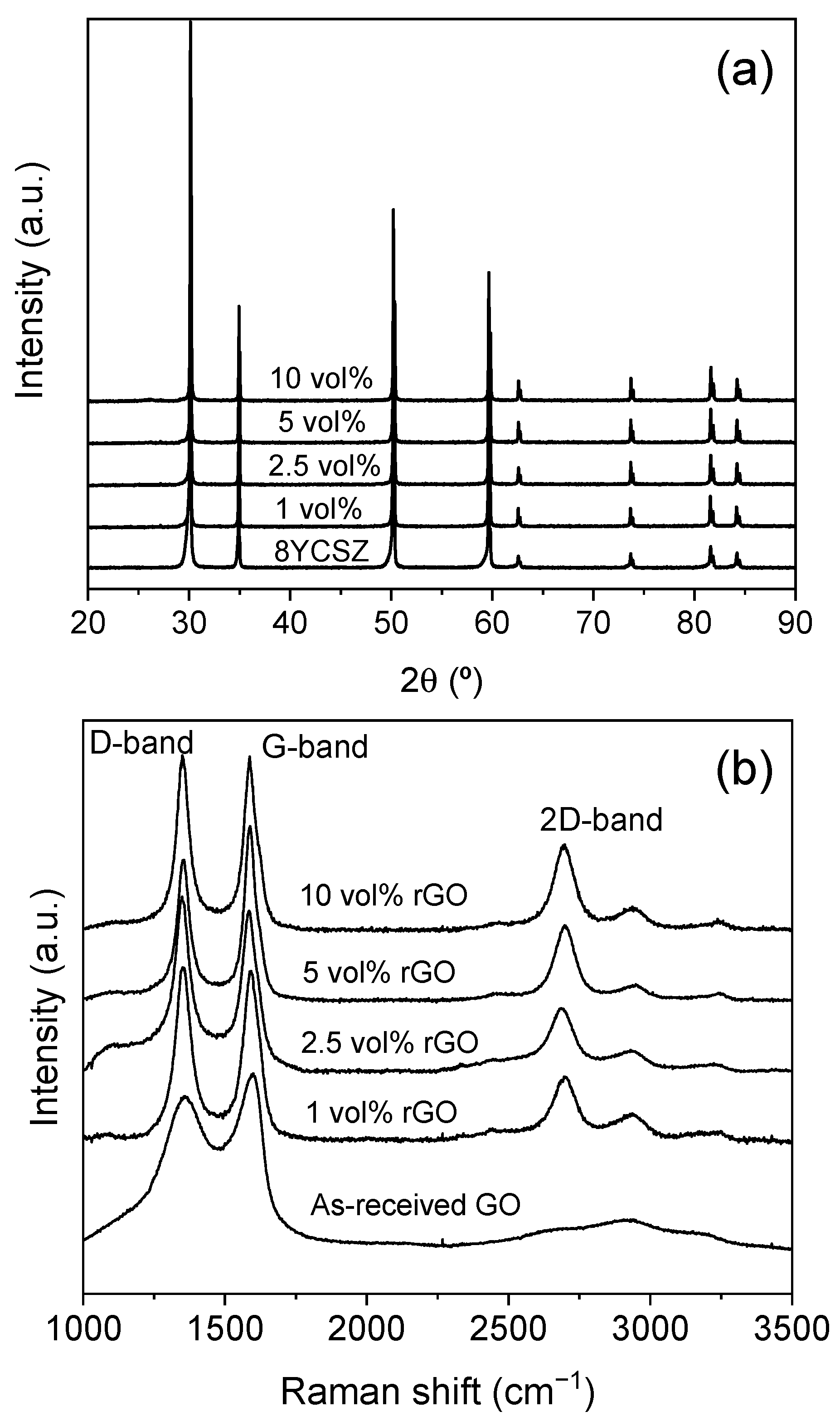
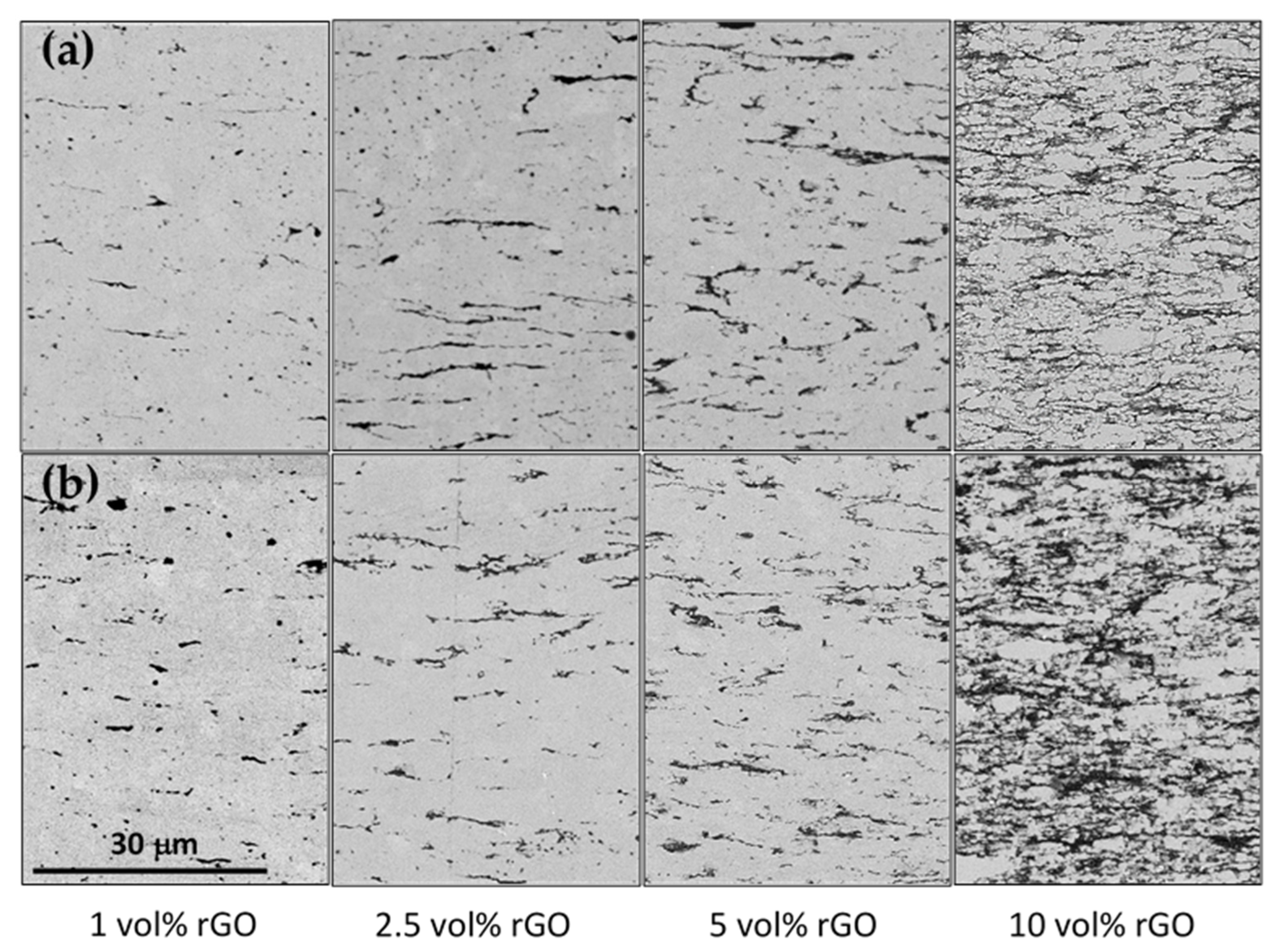



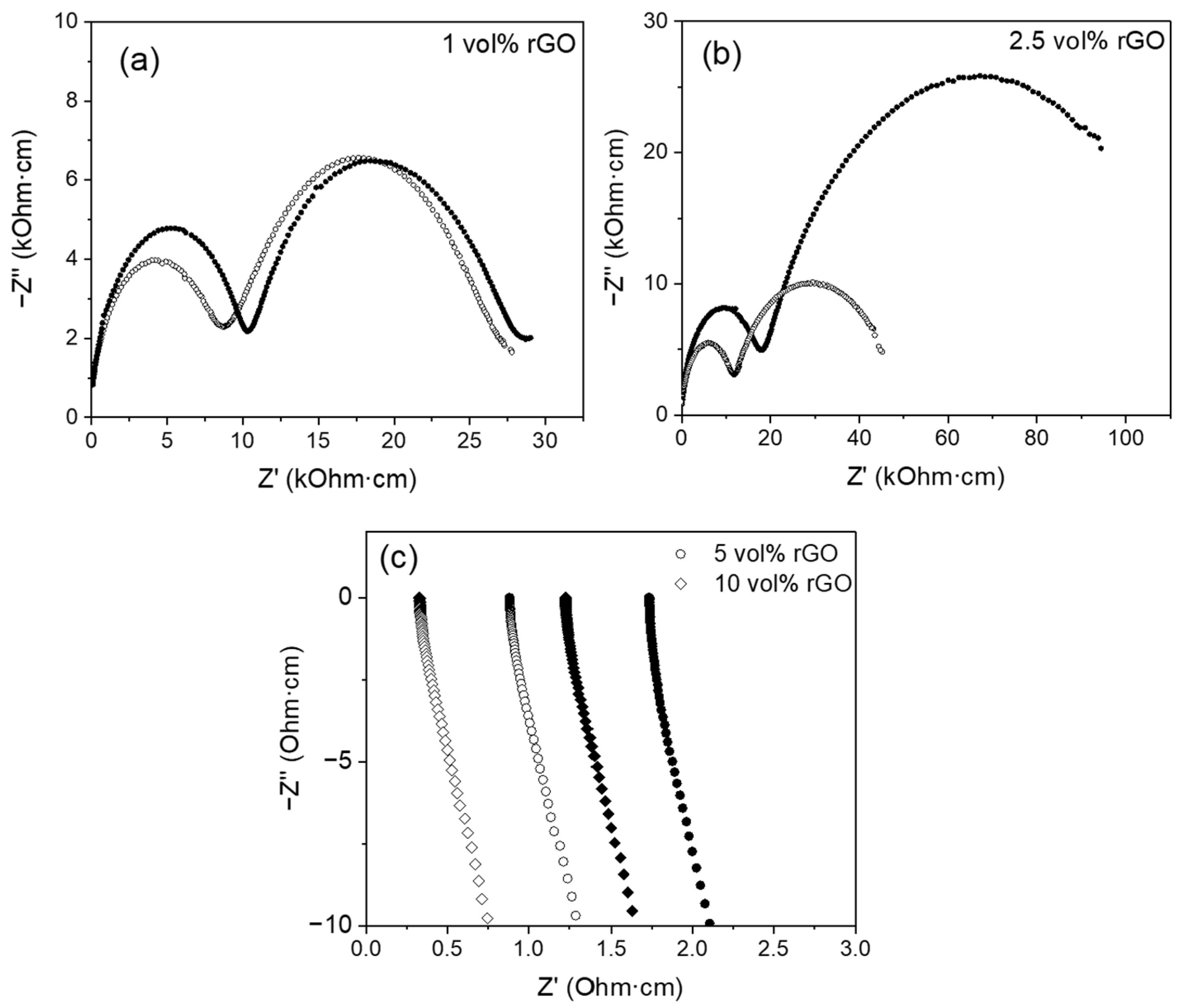
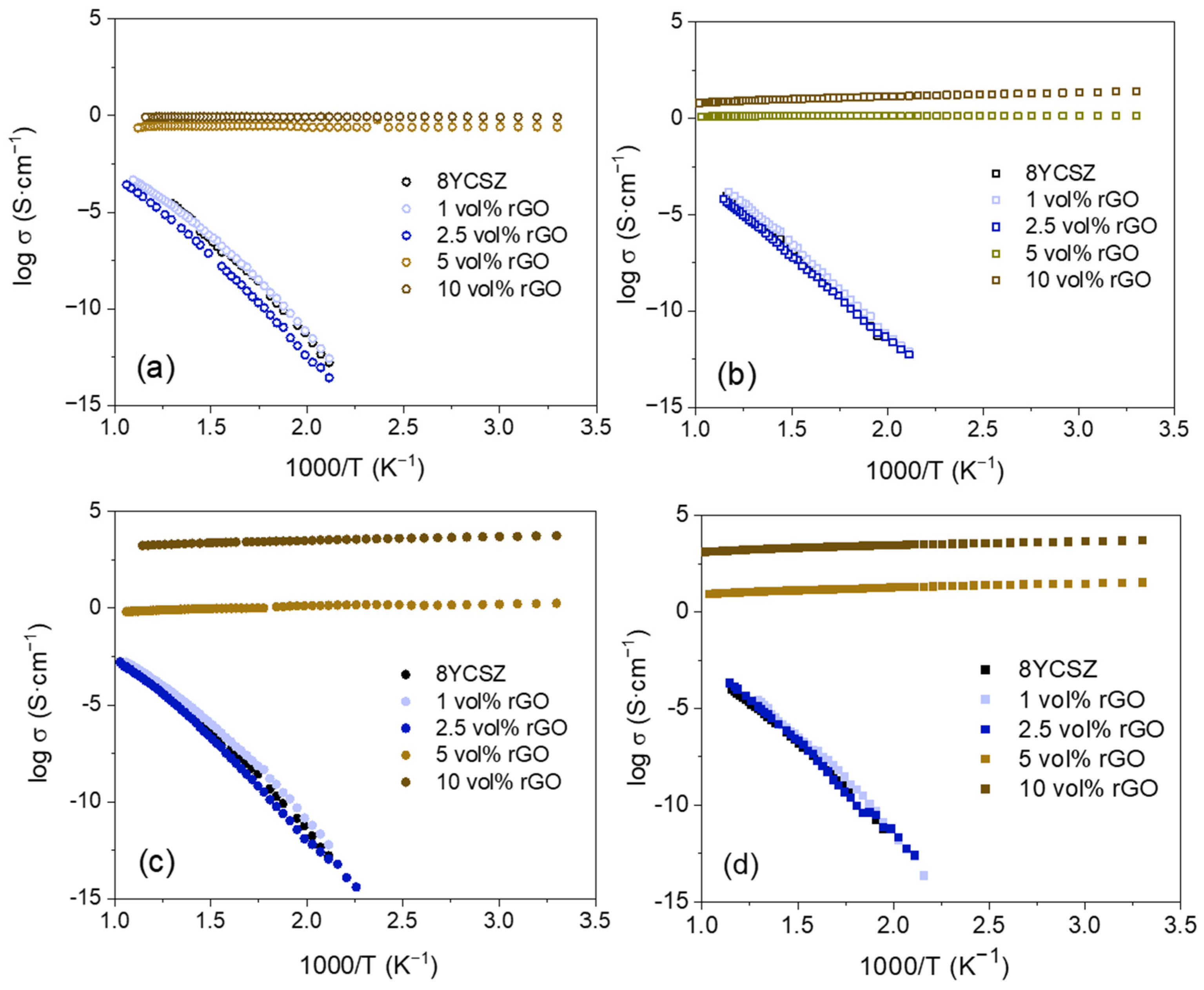
| rGO Vol% | Tsint (°C) | ρr (%) | <d> (μm) | σ<d> (μm) | A.R. | σA.R. |
|---|---|---|---|---|---|---|
| 0 | 1300 | 100 | 2.2 | 1.2 | 1.5 | 0.4 |
| 1 | 100 | 1.5 | 0.8 | 1.6 | 0.8 | |
| 2.5 | 99 | 1.3 | 1.4 | 1.6 | 0.6 | |
| 5 | 98 | 1.2 | 1.0 | 1.6 | 0.6 | |
| 10 | 94 | 0.5 | 0.1 | 1.6 | 0.5 | |
| 0 | 1350 | 100 | 3.7 | 2.2 | 1.5 | 0.4 |
| 1 | 100 | 2.9 | 1.6 | 1.5 | 0.3 | |
