Sustainable Recycling of Lithium-Ion Battery Cathodes: Life Cycle Assessment, Technologies, and Economic Insights
Abstract
1. Introduction
2. Cathode Materials in Lithium Battery Recycling Process and Technology
2.1. Pyrometallurgical-Based Recycling of Cathode Materials
2.2. Hydrometallurgical-Based Recycling of Cathode Materials
2.3. Direct Regeneration Recycling of Cathode Materials
2.4. Advanced Recycling of Cathode Materials
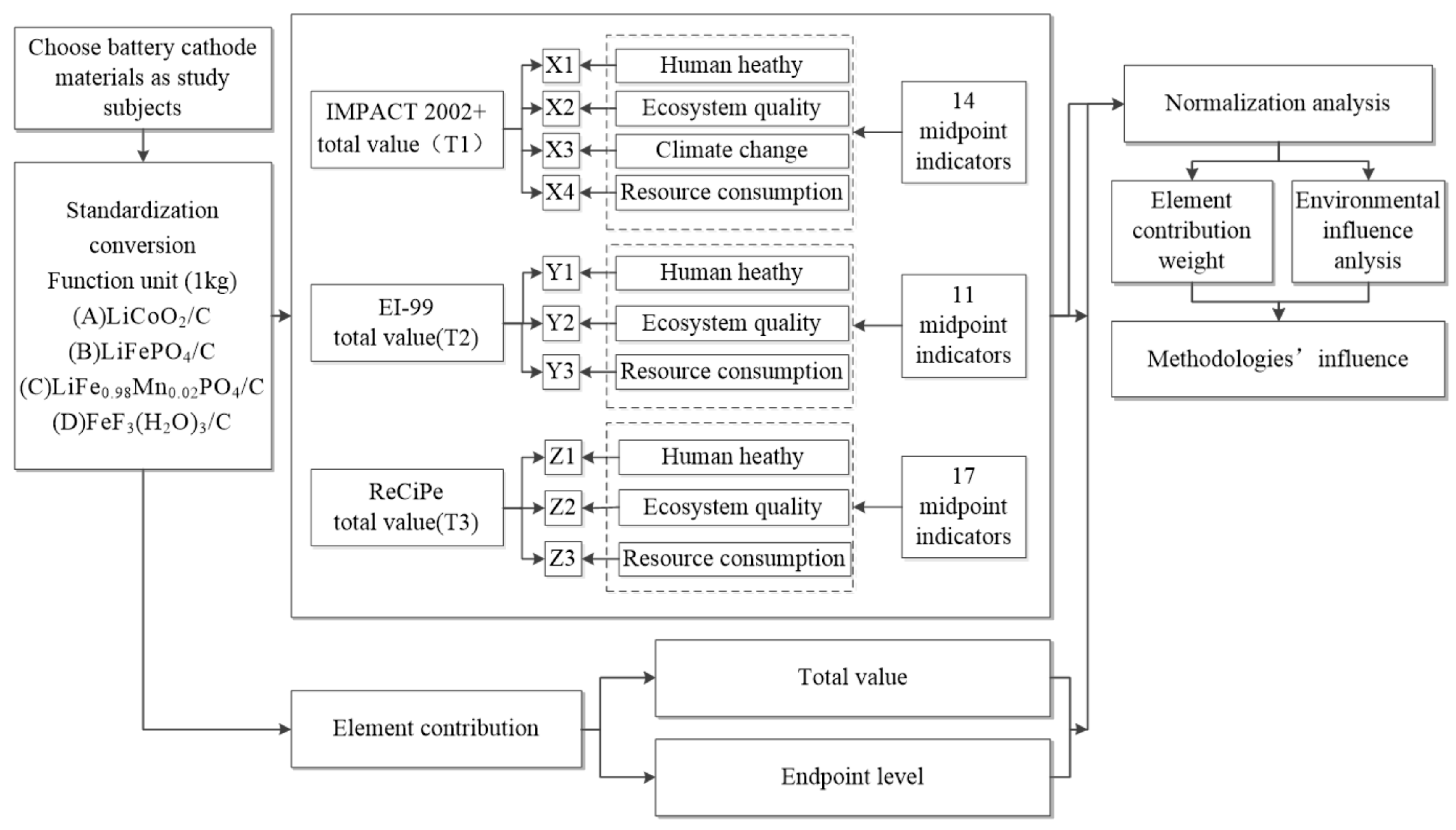
3. Life Cycle Assessment of Cathode Materials in Lithium Battery Recycling
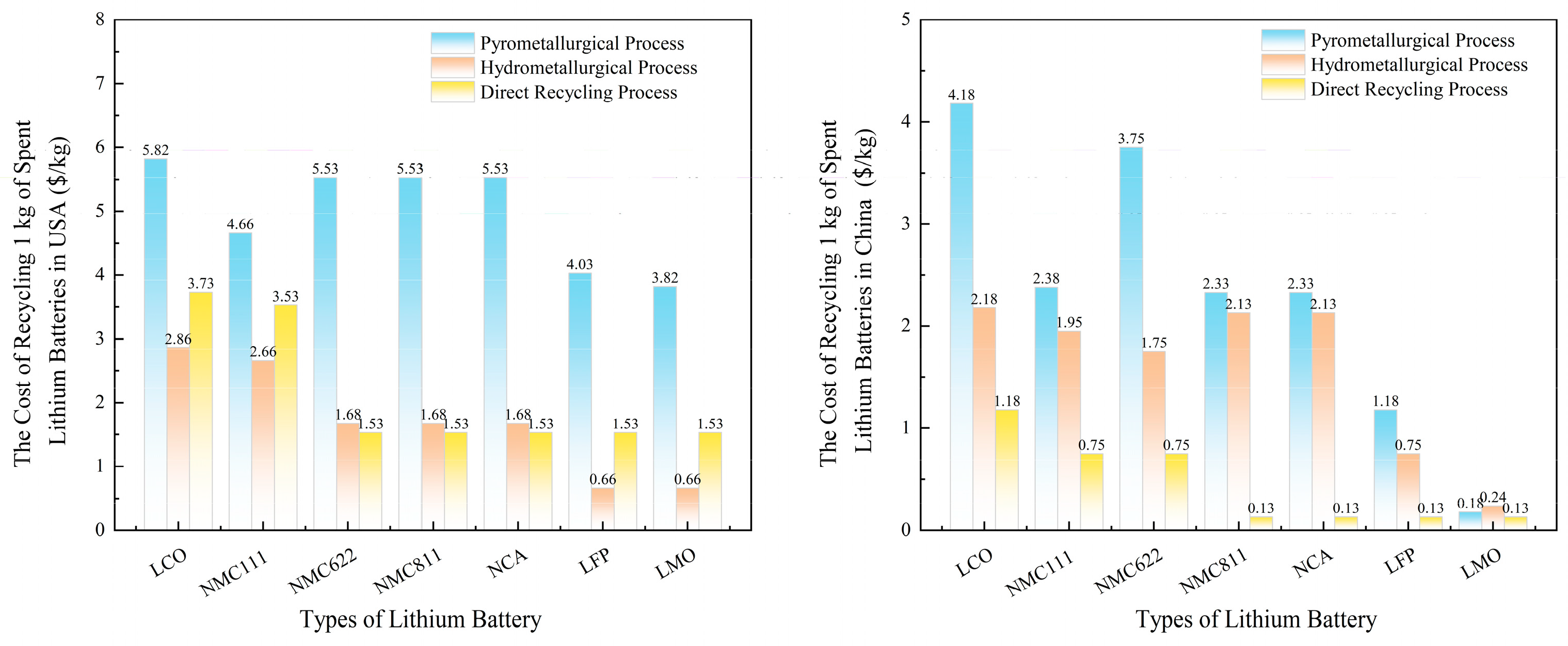
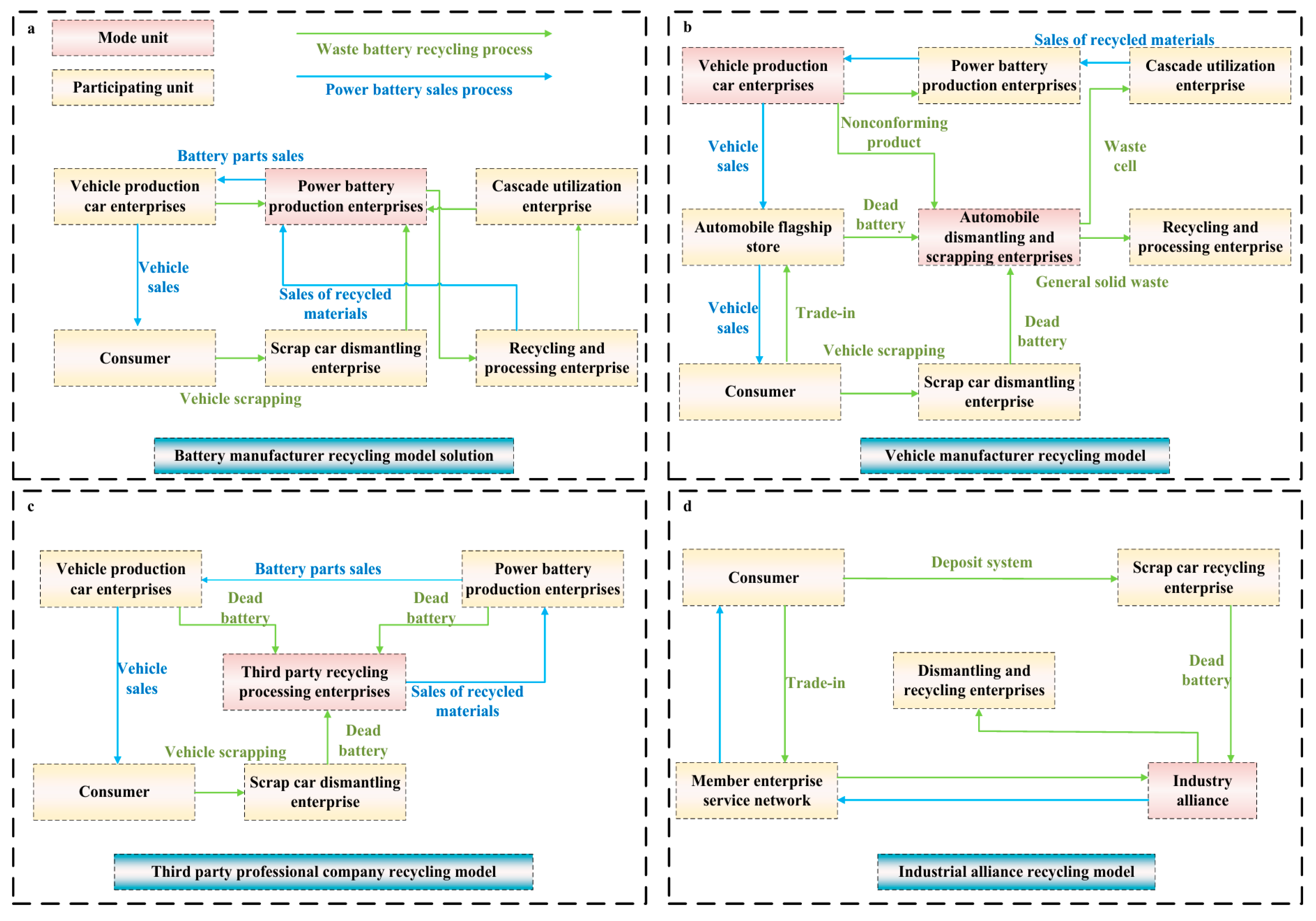
4. Economic Analysis of Cathode Materials in Lithium Battery Recycling
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Zhao, Y.; Ding, L.; Wang, D.; Guo, Q.; Li, Z.; Luo, H.; Zhang, D.; Yu, Y. Enhancing the Capacity and Stability by CoFe2O4 Modified g-C3N4 Composite for Lithium-Oxygen Batteries. Nanomaterials 2021, 11, 1088. [Google Scholar] [CrossRef]
- BloombergNef. Electric Vehicle Outlook 2025: Executive Summary; Bloomberg Finance L.P.: New York, NY, USA, 2025. [Google Scholar]
- Toba, A.-L.; Nguyen, R.T.; Cole, C.; Neupane, G.; Paranthaman, M.P. U.S. lithium resources from geothermal and extraction feasibility. Resour. Conserv. Recycl. 2021, 169, 105514. [Google Scholar] [CrossRef]
- Sun, X.; Liu, G.; Hao, H.; Liu, Z.; Zhao, F. Modeling potential impact of COVID-19 pandemic on global electric vehicle supply chain. iScience 2022, 25, 103903. [Google Scholar] [CrossRef]
- Borah, R.; Hughson, F.; Johnston, J.; Nann, T. On battery materials and methods. Mater. Today Adv. 2020, 6, 100046. [Google Scholar] [CrossRef]
- Bai, X.; Sun, Y.; Li, X.; He, R.; Liu, Z.; Pan, J.; Zhang, J. A Deep Dive into Spent Lithium-Ion Batteries: From Degradation Diagnostics to Sustainable Material Recovery. Electrochem. Energy Rev. 2024, 7, 1–48. [Google Scholar] [CrossRef]
- Kim, J.; Jeghan, S.M.N.; Lee, G. Superior fast-charging capability of graphite anode via facile surface treatment for lithium-ion batteries. Microporous Mesoporous Mater. 2020, 305, 110325. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.; Sun, J.; Jiang, Z.; Jia, P.S. Research progress on resource regeneration of cathode materials for spent ternary lithium batteries. J. Eng. Sci. 2023, 45, 1470–1481. (In Chinese) [Google Scholar] [CrossRef]
- Leal, V.; Ribeiro, J.; Coelho, E.; Freitas, M. Recycling of spent lithium-ion batteries as a sustainable solution to obtain raw materials for different applications. J. Energy Chem. 2022, 79, 118–134. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Dong, L.; Yang, Q.; Tian, S.; Ren, Z.; Zhou, Z. Study on selective recovery of lithium ions from lithium iron phosphate powder by electrochemical method. Sep. Purif. Technol. 2023, 310, 123133. [Google Scholar] [CrossRef]
