Advancements in Tumor-Targeted Nanoparticles: Design Strategies and Multifunctional Therapeutic Approaches
Abstract
1. Introduction
2. Methodology
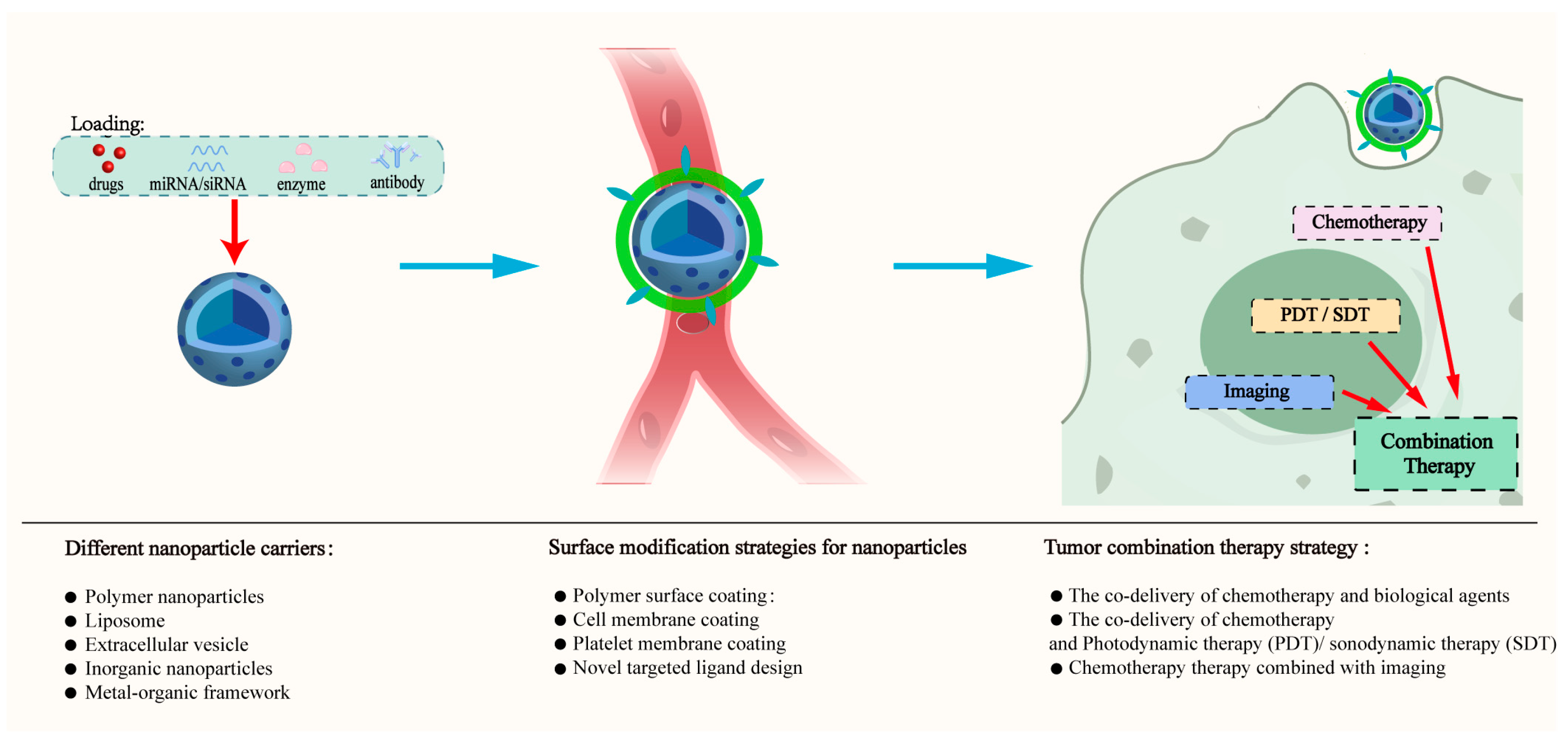
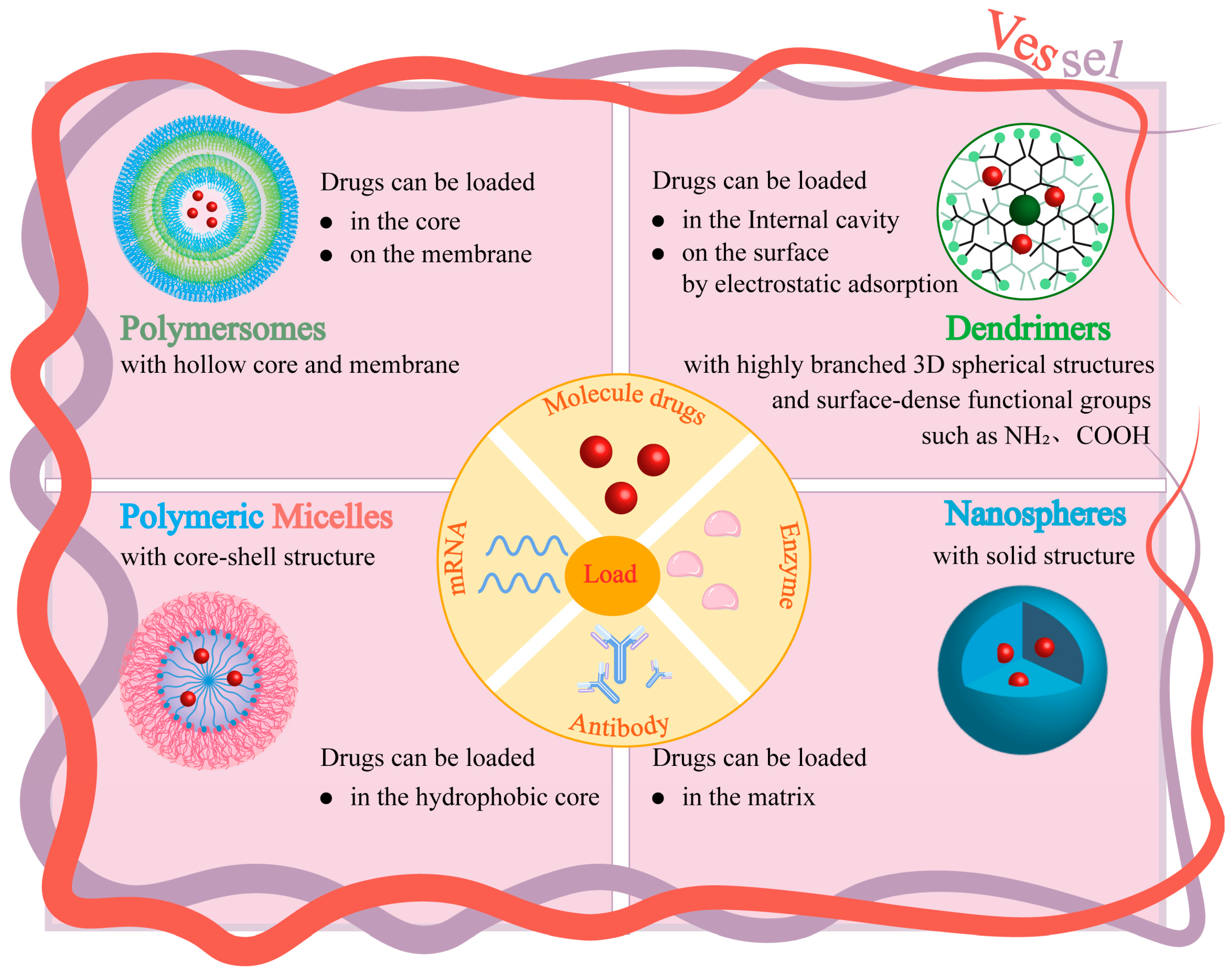
3. Different Nanoparticle Carriers
3.1. Polymer Nanoparticles
3.1.1. Natural Polymer Nanoparticles
3.1.2. Synthetic Polymer Nanoparticles
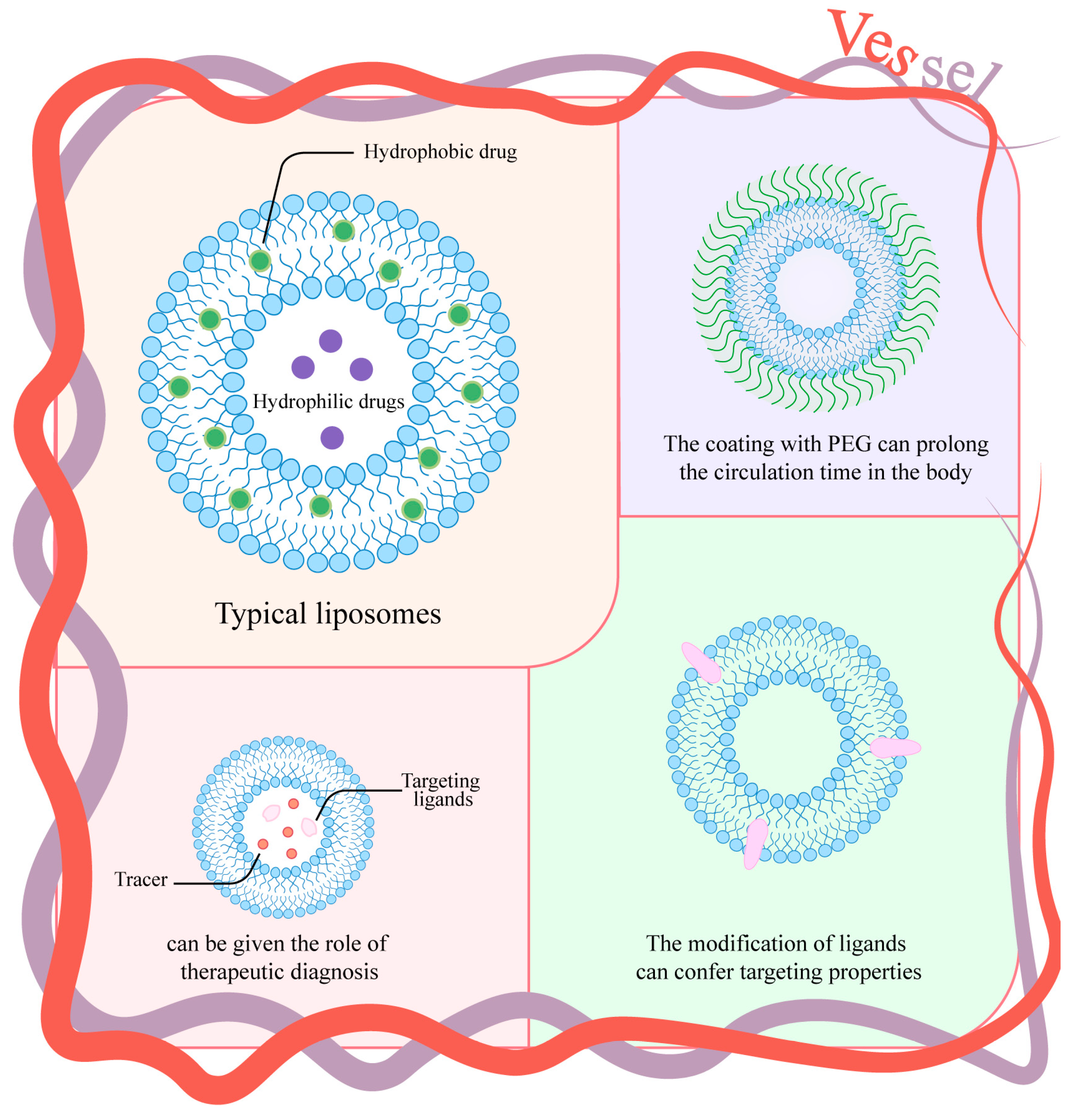
3.2. Liposomes
3.3. Extracellular Vesicles
3.4. Inorganic Nanoparticles
3.5. Metal–Organic Frameworks
4. Surface Modification Strategies for Nanoparticles
4.1. Polymer Surface Coating
4.2. Cell Membrane Coating
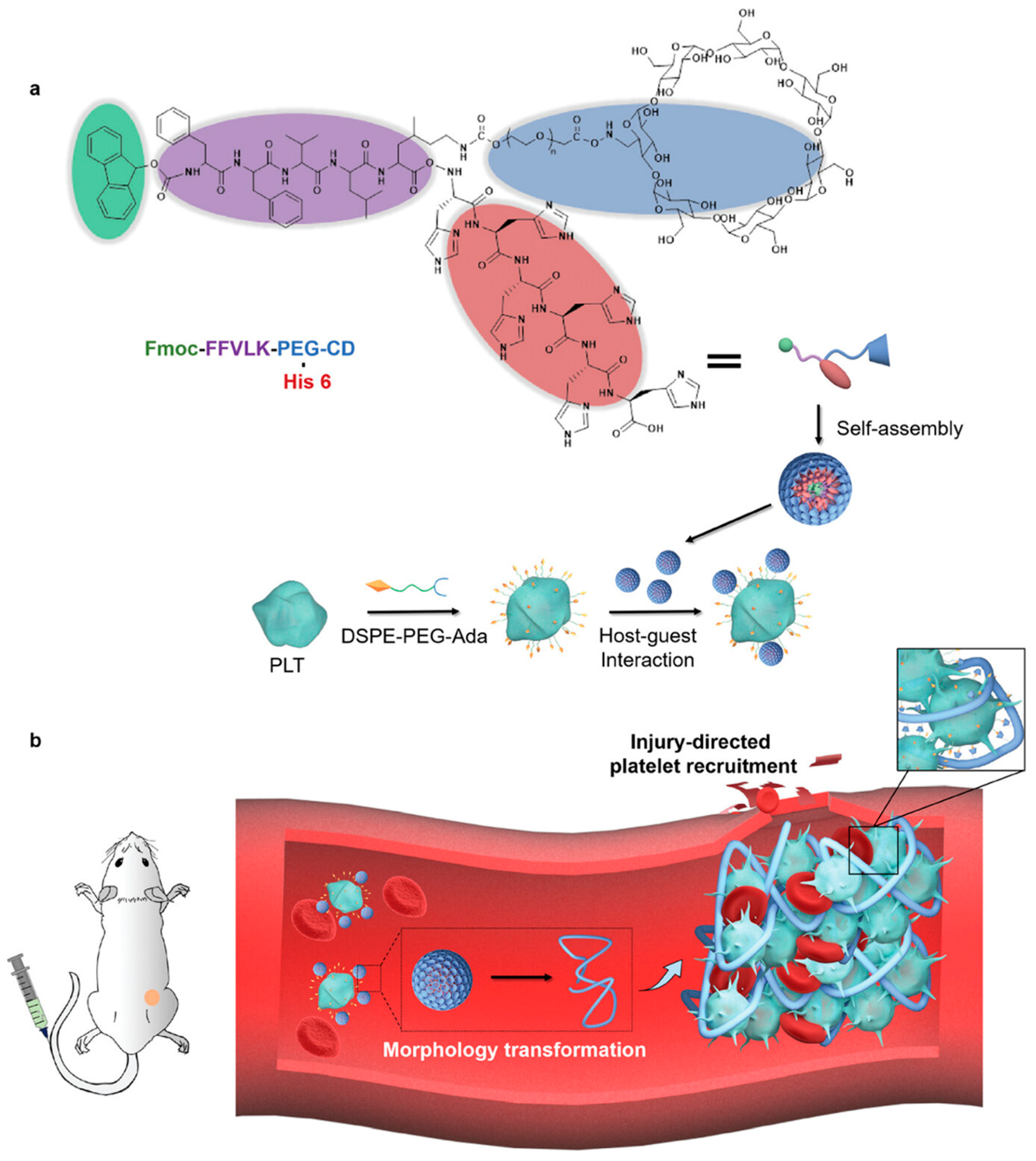
4.3. Platelet Membrane Coating
4.4. Targeted Ligand Coating
5. Combination Therapy Strategy Based on Multifunctional Nanoparticles
5.1. The Co-Delivery of Chemotherapy Agents and Biological Agents
5.2. The Co-Delivery of Chemotherapy Agents and Photosensitizers/Sonosensitizers
5.3. Chemotherapy Drugs and Imaging in Combination
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, B.; Chen, Y.; Shi, J. Nanocatalytic Medicine. Adv. Mater. 2019, 31, e1901778. [Google Scholar] [CrossRef]
- Chang, M.; Dong, C.; Huang, H.; Ding, L.; Feng, W.; Chen, Y. Nanobiomimetic Medicine. Adv. Funct. Mater. 2022, 32, 2204791. [Google Scholar] [CrossRef]
- Ren, Y.; Li, P.; Xie, Y.; Xu, J.; Luo, Q.; Chen, M.; Liu, R.; Feng, H.; Chen, Y.; Liu, Y.; et al. Dual-responsive nanoparticles for enhanced drug delivery in breast Cancer chemotherapy. J. Control. Release 2024, 377, 146–161. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Zeng, X.; Guo, W.; Jin, Y.; Wang, S.; Tian, R.; Han, Y.; Guo, L.; Han, J.; et al. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm. Sin. B 2019, 9, 167–176. [Google Scholar] [CrossRef]
- Chen, Q.; Chang, Y.; He, X.; Ding, Y.; Wang, R.; Luo, R.; Yuan, J.; Chen, J.; Zhong, G.; Yang, H.; et al. Targeted Delivery of mRNA with Polymer–Lipid Nanoparticles for In Vivo Base Editing. ACS Nano 2025, 19, 7835–7850. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Choi, J.; Hwang, J.; Kim, S.; Kim, Y.; Shim, M.K.; Park, W.; Yu, S.; Jung, S.; Yang, Y.; et al. Apolipoprotein Fusion Enables Spontaneous Functionalization of mRNA Lipid Nanoparticles with Antibody for Targeted Cancer Therapy. ACS Nano 2025, 19, 6412–6425. [Google Scholar] [CrossRef]
- Li, P.; Zhang, J.; Shao, T.; Jiang, J.; Tang, X.; Yang, J.; Li, J.; Fang, B.; Huang, Z.; Fang, H.; et al. NIR-II Photosensitizer-Based Nanoparticles Defunctionalizing Mitochondria to Overcome Tumor Self-Defense by Promoting Heat Shock Protein 40. ACS Nano 2025, 19, 15751–15766. [Google Scholar] [CrossRef] [PubMed]
- Abalos, R.N.; Aziz, I.A.; Caverzan, M.; Lochedino, A.S.; Ibarra, L.E.; Gallastegui, A.; Chesta, C.A.; Gomez, M.L.; Mecerreyes, D.; Palacios, R.E.; et al. Poly(3-hexylthiophene) nanoparticles as visible-light photoinitiators and photosensitizers in 3D printable acrylic hydrogels for photodynamic therapies. Mater. Horizons 2025, 12, 2524–2534. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, X.; Pan, L.; Tang, M.; Song, P.; Long, B.; Sun, X.; Li, Y.; Zhang, H.; Wang, S.; et al. Single-Atom W Anchored Hexagonal Boron Nitride Piezoelectric Nanosonosensitizer Remodels Tumor Microenvironment for Enhanced Sonodynamic Therapy. Adv. Funct. Mater. 2025, 35, 2420974. [Google Scholar] [CrossRef]
- Liu, R.-G.; Zhao, R.-R.; Yu, Z.-W.; Liu, F.-J.; Liu, C.-Z.; Wu, X. Metal-organic coordinated self-assembled nanomedicine for enhanced cuproptosis-mediated chemo-photo-chemodynamic synergistic therapy of non-small cell lung cancer. Chem. Eng. J. 2025, 509, 161305. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, N.; Li, R.; Foiret, J.; Ferrara, K.W.; Yue, X.; Dai, Z. Multimodal imaging-guided sonodynamic therapy for orthotopic liver cancer using a functionalized sonosensitizer. Nano Today 2025, 61, 102618. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, Q.; Zhou, Y.; Yan, S.; Li, J.; Yang, C.; Xu, J.; Li, C.; Zhang, C.; Sun, Y. Acid-Responsive Nanoregulators Elicit Hydrogen Sulfide-Mediated Tumor Oxygenation and Selective Sonosensitization for Hypoxic Tumors. Adv. Funct. Mater. 2025, 35, 2419386. [Google Scholar] [CrossRef]
- Venkateswaran, S.V.; Kreuzaler, P.; Maclachlan, C.; McMahon, G.; Greenidge, G.; Collinson, L.; Bunch, J.; Yuneva, M. A multimodal imaging pipeline to decipher cell-specific metabolic functions and tissue microenvironment dynamics. Nat. Protoc. 2025, 20, 1678–1699. [Google Scholar] [CrossRef]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Shakeri, S.; Ashrafizadeh, M.; Zarrabi, A.; Roghanian, R.; Afshar, E.G.; Pardakhty, A.; Mohammadinejad, R.; Kumar, A.; Thakur, V.K. Multifunctional Polymeric Nanoplatforms for Brain Diseases Diagnosis, Therapy and Theranostics. Biomedicines 2020, 8, 13. [Google Scholar] [CrossRef]
- Song, X.; Chen, Y.; Zhao, G.; Sun, H.; Che, H.; Leng, X. Effect of molecular weight of chitosan and its oligosaccharides on antitumor activities of chitosan-selenium nanoparticles. Carbohydr. Polym. 2020, 231, 115689. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Faujdar, E.; Singh, R.K.; Paul, S.; Kukrety, A.; Chhibber, V.K.; Ray, S.S. High CO2 absorption of O-carboxymethylchitosan synthesised from chitosan. Environ. Chem. Lett. 2018, 16, 1025–1031. [Google Scholar] [CrossRef]
- Yu, F.; Zheng, M.; Zhang, A.Y.; Han, Z. A cerium oxide loaded glycol chitosan nano-system for the treatment of dry eye disease. J. Control. Release 2019, 315, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Dually functional hollow ceria nanoparticle platform for intraocular drug delivery: A push beyond the limits of static and dynamic ocular barriers toward glaucoma therapy. Biomaterials 2020, 243, 119961. [Google Scholar] [CrossRef]
- Selvasudha, N.; Koumaravelou, K. The multifunctional synergistic effect of chitosan on simvastatin loaded nanoparticulate drug delivery system. Carbohydr. Polym. 2017, 163, 70–80. [Google Scholar] [CrossRef]
- Dai, Z.; Yin, W.; Li, J.; Ma, L.; Chen, F.; Shen, Q.; Hu, X.; Xue, Y.; Ji, J. Zein and Trimethyl Chitosan-Based Core–Shell Nanoparticles for Quercetin Oral Delivery to Enhance Absorption by Paracellular Pathway in Obesity Mice. Biomater. Res. 2025, 29, 0193. [Google Scholar] [CrossRef]
- Castro, F.; Pinto, M.L.; Leite Pereira, C.; Serre, K.; Costa, Â.M.; Cavadas, B.; Barbosa, M.A.; Vermaelen, K.; León, S.; Serrano, D.; et al. Chitosan/γ-PGA Nanoparticles and IFN-γ Immu-notherapy: A Dual Approach for Triple-Negative Breast Cancer Treatment. J. Control. Release 2025, 379, 621–635. [Google Scholar] [CrossRef]
- Castro, F.; Pinto, M.L.; Silva, A.M.; Pereira, C.L.; Teixeira, G.Q.; Gomez-Lazaro, M.; Santos, S.G.; Barbosa, M.A.; Gonçalves, R.M.; Oliveira, M.J. Pro-inflammatory chitosan/poly(γ-glutamic acid) nanoparticles modulate human antigen-presenting cells phenotype and revert their pro-invasive capacity. Acta Biomater. 2017, 63, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Liu, L.; Huang, C.; Zhou, D.; Fu, L. Characterization and toxicology evaluation of chitosan nanoparticles on the embryonic development of zebrafish, Danio rerio. Carbohydr. Polym. 2016, 141, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Yhee, J.Y.; Jeon, S.; Shim, M.K.; Yoon, H.Y.; Lee, S.; Kim, K. In vivo toxicity evaluation of tumor targeted glycol chitosan nanoparticles in healthy mice: Repeated high-dose of glycol chitosan nanoparticles potentially induce cardiotoxicity. J. Nanobiotechnol. 2023, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Wang, S.; Yan, X.; Zhou, H.; Zhan, J.; Liu, S.; Sharma, V.; Jiang, G.; Zhu, H.; Yan, B. Regulation of Cell Uptake and Cytotoxicity by Nanoparticle Core under the Controlled Shape, Size, and Surface Chemistries. ACS Nano 2019, 14, 289–302. [Google Scholar] [CrossRef]
