Biomass-Derived Carbon Dots: Preparation, Properties, and Applications
Abstract
1. Introduction
1.1. Carbon Dots


1.2. Biomass Derived Carbon Dots
2. Synthetic Methods
3. Raw Materials and Formation Mechanisms
3.1. Cellulose
3.1.1. Introduction of Cellulose
3.1.2. Cellulose-Based Carbon Dots
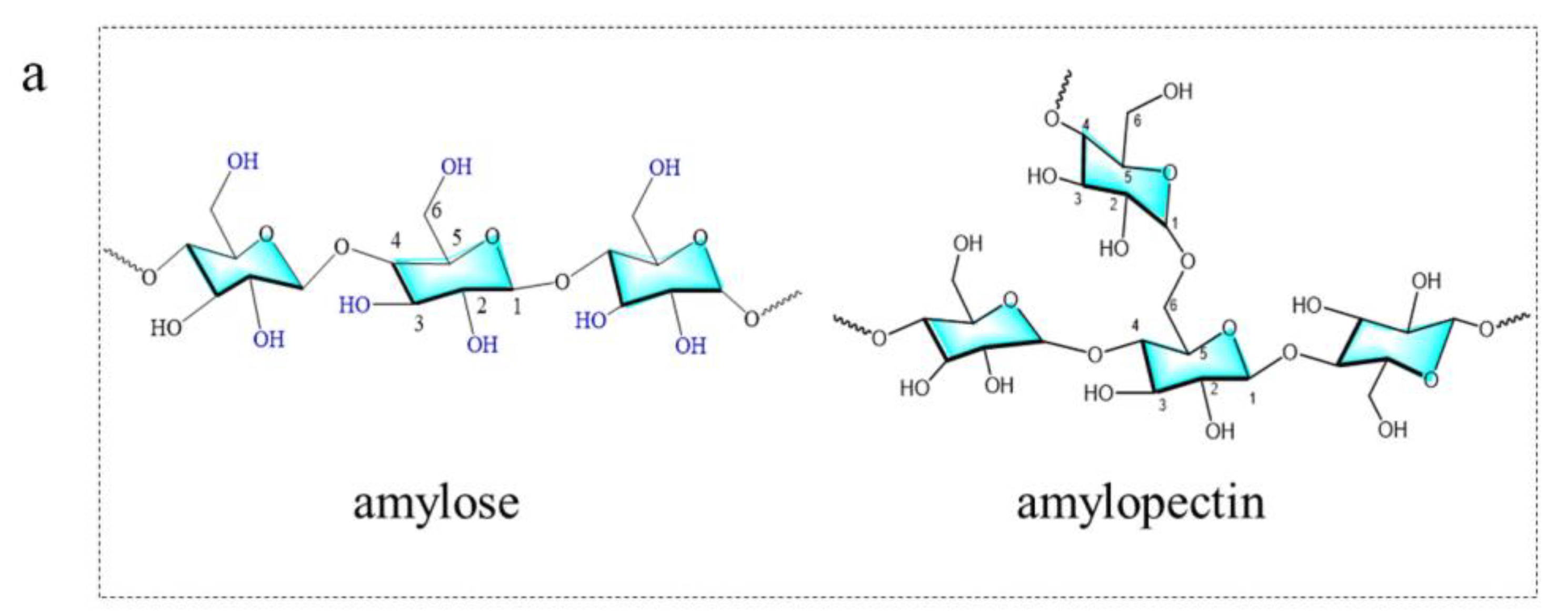
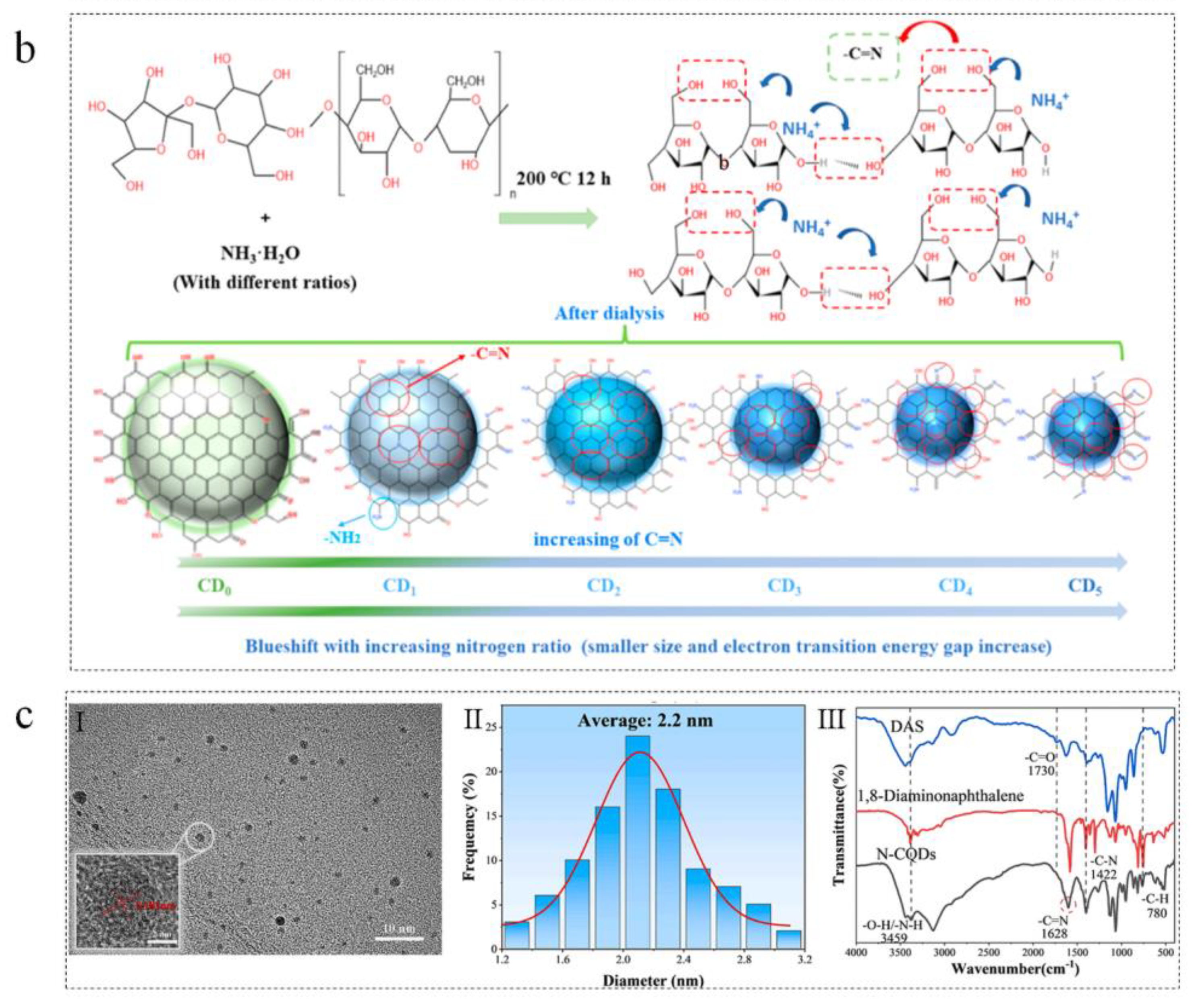
3.2. Starch
3.2.1. Introduction of Starch
3.2.2. Starch-Based Carbon Dots
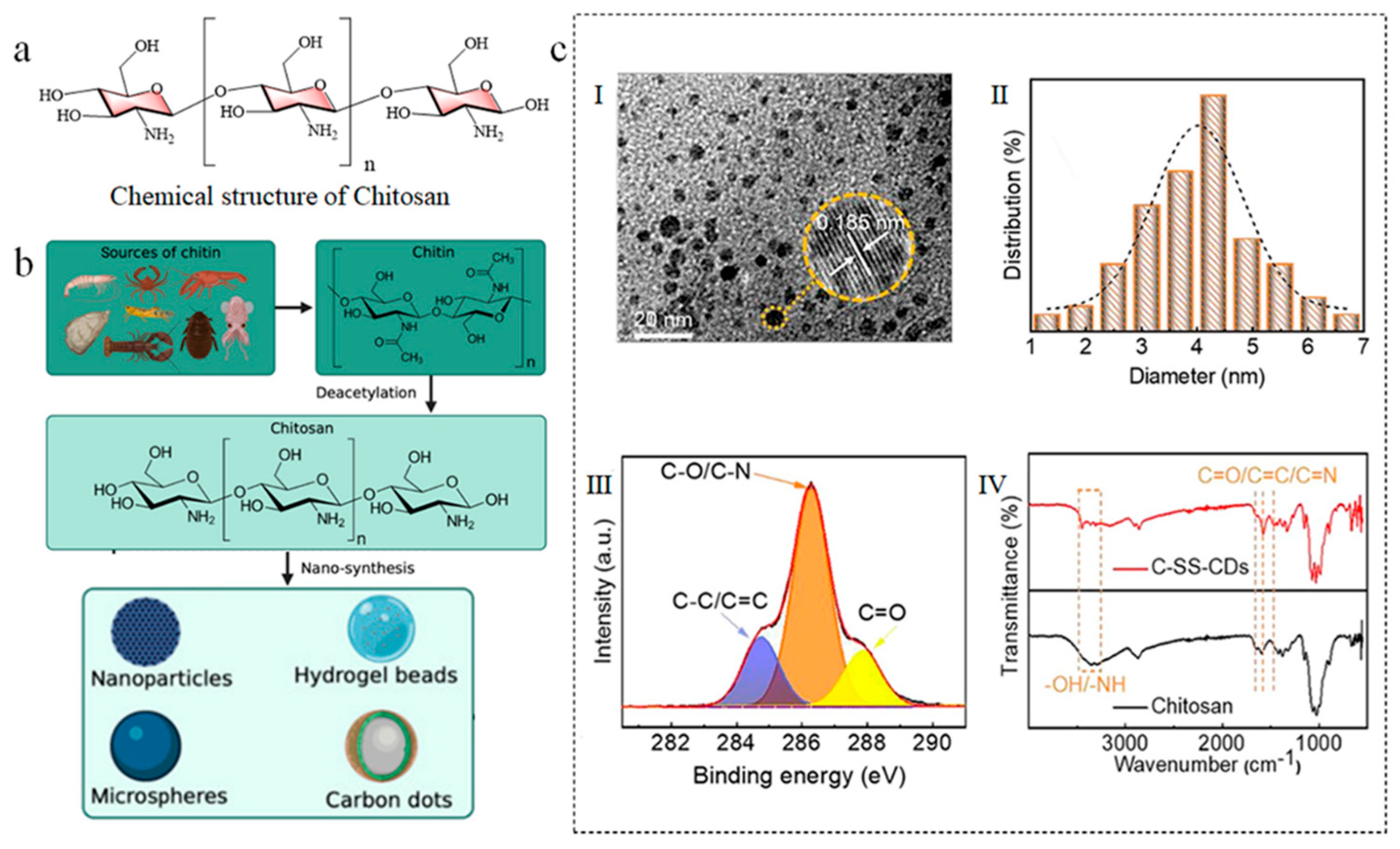
3.3. Chitosan
3.3.1. Introduction of Chitosan
3.3.2. Chitosan-Based Carbon Dots
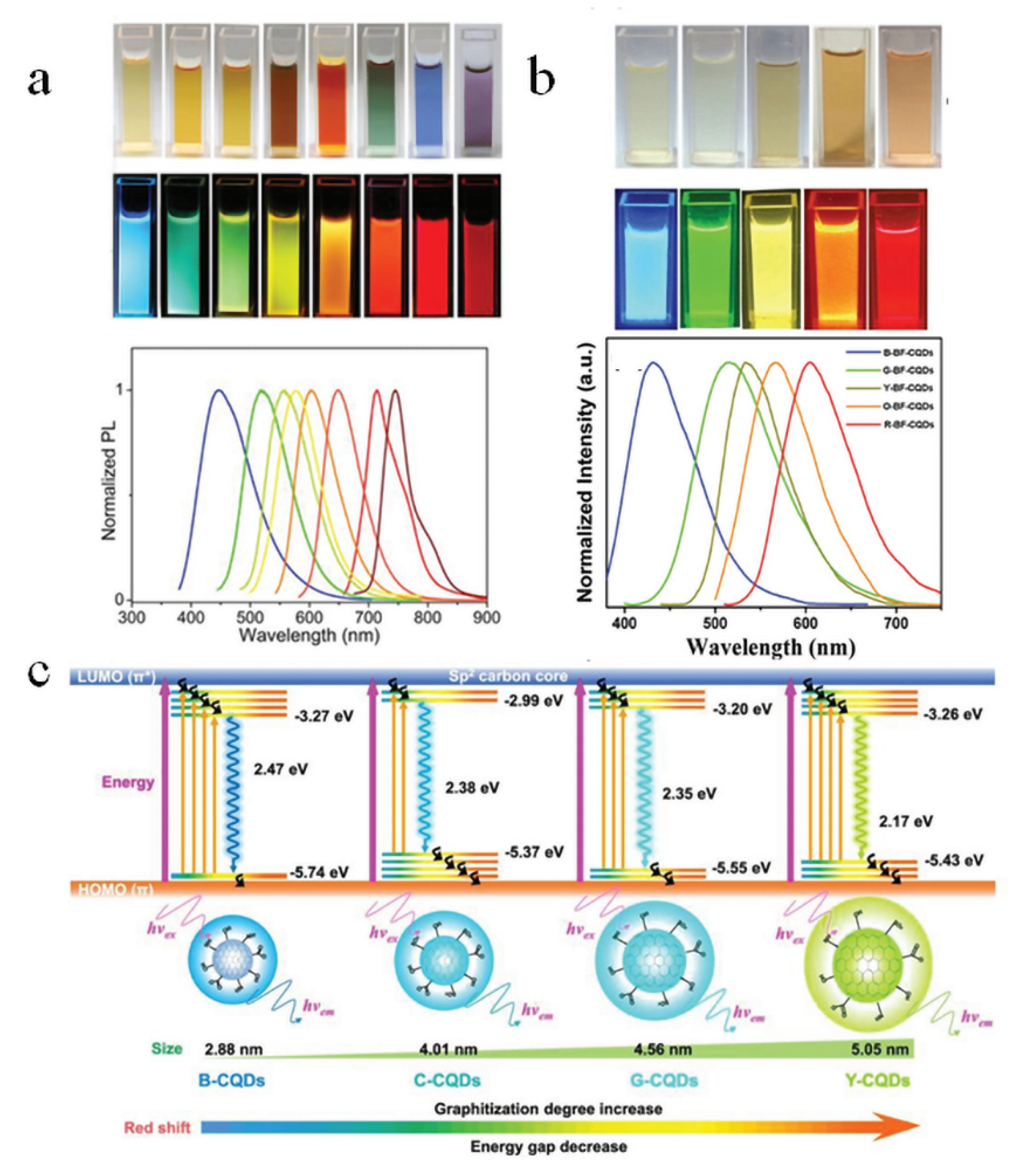
4. PL Properties of Biomass CDs
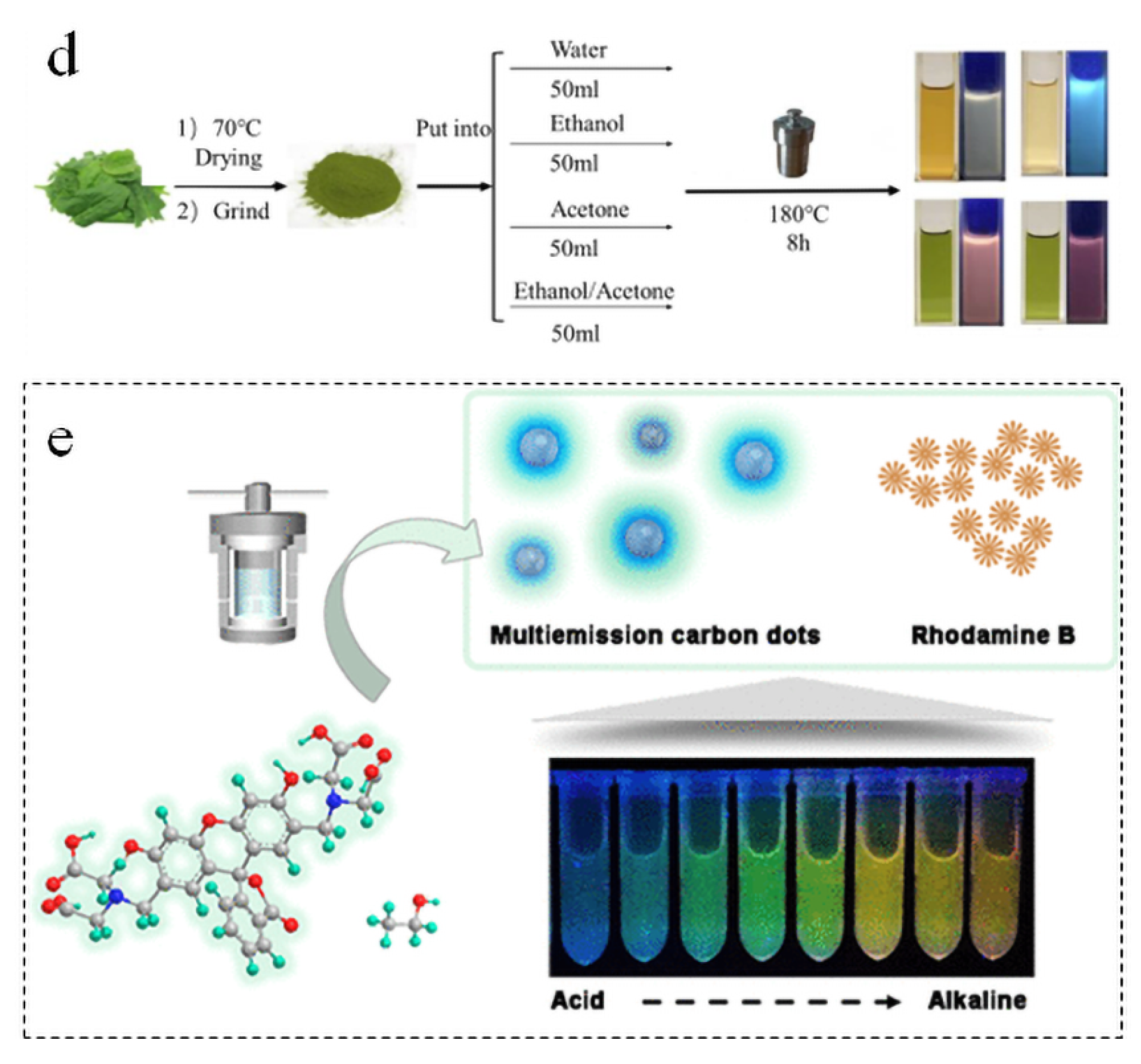
4.1. Tunable Fluorescence Emission
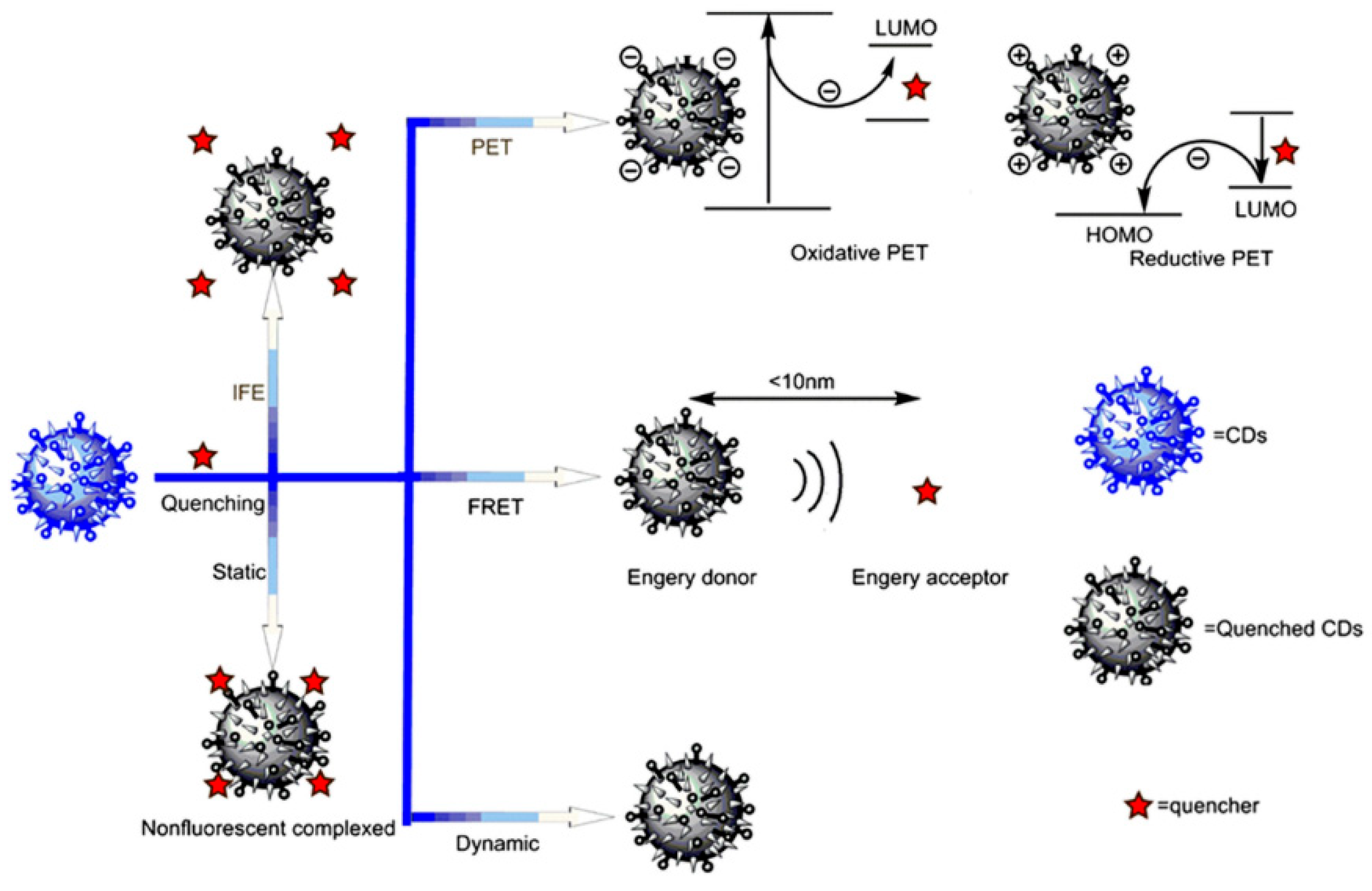
4.2. Fluorescence Quenching Phenomenon
4.3. Quantum Yield
4.4. Chiral Carbon Dots
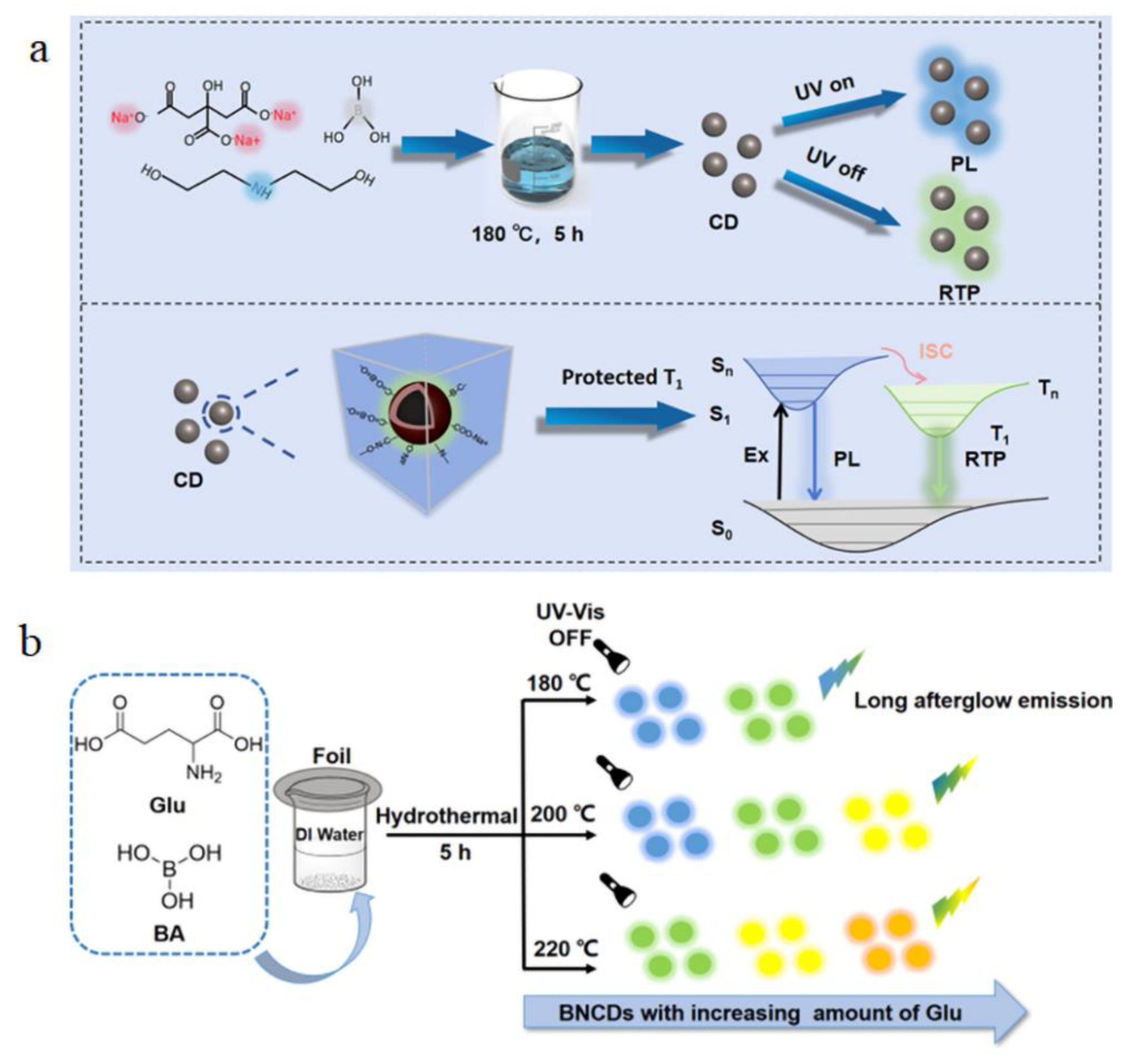
4.5. Room-Temperature Phosphorescence
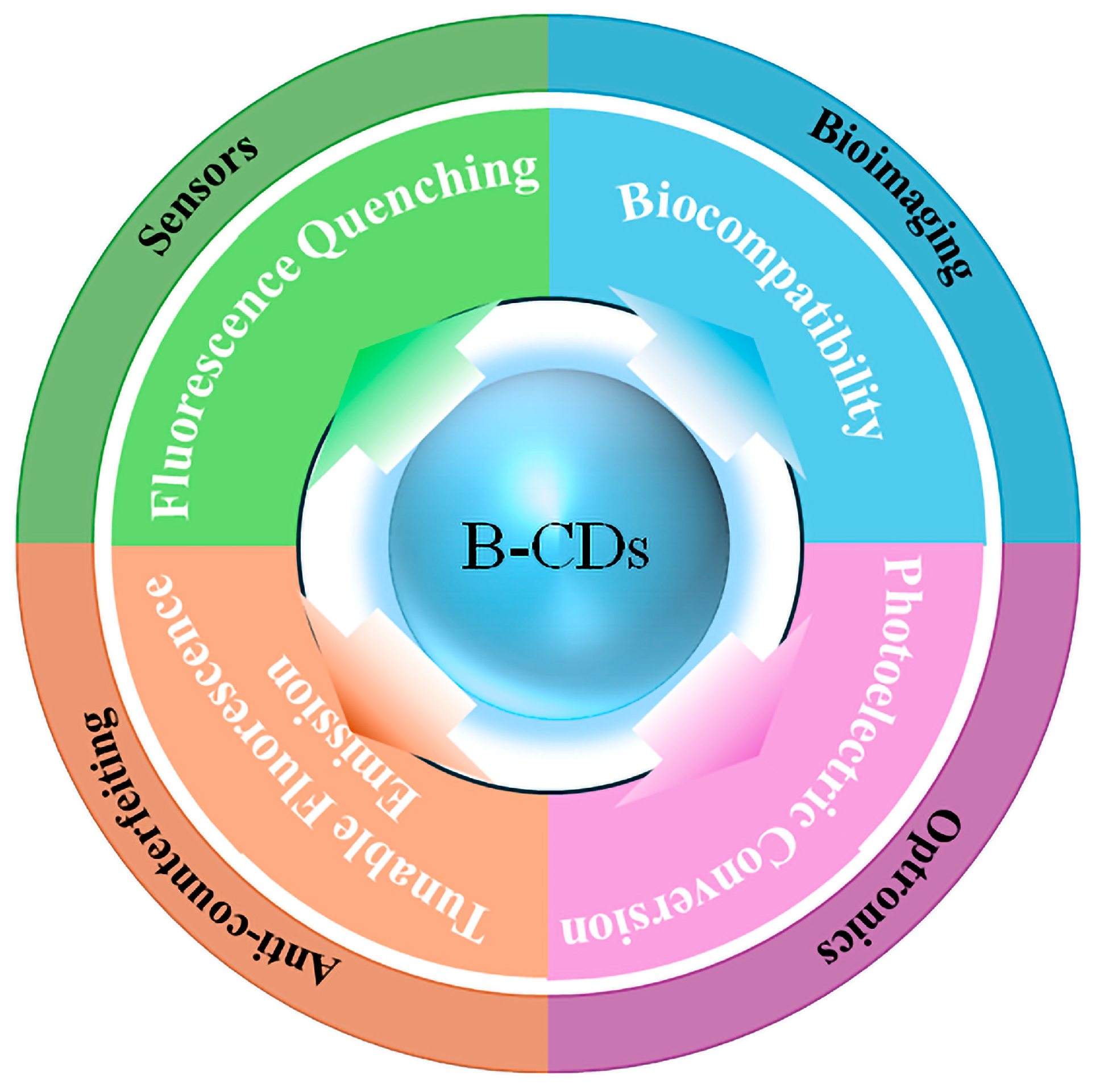
5. Functional Applications
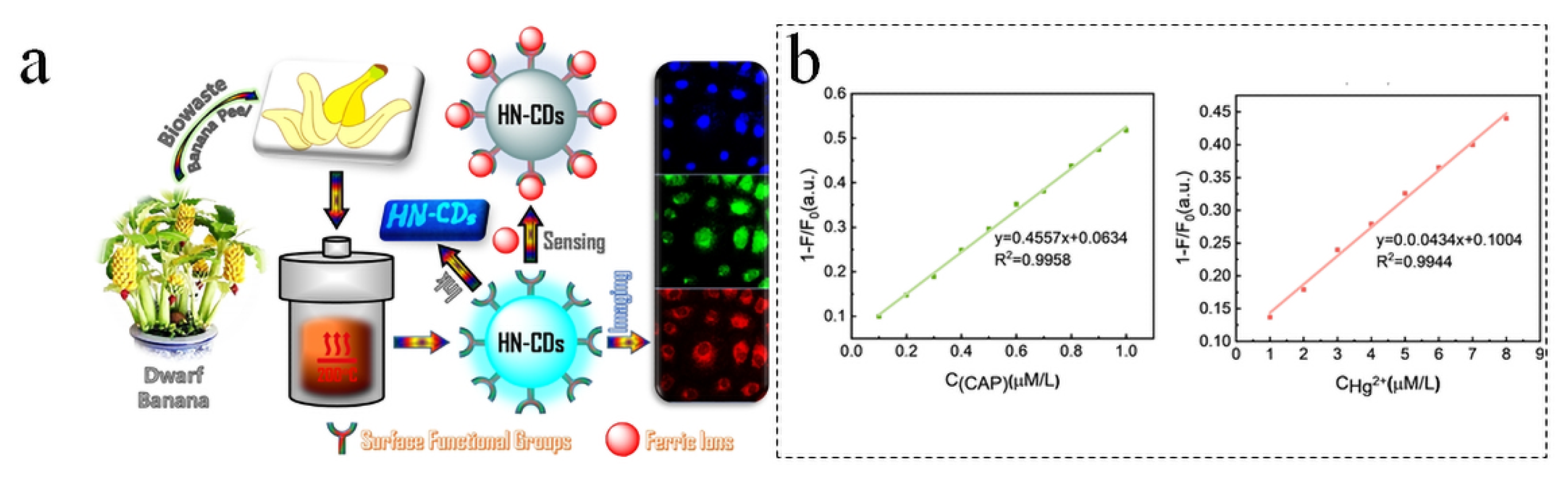
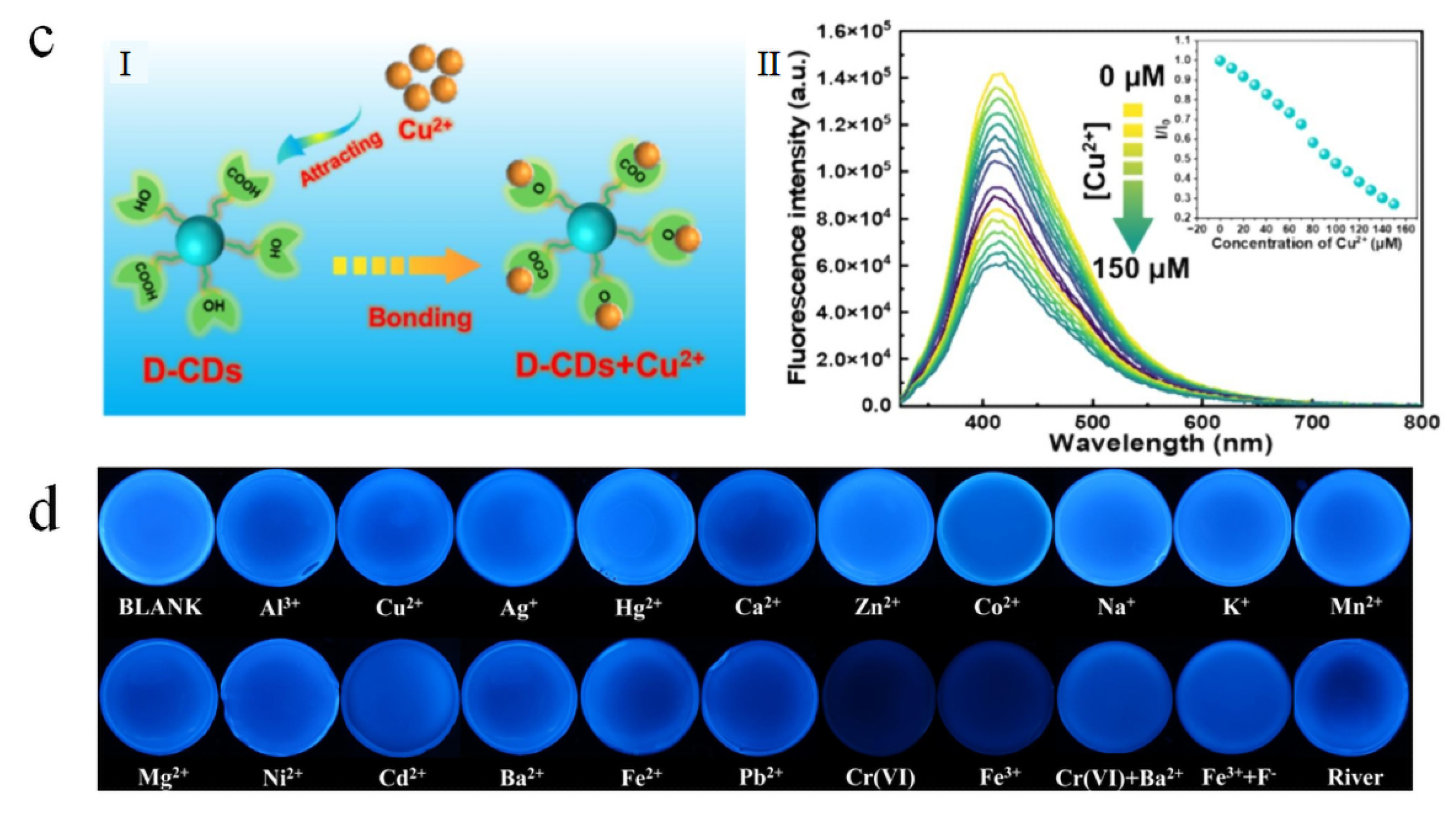
5.1. Sensors
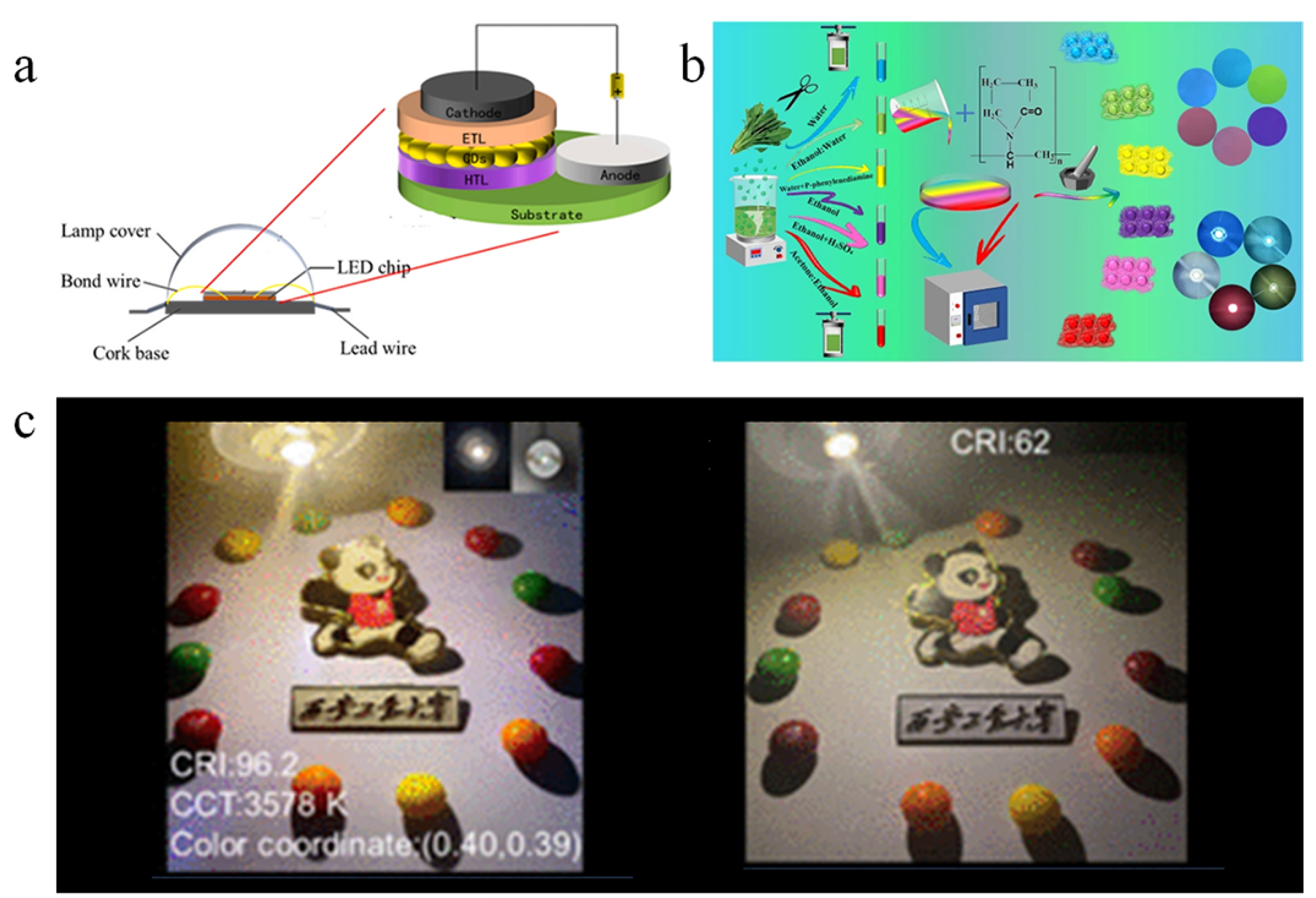
5.2. Anti-Counterfeiting
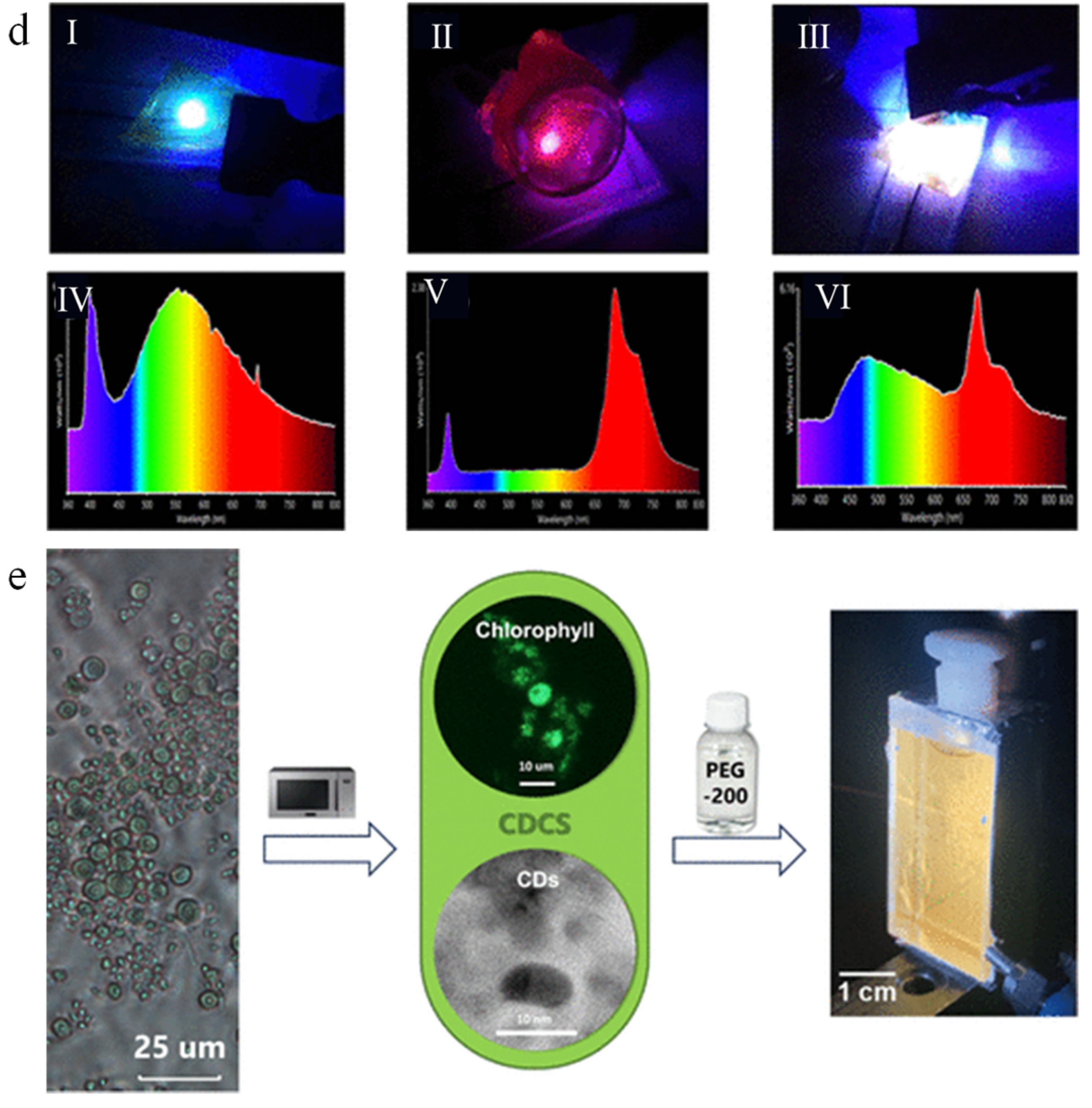
5.3. Bioimaging
5.4. Optronics
6. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Fang, M.; Wang, B.; Qu, X.; Li, S.; Huang, J.; Li, J.; Lu, S.; Zhou, N. State-of-the-Art of Biomass-Derived Carbon Dots: Preparation, Properties, and Applications. Chin. Chem. Lett. 2024, 35, 108423. [Google Scholar] [CrossRef]
- Wang, B.; Lu, S. The Light of Carbon Dots: From Mechanism to Applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Sagbas, S.; Sahiner, N. Carbon Dots: Preparation, Properties, and Application. In Nanocarbon and Its Composites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 651–676. ISBN 978-0-08-102509-3. [Google Scholar]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Shi, W.; Han, Q.; Wu, J.; Ji, C.; Zhou, Y.; Li, S.; Gao, L.; Leblanc, R.M.; Peng, Z. Synthesis Mechanisms, Structural Models, and Photothermal Therapy Applications of Top-down Carbon Dots from Carbon Powder, Graphite, Graphene, and Carbon Nanotubes. Int. J. Mol. Sci. 2022, 23, 1456. [Google Scholar] [CrossRef]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Han, C.; Zhang, X.; Wang, F.; Yu, Q.; Chen, F.; Shen, D.; Yang, Z.; Wang, T.; Jiang, M.; Deng, T. Duplex Metal Co-Doped Carbon Quantum Dots-Based Drug Delivery System with Intelligent Adjustable Size as Adjuvant for Synergistic Cancer Therapy. Carbon 2021, 183, 789–808. [Google Scholar] [CrossRef]
- Nguyen, V.; Si, J.; Yan, L.; Hou, X. Electron–Hole Recombination Dynamics in Carbon Nanodots. Carbon 2015, 95, 659–663. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Yao, X.; Lewis, R.E.; Haynes, C.L. Synthesis Processes, Photoluminescence Mechanism, and the Toxicity of Amorphous or Polymeric Carbon Dots. Acc. Chem. Res. 2022, 55, 3312–3321. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-L.; Bai, L.-F.; Geng, Z.-R.; Chen, H.; Xu, L.-T.; Xie, Y.-C.; Wang, D.-J.; Gu, H.-W.; Wang, X.-M. Carbon Quantum Dots: Preparation, Optical Properties, and Biomedical Applications. Mater. Today Adv. 2023, 18, 100376. [Google Scholar] [CrossRef]
