Waterborne Phosphated Alkynediol-Modified Mica Nanosheet/Acrylic Nanocomposite Coatings with Superior Anticorrosive Performance
Abstract
1. Introduction
2. Results and Discussion
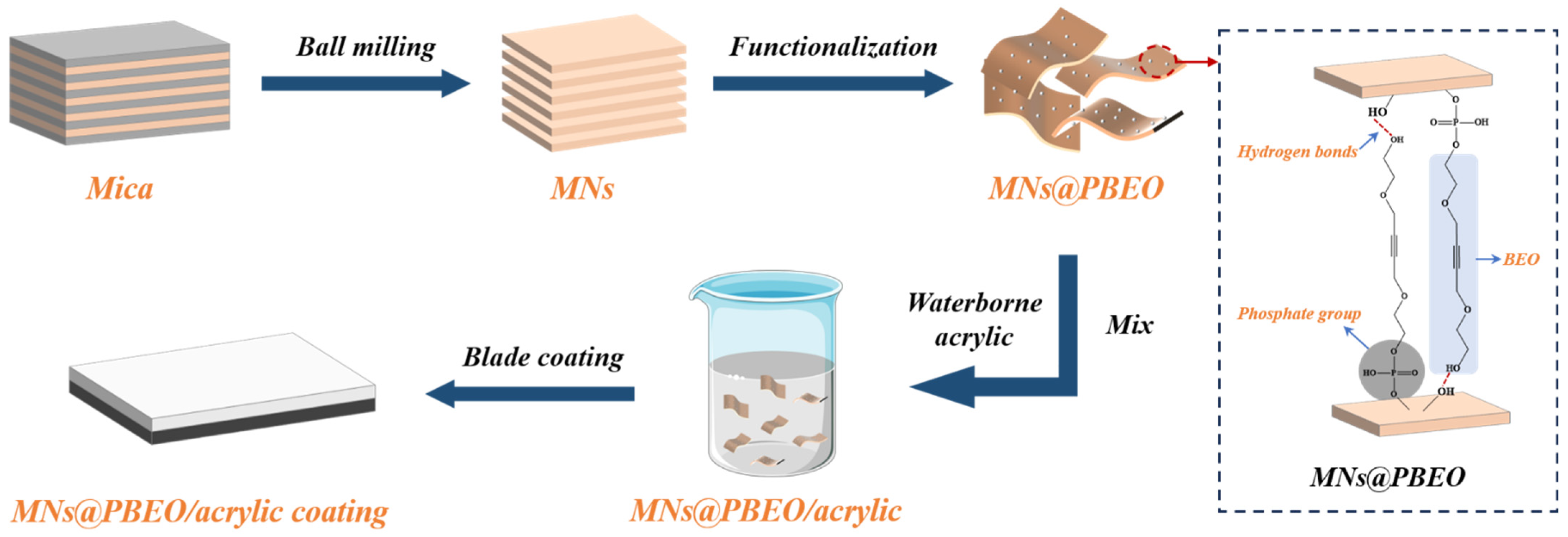
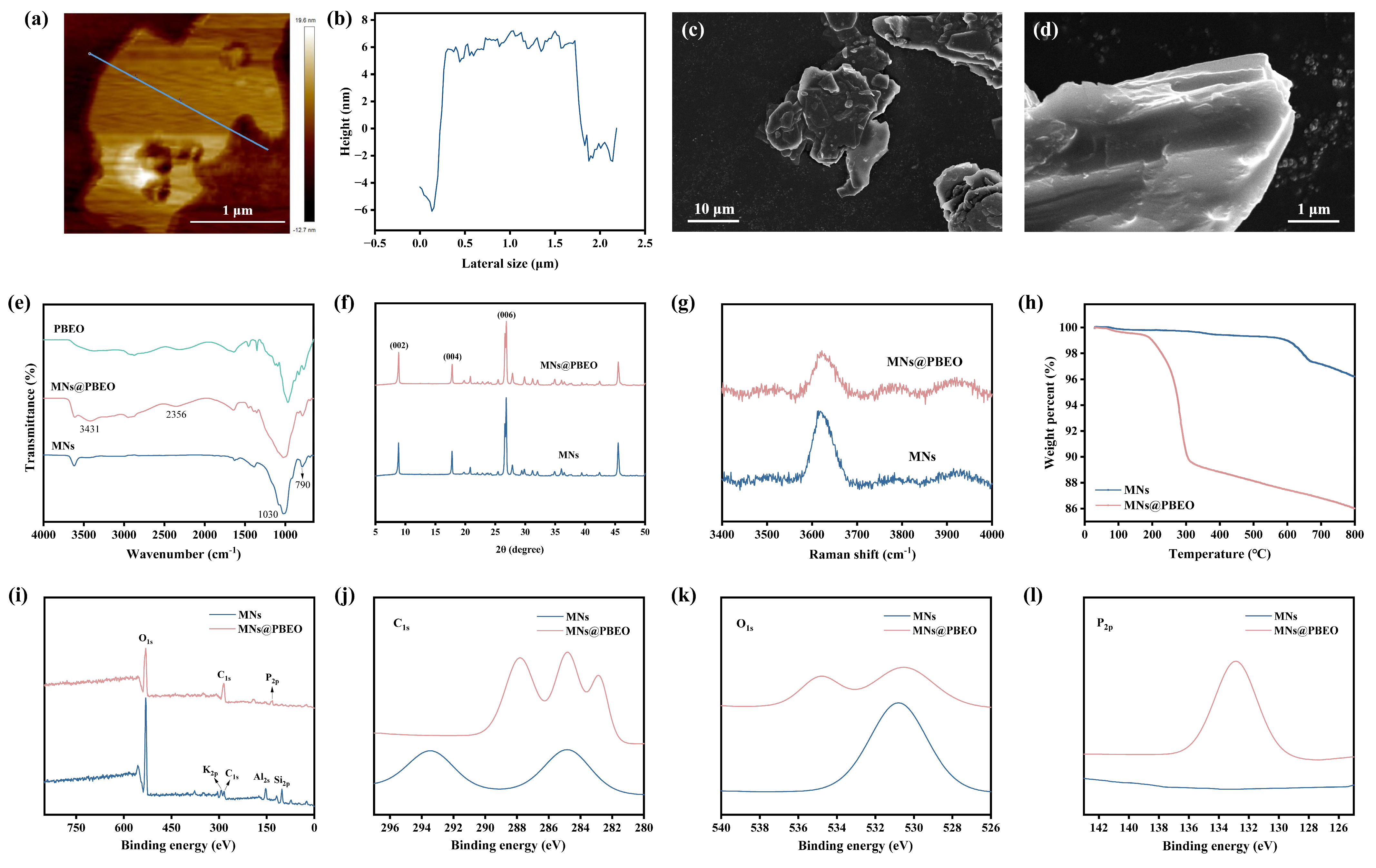
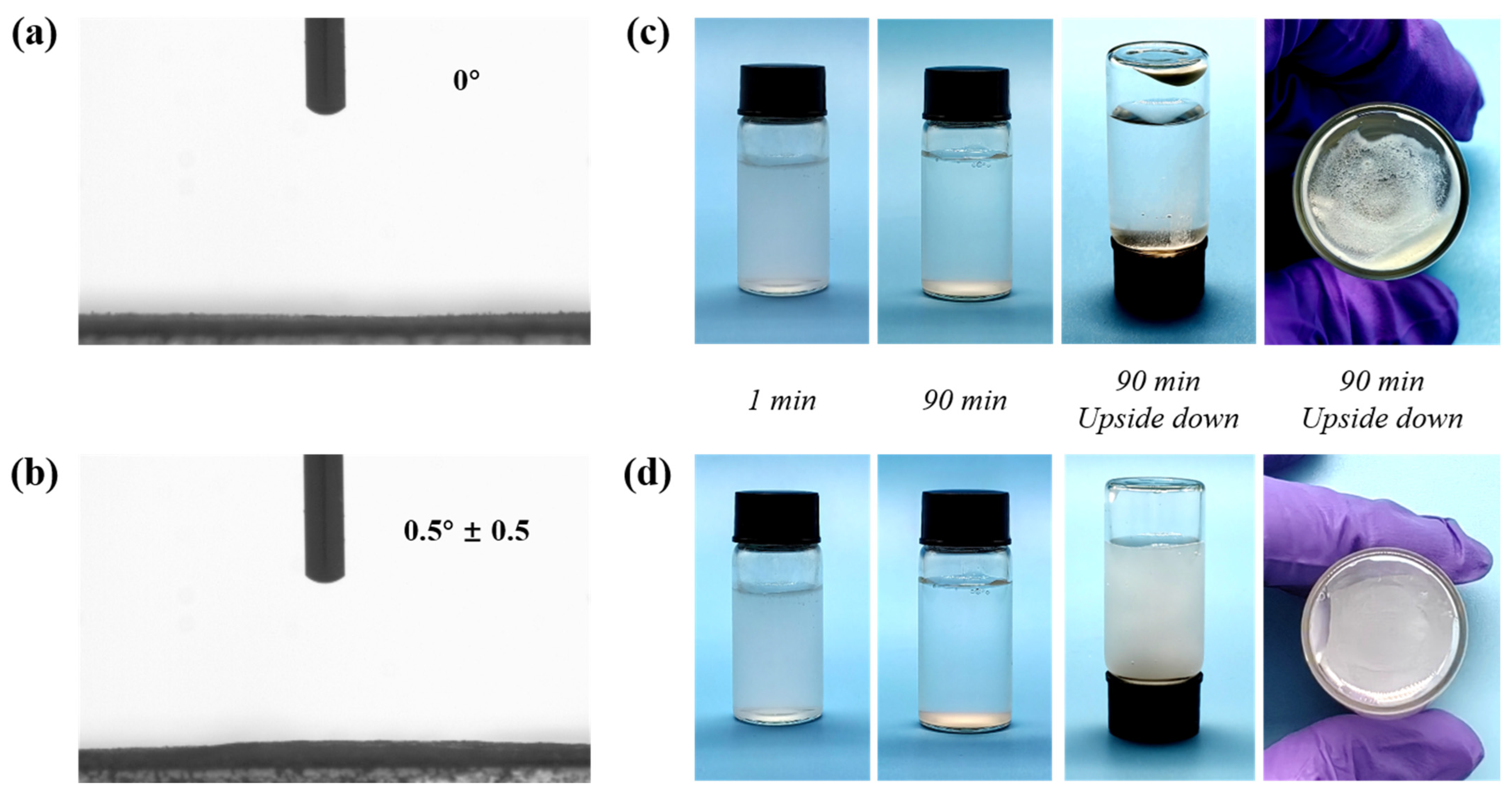
2.1. Preparation and Characterization of MN@PBEO
2.2. Morphologies of the MN@PBEO/Acrylic Coatings
2.3. Corrosion Protection Performance of MN@PBEO/Acrylic Composite Coatings
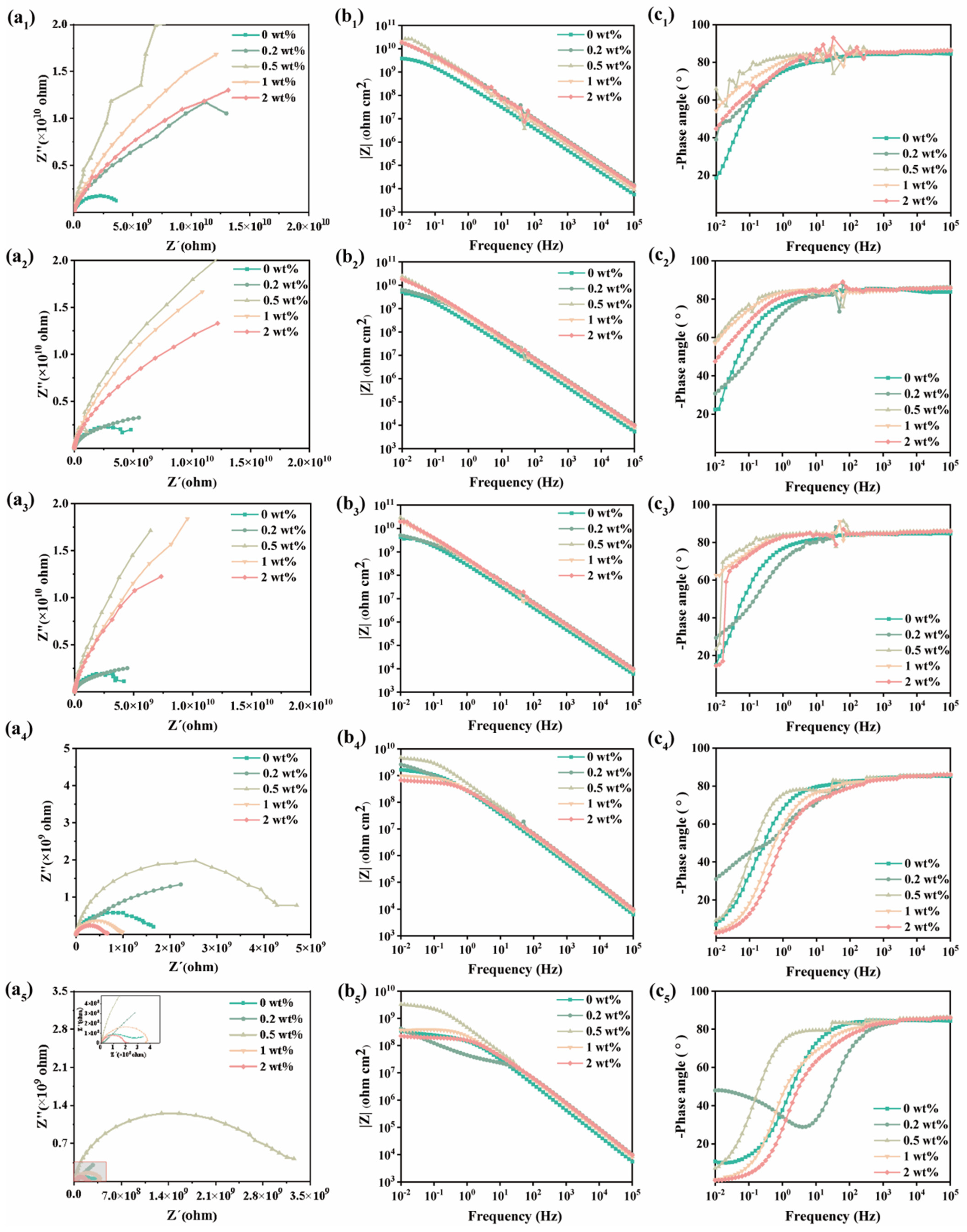
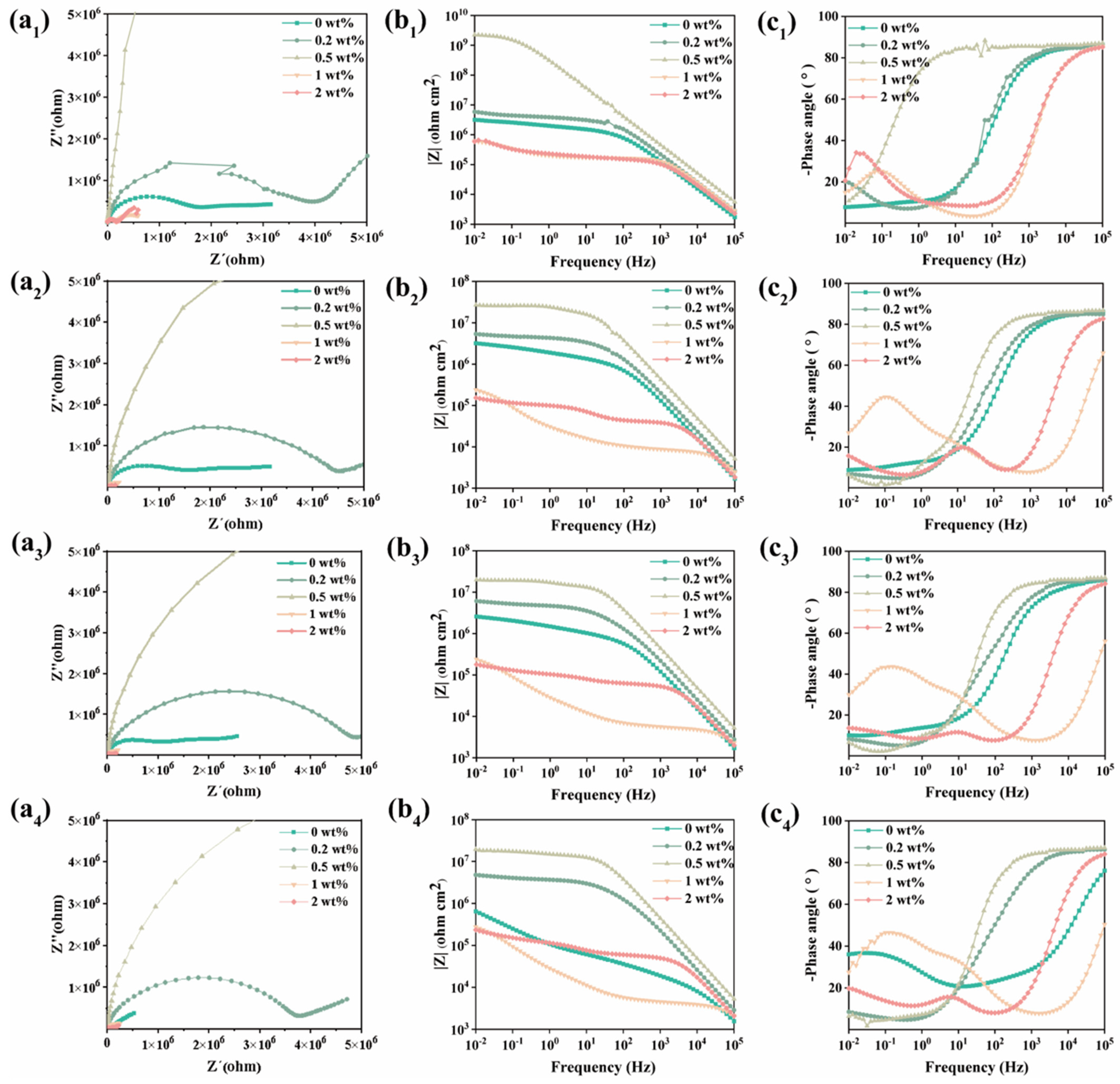
2.3.1. Electrochemical Characterization
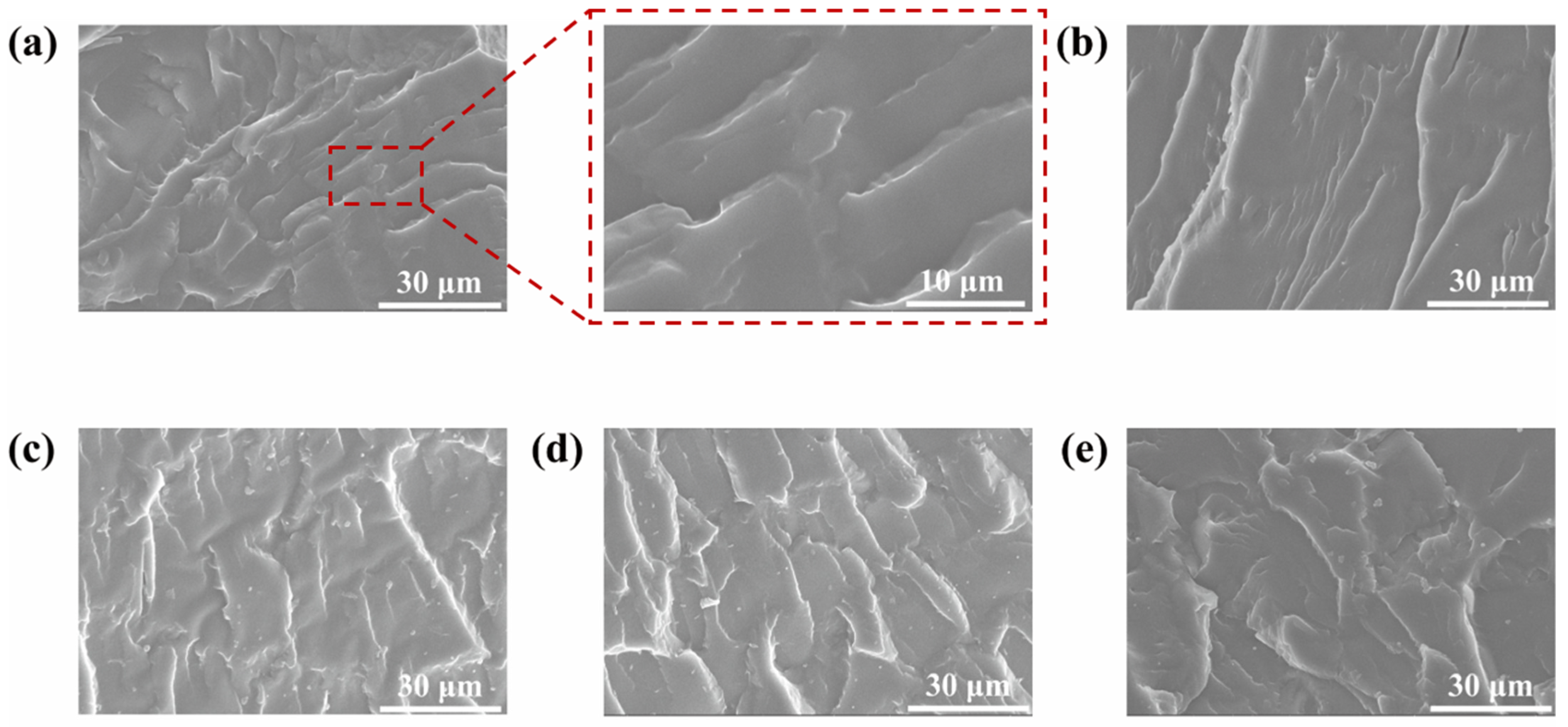
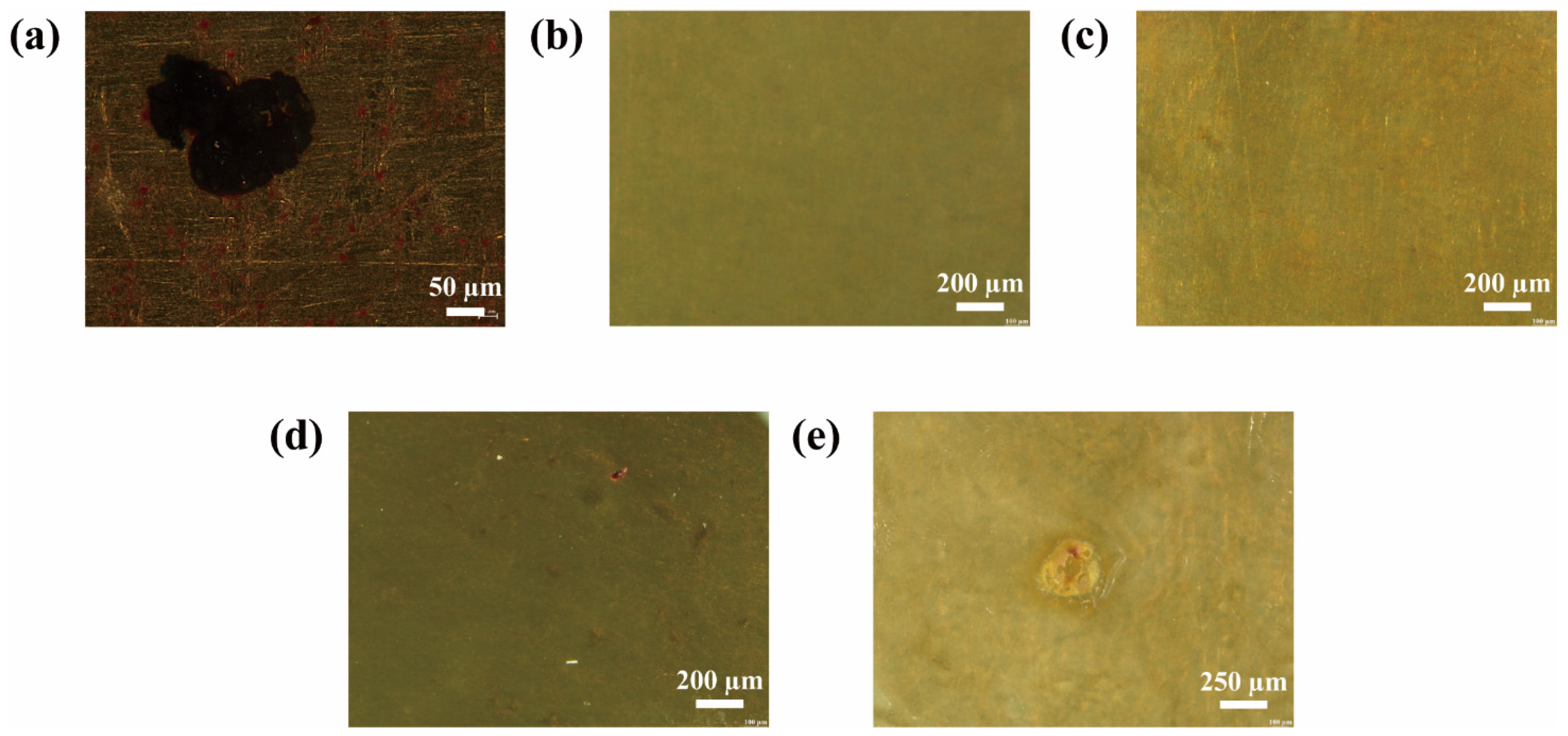
2.3.2. Morphologies of MN@PBEO/Acrylic Composite Coatings After Immersion
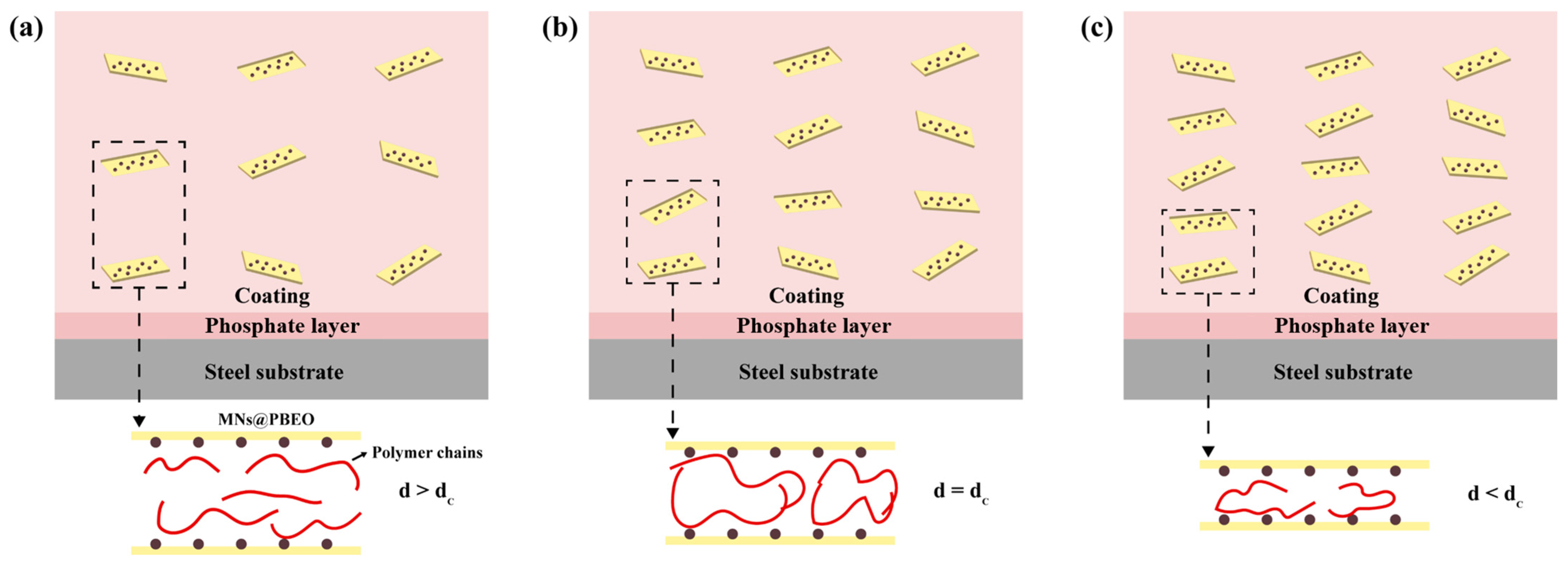
2.3.3. Corrosion Protection Mechanism of MN@PBEO/Acrylic Composite Coatings
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Harb, S.V.; Trentin, A.; de Souza, T.A.C.; Magnani, M.; Pulcinelli, S.H.; Santilli, C.V.; Hammer, P. Effective corrosion protection by eco-friendly self-healing PMMA-cerium oxide coatings. Chem. Eng. J. 2020, 383, 123219. [Google Scholar] [CrossRef]
- Ma, L.; Ren, C.; Wang, J.; Liu, T.; Yang, H.; Wang, Y.; Huang, Y.; Zhang, D. Self-reporting coatings for autonomous detection of coating damage and metal corrosion: A review. Chem. Eng. J. 2021, 421, 127854. [Google Scholar] [CrossRef]