- Bar-Ilan, O.; Albrecht, R.M.; Fako, V.E.; Furgeson, D.Y. Toxicity Assessments of Multisized Gold and Silver Nanoparticles in Zebrafish Embryos. Small 2009, 5, 1897–1910. [Google Scholar] [CrossRef]
- Talamini, L.; Violatto, M.B.; Cai, Q.; Monopoli, M.P.; Kantner, K.; Krpetić, Ž.; Perez-Potti, A.; Cookman, J.; Garry, D.; Silveira, C.P.; et al. Influence of Size and Shape on the Anatomical Distribution of Endotoxin-Free Gold Nanoparticles. ACS Nano 2017, 11, 5519–5529. [Google Scholar] [CrossRef]
- di Masi, A.; Trezza, V.; Leboffe, L.; Ascenzi, P. Human Plasma Lipocalins and Serum Albumin: Plasma Alternative Carriers? J. Control. Release 2016, 228, 191–205. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Y.; Duan, H.; Hou, J.; He, S. A new NCL-targeting aptamer-drug conjugate as a promising therapy against esophageal cancer. J. Nanobiotechnol. 2025, 23, 52. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Park, S.; Wu, S.; Guo, Y.; Nam, K.T.; Kwon, N.; Yoon, J.; Hu, Q. Photodynamic and Photothermal therapy via human serum albumin delivery. Coord. Chem. Rev. 2024, 520, 216142. [Google Scholar] [CrossRef]
- Jang, H.J.; Song, M.G.; Park, C.R.; Youn, H.; Lee, Y.-S.; Cheon, G.J.; Kang, K.W. Imaging of Indocyanine Green-Human Serum Albumin (ICG-HSA) Complex in Secreted Protein Acidic and Rich in Cysteine (SPARC)-Expressing Glioblastoma. Int. J. Mol. Sci. 2023, 24, 850. [Google Scholar] [CrossRef]
- He, S.; Zhao, C.; Tao, H.; Sheng, W.; Gao, R.; Liu, X.; Zhen, Y. A recombinant scFv antibody-based fusion protein that targets EGFR associated with IMPDH2 downregulation and its drug conjugate show therapeutic efficacy against esophageal cancer. Drug Deliv. 2022, 29, 1243–1256. [Google Scholar] [CrossRef]
- Xiong, G.; Schätzlein, A.G.; Uchegbu, I.F. Acetyl-lysine human serum albumin nanoparticles activate CD44 receptors, with preferential uptake by cancer stem cells, leading to tumor eradication. J. Control. Release 2025, 382, 113632. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xu, X.; Li, A.; Liu, H.; Zhu, J.; Fei, H. GSH-activable and cytolytic iPep-coupled immune nanoagonist for cancer synergetic therapy. Biomaterials 2025, 322, 123402. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Gigmes, D.; Trimaille, T. Advances in amphiphilic polylactide/vinyl polymer based nano-assemblies for drug delivery. Adv. Colloid Interface Sci. 2021, 294, 102483. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, Y.; He, X.; Yang, F.; Han, R.; Yang, C.; Li, W.; Qian, Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020, 5, 542–552. [Google Scholar] [CrossRef]
- Shang, H.; Xia, D.; Geng, R.; Wu, J.; Deng, W.; Tong, Y.; Ba, X.; Zhong, Z.; He, Y.; Huang, Q.; et al. Thermosensitive Resiquimod-Loaded Lipid Nanoparticles Promote the Polarization of Tumor-Associated Macrophages to Enhance Bladder Cancer Immunotherapy. ACS Nano 2025, 19, 19599–19621. [Google Scholar] [CrossRef]
- Cai, Z.; Fu, F.; Zhu, S.; Wu, J.; Han, X.; Jiang, S.; Qian, Z.; Wang, C. Novel polymersomes based on Poly(2-methyl-2-oxazoline) polymers with excellent pharmacokinetic behavior and antitumor effect by modulating dynamic protein corona. Chem. Eng. J. 2025, 512, 162443. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Lee, S.-H.; Kasala, D.; Zhao, Y.; Jiao, A.; Hong, J.; Kim, J.S.; Yoon, A.-R.; Yun, C.-O. Potent therapeutic efficacy of intranasally deliverable paclitaxel modified with pH-sensitive and PEGylated polymeric micelle against glioblastoma. J. Control. Release 2025, 382, 113711. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; May, J.-N.; Klinkenberg, G.; Besse, H.C.; Buhl, E.M.; Moeckel, D.; Mihyar, R.; Peña, Q.; Shalmani, A.A.; Hark, C.; et al. Multidrug micelles and sonopermeation for chemotherapy co-delivery to brain tumors. J. Control. Release 2025, 380, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Bennett, Z.T.; Krishnamurthy, A.; Ye, S.; Basava, V.S.; Feng, Q.; Huang, G.; Sumer, B.D.; Gao, J. A Multi-Threshold Micelle Improves Tumor Accumulation and STING Immunotherapy. J. Am. Chem. Soc. 2025, 147, 19547–19558. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.Y.; Hou, X.X.; Liu, J.F.; Huang, F. Advances in Polymer Nanomaterials Targeting cGAS-STING Pathway for En-hanced Cancer Immunotherapy. J. Control. Release 2025, 381, 113560. [Google Scholar] [CrossRef]
- Liu, F.; Howard, C.B.; Huda, P.; Fletcher, N.L.; Bell, C.A.; Blakey, I.; Agrez, M.; Thurecht, K.J. Immune-modulating nanomedicines for enhanced drug delivery to non-small-cell lung cancer. Biomaterials 2025, 317, 123089. [Google Scholar] [CrossRef]
- Cai, Y.; Qi, J.; Lu, Y.; He, H.; Wu, W. The in vivo fate of polymeric micelles. Adv. Drug Deliv. Rev. 2022, 188, 114463. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Zhu, A.; Lv, R.; Liu, B.; Zhao, L.; Yin, T.; Zhang, Y.; He, H.; Gou, J.; et al. The self-assembled cabazitaxel-carboxymethylcellulose-mPEG polymer micelles for efficient antitumor activity and low systemic toxicity. Carbohydr. Polym. 2025, 363, 123745. [Google Scholar] [CrossRef]
- Xing, Y.; Zhu, J.Y.; Zhao, L.Z.; Xiong, Z.J.; Li, Y.J.; Wu, S.; Chand, G.S.; Shi, X.Y.; Zhao, J.H. SPECT/CT Imaging of Chem-otherapy-Induced Tumor Apoptosis Using 99mTc-Labeled Dendrimer-Entrapped Gold Nanoparticles. Drug Deliv. 2018, 25, 1384–1393. [Google Scholar] [CrossRef]
- Liu, J.; Minchom, A.R.; Greystoke, A.; Evans, T.R.J.; Sarker, D.; Joshua, A.M.; Morton, C.; Aktas, B.Y.; Cosman, R.; Chwialkowska, D.; et al. Dendrimer-Enhanced (DEP) SN38 (DEP Irinotecan) in Patients (Pts) with Advanced Solid Tumors: A Phase 1/2 Trial. JCO 2024, 42, 3014. [Google Scholar] [CrossRef]
- Spicer, J.F.; Pinato, D.J.; Forster, M.; Joshua, A.M.; Korolewicz, J.; Aboud, K.; Morton, C.; Liu, J.; Cosman, R.; Main, N.J.; et al. Efficacy and safety of dendrimer-enhanced (DEP) cabazitaxel (DEP CTX) in patients with advanced solid cancers in a phase 1/2 trial (P1/2). J. Clin. Oncol. 2024, 42, 3004. [Google Scholar] [CrossRef]
- del Olmo, N.S.; García, J.S.J.; Yin, Y.; Zhao, Y.; Hassan, M.; Malkoch, M. Responsive Organoselenium Dendritic Polymers: From Monodisperse Dendrimers to Self-Assembled Micelles for Advanced Therapeutic Applications. J. Am. Chem. Soc. 2025, 147, 18626–18636. [Google Scholar] [CrossRef]
- Qin, Y.; Lin, Y.; Tian, C.; Qi, Y.; Wang, S.; Chen, X.; Gu, W. pH-responsive nanocomplex for active transport of aPD-1 and PTX to enhance cancer chemoimmunotherapy. Nano Today 2025, 62, 102710. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Barenholz, Y. Liposomes: Preparation, Characterization, and Preservation. Methods Biochem. Anal. 1988, 33, 337–462. [Google Scholar]
- Mitsou, E.; Klein, J. Liposome-Based Interventions in Knee Osteoarthritis. Small 2025, 21, e2410060. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, Y.; Huang, W.; Li, J.; Zhang, B.; Li, Z.; Yang, X. Hyperbaric Oxygen Potentiates Doxil Antitumor Efficacy by Promoting Tumor Penetration and Sensitizing Cancer Cells. Adv. Sci. 2018, 5, 1700859. [Google Scholar] [CrossRef]