- Jiang, M.; Sun, Y.; Chen, M.; Ji, H.; Liu, Y.; Qin, R.; Li, X.; Gao, H.; Zhang, R.; Zhang, L. Multicolor Luminescence of Carbon Dots: From Mechanisms to Applications. Chem. Eng. J. 2024, 496, 153761. [Google Scholar] [CrossRef]
- Qu, Y.; Bai, X.; Li, D.; Zhang, X.; Liang, C.; Zheng, W.; Qu, S. Solution-Processable Carbon Dots with Efficient Solid-State Red/near-Infrared Emission. J. Colloid. Interface Sci. 2022, 613, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Xu, W.; Deng, J.; Zhang, X.; Li, J.; Peng, Z. Multicolor Carbon Dots for Warm White Light-Emitting Diodes with a High Color Rendering Index of 93.2. ACS Appl. Nano Mater. 2023, 6, 16373–16382. [Google Scholar] [CrossRef]
- Xue, S.; Li, P.; Sun, L.; An, L.; Qu, D.; Wang, X.; Sun, Z. The Formation Process and Mechanism of Carbon Dots Prepared from Aromatic Compounds as Precursors: A Review. Small 2023, 19, 2206180. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Green Synthesis, Biomedical and Biotechnological Applications of Carbon and Graphene Quantum Dots. A Review. Environ. Chem. Lett. 2020, 18, 703–727. [Google Scholar] [CrossRef]
- Yang, X.; Sui, L.; Wang, B.; Zhang, Y.; Tang, Z.; Yang, B.; Lu, S. Red-Emitting, Self-Oxidizing Carbon Dots for the Preparation of White LEDs with Super-High Color Rendering Index. Sci. China Chem. 2021, 64, 1547–1553. [Google Scholar] [CrossRef]
- Bai, Y.; Zhao, J.; Wang, S.; Lin, T.; Ye, F.; Zhao, S. Carbon Dots with Absorption Red-Shifting for Two-Photon Fluorescence Imaging of Tumor Tissue pH and Synergistic Phototherapy. ACS Appl. Mater. Interfaces 2021, 13, 35365–35375. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, J.; Ren, T.; Luo, Q.; Luo, Y.; Zhang, N.; Huang, Y.; Guo, X.; Wu, Y. Novel Fluorescent Lignin-Based Hydrogel with Cellulose Nanofibers and Carbon Dots for Highly Efficient Adsorption and Detection of Cr (VI). Sci. Total Environ. 2021, 760, 143395. [Google Scholar] [CrossRef]
- Mukherjee, I.; Cilamkoti, V.; Dutta, R.K. Sunlight-Driven Photocatalytic Degradation of Ciprofloxacin by Carbon Dots Embedded in ZnO Nanostructures. ACS Appl. Nano Mater. 2021, 4, 7686–7697. [Google Scholar] [CrossRef]
- Calabrese, G.; De Luca, G.; Nocito, G.; Rizzo, M.G.; Lombardo, S.P.; Chisari, G.; Forte, S.; Sciuto, E.L.; Conoci, S. Carbon Dots: An Innovative Tool for Drug Delivery in Brain Tumors. Int. J. Mol. Sci. 2021, 22, 11783. [Google Scholar] [CrossRef]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, Properties and Biomedical Applications of Carbon-Based Quantum Dots: An Updated Review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, Z.; Chen, M.; Zhou, X.; Chen, W. Microwave-Assisted Synthesis of Polyamine-Functionalized Carbon Dots from Xylan and Their Use for the Detection of Tannic Acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 213, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Waterhouse, G.I.; Lu, S. Carbon Dots: Mysterious Past, Vibrant Present, and Expansive Future. Trends Chem. 2023, 5, 76–87. [Google Scholar] [CrossRef]
