Coating Metal–Organic Frameworks (MOFs) and Associated Composites on Electrodes, Thin Film Polymeric Materials, and Glass Surfaces
Abstract
1. Introduction
2. Approaches and Strategies for Coating MOFs on Various Surfaces
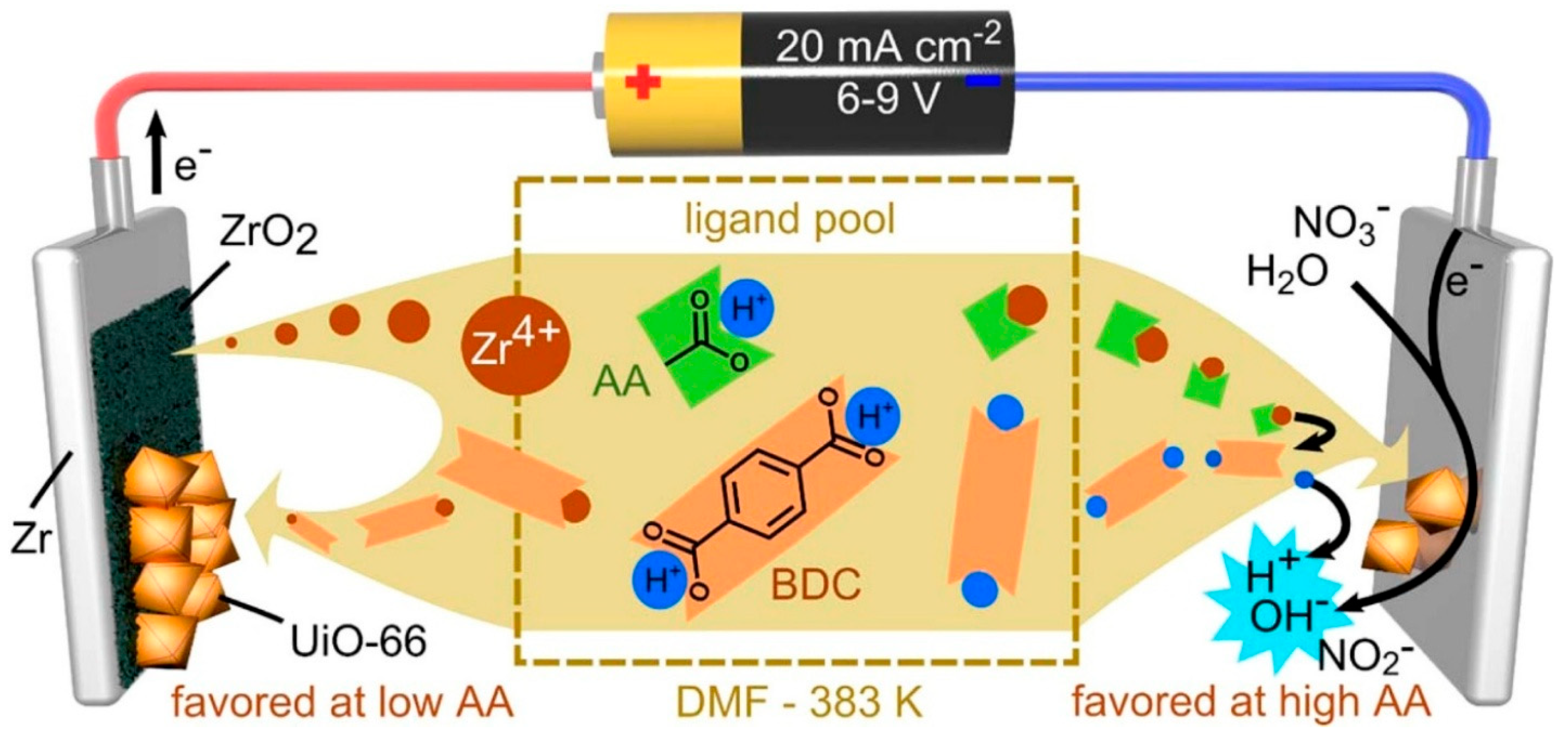
2.1. Electrochemical Deposition
2.2. In Situ (Solvothermal/Hydrothermal) Growth
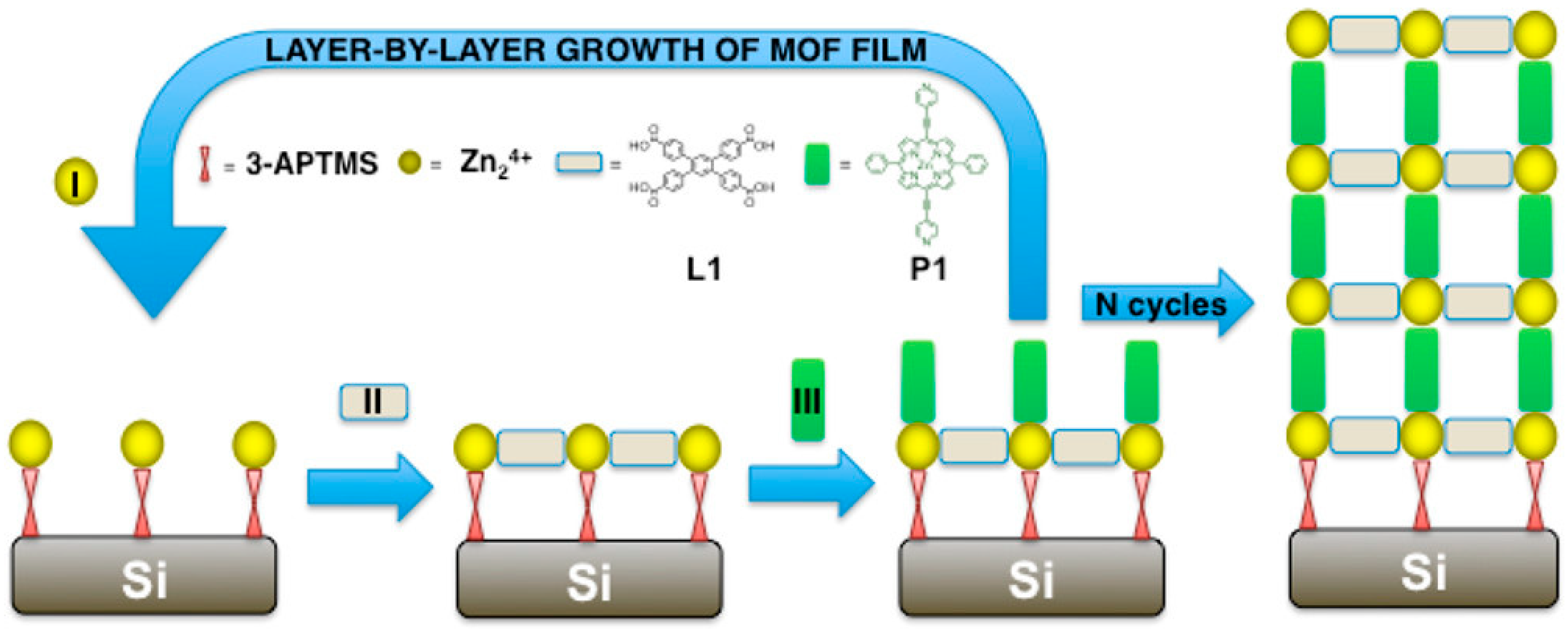
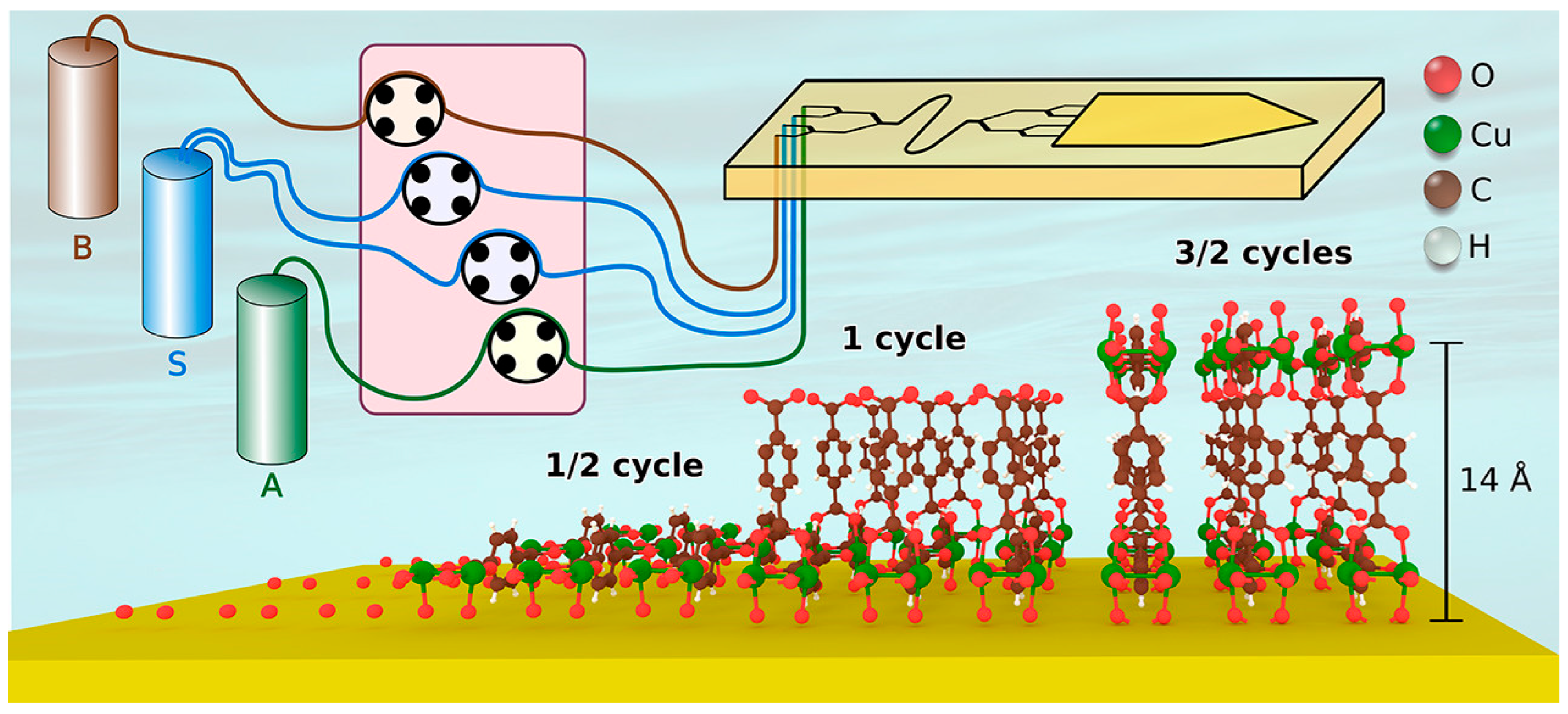
2.3. Layer-by-Layer Deposition
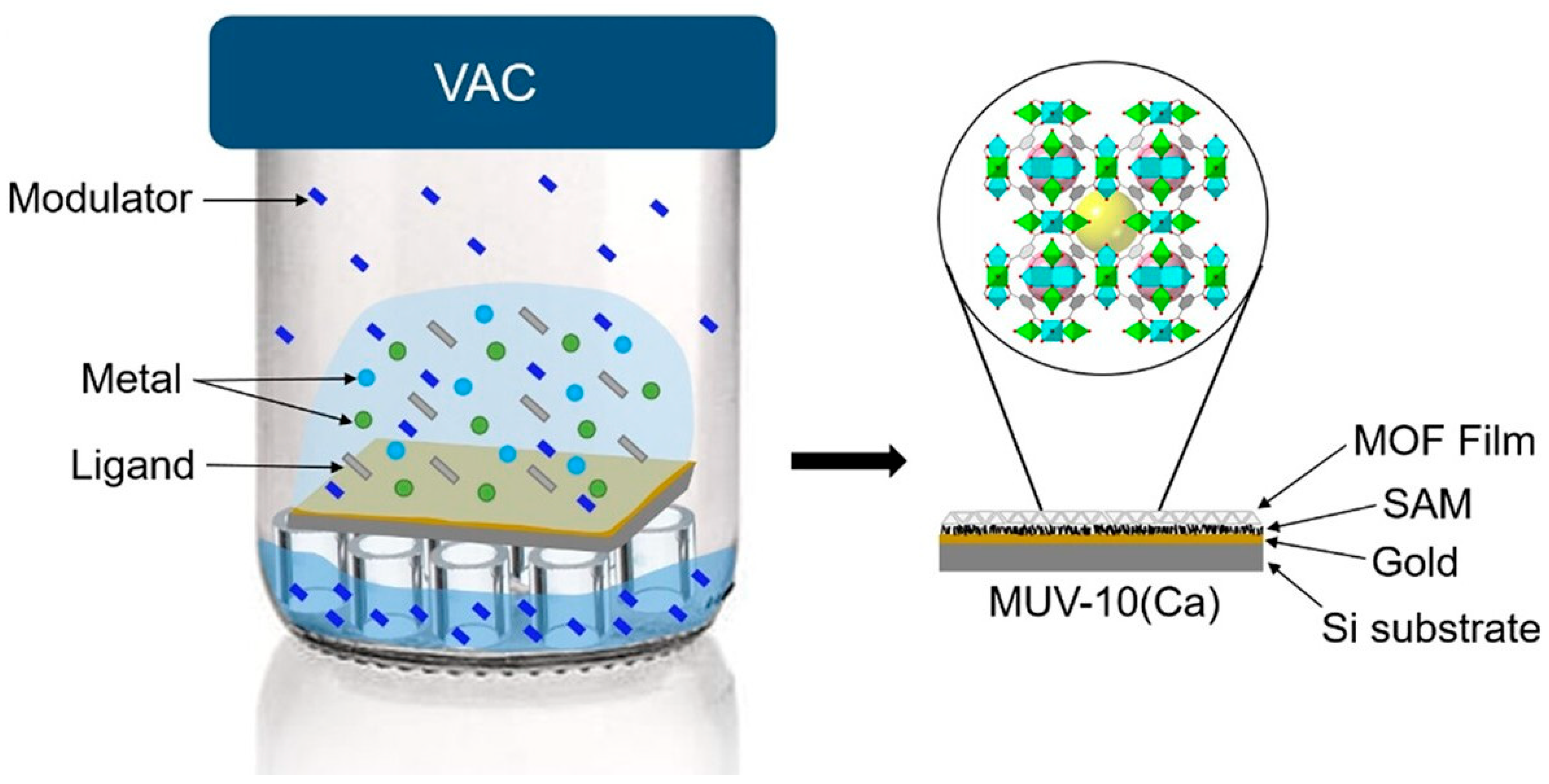
2.4. Vapor Phase Deposition
2.5. Other Coating Methods
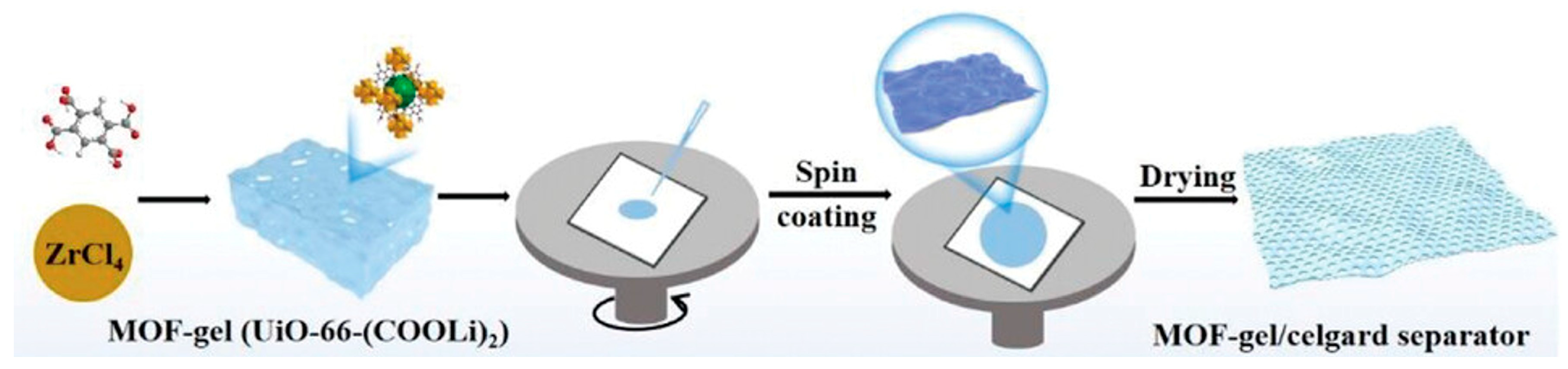
2.5.1. Spin-Coating
2.5.2. Dip-Coating
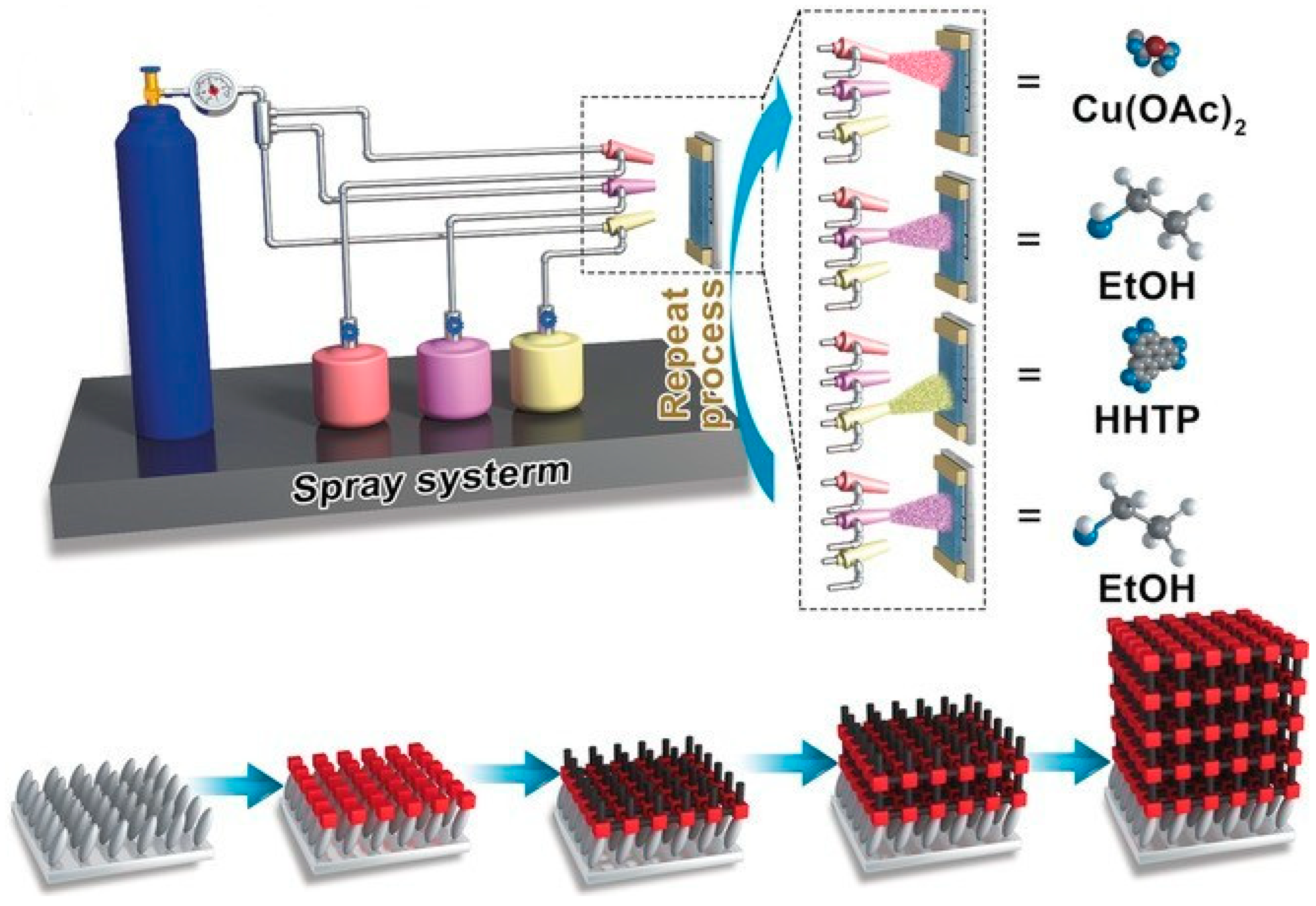
2.5.3. Spray-Coating
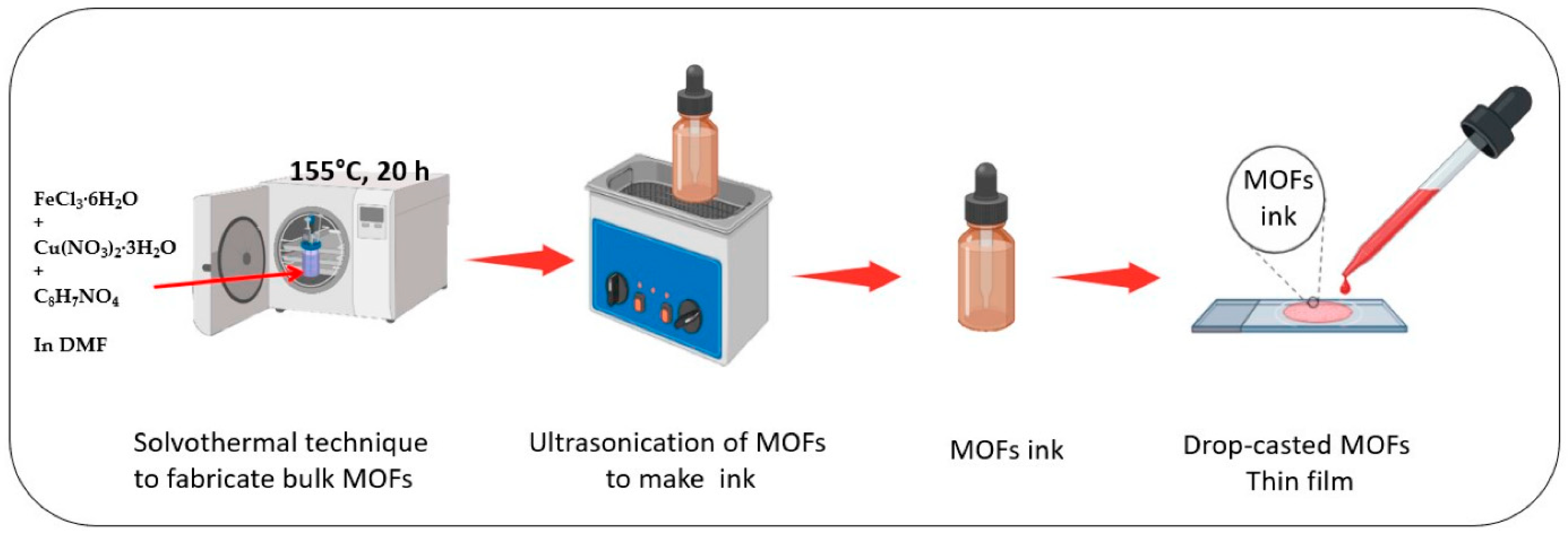
2.5.4. Drop-Casting
2.6. MOF/Substrate Interface Engineering
3. Representative Applications of MOF Coatings
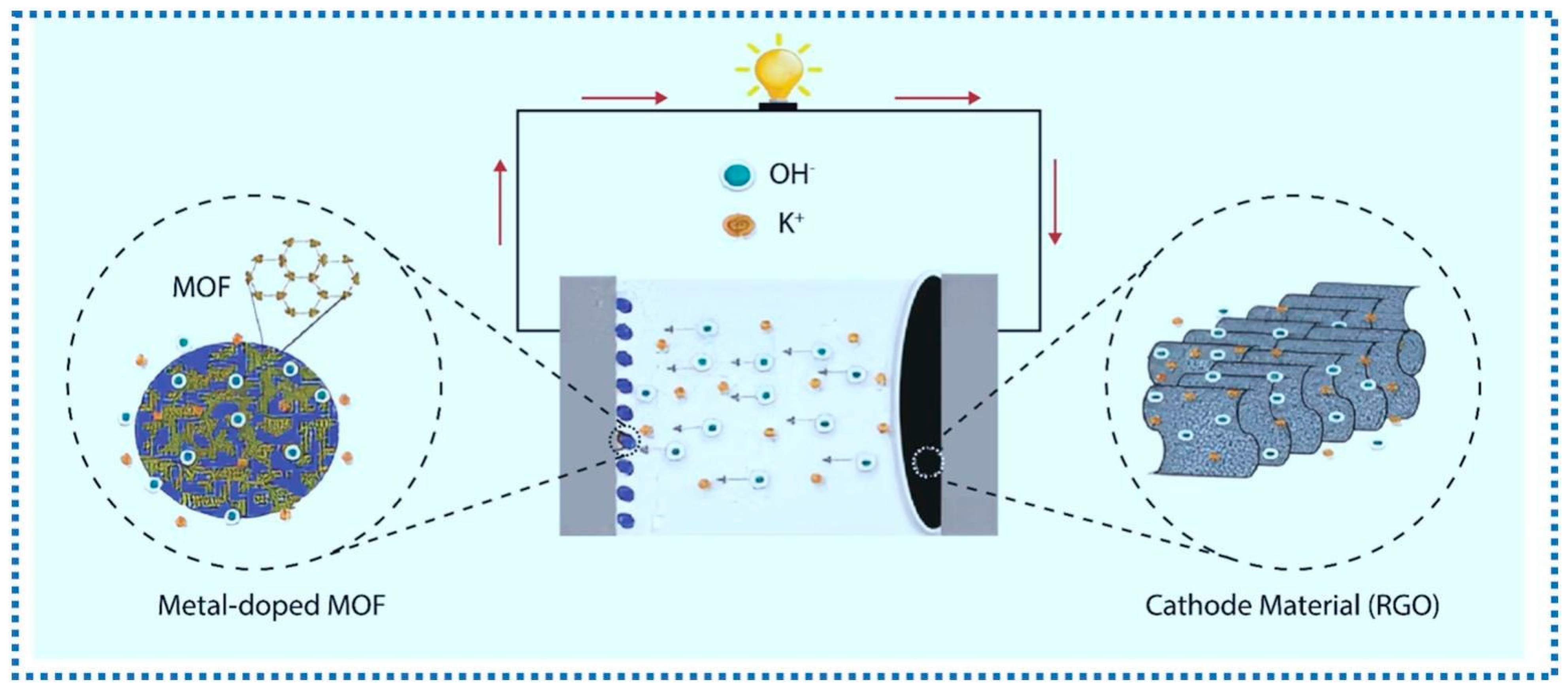
3.1. Batteries
3.2. Supercapacitor
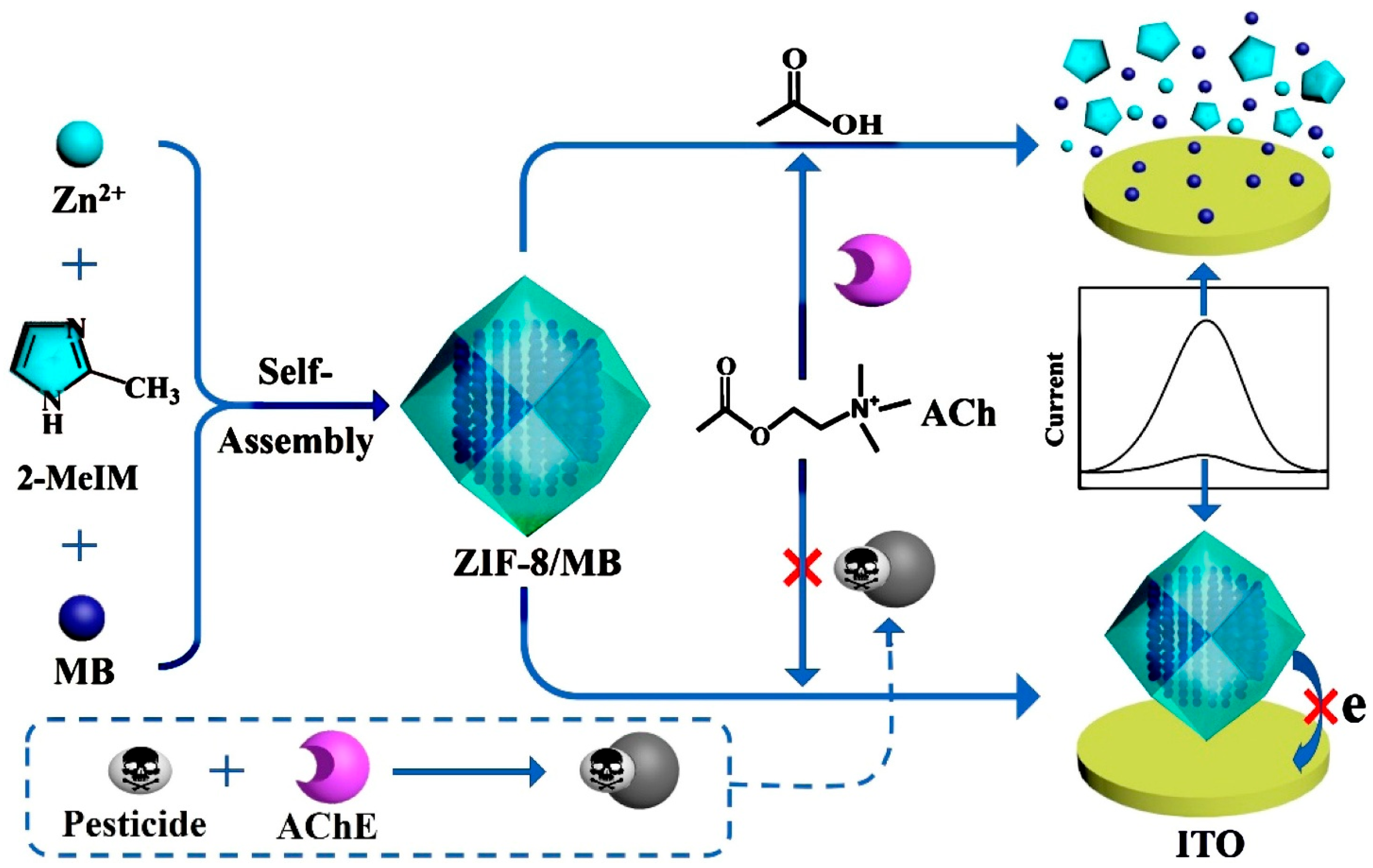
3.3. Electrochemical Sensor
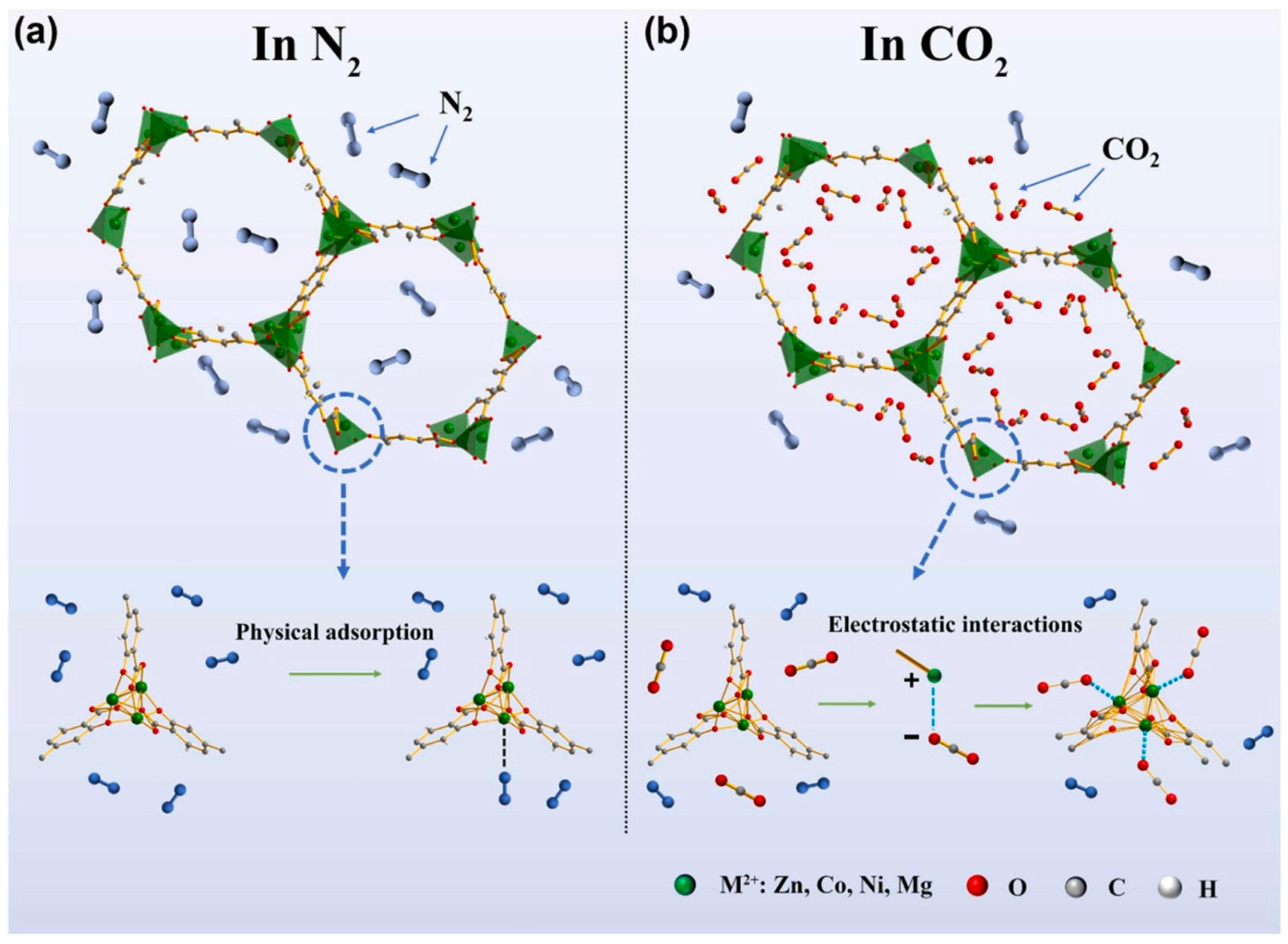
3.4. Gas Sensor
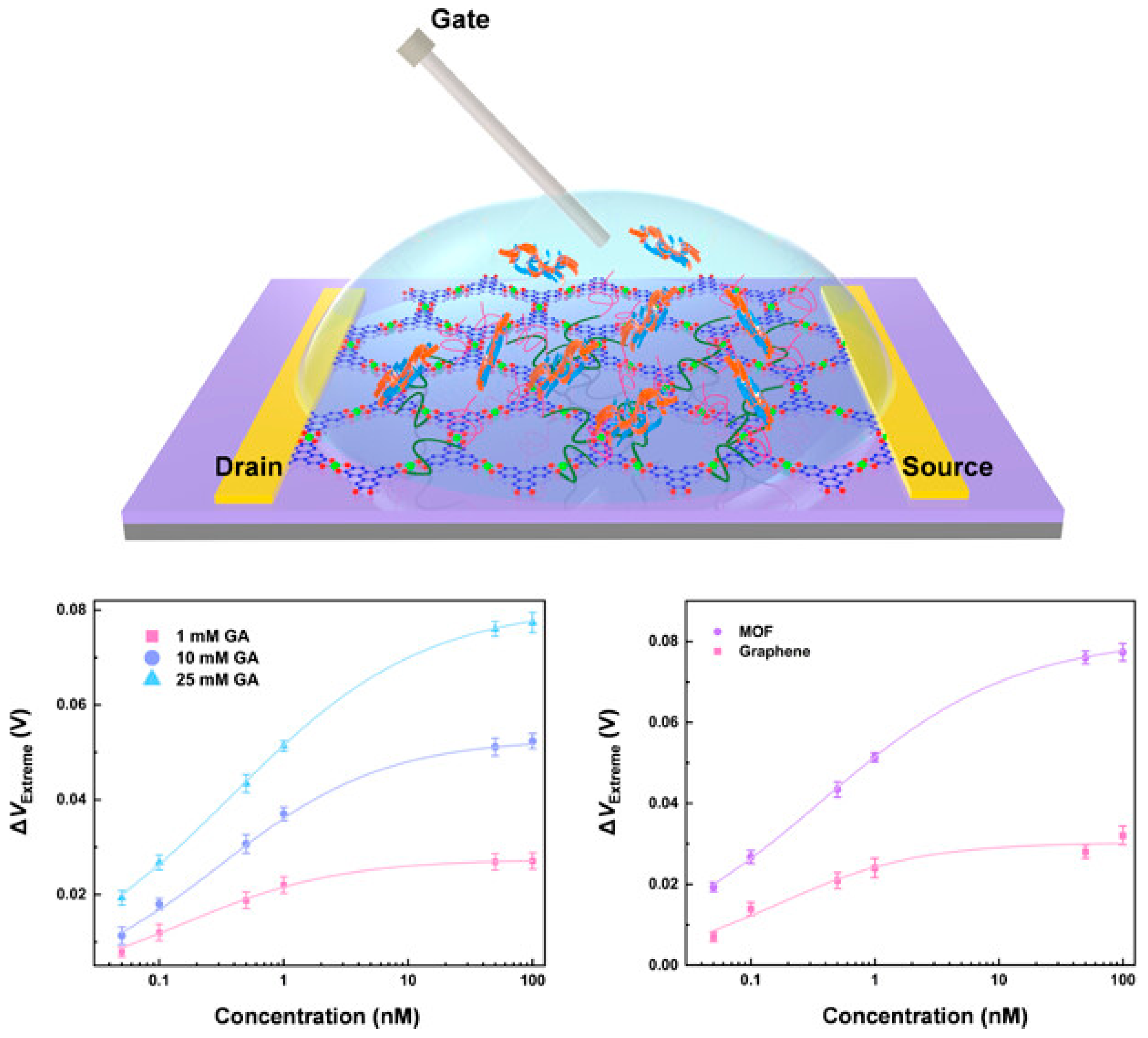
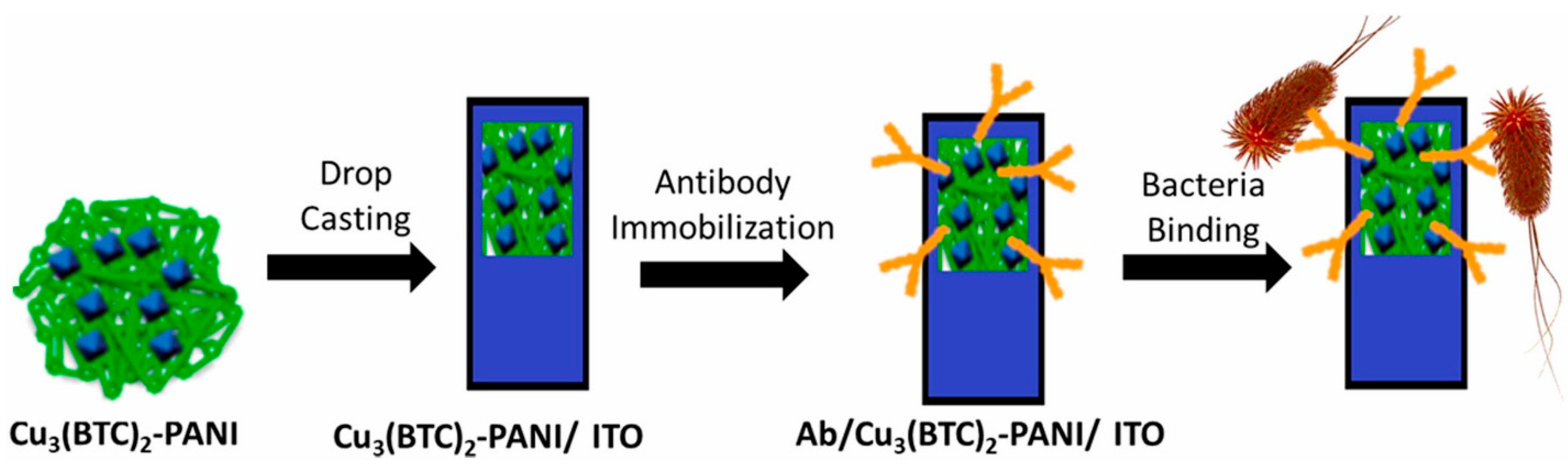
3.5. Biosensor
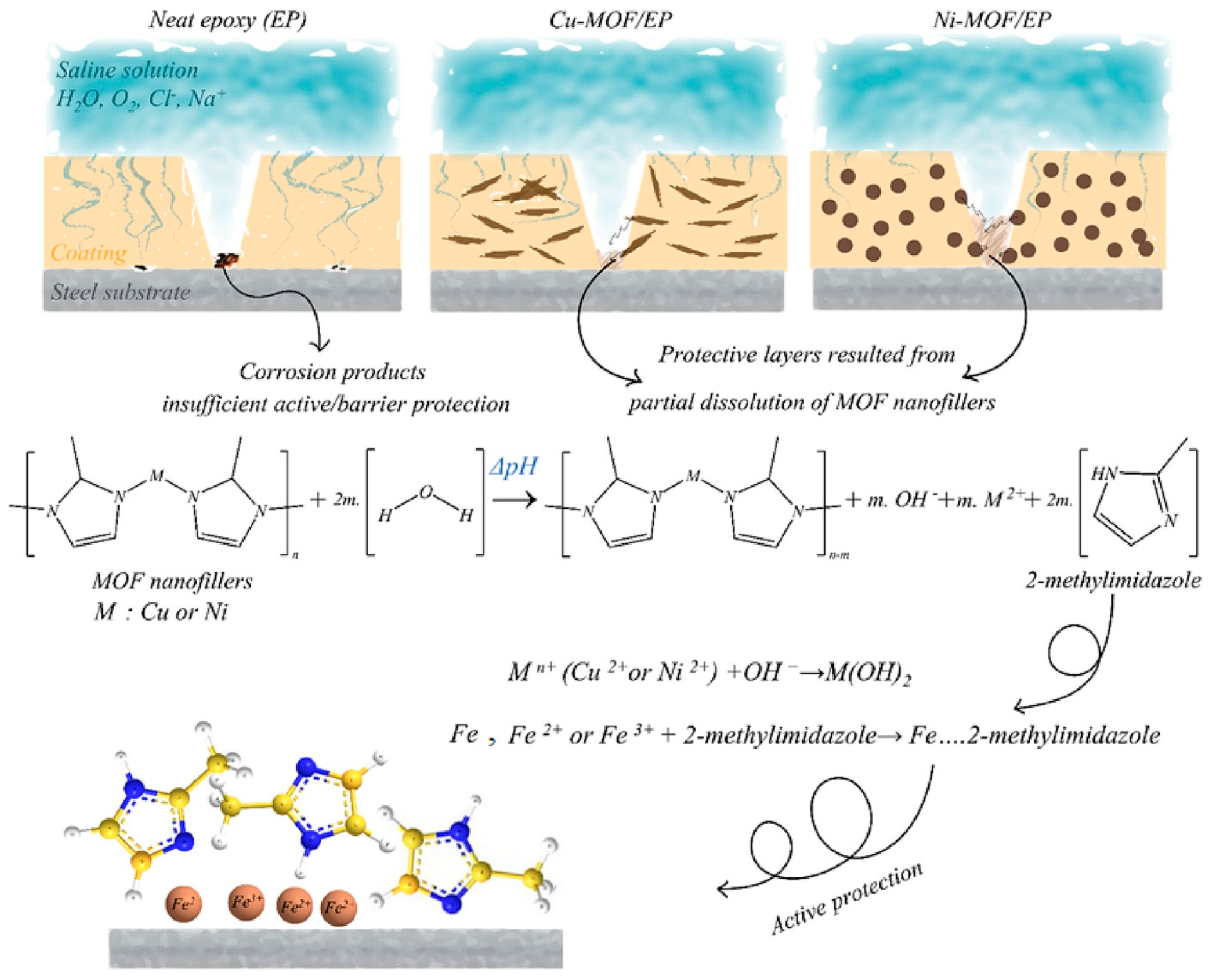
3.6. Corrosion Resistance
3.7. Biomedical Applications
3.8. Heat Exchangers
3.9. Sustainable Scale Resistance
4. Limitations and Future Perspectives
4.1. Challenges and Limitations
4.2. Biocompatibility of MOF-Based Coatings
4.3. Future Perspectives and Research Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gándara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-Pore Apertures in a Series of Metal-Organic Frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Zhou, H.-C.J.; Kitagawa, S. Metal–Organic Frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar] [CrossRef]
- Du, Y.; Jia, X.; Zhong, L.; Jiao, Y.; Zhang, Z.; Wang, Z.; Feng, Y.; Bilal, M.; Cui, J.; Jia, S. Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@MOF composites. Coord. Chem. Rev. 2022, 454, 214327. [Google Scholar] [CrossRef]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef] [PubMed]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611–8638. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme-MOF (metal-organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, P.C.; Ma, S. Metal-Organic Frameworks for Enzyme Immobilization: Beyond Host Matrix Materials. ACS Cent. Sci. 2020, 6, 1497–1506. [Google Scholar] [CrossRef]
- Ding, M.; Cai, X.; Jiang, H.-L. Improving MOF stability: Approaches and applications. Chem. Sci. 2019, 10, 10209–10230. [Google Scholar] [CrossRef] [PubMed]
- Freund, R.; Zaremba, O.; Arnauts, G.; Ameloot, R.; Skorupskii, G.; Dincă, M.; Bavykina, A.; Gascon, J.; Ejsmont, A.; Goscianska, J.; et al. The Current Status of MOF and COF Applications. Angew. Chem. Int. Ed. 2021, 60, 23975–24001. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef]
- Shi, R.; Yu, Y.-X.; Chibisov, A.N. Electrochemical reduction of cyanide on conjugated copper-organic framework Cu3(HHTP)2 monolayer: A dispersion-corrected DFT investigation. Int. J. Hydrogen Energy 2025, 107, 52–62. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Li, M.; Zhao, Z. Metal–organic framework thin films: Review of their room-temperature synthesis and applications. J. Mater. Chem. C 2024, 12, 3394–3409. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Gafari, S.; Jamali, S.; Kazemzad, M. Concepts, fabrication and applications of MOF thin films in optoelectronics: A review. Appl. Mater. Today 2024, 38, 102153. [Google Scholar] [CrossRef]
- Jeong, H.; Park, G.; Jeon, J.; Park, S.S. Fabricating Large-Area Thin Films of 2D Conductive Metal–Organic Frameworks. Acc. Chem. Res. 2024, 57, 2336–2346. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, J.; Xu, J.; Ma, J.; Bai, Y.; Cao, S.; Zhang, S.; Pang, H. Metal-organic framework (MOF) composites as promising materials for energy storage applications. Adv. Colloid Interface Sci. 2022, 307, 102732. [Google Scholar] [CrossRef]
- Shen, M.; Ma, H. Metal-organic frameworks (MOFs) and their derivative as electrode materials for lithium-ion batteries. Coord. Chem. Rev. 2022, 470, 214715. [Google Scholar] [CrossRef]
- Zhou, C.; Pan, M.; Li, S.; Sun, Y.; Zhang, H.; Luo, X.; Liu, Y.; Zeng, H. Metal organic frameworks (MOFs) as multifunctional nanoplatform for anticorrosion surfaces and coatings. Adv. Colloid Interface Sci. 2022, 305, 102707. [Google Scholar] [CrossRef]
- Choudhary, P.; Ola, S.K.; Chopra, I.; Dhayal, V.; Shekhawat, S.D. Metal–organic framework (MOF)/graphene–oxide (GO) nanocomposites materials: A potential formulation for anti-corrosive coatings- a review. Mater. Today Proc. 2023, 79, 172–178. [Google Scholar] [CrossRef]