- Kumar, K.; Butowski, N.; Aghi, M.; Bankiewicz, K.; Bringas, J.; Bush, N.A.O.; Chang, S.; Clarke, J.; Taylor, J.; Martin, A.; et al. Scidot-A Phase I Study of Convection-Enhanced Delivery of Liposomal-Irinotecan (Onivyde) Using Real-Time Imaging with Gadolinium in Patients with Recurrent High Grade Gliomas: Results thus Far. Neuro-Oncology 2019, 21, vi272. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, Q.; Ma, H.; Xu, H.; Xiao, D.; Li, Y.; Xiong, S.; Li, Y. Slow intravenous infusion reduces the accelerated blood clearance of PEGylated liposomes by removing anti-PEG antibodies. J. Control. Release 2025, 382, 113762. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Gan, Z.; Zhang, J.; Dong, M.; Liu, S.; Cui, B.; Guo, P.; Pang, Z.; Lu, T.; Gu, N.; et al. Geometric-aware deep learning enables discovery of bifunctional ligand-based liposomes for tumor targeting therapy. Nano Today 2025, 61, 102668. [Google Scholar] [CrossRef]
- Li, J.; Lu, J.; Huang, X.; Ding, Y.; Zhou, J.; Chen, R.; Jiang, Z.; Fan, X.; Yang, Y.; Liao, C.; et al. Anti-CD33 antibody enhances liposomal co-delivery of cytarabine and daunorubicin for targeted combination chemotherapy. J. Control. Release 2025, 384, 113899. [Google Scholar] [CrossRef]
- Gil You, D.; Oh, B.H.; Nguyen, V.Q.; Lim, G.T.; Um, W.; Jung, J.M.; Jeon, J.; Choi, J.S.; Choi, Y.C.; Jung, Y.J.; et al. Vitamin A-coupled stem cell-derived extracellular vesicles regulate the fibrotic cascade by targeting activated hepatic stellate cells in vivo. J. Control. Release 2021, 336, 285–295. [Google Scholar] [CrossRef]
- Cui, F.; Song, Y.; Ji, H.; Li, M.; Zhuang, X.; Zeng, C.; Qu, B.; Mao, H.; Zhang, J.; Zhou, H.S.; et al. GlycoEVLR: Glycosylated extracellular vesicle-like receptors for targeting and sensing viral antigen. Chem. Eng. J. 2023, 469, 143844. [Google Scholar] [CrossRef]
- Chen, X.H.; Jia, M.; Liu, L.H.; Qiu, X.P.; Zhang, H.; Yu, X.L.; Gu, W.; Qing, G.C.; Li, Q.M.; Hu, X.L.; et al. High-fidelity Determination and Tracing of Small Ex-tracellular Vesicle Cargoes. Small 2020, 16, 2002800. [Google Scholar] [CrossRef]
- Pamperl, H.; Kleinberger, G. Morphologic Changes of Intralipid® 20% Liposomes in All-in-One Solutions during Prolonged Storage. Transfus. Med. Hemotherapy 1982, 9, 86–91. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Cvjetkovic, A.; Jang, S.C.; Crescitelli, R.; Hosseinpour Feizi, M.A.; Nieuwland, R.; Lötvall, J.; Lässer, C. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell. Mol. Life Sci. 2018, 75, 2873–2886. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [PubMed]
- Mateescu, B.; Kowal, E.J.K.; Van Balkom, B.W.M.; Bartel, S.; Bhattacharyya, S.N.; Buzás, E.I.; Buck, A.H.; de Candia, P.; Chow, F.W.N.; Das, S.; et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—An ISEV position paper. J. Extracell. Vesicles 2017, 6, 1286095. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Vergani, E.; Daveri, E.; Vallacchi, V.; Bergamaschi, L.; Lalli, L.; Castelli, C.; Rodolfo, M.; Rivoltini, L.; Huber, V. Extracellular vesicles in anti-tumor immunity. Semin. Cancer Biol. 2022, 86, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Birch, P.R.; Jin, H. Extracellular vesicles: A new avenue for mRNA delivery. Trends Plant Sci. 2024, 29, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Del Paggio, J.C. Cancer immunotherapy and the value of cure. Nat. Rev. Clin. Oncol. 2018, 15, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Hu, S.; Sun, H.; Tuo, B.; Jia, B.; Chen, C.; Wang, W.; Liu, J.; Liu, Y.; Sun, Z.; et al. Extracellular vesicles remodel tumor environment for cancer immunotherapy. Mol. Cancer 2023, 22, 203. [Google Scholar] [CrossRef]
- Park, K.; Svennerholm, K.; Crescitelli, R.; Lässer, C.; Gribonika, I.; Lötvall, J. Synthetic bacterial vesicles combined with tumour extracellular vesicles as cancer immunotherapy. J. Extracell. Vesicles 2021, 10, e12120. [Google Scholar] [CrossRef]
- Qing, Y.; Jiang, K.; Jiang, H.; Zhao, Y.; Lai, C.; Aicher, A.; Li, Z.; Heeschen, C. CLDN18.2 CAR-derived Extracellular Vesicle Immunotherapy Improves Outcome in Murine Pancreatic Cancer. Adv. Health Mater. 2025, 14, e2500546. [Google Scholar] [CrossRef]
- Wu, Q.; Zeng, Y.; Wang, W.; Liu, S.; Huang, Y.; Zhang, Y.; Chen, X.; You, Z.; Zhang, C.; Wang, T.; et al. Profiling Nascent Tumor Extracellular Vesicles via Metabolic Timestamping and Aptamer-Driven Specific Click Chemistry. J. Am. Chem. Soc. 2025, 147, 10737–10749. [Google Scholar] [CrossRef]
- Yuana, Y.; Levels, J.; Grootemaat, A.; Sturk, A.; Nieuwland, R. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J. Extracell. Vesicles 2014, 3, 23262. [Google Scholar] [CrossRef]
- Parsamian, P.; Liu, Y.; Xie, C.; Chen, Z.; Kang, P.; Wijesundara, Y.H.; Al-Kharji, N.M.; Ehrman, R.N.; Trashi, O.; Randrianalisoa, J.; et al. Enhanced Nanobubble Formation: Gold Nanoparticle Conjugation to Qβ Virus-like Particles. ACS Nano 2023, 17, 7797–7805. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, W.; Chen, H.; Wu, J.; Wang, X.; Yang, X.; Liang, X.-J.; Zhang, J.; Liu, D. An iron oxyhydroxide-based nanosystem sensitizes ferroptosis by a “Three-Pronged” strategy in breast cancer stem cells. Acta Biomater. 2023, 160, 281–296. [Google Scholar] [CrossRef]
- Cun, J.; He, Z.; Fan, X.; Pan, Q.; Luo, K.; He, B.; Pu, Y. Copper-Based Bio-Coordination Nanoparticle for Enhanced Pyroptosis-Cuproptosis Cancer Immunotherapy through Redox Modulation and Glycolysis Inhibition. Small 2025, 21, e2409875. [Google Scholar] [CrossRef] [PubMed]
- Picchetti, P.; Volpi, S.; Rossetti, M.; Dore, M.D.; Trinh, T.; Biedermann, F.; Neri, M.; Bertucci, A.; Porchetta, A.; Corradini, R.; et al. Responsive Nucleic Acid-Based Organosilica Nanoparticles. J. Am. Chem. Soc. 2023, 145, 22896–22902. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Park, M.; Kim, J.; Byun, H.; Kim, J.; Kim, W.J. Inorganic nanoparticle-based cancer immunotheranostics. Coord. Chem. Rev. 2025, 542. [Google Scholar] [CrossRef]
- Kesharwani, P.; Ma, R.Y.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.S.; Zhou, Y. Gold Na-noparticles and Gold Nanorods in the Landscape of Cancer Therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Wang, Q.; Ni, N.; Tee, J.K.; Ariga, K.; Ke, P.C.; Ho, H.K.; Wang, Y.; Leong, D.T. Engineering tumoral vascular leakiness with gold nanoparticles. Nat. Commun. 2023, 14, 4269. [Google Scholar] [CrossRef]
- Zhang, Z.; Lo, H.; Zhao, X.; Li, W.; Wu, K.; Zeng, F.; Li, S.; Sun, H. Mild photothermal/radiation therapy potentiates ferroptosis effect for ablation of breast cancer via MRI/PA imaging guided all-in-one strategy. J. Nanobiotechnol. 2023, 21, 150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, X.; Huang, W.; Chen, R.; Chen, K.; Nie, L.; Fang, C. A microenvironment-responsive FePt probes for imaging-guided Fenton-enhanced radiotherapy of hepatocellular carcinoma. J. Nanobiotechnol. 2022, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Xuan, F.; Zhao, X.; Pang, W.; Li, Z.; Yin, X.; Xie, W.; Zeng, X.; Nie, L.; Yang, J.; Li, S.; et al. Biomimetic Co-delivery of Lenvatinib and FePt Nanoparticles for Enhanced Ferroptosis/Apoptosis Treatment of Hepatocellular Carcinoma. Adv. Health Mater. 2025, 14, e2401747. [Google Scholar] [CrossRef]
