Inspired Fluorinated BDD Film for Multifunctional Protection of Downhole Sensor Electrodes
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization
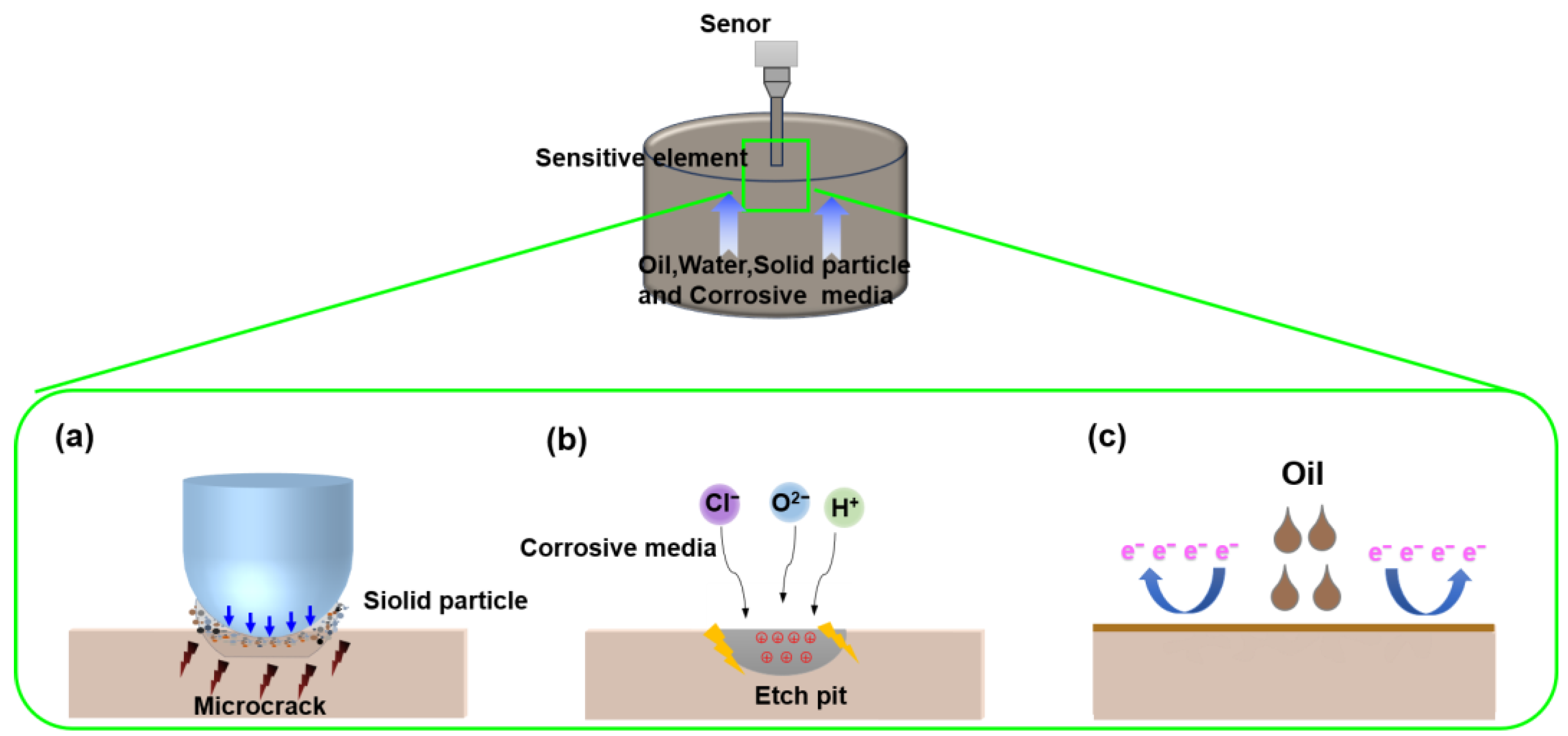
3. Fabrication of Fish Scale-Inspired FBDD Film Based on Failure Analysis of the Sensor
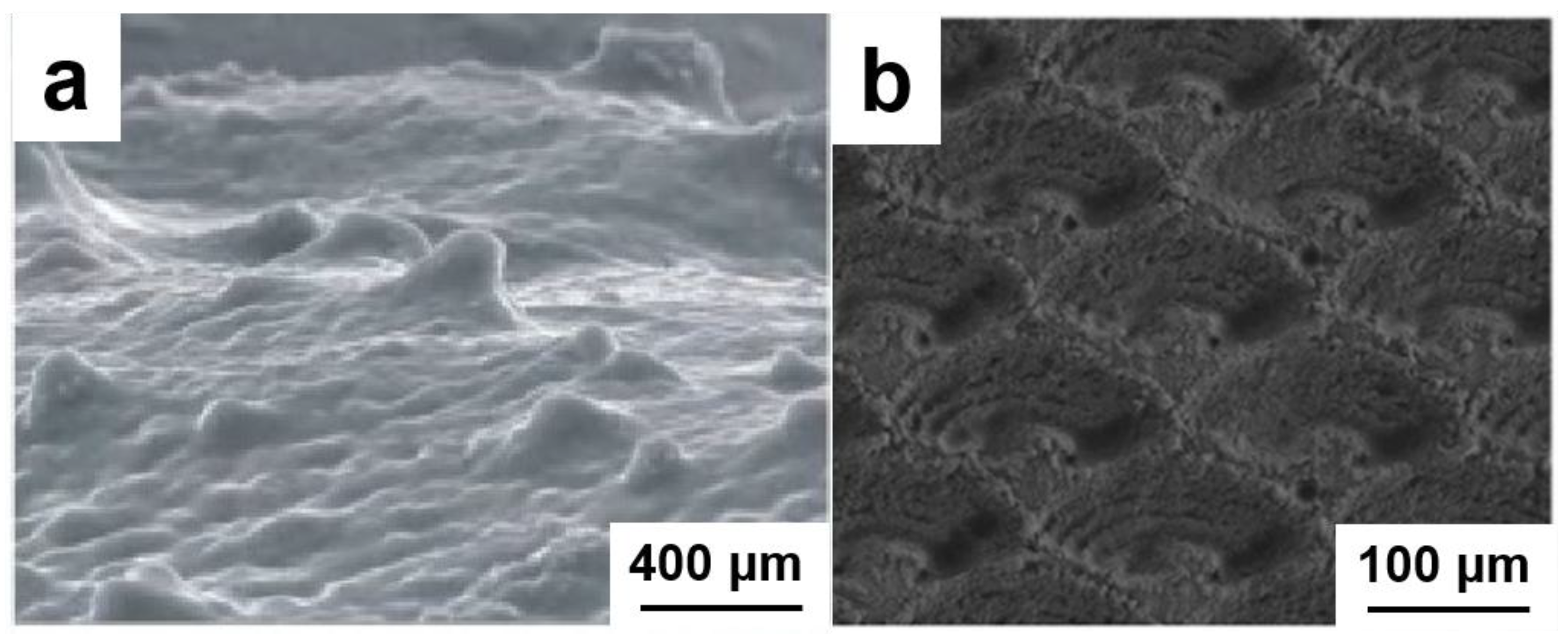
3.1. Failure Analysis of Conductivity Sensor