- He, R.; He, Y.; Xie, W.; Guo, B.; Yang, S. Multi-scale analysis for accelerated degradation mechanisms of cylindrical LiFePO4/graphite batteries under distinct positions of jelly roll. Electrochim. Acta 2023, 444, 142048. [Google Scholar] [CrossRef]
- El Moutchou, S.; Aziam, H.; Mansori, M.; Saadoune, I. Thermal stability of Lithium-ion batteries: Case study of NMC811 and LFP cathode materials. Mater. Today: Proc. 2022, 51, A1–A7. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Yim, C.-H.; Karkar, Z.; Abu-Lebdeh, Y. On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review. Batteries 2022, 8, 70. [Google Scholar] [CrossRef]
- Adenusi, H.; Chass, G.A.; Passerini, S.; Tian, K.V.; Chen, G. Lithium Batteries and the Solid Electrolyte Interphase (SEI)—Progress and Outlook. Adv. Energy Mater. 2023, 13, 2203307. [Google Scholar] [CrossRef]
- Wang, X.; Gaustad, G.; Babbitt, C.W.; Richa, K. Economies of scale for future lithium-ion battery recycling infrastructure. Resour. Conserv. Recycl. 2014, 83, 53–62. [Google Scholar] [CrossRef]
- Biswal, B.K.; Zhang, B.; Tran, P.T.M.; Zhang, J.; Balasubramanian, R. Recycling of spent lithium-ion batteries for a sustainable future: Recent advancements. Chem. Soc. Rev. 2024, 53, 5552–5592. [Google Scholar] [CrossRef]
- Wang, P.; Gu, X.; Xue, M.; Li, Y.; Dong, S.; Chen, G.; Zhang, J. Resource utilization of medical waste under COVID-19: Waste mask used as crude oil fluidity improver. J. Clean. Prod. 2022, 358, 131903. [Google Scholar] [CrossRef] [PubMed]
- Jose, S.A.; Stoll, J.L.; Smith, T.; Jackson, C.; Dieleman, T.; Leath, E.; Eastwood, N.; Menezes, P.L. Critical Review of Lithium Recovery Methods: Advancements, Challenges, and Future Directions. Processes 2024, 12, 2203. [Google Scholar] [CrossRef]
- Batrec Industrie AG. Recycling Services. Available online: https://batrec.ch/recycling-services/ (accessed on 7 June 2021).
- Duesenfeld. Available online: https://www.duesenfeld.com/index.html (accessed on 7 June 2021).
- GEM. Available online: http://en.gem.com.cn (accessed on 7 June 2021).
- Glencore. Glencore Recycling. Available online: https://www.glencore.com/what-we-do/recycling (accessed on 7 June 2021).
- Inmetco. Available online: https://metalzenith.com/ja/blogs/steel-production-processing-terms/inmetco-key-process-in-steel-recycling-and-primary-production (accessed on 7 June 2021).
- OnTo Technology. Available online: https://www.onto-technology.com/ (accessed on 7 June 2021).
- Recupyl. Available online: http://www.recupyl.com/ (accessed on 7 June 2021).