- Chen, D.-H.; Gliemann, H.; Wöll, C. Layer-by-layer assembly of metal-organic framework thin films: Fabrication and advanced applications. Chem. Phys. Rev. 2023, 4, 011305. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, L.; Ding, Y.; Xiao, H.; Shi, Q.; Li, X.; Li, A.; Fang, G. Atomic layer deposition meets metal–organic frameworks. Prog. Mater. Sci. 2023, 138, 101159. [Google Scholar] [CrossRef]
- Babu, A.M.; Varghese, A. Electrochemical deposition for metal organic Frameworks: Advanced Energy, Catalysis, sensing and separation applications. J. Electroanal. Chem. 2023, 937, 117417. [Google Scholar] [CrossRef]
- Ulaeto, S.B.; Ravi, R.P.; Udoh, I.I.; Mathew, G.M.; Rajan, T.P. Polymer-Based Coating for Steel Protection, Highlighting Metal–Organic Framework as Functional Actives: A Review. Corros. Mater. Degrad. 2023, 4, 284–316. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Van Assche, T.; Campagnol, N.; Fransaer, J.; Denayer, J.; Tan, J.-C.; Falcaro, P.; De Vos, D.; Ameloot, R. Electrochemical Film Deposition of the Zirconium Metal–Organic Framework UiO-66 and Application in a Miniaturized Sorbent Trap. Chem. Mater. 2015, 27, 1801–1807. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, L.; Lu, S.; Wen, Y.; Shang, W. Construction of porous anodic oxide/Ce-MOFs film by induced electrodeposition and its corrosion resistance. J. Ind. Eng. Chem. 2023, 122, 378–388. [Google Scholar] [CrossRef]
- Borralho, D.; Realista, S.; Nunes, T.; Jorge, M.E.M.; Martinho, P.N. Immobilisation of Ni(II) and Zr(IV) metal-organic frameworks on electrodes using electrophoretic deposition. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Ito, T.; Jenkins, S.G.; Seifert, S.; Uysal, A. Electrochemistry-Induced Direct Deposition of Nanoscale Thin Zeolitic Imidazolate Framework-8 Films on Insulator Substrates. Cryst. Growth Des. 2023, 23, 6369–6377. [Google Scholar] [CrossRef]
- Van Assche, T.R.C.; Desmet, G.; Ameloot, R.; De Vos, D.E.; Terryn, H.; Denayer, J.F.M. Electrochemical synthesis of thin HKUST-1 layers on copper mesh. Microporous Mesoporous Mater. 2012, 158, 209–213. [Google Scholar] [CrossRef]
- Joaristi, A.M.; Juan-Alcañiz, J.; Serra-Crespo, P.; Kapteijn, F.; Gascon, J. Electrochemical Synthesis of Some Archetypical Zn2+, Cu2+, and Al3+ Metal Organic Frameworks. Cryst. Growth Des. 2012, 12, 3489–3498. [Google Scholar] [CrossRef]
- Campagnol, N.; Van Assche, T.R.C.; Li, M.; Stappers, L.; Dincă, M.; Denayer, J.F.M.; Binnemans, K.; De Vos, D.E.; Fransaer, J. On the electrochemical deposition of metal–organic frameworks. J. Mater. Chem. A 2016, 4, 3914–3925. [Google Scholar] [CrossRef]
- Zhang, X.; Luo, J.; Tang, P.; Ye, X.; Peng, X.; Tang, H.; Sun, S.-G.; Fransaer, J. A universal strategy for metal oxide anchored and binder-free carbon matrix electrode: A supercapacitor case with superior rate performance and high mass loading. Nano Energy 2017, 31, 311–321. [Google Scholar] [CrossRef]
- Bailmare, D.B.; Malozyomov, B.V.; Deshmukh, A.D. Electrodeposition of porous metal-organic frameworks for efficient charge storage. Commun. Chem. 2024, 7, 178. [Google Scholar] [CrossRef]
- Yan, C.; Shang, J.; Wan, S.-Y.; Cui, X.-T.; Liu, Z.-G.; Guo, Z. In-situ Electrodeposition of FeCo-MOF on Au Ultramicroelectrode for Highly Sensitive Detection of Epinephrine. J. Electrochem. 2025, 31, 2417001. [Google Scholar] [CrossRef]
- Araújo-Cordero, A.M.; Caddeo, F.; Mahmoudi, B.; Bron, M.; Maijenburg, A.W. Direct Electrochemical Synthesis of Metal-Organic Frameworks: Cu3(BTC)2 and Cu(TCPP) on Copper Thin films and Copper-Based Microstructures. ChemPlusChem 2024, 89, e202300378. [Google Scholar] [CrossRef]
- Varsha, M.V.; Nageswaran, G. Review—Direct Electrochemical Synthesis of Metal Organic Frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar] [CrossRef]
- Li, W.-J.; Tu, M.; Cao, R.; Fischer, R.A. Metal–organic framework thin films: Electrochemical fabrication techniques and corresponding applications & perspectives. J. Mater. Chem. A 2016, 4, 12356–12369. [Google Scholar] [CrossRef]
- Zhang, X.; Wan, K.; Subramanian, P.; Xu, M.; Luo, J.; Fransaer, J. Electrochemical deposition of metal–organic framework films and their applications. J. Mater. Chem. A 2020, 8, 7569–7587. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, X.; Tan, X.; Zhang, W.; Guo, W.; Han, N.; Zhou, Z.; Jiang, Y.; Vankelecom, I.F.J.; Fransaer, J. One-Step Reductive Electrodeposition of MOF Film on Polymer Membrane. ACS Mater. Lett. 2022, 4, 1721–1725. [Google Scholar] [CrossRef]
