Band Engineering Induced by Sulphur Vacancies in MoS2/g-C3N4 or Selective CO2 Photoreduction to CH3OH
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Catalysts
2.2. Structural Characterisation of Catalysts
2.3. Photocatalytic Performance Test
3. Results and Discussion
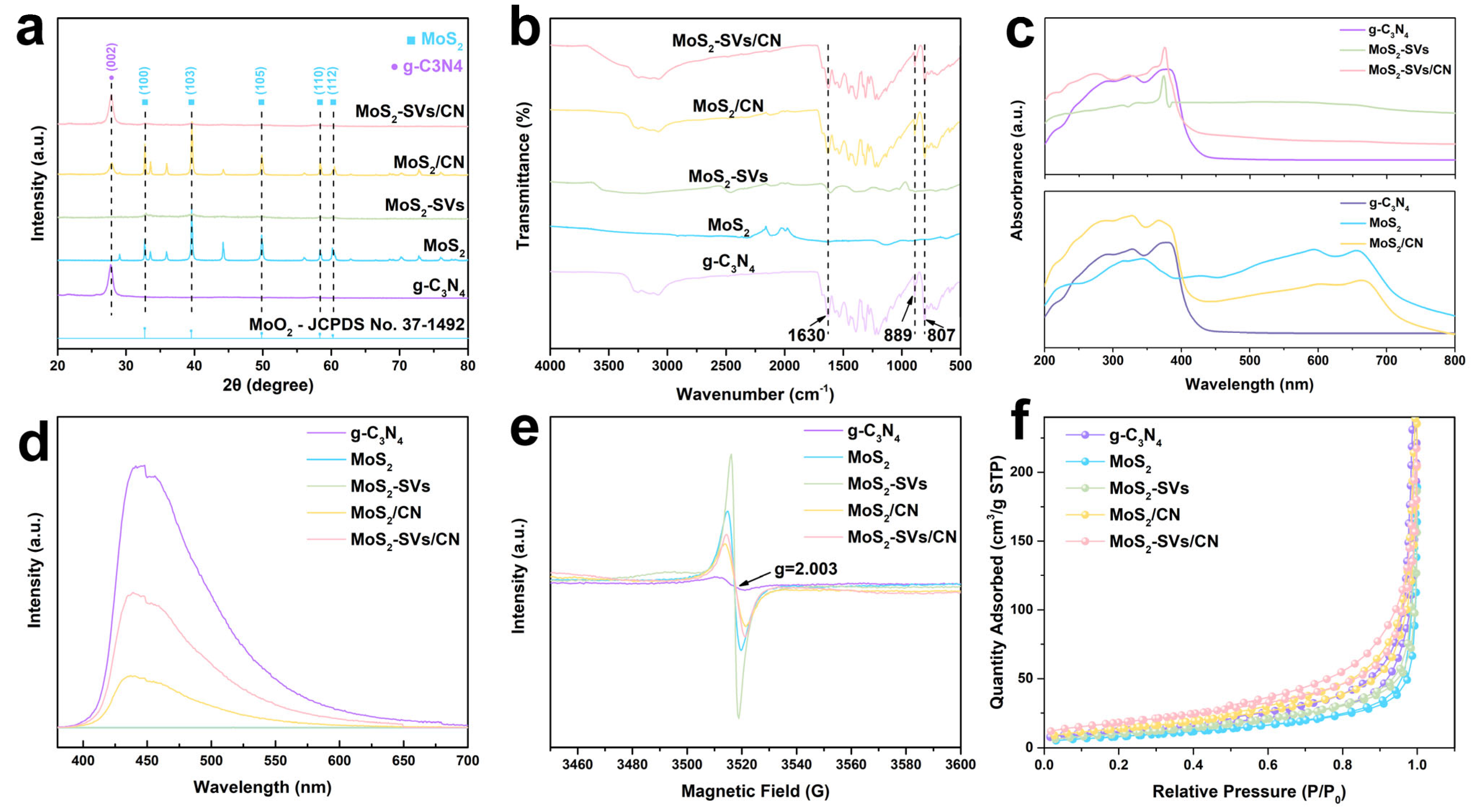
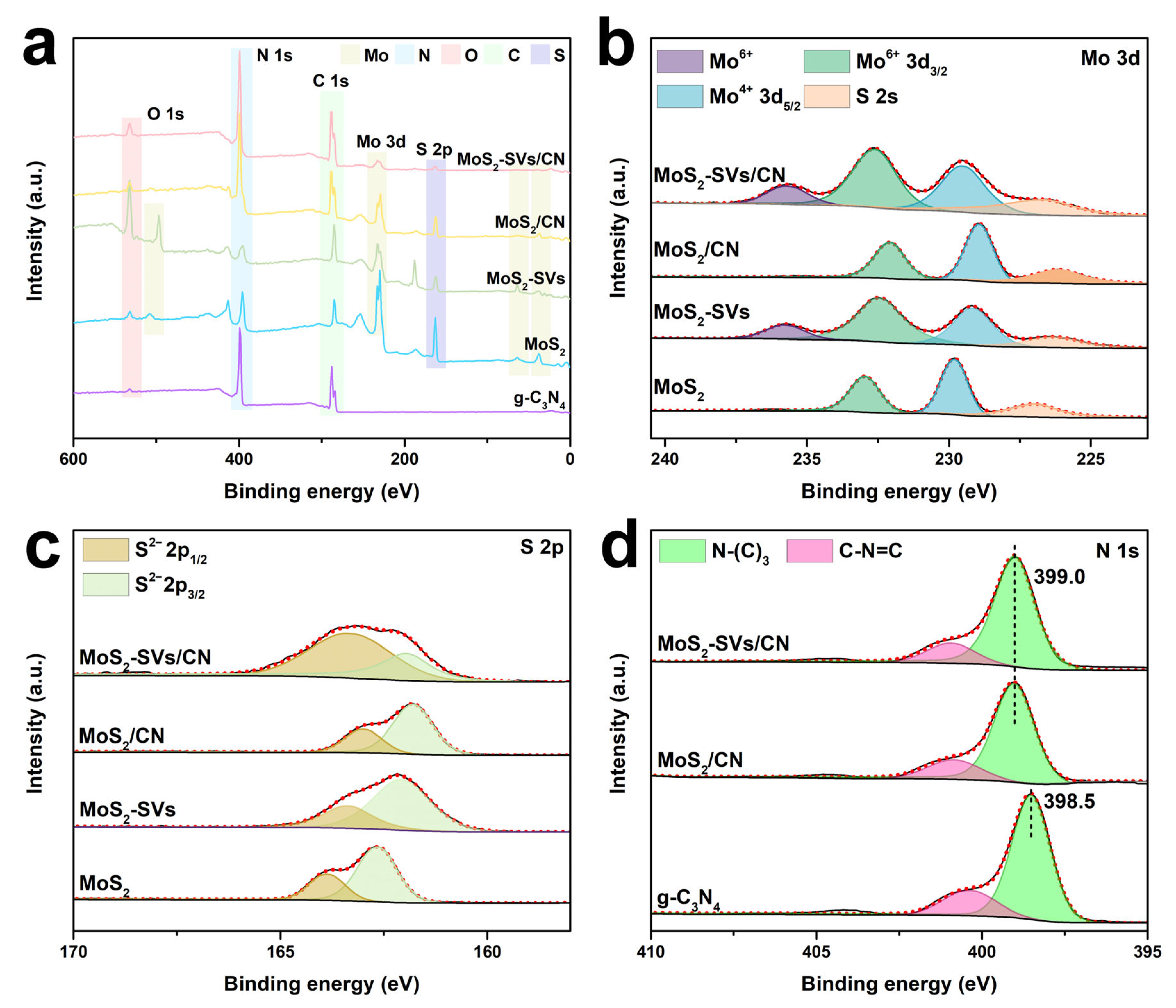
3.1. Structural and Optical Characteristics
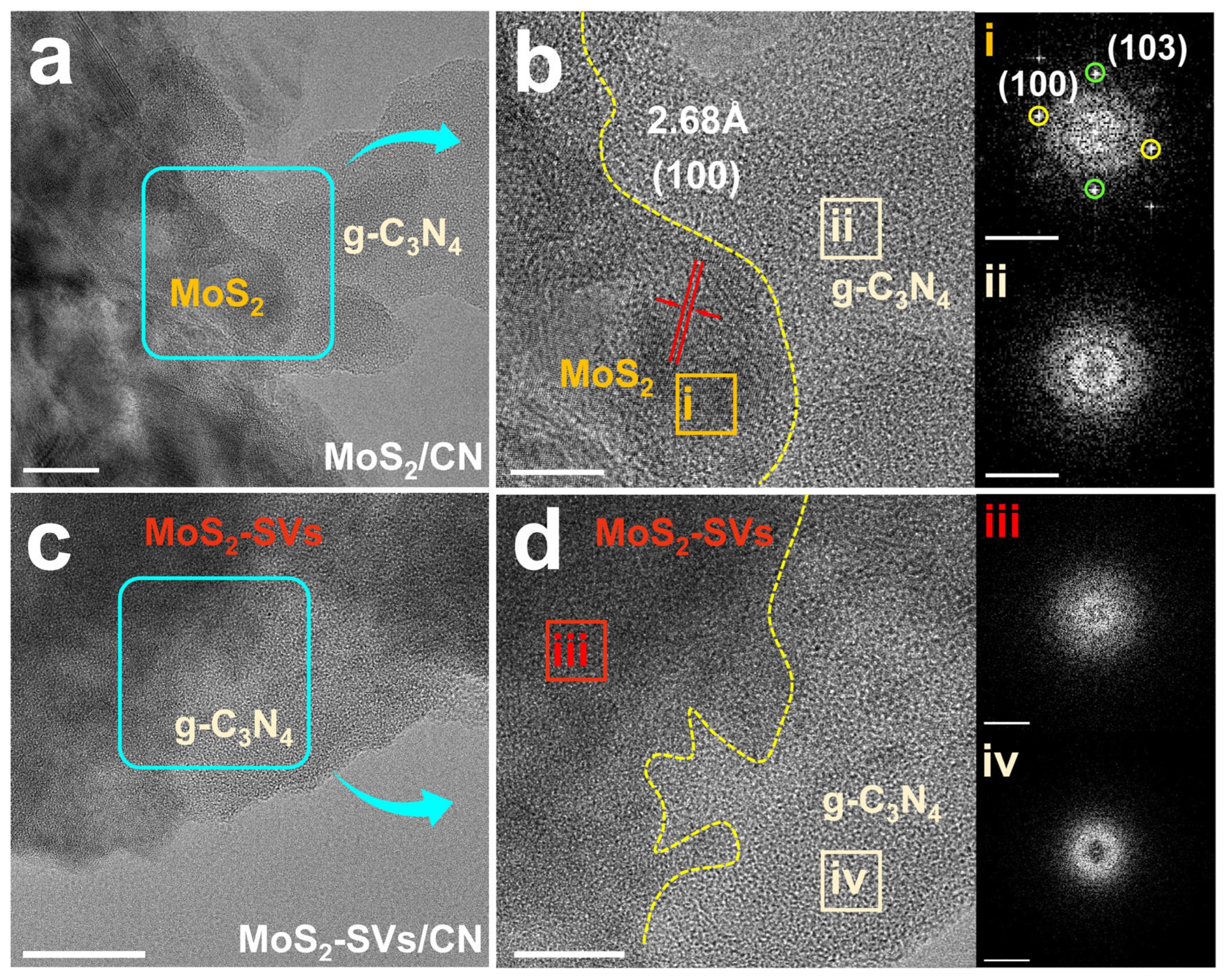
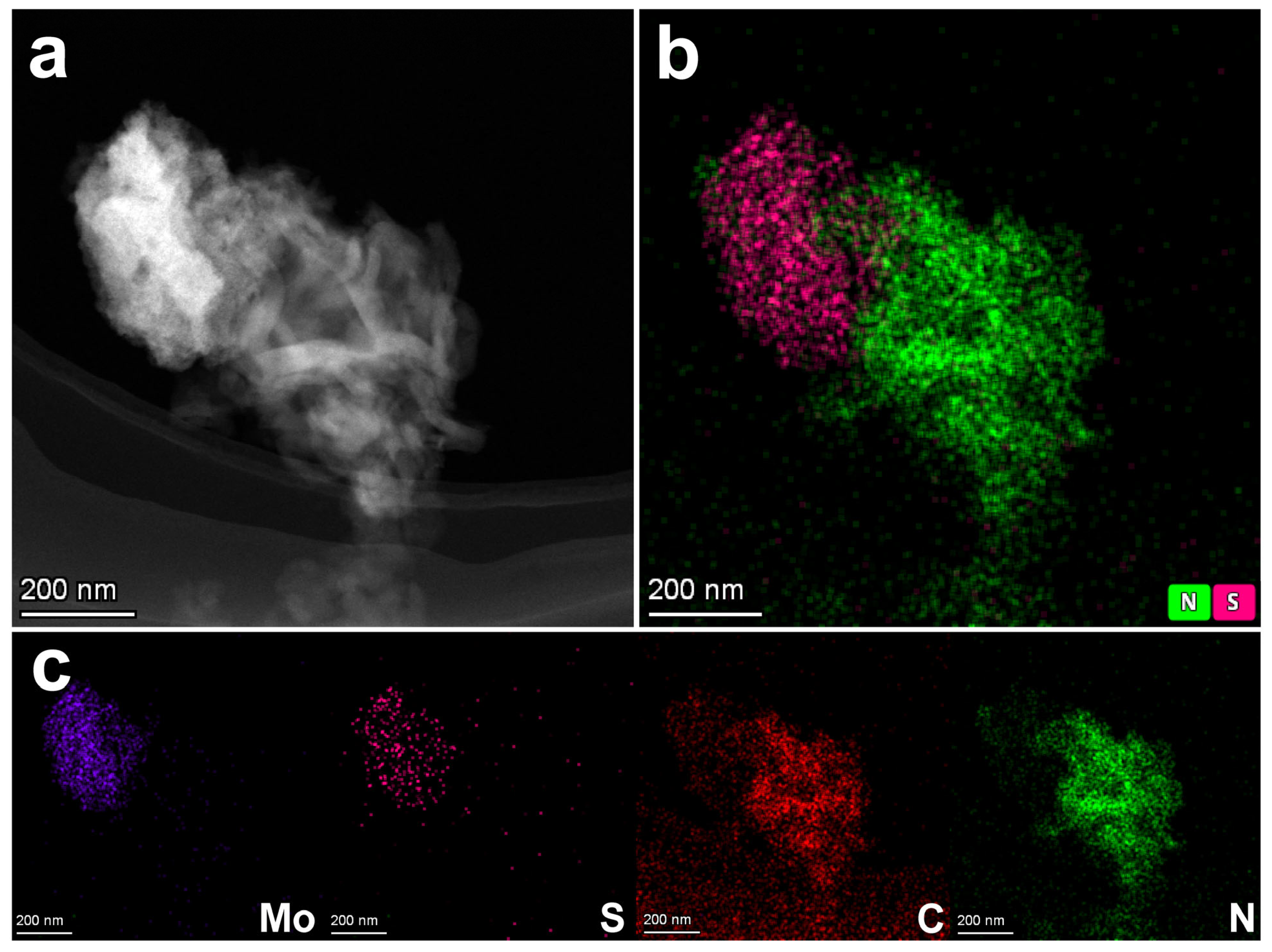
3.2. Morphological and Structural Characterisation
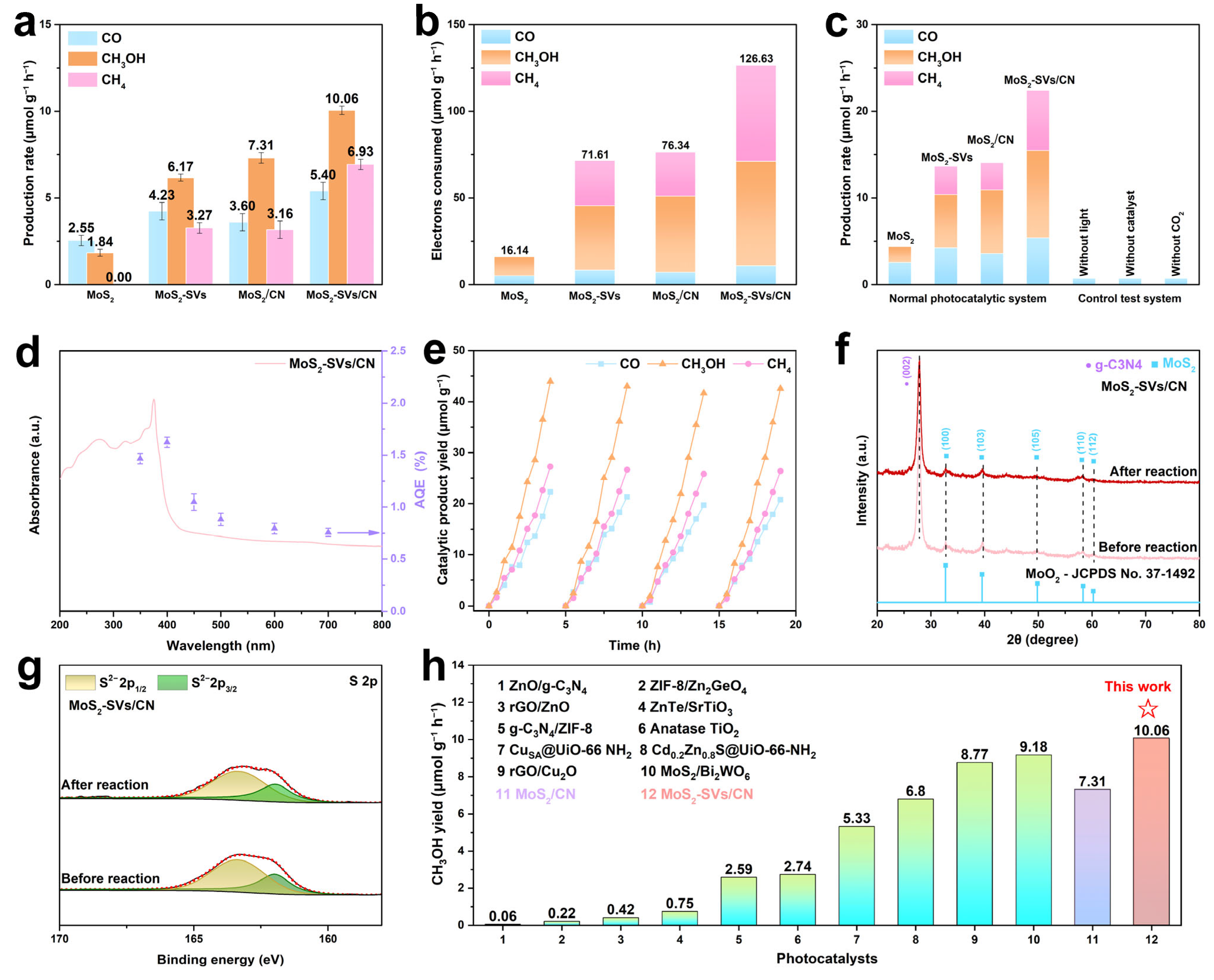
3.3. Photocatalytic Performance Evaluation
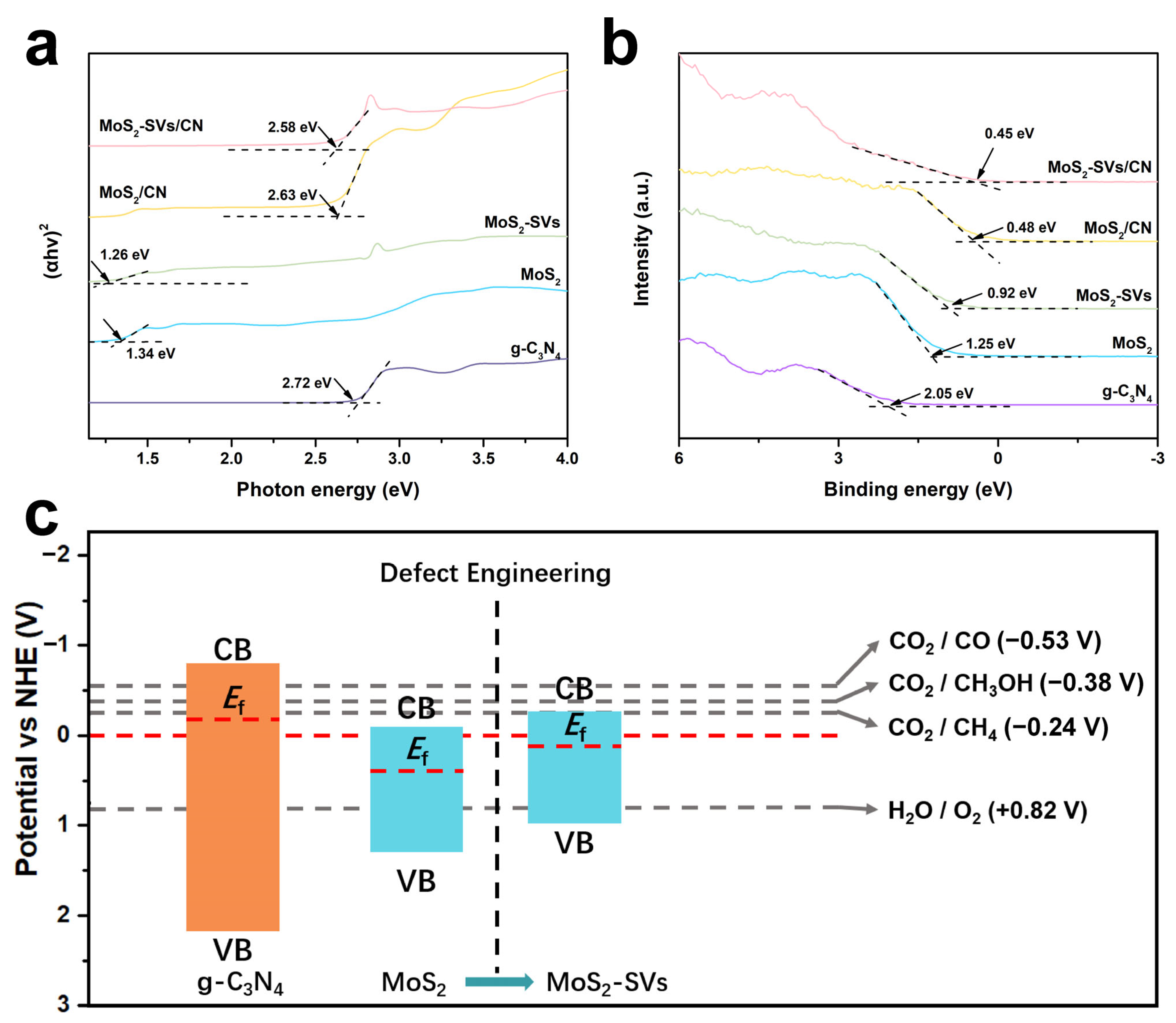
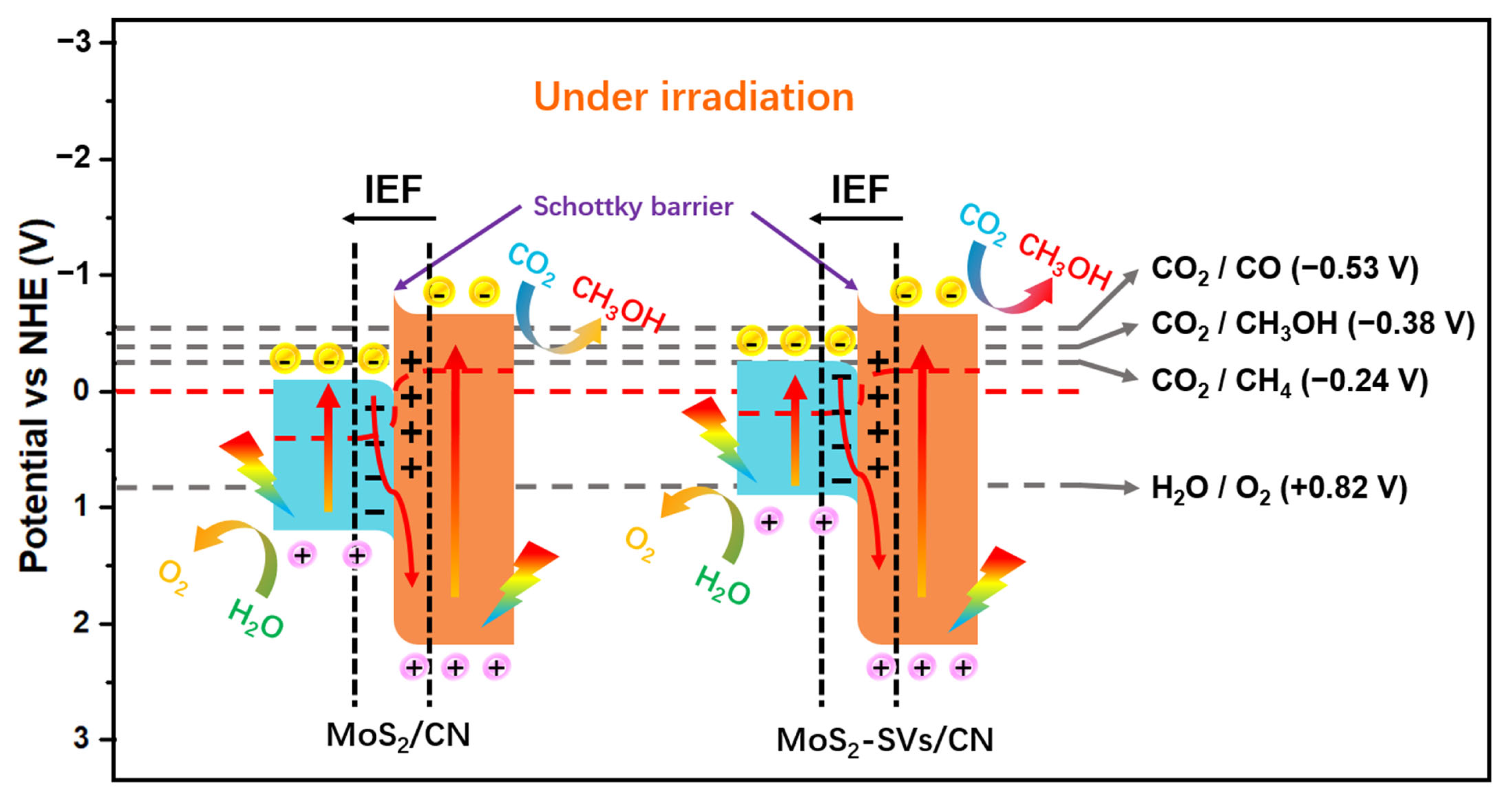
3.4. Electronic Structure and Band Modulation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, J.; Ma, Y.; Han, X.; Ergu, A.; Zhang, C.; Chen, P.; Liu, W.; Luo, Q.; Shi, Z.; Xu, H.; et al. Sun-simulated-driven production of high-purity methanol from carbon dioxide. Nat. Commun. 2025, 16, 857. [Google Scholar] [CrossRef]
- Song, L.; Wang, H.; Wang, S.; Qu, Z. Dual-site activation of H2 over Cu/ZnAl2O4 boosting CO2 hydrogenation to methanol. Appl. Catal. B Environ. 2023, 322, 122137. [Google Scholar] [CrossRef]
- Zhao, D.; Ortner, N.; Holena, M.; Wohlrab, S.; Kondratenko, E.V. Identifying Catalyst Property Descriptors for CO2 Hydrogenation to Methanol via Big-Data Analysis. ACS Catal. 2023, 13, 10547–10559. [Google Scholar] [CrossRef]
- Cao, M.; Chen, Y.; Liu, B.; Zhang, Z. CO2 hydrogenation to methanol on efficient Pd modified α-MoC1−x catalysts. Fuel 2024, 375, 132587. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, Y.; Gu, M.; Hu, Z.; Chang, Y.-C.; Pao, C.-W.; Xu, Y.; Huang, X. A Derivative of ZnIn2S4 Nanosheet Supported Pd Boosts Selective CO2 Hydrogenation. Adv. Funct. Mater. 2023, 33, 2215148. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Pant, K.K.; Kumar, S.; Joshi, D.; Biswas, P. Mechanistic exploration in controlling the product selectivity via metals in TiO2 for photocatalytic carbon dioxide reduction. Appl. Catal. B Environ. Energy 2024, 352, 124054. [Google Scholar] [CrossRef]