- Glover, C.F.; Cain, T.W.; Scully, J.R. Performance of Mg-Sn surface alloys for the sacrificial cathodic protection of Mg alloy AZ31B-H24. Corros. Sci. 2019, 149, 195–206. [Google Scholar] [CrossRef]
- Ye, Z.; Tang, Z.; Meng, G.; Yuan, X.; Gu, L. Mussel-inspired preparation of dispersible mica nanosheets for waterborne epoxy coatings with reinforced anticorrosive performance. Prog. Org. Coat. 2023, 175, 107379. [Google Scholar] [CrossRef]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Yan, D.; Liu, J.; Zhang, Z.; Wang, Y.; Zhang, M.; Song, D.; Zhang, T.; Liu, J.; He, F.; Wang, J. Dual-functional graphene oxide-based nanomaterial for enhancing the passive and active corrosion protection of epoxy coating. Compos. Part B Eng. 2021, 222, 109075. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Lu, J.; Chen, Z.; Li, L.; Guo, F.; Shi, W. Tannic acid-modified g-C3N4 nanosheets/polydimethylsiloxane as a photothermal-responsive self-healing composite coating for smart corrosion protection. Chem. Eng. J. 2024, 483, 149232. [Google Scholar] [CrossRef]
- Xu, C.-A.; Li, X.; Tong, Z.; Chu, Z.; Fang, H.; Hu, Y.; Chen, X.; Yang, Z. Mimosa inspired intelligent anti-corrosive composite coating by incorporating lignin and pyridine derivatives grafted graphene oxide. Chem. Eng. J. 2024, 483, 149376. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, X.; Meng, G.; Gu, L. Mussel-inspired hydrophobic modification of boron nitride nanosheets to enhance the dispersion and anticorrosive performance in polymeric coatings. Prog. Org. Coat. 2022, 170, 106986. [Google Scholar] [CrossRef]
- Sun, J.; Tang, Z.; Meng, G.; Gu, L. Silane functionalized plasma-treated boron nitride nanosheets for anticorrosive reinforcement of waterborne epoxy coatings. Prog. Org. Coat. 2022, 167, 106831. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Yin, Q.; Xiang, X.; Fu, X.-Z.; Wang, X.-Z.; Luo, J.-L. A facile approach to fabricating graphene/waterborne epoxy coatings with dual functionalities of barrier and corrosion inhibitor. J. Mater. Sci. Technol. 2022, 112, 263–276. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Xiao, G.; Zhong, F.; Xia, Y.; Wu, Y. Graphic C3N4-assisted dispersion of graphene to improve the corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2020, 139, 105448. [Google Scholar] [CrossRef]
- Xu, W.; An, Q.; Hao, L.; Zhang, D.; Zhang, M. Synthesis and characterization of self-crosslinking fluorinated polyacrylate soap-free latices with core–shell structure. Appl. Surf. Sci. 2013, 268, 373–380. [Google Scholar] [CrossRef]