- Usman, M.; Yang, A.-C.; Inamdar, A.I.; Kamal, S.; Hsu, J.-C.; Kang, D.-Y.; Tseng, T.-W.; Hung, C.-H.; Lu, K.-L. Thin Film Growth of 3D Sr-based Metal-Organic Framework on Conductive Glass via Electrochemical Deposition. ChemistryOpen 2022, 11, e202100295. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Song, Y.; Huang, Y.; Zhu, H.; Yue, C.; Liu, F.; Zhao, J. One-Step Synthesis of Ultrathin Zeolitic Imidazole Framework-8 (ZIF-8) Membrane on Unmodified Porous Support via Electrophoretic Deposition. Membranes 2022, 12, 1062. [Google Scholar] [CrossRef]
- Ji, H.; Hwang, S.; Kim, K.; Kim, C.; Jeong, N.C. Direct in Situ Conversion of Metals into Metal–Organic Frameworks: A Strategy for the Rapid Growth of MOF Films on Metal Substrates. ACS Appl. Mater. Interfaces 2016, 8, 32414–32420. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Li, J.; Li, J.; Yan, Y.; Li, R. Synthesis of Ni3S2 and MOF-Derived Ni(OH)2 Composite Electrode Materials on Ni Foam for High-Performance Supercapacitors. Nanomaterials 2023, 13, 493. [Google Scholar] [CrossRef]
- Shen, W.; Guo, X.; Pang, H. Effect of Solvothermal Temperature on Morphology and Supercapacitor Performance of Ni-MOF. Molecules 2022, 27, 8226. [Google Scholar] [CrossRef] [PubMed]
- Ravipati, M.; Badhulika, S. Solvothermal synthesis of hybrid nanoarchitectonics nickel-metal organic framework modified nickel foam as a bifunctional electrocatalyst for direct urea and nitrate fuel cell. Adv. Powder Technol. 2023, 34, 104087. [Google Scholar] [CrossRef]
- Han, G.; Zhao, S.; Sui, S.; Feng, B.; Wang, Y.; Zhou, J.; Li, Z.; Tian, X.; Jia, Y.; Wang, J.; et al. A New Type Microwave Absorption Materials: Metal–Organic-Frameworks (MOFs) Used as Non-Conductive Material Combination with P-C-800. Adv. Electron. Mater. 2025, 11, 2400738. [Google Scholar] [CrossRef]
- Li, J.; Kumar, A.; Ott, S. Diffusional Electron Transport Coupled to Thermodynamically Driven Electron Transfers in Redox-Conductive Multivariate Metal–Organic Frameworks. J. Am. Chem. Soc. 2024, 146, 12000–12010. [Google Scholar] [CrossRef]
- Bhoite, A.A.; Patil, K.V.; Redekar, R.S.; Patil, P.S.; Sawant, V.A.; Tarwal, N.L. Solvothermal synthesis of binder free Ni-MOF thin films for supercapacitor electrodes. J. Solid State Chem. 2023, 326, 124192. [Google Scholar] [CrossRef]
- Bhoite, A.A.; Sawant, V.A.; Tarwal, N.L. Solvothermal synthesis of Ni/Co-based metal-organic framework with nanosheets-like structure for high-performance supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2024, 702, 134814. [Google Scholar] [CrossRef]
- Suremann, N.F.; Laporte, A.A.H.; Greenwell, F.; Ott, S. Pseudomorphic replication as enabling technology for porphyrinic metal–organic framework thin film growth. Polyhedron 2025, 275, 117519. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, L.; Kuang, Y.; Ouyang, G. Universal Strategy for Metal-Organic Framework Growth: From Cascading-Functional Films to MOF-on-MOFs. Small 2024, 20, 2307976. [Google Scholar] [CrossRef]
- So, M.C.; Jin, S.; Son, H.-J.; Wiederrecht, G.P.; Farha, O.K.; Hupp, J.T. Layer-by-Layer Fabrication of Oriented Porous Thin Films Based on Porphyrin-Containing Metal–Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 15698–15701. [Google Scholar] [CrossRef]
- Shekhah, O.; Wang, H.; Kowarik, S.; Schreiber, F.; Paulus, M.; Tolan, M.; Sternemann, C.; Evers, F.; Zacher, D.; Fischer, R.A.; et al. Step-by-Step Route for the Synthesis of Metal−Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 15118–15119. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.K.; Shekhah, O.; Wieland, D.C.F.; Paulus, M.; Sternemann, C.; Schroer, M.A.; Tiemeyer, S.; Tolan, M.; Fischer, R.A.; Wöll, C. Intercalation in Layered Metal–Organic Frameworks: Reversible Inclusion of an Extended π-System. J. Am. Chem. Soc. 2011, 133, 8158–8161. [Google Scholar] [CrossRef]
- Arslan, H.K.; Shekhah, O.; Wohlgemuth, J.; Franzreb, M.; Fischer, R.A.; Wöll, C. High-Throughput Fabrication of Uniform and Homogenous MOF Coatings. Adv. Funct. Mater. 2011, 21, 4228–4231. [Google Scholar] [CrossRef]
- Chernikova, V.; Shekhah, O.; Eddaoudi, M. Advanced Fabrication Method for the Preparation of MOF Thin Films: Liquid-Phase Epitaxy Approach Meets Spin Coating Method. ACS Appl. Mater. Interfaces 2016, 8, 20459–20464. [Google Scholar] [CrossRef]
- Li, M.-H.; Yang, Z.; Li, Z.; Wu, J.-R.; Yang, B.; Yang, Y.-W. Construction of Hydrazone-Linked Macrocycle-Enriched Covalent Organic Frameworks for Highly Efficient Photocatalysis. Chem. Mater. 2022, 34, 5726–5739. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, Z.; You, M.; Zhang, Y.; Feng, X.; Ma, X.; Meng, J. Formation of a thin and continuous MOF membrane with 2-D MOF nanosheets as seeds via layer-by-layer growth. Chem. Commun. 2019, 55, 10146–10149. [Google Scholar] [CrossRef]