- Shi, L.; Yu, Y.; Li, J.; Ma, B.; Zhang, X.; Yang, P.; Chen, P.; Qu, Z.; Zhang, F.; Liu, K.; et al. NIR-responsive Cu2 − xSe@Fc nanoparticles for photothermal- ferroptosis combination therapy in esophageal cancer. J. Nanobiotechnol. 2025, 23, 356. [Google Scholar] [CrossRef]
- Yu, Z.; Cao, L.; Shen, Y.; Chen, J.; Li, H.; Li, C.; Yin, J.; Li, Y.; Meng, Y.; Li, X. Inducing Cuproptosis with Copper Ion-Loaded Aloe Emodin Self-Assembled Nanoparticles for Enhanced Tumor Photodynamic Immunotherapy. Adv. Health Mater. 2025, 14, e2404612. [Google Scholar] [CrossRef]
- Jiang, C.; Li, X.; Wan, S.; Ji, S.; Wang, Q.; Hu, S.; Chen, P.; Wang, B.; Ge, T.; Zhang, J.; et al. Copper-Doped Polydopamine Nanoparticles-Mediated GSH/GPX4-Depleted Ferroptosis and Cuproptosis Sensitizes Lung Tumor to Checkpoint Blockade Immunotherapy. Small 2025, 21, e2503208. [Google Scholar] [CrossRef]
- Fa, Y.-C.; Chen, C.-C.; Liu, Y.-C.; Lu, Y.-H.; Wang, X.-H.; Kuo, Y.-Y.; Yang, C.-M.; Wu, L.-C.; Ho, J.-A.A. Precise identification of bladder tumors utilizing mucoadhesive thiolated hollow mesoporous silica nanoparticles. J. Control. Release 2025, 380, 1127–1140. [Google Scholar] [CrossRef]
- Ramos-Valle, A.; Domínguez, A.; Navarro, N.; Márquez-López, A.; Aviñó, A.; Eritja, R.; Fàbrega, C.; García-Hevia, L.; Fanarraga, M.L. Targeted Tumor Microenvironment Delivery of Floxuridine Prodrug via Soluble Silica Nanoparticles in Malignant Melanoma as a Model for Aggressive Cancer Treatment. Small 2025, 21, e2407752. [Google Scholar] [CrossRef] [PubMed]
- Lafuente-Gómez, N.; Martínez-Mingo, M.; Díaz-Riascos, Z.V.; García-Prats, B.; de la Iglesia, I.; Dhanjani, M.; García-Soriano, D.; Campos, L.A.; Mancilla-Zamora, S.; Salas, G.; et al. Gemcitabine and miRNA34a mimic codelivery with magnetic nanoparticles enhanced anti-tumor effect against pancreatic cancer. J. Control. Release 2025, 383, 113791. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wang, R.; Zhang, H.; Zhang, Q.; Qin, Y.; Du, C.; Zhang, X.; Ye, J.; Shi, C.; Shen, H.; et al. Preclinical and First-in-Human Study of a Compact Radionuclide Labeled Self-Assembly Nanomedicine for Chemo-Radio-Theranostics of Cancer. ACS Nano 2025, 19, 3953–3965. [Google Scholar] [CrossRef]
- Muthiah, K.S.; Natarajan, S.R.; Jayaraman, S.; Dhawan, U.; Lin, Y.C.; Chung, R.J. Photo-Responsive g-C3N4/Copper Mo-lybdate Nanocomposites Enable Laser-Driven Multi-Modal Imaging, Photothermal and Chemodynamic Therapy for Hepatocellular Carcinoma Treatment. Chem. Eng. J. 2025, 507, 160751. [Google Scholar] [CrossRef]
- Das, P.; Chakraborty, G.; Kaur, J.; Mandal, S.K. Nano-Scale Anti-Cancer Drug Delivery by a Zn-Based Metal Organic Framework Carrier. Small 2025, 21, 2408810. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal–Organic Framework (MOF)-based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Zhang, L.; Shen, H.; Liu, T.; Li, B.; Chen, X.; Wang, H.; He, C.; Liu, Y.; Cao, G.; Yu, S. A pH/GSH Dual-Responsive Triple Synergistic Bimetallic Nanocatalyst for Enhanced Tumor Chemodynamic Therapy. Small 2025, 21, e2409836. [Google Scholar] [CrossRef]
- Jiang, Y.P.; Shao, K.; Zhang, F.L.; Wang, T.Y.; Han, L.; Kong, X.Y.; Shi, J.S. “Block and Attack” Strategy for Tumor Therapy through ZnO2/siRNA/NIR-mediating Zn2+ -overload and Amplified Oxidative Stress. Aggregate 2023, 4, e321. [Google Scholar] [CrossRef]
- Liu, S.; Meng, Q.; Liu, Z.; Wang, J.; Li, J.; Ma, X.; Hu, Y.; Wang, Z.; Ma, P.; Lin, J. Engineered Metal–Organic Framework with Stereotactic Anchoring and Spatial Separation of Porphyrins for Amplified Ultrasound-Mediated Pyroptosis and Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2024, 64, e202421402. [Google Scholar] [CrossRef]
- Li, J.H.; Lv, W.X.; Han, Z.W.; Li, Y.K.; Deng, J.Q.; Huang, Y.J.; Wan, S.; Sun, J.S.; Dai, B. Mitoxantrone-encapsulated ZIF-8 Enhances Chemo-immunotherapy via Amplified Immunogenic Cell Death. Adv. Sci. 2025, 12, 2501542. [Google Scholar] [CrossRef]
- Chang, Y.; Huang, K.; Tang, H.; Yao, Y.; Min, J.; Quan, H.; Xu, K.; Wang, H.; Zhang, J.; Zhao, Y. Biomimetic MOF nanoplatform for dual-targeted co-delivery of FAK inhibitor and bismuth to enhance cervical cancer radiosensitivity. Adv. Compos. Hybrid Mater. 2025, 8, 147. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, F.; Li, Y.; Jiang, M.; Zhang, Y.; Kan, C.; Han, L.; Xue, S.; Wang, K.; Fan, Q.; et al. MOF-based nanoparticles for tumor-targeted protein degradation and photodynamic therapy induce enhanced anti-tumor immunity. Nano Today 2024, 56, 102308. [Google Scholar] [CrossRef]
- Rao, Y.; Fan, T.; Zhou, L.; Fang, K.; Sun, Y.; Hu, X.; Wang, A.; Li, R.; Zhu, Z.; Dong, C.; et al. A positive self-amplified H2O2 and acidity circulation for boosting CDT-PTT-starvation therapy. J. Control. Release 2023, 354, 701–712. [Google Scholar] [CrossRef]
- Peng, X.; Tang, S.; Tang, D.; Zhou, D.; Li, Y.; Chen, Q.; Wan, F.; Lukas, H.; Han, H.; Zhang, X.; et al. Autonomous metal-organic framework nanorobots for active mitochondria-targeted cancer therapy. Sci. Adv. 2023, 9, eadh1736. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, W.; Yu, N.; Li, X.; Feng, Y.; Geng, P.; Wen, M.; Li, M.; Zhang, H.; Chen, Z. Construction of CuS@Fe-MOF nanoplatforms for MRI-guided synergistic photothermal-chemo therapy of tumors. Chem. Eng. J. 2020, 400, 125877. [Google Scholar] [CrossRef]
- An, G.; Zheng, H.; Guo, L.; Huang, J.; Yang, C.; Bai, Z.; Wang, N.; Yang, W.; Zhu, Y. A metal-organic framework (MOF) built on surface-modified Cu nanoparticles eliminates tumors via multiple cascading synergistic therapeutic effects. J. Colloid Interface Sci. 2024, 662, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Du, S.; Ren, J.; Jiang, H.; Zhang, L.; Zhu, J. Mitochondria-Targeting Upconversion Nanoparticles@MOF for Multiple-Enhanced Photodynamic Therapy in Hypoxic Tumor. ACS Appl. Mater. Interfaces 2023, 15, 35884–35894. [Google Scholar] [CrossRef]
- Zhuang, H.L.; Wang, L.; Shao, S.J.; Jing, H.T.; Xue, P.P.; Bai, T.J.; Deng, J.P.; Zeng, X.M.; Qin, X.; Yan, S.Q. Photocataly-sis-Promoted Tumor Ferroptosis Enabled by MOF-Derived Black TiO2. Chem. Eng. J. 2024, 496, 154204. [Google Scholar] [CrossRef]
- Du, J.; Jia, T.; Li, F.; Li, Y.; Wang, Q.; He, L.; Ågren, H.; Chen, G. MOF-Coated Upconversion Nanoparticle Agents Enable Synergistic Photodynamic Therapy and Immunotherapy. Adv. Funct. Mater. 2024, 34. [Google Scholar] [CrossRef]
- Li, H.Y.; Zhang, Y.Y.; Zhao, Q.; Xu, R.S.; Yang, J.; Deng, K.Q.; Huang, H. Bioinspired Protein-mineralized Single-atom Nanozymes for Tumor-specific Cascade Therapy via Self-amplifying Catalytic Synergy. Small 2025, 31, 2500846. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Hou, Z.; Zhou, Q.; Chen, X.; Liu, W.; Xue, W.; Wang, Q.; Wang, J.; Zheng, T.; Tao, Z. Cu-optimized long-range interaction between Co nanoparticles and Co single atoms: Improved Fenton-like reaction activity. Sci. Bull. 2024, 69, 2529–2542. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wu, Z.; Zhou, H.; Wang, X.; Liu, Y.; Zhang, H.; Xiong, Z.; Lai, B. The structure-performance relationships in active center size-dependent Fenton-like catalysis: From nanoparticles to single atoms. Appl. Catal. B Environ. 2024, 355, 124157. [Google Scholar] [CrossRef]
- Lam, S.J.; Wong, E.H.H.; Boyer, C.; Qiao, G.G. Antimicrobial Polymeric Nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- Kumar, P.; Anand, B.; Tsang, Y.F.; Kim, K.-H.; Khullar, S.; Wang, B. Regeneration, degradation, and toxicity effect of MOFs: Opportunities and challenges. Environ. Res. 2019, 176, 108488. [Google Scholar] [CrossRef]