- Navarro-Jaén, S.; Virginie, M.; Bonin, J.; Robert, M.; Wojcieszak, R.; Khodakov, A.Y. Highlights and challenges in the selective reduction of carbon dioxide to methanol. Nat. Rev. Chem. 2021, 5, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Khaidar, D.M.; Isahak, W.N.R.W.; Ramli, Z.A.C.; Ahmad, K.N. Transition metal dichalcogenides-based catalysts for CO2 conversion: An updated review. Int. J. Hydrogen Energy 2024, 68, 35–50. [Google Scholar] [CrossRef]
- Zhou, Y.; Ye, Q.; Shi, X.; Zhang, Q.; Xie, Z.; Li, D.; Jiang, D. Regulating photocatalytic CO2 reduction selectivity via steering cascade multi-step charge transfer pathways in 1 T/2H-WS2/TiO2 heterojuncitons. Chem. Eng. J. 2022, 447, 137485. [Google Scholar] [CrossRef]
- Chen, C.; Ye, C.; Zhao, X.; Zhang, Y.; Li, R.; Zhang, Q.; Zhang, H.; Wu, Y. Supported Au single atoms and nanoparticles on MoS2 for highly selective CO2-to-CH3COOH photoreduction. Nat. Commun. 2024, 15, 7825. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Naden, A.B.; Skinner, W.S.J.; Kerherve, G.; Payne, D.J.; Irvine, J.T.S. Room Temperature Exsolution of Cds Nanodots on A-site Deficient Cotton-Ball Like Titanate Perovskite Nanoparticles for H2 Production Under Visible Light. Adv. Energy Mater. 2023, 13, 2301381. [Google Scholar] [CrossRef]
- Ding, X.; You, J.; Xue, Y.; Wang, J.; Qin, Y.; Tian, J.; Zhang, H.; Wang, X. Insights into the function of metallic 1T phase tungsten disulfide as cocatalyst decorated zinc indium sulfide for enhanced photocatalytic hydrogen production activity. J. Colloid Interface Sci. 2024, 673, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Yu, L.; Deng, J.; Wang, Y.; Cheng, K.; Ma, C.; Zhang, Q.; Wen, W.; Yu, S.; Pan, Y.; et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 2021, 4, 242–250. [Google Scholar] [CrossRef]
- Ling, W.; Ma, J.; Hong, M.; Sun, R. Enhance photocatalytic CO2 reduction and biomass selective oxidation via sulfur vacancy-enriched S-scheme heterojunction of MoS2@GCN. Chem. Eng. J. 2024, 493, 152729. [Google Scholar] [CrossRef]