- Gao, J.; Wei, W.; Yin, Y.; Liu, M.; Zheng, C.; Zhang, Y.; Deng, P. Continuous ultrathin UiO-66-NH2 coatings on a polymeric substrate synthesized by a layer-by-layer method: A kind of promising membrane for oil–water separation. Nanoscale 2020, 12, 6658–6663. [Google Scholar] [CrossRef]
- Chen, D.-H.; Haldar, R.; Neumeier, B.L.; Fu, Z.-H.; Feldmann, C.; Wöll, C.; Redel, E. Tunable Emission in Heteroepitaxial Ln-SURMOFs. Adv. Funct. Mater. 2019, 29, 1903086. [Google Scholar] [CrossRef]
- Fischer, J.C.; Steentjes, R.; Chen, D.-H.; Richards, B.S.; Zojer, E.; Wöll, C.; Howard, I.A. Determining Structures of Layer-by-Layer Spin-Coated Zinc Dicarboxylate-Based Metal-Organic Thin Films. Chem. A Eur. J. 2024, 30, e202400565. [Google Scholar] [CrossRef] [PubMed]
- Fratschko, M.; Zhao, T.; Fischer, J.C.; Werzer, O.; Gasser, F.; Howard, I.A.; Resel, R. Thin Film Formation Based on a Nanoporous Metal–Organic Framework by Layer-By-Layer Deposition. ACS Appl. Nano Mater. 2024, 7, 25645–25654. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Huang, X.; Ren, Y.; Hambsch, M.; Bodesheim, D.; Pohl, D.; Li, X.; Deconinck, M.; Zhang, B.; et al. On-liquid-gallium surface synthesis of ultra-smooth conductive metal-organic framework thin films. arXiv 2024, arXiv:2404.15357. [Google Scholar]
- Sindhu, P.; Ananthram, K.S.; Jain, A.; Tarafder, K.; Ballav, N. Charge-transfer interface of insulating metal-organic frameworks with metallic conduction. Nat. Commun. 2022, 13, 7665. [Google Scholar] [CrossRef]
- Goswami, S.; Rimoldi, M.; Anderson, R.; Lee, C.; Li, X.; Li, A.; Deria, P.; Chen, L.X.; Schaller, R.D.; Gómez-Gualdrón, D.A.; et al. Toward Ideal Metal–Organic Framework Thin-Film Growth via Automated Layer-by-Layer Deposition: Examples Based on Perylene Diimide Linkers. Chem. Mater. 2022, 34, 9446–9454. [Google Scholar] [CrossRef]
- Barr, M.K.S.; Nadiri, S.; Chen, D.-H.; Weidler, P.G.; Bochmann, S.; Baumgart, H.; Bachmann, J.; Redel, E. Solution Atomic Layer Deposition of Smooth, Continuous, Crystalline Metal–Organic Framework Thin Films. Chem. Mater. 2022, 34, 9836–9843. [Google Scholar] [CrossRef]
- Romero-Ángel, M.; Rubio-Giménez, V.; Gómez-Oliveira, E.P.; Verstreken, M.F.K.; Smets, J.; Gándara-Loe, J.; Padial, M.N.; Ameloot, R.; Tatay, S.; Martí-Gastaldo, C. Vapor-Assisted Conversion of Heterobimetallic Titanium–Organic Framework Thin Films. Chem. Mater. 2023, 35, 10394–10402. [Google Scholar] [CrossRef]
- Xue, L.; Luo, G.; Yang, X.-C.; Qin, Y.; Zhang, B. Vapor-phase methods for synthesizing metal-organic framework thin films. Innov. Mater. 2024, 2, 100047. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Mao, C.; Wang, F.; Yuan, S.; Wang, X.; Hu, Z. A MOF-Gel Based Separator for Suppressing Redox Mediator Shuttling in Li–O2 Batteries. Small 2024, 20, 2401231. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Woo, J.; Jeon, M.; Baek, J.W.; Shin, E.; Kim, J.; Park, S.; Kim, I.-D. Dual-MOF-Layered Films via Solution Shearing Approach: A Versatile Platform for Tunable Chemiresistive Sensors. ACS Nano 2025, 19, 11230–11240. [Google Scholar] [CrossRef]
- Zha, X.; Xi, R.; Wu, Y.; Xu, J.; Yang, Y. Synthesis of Good Electrical Conductivity of CoCe-BTC/PEDOT for Ultrahigh Selectivity of NO2 Detection. Sensors 2022, 22, 6891. [Google Scholar] [CrossRef] [PubMed]
- Zhestkij, N.A.; Povarov, S.A.; Volodin, L.; Chelmodeev, R.; Melkomukov, M.; Kenzhebayeva, Y.; Rzhevskiy, S.S.; Shipilovskikh, S.A.; Lubimova, A.V.; Timofeeva, M.V.; et al. One-step flashlight processing of MOF thin films for non-linear light absorption. Mater. Chem. Front. 2025, 9, 1355–1363. [Google Scholar] [CrossRef]
- Yang, H.; Giri, A.; Moon, S.; Shin, S.; Myoung, J.-M.; Jeong, U. Highly Scalable Synthesis of MoS2 Thin Films with Precise Thickness Control via Polymer-Assisted Deposition. Chem. Mater. 2017, 29, 5772–5776. [Google Scholar] [CrossRef]
- Abudayyeh, A.M.; Mahmoud, L.A.M.; Ting, V.P.; Nayak, S. Metal–Organic Frameworks (MOFs) and Their Composites for Oil/Water Separation. ACS Omega 2024, 9, 47374–47394. [Google Scholar] [CrossRef]
- Mondal, S.K.; Aina, P.O.; Rownaghi, A.A.; Rezaei, F. Design and development of UiO-67-coated PIM-1-based composites and demonstration of their detoxification performance. Chem. Eng. J. 2024, 493, 152269. [Google Scholar] [CrossRef]
- Gonzalez-Juarez, M.d.L.; Isaacs, M.A.; Bradshaw, D.; Nandhakumar, I. Enhanced Thermoelectric Properties of a Semiconducting Two-Dimensional Metal–Organic Framework via Iodine Loading. ACS Appl. Mater. Interfaces 2023, 15, 5478–5486. [Google Scholar] [CrossRef]
- Yao, M.-S.; Lv, X.-J.; Fu, Z.-H.; Li, W.-H.; Deng, W.-H.; Wu, G.-D.; Xu, G. Layer-by-Layer Assembled Conductive Metal–Organic Framework Nanofilms for Room-Temperature Chemiresistive Sensing. Angew. Chem. Int. Ed. 2017, 56, 16510–16514. [Google Scholar] [CrossRef]