- Chen, X.; Wen, S.F.; Feng, T.; Yuan, X.; Yue, Z.F. Investigating an effective model to estimate the water diffusion coefficient of a hybrid polymer-oxide coating. Prog. Org. Coat. 2020, 141, 105548. [Google Scholar] [CrossRef]
- Zou, R.; Xiao, G.; Chen, C.; Chen, C.; Yang, Z.; Zhong, F.; Wang, M.; Lan, Y.; Shang, S. Bionic coral-like PANI-modified graphene oxide for enhancing corrosion resistance of waterborne epoxy coatings: High barrier and self-healing properties. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131714. [Google Scholar] [CrossRef]
- Liu, H.; Xu, W.; Ren, H.; Li, D.; He, J.; Xia, L.; Xu, Y.; Zeng, B.; Yuan, C.; Dai, L. Integrated comprehensive protection coating achieved by ligand engineering modulated MXene@LDH heterojunction with anti-corrosion, electromagnetic wave absorption and fire safety. Chem. Eng. J. 2024, 486, 150444. [Google Scholar] [CrossRef]
- Xiao, X.; Xiao, M.; Gu, L. In situ 3D visualization and quantitative mapping of boron nitride nanosheet dispersion in anticorrosive coatings via cluster luminescence labeling. Chem. Eng. J. 2025, 520, 166036. [Google Scholar] [CrossRef]
- Lin, D.; Wang, X.; Zhang, M.; Yuan, S.; Xu, F.; Bao, D.; Wang, H. A robust and eco-friendly waterborne anti-corrosion composite coating with multiple synergistic corrosion protections. Compos. Part B Eng. 2022, 232, 109624. [Google Scholar] [CrossRef]