- Retriev Technologies. Available online: https://www.retrievtech.com/?_ga=2.188769579.184110765.1755660982-401937714.1755660981 (accessed on 7 June 2021).
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Umicore. The Battery Recycling of Umicore. Available online: https://csm.umicore.com/en/battery-recycling/our-recycling-process#tabs (accessed on 7 June 2021).
- Lee, H.; Kim, Y.-T.; Lee, S.-W. Optimization of the Electrochemical Discharge of Spent Li-Ion Batteries from Electric Vehicles for Direct Recycling. Energies 2023, 16, 2759. [Google Scholar] [CrossRef]
- Lu, Y.; Maftouni, M.; Yang, T.; Zheng, P.; Young, D.; Kong, Z.J.; Li, Z. A novel disassembly process of end-of-life lithium-ion batteries enhanced by online sensing and machine learning techniques. J. Intell. Manuf. 2022, 34, 2463–2475. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, J.; Ou, L. A comprehensive review on the separation and purification of valuable metals from the leachate of spent lithium-ion batteries. Sep. Purif. Technol. 2024, 360, 130847. [Google Scholar] [CrossRef]
- Li, L.; Zheng, P.; Yang, T.; Sturges, R.; Ellis, M.W.; Li, Z. Disassembly Automation for Recycling End-of-Life Lithium-Ion Pouch Cells. JOM 2019, 71, 4457–4464. [Google Scholar] [CrossRef]
- Recycle spent batteries. Nat. Energy 2019, 4, 253. [CrossRef]
- Gupta, D.; Zhang, Y.; Nie, Z.; Wang, J.; Jr, G.M.K. Chemical redox of lithium-ion solid electroactive material in a packed bed flow reactor. Chem. Eng. Sci. 2022, 251, 117443. [Google Scholar] [CrossRef]
- Kuwabara, A.; Enomoto, M.; Hosono, E.; Hamaguchi, K.; Onuma, T.; Kajiyama, S.; Kato, T. Nanostructured liquid-crystalline Li-ion conductors with high oxidation resistance: Molecular design strategy towards safe and high-voltage-operation Li-ion batteries. Chem. Sci. 2020, 11, 10631–10637. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Rahn, C.D.; Archer, L.A.; You, F. Second life and recycling: Energy and environmental sustainability perspectives for high-performance lithium-ion batteries. Sci. Adv. 2021, 7, eabi7633. [Google Scholar] [CrossRef]
- Xi, J.; Li, Z.; Yu, L.; Yin, B.; Wang, L.; Liu, L.; Qiu, X.; Chen, L. Effect of degree of sulfonation and casting solvent on sulfonated poly(ether ether ketone) membrane for vanadium redox flow battery. J. Power Sources 2015, 285, 195–204. [Google Scholar] [CrossRef]
- Assefi, M.; Maroufi, S.; Yamauchi, Y.; Sahajwalla, V. Pyrometallurgical recycling of Li-ion, Ni–Cd and Ni–MH batteries: A minireview. Curr. Opin. Green Sustain. Chem. 2020, 24, 26–31. [Google Scholar] [CrossRef]
- Mao, J.; Li, J.; Xu, Z. Coupling reactions and collapsing model in the roasting process of recycling metals from LiCoO2 batteries. J. Clean. Prod. 2018, 205, 923–929. [Google Scholar] [CrossRef]
- Liu, P.; Xiao, L.; Tang, Y.; Chen, Y.; Ye, L.; Zhu, Y. Study on the reduction roasting of spent LiNixCoyMnzO2 lithium-ion battery cathode materials. J. Therm. Anal. Calorim. 2018, 136, 1323–1332. [Google Scholar] [CrossRef]
- Shi, J.; Peng, C.; Chen, M.; Li, Y.; Eric, H.; Klemettinen, L.; Lundström, M.; Taskinen, P.; Jokilaakso, A. Sulfation Roasting Mechanism for Spent Lithium-Ion Battery Metal Oxides Under SO2-O2-Ar Atmosphere. JOM 2019, 71, 4473–4482. [Google Scholar] [CrossRef]
- Chang, D.; Chen, Y.M.; Xi, Y.; Chang, C.; Jie, Y.F.; Hu, F. Selective Recovery of Lithium from Ternary Spent Lithium-Ion Batteries Using Sulfate Roasting-Water Leaching Process. In Energy Technology 2020: Recycling, Carbon Dioxide Management, and Other Technologies; Springer International Publishing: Cham, Switzerland, 2020. [Google Scholar]
- Peng, C.; Liu, F.; Wang, Z.; Wilson, B.P.; Lundström, M. Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. J. Power Sources 2019, 415, 179–188. [Google Scholar] [CrossRef]
- Tang, Y.; Xie, H.; Zhang, B.; Chen, X.; Zhao, Z.; Qu, J.; Xing, P.; Yin, H. Recovery and regeneration of LiCoO2-based spent lithium-ion batteries by a carbothermic reduction vacuum pyrolysis approach: Controlling the recovery of CoO or Co. Waste Manag. 2019, 97, 140–148. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Lin, J.; Wu, J.; Yang, J.; Wu, F.; Chen, R. Low-Temperature Molten-Salt-Assisted Recovery of Valuable Metals from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 16144–16150. [Google Scholar] [CrossRef]
- Fu, Y.; He, Y.; Yang, Y.; Qu, L.; Li, J.; Zhou, R. Microwave reduction enhanced leaching of valuable metals from spent lithium-ion batteries. J. Alloy. Compd. 2020, 832, 154920. [Google Scholar] [CrossRef]
- Ren, G.-X.; Xiao, S.-W.; Xie, M.-Q.; Pan, B.; Chen, J.; Wang, F.-G.; Xia, X. Recovery of valuable metals from spent lithium ion batteries by smelting reduction process based on FeO–SiO2–Al2O3 slag system. Trans. Nonferrous Met. Soc. China 2017, 27, 450–456. [Google Scholar] [CrossRef]
- Diaz, F.; Wang, Y.; Moorthy, T.; Friedrich, B. Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis. Metals 2018, 8, 565. [Google Scholar] [CrossRef]
- Iturrondobeitia, M.; Vallejo, C.; Berroci, M.; Akizu-Gardoki, O.; Minguez, R.; Lizundia, E. Environmental Impact Assessment of LiNi1/3Mn1/3Co1/3O2 Hydrometallurgical Cathode Recycling from Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2022, 10, 9798–9810. [Google Scholar] [CrossRef]
- Xiao, S.; Ren, G.; Xie, M.; Pan, B.; Fan, Y.; Wang, F.; Xia, X. Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Smelting Reduction Process Based on MnO–SiO2–Al2O3 Slag System. J. Sustain. Met. 2017, 3, 703–710. [Google Scholar] [CrossRef]
- Lombardo, G.; Ebin, B.; St. Foreman, M.R.J.; Steenari, B.-M.; Petranikova, M. Chemical Transformations in Li-Ion Battery Electrode Materials by Carbothermic Reduction. ACS Sustain. Chem. Eng. 2019, 7, 13668–13679. [Google Scholar] [CrossRef]
- Alam Bhuyan, S.; Shin, H. Green Recovery of Cathode Active Materials from Li-Ion Battery Electrode Scraps Using Propylene Carbonate: A Novel Approach for Direct Recycling. ACS Sustain. Chem. Eng. 2023, 11, 10677–10687. [Google Scholar] [CrossRef]
- Chen, M.; Ma, X.; Chen, B.; Arsenault, R.; Karlson, P.; Simon, N.; Wang, Y. Recycling End-of-Life Electric Vehicle Lithium-Ion Batteries. Joule 2019, 3, 2622–2646. [Google Scholar] [CrossRef]
- Chagnes, A.; Pospiech, B. A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. J. Chem. Technol. Biotechnol. 2013, 88, 1191–1199. [Google Scholar] [CrossRef]
- Sattar, R.; Ilyas, S.; Bhatti, H.N.; Ghaffar, A. Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 2019, 209, 725–733. [Google Scholar] [CrossRef]
- Premathilake, D.S.; Ambaye, T.G.; Junior, A.B.B.; Lima, A.T.M.; Espinosa, D.C.R.; Vaccari, M. Comparative environmental and economic assessment of emerging hydrometallurgical recycling technologies for Li-ion battery cathodes. Sustain. Prod. Consum. 2024, 51, 327–344. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhou, T.; Liu, D.; Hu, H.; Fan, S. Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries. Waste Manag. 2015, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Niinae, M.; Komatsu, N.; Nakahiro, Y.; Wakamatsu, T.; Shibata, J. Preferential leaching of cobalt, nickel and copper from cobalt-rich ferromanganese crusts with ammoniacal solutions using ammonium thiosulfate and ammonium sulfite as reducing agents. Hydrometallurgy 1996, 40, 111–121. [Google Scholar] [CrossRef]