- Yang, X.; Lotfy, V.F.; Basta, A.H.; Liu, H.; Fu, S. Carbon Quantum Dots Derived from Rice Straw Doped with N and S and Its Nanocomposites with Hydroxypropyl Cellulose Nanocomposite. Int. J. Biol. Macromol. 2024, 278, 134925. [Google Scholar] [CrossRef]
- Liang, L.; Liu, Y.; Huang, C.; Han, M.; Yao, B.; Leng, Y.; Li, X.; Zhang, Y.; Cai, X. Fluorescent Carbon Dots Based on Nitrogen Doped Dialdehyde Starch for Highly Selective Fe3+/Glyphosate Detection and Its Applications. Microchem. J. 2024, 204, 111084. [Google Scholar] [CrossRef]
- Ni, J.; Huang, X.; Bai, Y.; Zhao, B.; Han, Y.; Han, S.; Xu, T.; Si, C.; Zhang, C. Resistance to Aggregation-Caused Quenching: Chitosan-Based Solid Carbon Dots for White Light-Emitting Diode and 3D Printing. Adv. Compos. Hybrid Mater. 2022, 5, 1865–1875. [Google Scholar] [CrossRef]
- Thara, C.R.; Korah, B.K.; Mathew, S.; John, B.K.; Mathew, B. Dual Mode Detection and Sunlight-Driven Photocatalytic Degradation of Tetracycline with Tailor-Made N-Doped Carbon Dots. Environ. Res. 2023, 216, 114450. [Google Scholar] [CrossRef]
- Wang, S.; Huo, X.; Zhao, H.; Dong, Y.; Cheng, Q.; Li, Y. One-Pot Green Synthesis of N, S Co-Doped Biomass Carbon Dots from Natural Grapefruit Juice for Selective Sensing of Cr (VI). Chem. Phys. Impact 2022, 5, 100112. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Chen, X.; Lai, Y.; Zhang, T. Hydrothermal Synthesis of Nitrogen-Doped Carbon Dots for Selective Fe3+ Detection. J. Fluoresc. 2025, 1–12. [Google Scholar] [CrossRef]
- Issa, M.A.; Zentou, H.; Jabbar, Z.H.; Abidin, Z.Z.; Harun, H.; Halim, N.A.A.; Alkhabet, M.M.; Pudza, M.Y. Ecofriendly Adsorption and Sensitive Detection of Hg (II) by Biomass-Derived Nitrogen-Doped Carbon Dots: Process Modelling Using Central Composite Design. Environ. Sci. Pollut. Res. 2022, 29, 86859–86872. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-Based Hydrogels: Present Status and Application Prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Gan, J.; Chen, L.; Chen, Z.; Zhang, J.; Yu, W.; Huang, C.; Wu, Y.; Zhang, K. Lignocellulosic Biomass-based Carbon Dots: Synthesis Processes, Properties, and Applications. Small 2023, 19, 2304066. [Google Scholar] [CrossRef]
- Arkin, K.; Zheng, Y.; Bei, Y.; Ma, X.; Che, W.; Shang, Q. Construction of Dual-Channel Ratio Sensing Platform and Molecular Logic Gate for Visual Detection of Oxytetracycline Based on Biomass Carbon Dots Prepared from Cherry Tomatoes Stalk. Chem. Eng. J. 2023, 464, 142552. [Google Scholar] [CrossRef]
- Naik, G.G.; Madavi, R.; Minocha, T.; Mohapatra, D.; Pratap, R.; Shreya, S.; Patel, P.K.; Yadav, S.K.; Parmar, A.; Patra, A. In Vitro Cytotoxic Potential of Cow Dung and Expired Tomato Sauces-Derived Carbon Nanodots against a-375 Human Melanoma Cell Line. Arab. J. Chem. 2024, 17, 105576. [Google Scholar] [CrossRef]
- Kasirajan, K.; Karunakaran, M.; Choi, H.K. Synthesis of Environmentally-Friendly Carbon Quantum Dots from Orange Juice for Selective Detection of Fe3+ Ions, Antibacterial Activity, and Bio-Imaging Applications. J. Environ. Chem. Eng. 2024, 12, 113535. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Guo, Z.; Lei, B.; Zhuang, J.; Zhang, X.; Liu, Z.; Hu, C. Hydrophobic Carbon Dots with Blue Dispersed Emission and Red Aggregation-Induced Emission. Nat. Commun. 2019, 10, 1789. [Google Scholar] [CrossRef]