- Mamo, T.T.; Qorbani, M.; Hailemariam, A.G.; Putikam, R.; Chu, C.-M.; Ko, T.-R.; Sabbah, A.; Huang, C.-Y.; Kholimatussadiah, S.; Billo, T.; et al. Enhanced CO2 photoreduction to CH4 via * COOH and * CHO intermediates stabilization by synergistic effect of implanted P and S vacancy in thin-film SnS2. Nano Energy 2024, 128, 109863. [Google Scholar] [CrossRef]
- Wang, J.; Bo, T.; Shao, B.; Zhang, Y.; Jia, L.; Tan, X.; Zhou, W.; Yu, T. Effect of S vacancy in Cu3SnS4 on high selectivity and activity of photocatalytic CO2 reduction. Appl. Catal. B Environ. 2021, 297, 120498. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, M.; Sun, Y.; Xiao, L.; Wu, W. Constructing Mo2C/MoS2 heterojunction nanostructure as outstanding catalysts for CO2 hydrogenation. J. Ind. Eng. Chem. 2025. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Deng, Y.; Zhang, Z.; Wang, H.; Wu, Y. Directed Inward Migration of S-Vacancy in Bi2S3 QDs for Selective Photocatalytic CO2 to CH3OH. Adv. Sci. 2025, 12, 2406925. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-Y.; Sun, K.; Liu, J.-X.; Ma, X.; Jian, M.; Sun, C.; Xu, Y.; Yin, H.; Li, W.-X. Bridge sulfur vacancies in MoS2 catalyst for reverse water gas shift: A first-principles study. Appl. Surf. Sci. 2021, 561, 149925. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Liang, H.; Hua, Y.; Chen, C.; Xiong, J.; Di, J. Tip-like copper sites on high curvature supports for non-covalent to covalent interaction tuning in CO2 photoreduction. Appl. Catal. B Environ. Energy 2025, 361, 124658. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Yu, Z.; Jiang, X.; Chen, J.; Tao, L.; Wang, M.; Shen, Y. Artificial photosynthesis of ethanol using type-II g-C3N4/ZnTe heterojunction in photoelectrochemical CO2 reduction system. Nano Energy 2019, 60, 827–835. [Google Scholar] [CrossRef]