- Mandal, P.; Singh, V.; Zhang, J.; Tiwari, M.K. Intercalated MOF nanocomposites: Robust, fluorine-free and waterborne amphiphobic coatings. Environ. Sci. Nano 2025, 12, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-X.; Li, C.; Chen, S.-M.; Gu, Z.-G.; Zhang, J. Layer by Layer Spraying Fabrication of Aggregation-Induced Emission Metal-Organic Frameworks Thin Film. Chem. A Eur. J. 2024, 30, e202400350. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Fu, Z.-H.; Deng, W.-H.; Wen, Y.; Wu, A.-Q.; Ye, X.-L.; Xu, G. The Growth Mechanism of a Conductive MOF Thin Film in Spray-based Layer-by-layer Liquid Phase Epitaxy. Angew. Chem. Int. Ed. 2022, 61, e202212797. [Google Scholar] [CrossRef]
- Patil, S.A.; Katkar, P.K.; Kaseem, M.; Nazir, G.; Lee, S.-W.; Patil, H.; Kim, H.; Magotra, V.K.; Thi, H.B.; Im, H.; et al. Cu@Fe-Redox Capacitive-Based Metal–Organic Framework Film for a High-Performance Supercapacitor Electrode. Nanomaterials 2023, 13, 1587. [Google Scholar] [CrossRef] [PubMed]
- Mu, A.U.; Cai, G.; Chen, Z. Metal–Organic Frameworks for the Enhancement of Lithium-Based Batteries: A Mini Review on Emerging Functional Designs. Adv. Sci. 2024, 11, 2305280. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, R.; Fang, J.; Liang, Z.; Gao, L.; Bian, J.; Zhu, J.; Zhao, Y. Metal-organic framework (MOF)-incorporated polymeric electrolyte realizing fast lithium-ion transportation with high Li+ transference number for solid-state batteries. Front. Chem. 2022, 10, 1013965. [Google Scholar] [CrossRef]
- Saleem, M.; Ahmad, F.; Fatima, M.; Shahzad, A.; Javed, M.S.; Atiq, S.; Khan, M.A.; Danish, M.; Munir, O.; Arif, S.M.B.; et al. Exploring new frontiers in supercapacitor electrodes through MOF advancements. J. Energy Storage 2024, 76, 109822. [Google Scholar] [CrossRef]
- Jia, H.; Lu, S.; Shin, S.H.R.; Sushko, M.L.; Tao, X.; Hummel, M.; Thallapally, P.K.; Liu, J.; Gu, Z. In situ anodic electrodeposition of two-dimensional conductive metal-organic framework@nickel foam for high-performance flexible supercapacitor. J. Power Sources 2022, 526, 231163. [Google Scholar] [CrossRef]
- Salunkhe, A.D.; Pawar, P.S.; Pagare, P.K.; Torane, A.P. Facile solvothermal synthesis of Ni-Co MOF/rGO nanoflakes for high-performance asymmetric supercapacitor. Electrochim. Acta 2024, 477, 143745. [Google Scholar] [CrossRef]
- Islam, R.; Tan, S.; Afroj, S.; Karim, N. Metal-Organic Framework (MOF)-based Smart E-textile Supercapacitors. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Gai, P.; Liu, X.; Li, F. Degradable metal-organic framework/methylene blue composites-based homogeneous electrochemical strategy for pesticide assay. Sens. Actuators B Chem. 2020, 323, 128701. [Google Scholar] [CrossRef]
- Janjani, P.; Bhardwaj, U.; Gupta, R.; Singh Kushwaha, H. Bimetallic Mn/Fe MOF modified screen-printed electrodes for non-enzymatic electrochemical sensing of organophosphate. Anal. Chim. Acta 2022, 1202, 339676. [Google Scholar] [CrossRef]
- De Chiara, B.; Del Duca, F.; Hussain, M.Z.; Kratky, T.; Banerjee, P.; Dummert, S.V.; Khoshouei, A.; Chanut, N.; Peng, H.; Al Boustani, G.; et al. Laser-Induced Metal–Organic Framework-Derived Flexible Electrodes for Electrochemical Sensing. ACS Appl. Mater. Interfaces 2025, 17, 3772–3784. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, M.; Wang, X.; Song, J.; Yang, X. Electrocatalysis in MOF Films for Flexible Electrochemical Sensing: A Comprehensive Review. Biosensors 2024, 14, 420. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Zhou, T.; Zhang, T. Nanoscale MOF-74-based QCM gas sensor for CO2 detection at room temperature. Sens. Actuators B Chem. 2024, 413, 135874. [Google Scholar] [CrossRef]
- Henkelis, S.E.; Percival, S.J.; Small, L.J.; Rademacher, D.X.; Nenoff, T.M. Continuous MOF Membrane-Based Sensors via Functionalization of Interdigitated Electrodes. Membranes 2021, 11, 176. [Google Scholar] [CrossRef]
- Dai, W.; Wen, K.; Meng, X.; Yu, S.; Zhao, J.; Lin, Q. Aptameric Metal–Organic Framework Nanobiosensor. ACS Appl. Nano Mater. 2024, 7, 17081–17091. [Google Scholar] [CrossRef]
- Ke, X.; Zhao, Z.; Huang, J.; Liu, C.; Huang, G.; Tan, J.; Zhu, H.; Xiao, Z.; Liu, X.; Mei, Y.; et al. Growth Control of Metal–Organic Framework Films on Marine Biological Carbon and Their Potential-Dependent Dopamine Sensing. ACS Appl. Mater. Interfaces 2023, 15, 12005–12016. [Google Scholar] [CrossRef]
- Majidi, R.; Farhadi, A.; Danaee, I.; Panah, N.B.; Zarei, D.; Nikmanesh, S. Investigation of synthesized planar Cu-MOF and spherical Ni-MOF nanofillers for improving the anti-corrosion performance of epoxy coatings. Prog. Org. Coat. 2023, 183, 107803. [Google Scholar] [CrossRef]