- Barik, S.; Prabaharan, G.; Kumar, L. Leaching and separation of Co and Mn from electrode materials of spent lithium-ion batteries using hydrochloric acid: Laboratory and pilot scale study. J. Clean. Prod. 2017, 147, 37–43. [Google Scholar] [CrossRef]
- Liu, Z.; Sederholm, J.G.; Lan, K.-W.; Hatzell, M.C.; Perry, N.H.; Miljkovic, N.; Braun, P.V.; Li, Y.; Wang, P. A Comprehensive Comparison for Battery Cathode Leaching Processes. In Proceedings of the 2023 IEEE Transportation Electrification Conference & Expo (ITEC), Detroit, MI, USA, 21–23 June 2023; pp. 1–5. [Google Scholar]
- Li, S.; Zhang, W.; Xia, Y.; Li, Q. Enhanced reducing capacity of citric acid for lithium-ion battery recycling under microwave-assisted leaching. Waste Manag. 2024, 189, 23–33. [Google Scholar] [CrossRef]
- Lei, Q.; Zhou, K.; Zhang, X.; Qiu, Z.; Peng, C.; He, D.; Chen, W. Recycling of spent LiNixCoyMn1−x-yO2 batteries by a glucose reduction-acid leaching approach: Performance and mechanism. Process. Saf. Environ. Prot. 2023, 180, 1094–1103. [Google Scholar] [CrossRef]
- Guo, Y.; Li, F.; Zhu, H.; Li, G.; Huang, J.; He, W. Leaching lithium from the anode electrode materials of spent lithium-ion batteries by hydrochloric acid (HCl). Waste Manag. 2016, 51, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, H.; Luo, C.; Zhou, T. Recovery of valuable metals from waste cathode materials of spent lithium-ion batteries using mild phosphoric acid. J. Hazard. Mater. 2017, 326, 77–86. [Google Scholar] [CrossRef]
- Zhuang, L.; Sun, C.; Zhou, T.; Li, H.; Dai, A. Recovery of valuable metals from LiNi0.5Co0.2Mn0.3O2 cathode materials of spent Li-ion batteries using mild mixed acid as leachant. Waste Manag. 2019, 85, 175–185. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of mild organic acid reagents to recover the Co and Li from spent Li-ion batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, Y.; Meng, Q.; Dong, P.; Fei, Z.; Li, Q. Recycling of LiCoO2 cathode material from spent lithium ion batteries by ultrasonic enhanced leaching and one-step regeneration. J. Environ. Manag. 2021, 277, 111426. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Y.; Dong, P. A combined process for cobalt recovering and cathode material regeneration from spent LiCoO2 batteries: Process optimization and kinetics aspects. Waste Manag. 2018, 71, 372–380. [Google Scholar] [CrossRef]
- Punt, T.; Akdogan, G.; Bradshaw, S.; van Wyk, P. Development of a novel solvent extraction process using citric acid for lithium-ion battery recycling. Miner. Eng. 2021, 173, 107204. [Google Scholar] [CrossRef]
- Cerrillo-Gonzalez, M.d.M.; Paz-Garcia, J.M.; Muñoz-Espinosa, M.; Rodriguez-Maroto, J.M.; Villen-Guzman, M. Extraction and selective precipitation of metal ions from LiCoO2 cathodes using citric acid. J. Power Sources 2023, 592, 233870. [Google Scholar] [CrossRef]
- Wu, J.; Ahn, J.; Lee, J. A Sustainable Complexation Leaching of Critical Metals from Spent Lithium-Ion Batteries by Glycine in a Neutral Solution. Mining, Met. Explor. 2024, 41, 1605–1617. [Google Scholar] [CrossRef]
- Chen, M.; Wang, R.; Qi, Y.; Han, Y.; Wang, R.; Fu, J.; Meng, F.; Yi, X.; Huang, J.; Shu, J. Cobalt and lithium leaching from waste lithium ion batteries by glycine. J. Power Sources 2021, 482, 228942. [Google Scholar] [CrossRef]
- Munir, H.; Srivastava, R.R.; Kim, H.; Ilyas, S.; Khosa, M.K.; Yameen, B. Leaching of exhausted LNCM cathode batteries in ascorbic acid lixiviant: A green recycling approach, reaction kinetics and process mechanism. J. Chem. Technol. Biotechnol. 2020, 95, 2286–2294. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel approach to recover cobalt and lithium from spent lithium-ion battery using oxalic acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef]
- Liang, J.; Chen, R.; Gu, J.-N.; Li, J.; Xue, Y.; Shi, F.; Huang, B.; Guo, M.; Jia, J.; Li, K.; et al. Sustainable recycling of spent ternary lithium-ion batteries via an environmentally friendly process: Selective recovery of lithium and non-hazardous upcycling of residue. Chem. Eng. J. 2024, 481, 148516. [Google Scholar] [CrossRef]
- Lan, Y.; Li, X.; Zhou, G.; Yao, W.; Cheng, H.; Tang, Y. Direct Regenerating Cathode Materials from Spent Lithium-Ion Batteries. Adv. Sci. 2023, 11, e2304425. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Zeng, W.; Chen, X.; Zhang, Y.; Zhang, S.; Li, Z.; Li, C.; Mu, S. Degradation mechanism, direct regeneration and upcycling of ternary cathode material for retired lithium-ion power batteries. J. Energy Chem. 2024, 102, 534–554. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, K.; Yuan, C. A Sustainable Direct Recycling Method for LMO/NMC Cathode Mixture from Retired Lithium-Ion Batteries in EV. Energy Environ. Mater. 2025, 8, 12863. [Google Scholar] [CrossRef]
- Yang, T.; Lu, Y.; Li, L.; Ge, D.; Yang, H.; Leng, W.; Zhou, H.; Han, X.; Schmidt, N.; Ellis, M.; et al. An Effective Relithiation Process for Recycling Lithium-Ion Battery Cathode Materials. Adv. Sustain. Syst. 2019, 4, 88. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, X.; Yu, W.; Shang, Z.; Xu, S. Towards Greener Recycling: Direct Repair of Cathode Materials in Spent Lithium-Ion Batteries. Electrochem. Energy Rev. 2024, 7, 1–30. [Google Scholar] [CrossRef]
- Gao, H.; Tran, D.; Chen, Z. Seeking direct cathode regeneration for more efficient lithium-ion battery recycling. Curr. Opin. Electrochem. 2022, 31, 100875. [Google Scholar] [CrossRef]
- Yang, T.; Luo, D.; Yu, A.; Chen, Z. Enabling Future Closed-Loop Recycling of Spent Lithium-Ion Batteries: Direct Cathode Regeneration. Adv. Mater. 2023, 35, 2203218. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Zhao, J.; Shao, Y.; Li, X.; Li, Z.; Zhang, Y.; Liu, S.; Lin, Z.; Bettels, F.; et al. Recycling and direct regeneration of valuable cathode materials from spent Li-ion batteries: A comprehensive review. J. Mater. Chem. A 2025, 13, 8968–9004. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, W.; Zhang, X.; He, M.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Spent lithium-ion battery recycling—Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Manag. 2017, 60, 680–688. [Google Scholar] [CrossRef]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.-M.; Sohn, J.; Kwon, K. Recycling of spent lithium-ion battery cathode materials by ammoniacal leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, Y.; Zhang, T.; Zhang, L.; Xu, S. Alkali Metal Salt Catalyzed Carbothermic Reduction for Sustainable Recovery of LiCoO2: Accurately Controlled Reduction and Efficient Water Leaching. ACS Sustain. Chem. Eng. 2019, 7, 16729–16737. [Google Scholar] [CrossRef]
- Wang, H.; Burke, S.; Yuan, R.; Whitacre, J.F. Effective direct recycling of inhomogeneously aged Li-ion battery cathode active materials. J. Energy Storage 2023, 60, 106616. [Google Scholar] [CrossRef]
- Ji, Y.; Jafvert, C.T.; Zhao, F. Recovery of cathode materials from spent lithium-ion batteries using eutectic system of lithium compounds. Resour. Conserv. Recycl. 2021, 170, 105551. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, X.; Xu, S. A Simplified Process for Recovery of Li and Co from Spent LiCoO2 Cathode Using Al Foil As the in Situ Reductant. ACS Sustain. Chem. Eng. 2019, 7, 12222–12230. [Google Scholar] [CrossRef]
