Clay Nanomaterials Sorbents for Cleaner Water: A Sustainable Application for the Mining Industry
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reactives
2.2. Synthesis of Mica-Na-4
2.3. Organofunctionalization of MMT and Mica-Na-4
2.4. Sample Collection
2.5. Sorption Process
2.6. Analysis of Water
2.7. Solid Characterization
3. Results and Discussion
3.1. Influence of Clays on the COD Values of the Water Samples Tested
3.2. Influence of Clays on the pH Values of the Tested Waters
3.3. Influence of Clays on the Nitrate and Sulphate Values of the Tested Waters
3.4. Influence of Clays on the Values of Ca2+, Na+, Cu2+ and Fe2+ in the Tested Waters
3.5. Structural Changes in Clays
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, M. Global Water Shortage and Potable Water Safety; Today’s Concern and Tomorrow’s Crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef] [PubMed]
- Jamil, T. Application of Ball Clay/MnO2 Based Catalytic Ozonation Process for Textile Wastewater Treatment. Desalin. Water Treat. 2024, 318, 100394. [Google Scholar] [CrossRef]
- Kovo, A.S.; Alaya-Ibrahim, S.; Abdulkareem, A.S.; Adeniyi, O.D.; Egbosiuba, T.C.; Tijani, J.O.; Saheed, M.; Okafor, B.O.; Adeyinka, Y.S. Column Adsorption of Biological Oxygen Demand, Chemical Oxygen Demand and Total Organic Carbon from Wastewater by Magnetite Nanoparticles-Zeolite A Composite. Heliyon 2023, 9, e13095. [Google Scholar] [CrossRef]
- El-Sofany, E.A.; Zaki, A.A.; Mekhamer, H.S. Kinetics and Thermodynamics Studies for the Removal of Co2+ and Cs+ from Aqueous Solution by Sand and Clay Soils. Rca-Radiochim. Acta 2009, 97, 23–32. [Google Scholar] [CrossRef]
- Park, J.H.; Oh, C.; Han, Y.S.; Ji, S.W. Optimizing the Addition of Flocculants for Recycling Mineral-Processing Wastewater. Geosyst. Eng. 2016, 19, 83–88. [Google Scholar] [CrossRef]
- Meng, S.; Wen, S.; Han, G.; Wang, X.; Feng, Q. Wastewater Treatment in Mineral Processing of Non-Ferrous Metal Resources: A Review. Water 2022, 14, 726. [Google Scholar] [CrossRef]
- Turk, S.; Asci, Y. Color and Chemical Oxygen Demand Removal Using Homogeneous and Heterogeneous Fenton Oxidation of Sugar Industry Wastewater. Desalin. Water Treat. 2023, 306, 112–121. [Google Scholar] [CrossRef]