- Mohammadi, N.K.; Fatemi, S. Efficient Photocatalytic CO2 Conversion to CO and CH4 by a rGO-Bridged g-C3N4/MoS2 Indirect Z-Scheme Heterojunction. Ind. Eng. Chem. Res. 2025, 64, 10399–10413. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, F.; Zhao, S.; Zhang, J.; Zhong, S.; Mao, H.; Zhao, L.; Bai, S. Efficient Charge and Proton Balance Enabled by a 2D/2D S-Scheme Heterojunction with a Nanochamber Design for Better Synergy of Photocatalytic CO2 Methanation and Benzylamine Oxidation. Adv. Funct. Mater. 2025, 35, 2413830. [Google Scholar] [CrossRef]
- Huu, H.T.; Thi, M.D.N.; Nguyen, V.P.; Thi, L.N.; Phan, T.T.T.; Hoang, Q.D.; Luc, H.H.; Kim, S.J.; Vo, V. One-pot synthesis of S-scheme MoS2/g-C3N4 heterojunction as effective visible light photocatalyst. Sci. Rep. 2021, 11, 14787. [Google Scholar]
- Li, X.; Wang, M.; Wang, R.; Wang, Y.; Zhu, M.; Zhang, L.; Shi, J. Construction of Ru Single-Atoms on Ceria to Reform the Products of CO2 Photoreduction. ACS Nano 2024, 18, 5741–5751. [Google Scholar] [CrossRef]
- Bashal, A.H.; Alkanad, K.; Al-Ghorbani, M.; Aoun, S.B.; Bajiri, M.A. Synergistic effect of cocatalyst and S-scheme heterojunction over 2D/2D g-C3N4/MoS2 heterostructure coupled Cu nanoparticles for selective photocatalytic CO2 reduction to CO under visible light irradiation. J. Environ. Chem. Eng. 2023, 11, 109545. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, N.; Yang, Y.; Wang, G.; Ng, D.H.L. High Efficiency Photocatalysis for Pollutant Degradation with MoS2/C3N4 Heterostructures. Langmuir 2014, 30, 8965–8972. [Google Scholar] [CrossRef]
- Cui, P.; Sun, R.; Xiao, L.; Wu, W. Exploring the Effects of the Interaction of Carbon and MoS2 Catalyst on CO2 Hydrogenation to Methanol. Int. J. Mol. Sci. 2022, 23, 5220. [Google Scholar] [CrossRef]
- Tonda, S.; Kumar, S.; Bhardwaj, M.; Yadav, P.; Ogale, S. g-C3N4/NiAl-LDH 2D/2D Hybrid Heterojunction for High-Performance Photocatalytic Reduction of CO2 into Renewable Fuels. ACS Appl. Mater. Interfaces 2018, 10, 2667–2678. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.J.; Wang, H.X.; Zhang, X.; Zhang, Y.T. MoS2 Nanosheets Functionalized Composite Mixed Matrix Membrane for Enhanced CO2 Capture via Surface Drop-Coating Method. ACS Appl. Mater. Interfaces 2016, 8, 23371–23378. [Google Scholar] [CrossRef]