3.2. Biomimetic Design Principles from Fish Scale Micro–Nano Structures
3.3. Fabrication of FBDD Film for Robust Sensor Protection
4. Results and Discussion
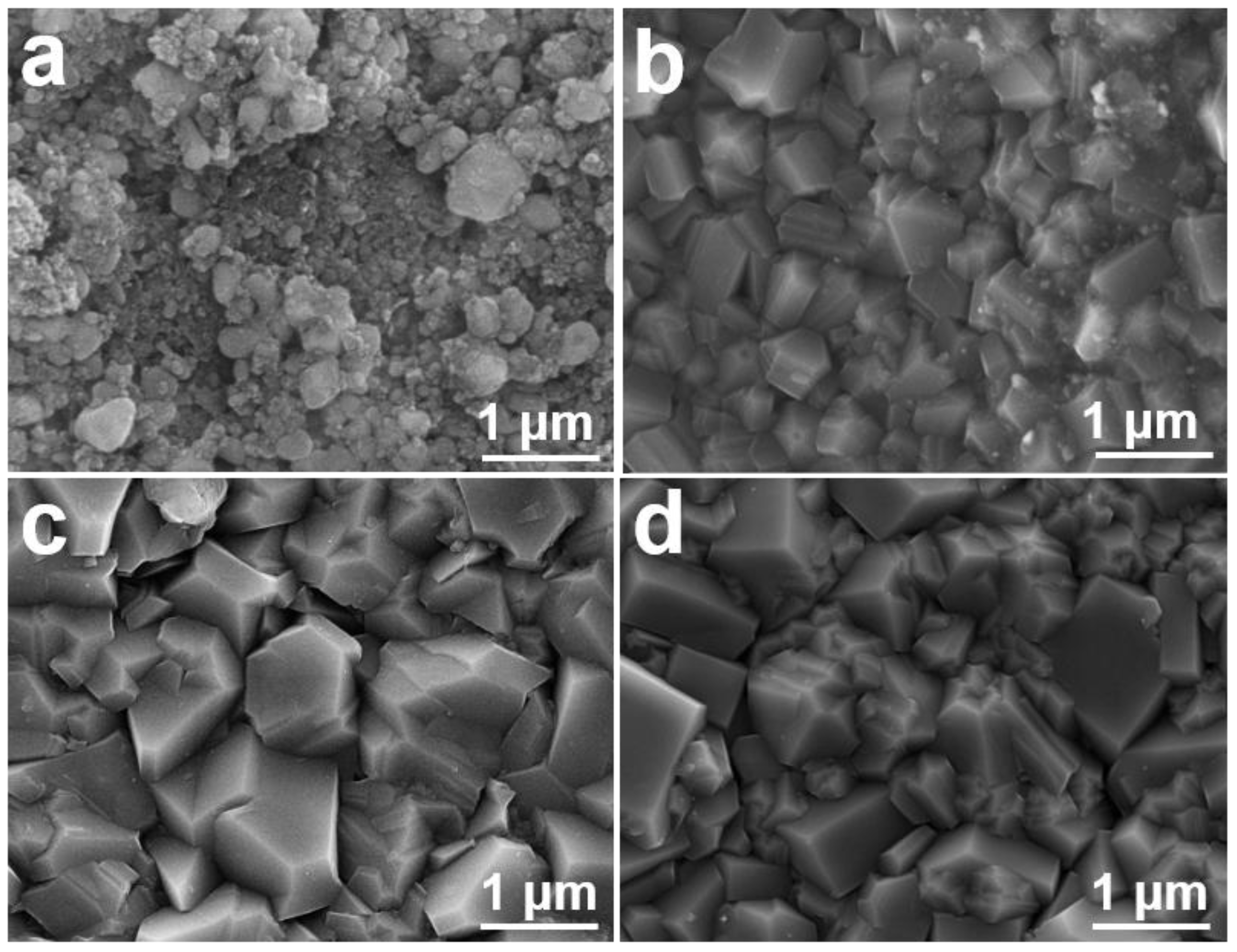
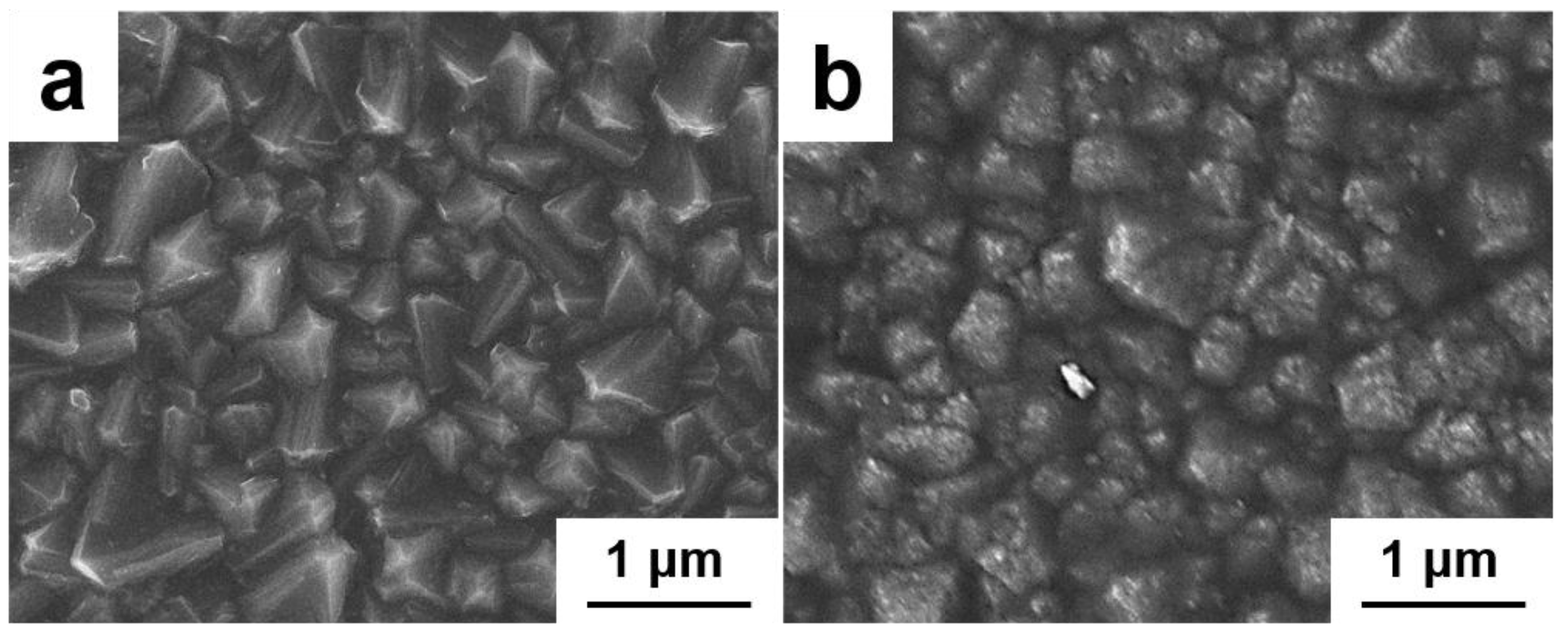
4.1. Morphology and Structural Characterization of FBDD Film
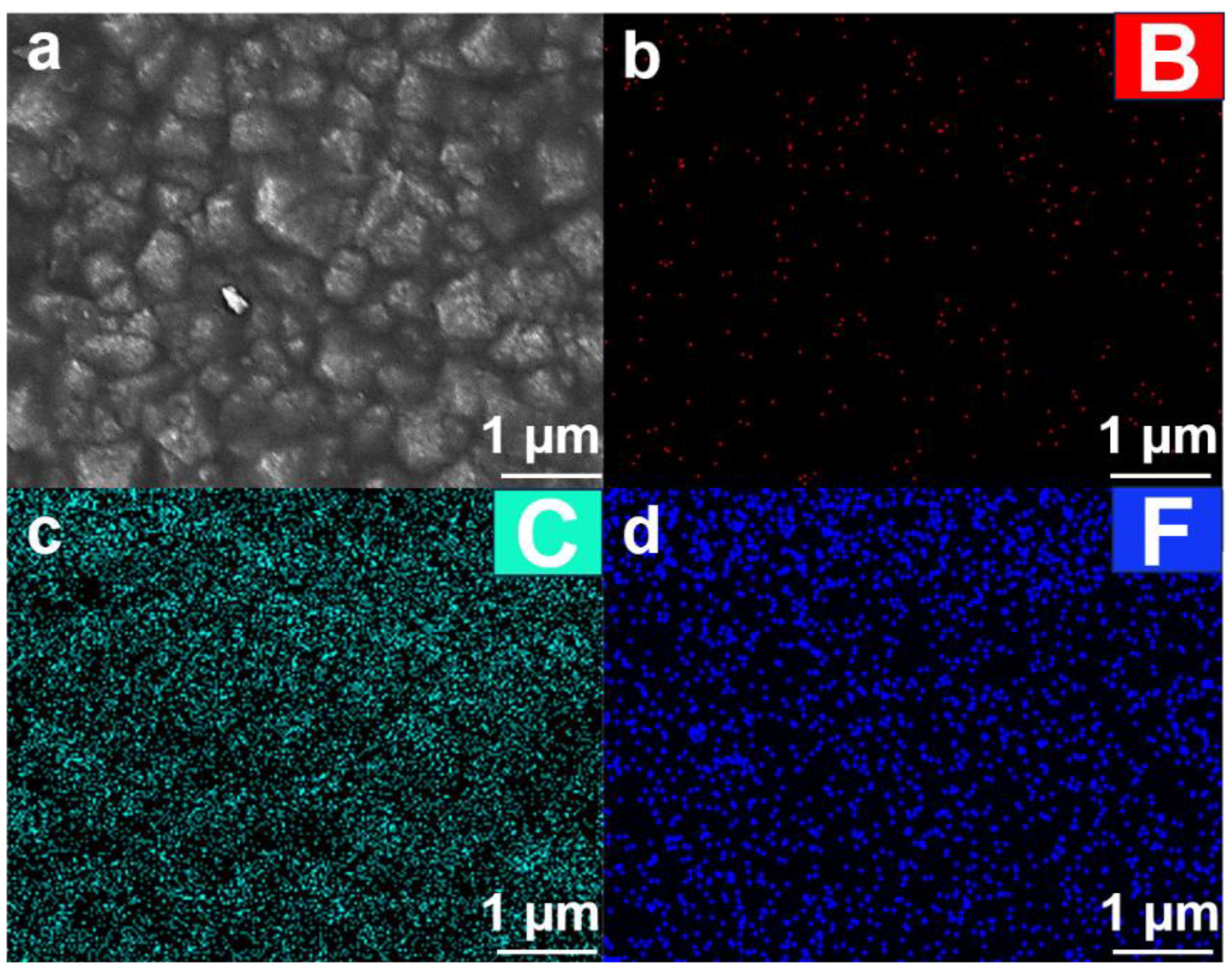