- Mazumder, M.A.J. Polymeric Adsorbents: Innovative Materials for Water Treatments. Curr. Anal. Chem. 2022, 19, 105–110. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient Techniques for the Removal of Toxic Heavy Metals from Aquatic Environment: A Review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Kabir, M.M.; Sabur, G.M.; Akter, M.M.; Nam, S.Y.; Im, K.S.; Tijing, L.; Shon, H.K. Electrodialysis Desalination, Resource and Energy Recovery from Water Industries for a Circular Economy. Desalination 2024, 569, 117041. [Google Scholar] [CrossRef]
- Ahmed, Y.; Maya, A.A.S.; Akhtar, P.; AlMohamadi, H.; Mohammad, A.W.; Ashekuzzaman, S.M.; Olbert, A.I.; Uddin, M.G. Advancements and Challenges in Fenton-Based Advanced Oxidation Processes for Antibiotic Removal in Wastewater: From the Laboratory to Practical Applications. J. Environ. Chem. Eng. 2025, 13, 115068. [Google Scholar] [CrossRef]
- Meese, A.F.; Kim, D.J.; Wu, X.; Le, L.; Napier, C.; Hernandez, M.T.; Laroco, N.; Linden, K.G.; Cox, J.; Kurup, P.; et al. Opportunities and Challenges for Industrial Water Treatment and Reuse. ACS EST Eng. 2022, 2, 465–488. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A Review on Emerging Water Contaminants and the Application of Sustainable Removal Technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, R.; Wang, J.; Hao, F. Optimization and Application of Acid-Modified Coal Gasification Fine Slag for Effective Removal of Chemical Oxygen Demand and Volatile Phenols in Semi-Coking Wastewater. Results Eng. 2025, 26, 104697. [Google Scholar] [CrossRef]
- Kainth, S.; Sharma, P.; Pandey, O.P. Green Sorbents from Agricultural Wastes: A Review of Sustainable Adsorption Materials. Appl. Surf. Sci. Adv. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Zafar, F.F.; Barati, B.; Sanaei, D.; Yousefzadeh, S.; Ahmadi, E.; Ansari, M.; Ghalhari, M.R.; Rasoulzadeh, H.; Zheng, X.; Wang, S.; et al. Application of Agricultural Waste-Based Activated Carbon for Antibiotic Removal in Wastewaters: A Comprehensive Review. Water 2025, 17, 1190. [Google Scholar] [CrossRef]
- Pavón, E.; Martín-Rodríguez, R.; Perdigón, A.C.; Alba, M.D. New Trends in Nanoclay-Modified Sensors. Inorganics 2021, 9, 43. [Google Scholar] [CrossRef]