- Jing, Q.; Zhang, J.; Liu, Y.; Zhang, W.; Chen, Y.; Wang, C. Direct Regeneration of Spent LiFePO4 Cathode Material by a Green and Efficient One-Step Hydrothermal Method. ACS Sustain. Chem. Eng. 2020, 8, 17622–17628. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, K.; Gong, R.; Meng, Q.; Zhang, Y.; Dong, P. Direct regeneration of spent LiFePO4 materials via a green and economical one-step hydrothermal process. J. Environ. Manag. 2023, 348, 119384. [Google Scholar] [CrossRef]
- Chen, B.; Liu, M.; Cao, S.; Hu, H.; Chen, G.; Guo, X.; Wang, X. Direct regeneration and performance of spent LiFePO4 via a green efficient hydrothermal technique. J. Alloy. Compd. 2022, 924, 166487. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Zhang, Y.; Dong, P.; Meng, Q.; Xu, M. Novel efficient regeneration of high-performance Li1.2[Mn0.56Ni0.16Co0.08]O2 cathode materials from spent LiMn2O4 batteries. J. Alloy. Compd. 2019, 783, 357–362. [Google Scholar] [CrossRef]
- Chen, X.; Yang, C.; Yang, Y.; Ji, H.; Yang, G. Co-precipitation preparation of Ni-Co-Mn ternary cathode materials by using the sources extracting directly from spent lithium-ion batteries. J. Alloy. Compd. 2022, 909, 164691. [Google Scholar] [CrossRef]
- Li, L.; Bian, Y.; Zhang, X.; Xue, Q.; Fan, E.; Wu, F.; Chen, R. Economical recycling process for spent lithium-ion batteries and macro- and micro-scale mechanistic study. J. Power Sources 2018, 377, 70–79. [Google Scholar] [CrossRef]
- Li, L.; Fan, E.; Guan, Y.; Zhang, X.; Xue, Q.; Wei, L.; Wu, F.; Chen, R. Sustainable Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using Lactic Acid Leaching System. ACS Sustain. Chem. Eng. 2017, 5, 5224–5233. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, J.; Li, H.; Chen, Y.; Wang, C. A promising approach for the recovery of high value-added metals from spent lithium-ion batteries. J. Power Sources 2017, 351, 192–199. [Google Scholar] [CrossRef]
- Yoo, E.; Lee, U.; Kelly, J.C.; Wang, M. Life-cycle analysis of battery metal recycling with lithium recovery from a spent lithium-ion battery. Resour. Conserv. Recycl. 2023, 196, 107040. [Google Scholar] [CrossRef]
- Muthu, S.S. Assessing the Environmental Impact of Textiles and the Clothing Supply Chain; Woodhead Publishing: Cambridge, UK, 2014; pp. 105–131. [Google Scholar]
- Hao, H.; Mu, Z.; Jiang, S.; Liu, Z.; Zhao, F. GHG Emissions from the Production of Lithium-Ion Batteries for Electric Vehicles in China. Sustainability 2017, 9, 504. [Google Scholar] [CrossRef]
- Yin, R.; Hu, S.; Yang, Y. Life cycle inventories of the commonly used materials for lithium-ion batteries in China. J. Clean. Prod. 2019, 227, 960–971. [Google Scholar] [CrossRef]
- Wang, L.; Wu, H.; Hu, Y.; Yu, Y.; Huang, K. Environmental Sustainability Assessment of Typical Cathode Materials of Lithium-Ion Battery Based on Three LCA Approaches. Processes 2019, 7, 83. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.; Wang, W.; Chen, M.; Zheng, W.; Yang, W.; Zou, H.; Chen, S. LCA for lithium battery recycling technology-recent progress. Ionics 2024, 30, 4417–4428. [Google Scholar] [CrossRef]
- Perocillo, Y.K.; Pirard, E.; Léonard, A. Process simulation-based LCA: Li-ion battery recycling case study. Int. J. Life Cycle Assess. 2025, 1–25. [Google Scholar] [CrossRef]
- Šimaitis, J.; Allen, S.; Vagg, C. Are future recycling benefits misleading? Prospective life cycle assessment of lithium-ion batteries. J. Ind. Ecol. 2023, 27, 1291–1303. [Google Scholar] [CrossRef]
- Gutsch, M.; Leker, J. Costs, carbon footprint, and environmental impacts of lithium-ion batteries—From cathode active material synthesis to cell manufacturing and recycling. Appl. Energy 2023, 353, 122132. [Google Scholar] [CrossRef]
- Castro, F.D.; Mehner, E.; Cutaia, L.; Vaccari, M. Life cycle assessment of an innovative lithium-ion battery recycling route: A feasibility study. J. Clean. Prod. 2022, 368, 133130. [Google Scholar] [CrossRef]
- Raugei, M.; Winfield, P. Prospective LCA of the production and EoL recycling of a novel type of Li-ion battery for electric vehicles. J. Clean. Prod. 2019, 213, 926–932. [Google Scholar] [CrossRef]
- Sun, X.; Luo, X.; Zhang, Z.; Meng, F.; Yang, J. Life cycle assessment of lithium nickel cobalt manganese oxide (NCM) batteries for electric passenger vehicles. J. Clean. Prod. 2020, 273, 123006. [Google Scholar] [CrossRef]
- Gonzales-Calienes, G.; Kannangara, M.; Bensebaa, F. Economic and Environmental Viability of Lithium-Ion Battery Recycling—Case Study in Two Canadian Regions with Different Energy Mixes. Batteries 2023, 9, 375. [Google Scholar] [CrossRef]
- Thompson, D.; Hyde, C.; Hartley, J.M.; Abbott, A.P.; Anderson, P.A.; Harper, G.D. To shred or not to shred: A comparative techno-economic assessment of lithium ion battery hydrometallurgical recycling retaining value and improving circularity in LIB supply chains. Resour. Conserv. Recycl. 2021, 175, 105741. [Google Scholar] [CrossRef]
- Kar, U.; Fahimi, A.; Suponik, T.; Kaya, M.; Chu, P. Comparison of different pretreatment processes for lithium-ion battery recycling from a life cycle assessment perspective. J. Environ. Manag. 2025, 392, 126798. [Google Scholar] [CrossRef]
- Zhang, X.; He, Y.; Wang, Y.; Yan, W.; Subramanian, N. Assessing the GHG Emissions and Savings during the Recycling of NMC Lithium-Ion Batteries Used in Electric Vehicles in China. Processes 2022, 10, 342. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hsieh, I.-Y.L. Techno-economic analysis of lithium-ion battery price reduction considering carbon footprint based on life cycle assessment. J. Clean. Prod. 2023, 425, 139045. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, X.; Ma, M.; Liu, Y.; Zhang, J.; Wu, X. Progress and prospect on the recycling of spent lithium-ion batteries: Ending is beginning. Carbon Neutralization 2022, 1, 247–266. [Google Scholar] [CrossRef]
- Yu, M.; Bai, B.; Xiong, S.; Liao, X. Evaluating environmental impacts and economic performance of remanufacturing electric vehicle lithium-ion batteries. J. Clean. Prod. 2021, 321, 128935. [Google Scholar] [CrossRef]
- Xiong, S.; Ji, J.; Ma, X. Environmental and economic evaluation of remanufacturing lithium-ion batteries from electric vehicles. Waste Manag. 2020, 102, 579–586. [Google Scholar] [CrossRef]
- Zhou, Z.; Lai, Y.; Peng, Q.; Li, J. Comparative Life Cycle Assessment of Merging Recycling Methods for Spent Lithium Ion Batteries. Energies 2021, 14, 6263. [Google Scholar] [CrossRef]
- Quan, J.; Zhao, S.; Song, D.; Wang, T.; He, W.; Li, G. Comparative life cycle assessment of LFP and NCM batteries including the secondary use and different recycling technologies. Sci. Total. Environ. 2022, 819, 153105. [Google Scholar] [CrossRef]
- Yu, W.; Guo, Y.; Xu, S.; Yang, Y.; Zhao, Y.; Zhang, J. Comprehensive recycling of lithium-ion batteries: Fundamentals, pretreatment, and perspectives. Energy Storage Mater. 2022, 54, 172–220. [Google Scholar] [CrossRef]