- Cao, L.; Zan, M.; Chen, F.; Kou, X.; Liu, Y.; Wang, P.; Mei, Q.; Hou, Z.; Dong, W.-F.; Li, L. Formation Mechanism of Carbon Dots: From Chemical Structures to Fluorescent Behaviors. Carbon 2022, 194, 42–51. [Google Scholar] [CrossRef]
- Pérez, S.; Bertoft, E. The Molecular Structures of Starch Components and Their Contribution to the Architecture of Starch Granules: A Comprehensive Review. Starch Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Vanier, N.L.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Molecular Structure, Functionality and Applications of Oxidized Starches: A Review. Food Chem. 2017, 221, 1546–1559. [Google Scholar] [CrossRef]
- Shibata, H.; Abe, M.; Sato, K.; Uwai, K.; Tokuraku, K.; Iimori, T. Microwave-Assisted Synthesis and Formation Mechanism of Fluorescent Carbon Dots from Starch. Carbohydr. Polym. Technol. Appl. 2022, 3, 100218. [Google Scholar] [CrossRef]
- Liu, C.; Wen, M.; Zhou, X.; Huang, X.; Zhang, S.; Liu, H. Starch-Derived Carbon Dots with Enhanced Photoluminescence and Tunable Emission for Multilevel Anticounterfeiting. ACS Sustain. Chem. Eng. 2024, 12, 12354–12364. [Google Scholar] [CrossRef]
- Pillai, C.K.; Paul, W.; Sharma, C.P. Chitin and Chitosan Polymers: Chemistry, Solubility and Fiber Formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kumar, S.; Aziz, S.T.; Girshevitz, O.; Nessim, G.D. One-Step Synthesis of N-Doped Graphene Quantum Dots from Chitosan as a Sole Precursor Using Chemical Vapor Deposition. J. Phys. Chem. C 2018, 122, 2343–2349. [Google Scholar] [CrossRef]
- Villalba-Rodríguez, A.M.; González-González, R.B.; Martínez-Ruiz, M.; Flores-Contreras, E.A.; Cárdenas-Alcaide, M.F.; Iqbal, H.M.N.; Parra-Saldívar, R. Chitosan-Based Carbon Dots with Applied Aspects: New Frontiers of International Interest in a Material of Marine Origin. Mar. Drugs 2022, 20, 782. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.; Zhang, P.; Zhou, Z.; Gao, Q.; Xiong, H. Solvent-controlled Synthesis of Highly Luminescent Carbon Dots with a Wide Color Gamut and Narrowed Emission Peak Widths. Small 2018, 14, 1800612. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Wang, Z.; Li, X.; Li, Y.; Tan, Z.; Fan, L.; Yang, S. Bright Multicolor Bandgap Fluorescent Carbon Quantum Dots for Electroluminescent Light-emitting Diodes. Adv. Mater. 2017, 29, 1604436. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Wang, Q.; Luo, K.H. Green Synthesis of Tunable Fluorescent Carbon Quantum Dots from Lignin and Their Application in Anti-Counterfeit Printing. ACS Appl. Mater. Interfaces 2021, 13, 56465–56475. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, H.; Yang, J.; Dong, Y.; Guo, S.; Cheng, Q.; Li, Y.; Liu, S. Preparation of Multicolor Biomass Carbon Dots Based on Solvent Control and Their Application in Cr(VI) Detection and Advanced Anti-Counterfeiting. ACS Omega 2023, 8, 6550–6558. [Google Scholar] [CrossRef]