- Hsia, H.-H.; Chen, Y.-L.; Tai, Y.-T.; Tian, H.-K.; Kung, C.-W.; Liu, W.-R. Two-Dimensional Metal–Organic Frameworks/Epoxy Composite Coatings with Superior O2/H2O Resistance for Anticorrosion Applications. ACS Appl. Mater. Interfaces 2024, 16, 41421–41434. [Google Scholar] [CrossRef]
- Damian-Buda, A.-I.; Alipanah, N.; Bider, F.; Sisman, O.; Neščáková, Z.; Boccaccini, A.R. Metal-organic framework (MOF)-bioactive glass (BG) systems for biomedical applications—A review. Mater. Today Bio 2025, 30, 101413. [Google Scholar] [CrossRef]
- Gupta, A.; Bhardwaj, S.K.; Sharma, A.L.; Kim, K.-H.; Deep, A. Development of an advanced electrochemical biosensing platform for E. coli using hybrid metal-organic framework/polyaniline composite. Environ. Res. 2019, 171, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Domke, A.; Jakubowski, M.; Ławniczak, Ł.; Ratajczak, M.; Voelkel, A.; Sandomierski, M. Modification of titanium implants by bioactive and antibacterial zinc gallate metal organic framework. Surf. Coat. Technol. 2024, 479, 130581. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Tang, H.; Niu, X.; Yang, H.; Bai, Y.; Gu, X.; Zheng, Y. Fabrication of a Nanoscale Magnesium/Copper Metal–Organic Framework on Zn-Based Guided Bone Generation Membranes for Enhancing Osteogenesis, Angiogenesis, and Bacteriostasis Properties. ACS Appl. Mater. Interfaces 2024, 16, 5648–5665. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, T.B.; Imran, M.; Tandis, E. Applications of MOF-Based Nanocomposites in Heat Exchangers: Innovations, Challenges, and Future Directions. Nanomaterials 2025, 15, 205. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Feng, Y.; Wu, J.; Wang, R.; Ge, T. Performance evaluation of MIL-101(Cr) based desiccant-coated heat exchangers for efficient dehumidification. Energy 2024, 289, 130049. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, Z.; Chen, G. Constructing flexible metal-organic framework/polymer/carbon nanotubes ternary composite films with enhanced thermoelectric properties for heat-to-electricity conversion. Compos. Commun. 2022, 29, 100997. [Google Scholar] [CrossRef]
- Kim, H.K.; Yun, W.S.; Kim, M.-B.; Kim, J.Y.; Bae, Y.-S.; Lee, J.; Jeong, N.C. A Chemical Route to Activation of Open Metal Sites in the Copper-Based Metal–Organic Framework Materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 137, 10009–10015. [Google Scholar] [CrossRef]
- Peterson, G.W.; Lee, D.T.; Barton, H.F.; Epps, T.H.; Parsons, G.N. Fibre-based composites from the integration of metal–organic frameworks and polymers. Nat. Rev. Mater. 2021, 6, 605–621. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Derakhshandeh, P.G.; Depauw, H.; Coudert, F.-X.; Vrielinck, H.; Van Der Voort, P.; Leus, K. Mixed-metal metal–organic frameworks. Chem. Soc. Rev. 2019, 48, 2535–2565. [Google Scholar] [CrossRef]
- Elrasheedy, A.; Nady, N.; Bassyouni, M.; El-Shazly, A. Metal Organic Framework Based Polymer Mixed Matrix Membranes: Review on Applications in Water Purification. Membranes 2019, 9, 88. [Google Scholar] [CrossRef]
- Albdoor, A.K.; Ma, Z.; Cooper, P.; Al-Ghazzawi, F.; Liu, J.; Richardson, C.; Wagner, P. Air-to-air enthalpy exchangers: Membrane modification using metal-organic frameworks, characterisation and performance assessment. J. Clean. Prod. 2021, 293, 126157. [Google Scholar] [CrossRef]
- Li, H.; Yan, M.; Zhao, W. Designing a MOF-based slippery lubricant-infused porous surface with dual functional anti-fouling strategy. J. Colloid Interface Sci. 2022, 607, 1424–1435. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Zhang, X.; Li, Q.; Zhang, G.; Pu, S.; Liu, F.-Q. UV-curable PBMA coating containing CuZn-MOF-74 for fouling-resistance. Microporous Mesoporous Mater. 2024, 368, 113020. [Google Scholar] [CrossRef]
- Moaness, M.; El-Sayed, S.A.M.; Beherei, H.H.; Mabrouk, M. Enhancing the Antifouling Properties of Alumina Nanoporous Membranes by GO/MOF Impregnated Polymer Coatings: In Vitro Studies. J. Funct. Biomater. 2024, 15, 50. [Google Scholar] [CrossRef]
- Wang, M.; Zi, Y.; Zhu, J.; Huang, W.; Zhang, Z.; Zhang, H. Construction of super-hydrophobic PDMS@MOF@Cu mesh for reduced drag, anti-fouling and self-cleaning towards marine vehicle applications. Chem. Eng. J. 2021, 417, 129265. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Xiao, X.; Klomkliang, N.; Yusubov, M.S.; Verpoort, F. Tunable Metal–Organic Frameworks for Heat Transformation Applications. Nanomaterials 2018, 8, 661. [Google Scholar] [CrossRef]
- Shahvari, S.Z.; Kalkhorani, V.A.; Clark, J.D. Performance evaluation of a metal organic frameworks based combined dehumidification and indirect evaporative cooling system in different climates. Int. J. Refrig. 2022, 140, 186–197. [Google Scholar] [CrossRef]