- Aguado, F.; Martín-Rodríguez, R.; Pesquera, C.; Valiente, R.; Perdigón, A.C. Adsorptive Capture of Ionic and Non-Ionic Pollutants Using a Versatile Hybrid Amphiphilic-Nanomica. Nanomaterials 2021, 11, 3167. [Google Scholar] [CrossRef]
- Ihekweme, G.O.; Shondo, J.N.; Orisekeh, K.I.; Kalu-Uka, G.M.; Nwuzor, I.C.; Onwualu, A.P. Characterization of Certain Nigerian Clay Minerals for Water Purification and Other Industrial Applications. Heliyon 2020, 6, e03783. [Google Scholar] [CrossRef]
- Ligaray, M.; Futalan, C.M.; de Luna, M.D.; Wan, M.W. Removal of Chemical Oxygen Demand from Thin-Film Transistor Liquid-Crystal Display Wastewater Using Chitosan-Coated Bentonite: Isotherm, Kinetics and Optimization Studies. J. Clean. Prod. 2018, 175, 145–154. [Google Scholar] [CrossRef]
- Ewis, D.; Ba-Abbad, M.M.; Benamor, A.; El-Naas, M.H. Adsorption of Organic Water Pollutants by Clays and Clay Minerals Composites: A Comprehensive Review. Appl. Clay Sci. 2022, 229, 106686. [Google Scholar] [CrossRef]
- Oliveira, L.H.; França, D.B.; Moraes, A.I.S.; Medina-Carrasco, S.; Fonseca, M.G.; Osajima, J.A.; Silva-filho, E.C.; Orta, M. del M. An Overview about Synthetic High Charge Micas and Their Uses. Appl. Clay Sci. 2024, 251, 107325. [Google Scholar] [CrossRef]
- Osuna, F.J.; Pavón, E.; Alba, M.D. Pb2+, Cd2+ and Hg2+ Removal by Designed Functionalized Swelling High-Charged Micas. Sci. Total Environ. 2021, 764, 142811. [Google Scholar] [CrossRef] [PubMed]
- Pranoto; Inayati; Firmansyah, F. Effectiveness Study of Drinking Water Treatment Using Clays/Andisol Adsorbent in Lariat Heavy Metal Cadmium (Cd) and Bacterial Pathogens. IOP Conf. Ser. Mater. Sci. Eng. 2018, 349, 012047. [Google Scholar] [CrossRef]
- Martín, J.; Orta, M.; Medina-carrasco, S.; Luis, J.; Aparicio, I.; Alonso, E. Evaluation of a Modified Mica and Montmorillonite for the Adsorption of Ibuprofen from Aqueous Media. Appl. Clay Sci. 2019, 171, 29–37. [Google Scholar] [CrossRef]
- Satouh, S.; Martín, J.; Orta, M.; Medina-carrasco, S.; Messikh, N.; Bougdah, N.; Santos, J.L.; Aparicio, I.; Alonso, E. Adsorption of Polycyclic Aromatic Hydrocarbons by Natural, Synthetic and Modified Clays. Environments 2021, 8, 124. [Google Scholar] [CrossRef]