- Zhu, P.; Li, J.; Gao, L.; Xiong, J.; Tan, K. Strategy to Synthesize Tunable Multiemission Carbon Dots and Their Multicolor Visualization Application. ACS Appl. Mater. Interfaces 2021, 13, 33354–33362. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The Quenching of the Fluorescence of Carbon Dots: A Review on Mechanisms and Applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Han, S.; Chen, X.; Hu, Y.; Han, L. Solid-State N,P-Doped Carbon Dots Conquer Aggregation-Caused Fluorescence Quenching and Couple with Europium Metal-Organic Frameworks toward White Light-Emitting Diodes. Dye. Pigment. 2021, 187, 109090. [Google Scholar] [CrossRef]
- Mahto, M.K.; Samanta, D.; Shaw, M.; Shaik, M.A.S.; Basu, R.; Mondal, I.; Bhattacharya, A.; Pathak, A. Blue-Emissive Nitrogen-Doped Carbon Dots for Picric Acid Detection: Molecular Fluorescence Quenching Mechanism. ACS Appl. Nano Mater. 2023, 6, 8059–8070. [Google Scholar] [CrossRef]
- Yang, X.; Hou, S.; Chu, T.; Han, J.; Li, R.; Guo, Y.; Gong, Y.; Li, H.; Wan, Z. Preparation of Magnesium, Nitrogen-Codoped Carbon Quantum Dots from Lignin with Bright Green Fluorescence and Sensitive pH Response. Ind. Crops Prod. 2021, 167, 113507. [Google Scholar] [CrossRef]
- Prado, M.B.; Truong, N.T.; Wanekaya, A.K. Improving the Quantum Yield of Nitrogen-Doped Carbon Dots by Varying Dopant Ratios and pH. Sens. Actuators Rep. 2023, 6, 100165. [Google Scholar] [CrossRef]
- Hua, J.; Hua, P.; Qin, K. Highly Fluorescent N, F Co-Doped Carbon Dots with Tunable Light Emission for Multicolor Bio-Labeling and Antibacterial Applications. J. Hazard. Mater. 2023, 459, 132331. [Google Scholar] [CrossRef]
- Zhou, W.; Mo, F.; Sun, Z.; Luo, J.; Fan, J.; Zhu, H.; Zhu, Z.; Huang, J.; Zhang, X. Bright Red-Emitting P, Br Co-Doped Carbon Dots as “OFF-ON” Fluorescent Probe for Cu2+ and L-Cysteine Detection. J. Alloys Compd. 2022, 897, 162731. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.; Yu, K.; Wu, Z.; Wang, N.; Yu, X. Nitrogen and Sulfur Co-Doped Carbon Dots: Facile Synthesis and Multifunctional Applications for pH Sensing, Temperature Sensing and RNA-Selective Imaging. Microchem. J. 2021, 168, 106248. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Li, Q.; Zhang, T.; Fu, Y. Nitrogen-Doped Carbon Quantum Dots as Dual Mode Fluorescence Sensors for the Determination of Food Colorant Quinoline Yellow. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 315, 124285. [Google Scholar] [CrossRef]
- Chen, X.; Yu, M.; Li, P.; Xu, C.; Zhang, S.; Wang, Y.; Xing, X. Recent Progress on Chiral Carbon Dots: Synthetic Strategies and Biomedical Applications. ACS Biomater. Sci. Eng. 2023, 9, 5548–5566. [Google Scholar] [CrossRef]
- Shi, Y.; Su, W.; Teng, Q.; Li, C.; Yuan, T.; Xu, H.; Song, X.; Han, Y.; Wei, S.; Zhang, Y.; et al. Opportunity and Application of Chiral Carbon Dots. Matter 2023, 6, 2776–2806. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Ma, Y.; Wu, J.; Wang, Y.; Huang, H.; Liu, Y.; Kang, Z. Carbon Dots with Chiral Surface Selectively Inhibit the Activity of Laccase. Appl. Surf. Sci. 2022, 583, 152540. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Samanta, D.; Shaw, M.; Shaik, M.A.S.; Basu, R.; Mondal, I.; Pathak, A. Sensitive Detection of Hg2+ and l-Cysteine through Optical Asymmetry-Tuned Fluorescence Switch Off-On Behavior in N-Doped Chiral Carbon Dot. ACS Appl. Bio Mater. 2025, 8, 503–518. [Google Scholar] [CrossRef]