- Hou, P.; Zu, K.; Qin, M.; Cui, S. A novel metal-organic frameworks based humidity pump for indoor moisture control. Build. Environ. 2021, 187, 107396. [Google Scholar] [CrossRef]
- Zu, K.; Qin, M. Optimization of the hygrothermal performance of novel metal-organic framework (MOF) based humidity pump: A CFD approach. Energy 2022, 259, 125073. [Google Scholar] [CrossRef]
- Kummer, H.; Jeremias, F.; Warlo, A.; Füldner, G.; Fröhlich, D.; Janiak, C.; Gläser, R.; Henninger, S.K. A Functional Full-Scale Heat Exchanger Coated with Aluminum Fumarate Metal–Organic Framework for Adsorption Heat Transformation. Ind. Eng. Chem. Res. 2017, 56, 8393–8398. [Google Scholar] [CrossRef]
- Aziz, A.N.; Mahmoud, S.; Al-Dadah, R.; Taskin, A.; Ismail, M.A.; Fahmy, Y.M.; Rashid, M.M. Novel MOF-303/G coated wire-finned heat exchanger for dehumidification applications–Experimental investigation. Energy 2024, 305, 132333. [Google Scholar] [CrossRef]
- Albaik, I.; Al-Dadah, R.; Mahmoud, S.; Solmaz, İ. Non-equilibrium numerical modelling of finned tube heat exchanger for adsorption desalination/cooling system using segregated solution approach. Appl. Therm. Eng. 2021, 183, 116171. [Google Scholar] [CrossRef]
- Wang, S.; Fan, Y.; Wang, Y.; Gui, L.; Huang, S.; Wu, T.; Tian, X. Light-Responsive Metal–Organic Framework (MOF)-Based Liquid-Like Coating for Sustainable Scale Resistance. Adv. Funct. Mater. 2024, 34, 2311036. [Google Scholar] [CrossRef]



| Coating Method | Principle of Operation | Advantages | Limitations | Coated MOFs | Substrates | Key Parameters/Consideration | Refs. |
|---|---|---|---|---|---|---|---|
| Anodic electro-deposition | Oxidation at the anode releases metal ions to react with ligands in solution and form MOFs on anode surface. | Controlled growth; tunable thickness; compatible with electronic devices. | Limited to conductive, sacrificial anode materials. | Cu-BTC; Ce-MOFs; Ni-MOF-74; HKUST-1 MOF. | Electrodes; metal surfaces. | The voltage applied varies by metal. | [24,25,26,27,28,29,30,31] |
| Cathodic electro-deposition | Reduction at the cathode generates OH− to increase pH and to trigger ligand deprotonation; metal ions in solution then react with deprotonated ligands to form MOFs on the cathode. | Better control over MOF composition; applicable to non-conductive substrates including thin films and glass. | Side-reactions may occur if pH is not well-controlled; adhesion may vary. | ZIF-8; ZIF-67; Co-MOF; Fe/Co-MOF; MOF-5; MIL-100. | Electrodes; inert polymeric thin films; non-conductive glass. | Voltage applied is ~1.5 V; metal ion concentration needs to be regulated in solution. | [24,32,33,34,35,36,37,38,39] |
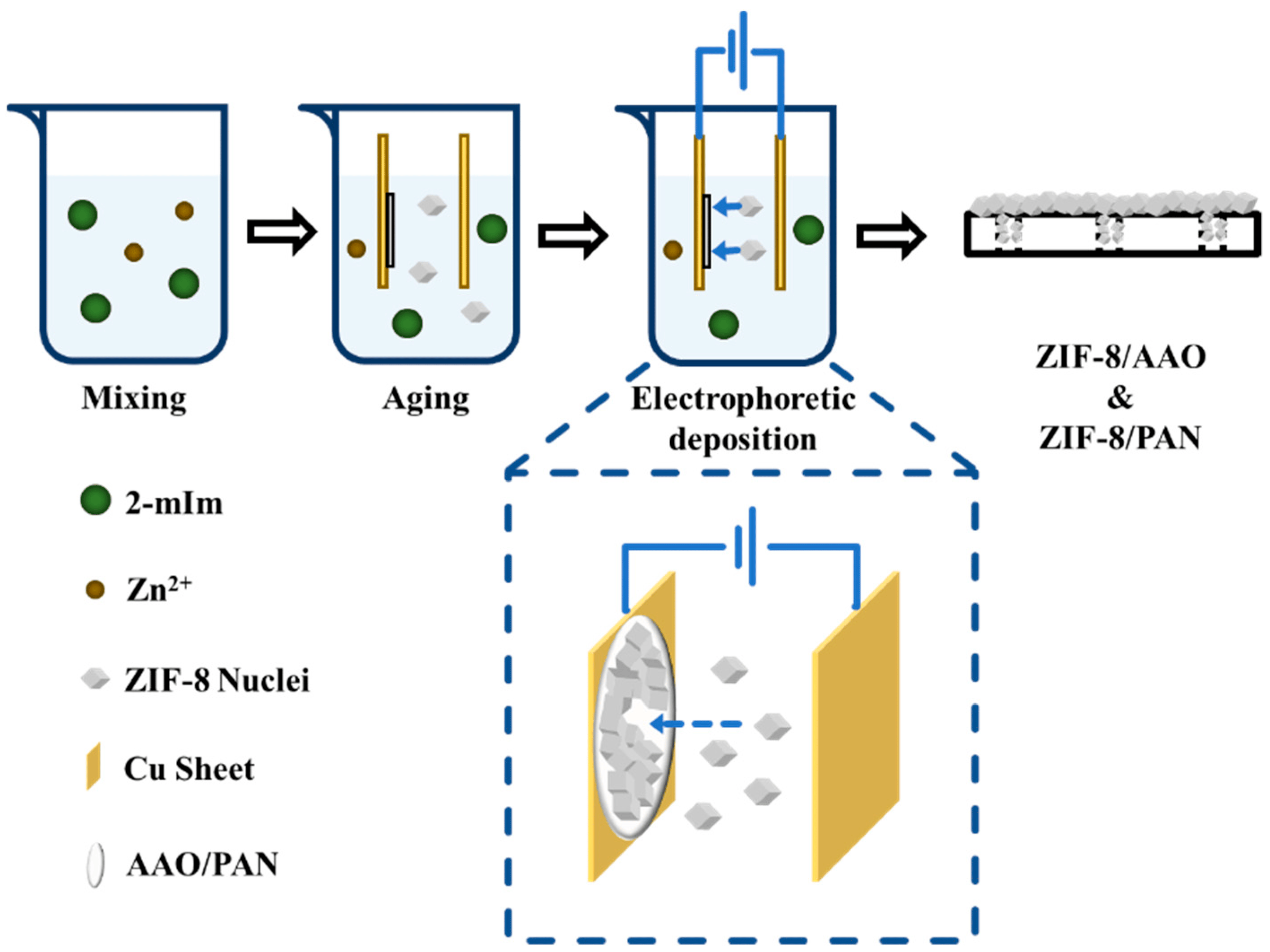
| Electro-phoretic deposition | Charged, presynthesized MOFs are immobilized onto an oppositely charged electrode surface under an electric field. | Simple operation; universal applicability. | Film/coating stability may require extra design: poor uniformity if MOFs suspension is unstable. | UiO-66; UiO-66-NH2; Ni-MOF-74. | Negatively charged surfaces, such as the FTO glass. | It requires stable MOF suspension as well as control over voltage and deposition time. | [26,40] |
| Solvothermal /hydrothermal in situ growth | Metal ions and ligands react in organic solvents or water at high temperature and pressure to grow MOFs directly on the immersed substrate surfaces. | Strong adhesion; uniform coating; high crystallinity. | MOFs and substrates sensitive to temperature are not adaptable; reaction rate is slow; it often uses toxic solvents; it is difficult to control thickness/morphology. | Cu-BTC; Ni-MOF; Ni/Co MOFs; multi-variant MOFs. | FTO; metal films (Cu, Ni); stainless steel; glass; stable polymers. | Critical surface cleaning; pretreatment (plasma etching, salinization); control of precursor concentration, temperature, and duration; modulators are needed. | [41,42,43,44,45,46,47,48,49,50] |
| Layer-by-Layer (LbL) deposition | Sequential deposition of precursor layers, such as metal ions and organic linkers, followed by rinsing steps builds up one MOF layer at a time. | Atomic level thickness control; exceptional uniformity; tunable composition; formation of heteroepitaxial interfaces. | It is time-consuming for thick films; it may require vacuum and volatile precursors; it is limited by precursor suitability and film stability. | Zn-BPDC; Cu-BDC; PDI-linker MOFs. | Silicon; glass; FTO; liquid gallium. | Alternating precursor exposure and rinsing; proper substrate pretreatment and temperature control. | [20,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65] |