- del Mar Orta, M.; Martín, J.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Novel Synthetic Clays for the Adsorption of Surfactants from Aqueous Media. J. Environ. Manag. 2018, 206, 357–363. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Tallone, L.; Rosa, C.A.R.; Dalcero, A.M.; Chiacchiera, S.M.; Torres Sanchez, R.M. Commercial Bentonites as Detoxifier of Broiler Feed Contaminated with Aflatoxin. Appl. Clay Sci. 2008, 40, 63–71. [Google Scholar] [CrossRef]
- Gamba, M.; Flores, F.M.; Madejová, J.; Sánchez, R.M.T. Comparison of Imazalil Removal onto Montmorillonite and Nanomontmorillonite and Adsorption Surface Sites Involved: An Approach for Agricultural Wastewater Treatment. Ind. Eng. Chem. Res. 2015, 54, 1529–1538. [Google Scholar] [CrossRef]
- Alba, M.D.; Castro, M.A.; Naranjo, M.; Pavón, E. Hydrothermal Reactivity of Na-n-Micas (n = 2, 3, 4). Chem. Mater. 2006, 18, 2867–2872. [Google Scholar] [CrossRef]
- Pazos, M.C.; Castro, M.A.; Orta, M.M.; Pavón, E.; Rios, J.S.V.; Alba, M.D. Synthetic High-Charge Organomica: Effect of the Layer Charge and Alkyl Chain Length on the Structure of the Adsorbed Surfactants. Langmuir 2012, 28, 7325–7332. [Google Scholar] [CrossRef] [PubMed]
- Alba, M.D.; Castro, M.A.; Orta, M.M.; Pavón, E.; Pazos, M.C.; Valencia Rios, J.S. Formation of Organo-Highly Charged Mica. Langmuir 2011, 27, 9711–9718. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.M.; Undabeytia, T.; Morillo, E.; Torres Sánchez, R.M. Technological Applications of Organo-Montmorillonites in the Removal of Pyrimethanil from Water: Adsorption/Desorption and Flocculation Studies. Environ. Sci. Pollut. Res. 2017, 24, 14463–14476. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; del Mar Orta, M.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Removal of Priority and Emerging Pollutants from Aqueous Media by Adsorption onto Synthetic Organo-Funtionalized High-Charge Swelling Micas. Environ. Res. 2018, 164, 488–494. [Google Scholar] [CrossRef]