- Woeste, R.; Drude, E.-S.; Vrucak, D.; Klöckner, K.; Rombach, E.; Letmathe, P.; Friedrich, B. A techno-economic assessment of two recycling processes for black mass from end-of-life lithium-ion batteries. Appl. Energy 2024, 361, 122921. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Lim, J.; Moon, I.; Kim, J. Sequential flue gas utilization for sustainable leaching and metal precipitation of spent lithium-ion battery cathode material: Process design and techno-economic analysis. J. Clean. Prod. 2022, 380, 134988. [Google Scholar] [CrossRef]
- Lee, J.; Choi, H.; Kim, J. Environmental and economic impacts of e-waste recycling: A systematic review. Chem. Eng. J. 2024, 494, 152917. [Google Scholar] [CrossRef]
- Dunn, J.; Kendall, A.; Slattery, M. Electric vehicle lithium-ion battery recycled content standards for the US—targets, costs, and environmental impacts. Resour. Conserv. Recycl. 2022, 185, 106488. [Google Scholar] [CrossRef]
- Zhang, J.; Ilyas, S. Economic benefit analysis of lithium battery recycling based on machine learning algorithm. PLoS ONE 2024, 19, e0303933. [Google Scholar] [CrossRef]
- Wasesa, M.; Hidayat, T.; Andariesta, D.T.; Natha, M.G.; Attazahri, A.K.; Afrianto, M.A.; Mubarok, M.Z.; Zulhan, Z.; Putro, U.S. Economic and environmental assessments of an integrated lithium-ion battery waste recycling supply chain: A hybrid simulation approach. J. Clean. Prod. 2022, 379, 134625. [Google Scholar] [CrossRef]
- Dai, Q.; Spangenberger, J.; Ahmed, S.; Gaines, L.; Kelly, J.C.; Wang, M. EverBatt: A Closed-loop Battery Recycling Cost and Environmental Impacts Model; Argonne National Laboratory (ANL): Argonne, IL, USA, 2019. [Google Scholar]
- Bruno, M.; Francia, C.; Fiore, S. Selective Leaching for the Recycling of Lithium, Iron, and Phosphorous from Lithium-Ion Battery Cathodes’ Production Scraps. Batteries 2024, 10, 415. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef]
- Yang, Y.; Okonkwo, E.G.; Huang, G.; Xu, S.; Sun, W.; He, Y. On the sustainability of lithium ion battery industry—A review and perspective. Energy Storage Mater. 2021, 36, 186–212. [Google Scholar] [CrossRef]
- Latini, D.; Vaccari, M.; Lagnoni, M.; Orefice, M.; Mathieux, F.; Huisman, J.; Tognotti, L.; Bertei, A. A comprehensive review and classification of unit operations with assessment of outputs quality in lithium-ion battery recycling. J. Power Sources 2022, 546, 231979. [Google Scholar] [CrossRef]
- Bhuyan, A.; Tripathy, A.; Padhy, R.; Gautam, A. Evaluating the lithium-ion battery recycling industry in an emerging economy: A multi-stakeholder and multi-criteria decision-making approach. J. Clean. Prod. 2022, 331, 130007. [Google Scholar] [CrossRef]
- Sederholm, J.G.; Li, L.; Liu, Z.; Lan, K.-W.; Cho, E.J.; Gurumukhi, Y.; Dipto, M.J.; Ahmari, A.; Yu, J.; Haynes, M.; et al. Emerging Trends and Future Opportunities for Battery Recycling. ACS Energy Lett. 2024, 10, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Liu, W.; Kong, S.; Li, H.; Deng, Y.; Ma, H. End-of-Use Management of Spent Lithium-Ion Batteries From Sustainability Perspective: A Review. J. Manuf. Sci. Eng. 2021, 143, 100801. [Google Scholar] [CrossRef]
- Dalini, E.A.; Karimi, G.; Zandevakili, S.; Goodarzi, M. A Review on Environmental, Economic and Hydrometallurgical Processes of Recycling Spent Lithium-ion Batteries. Miner. Process. Extr. Met. Rev. 2020, 42, 451–472. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M. Policies and perspective on end-of-life vehicles in China. J. Clean. Prod. 2013, 44, 168–176. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, L. Screening Contract Excitation Models Involving Closed-Loop Supply Chains Under Asymmetric Information Games: A Case Study with New Energy Vehicle Power Battery. Appl. Sci. 2019, 9, 146. [Google Scholar] [CrossRef]
- Deng, Q.; Li, X.; Wang, Y.; Li, W. Analysis of End-of-Life Vehicle Recycling Based on Theory of Planned Behavior. Environ. Eng. Sci. 2017, 34, 627–637. [Google Scholar] [CrossRef]



| Recycling Product | Recovery Rate | Market Price (USD/kg) |
|---|---|---|
| Cu | - | 7.54 |
| Ni | 95–98% | 21.72 |
| Co | 95–98% | 46.3 |
| Mn | - | 0.01 |
| Li | 90–95% | 62.26 |
| Company | Location | Technology | Main Products | Ref. |
|---|---|---|---|---|
| Accurec | Germany | Pyrometallurgy and Hydrometallurgy | Co alloy, Li2CO3 | [18] |
| Batrec | Switzerland | Pyrometallurgy and Hydrometallurgy | Battery scraps | [19] |
| Duesenfeld | Germany | Mechanical treatment and Hydrometallurgy | Co, Li salt | [20] |
| GEM | China | Hydrometallurgy | Co, Ni powder, Ni/Co alloy, Co3O4 | [21] |
| Glencore (former Xstrata) | Switzerland | Pyrometallurgy and Hydrometallurgy | Alloy (Co/Ni/Cu) | [22] |
| Inmetco | USA | Pyrometallurgy and Hydrometallurgy | Co, Ni, and Fe alloy | [23] |
| OnTo Technology | USA | Mechanical treatment and Hydrometallurgy | Cathode powder | [24] |
| Recupyl | France | Hydrometallurgy | Co(OH)2 and Li2PO4, Li2CO3 | [25] |
| Retriev Technologies & Toxco (Canada) | USA and Canada | Hydrometallurgy | CoO, Li2CO3, mixed metal oxides | [26] |
| Sumitomo-Sony | Japan | Pyrometallurgy and Hydrometallurgy | CoO | [27] |
| Umicore | Belgium | Pyrometallurgy and Hydrometallurgy | Co salt, Ni salt, Cu salt, mixed metal oxides | [28] |
| Recycling Stage | Technology Type | Representative Methods | Main Products | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Pretreatment | Battery Discharge | NaCl solution immersion, Freezing | Discharged batteries | Safe deactivation | Limited to early-stage processing |
| Mechanical Processing | Physical Separation | Crushing, Screening, Manual dismantling | Al, Cu foil, shell, active material | Simple operation, high separation efficiency | High energy use, gas/solvent emissions |
| Cathode Recovery | Pyrometallurgy | Reduction smelting, Carbothermal, Roasting | Slag, metal ion filtrate | Simple, scalable, high metal tolerance | High temperature, lithium loss, gas emissions |
| Hydrometallurgy | Inorganic/organic acid, ammonia leaching | Metal ion filtrate | High efficiency, low energy, flexible conditions | Wastewater, toxic gas risks | |
| Metal Refining | Separation and Purification | Solvent extraction, Precipitation, Sol–gel | Purified metals or compounds | High purity, high recovery | Complex process, variable product stability |
| Process | Temp. | Additive | Pretreatment | Conditions | Separated Materials | Secondary Treatment | Recovery Rate | Ref. |
|---|---|---|---|---|---|---|---|---|
| Reduction Roasting | 650 °C | Carbon | Not specifically mentioned | 650 °C, 30 min | Li2CO3, Co, Ni, NiO, MnO | Water and acid leaching (H2SO4) | Li: 93.67%, Ni: 93.33%, Co: 98.08%, Mn: 98.68% | [41] |
| Sulfation Roasting | 700 °C | SO2(g) | Not specifically mentioned | 700 °C, 120 min | Li2SO4, Li2Co(SO4)2, CoO | Water leaching | Li: 99.5%, Co: 17.4% | [42] |
| Sulfation Roasting | 750 °C | Na2SO4 | NaCl immersion, Manual dismantling, Calcination | 750 °C, 90 min | Li2SO4, MnO, NiO, CoO, CuO2 | Water leaching | Li: 85.43%, Ni, Co, Mn all 84.93% | [43] |
| Nitration Roasting | 250 °C | HNO3 | Mechanical pretreatment | 250 °C, 60 min | LiNO3, Co(NO3)2, | Water leaching | Li: 93%, Co, Ni, Cu all 92.9% | [44] |
| Vacuum Pyrolysis | 600 °C | Carbon | NaCl discharging, Manual dismantling, Vacuum pyrolysis | 600 °C | Co, CoO, Li2CO3 | Water leaching | Li: 93%, Co: 99% | [45] |