- Zheng, C.; Tao, S.; Yang, B. Polymer–Structure-Induced Room-Temperature Phosphorescence of Carbon Dot Materials. Small Struct. 2023, 4, 2200327. [Google Scholar] [CrossRef]
- Ye, S.; Ji, S.; Kang, M.; Liu, L.; Qu, J.; Song, J.; Guo, J. Aggregated Carbon Dots Endow Them with Room-Temperature Phosphorescence in Aqueous Environments: Mechanisms, Properties, and Applications. Chem. Eng. J. 2024, 495, 153653. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, X.; Mao, X.; Dong, Y.; Li, W.; Li, Z.; Gao, W. Na-Doped Carbon Dots with Room-Temperature Phosphorescence for Information Encryption. ACS Appl. Nano Mater. 2024, 7, 22525–22533. [Google Scholar] [CrossRef]
- Sun, X.; Ouyang, M.; Chen, X. Color-Tunable and Time-Dependent Room-Temperature Phosphorescence Carbon Dots for Dynamic Multi-Information Encryption. ACS Appl. Nano Mater. 2025, 8, 5527–5534. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Muthuchamy, N.; Lee, Y.R. Hydrophilic Nitrogen-Doped Carbon Dots from Biowaste Using Dwarf Banana Peel for Environmental and Biological Applications. Fuel 2020, 275, 117821. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Zhou, L.; Chen, Z.; Cui, X.; Xiao, H.; Yang, A.; Minxie; Xiong, R.; Cheng, W.; et al. Development of Sensitive Biomass Xylan-Based Carbon Dots Fluorescence Sensor for Quantification Detection Cu2+ in Real Water and Soil. Int. J. Biol. Macromol. 2024, 278, 135037. [Google Scholar] [CrossRef]
- Liu, H.; Wu, S. Blue Fluorescent Carbon Dots Doped with Nitrogen and Sulfur as a Dual-Functional Fluorescent Probe for the Detection of Hg2+ and Chloramphenicol. J. Mol. Struct. 2025, 1329, 141459. [Google Scholar] [CrossRef]
- Xia, L.; Li, X.; Zhang, Y.; Zhou, K.; Yuan, L.; Shi, R.; Zhang, K.; Fu, Q. Sustainable and Green Synthesis of Waste-Biomass-Derived Carbon Dots for Parallel and Semi-Quantitative Visual Detection of Cr(VI) and Fe3+. Molecules 2022, 27, 1258. [Google Scholar] [CrossRef]
- Gao, Q.; Guo, X.; Meng, L.; Liu, M.; Chen, L.; Li, H.; Hu, J. N, S/P Co-Doped Hemicellulose-Based Carbon Dots with Tunable Fluorescence for Anti-Counterfeiting. Int. J. Biol. Macromol. 2023, 253, 126332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, L.; Yan, M.; Ma, J.; Wang, J.; Liu, C.; Bao, Y.; Jin, H.; Fan, Q. Turning Waste into Treasure: Multicolor Carbon Dots Synthesized from Waste Leather Scrap and Their Application in Anti-Counterfeiting. ACS Sustain. Chem. Eng. 2023, 11, 5082–5092. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Jia, L.; Zhang, X.; Xu, J. Dynamic Anti-Counterfeiting and Reversible Multi-Level Encryption-Decryption Based on Spirulina Derived pH-Responsive Dual-Emissive Carbon Dots. J. Lumin. 2023, 257, 119727. [Google Scholar] [CrossRef]
- Guo, X.; Yang, R.; Wang, Y.; Cheng, C.; Fu, D.; Sheng, J. Molecularly Designed and Synthesized of Bright Blue Nitrogen-Doped Lignin-Derived Carbon Dots Applied in Printable Anti-Counterfeiting. Int. J. Biol. Macromol. 2023, 253, 126723. [Google Scholar] [CrossRef] [PubMed]
- Atchudan, R.; Gangadaran, P.; Perumal, S.; Edison, T.N.J.I.; Sundramoorthy, A.K.; Rajendran, R.L.; Ahn, B.-C.; Lee, Y.R. Green Synthesis of Multicolor Emissive Nitrogen-Doped Carbon Dots for Bioimaging of Human Cancer Cells. J. Clust. Sci. 2023, 34, 1583–1594. [Google Scholar] [CrossRef]