- del Mar Orta, M.; Flores, F.M.; Morantes, C.F.; Curutchet, G.; Torres Sánchez, R.M. Interrelations of Structure, Electric Surface Charge, and Hydrophobicity of Organo-Mica and –Montmorillonite, Tailored with Quaternary or Primary Amine Cations. Preliminary Study of Pyrimethanil Adsorption. Mater. Chem. Phys. 2019, 223, 325–335. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Khodadoust, A.P.; Reddy, K.R.; Maturi, K. Effect of Different Extraction Agents on Metal and Organic Contaminant Removal from a Field Soil. J. Hazard. Mater. 2005, 117, 15–24. [Google Scholar] [CrossRef]
- Morillo-Velarde Pérez, J.I. REPORT: The Discharge Authorization Procedure Contained in the Regulation of Discharges to the Public Hydraulic Domain and to the Maritime-Terrestrial Public Domain of Andalusia. Rev. Andaluza Adm. Pública 2015, 91, 407–413. [Google Scholar] [CrossRef]
- Aziz, H.A.; Umar, M.; Yusoff, M.S. Variability of Parameters Involved in Leachate Pollution Index and Determination of LPI from Four Landfills in Malaysia. Int. J. Chem. Eng. 2010, 2010, 747953. [Google Scholar] [CrossRef]
- Malvar, J.L.; Martín, J.; del Mar Orta, M.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Simultaneous and Individual Adsorption of Ibuprofen Metabolites by a Modified Montmorillonite. Appl. Clay Sci. 2020, 189, 105529. [Google Scholar] [CrossRef]
- Pazos, M.C.; Castro, M.A.; Cota, A.; Osuna, F.J.; Pavón, E.; Alba, M.D. New Insights into Surface-Functionalized Swelling High Charged Micas: Their Adsorption Performance for Non-Ionic Organic Pollutants. J. Ind. Eng. Chem. 2017, 52, 179–186. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Uddin, M.K. A Review on the Adsorption of Heavy Metals by Clay Minerals, with Special Focus on the Past Decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Gatti, M.N.; Fernández, L.G.; Sánchez, M.P.; Parolo, M.E. Aminopropyltrimethoxysilane- and Aminopropyltrimethoxysilane-Silver-Modified Montmorillonite for the Removal of Nitrate Ions. Environ. Technol. 2016, 37, 2658–2668. [Google Scholar] [CrossRef]
- Jaworski, M.A.; Flores, F.M.; Fernández, M.A.; Casella, M.; Torres Sánchez, R.M. Use of Organo-Montmorillonite for the Nitrate Retention in Water: Influence of Alkyl Length of Loaded Surfactants. SN Appl. Sci. 2019, 1, 1318. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A Review of the Biochemistry of Heavy Metal Biosorption by Brown Algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.J.; Park, M. Stabilization of Alkali Earth Metal Cations in Na-4-Mica. Appl. Clay Sci. 2014, 101, 272–276. [Google Scholar] [CrossRef]