- Xiao, X.; Ye, Z.; Meng, G.; Gu, L. Mussel-inspired preparation of superhydrophobic mica nanosheets for long-term anticorrosion and self-healing performance of epoxy coatings. Prog. Org. Coat. 2023, 178, 107456. [Google Scholar] [CrossRef]
- Gu, L.; Ding, J.; Yu, H. Research in graphene-based anticorrosion coatings. Prog. Chem. 2016, 28, 737–743. [Google Scholar]
- Xiao, M.; Zhang, Y.; Xiao, X.; Dai, L.; Wu, Q.Y.; Liu, S.; Gu, L. Visualizing the macroscale dispersion of graphene based sheets in epoxy anticorrosive coatings by fluorescence quenching. Chem. Eng. J. 2025, 515, 163504. [Google Scholar] [CrossRef]
- Ding, J.-H.; Zhao, H.-R.; Zheng, Y.; Zhao, X.; Yu, H.-B. A long-term anticorrsive coating through graphene passivation. Carbon 2018, 138, 197–206. [Google Scholar] [CrossRef]
- Qiu, S.; Li, W.; Zheng, W.; Zhao, H.; Wang, L. Synergistic Effect of Polypyrrole-Intercalated Graphene for Enhanced Corrosion Protection of Aqueous Coating in 3.5% NaCl Solution. ACS Appl. Mater. Interfaces 2017, 9, 34294–34304. [Google Scholar] [CrossRef] [PubMed]
- Xi, K.; Wu, H.; Zhou, C.; Qi, Z.; Yang, K.; Fu, R.K.Y.; Xiao, S.; Wu, G.; Ding, K.; Chen, G.; et al. Improved corrosion and wear resistance of micro-arc oxidation coatings on the 2024 aluminum alloy by incorporation of quasi-two-dimensional sericite microplates. Appl. Surf. Sci. 2022, 585, 152693. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, H.; Yu, H. Superior to graphene: Super-anticorrosive natural mica nanosheets. Nanoscale 2020, 12, 16253–16261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, J.; Xiao, X.; Wang, N.; Meng, G.; Gu, L. Graphene-like two-dimensional nanosheets-based anticorrosive coatings: A review. J. Mater. Sci. Technol. 2022, 129, 139–162. [Google Scholar] [CrossRef]
- Cai, Y.; Meng, F.; Liu, L.; Liu, R.; Cui, Y.; Zheng, H.; Wang, F. The Effect of the Modification of Mica by High-Temperature Mechanochemistry on the Anticorrosion Performance of Epoxy Coatings. Polymers 2021, 13, 378. [Google Scholar] [CrossRef]
- Wu, H.; Xi, K.; Huang, Y.; Zheng, Z.; Wu, Z.; Liu, R.; Zhou, C.; Xu, Y.; Du, H.; Yin, Y. Highly Orientated Sericite Nanosheets in Epoxy Coating for Excellent Corrosion Protection of AZ31B Mg Alloy. Nanomaterials 2023, 13, 2310. [Google Scholar] [CrossRef]
- Chimenti, S.; Vega, J.M.; García-Lecina, E.; Grande, H.-J.; Paulis, M.; Leiza, J.R. In-situ phosphatization and enhanced corrosion properties of films made of phosphate functionalized nanoparticles. React. Funct. Polym. 2019, 143, 104334. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Qin, S.; Xue, Q.; Zhao, H.; Wang, L. Processable poly(2-butylaniline)/hexagonal boron nitride nanohybrids for synergetic anticorrosive reinforcement of epoxy coating. Corros. Sci. 2018, 131, 187–198. [Google Scholar] [CrossRef]