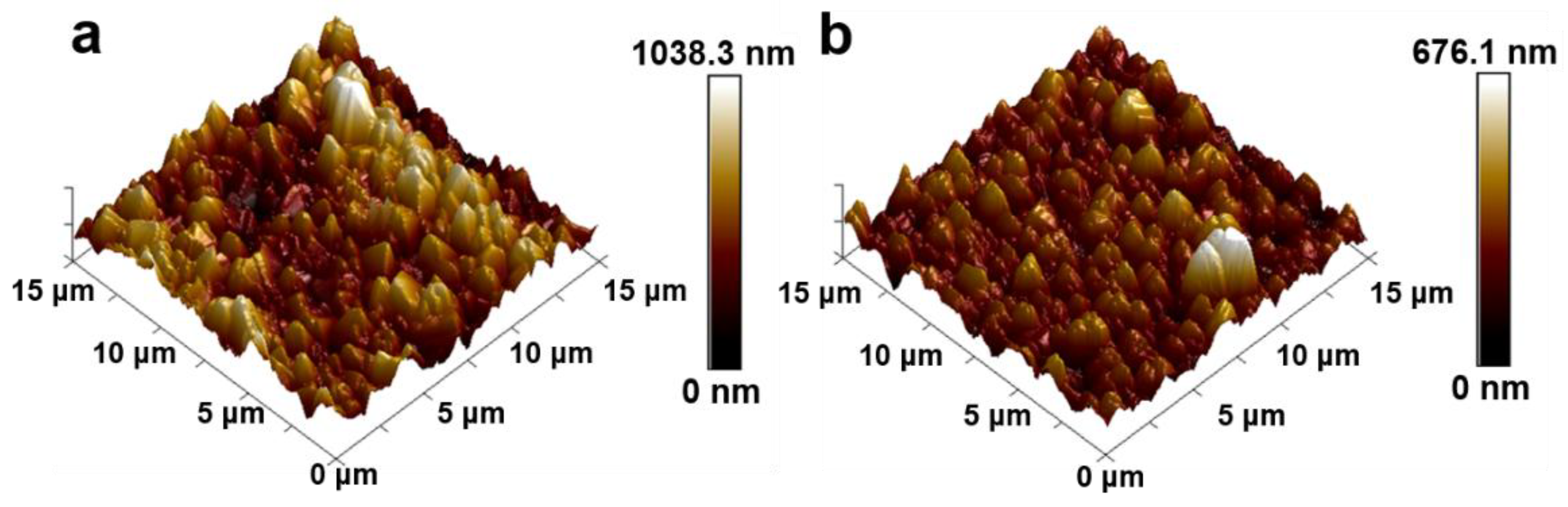
4.1.1. Microscopic Morphology
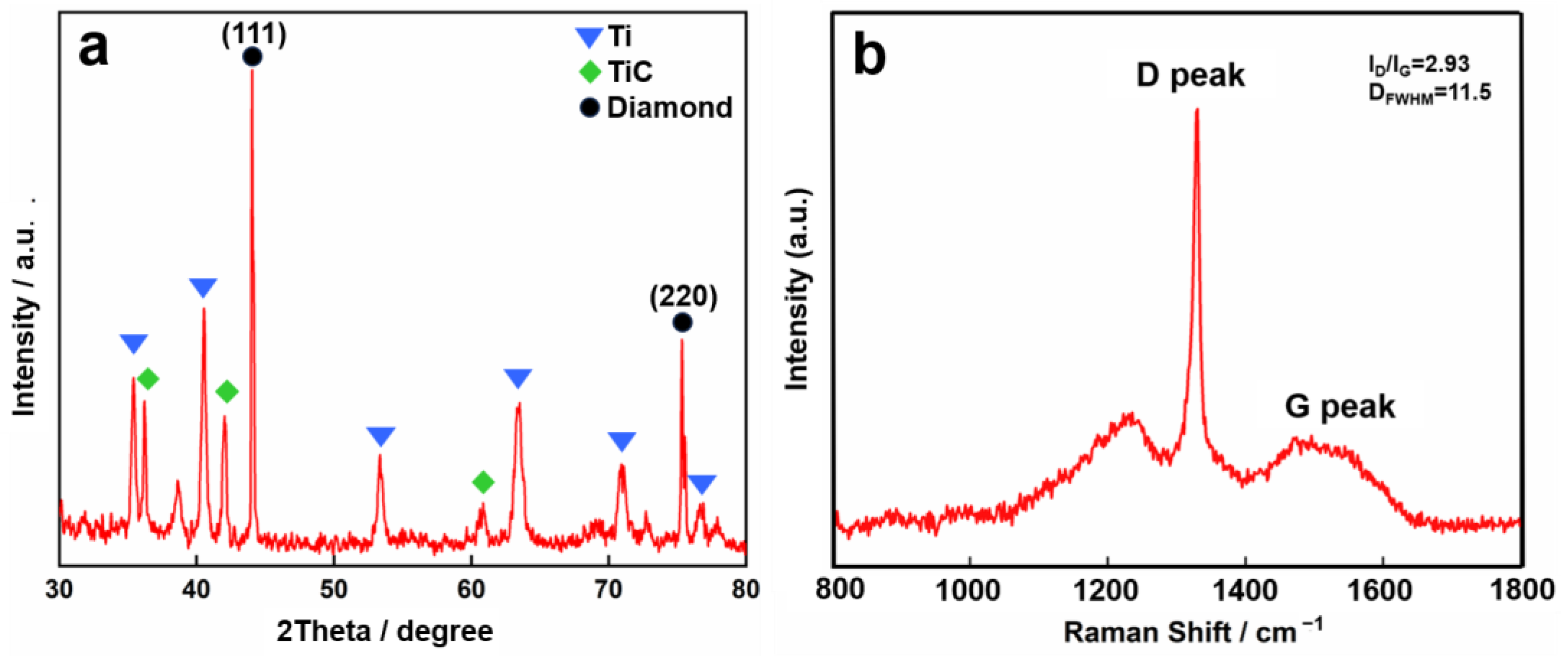
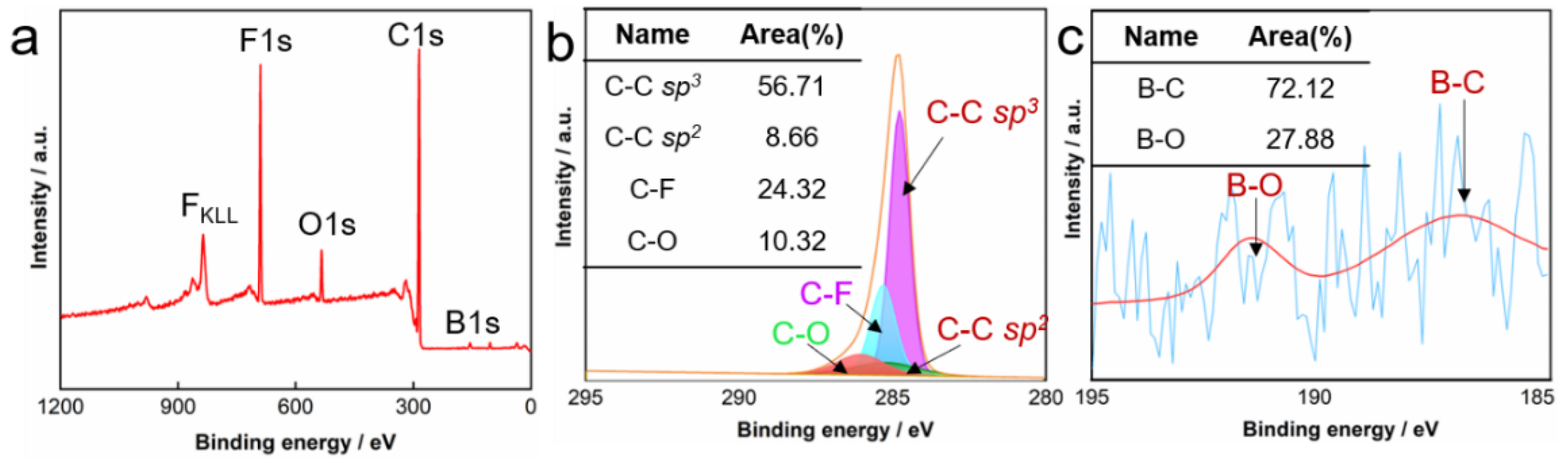
4.1.2. Structural Characterization
4.2. Performance Evaluation
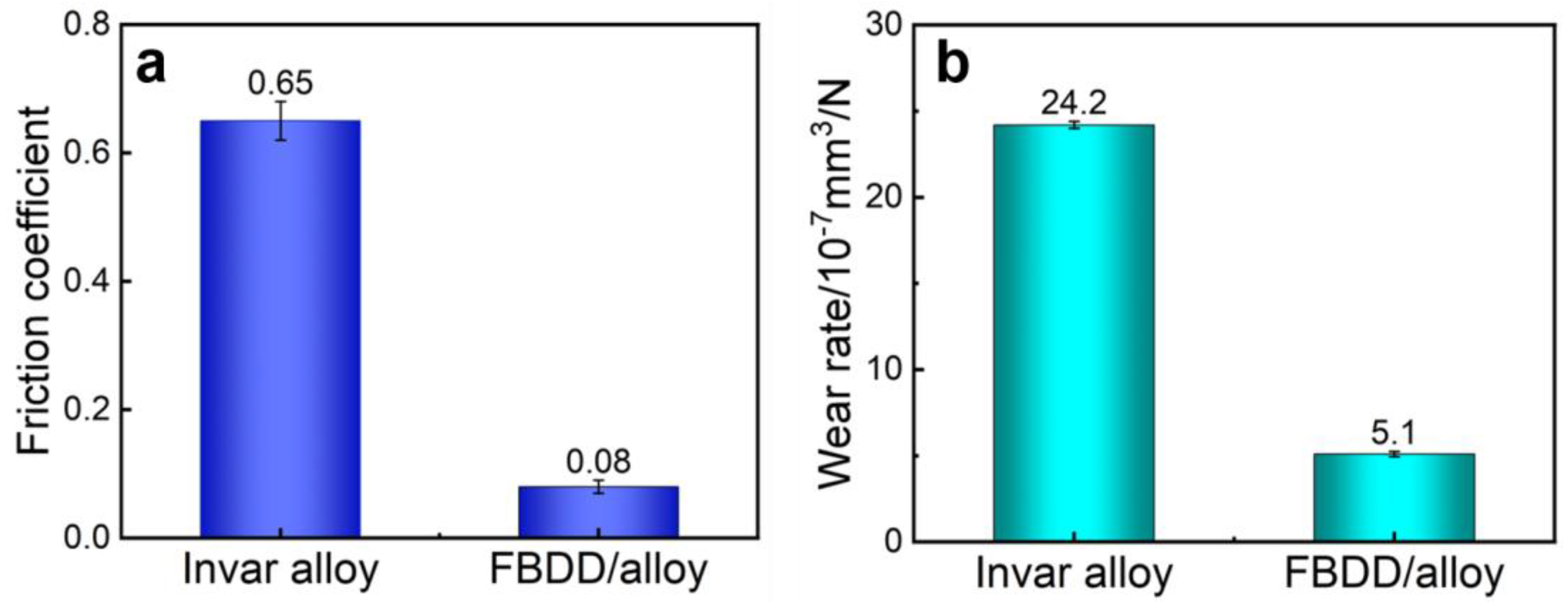
4.2.1. Wear Resistance Enhancement
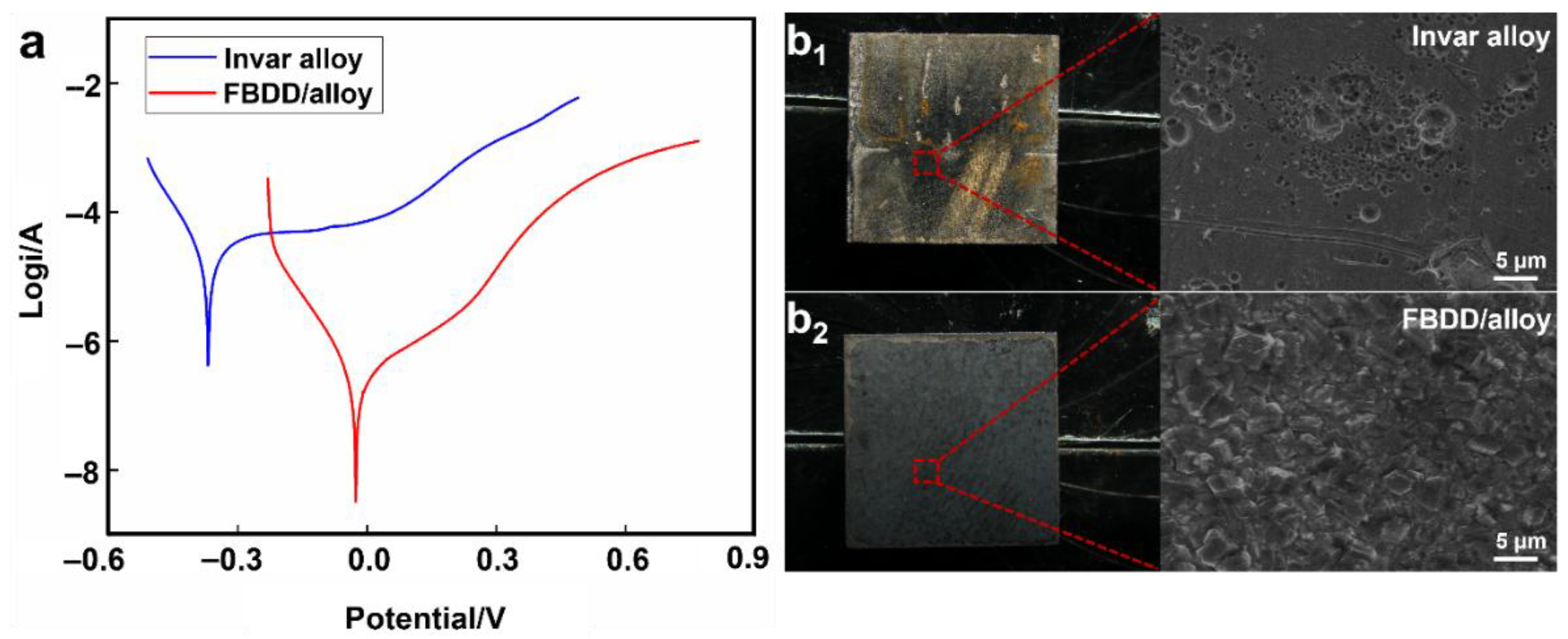
4.2.2. Electrochemical Corrosion Resistance