- Araníbar, H.K.; Rojas-Villanueva, A.; Bermejo-Severino, F.; Araníbar, M.J. Utilización de Una Arcilla Modificada Para La Adsorción de Metales Pesados En Un Efluente Minero. ÑAWPARISUN Rev. Investig. Científica 2018, 1, 63–68. [Google Scholar]
- Zhu, J.; Cozzolino, V.; Pigna, M.; Huang, Q.; Caporale, A.G.; Violante, A. Sorption of Cu, Pb and Cr on Na-Montmorillonite: Competition and Effect of Major Elements. Chemosphere 2011, 84, 484–489. [Google Scholar] [CrossRef]
- Huang, P.; Feng, W.; Li, W.; Zhuo, Y.; Yang, Z.; Yang, S.; Han, D. A Stable and Reusable Z-Scheme ZnO/Ag2CO3 Photocatalyst for Efficient Degradation of Emerging Water Pollutants. Mater. Res. Bull. 2025, 193, 113663. [Google Scholar] [CrossRef]
- Ahmad, R.; Mirza, A. Adsorption of Pb(II) and Cu(II) by Alginate-Au-Mica Bionanocomposite: Kinetic, Isotherm and Thermodynamic Studies. Process Saf. Environ. Prot. 2017, 109, 1–10. [Google Scholar] [CrossRef]
- Tahir, S.S.; Rauf, N. Removal of Fe(II) from the Wastewater of a Galvanized Pipe Manufacturing Industry by Adsorption onto Bentonite Clay. J. Environ. Manag. 2004, 73, 285–292. [Google Scholar] [CrossRef]
- Setshedi, K.Z.; Bhaumik, M.; Songwane, S.; Onyango, M.S.; Maity, A. Exfoliated Polypyrrole-Organically Modified Montmorillonite Clay Nanocomposite as a Potential Adsorbent for Cr(VI) Removal. Chem. Eng. J. 2013, 222, 186–197. [Google Scholar] [CrossRef]
- Houmia, I.; Fardioui, M.; El Amri, A.; Houmia, B.; Kaibous, N.; Bazhar, K.; Hammani, O.; Arhoutane, M.R.; Guedira, T. Structural Characterization and Ecological Evaluation of Natural Clay Mixtures for the Removal of Heavy Metals (Cu(II), Co(II), and Zn(II)) from Aqueous Solutions: Experimental Study Combined with RSM Process Optimization. J. Mol. Struct. 2025, 1344, 142917. [Google Scholar] [CrossRef]
- Madejová, J.; Kovadel, P. Baseline Studies of the Clay Minerals Society Source Clays: Introduction. Clays Clay Miner. 2001, 49, 372–373. [Google Scholar] [CrossRef]