- Bahrami, A.; Delshadi, R.; Jafari, S.M. Active delivery of antimicrobial nanoparticles into microbial cells through surface functionalization strategies. Trends Food Sci. Technol. 2020, 99, 217–228. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, T.; Wu, C.; Qiu, L.; Hu, R.; Li, J.; Cansiz, S.; Zhang, L.; Cui, C.; Zhu, G.; et al. Facile Surface Functionalization of Hydrophobic Magnetic Nanoparticles. J. Am. Chem. Soc. 2014, 136, 12552–12555. [Google Scholar] [CrossRef]
- Escriche-Navarro, B.; Garrido, E.; Escudero, A.; Montoya-Méndez, I.; Sancenón, F.; García-Fernández, A.; Martínez-Máñez, R. Targeting the senescent surfaceome through DPP4 antibody-functionalized nanoparticles. An application to cancer therapy. Biomaterials 2025, 324, 123461. [Google Scholar] [CrossRef]
- Sheng, Y.; Liu, C.; Yuan, Y.; Tao, X.; Yang, F.; Shan, X.; Zhou, H.; Xu, F. Long-circulating polymeric nanoparticles bearing a combinatorial coating of PEG and water-soluble chitosan. Biomaterials 2009, 30, 2340–2348. [Google Scholar] [CrossRef]
- Toro-Mendoza, J.; Maio, L.; Gallego, M.; Otto, F.; Schulz, F.; Parak, W.J.; Sanchez-Cano, C.; Coluzza, I. Bioinspired Poly-ethylene Glycol Coatings for Reduced Nanoparticle–Protein Interactions. ACS Nano 2023, 17, 955–965. [Google Scholar] [CrossRef]
- Mamnoon, B.; Souza, A.P.M.; Korzun, T.; Baldwin, M.K.; Sharma, K.S.; Goo, Y.T.; Singh, P.; Grigoriev, V.; Lakhanpal, A.; Taratula, O.R. ENT-1-Targeted Polymersomes to Enhance the Efficacy of Methotrexate in Choriocarcinoma Treatment. Small Sci. 2025, 5, 2400361. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, X.; Chen, L.; Zhang, L.; Wang, F.; Zhang, Y.; Pan, G.; Zhang, Y.; Zhang, L.; Cui, W. Nano-in-micro electronspun membrane: Merging nanocarriers and microfibrous scaffold for long-term scar inhibition. Chem. Eng. J. 2020, 397, 125405. [Google Scholar] [CrossRef]
- Zewail, M.B.; Yang, G.Z.; Fan, Y.L.; Hui, Y.; Zhao, C.X.; Liu, Y. Cell Membrane-Coated Lipid Nanoparticles for Drug De-livery. Aggregate 2025, 6, e70054. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, P.; Luo, Z.; Zheng, M.; Tian, H.; Gong, P.; Gao, G.; Pan, H.; Liu, L.; Ma, A.; et al. Cancer Cell Membrane–Biomimetic Nanoparticles for Homologous-Targeting Dual-Modal Imaging and Photothermal Therapy. ACS Nano 2016, 10, 10049–10057. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Cui, Y.; Hao, W.; Chen, M.; Liu, Q.; Wang, Y.; Yang, M.; Li, Z.; Gong, W.; Song, S.; et al. Carrier-free highly drug-loaded biomimetic nanosuspensions encapsulated by cancer cell membrane based on homology and active targeting for the treatment of glioma. Bioact. Mater. 2021, 6, 4402–4414. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, F.; Yan, D.Y.; Han, T.; Wang, L.; Wang, D.; Tang, B.Z. “Trojan Horse” Phototheranostics: Fine-Engineering NIR-II AIEgen Camouflaged by Cancer Cell Membrane for Homologous-Targeting Multimodal Imaging-Guided Photo-therapy. Adv. Mater. 2023, 35, 2302639. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Wang, M.; Liu, R.; Song, H.; Li, N.; Lu, Q.; Zhang, W.; Du, Y.; Yang, W.; et al. Bioinspired nanoplatelets for chemo-photothermal therapy of breast cancer metastasis inhibition. Biomaterials 2019, 206, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Huang, H.; Jia, M.; Chen, S.; Wang, F.; He, G.; Wu, C.; Lou, K.; Zheng, X.; Zhang, H.; et al. Autologous platelet delivery of siRNAs by autologous plasma protein self-assembled nanoparticles for the treatment of acute kidney injury. J. Nanobiotechnol. 2025, 23, 256. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.; Luo, R.; Quan, X.; Fong, H.U.; Cheng, Q.; Wei, J.; Wang, L.; Zhao, Y.; Wang, R. Tumor Microenvironment Triggered In Situ Coagulation of Supramolecularly Engineered Platelets for Precise Tumor Embolization. Adv. Sci. 2025, 12, e2414879. [Google Scholar] [CrossRef]
- Chu, H.; Xu, Y.; Shan, Y.; Sun, M.; Zhao, W.; Fang, X.; Shen, N.; Tang, Z. Platelet hitchhiking vascular-disrupting agents for self-amplified tumor-targeting therapy. J. Nanobiotechnol. 2025, 23, 197. [Google Scholar] [CrossRef]
- Zhang, J.A.; Feng, K.; Shen, W.-T.; Gao, W.; Zhang, L. Research Advances of Cellular Nanoparticles as Multiplex Countermeasures. ACS Nano 2024, 18, 30211–30223. [Google Scholar] [CrossRef]
- Huang, P.; Wang, C.; Deng, H.; Zhou, Y.; Chen, X. Surface Engineering of Nanoparticles toward Cancer Theranostics. Accounts Chem. Res. 2023, 56, 1766–1779. [Google Scholar] [CrossRef]
- Chen, Z.-A.; Wu, C.-H.; Wu, S.-H.; Huang, C.-Y.; Mou, C.-Y.; Wei, K.-C.; Yen, Y.; Chien, I.-T.; Runa, S.; Chen, Y.-P.; et al. Receptor Ligand-Free Mesoporous Silica Nanoparticles: A Streamlined Strategy for Targeted Drug Delivery across the Blood–Brain Barrier. ACS Nano 2024, 18, 12716–12736. [Google Scholar] [CrossRef]
- Moreira, R.; Nóbrega, C.; de Almeida, L.P.; Mendonça, L. Brain-targeted drug delivery—Nanovesicles directed to specific brain cells by brain-targeting ligands. J. Nanobiotechnol. 2024, 22, 260. [Google Scholar] [CrossRef]
- Nie, H.; Huang, R.; Jiang, G.; Li, W.; Yang, L.; Zhang, M.; Qian, M.; Guo, W.; Ye, T.; Huang, R. Modulating active targeting nanoparticle design according to tumor progressions. Acta Pharm. Sin. B 2024, 15, 1143–1158. [Google Scholar] [CrossRef]
- Alonso-Valenteen, F.; Mikhael, S.; Wang, H.; Sims, J.; Taguiam, M.; Teh, J.; Sances, S.; Wong, M.; Miao, T.; Srinivas, D.; et al. Systemic HER3 ligand-mimicking nanobioparticles enter the brain and reduce intracranial tumour growth. Nat. Nanotechnol. 2025, 20, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Hu, L.; Chen, G.; Ye, J.; Xiao, Z.; Guan, T.; Guo, S.; Xia, W.; Cheng, D.; Wan, X.; et al. A PD-L1 siRNA-Loaded Boron Nanoparticle for Targeted Cancer Radiotherapy and Immunotherapy. Adv. Mater. 2025, 37, e2419418. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, C.; Ma, W.; Ba, Z.; Hu, Z.; Willner, I.; Wang, C. An Activatable Caged Palladium Nanocomposite for Targeted Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2025, 64, e202503485. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.C.; Bruno, P.M.; Nguyen, H.V.-T.; Liao, L.; Liu, J.; Hemann, M.T.; Johnson, J.A. Using an RNAi Signature Assay To Guide the Design of Three-Drug-Conjugated Nanoparticles with Validated Mechanisms, In Vivo Efficacy, and Low Toxicity. J. Am. Chem. Soc. 2016, 138, 12494–12501. [Google Scholar] [CrossRef]
- Zhao, Y.; Shirasu, T.; Yodsanit, N.; Kent, E.; Ye, M.; Wang, Y.; Xie, R.S.; Gregg, A.C.; Huang, Y.T.; Kent, K.C.; et al. Biomimetic, ROS-Detonable Nanoclusters—A Multimodal Nanoplatform for Anti-Restenotic Therapy. J. Control. Release 2021, 338, 295–306. [Google Scholar] [CrossRef]
- Xu, W.; Zeng, Z.; Tang, Y.; Tian, J.; Hao, X.; Sun, P.; Peng, Y.; Tian, T.; Xiang, D.; Wang, R.; et al. Spatiotemporal-controllable ROS-responsive camptothecin nano-bomb for chemo/photo/immunotherapy in triple-negative breast cancer. J. Nanobiotechnol. 2024, 22, 798. [Google Scholar] [CrossRef]
- Shen, M.; Wang, Y.; Bing, T.; Tang, Y.; Liu, X.; Yu, Y. Alendronate Triggered Dual-Cascade Targeting Prodrug Nanoparticles for Enhanced Tumor Penetration and STING Activation of Osteosarcoma. Adv. Funct. Mater. 2023, 33, 2307013. [Google Scholar] [CrossRef]