| 2.5 | 98 | 3.1 | 2.3 | 1.5 | 0.4 | |
| 5 | 98 | 1.1 | 0.9 | 1.5 | 0.4 | |
| 10 | 94 | 0.7 | 0.5 | 1.6 | 0.5 |
| rGO Vol% | Tsint (°C) | CHF (F) | CLF (F) |
|---|---|---|---|
| 1 | 1300 | ~10−11 | ~10−8–10−9 |
| 2.5 | ~10−11 | ~10−7–10−8 | |
| 1 | 1350 | ~10−11 | ~10−8 |
| 2.5 | ~10−11 | ~10−8 |
| rGO Vol% | Tsint (°C) | CHF (F) | CLF (F) |
|---|---|---|---|
| 1 | 1300 | ~10−11 | ~10−8–10−9 |
| 2.5 | ~10−10 | ~10−7 | |
| 1 | 1350 | ~10−10–10−11 | ~10−7–10−8 |
| 2.5 | ~10−10–10−11 | ~10−6–10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coto-Ruiz, F.J.; de la Cruz-Blanco, A.; Moriche, R.; Morales-Rodríguez, A.; Poyato, R. Graphene/Zirconia Composites for Components in Solid Oxide Fuel Cells: Microstructure and Electrical Conductivity. Nanomaterials 2025, 15, 1314. https://doi.org/10.3390/nano15171314
Coto-Ruiz FJ, de la Cruz-Blanco A, Moriche R, Morales-Rodríguez A, Poyato R. Graphene/Zirconia Composites for Components in Solid Oxide Fuel Cells: Microstructure and Electrical Conductivity. Nanomaterials. 2025; 15(17):1314. https://doi.org/10.3390/nano15171314
Chicago/Turabian StyleCoto-Ruiz, Francisco J., Ana de la Cruz-Blanco, Rocío Moriche, Ana Morales-Rodríguez, and Rosalía Poyato. 2025. "Graphene/Zirconia Composites for Components in Solid Oxide Fuel Cells: Microstructure and Electrical Conductivity" Nanomaterials 15, no. 17: 1314. https://doi.org/10.3390/nano15171314
APA StyleCoto-Ruiz, F. J., de la Cruz-Blanco, A., Moriche, R., Morales-Rodríguez, A., & Poyato, R. (2025). Graphene/Zirconia Composites for Components in Solid Oxide Fuel Cells: Microstructure and Electrical Conductivity. Nanomaterials, 15(17), 1314. https://doi.org/10.3390/nano15171314