- Pan, Y.L.; Gong, L.L.; Cheng, X.D.; Zhou, Y.; Fu, Y.B.; Feng, J.; Ahmed, H.; Zhang, H.P. Layer-Spacing-Enlarged MoS2 Superstructural Nanotubes with Further Enhanced Catalysis and Immobilization for Li-S Batteries. ACS Nano 2020, 14, 5917–5925. [Google Scholar] [CrossRef] [PubMed]
- Parzinger, E.; Miller, B.; Blaschke, B.; Garrido, J.A.; Ager, J.W.; Holleitner, A.; Wurstbauer, U. Photocatalytic Stability of Single- and Few-Layer MoS2. ACS Nano 2015, 9, 11302–11309. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.M.A.; Mohamed, H.S.H.; Khabiri, G. In situ growth of semiconducting 1T/3R-MoS2 nanosheets on spindle Mil88a as a novel heterostructure for outstanding photocatalytic performance. Sep. Purif. Technol. 2024, 339, 126712. [Google Scholar] [CrossRef]
- Qin, H.; Guo, R.T.; Liu, X.Y.; Pan, W.G.; Wang, Z.Y.; Shi, X.; Tang, J.Y.; Huang, C.Y. Z-Scheme MoS2/g-C3N4 heterojunction for efficient visible light photocatalytic CO2 reduction. Dalton Trans. 2018, 47, 15155–15163. [Google Scholar] [CrossRef]
- Qi, Y.H.; Xu, Q.; Wang, Y.; Yan, B.; Ren, Y.M.; Chen, Z.M. CO2-Induced Phase Engineering: Protocol for Enhanced Photoelectrocatalytic Performance of 2D MoS2 Nanosheets. ACS Nano 2016, 10, 2903–2909. [Google Scholar] [CrossRef]
- He, Y.M.; Wang, Y.; Zhang, L.H.; Teng, B.T.; Fan, M.H. High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl. Catal. B-Environ. 2015, 168, 1–8. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.S.; Cheng, B.; Ho, W.K.; Yu, J.G. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B-Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Kim, R.; Kim, J.; Do, J.Y.; Seo, M.W.; Kang, M. Carbon Dioxide Photoreduction on the Bi2S3/MoS2 Catalyst. Catalysts 2019, 9, 998. [Google Scholar] [CrossRef]
- Zhao, X.X.; Guan, J.R.; Li, J.Z.; Li, X.; Wang, H.Q.; Huo, P.W.; Yan, Y.S. CeO2/3D g-C3N4 heterojunction deposited with Pt cocatalyst for enhanced photocatalytic CO2 reduction. Appl. Surf. Sci. 2021, 537, 147891. [Google Scholar] [CrossRef]
- Cao, S.W.; Li, Y.; Zhu, B.C.; Jaroniec, M.; Yu, J.G. Facet effect of Pd cocatalyst on photocatalytic CO2 reduction over g-C3N4. J. Catal. 2017, 349, 208–217. [Google Scholar] [CrossRef]