- Thota, S.P.; Kurdekar, A.; Vadlani, P.V.; Kumar, B.S. Green Synthesis of Highly Fluorescent Carbon Dots from Groundnut Shell Biomass for Bioimaging Applications. J. Fluoresc. 2024, 1–11. [Google Scholar] [CrossRef]
- Hua, J.; Hua, P.; Qin, K. Tunable Fluorescent Biomass-Derived Carbon Dots for Efficient Antibacterial Action and Bioimaging. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132672. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Y.; Xu, Q.; Cao, Y.; Wei, J.; Liang, Z.; Jiang, X.; Wu, Y.; Guo, C. Solvothermal Regulation of Fluorescence Quantum Yield of Carbon Dots Derived from Biomass for Zebrafish Imaging. J. Lumin. 2024, 271, 120583. [Google Scholar] [CrossRef]
- Ji, C.; Xu, W.; Han, Q.; Zhao, T.; Deng, J.; Peng, Z. Light of Carbon: Recent Advancements of Carbon Dots for LEDs. Nano Energy 2023, 114, 108623. [Google Scholar] [CrossRef]
- Kumar, A.; Sahoo, S.; Singh, A.; Sahu, S.K. High-Performance Biomass-Derived Red- and Green-Emissive Carbon Dots with Narrow Emission for White-Light-Emitting Diodes Comprising a 97 Color Rendering Index. ACS Sustain. Resour. Manag. 2025, acssusresmgt.5c00024. [Google Scholar] [CrossRef]
- Wang, H.; Jin, X.; He, Y.; Bai, H.; Ma, L.; Zhao, L.; Ding, L.; Zhou, H.; Si, Q.; Chen, W. The Modification Strategy of Biomass-Derived Full-Color Carbon Dots and Its Applications to Warm White Light-Emitting Diodes with Ultra-High CRI-96.2. ACS Sustain. Resour. Manag. 2024, 1, 2255–2265. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; He, Y.; Wang, S.; Cheng, Q.; Li, Y. Full-Color Biomass Carbon Dots for High-Level Information Encryption and Multi-Color Light Emitting Diode Applications. Microchim. Acta 2024, 191, 538. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.; Duarte, T.A.G.; Correia, S.F.H.; Pereira, R.F.P.; Conde, A.; Ribeiro, Á.R.; Braga, S.S.; Ventura, S.P.M.; Ferreira, R.A.S.; De Zea Bermudez, V.; et al. Biphosphor Carbon Dots/Chlorophyll System Entirely Derived from Chlorella Microalgae for Luminescent Solar Concentrators. ACS Nanosci. Au 2025, 5, 137–152. [Google Scholar] [CrossRef]






| Synthesis Method | Topic | Advantages | Disadvantages |
|---|---|---|---|
| Arc-Discharge Method | High-temperature (50–200 A) and high-pressure (0.05–40 kPa) arc evaporation decomposition. | Simple process; diverse carbon sources; large scale. | Complex process; poor size control; low yield. |
| Laser Ablation Method | Laser beam focusing resolution. | Fast; effective; highly adjustable. | Low yield; poor size control; high equipment requirements. |
| Chemical Etching Method | Strong acid carbonizes small organic molecules. | Simple process. | Complex process; harsh conditions, many steps; poor control over size. |
| Synthesis Method | Topic | Advantages | Disadvantages |
|---|---|---|---|
| Hydrothermal Synthesis | Reaction with solvent in high temperature (180–280 °C) and high pressure (0.1–4.0 MPa) water environment. | Environmentally friendly; easy operation; mild conditions. | Longer reaction time; harsh conditions. |