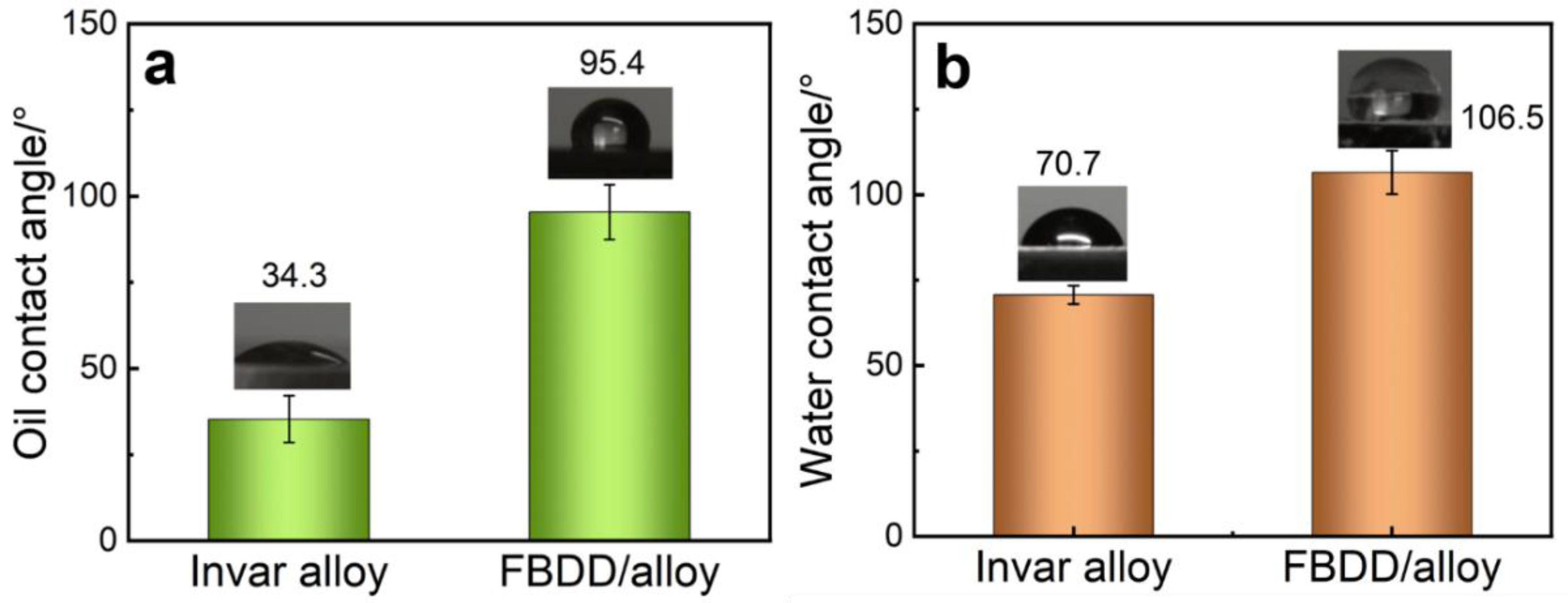
4.2.3. Surface Wettability
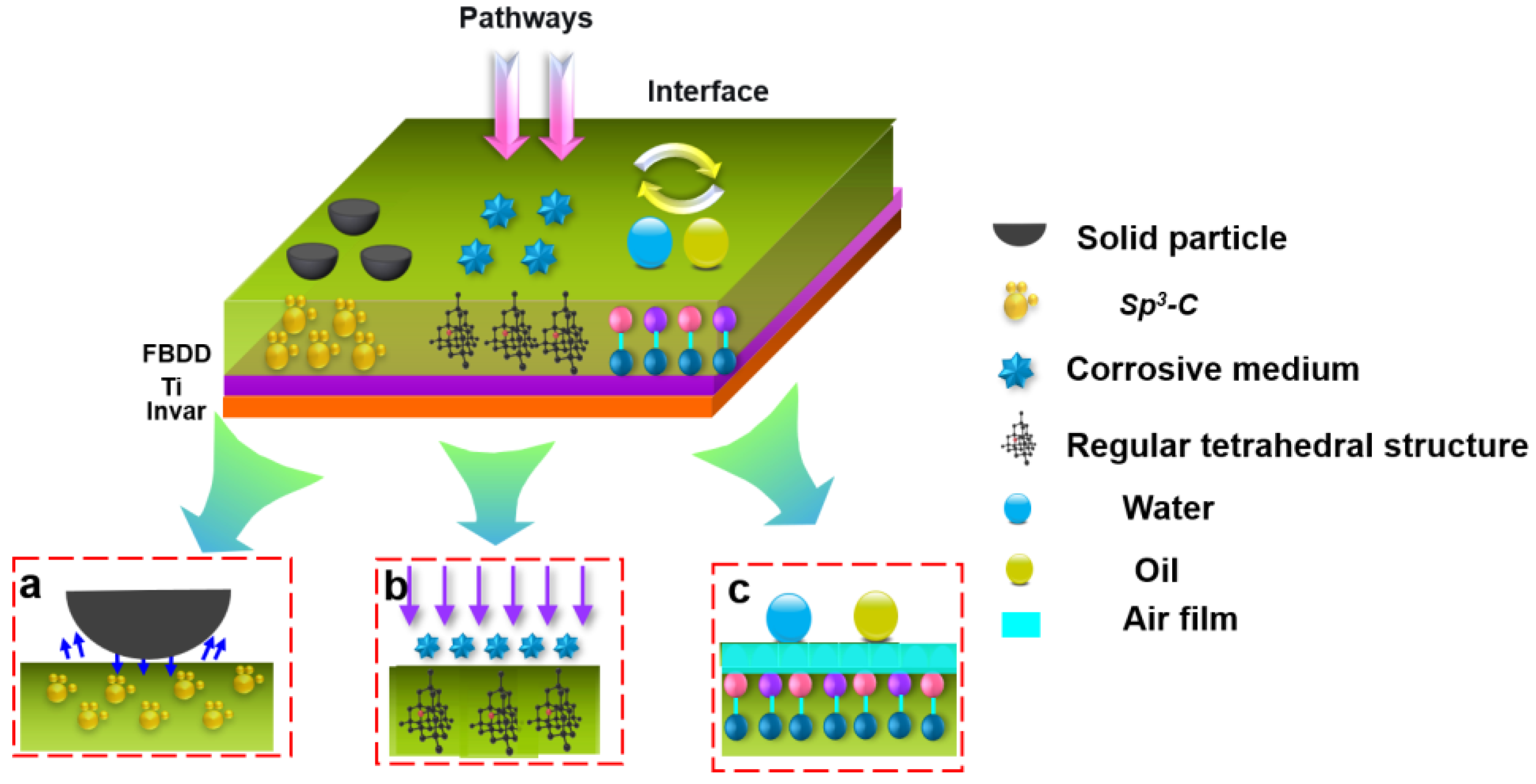
4.3. Multifunctional Protection Mechanisms of FBDD Film
5. Conclusions
- Wear Resistance: The FBDD coating reduced the friction coefficient by 88% (from 0.65 to 0.08) and lowered the wear rate by 79% (from 24.2 × 10−7 to 5.1 × 10−7 mm3/(N·mm)) compared with the bare Invar alloy. This superior tribological performance is attributed to the synergistic effect of the hard sp3-diamond matrix, the crack-deflecting role of grain boundaries, and the in situ formation of a low-shear, fluorinated carbon transfer film.
- Corrosion Resistance: The FBDD coating decreased the corrosion rate by 98.8%, achieving a rate of 3.581 × 10−3 mm/a, which is two orders of magnitude lower than that of uncoated Invar. This remarkable improvement stems from the dense, columnar microstructure of the diamond film acting as an inert physical barrier, combined with the electrochemically stable, fluorinated surface that minimizes charge transfer and the adsorption of corrosive species.