| Chlorination Calcination | 350 °C | NH4Cl | Discharging, Manual dismantling, NaOH dissolution | 350 °C for 20 min | LiCl, CoCl2, | Water leaching | Li: 99.18%, Co: 99.3% | [46] |
| Microwave Carbothermic Reduction | 900 °C | Carbon | NaCl discharge, Manual dismantling, comminution | 900 °C, 500 W, 30 min | Not specifically mentioned | Acid leaching (HCl(aq)) | Li: 99.68%, Ni: 97.65%, Co: 97.85%, Mn: 96.73% | [47] |
| Carbothermic Reduction Smelting | 1450 °C | Cu slag (slag former) | Not specifically mentioned | 1450 °C for 30 min | Co, Ni, Cu and Fe alloy and slag | Manual separation of slag and alloy, comminution | Co: 98.83%, Ni: 98.39%, Cu: 93.57% | [48] |
| Reduction Smelting | 1475 °C | Pyrolusite slag former, SiO2, CaO | Roasting at 800 °C for 120 min to remove carbon | 1475 °C, 30 min | Co-Ni-Cu-Fe alloy | Manual separation, comminution, acid leaching | Li: 79.86%, Mn: 94.85% | [49] |
| Inorganic Leaching Agent | Reducing Agent | Temp. (°C) | Time | Efficiency (%) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Li | Co | Mn | |||||
| 60 | - | 98.9 | 98.4 | 98.6 | [61] | ||
| Citric Acid | 120 | 2 h | 99.5 | 99.5 | 99.7 | [62] | |
| Glucose | 90 | 2 h | 99.54 | 99.58 | 99.1 | [63] | |
| HCl | 80 | 1.5 h | 99.4 | - | - | [64] | |
| 40 | 1 h | 99 | 99 | - | [65] | ||
| Citric Acid (0.4 mol/) | 90 | 0.5 h | 100 | 91.63 | 92 | [66] | |
| Inorganic Leaching Agent | Reducing Agent | Temp. (°C) | Time | Efficiency (%) | Ref. | ||
|---|---|---|---|---|---|---|---|
| Li | Co | Mn | |||||
| DL-malic Acid (1.5 mol/L) | 80 | 25 min | 98.13 | 98.86 | - | [69] | |
| DL-malic Acid (1.25 mol/L) | Glucose | 80 | 3 h | 100 | 99.87 | - | [70] |
| Citric acid | 95 | 20 min | 96 | 90 | 94 | [71] | |
| Citric acid | Cu | 70 | 24 h | 97.8 | 81.3 | - | [72] |
| Glycine ) | 80 | 3 h | 99.8 | 100 | - | [73] | |
| Glycine ) | 80 | 7 h | 90.95 | 97.07 | - | [74] | |
| Ascorbic acid (1.25 mol/L) | - | 70 | 20 min | 98.5 | 94.8 | - | [67] |
| Ascorbic acid (2 mol/L) | - | 75 | 1.5 h | 96.3 | 94.8 | 95.6 | [75] |
| Oxalic acid (1 mol/L) | - | 95 | 2.5 h | 98 | 97 | - | [76] |
| Oxalic acid (0.5 mol/L) | - | 80 | 20 min | 95.7 | - | - | [77] |
| Method Type | Treated Material | Temperature | Lithium Source/Medium | Reaction Time | Post-Treatment | Regenerated Performance (Initial Capacity/Cycle Retention) | Ref. |
|---|---|---|---|---|---|---|---|
| High-temperature solid-state method | LFP | 600–800 °C | - | Sintering in reductive atmosphere | 147.3 mAh g−1/95.32% (after 100 cycles) | [78] | |
| Hydrothermal method | LCO | 220 °C | 4 M LiOH solution | 4 h | Annealing at 800 °C | 153.1 mAh g−1/91.2% (after 100 cycles) | [78] |
| Molten salt thermochemistry | NCM523 | 300 °C | LiNO3-LiOH eutectic salt | 4 h | Annealing at 850 °C | 149.3 mAh g−1/90.15% (after 100 cycles) | [78] |
| Low-temperature sintering method | LMO/NMC mixture cathode | 300 °C | 4 h | - | 144.0 mAh g−1/95.1% (after 250 cycles); 83 mAh g−1 at 2 C | [80] | |
| Deep eutectic solvent (DES) method | LCO | 120 °C | LiCl–urea DES | 4 h | Annealing at 850 °C | 133.1 mAh g−1/72.7% (after 100 cycles); DES recovery rate 98.7% | [78] |
| Electrochemical method | LCO | Room temperature | solution | - | Annealing at 700 °C for 6 h | 140 mAh g−1/93% (after 100 cycles) | [81] |
| Chemical lithiation method | LFP | Room temperature | Pyrene (reducing agent + lithium source) | 10 min | - | Repaired Li vacancies, restored Fe2+ valence state, improved cycle stability | [82] |
| Low-temperature hydrothermal method (Co-free) | LFP | 80 °C | LiOH solution + citric acid (reducing agent) | - | Ambient pressure operation | 159 mAh g−1/93.7% (after 100 cycles) | [83] |
| Method | Reductant and Lithium Source | Temp. | Time | Electrochemical Performance | Technical Readiness Level (TRL) | Capital Expenditure (CAPEX) | Operational Expenditure (OPEX) | Practical Implementation Barriers | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| One-step hydrothermal method | N2H4·H2O + Li2SO4·H2O | 200 °C | 3 h | 146.2 mAh/g (0.2 C), 128.2 mAh/g (5 C); 98.6% after 200 cycles | 3–4 (Lab-scale validation) | Lower: Relies on hydrothermal autoclaves, filtration, and drying units; no complex wastewater treatment systems required | Lower: Main costs from Li2SO4·H2O (lithium source), N2H4·H2O (reductant), and low energy consumption; total ~USD 1130/ton (22.2% of LFP price) | 1. Controlling the uniformity of temperature and pressure. 2. Residual impurities (carbon black and PVDF) | [92] |
| DL-malic acid + LiOH·H2O | 100 °C | 6 h | 138.4 → 136.6 mAh/g @1 C (200 cycles); CE > 97.2% | 3 (Lab-scale validation) | Lower: Similarly to above, using standard hydrothermal equipment; no toxic waste treatment required | Lower: DL-malic acid is low cost (~USD 2.1/kg, cheaper than N2H4·H2O); LiOH·H2O as Li source; energy consumption reduced due to lower temperature | [93] | ||
| Tartaric acid + LiOH·H2O | 200 °C | 3 h | 165.9 → 114.96 mAh/g (0.1 C–5 C); 99.1% after 200 cycles | 3 (Lab-scale validation) | Lower: Standard hydrothermal setup, no hazardous waste treatment | Lower: Uses green reductants, reducing chemical costs and environmental impact | [94] | ||
| Co-precipitation | H2O2/Na2CO3 + Li2CO3, LiOH·H2O | 25 °C | — | 239.4 mAh/g (0.1 C); 81.0% retention (100 cycles); 105.1 mAh/g @5 C | 3–4 (Lab-scale validation) | Moderate: Requires co-precipitation reactors, sintering furnaces, and purification systems; higher than hydrothermal methods but lower than hydrometallurgy | Moderate: Costs include leaching reagents (H2SO4, H2O2), Li2CO3, and energy for sintering; lower chemical consumption than hydrometallurgy | 1. Temperature affects crystallinity/loss 2. Diffusion causes segregation/mixing | [95,96] |
| Sol–Gel | H2O2 + CH3COOLi·2H2O | 70 °C (leaching), 900 °C (sintering) | 60 min (leaching), 12 h (sintering) | NCM-Ma: 151.6 mAh/g (0.2 C), 120.2 mAh/g (5 C); 84% retention after 150 cycles at 0.2 C | 3–4 (Lab-scale validation) | Moderate: Leaching tanks, sol–gel reactors, and sintering equipment; similar to co-precipitation | Lower than hydrometallurgy: Maleic acid (~USD 2.1/kg) and H2O2 costs are lower than inorganic acids; energy dominated by sintering | Gel uniformity sensitive to conditions | [97] |
| Acetic/maleic acid + acetate salts | 70 °C (leaching), 900 °C (sintering) | 20 min (leaching), 12 h (sintering) | R-NCM: 138.2 mAh/g (0.5 C) after 100 cycles, 96% retention; 120.6 mAh/g (5 C) | 3–4 (Lab-scale validation) | Moderate: Similarly to maleic acid process; leaching and sol–gel equipment | Lower: Lactic acid is biodegradable and low cost; H2O2 usage (0.5 vol%) minimizes reductant costs; short leaching | [98] |
| Tool | Type | Application Stage | Key Features | Ref. |
|---|---|---|---|---|
| Simapro v9.6 | Commercial LCA software | LCIA phase | Supports EF 3.0, ReCiPe, Eco-Indicator; widely used in EU and Asia | [106] |
| HSC Chemistry v10.5 | Process simulator | Foreground data modeling | Thermodynamic simulation for recovery routes | [106] |
| GaBi | Commercial LCA software | LCA modeling and impact assessment | Used for attributional LCA modeling, integrates with Ecoinvent database for background processes | [105] |