- Blanchet, E.; Duquenne, F.; Rafrafi, Y.; Etcheverry, L.; Erable, B.; Bergel, A. Importance of the hydrogen route in up-scaling electrosynthesis for microbial CO2 reduction. Energy Environ. Sci. 2015, 8, 3731–3744. [Google Scholar] [CrossRef]
- Liu, Q.; Low, Z.-X.; Li, L.; Razmjou, A.; Wang, K.; Yao, J.; Wang, H. ZIF-8/Zn2GeO4 nanorods with an enhanced CO2 adsorption property in an aqueous medium for photocatalytic synthesis of liquid fuel. J. Mater. Chem. A 2013, 1, 11563–11569. [Google Scholar] [CrossRef]
- Zhang, L.; Li, N.; Jiu, H.; Qi, G.; Huang, Y. ZnO-reduced graphene oxide nanocomposites as efficient photocatalysts for photocatalytic reduction of CO2. Ceram. Int. 2015, 41, 6256–6262. [Google Scholar] [CrossRef]
- Ehsan, M.F.; Ashiq, M.N.; Bi, F.; Bi, Y.; Palanisamy, S.; He, T. Preparation and characterization of SrTiO3–ZnTe nanocomposites for the visible-light photoconversion of carbon dioxide to methane. RSC Adv. 2014, 4, 48411–48418. [Google Scholar] [CrossRef]
- Liu, S.; Chen, F.; Li, S.; Peng, X.; Xiong, Y. Enhanced photocatalytic conversion of greenhouse gas CO2 into solar fuels over g-C3N4 nanotubes with decorated transparent ZIF-8 nanoclusters. Appl. Catal. B Environ. 2017, 211, 1–10. [Google Scholar] [CrossRef]
- An, B.; Zhang, J.; Cheng, K.; Ji, P.; Wang, C.; Lin, W. Confinement of Ultrasmall Cu/ZnOx Nanoparticles in Metal–Organic Frameworks for Selective Methanol Synthesis from Catalytic Hydrogenation of CO2. J. Am. Chem. Soc. 2017, 139, 3834–3840. [Google Scholar] [CrossRef]
- Akhter, P.; Hussain, M.; Saracco, G.; Russo, N. Novel nanostructured-TiO2 materials for the photocatalytic reduction of CO2 greenhouse gas to hydrocarbons and syngas. Fuel 2015, 149, 55–65. [Google Scholar] [CrossRef]
- Wang, G.; He, C.-T.; Huang, R.; Mao, J.; Wang, D.; Li, Y. Photoinduction of Cu Single Atoms Decorated on UiO-66-NH2 for Enhanced Photocatalytic Reduction of CO2 to Liquid Fuels. J. Am. Chem. Soc. 2020, 142, 19339–19345. [Google Scholar] [CrossRef]