- Xiao, X.; Dong, Y.; Tang, Z.; Shi, S.; Gu, L. Degradable, anticorrosive, and fluorescent waterborne polyurethanes from vegetable oil internal emulsifiers for adhesives and strain sensor. Ind. Crops Prod. 2023, 200, 116865. [Google Scholar] [CrossRef]
- Song, S.; Yan, H.; Cai, M.; Huang, Y.; Fan, X.; Zhu, M. Multilayer structural epoxy composite coating towards long-term corrosion/wear protection. Carbon 2021, 183, 42–52. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, F.; Qiang, Y.; Zhao, W. Synthesizing a novel fluorinated reduced graphene oxide-CeO2 hybrid nanofiller to achieve highly corrosion protection for waterborne epoxy coatings. Carbon 2021, 176, 39–51. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Wang, C.; Zhang, M.; Liu, P.; Wang, J. Fabrication of ZnO/epoxy resin superhydrophobic coating on AZ31 magnesium alloy. Chem. Eng. J. 2019, 368, 261–272. [Google Scholar] [CrossRef]
- Eyann, L.; Ariff, Z.M.; Shafiq, M.D.; Shuib, R.K.; Musa, M.S. Investigating the effect of methacrylic acid on the properties of waterborne epoxy-acrylate core-shell emulsion, film and coating. Prog. Org. Coat. 2024, 187, 108096. [Google Scholar] [CrossRef]
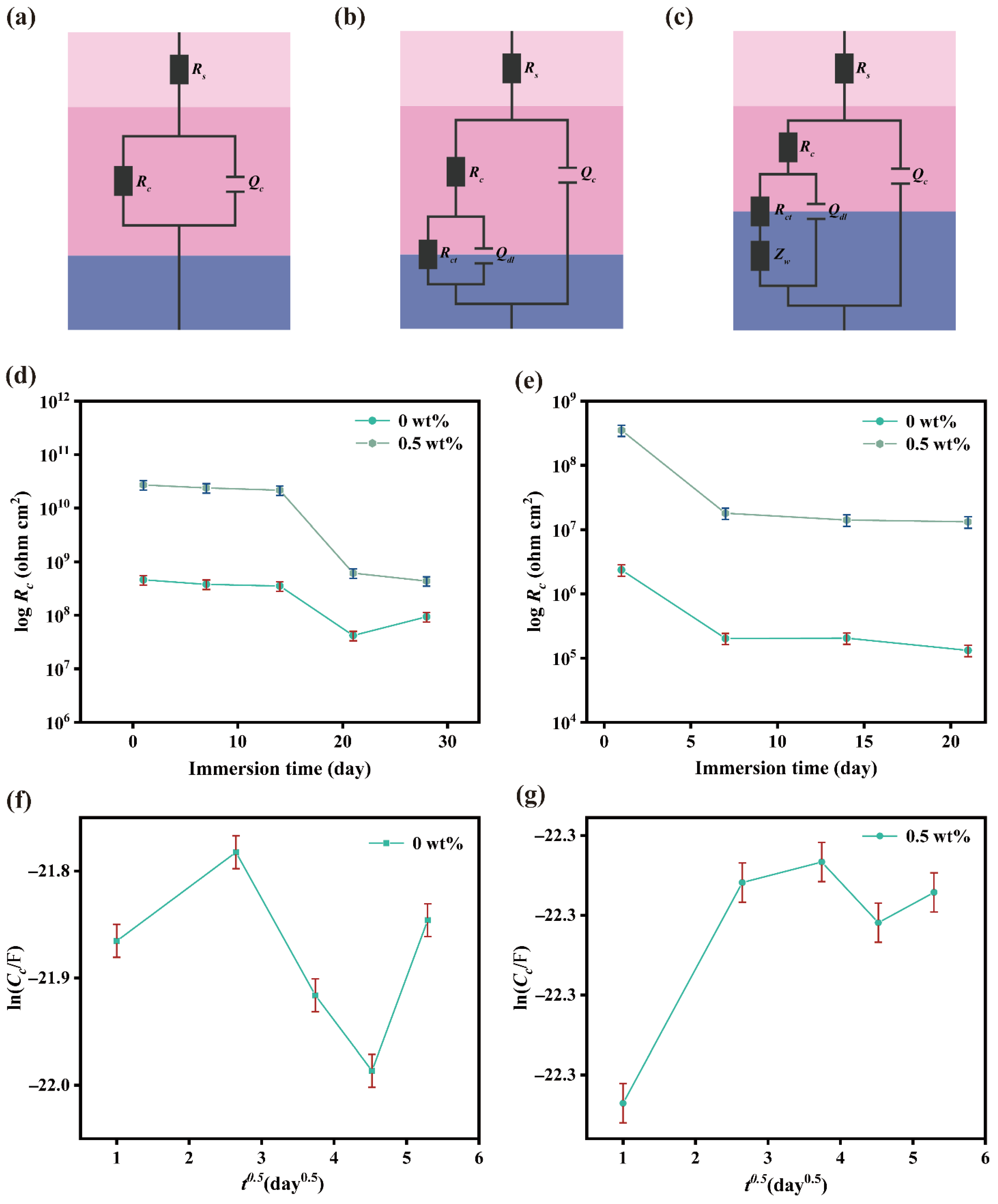

| Coating | lnC0 (F) | lnC∞ (F) | L (μm) | D (m2/s) |