| Microwave Synthesis | Microwave radiation heating (150–300 °C). | Fast reaction; high yield; enables rapid synthesis. | High equipment requirements; poor size control. |
| Pyrolysis Synthesis | High-temperature (200–900 °C) pyrolytic carbonization of organic precursors. | Simple procedure; high yield. | High energy cost. |
| Carbon Source | Synthesis Method | Heteroatom Dopant(s) | Carbon Dot C Content (at%) | Scalability | Reference |
|---|---|---|---|---|---|
| Rice straw | One-step hydrothermal | N: ethylenediamine, S: lipoic acid | 67.71 | Low | [25] |
| Dialdehyde starch | One-step hydrothermal | N: 1,8-diaminonaphthalene | 67.86 | Low | [26] |
| Chitosan | One-step hydrothermal | N: ethylenediamine | Not reported | Low | [27] |
| Glucose | One-step hydrothermal | N: Urea | Not reported | Low | [28] |
| Grapefruit juice | One-step hydrothermal | N: Urea | 58.91 | Low | [29] |
| Citric acid | One-step hydrothermal | N: Urea | Not reported | Low | [30] |
| Carboxymethyl cellulose | One-step hydrothermal | N: Urea | Not reported | Low | [31] |
| Raw Material | Solvent | Dopant | Ex/Em (nm) | Meas. Temp. | QY | Reference |
|---|---|---|---|---|---|---|
| Rice straw | Water | N: Ethylenediamine; S: Lipoic acid | 350/440 | Room temperature | 62.8% | [25] |
| Glucose | Water | N: Urea | 340/435 | Room temperature | 14.9% | [28] |
| o-Phenylenediamine | Water | N: o-Phenylenediamine; F: 2,3,5,6-Tetrafluoroterephthalic acid | 428/540 | Room temperature | 52.2% | [58] |
| o-Phenylenediamine | Water | P: Ammonium dihydrogen phosphate; Br: KBr | 450/610 | Room temperature | 11.3% | [59] |
| Grapefruit juice | Water | N: Urea | 430/512 | Room temperature | 84.9% | [29] |
| Methyl cellulose | Water | N: Ethylenediamine; S: L-Cysteine | 330/370 | Room temperature | 12.3% | [60] |
| Citric acid | Water | N: Urea | 360/435 | Room temperature | 26.6% | [30] |
| Carboxymethyl cellulose | Water | N: Urea | 350/470 | Room temperature | 35.5% | [31] |
| Citric acid | Water | N: Triethylenetetramine | 360/440 | Room temperature | 70% | [61] |
| Spinach | Ethanol/Acetone | / | 365/493 | Room temperature | 10.8% | [51] |
| Calcein | Ethanol/NaOH | / | 490/530 | Room temperature | 21.9% | [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Chen, H.; Mi, R.; Min, X.; Fang, M.; Wu, X.; Huang, Z.; Liu, Y. Biomass-Derived Carbon Dots: Preparation, Properties, and Applications. Nanomaterials 2025, 15, 1279. https://doi.org/10.3390/nano15161279
Liu Q, Chen H, Mi R, Min X, Fang M, Wu X, Huang Z, Liu Y. Biomass-Derived Carbon Dots: Preparation, Properties, and Applications. Nanomaterials. 2025; 15(16):1279. https://doi.org/10.3390/nano15161279
Chicago/Turabian StyleLiu, Qinfeng, Huan Chen, Ruiyu Mi, Xin Min, Minghao Fang, Xiaowen Wu, Zhaohui Huang, and Yangai Liu. 2025. "Biomass-Derived Carbon Dots: Preparation, Properties, and Applications" Nanomaterials 15, no. 16: 1279. https://doi.org/10.3390/nano15161279
APA StyleLiu, Q., Chen, H., Mi, R., Min, X., Fang, M., Wu, X., Huang, Z., & Liu, Y. (2025). Biomass-Derived Carbon Dots: Preparation, Properties, and Applications. Nanomaterials, 15(16), 1279. https://doi.org/10.3390/nano15161279