- Surface Wettability: The FBDD coating fundamentally transformed the surface wettability from oleophilic/hydrophilic (oil/water contact angles of 34.3°/70.7°) to oleophobic/hydrophobic (oil/water contact angles of 95.3°/106.5°). This shift is crucial for preventing oil fouling and aqueous corrosion, and it originates from the combination of the biomimetic micro–nano structure, which traps air pockets, and the chemical modification with low-surface energy C-F terminals.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aalsalem, M.Y.; Khan, W.Z.; Gharibi, W.; Khan, M.K.; Arshad, Q. Wireless Sensor Networks in oil and gas industry: Recent advances, taxonomy, requirements, and open challenges. J. Netw. Comput. Appl. 2018, 113, 87–97. [Google Scholar] [CrossRef]
- Teixeira, B.O.S.; Castro, W.S.; Teixeira, A.F.; Aguirre, L.A. Data-driven soft sensor of downhole pressure for a gas-lift oil well. Control Eng. Pract. 2014, 22, 34–43. [Google Scholar] [CrossRef]
- Padidar, S.; Ahmadi, V.; Ebnali-Heidari, M. Design of High Sensitive Pressure and Temperature Sensor Using Photonic Crystal Fiber for Downhole Application. IEEE Photonics J. 2012, 4, 1590–1599. [Google Scholar] [CrossRef]
- Wright, R.F.; Lu, P.; Devkota, J.; Lu, F.; Ziomek-Moroz, M.; Ohodnicki, P.R. Corrosion Sensors for Structural Health Monitoring of Oil and Natural Gas Infrastructure: A Review. Sensors 2019, 19, 3964. [Google Scholar] [CrossRef]
- Shultz, J.M.; Walsh, L.; Garfin, D.R.; Wilson, F.E.; Neria, Y. The 2010 Deepwater Horizon Oil Spill: The Trauma Signature of an Ecological Disaster. J. Behav. Health Serv. Res. 2015, 42, 58–76. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Oey, L.; Xu, F.-H.; Lu, H.-F.; Fujisaki, A. 2010 oil spill: Trajectory projections based on ensemble drifter analyses. Ocean. Dyn. 2011, 61, 829–839. [Google Scholar] [CrossRef]
- Wu, T.; Wu, H.; Du, Y.; Peng, Z. Progress and trend of sensor technology for on-line oil monitoring. Sci. China Technol. Sci. 2013, 56, 2914–2926. [Google Scholar] [CrossRef]
- Zhai, W.; Bai, L.; Zhou, R.; Fan, X.; Kang, G.; Liu, Y.; Zhou, K. Recent Progress on Wear-Resistant Materials: Designs, Properties, and Applications. Adv. Sci. 2021, 8, 2003739. [Google Scholar] [CrossRef]
- Montemor, M.F. Functional and smart coatings for corrosion protection: A review of recent advances. Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar] [CrossRef]
- Brown, P.S.; Bhushan, B. Bioinspired, roughness-induced, water and oil super-philic and super-phobic coatings prepared by adaptable layer-by-layer technique. Sci. Rep. 2015, 5, 14030. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Z.; Qiu, Z.; Feng, S.; Xie, Y.; Leng, D.; Tian, X. A brief review of bio-inspired surface technology and application toward underwater drag reduction. Ocean. Eng. 2020, 199, 106962. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Song, H.; Wang, F.; Su, X.; Zhang, D.; Xu, J. Biomimetic Superhydrophobic Surfaces: From Nature to Application. Materials 2025, 18, 2772. [Google Scholar] [CrossRef]
- Lian, Z.; Zhou, J.; Ren, W.; Chen, F.; Xu, J.; Tian, Y.; Yu, H. Recent progress in bio-inspired macrostructure array materials with special wettability—From surface engineering to functional applications. Int. J. Extreme Manuf. 2024, 6, 012008. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhou, H.; Huang, J.; Guo, Z. Review on the recent development of durable superhydrophobic materials for practical applications. Nanoscale 2021, 13, 11734–11764. [Google Scholar] [CrossRef] [PubMed]
- Waghmare, P.R.; Gunda, N.S.K.; Mitra, S.K. Under-water superoleophobicity of fish scales. Sci. Rep. 2014, 4, 7454. [Google Scholar] [CrossRef] [PubMed]
- Bhat, I.M.; Ara, T.; Lone, S. Fish-Scale-Inspired Underwater Superoleophobic Nanosurface for Efficient Oil–Water Separation and Manipulation. ACS Appl. Eng. Mater. 2024, 2, 2414–2424. [Google Scholar] [CrossRef]
- Su, B.; Tian, Y.; Jiang, L. Bioinspired Interfaces with Superwettability: From Materials to Chemistry. J. Am. Chem. Soc. 2016, 138, 1727–1748. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Shen, B.; Sun, F. Friction and wear performance of boron doped, undoped microcrystalline and fine grained composite diamond films. Chin. J. Mech. Eng. 2015, 28, 155–163. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, X.; Zhao, Z.-y.; Zhang, Z.; Li, J. Influence of pore size of Ti substrate on structural and capacitive properties of Ti/boron doped diamond electrode. J. Alloys Compd. 2019, 777, 84–93. [Google Scholar] [CrossRef]
- Cai, Y.; Li, J.; Yi, L.; Yan, X.; Li, J. Fabricating superhydrophobic and oleophobic surface with silica nanoparticles modified by silanes and environment-friendly fluorinated chemicals. Appl. Surf. Sci. 2018, 450, 102–111. [Google Scholar] [CrossRef]
- Hossain, M.S.; Ebrahimi, H.; Ghosh, R. Fish scale inspired structures—A review of materials, manufacturing and models. Bioinspir. Biomim. 2022, 17, 061001. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ji, R.; Dong, T.; Liu, S.; Zhang, F.; Zhao, L.; Ma, C.; Cai, B.; Liu, Y. Efficient fabrication and characterization of Ni-Fe-WC composite coatings with high corrosion resistance. J. Mater. Res. Technol. 2022, 16, 152–167. [Google Scholar] [CrossRef]
- Lu, X.R.; Ding, M.H.; Zhang, L.; Yang, Z.L.; Lu, Y.; Tang, W.Z. Optimizing the Microstructure and Corrosion Resistance of BDD Coating to Improve the Service Life of Ti/BDD Coated Electrode. Materials 2019, 12, 3188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, J.; Gu, Z.; Wan, X.; Jiang, L.; Wang, S. Bioinspired Multiscale Wet Adhesive Surfaces: Structures and Controlled Adhesion. Adv. Funct. Mater. 2020, 30, 1905287. [Google Scholar] [CrossRef]
- Quan, H.; Yang, W.; Lapeyriere, M.; Schaible, E.; Ritchie, R.O.; Meyers, M.A. Structure and Mechanical Adaptability of a Modern Elasmoid Fish Scale from the Common Carp. Matter 2020, 3, 842–863. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Du, J.; Ren, W.; Guo, P.; Xu, S.; Wang, J. A robust and coarse surface mesh modified by interpenetrating polymer network hydrogel for oil-water separation. J. Appl. Polym. Sci. 2015, 132, 41949. [Google Scholar] [CrossRef]
- Rong, W.; Zhang, H.; Mao, Z.; Chen, L.; Liu, X. Stable drag reduction of anisotropic superhydrophobic/hydrophilic surfaces containing bioinspired micro/nanostructured arrays by laser ablation. Colloids Surf. A 2021, 622, 126712. [Google Scholar] [CrossRef]
- Li, H.; Miao, S.; Chen, W.; Yang, X.; Li, M.; Xing, T.; Zhao, Y.; Chen, G. Durable superhydrophobic and oleophobic cotton fabric based on the grafting of fluorinated POSS through silane coupling and thiol-ene click reaction. Colloid Surf. A. 2021, 630, 127566. [Google Scholar] [CrossRef]
- Ciarsolo, I.; Fernández, X.; de Gopegui, U.R.; Zubizarreta, C.; Abad, M.D.; Mariscal, A.; Caretti, I.; Jiménez, I.; Sánchez-López, J.C. Tribological comparison of different C-based coatings in lubricated and unlubricated conditions. Surf. Coat. Technol. 2014, 257, 278–285. [Google Scholar] [CrossRef][Green Version]