| Ecoinvent v3.8 | LCI database | Background data | Power grid mix, logistics, chemical reagents | [107] |
| Initial Feeding | Recycling Step | Metal Recovery | Energy Consumption | System Boundaries | Functional Units | Impact Categories | GHG Emissions | Ref. |
|---|---|---|---|---|---|---|---|---|
| SLIBs/NMC | Hydro/solvent extraction | Ni 92.4%, Co 92.3%, Mn 30.1%, Li 89.3% | – | Grave-to-gate: upstream (collection, transportation, dismantling), midstream (hydrometallurgical recycling), downstream (precursor CAM synthesis, calcination) | 1 kg of cathode active material (CAM) | Global Warming Potential (GWP, kg CO2e) | 6.46 kgCO2/kg CAM | [112] |
| SLIBs/NMC&NCA | Hydro | – | – | Cradle-to-gate: mineral acquisition, cell production, module/pack assembly, end-of-life treatment (excludes use phase and transportation) | 1 kgCO2eq per kWh of battery capacity | Global Warming Potential (GWP100, kgCO2eq) | 49.2 kgCO2/kWh | [116] |
| Direct Recycling | 65.9 kgCO2/kWh | |||||||
| Pyro | 70 kgCO2/kWh | |||||||
| SLIBs | Shredding-hydro/precipitation | Co 97%, Mn 98%, Li 80% | – | Shredding, leaching, precipitation (focus on wet processing stage) | 1 kg of input electrode material | Resource recovery efficiency, chemical usage impact, waste generation from shredding | – | [113] |
| SLIBs | Shredding-hydro/solvent extraction | Li 85%, Ni 97%, Mn 99%, Co 98% | Shredding, acid leaching, solvent extraction (includes solvent regeneration) | Solvent recyclability, metal purity, energy for extraction processes | ||||
| SLIBs | Disassembly hydro/calcination | NMC 95%, LMO 95%, Al 100% | Disassembly, delamination, calcination (excludes manual disassembly labor costs) | Material purity, thermal energy for calcination, reduction in cross-contamination | ||||
| SLIBs | Hydro | – | 160.7 MJ/kg battery | Cradle-to-gate for recycling: leaching, solvent extraction, precipitation (excludes upstream mining and battery production) | 1 kg of battery | Global Warming Potential (GWP, kgCO2/kg battery), energy consumption (MJ/kg battery) | 10.811 kgCO2/kg battery | [117] |
| SLIBs | Pyro | 152.5 MJ/kg battery | 11.342 kgCO2/kg battery | |||||
| SLIBs | Pyro | – | 0.0536 (kwh/t) + 35.68 (kwh/t) | Cradle-to-gate for recycling: collection, transportation, pretreatment (dismantling), cascade utilization (remanufacturing), recovery utilization (smelting) | 1 ton of spent LIBs | Global Warming Potential (GWP, kgCO2-eq/t), energy consumption (kWh/t, diesel L/t) | 713.3 kgCO2/t | [115] |
| SLIBs/NMC | Direct Recycling | – | – | Recycling (supercritical CO2 extraction, relithiation), cell remanufacturing | 1 kg of battery | Global Warming Potential (GWP), water consumption | 29.27 kgCO2/t | [118] |
| SLIBs/NCM111 | Hydro | Cu 70%, Al 70%, Co 90%, Ni 90%, Mn 90% | 149.80 MJ/kg battery | Recycling (discharge, disassembly, shredding, leaching), cathode remanufacturing, cell remanufacturing (excludes collection and transportation) | 1 kg of battery cell | Energy consumption (MJ/kg), Global Warming Potential (GWP, kgCO2eq/kg) | 10.53 kgCO2/kg | [119] |
| SLIBs/NMC&NCA | Ultra-High Temperature (UHT) | Li and Co; 284.2 kg of LCO per FU | 43 MJ/kg LCO | Collection and transportation, pretreatment (discharge, crushing), UHT smelting, hydrometallurgical purification, regeneration | 1 ton of spent EV LIBs | Energy consumption (MJ/t), Global Warming Potential (GWP, kgCO2-eq/t) | 1371 kgCO2-eq/t | [105,120] |
| Hydrometallurgy | Co, Li, etc.; | 19,637 MJ/FU | Energy consumption (MJ/t), GWP, acidification potential (AP) | 1393 kgCO2-eq/t |
| Battery Type | Technology | Main Inputs | Energy Input | Main Product | Atmospheric Emissions | Waste |
|---|---|---|---|---|---|---|
| LFP | Hydrometallurgy | EoL LFP, HCl, H2O2, NaOH, CaCl, Water | Electricity: 0.485 kWh | LiCl (solid) | Dust, HF, VOCs, HCl mist | Plastic, etc. |
| NCM Battery | Hydrometallurgy | EoL NCM, H2SO4, HCl, Na2CO3, NH3, H2O2, Li2CO3 | Gas: 0.28 m3 | NCM cathode material | Ni, CO2 | Wastewater, ammonia-N, etc. |
| Pyrometallurgy | EoL NCM, H2SO4, Na2CO3, NH3, Li2CO3 | Coal: 0.93 kg | NCM cathode material | Ni, CO2 | Wastewater, ammonia, etc. |
| Recovery Stage | Necessary Operations | Recycle Method | Cost (USD/ton) | Ref. |
|---|---|---|---|---|
| Collection and Transportation | Retrieval from EV stations, retail sites; transport to central hubs | - | 5–15 | [112] |
| Pretreatment | Discharge, disassembly, crushing, casing removal | - | 10–20 | [122] |
| Material Sorting and Separation | Extraction of valuable metals (Co, Ni, Li, Mn) | Pyrometallurgy | 200–300 | [123] |
| Hydrometallurgy | 150–250 | [124] | ||
| Metal Refining and Purification | Final refinement to achieve battery-grade purity | - | 30–80 | [125] |
| Model Name | Developer | Key Features | Application for Lithium Battery Recycling | Example of Application | Limitations |
|---|---|---|---|---|---|
| EVERBATT | Argonne National Laboratory, USA | Full life cycle modeling, cost estimation, environmental impact evaluation | Scenario comparison for LIB recycling | Lacks transparency in some parameters | Full life cycle modeling, cost estimation, environmental impact evaluation |
| GREET | Focuses on energy-related emissions, less on cost | GHG analysis of recycling vs. mining | Does not evaluate cost structures | Focuses on energy-related emissions, less on cost |
| Category | Element | Description |
|---|---|---|
| Direct Cost | CAPEX (Capital Expenditure) | Initial investments in equipment, facilities, transport fleet |
| OPEX (Operational Expenditure) | Labor, utilities (electricity, water), reagents (e.g., H2SO4, NaOH), maintenance | |
| Revenue | Material Recovery Value | Income from resale of Li2CO3, Co(OH)2, Ni(OH)2, Cu, Al |
| Environmental and Social | Environmental Benefits | Emission reduction, energy savings, landfill reduction |
| Social Benefits | Employment creation, regional economic stimulation | |
| Financial Indicators | NPV (Net Present Value) | Discounted future net cash flows of the project |
| CBR (Cost–Benefit Ratio) | Ratio of total benefits to total costs | |
| RMSE, AE, R2 (from ML models) | Used in data-driven prediction of economic returns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, D.; Wang, H.; Zeng, Y.; Han, X.; Zheng, Y. Sustainable Recycling of Lithium-Ion Battery Cathodes: Life Cycle Assessment, Technologies, and Economic Insights. Nanomaterials 2025, 15, 1283. https://doi.org/10.3390/nano15161283
Pang D, Wang H, Zeng Y, Han X, Zheng Y. Sustainable Recycling of Lithium-Ion Battery Cathodes: Life Cycle Assessment, Technologies, and Economic Insights. Nanomaterials. 2025; 15(16):1283. https://doi.org/10.3390/nano15161283
Chicago/Turabian StylePang, Dongjie, Haoyu Wang, Yimin Zeng, Xue Han, and Ying Zheng. 2025. "Sustainable Recycling of Lithium-Ion Battery Cathodes: Life Cycle Assessment, Technologies, and Economic Insights" Nanomaterials 15, no. 16: 1283. https://doi.org/10.3390/nano15161283
APA StylePang, D., Wang, H., Zeng, Y., Han, X., & Zheng, Y. (2025). Sustainable Recycling of Lithium-Ion Battery Cathodes: Life Cycle Assessment, Technologies, and Economic Insights. Nanomaterials, 15(16), 1283. https://doi.org/10.3390/nano15161283