- Wang, A.; Li, X.; Zhao, Y.; Wu, W.; Chen, J.; Meng, H. Preparation and characterizations of Cu2O/reduced graphene oxide nanocomposites with high photo-catalytic performances. Powder Technol. 2014, 261, 42–48. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.; Deng, Y.; Hu, X.; Wang, T.; Luo, X. Facile synthesis of MoS2/Bi2WO6 nanocomposites for enhanced CO2 photoreduction activity under visible light irradiation. Appl. Surf. Sci. 2017, 403, 230–239. [Google Scholar] [CrossRef]
- Wang, H.M.; Li, C.H.; Fang, P.F.; Zhang, Z.L.; Zhang, J.Z. Synthesis, properties, and optoelectronic applications of two-dimensional MoS2 and MoS2-based heterostructures. Chem. Soc. Rev. 2018, 47, 6101–6127. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Mukherjee, S.; Mitra, R.K.; Ray, S.K. Size-dependent optical properties of MoS2 nanoparticles and their photo-catalytic applications. Nanotechnology 2020, 31, 145701. [Google Scholar] [CrossRef]
- Zhang, G.; Song, C.; Meng, X.; Wang, H.; Wang, Z.; Gao, H.; Wang, M.; Guo, X. Switching the product selectivity from methane to methanol in CO2 hydrogenation via Cu-modified vacancy engineering at MoS2 edge sites. J. Energy Chem. 2025, 108, 286–296. [Google Scholar] [CrossRef]
- Ye, L.Q.; Wu, D.; Chu, K.H.; Wang, B.; Xie, H.Q.; Yip, H.Y.; Wong, P.K. Phosphorylation of g-C3N4 for enhanced photocatalytic CO2 reduction. Chem. Eng. J. 2016, 304, 376–383. [Google Scholar] [CrossRef]
- Singh, S.; Modak, A.; Pant, K.K. CO2 Reduction to Methanol Using a Conjugated Organic-Inorganic Hybrid TiO2-C3N4 NanoAssembly. Trans. Indian Natl. Acad. Eng. 2021, 6, 395–404. [Google Scholar] [CrossRef]
- Fu, J.W.; Zhu, B.C.; Jiang, C.J.; Cheng, B.; You, W.; Yu, J.G. Hierarchical Porous O-Doped g-C3N4 with Enhanced Photocatalytic CO2 Reduction Activity. Small 2017, 13, 1603938. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.J.; Garg, A.; Sutter, B.; Kuhn, J.N.; Bhethanabotla, V.R. MoS2 Nanoflowers as a Gateway for Solar-Driven CO2 Photoreduction. ACS Sustain. Chem. Eng. 2018, 7, 265–275. [Google Scholar] [CrossRef]