- Trigueiro, P.; Rodrigues, F.; Rigaud, B.; Balme, S.; Janot, J.M.; dos Santos, I.M.G.; Fonseca, M.G.; Osajima, J.; Walter, P.; Jaber, M. When Anthraquinone Dyes Meet Pillared Montmorillonite: Stability or Fading upon Exposure to Light? Dye. Pigment. 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Carriazo, J.; Rafael, M.; Moreno, S. Caracterización Estructural y Textural de una Bentonita Colombiana. Rev. Colomb. Química 2007, 36, 213–225. [Google Scholar]
- Cai, Y.; Huang, F.; Xia, X.; Wei, Q.; Tong, X.; Wei, A. Weidong Gao Comparison between Structures and Properties of ABS Nanocomposites Derived from Two Different Kinds of OMT. J. Mater. Eng. Perform. 2010, 19, 171–176. [Google Scholar] [CrossRef]
- Gregorkiewitz, M.; Rausell-Colom, J.A. Characterization and Properties of a New Synthetic Silicate with Highly Charged Mica-Type Layers. Am. Mineral. 1987, 72, 515–527. [Google Scholar]
- Laoot, P.; Rangsriwatananon, K.; Chaisena, A. Synthesis of Sodium-Type Fluorophlogopite Mica from Perlite and Diatomite. J. Ceram. Process. Res. 2011, 12, 273–278. [Google Scholar]
- Liang, X.; Wang, W.; Tang, M.; Kang, Y.; Cui, M.; Zhao, H. Preparation, Characterization and Antibacterial Activities of Bio-Based Schiff Base Copper Complex Intercalated Montmorillonite. Appl. Clay Sci. 2025, 271, 107809. [Google Scholar] [CrossRef]
- Chu, Q.; Gong, Y.; Xu, J.; Liu, Y.; Chu, W. Synergistic Removal of Atrazine and Cr (VI) from Water by FeS-Loaded Modified Montmorillonite Activated Persulfates. J. Ind. Eng. Chem. 2025, in press. [Google Scholar] [CrossRef]
- Lim, J.H.X.; Goh, K.; Ng, D.Y.F.; Jiang, X.; Chuah, C.Y.; Chew, J.W.; Wang, R. Alternating Spin-and-Spray Electrospun Scaffold Membranes with Fractionated MIL-101(Cr) Adsorbent for High-Performance Single-Pass Dye Adsorption Process. Chem. Eng. J. 2022, 450, 137963. [Google Scholar] [CrossRef]
- Abutaleb, A.; Eldoma, M.A.; Imran, M.; Taha, K.K.; Bakather, O.Y.; Zouli, N.; Hegazi, S.E.F.; Hassan, M. Active Adsorption Performance of Planetary Ball Milled Saudi Arabian Bentonite Clay for the Removal of Copper Ions from Aqueous Solution. Europhys. Lett. 2021, 135, 30005. [Google Scholar] [CrossRef]