- Chae, S.Y.; Kim, G.Y.; Choe, H.-S.; Kwon, Y.W.; Hong, S.W.; Kim, J.-H. Photothermal cancer treatment with laser-activated yolk-shell nanoparticles and synergistic combination of chemotherapy. Chem. Eng. J. 2025, 513, 162919. [Google Scholar] [CrossRef]
- Herzog, B.H.; Devarakonda, S.; Govindan, R. Overcoming Chemotherapy Resistance in SCLC. J. Thorac. Oncol. 2021, 16, 2002–2015. [Google Scholar] [CrossRef]
- Siemer, S.; Bauer, T.A.; Scholz, P.; Breder, C.; Fenaroli, F.; Harms, G.; Dietrich, D.; Dietrich, J.; Rosenauer, C.; Barz, M.; et al. Targeting Cancer Chemotherapy Resistance by Precision Medicine-Driven Nanoparticle-Formulated Cisplatin. ACS Nano 2021, 15, 18541–18556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, M.; Feng, J.; Qin, B.; Zhang, C.; Zhu, C.; Liu, W.; Wang, Y.; Liu, W.; Huang, L.; et al. Multi-functional Nanoparticles Co-Loaded with Adriamycin and MDR-Targeting siRNAs for Treatment of Chemothera-py-Resistant Esophageal Cancer. J. Nanobiotechnol. 2022, 20, 166. [Google Scholar]
- Kejun, D.; Hao, H.; Shuangshuang, C.; Yaoqin, M.; Wei, Z.; Ting, Z.; Jiarui, Z.; Wan, S.; Xiaoyu, S.; Hongbo, W.; et al. Multifunctional DNA nano-sponge system for targeted sensitization of ovarian cancer chemotherapy via metabolic reprogramming and ferroptosis induction. J. Control. Release 2025, 382, 113663. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, B.; Ge, M.; Yu, J.; Ju, F.; Sun, J.; Zhou, Y.L.; Wang, L.; Jia, Z. Microglial membrane-coated nanoparticles for ACSL4-siRNA delivery in Parkinson’s disease. Chem. Eng. J. 2025, 509. [Google Scholar] [CrossRef]
- Yao, Z.; Liu, T.; Wang, J.; Fu, Y.; Zhao, J.; Wang, X.; Li, Y.; Yang, X.; He, Z. Targeted delivery systems of siRNA based on ionizable lipid nanoparticles and cationic polymer vectors. Biotechnol. Adv. 2025, 81, 108546. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xie, K.; Zhang, X.-Q.; Pridgen, E.M.; Park, G.Y.; Cui, D.S.; Shi, J.; Wu, J.; Kantoff, P.W.; Lippard, S.J.; et al. Enhancing tumor cell response to chemotherapy through nanoparticle-mediated codelivery of siRNA and cisplatin prodrug. Proc. Natl. Acad. Sci. USA 2013, 110, 18638–18643. [Google Scholar] [CrossRef]
- Dubrova, A.; Cavaniol, C.; Van de Walle, A.; Mathieu, P.; Fusilier, Z.; Yaacoub, N.; Lalatonne, Y.; Descroix, S.; Wilhelm, C. Magnetite Nanoparticle Photothermal Therapy in a Pancreatic Tumor-on-Chip: A Dual-Action Approach Targeting Cancer Cells and their Microenvironment. ACS Nano 2025, 19, 19790–19805. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, J.; Sun, L.; Xu, S.; Ma, S.S.; Wang, H.; Li, X.; Xiang, Q.; Cui, L.; Liang, X. Ultrasound-triggered topical oxygen delivery enhances synergistic sonodynamic and antibody therapies against hypoxic gastric cancer. J. Control. Release 2025, 380, 736–750. [Google Scholar] [CrossRef]
- Huang, J.; Hu, F.; Zhang, H.; Cao, Z.; Xiao, H.; Yang, Z.; Jin, Q.; Shang, K. Ultrasound-Triggered Nanoparticles Induce Cuproptosis for Enhancing Immunogenic Sonodynamic Therapy. Adv. Mater. 2025, e2504228. [Google Scholar] [CrossRef]
- Wu, P.; Dong, W.; Guo, X.; Qiao, X.; Guo, S.; Zhang, L.; Wan, M.; Zong, Y. ROS-Responsive Blended Nanoparticles: Cascade-Amplifying Synergistic Effects of Sonochemotherapy with On-demand Boosted Drug Release During SDT Process. Adv. Healthc. Mater. 2019, 8, 1900720. [Google Scholar] [CrossRef]
- Jiao, X.; Li, X.; Du, Y.; Cong, Y.; Yang, S.; Chen, D.; Zhang, T.; Feng, M.; Hong, H. Positron emission tomography guided synergistic treatment of melanoma using multifunctional zirconium-hematoporphyrin nanosonosensitizers. J. Control. Release 2024, 370, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xu, P.; Fang, Q.; Zhu, Y.; Cao, F.; Zhao, Z.; Wu, D.; Yang, X.; Li, D.; Liu, X. Biomimetic Nanoparticle with Glutathione Depletion and Amplified ROS Generation Capabilities for Synergistic Chemo-Sonodynamic Therapy in Squamous Cell Carcinomas. ACS Appl. Mater. Interfaces 2023, 15, 27183–27194. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.M.; Wang, Z.; Long, W.; Xu, M.Z.; Liu, A.L.; Gao, Y.F.; Wen, Z.; Liu, C.; He, J.C.; Cheng, Y.X.; et al. Nanosonosensitizer-augmented Sono-immunotherapy for Glioblastoma by Non-invasive Opening of the Blood–Brain Barrier. Adv. Funct. Mater. 2023, 33, 2209219. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, L.; Zhu, G.; Sun, Q.; Xie, Y.; Wang, M.; Xu, Y.; Li, C. Biodegradable pyroptosis inducer with multienzyme-mimic activity kicks up reactive oxygen species storm for sensitizing immunotherapy. J. Control. Release 2024, 370, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Chen, H.; Cui, Y.; Luo, Z.; Liang, R.; Wu, Z.; Chen, Z.; Yin, T.; Ni, J.; Zheng, M.; et al. Metalloporphyrin Complex-Based Nanosonosensitizers for Deep-Tissue Tumor Theranostics by Noninvasive Sonodynamic Therapy. Small 2018, 15, e1804028. [Google Scholar] [CrossRef]
- Yang, Z.; Jiao, Z.P.; Chen, Z.; Qiao, C.Q.; Huang, C.T.; Wang, L.Y.; Rao, Z.P.; Zhang, R.L.; Wang, Z.L. Programmable Bac-terial Architects Crafting Sonosensitizers for Tumor-specific Sonodynamic Immunotherapy. Adv. Mater. 2025, 2504206. [Google Scholar] [CrossRef]
- Bai, Q.; Wang, M.; Wang, K.; Liu, J.; Qu, F.; Lin, H. CuPc-Fe@BSA nanocomposite: Intracellular acid-sensitive aggregation for enhanced sonodynamic and chemo-therapy. J. Colloid Interface Sci. 2024, 671, 577–588. [Google Scholar] [CrossRef]
- Li, C.; Gao, Y.; Wang, Y.; Wang, J.; Lin, J.; Du, J.; Zhou, Z.; Liu, X.; Yang, S.; Yang, H. Bifunctional Nano-Assembly of Iridium(III) Phthalocyanine Complex Encapsulated with BSA: Hypoxia-relieving/Sonosensitizing Effects and their Immunogenic Sonodynamic Therapy. Adv. Funct. Mater. 2022, 33, 2210348. [Google Scholar] [CrossRef]
- Huang, H.; Du, L.; Su, R.; Li, Z.; Shao, Y.; Yuan, Y.; Wang, C.; Lu, C.; He, Y.; He, H.; et al. Albumin-based co-loaded sonosensitizer and STING agonist nanodelivery system for enhanced sonodynamic and immune combination antitumor therapy. J. Control. Release 2024, 375, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Hoang, Q.T.; Cao, T.G.N.; Kang, S.J.; Lee, M.; Kang, J.H.; Park, H.S.; Kim, J.-E.; Bhang, S.H.; Ko, Y.T.; Rhee, W.J.; et al. Exosome membrane-sheathed and multi-stimuli-responsive MnO2 nanoparticles with self-oxygenation and energy depletion abilities potentiate the sonodynamic therapy of hypoxic tumors. Chem. Eng. J. 2023, 472. [Google Scholar] [CrossRef]
- Zhang, L.; Yi, H.; Song, J.; Huang, J.; Yang, K.; Tan, B.; Wang, D.; Yang, N.; Wang, Z.-G.; Li, X. Mitochondria-Targeted and Ultrasound-Activated Nanodroplets for Enhanced Deep-Penetration Sonodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2019, 11, 9355–9366. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, T.; Zhou, Z.; Hu, A.; Li, M.; Zhang, Z.; Yu, G.; Feng, H.; An, Y.; Peng, J.; et al. Berberine nanoparticles for promising sonodynamic therapy of a HeLa xenograft tumour. RSC Adv. 2019, 9, 10528–10535. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, W.; Ge, J.; Jia, Q.; Nan, F.; Ding, Y.; Wu, J.; Zhang, W.; Lee, C.-S.; Wang, P. Biodegradable Natural Product-Based Nanoparticles for Near-Infrared Fluorescence Imaging-Guided Sonodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 18178–18185. [Google Scholar] [CrossRef]