- Zhu, B.C.; Zhang, L.Y.; Xu, D.F.; Cheng, B.; Yu, J.G. Adsorption investigation of CO2 on g-C3N4 surface by DFT calculation. J. CO2 Util. 2017, 21, 327–335. [Google Scholar] [CrossRef]
- Mano, P.; Jitwatanasirikul, T.; Roongcharoen, T.; Takahashi, K.; Namuangruk, S. Tuning covalent bonding of single transition metal atom doped in S vacant MoS2 for catalytic CO2 reduction reaction product selectivity. Appl. Surf. Sci. 2025, 688, 162339. [Google Scholar] [CrossRef]
- Jin, M.; Yang, X.; Wang, X.; Zhang, Z. UV-visible-infrared light driven photothermal synergistic catalytic reduction of CO2 over Cs3Bi2Br9/MoS2 S-scheme photocatalyst. J. Colloid Interface Sci. 2025, 680, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.R.; Huang, J.J.; Yang, Y.; Wu, J.J.; Shen, J.Y.; Liu, X.D.; Sun, A.W.; Liu, Z.L. In-situ construction of urchin-like hierarchical g-C3N4/NiAl-LDH hybrid for efficient photoreduction of CO2. Mater. Lett. 2020, 268, 127560. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Joo, M.H.; Maeng, J.Y.; Park, G.E.; Yang, S.Y.; Rhee, C.K.; Sohn, Y. Electrochemical CO2 Reduction over a MoS2/Mo Electrode. Appl. Sci. Converg. Technol. 2023, 32, 48–53. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Yu, J.; Chen, X.; Li, N.; Zhou, Q. Band Engineering Induced by Sulphur Vacancies in MoS2/g-C3N4 or Selective CO2 Photoreduction to CH3OH. Nanomaterials 2025, 15, 1294. https://doi.org/10.3390/nano15171294
Liu S, Yu J, Chen X, Li N, Zhou Q. Band Engineering Induced by Sulphur Vacancies in MoS2/g-C3N4 or Selective CO2 Photoreduction to CH3OH. Nanomaterials. 2025; 15(17):1294. https://doi.org/10.3390/nano15171294
Chicago/Turabian StyleLiu, Shicheng, Junbo Yu, Xiangyu Chen, Na Li, and Qulan Zhou. 2025. "Band Engineering Induced by Sulphur Vacancies in MoS2/g-C3N4 or Selective CO2 Photoreduction to CH3OH" Nanomaterials 15, no. 17: 1294. https://doi.org/10.3390/nano15171294
APA StyleLiu, S., Yu, J., Chen, X., Li, N., & Zhou, Q. (2025). Band Engineering Induced by Sulphur Vacancies in MoS2/g-C3N4 or Selective CO2 Photoreduction to CH3OH. Nanomaterials, 15(17), 1294. https://doi.org/10.3390/nano15171294