|---|---|---|---|---|
| Pure acrylic | −21.87 ± 0.05 | −21.85 ± 0.03 | 123 ± 2 | (8.727 ± 0.212) ×10−13 |
| 0.5 wt% MN@PBEO/acrylic | −22.34 ± 0.07 | −22.29 ± 0.04 | 116 ± 3 | (5.787 ± 0.145) ×10−14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, R.; Tang, Z.; Xiao, M.; Cai, M.; Yuan, X.; Gu, L. Waterborne Phosphated Alkynediol-Modified Mica Nanosheet/Acrylic Nanocomposite Coatings with Superior Anticorrosive Performance. Nanomaterials 2025, 15, 1266. https://doi.org/10.3390/nano15161266
Yuan R, Tang Z, Xiao M, Cai M, Yuan X, Gu L. Waterborne Phosphated Alkynediol-Modified Mica Nanosheet/Acrylic Nanocomposite Coatings with Superior Anticorrosive Performance. Nanomaterials. 2025; 15(16):1266. https://doi.org/10.3390/nano15161266
Chicago/Turabian StyleYuan, Rui, Zhixing Tang, Mindi Xiao, Minzhao Cai, Xin Yuan, and Lin Gu. 2025. "Waterborne Phosphated Alkynediol-Modified Mica Nanosheet/Acrylic Nanocomposite Coatings with Superior Anticorrosive Performance" Nanomaterials 15, no. 16: 1266. https://doi.org/10.3390/nano15161266
APA StyleYuan, R., Tang, Z., Xiao, M., Cai, M., Yuan, X., & Gu, L. (2025). Waterborne Phosphated Alkynediol-Modified Mica Nanosheet/Acrylic Nanocomposite Coatings with Superior Anticorrosive Performance. Nanomaterials, 15(16), 1266. https://doi.org/10.3390/nano15161266