- Lin, X.; Liu, S.; Zhang, X.; Zhu, R.; Chen, S.; Chen, X.; Song, J.; Yang, H. An Ultrasound Activated Vesicle of Janus Au-MnO Nanoparticles for Promoted Tumor Penetration and Sono-Chemodynamic Therapy of Orthotopic Liver Cancer. Angew. Chem. Int. Ed. Engl. 2019, 59, 1682–1688. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.-Q.; An, J.; Cheng, K.; Hou, X.-L.; Zhang, X.-S.; Hu, Y.-G.; Liu, B.; Zhao, Y.-D. Red blood cell membrane-enveloped O2 self-supplementing biomimetic nanoparticles for tumor imaging-guided enhanced sonodynamic therapy. Theranostics 2020, 10, 867–879. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Wang, X.W.; Cheng, L.; Tang, Y.A.; Zhan, G.T.; Gong, F.; Zhang, R.; Hu, J.; Liu, Z.; Yang, X.L. GSH-depleted PtCu3 Nanocages for Chemodynamic- Enhanced Sonodynamic Cancer Therapy. Adv. Funct. Mater. 2020, 30, 1907954. [Google Scholar] [CrossRef]
- Sun, T.; Wang, R.; Lu, W.; Shi, X.; Gao, F.; Wu, T.; Wang, G.; Su, X.; Teng, Z. Platinum nanoparticle-anchored metal–organic complex nanospheres by a coordination–crystallization approach for enhanced sonodynamic therapy of tumors. J. Mater. Chem. B 2023, 11, 11280–11289. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhong, X.Y.; Bai, L.X.; Xu, J.; Gong, F.; Dong, Z.L.; Yang, Z.J.; Zeng, Z.J.; Liu, Z.; Cheng, L. Ultrafine Titanium Monoxide (TiO1+x) Nanorods for Enhanced Sonodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 6527–6537. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, Y.; Wang, H.; Hao, J.; Cao, Y.; Liu, Z. Titanium boride nanosheets with photo-enhanced sonodynamic efficiency for glioblastoma treatment. Acta Biomater. 2024, 188, 344–357. [Google Scholar] [CrossRef]
- Sun, L.; Cao, Y.; Li, W.; Wang, L.; Ding, P.; Lu, Z.; Ma, F.; Wang, Z.; Pei, R. Perovskite-Type Manganese Vanadate Sonosensitizers with Biodegradability for Enhanced Sonodynamic Therapy of Cancer. Small 2023, 19, e2300101. [Google Scholar] [CrossRef]
- Hu, Z.; Song, X.; Ding, L.; Cai, Y.; Yu, L.; Zhang, L.; Zhou, Y.; Chen, Y. Engineering Fe/Mn-doped zinc oxide nanosonosensitizers for ultrasound-activated and multiple ferroptosis-augmented nanodynamic tumor suppression. Mater. Today Bio 2022, 16, 100452. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, Y.; You, Q.; Lei, Z.; Ji, J.; Zhang, F.; Dong, W.; Li, L. Metal-Doping Strategy for Carbon-Based Sonosensitizer in Sonodynamic Therapy of Glioblastoma. Adv. Sci. 2024, 11, e2404230. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Gong, F.; Yin, K.; Zhu, W.; Yang, N.; Bai, S.; Liao, F.; Shao, M.; Cheng, L. Silicon nanowires decorated with platinum nanoparticles were applied for photothermal-enhanced sonodynamic therapy. Theranostics 2021, 11, 9234–9242. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhong, D.; Hua, S.Y.; Du, Z.; Zhou, M. Biomineralized Biohybrid Algae for Tumor Hypoxia Modulation and Cascade Radio-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 44541–44553. [Google Scholar] [CrossRef]
- Hu, J.; Jia, X.; Li, M.; Duan, G.; Man, K.; Dai, H.; Wen, L.; Geng, H. Enhanced Delivery of Photothermal Gelatin Nanoparticle for Redox Balanced Nanocatalytic Tumor Chemotherapy. Small 2025, 21, e2411018. [Google Scholar] [CrossRef]
- Chen, Z.; Sang, L.; Qixi, Z.; Li, X.; Liu, Y.; Bai, Z. Ultrasound-responsive nanoparticles for imaging and therapy of brain tumors. Mater. Today Bio 2025, 32, 101661. [Google Scholar] [CrossRef]
- Wang, X.Y.; Fang, H.Y.; Hu, W.Z.; Feng, Y.; Zhou, Z.Y.X.; Hu, M.Y.; Jiang, D.; Zhang, Y.X.; Lan, X.L. Oxygen-Delivery Nanoparticles Enhanced Immunotherapy Efficacy Monitored by Granzyme B PET Imaging in Malignant Tumors. J. Nanobiotechnol. 2025, 23, 186. [Google Scholar] [CrossRef]
- Azizi, M.; Dianat-Moghadam, H.; Salehi, R.; Farshbaf, M.; Iyengar, D.; Sau, S.; Iyer, A.K.; Valizadeh, H.; Mehrmohammadi, M.; Hamblin, M.R. Interactions Between Tumor Biology and Targeted Nanoplatforms for Imaging Applications. Adv. Funct. Mater. 2020, 30, 1910402. [Google Scholar] [CrossRef]
- Chen, L.; Li, Q. Nanomaterials in the diagnosis and treatment of gastrointestinal tumors: New clinical choices and treatment strategies. Mater. Today Bio 2025, 32, 101782. [Google Scholar] [CrossRef]
- Lozano-García, M.; Dikici, E.; Bilbao, D.; Mohan, P.; Deo, S.; Daunert, S. Multifunctional Delivery Strategies and Nanoplat-forms of SN-38 in Cancer Therapeutics. J. Control. Release 2025, 384, 113937. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, Q.; Wang, L.; Zhang, Y.; Zhu, L. Engineered nanoparticles for imaging and targeted drug delivery in hepatocellular carcinoma. Exp. Hematol. Oncol. 2025, 14, 62. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Xing, J.Q.; Jiang, J.; Liao, M.L.; Pan, G.J.; Wang, Y.F. Hypoxia-Responsive Nanoparticles for Fluorescence Di-agnosis and Therapy of Cancer. Theranostics 2025, 15, 1353–1375. [Google Scholar] [CrossRef] [PubMed]
- Suman, S.K.; Balakrishnan, B.; Mallia, M.B.; Mukherjee, A. Tumor-Targeted Radioiodinated Glyconanoparticles for Doxorubicin Delivery and Auger-Chemotherapy in Triple-Negative Breast Cancer. Small 2025, e2502419. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gao, W.; Chang, S.; He, B.; Zhang, C.; Liu, M.; Ye, X. Mitochondria-targeting nanomedicines with autophagy inhibitor to enhance cancer photothermal-chemotherapy. Regen. Biomater. 2025, 12, rbae141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Ji, Y.; Yang, M.; Li, C.; Li, M.; Yang, J.; Tang, H.; Luo, X.; Hao, H.; et al. Magnetically driven bionic nanorobots enhance chemotherapeutic efficacy and the tumor immune response via precise targeting. Innovation 2025, 6, 100777. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Hang, L.; Diao, Y.; Wang, H.; Fang, L.; Liu, W.; Liu, J.; Sun, H.; Wang, J.; Meng, X.; et al. Mn-doped MOF nanoparticles mitigating hypoxia via in-situ substitution strategy for dual-imaging guided combination treatment of microwave dynamic therapy and chemotherapy. J. Colloid Interface Sci. 2025, 685, 912–926. [Google Scholar] [CrossRef]
- Jia, P.; Tu, J.; Shen, H.; Jiang, Y.; Zhang, Q.; Xue, W.; Liu, M.; Liu, J.; Miao, Y.; Ouyang, R.; et al. Defect-engineered magnetic bismuth nanomedicine for dual-modal imaging and synergistic lung tumor therapy. Mater. Today Bio 2025, 32, 101680. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Meng, Y.; An, Y.; Jia, J.; Wang, Y.; Li, G.; Li, Y.; Wu, S.; Geng, C.; Chen, Y.; et al. In Situ and Real-Time Multi-Modality Imaging Guided Orderly Triple-Therapy of Tumors with a Multifunctional Nanodrug. Adv. Sci. 2025, 12, e2501048. [Google Scholar] [CrossRef]
- Haye, L.; Pini, F.; Soro, L.K.; Knighton, R.C.; Fayad, N.; Benard, M.; Gagliazzo, F.; Light, M.E.; Natile, M.M.; Charbonnière, L.J.; et al. Molecular Upconversion Nanoparticles for Live-Cell Imaging. ACS Nano 2025, 19, 7178–7187. [Google Scholar] [CrossRef] [PubMed]
- Muradova, Z.; Carmès, L.; Brown, N.; Rossetti, F.; Guthier, R.; Yasmin-Karim, S.; Lavelle, M.; Morris, T.; Guidelli, E.J.; Isikawa, M.; et al. Targeted-theranostic nanoparticles induce anti-tumor immune response in lung cancer. J. Nanobiotechnol. 2025, 23, 466. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, J.; Li, Y.; Hu, Y.; He, D.; Ni, H.; Zhang, J.; Wu, F.; Tang, Y.; Wang, S. Interpretable Radiomics Model Predicts Nanomedicine Tumor Accumulation Using Routine Medical Imaging. Adv. Mater. 2025, 37, e2416696. [Google Scholar] [CrossRef]