| Vapor Phase Deposition: vapor-assisted conversion (VAC) | Evaporated precursors react with solvent vapor to form crystalline MOF films. | Highly crystalline films; mild reaction conditions. | It requires a solvent; there is a potential for corrosion, contaminant, or non-uniform coating. | ZIF-8; ZIF-67, PCN-6; CAT-1; Co-MOF-74; Ni-MOF-74; Mg-MOF-74; PCN-222; UiO series. | Compatible precursors. | Controlled precursors and solvent ratio; moderate temperature. | [66,67] |
| Vapor Phase Deposition: vapor-phase transformation (VPT) | Metal oxide precursor film is transformed into MOF film by reaction with vapor-phase organic linkers. | Solvent-free; superior shape retention and controllability. | Demanding conditions; thickness deformation; reduced mechanical stability. | ZIF-8; ZIF-61; ZIF-67; ZIF-72; Cu-CDC; Cu-BDC; HKUST-1. | Substrates precoated with metal oxide films. | CVD/PVD for oxide film; controlled organic linker vapor exposure. | |
| Vapor Phase Deposition: vapor-phase linker exchange (VPLE) | Existing MOF or hybrid film reacts with organic linker vapor to exchange linkers. | Allows property modification; minimal film deformation. | Mechanism or degree of exchange needs more study. | ZIF-8; ZIF-8/I; ZIF-8/Br; carboxy-late MOFs. | Substrates with preexisting MOF/hybrid films. | Controlled exposure to linker vapor. | |
| Vapor Phase Deposition: atomic layer deposition (ALD)/ molecular layer deposition (MLD) | Sequential exposure of substrate to volatile precursors in the vapor phase to form MOFs. | Atomic level thickness control; exceptional uniformity; tunable composition; formation of heteroepitaxial interfaces. | Challenges in crystallinity; a limited range of MOFs; it requires volatile/stable precursors. | MOF-5 nanofilm; NU-1000; UiO-66; ZIF-8, Cu-TPA; Mn; Co-based MOF films. | Various, suitable for vacuum. | Controlled precursor pulses; temperature cycles. | [65] |
| Spin-coating | MOF solution or slurry is spread on a rotating substrate by centrifugal force while solvent evaporating. | Simple; rapid; scalable; cost-effective; good uniformity; can be combined with LPE. | Limited surface size/curvature; high viscosity; spin rate control are needed. | Cu2(bdc)2•xH2O; Zn2(bdc)2•xH2O; HKUST1; ZIF8. | Gold; silicon; glass; porous stainless steel; aluminum oxide. | Substrate preprocessing; control over spin speed/time and solution viscosity. | [55,68,69,70,71,72] |
| Dip-coating | Substrates are dipped into an MOF solution or suspension and withdrawn at a controlled rate. | Simple; scalable; applicable to complex shapes. | Inconsistent quality (gravimetric effects); thermal stability of substrate during drying. | UiO-67; Cu-HHTP MOFs. | FTO glass; polymer sheets. | Controlled withdrawal speed; solution stability; drying conditions. | [73,74,75] |
| Spray-coating | Fine droplets of MOF solution or suspension are sprayed onto the substrate. | Rapid; suitable for large or complex surfaces; can be combined with LbL or LPE. | Uniformity depends on technique; potential material waste. | UiO-66; Zn-TPE; Cu3(HHTP)2. | Glass; metals; plastics; quartz; sapphire; Si/SiO2. | Control over spray pressure, distance, and nozzle; substrate temperature; precursor solution stability. | [76,77,78,79] |
| Drop-casting | Droplets of MOF solution or suspension are placed on the surface and dried. | Easy operation; coats complex materials. | Difficult to control thickness or uniformity; potential cracking; small scale. | UiO-67; Mn/Fe-BDC MOFs. | Polymer sheets; screen-printed electrodes. | Solution concentration or volume; drying conditions; surface wettability. | [74,75,76,77,78,79,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.Z.; Dipti, T.T.; Liu, L.; Wan, C.; Feng, L.; Yang, Z. Coating Metal–Organic Frameworks (MOFs) and Associated Composites on Electrodes, Thin Film Polymeric Materials, and Glass Surfaces. Nanomaterials 2025, 15, 1187. https://doi.org/10.3390/nano15151187
Hasan MZ, Dipti TT, Liu L, Wan C, Feng L, Yang Z. Coating Metal–Organic Frameworks (MOFs) and Associated Composites on Electrodes, Thin Film Polymeric Materials, and Glass Surfaces. Nanomaterials. 2025; 15(15):1187. https://doi.org/10.3390/nano15151187
Chicago/Turabian StyleHasan, Md Zahidul, Tyeaba Tasnim Dipti, Liu Liu, Caixia Wan, Li Feng, and Zhongyu Yang. 2025. "Coating Metal–Organic Frameworks (MOFs) and Associated Composites on Electrodes, Thin Film Polymeric Materials, and Glass Surfaces" Nanomaterials 15, no. 15: 1187. https://doi.org/10.3390/nano15151187
APA StyleHasan, M. Z., Dipti, T. T., Liu, L., Wan, C., Feng, L., & Yang, Z. (2025). Coating Metal–Organic Frameworks (MOFs) and Associated Composites on Electrodes, Thin Film Polymeric Materials, and Glass Surfaces. Nanomaterials, 15(15), 1187. https://doi.org/10.3390/nano15151187