- Orta, M.M.; Maisanaba, S.; Medina-Carrasco, S.; Jos, A. Potential Application of a Synthetic Organo-Funtionalized High Load Expandable Mica as Drug Carrier for Controlled Release. Curr. Drug Deliv. 2020, 18, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zuo, W.; Tian, Y.; Kong, L.; Cai, G.; Zhang, H.; Li, L.; Zhang, J. Flexible Brushite/Nanofibrillated Cellulose Aerogels for Efficient and Selective Removal of Copper(II). Chem. Eng. J. 2022, 450, 138262. [Google Scholar] [CrossRef]
- Ahmed, M.K.; Afifi, M.; Mansour, S.F.; Ibrahium, H.A.; Aldulmani, S.A.A.; Awwad, N.S. Morphological Behaviors of Brushite/Vivianite Nanocomposites and Their Potency for Se(IV) and Cd(II) Removal from Aqueous Solutions. Mater. Chem. Phys. 2021, 259, 124057. [Google Scholar] [CrossRef]


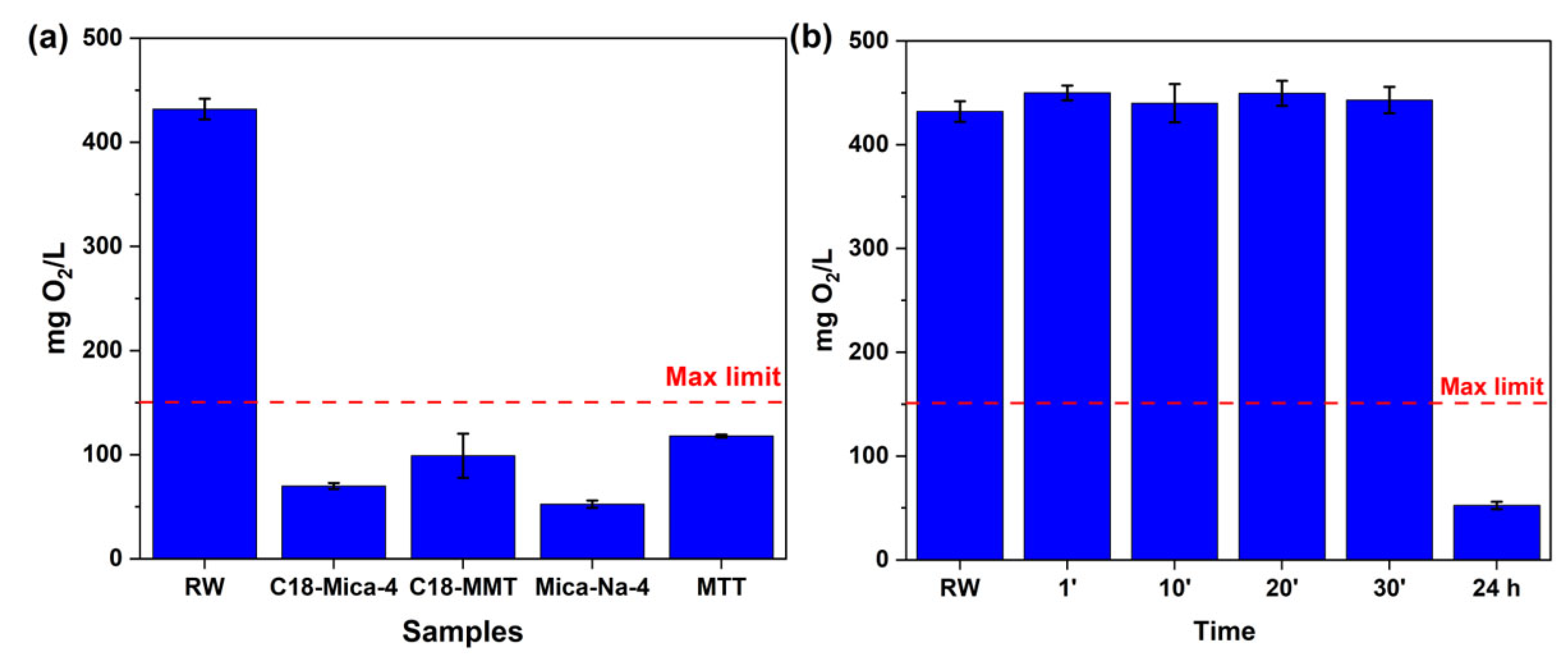

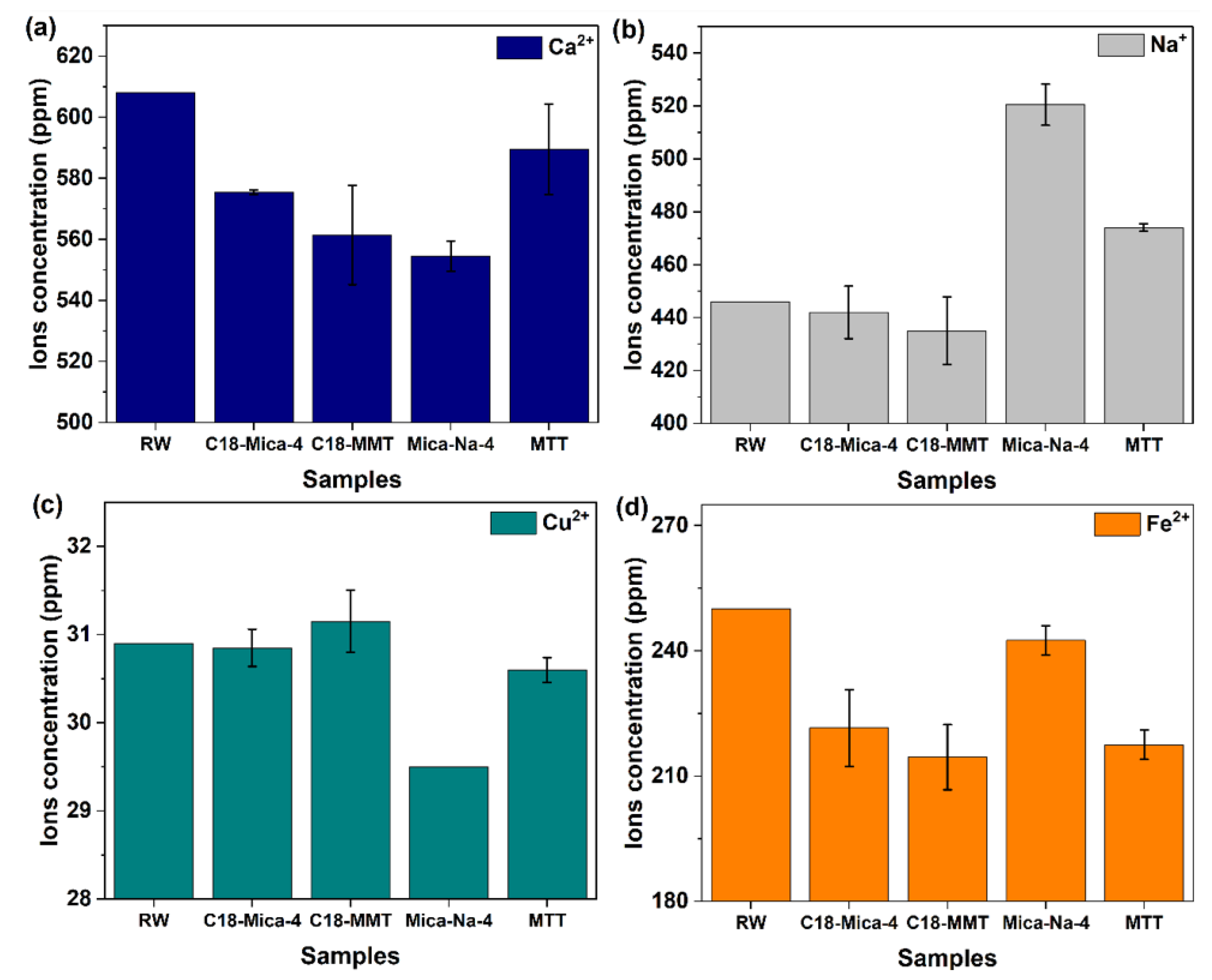
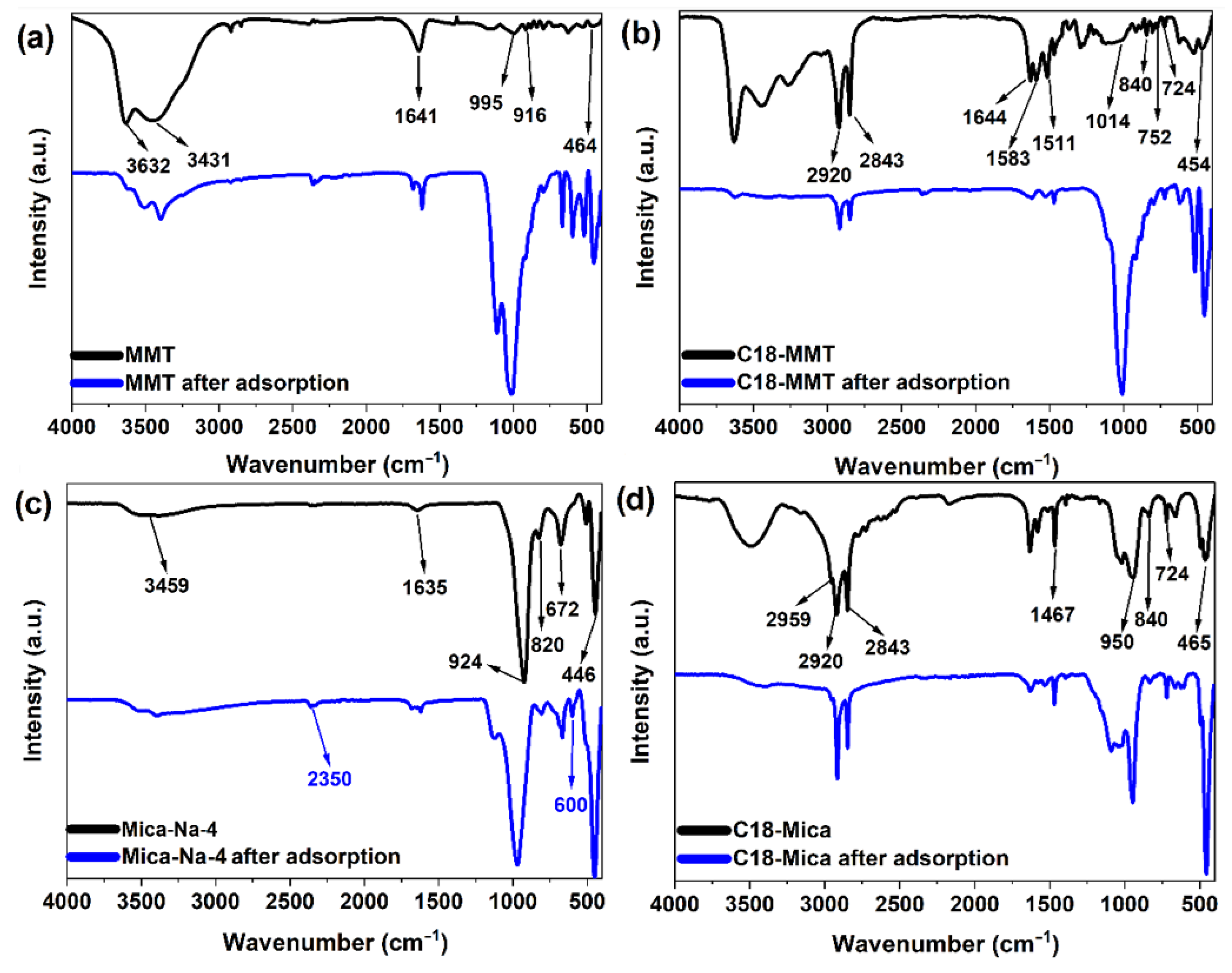

| RW Raw | C18-Mica-4 | C18-MMT | Mica-Na-4 | MMT | |
|---|---|---|---|---|---|
| pH | 2.53 | 2.48 | 2.55 | 2.43 | 2.58 |
| RW Raw | 1 min | 10 min | 20 min | 30 min | 24 h | |
|---|---|---|---|---|---|---|
| pH | 2.46 | 2.40 | 2.52 | 2.47 | 2.51 | 2.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Fernández, M.; Santos Silva, A.; Prado Feitosa, R.; Silva-Filho, E.C.; Osajima, J.A.; Medina-Carrasco, S.; Orta Cuevas, M.d.M. Clay Nanomaterials Sorbents for Cleaner Water: A Sustainable Application for the Mining Industry. Nanomaterials 2025, 15, 1211. https://doi.org/10.3390/nano15151211
Molina-Fernández M, Santos Silva A, Prado Feitosa R, Silva-Filho EC, Osajima JA, Medina-Carrasco S, Orta Cuevas MdM. Clay Nanomaterials Sorbents for Cleaner Water: A Sustainable Application for the Mining Industry. Nanomaterials. 2025; 15(15):1211. https://doi.org/10.3390/nano15151211
Chicago/Turabian StyleMolina-Fernández, María, Albert Santos Silva, Rodrigo Prado Feitosa, Edson C. Silva-Filho, Josy A. Osajima, Santiago Medina-Carrasco, and María del Mar Orta Cuevas. 2025. "Clay Nanomaterials Sorbents for Cleaner Water: A Sustainable Application for the Mining Industry" Nanomaterials 15, no. 15: 1211. https://doi.org/10.3390/nano15151211
APA StyleMolina-Fernández, M., Santos Silva, A., Prado Feitosa, R., Silva-Filho, E. C., Osajima, J. A., Medina-Carrasco, S., & Orta Cuevas, M. d. M. (2025). Clay Nanomaterials Sorbents for Cleaner Water: A Sustainable Application for the Mining Industry. Nanomaterials, 15(15), 1211. https://doi.org/10.3390/nano15151211