- Shi, B.; Wu, Y.; Liu, Y.; Wang, L.; Gao, J.; Hei, H.; Zheng, K.; Yu, S. A review on diamond-like carbon-based films for space tribology. Mater. Sci. Technol. 2022, 38, 1151–1167. [Google Scholar] [CrossRef]
- Wang, X.; Chengchuan, W.; Sun, F. Development and growth time optimization of boron-doped micro-crystalline, undoped micro-crystalline and undoped nano-crystalline composite diamond film. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2018, 232, 1244–1258. [Google Scholar] [CrossRef]
- Schade, A.; Rosiwal, S.M.; Singer, R.F. Tribological behaviour of <100> and <111> fibre textured CVD diamond films under dry planar sliding contact. Diamond Relat. Mater. 2006, 15, 1682–1688. [Google Scholar]
- Ma, Z.; Zhang, S.; Chen, G.; Xiao, K.; Li, M.; Gao, Y.; Liang, S.; Huang, X. Superhydrophilic and oleophobic membrane functionalized with heterogeneously tailored two-dimensional layered double hydroxide nanosheets for antifouling. J. Membrane Sci. 2019, 577, 165–175. [Google Scholar] [CrossRef]
- Wang, B.; Wu, C.; Chang, H.; Yang, W. Efficient protection of Cu surfaces by polydopamine/1-bromohexadecane coating with long-term corrosion resistance. Surf. Interfaces 2025, 60, 106027. [Google Scholar] [CrossRef]
- Kovač, N.; Može, M.; Kapun, B.; Golobič, I.; Milošev, I.; Rodič, P. Enhanced corrosion resistance and self-cleaning of AlSi7Mg0.3 via superhydrophobic surface using laser structuring and stearic acid grafting. Surf. Interfaces 2025, 61, 106089. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, G.; Wang, Q.; Zhang, Q.; Zhan, X.; Chen, F. Novel Fluorinated Polymers Containing Short Perfluorobutyl Side Chains and Their Super Wetting Performance on Diverse Substrates. ACS Appl. Mater. Interfaces 2016, 8, 10513–10523. [Google Scholar] [CrossRef]
- Ramasubramanian, K.; Arunachalam, N.; Ramachandra Rao, M.S. Investigation on tribological behaviour of boron doped diamond coated cemented tungsten carbide for cutting tool applications. Surf. Coat. Technol. 2017, 332, 332–340. [Google Scholar] [CrossRef]
- Wei, J.J.; Li, C.M.; Gao, X.H.; Hei, L.F.; Lvun, F.X. The influence of boron doping level on quality and stability of diamond film on Ti substrate. Appl. Surf. Sci. 2012, 258, 6909–6913. [Google Scholar] [CrossRef]
- Cobb, S.J.; Ayres, Z.J.; Macpherson, J.V. Boron Doped Diamond: A Designer Electrode Material for the Twenty-First Century. Annu. Rev. Anal. Chem. 2018, 11, 463–484. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Zhang, Z.-q.; Zhao, Z.-y. Exploring a diamond film to improve wear resistance of the hydraulic drilling impactor. Surf. Coat. Technol. 2019, 360, 297–306. [Google Scholar] [CrossRef]
- Berman, D.; Erdemir, A.; Sumant, A.V. Approaches for Achieving Superlubricity in Two-Dimensional Materials. ACS Nano 2018, 12, 2122–2137. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Martínez-Huitle, C.A. Nature, Mechanisms and Reactivity of Electrogenerated Reactive Species at Thin-Film Boron-Doped Diamond (BDD) Electrodes During Electrochemical Wastewater Treatment. ChemElectroChem 2019, 6, 2379–2392. [Google Scholar] [CrossRef]
- Lv, W.; Yan, L.; Pang, X.; Yang, H.; Qiao, L.; Su, Y.; Gao, K. Study of the stability of α-Fe/MnS interfaces from first principles and experiment. Appl. Surf. Sci. 2020, 501, 144017. [Google Scholar] [CrossRef]
- Rani, R.; Sankaran, K.J.; Panda, K.; Kumar, N.; Ganesan, K.; Chakravarty, S.; Lin, I.N. Tribofilm formation in ultrananocrystalline diamond film. Diamond Relat. Mater. 2017, 78, 12–23. [Google Scholar] [CrossRef]
- Lee, S.-J.; Segu, D.Z.; Kim, C.-L. Synergistic effects of hierarchical micro/nanostructures and PDMS/lubricant composites for superior tribological and wetting performance on aluminum. RSC Adv. 2024, 14, 37062–37073. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.L.C.; Fricano, L.; Brown-Tseng, E.; Tan, K.T. Coating Performance Assessment on AA 6111-T4 via Standard Accelerated Corrosion Tests. In Proceedings of the NACE CORROSION 2019, Nashville, TN, USA, 24–28 March 2019; pp. 1–10. [Google Scholar]
- Muthuramalingam, M.; Villemin, L.S.; Bruecker, C. Streak formation in flow over biomimetic fish scale arrays. J. Exp. Biol. 2019, 222, jeb205963. [Google Scholar] [CrossRef]
- Oviedo, C.; Salamanca-Flores, M.; Fernández-Pérez, A. Optical and electrical properties of cadmium sulphide thin films coated with fungal-derived core/shell silver nanoparticles. Surf. Interfaces 2025, 62, 106166. [Google Scholar] [CrossRef]
- de Leon, A.C.C.; da Silva, Í.G.M.; Pangilinan, K.D.; Chen, Q.; Caldona, E.B.; Advincula, R.C. High performance polymers for oil and gas applications. React. Funct. Polym. 2021, 162, 104878. [Google Scholar] [CrossRef]
- Yence, M.; Cetinkaya, A.; Ozcelikay, G.; Kaya, S.I.; Ozkan, S.A. Boron-Doped Diamond Electrodes: Recent Developments and Advances in View of Electrochemical Drug Sensors. Crit. Rev. Anal. Chem. 2022, 52, 1122–1138. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Wang, X.; Liu, X.; Li, H.; Ren, P.; Sun, C. Tribological Properties and Corrosion Resistance of Multilayer a-C:H:Ti Films at Different Target Currents. Metals 2023, 13, 1274. [Google Scholar] [CrossRef]
- Hussain, S.; Sayem, S.M.; Basurrah, A.; Rashed, T.; Watanabe, F.; Siraj, N.; Karabacak, T. Glancing Angle Deposited Nanostructured Tellurium Layer Against Dendrite Formation and Side Reactions in Aqueous Zn-Ion Battery Anode. Nanomaterials 2025, 15, 952. [Google Scholar] [CrossRef]
- Kredel, J.; Schmitt, D. Cross-Linking Strategies for Fluorine-Containing Polymer Coatings for Durable Resistant Water- and Oil-Repellency. Polymers 2021, 13, 723. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, X. Special oleophobic and hydrophilic surfaces: Approaches, mechanisms, and applications. J. Mater. Chem. A 2017, 5, 3759–3773. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zhao, S.; Wang, J.; Liu, J.; Yu, X.; Zhang, J. Inspired Fluorinated BDD Film for Multifunctional Protection of Downhole Sensor Electrodes. Nanomaterials 2025, 15, 1647. https://doi.org/10.3390/nano15211647
Liu J, Zhao S, Wang J, Liu J, Yu X, Zhang J. Inspired Fluorinated BDD Film for Multifunctional Protection of Downhole Sensor Electrodes. Nanomaterials. 2025; 15(21):1647. https://doi.org/10.3390/nano15211647
Chicago/Turabian StyleLiu, Jiahao, Shuo Zhao, Jincan Wang, Jiaxi Liu, Xiang Yu, and Jing Zhang. 2025. "Inspired Fluorinated BDD Film for Multifunctional Protection of Downhole Sensor Electrodes" Nanomaterials 15, no. 21: 1647. https://doi.org/10.3390/nano15211647
APA StyleLiu, J., Zhao, S., Wang, J., Liu, J., Yu, X., & Zhang, J. (2025). Inspired Fluorinated BDD Film for Multifunctional Protection of Downhole Sensor Electrodes. Nanomaterials, 15(21), 1647. https://doi.org/10.3390/nano15211647