- Spaanderman, D.J.; Marzetti, M.; Wan, X.; Scarsbrook, A.F.; Robinson, P.; Oei, E.H.; Visser, J.J.; Hemke, R.; van Langevelde, K.; Hanff, D.F.; et al. AI in radiological imaging of soft-tissue and bone tumours: A systematic review evaluating against CLAIM and FUTURE-AI guidelines. EBioMedicine 2025, 114, 105642. [Google Scholar] [CrossRef]
- Gong, X.; Dai, X.; Cheng, W.; Li, B.; Liu, J.; Chen, H.; Wu, M.; Yang, J.; Liu, B. Targeted High-Resolution 3D Imaging of Tumor Vasculatures at Different Stages Using Far-Red AIE Nanoparticles Compatible with Tissue Clearing. Adv. Mater. 2025, 37, e2501144. [Google Scholar] [CrossRef]
- Chang, X.; Wang, H.; Chen, X. Tumor Diagnosis and Treatment Based on Stimuli-Responsive Aggregation of Gold Nanoparticles. Exploration 2025, 5, 270006. [Google Scholar] [CrossRef]


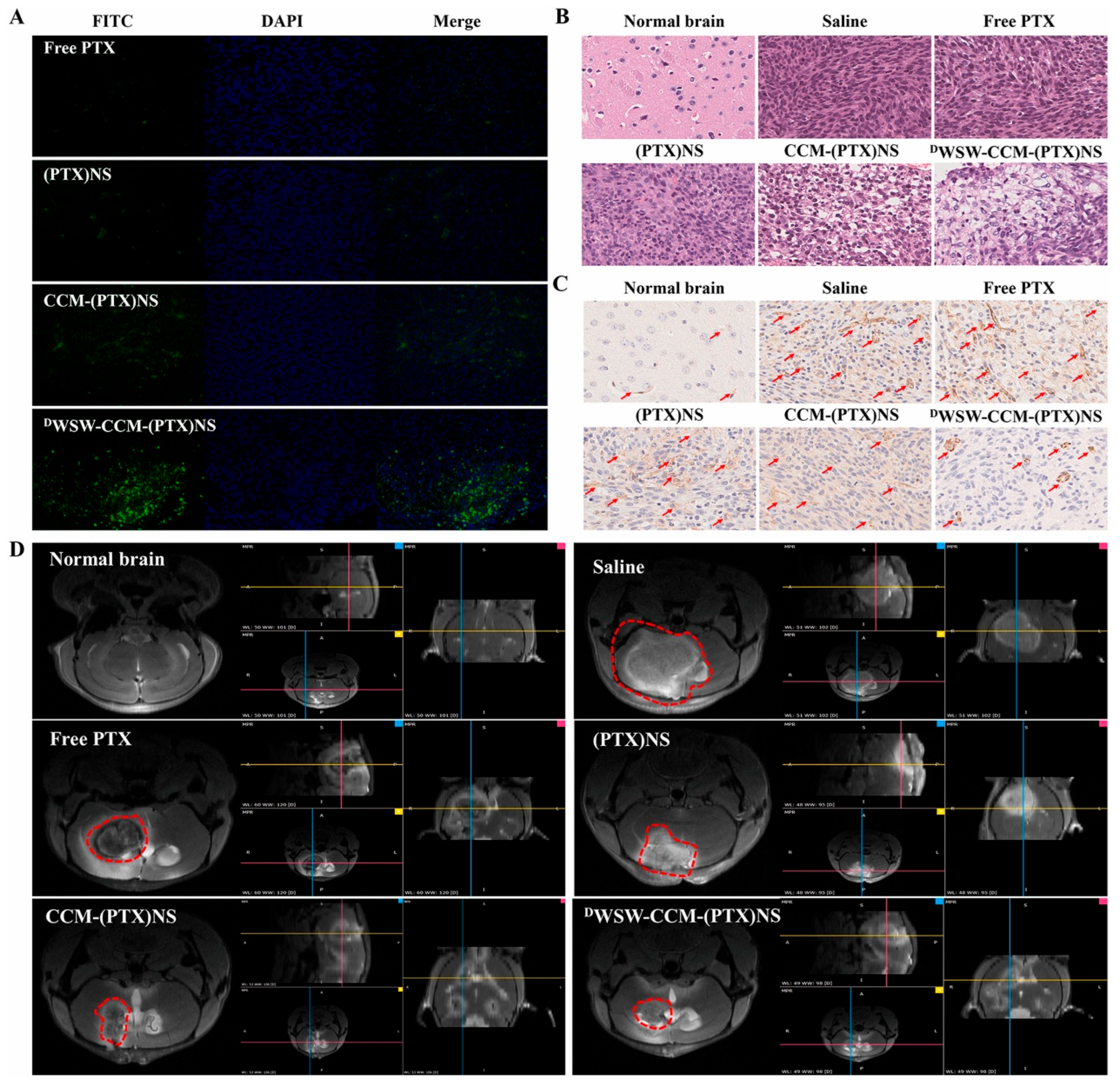
| Name | In-Base | Character | Function | Ref. |
|---|---|---|---|---|
| Fe(TCPP)-MOF | Fe | Amplify ROS generation | Pyroptosis | [101] |
| ZIF-8 | Zn | pH response | Drug release | [102,103] |
| ZMCH | Zn | Tumor-specific delivery and local drug release | Inhibiting tumor metastasis | [104] |
| ZIF-67 | Co | Catalyze hydrogen peroxide (H2O2) | Damage cell structure | [105,106] |
| MIL-88A | Fe | Magnetic resonance imaging (MRI) properties | MRI contrast | [107] |
| Cu@MIL-101@PMTPC | Fe | Multiple cascading synergistic therapeutic | Precision tumor intervention | [108] |
| TPP-UCNPs@MOF-Pt | Zr | Catalytically convert intracellular H2O2 to generate O2 | Improvement in the hypoxic microenvironment | [109] |
| bTiO2@TA/Fe | Ti | GSH depletion and chemodynamic therapy | Induce cancer cell ferroptosis | [110] |
| UCNP@MOF | Cu | Amplified ROS effects and unlocking infiltrating T cells | Tumor eradication | [111] |
| Category | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Polymer nanoparticles | Sustained release; controlled drug release | Highly cationic nature results in poor biocompatibility | [113] |
| Liposomes | Good biocompatibility | Difficulties in mass production and storage | [65] |
| Extracellular vesicles | Good biocompatibility; good targeting | Difficulties in isolation, purification, and mass production; differences among products of the same batch The recognition and function of the surface substances are not clear | [14,69,80] |
| Inorganic nanoparticles | Outstanding photothermal/magnetic performance; multimodal diagnosis and treatment | Metal ion toxicity caused by off-target behavior | [86] |
| Metal-organic frameworks | High drug loading; pH-responsive | Metal ion toxicity caused by off-target behavior | [114] |
| Category | Nano Sonosensitizer | Efficacy | Ref. | |
|---|---|---|---|---|
| Organic sonosensitizers | Porphyrins | Zr-HMME-PEG-F3 | Promotes anti-tumor immunity by suppressing tumor metastasis | [156] |
| R@S/SS-NPH&D | Provides GSH depletion and amplified ROS generation capabilities | [157] | ||
| HP/CP | Excellent stability, acoustic responsiveness, good tumor targeting and permeability, and efficient sonotoxicity and immune activation | [158] | ||
| CuTA-Ce6 | Enhanced tumor cytotoxicity and immunotherapy effect by US | [159] | ||
| MnTTP-HSA | Realizes real-time monitoring of molecular accumulation and tumor targeting for precision theranostic SDT | [160] | ||
| 5-Aminolevulinic acid | SPEC5 | Achieves robust sensitizer accumulation and enhances SDT efficacy | [161] | |
| Phthalocyanines | PAMSN | This platform can effectively treat orthotopic liver cancer in a murine model while in vivo monitoring of ROS and detection of cavitation are enabled | [11] | |
| CuPc-Fe@BSA | Not only has great anticancer effects but also stimulates an anticancer immune response to fight against metastasis and cancer recurrence | [162] | ||
| IrPc NPs | It exhibits good biocompatibility in vitro, can inhibit tumor growth in 4T1 tumor-bearing mice, and enables controllable response by timely ultrasound (US) irradiation during treatment | [163] | ||
| Indocyanines | FA-ICG&MnOx@HSA | Targeting and alleviating tumor hypoxia and improving the tumor immune microenvironment | [164] | |
| Exo-M (ICG/FX11) | Effectively treats hypoxic tumor cells via combined SDT and energy-depleting chemotherapy | [165] | ||
| IR780-NDs | Mitochondria-targeted and multimodal imaging-guided SDT can be achieved | [166] | ||
| Natural products | BBR NPs | Showing anti-tumor effects both in vitro and in vivo, and its potential mechanisms might be related to inhibiting PI3K-AKT-mTOR signaling pathways and blocking tumor blood vessels | [167] | |
| APHB NPs | Novel safe and precise NIR FL imaging and SDT agents for deep-seated tumor therapy | [168] | ||
| Inorganic Sonosensitizers | Noble metal-based | Janus Au-MnO | Effectively guided synergistic SDT/CDT for deep orthotopic liver tumors | [169] |
| (QD@P)R | Utilized the catalase enzyme of the RBC membrane to relieve tumor hypoxia, thereby further enhancing the SDT effect on the tumor under the guidance of fluorescence imaging | [170] | ||
| PtCu3 | Can pave a new way for imaging-guided in situ TME-responsive CDT-enhanced SDT triggered by US irradiation for deep-seated tumors | [171] | ||
| Pt-MOCs | Effectively produces reactive oxygen species and exhibits superior cytotoxicity for tumor cells | [172] | ||
| Transition metal-based | PEG-TiO1 + x NRs | Because of their efficient passive retention in tumors post intravenous injection, PEG-TiO1 + x NRs can be used as sonosensitizers and CDT agents for highly effective tumor ablation under US treatment | [173] | |
| TiB2@CM-RGD | Effectively crosses the BBB and accumulates in tumor sites, and significantly inhibits tumor growth after US irradiation | [174] | ||
| MnVO3 | MnVO3 may serve as a highly efficient, low-toxicity, and biodegradable sonosensitizer for cancer SDT | [175] | ||
| D-ZnO-PEG NPs | The simultaneously endowed multiple ferroptosis and synergistically enhanced SDT achieved high in vivo tumor suppression efficiency | [176] | ||
| Carbon-based Si-based | Cu-CDs | Exhibit excellent permeability through the blood–brain barrier and potent anti-tumor activity | [177] | |
| Si-Pt NCs | The mild photothermal effect of Si-Pt NCs further improves SDT and CDT activity and improves the combined cancer therapy | [178] | ||
| Algae@SiO2 | The significant suppression of tumor growth in mice bearing a 4T1 tumor successfully demonstrates the promising anti-tumor effect of Algae@SiO2-mediated synergistic therapy | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Zhou, S.; Zhang, Y.; Li, J.; Zhang, K. Advancements in Tumor-Targeted Nanoparticles: Design Strategies and Multifunctional Therapeutic Approaches. Nanomaterials 2025, 15, 1262. https://doi.org/10.3390/nano15161262
Li M, Zhou S, Zhang Y, Li J, Zhang K. Advancements in Tumor-Targeted Nanoparticles: Design Strategies and Multifunctional Therapeutic Approaches. Nanomaterials. 2025; 15(16):1262. https://doi.org/10.3390/nano15161262
Chicago/Turabian StyleLi, Mengya, Shengxi Zhou, Yan Zhang, Jingan Li, and Kun Zhang. 2025. "Advancements in Tumor-Targeted Nanoparticles: Design Strategies and Multifunctional Therapeutic Approaches" Nanomaterials 15, no. 16: 1262. https://doi.org/10.3390/nano15161262
APA StyleLi, M., Zhou, S., Zhang, Y., Li, J., & Zhang, K. (2025). Advancements in Tumor-Targeted Nanoparticles: Design Strategies and Multifunctional Therapeutic Approaches. Nanomaterials, 15(16), 1262. https://doi.org/10.3390/nano15161262









