A Stable Metal Chalcogenide Cluster-Based Framework Decorated with Transition Metal Complexes for an Efficient Electrocatalytic O2 Reduction Reaction
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
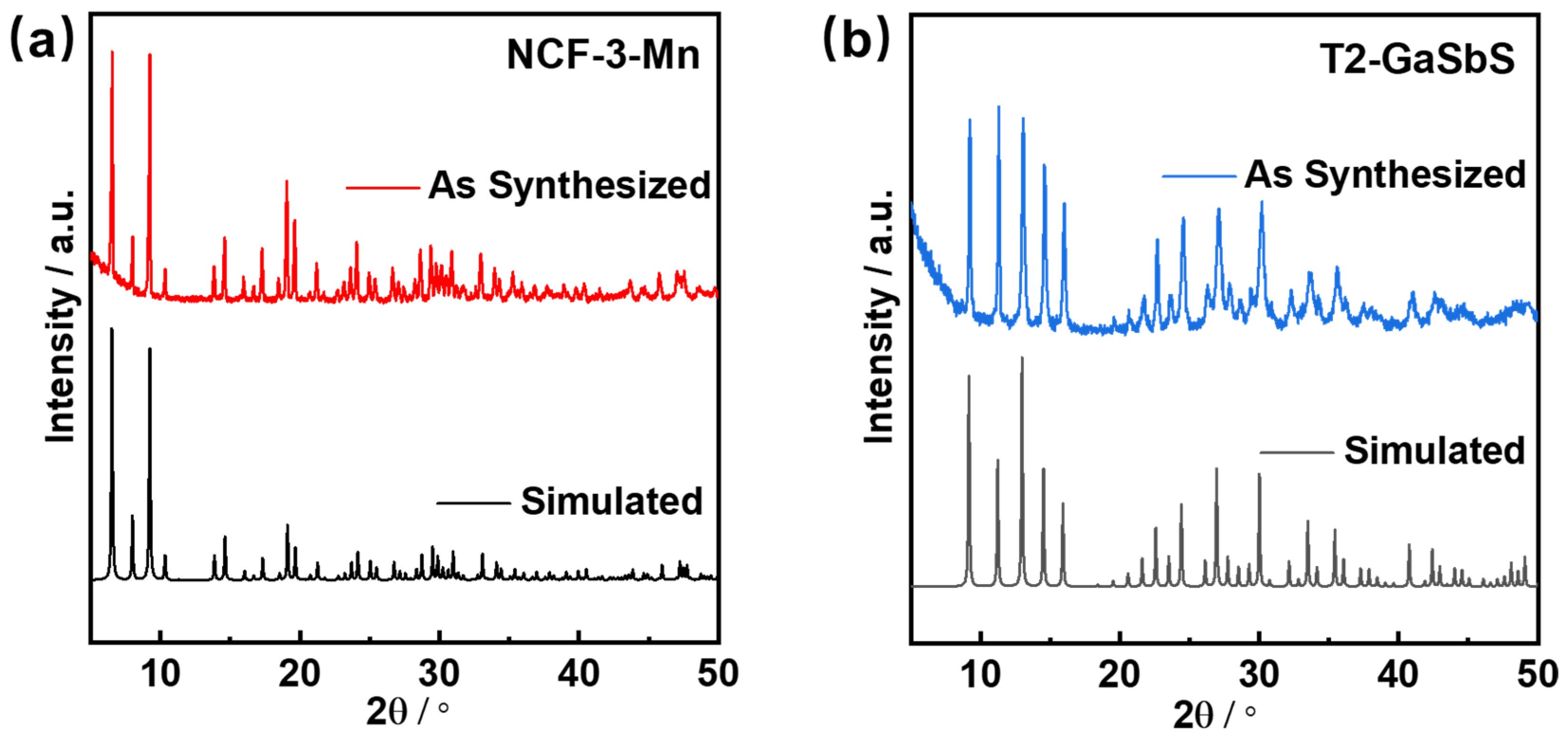
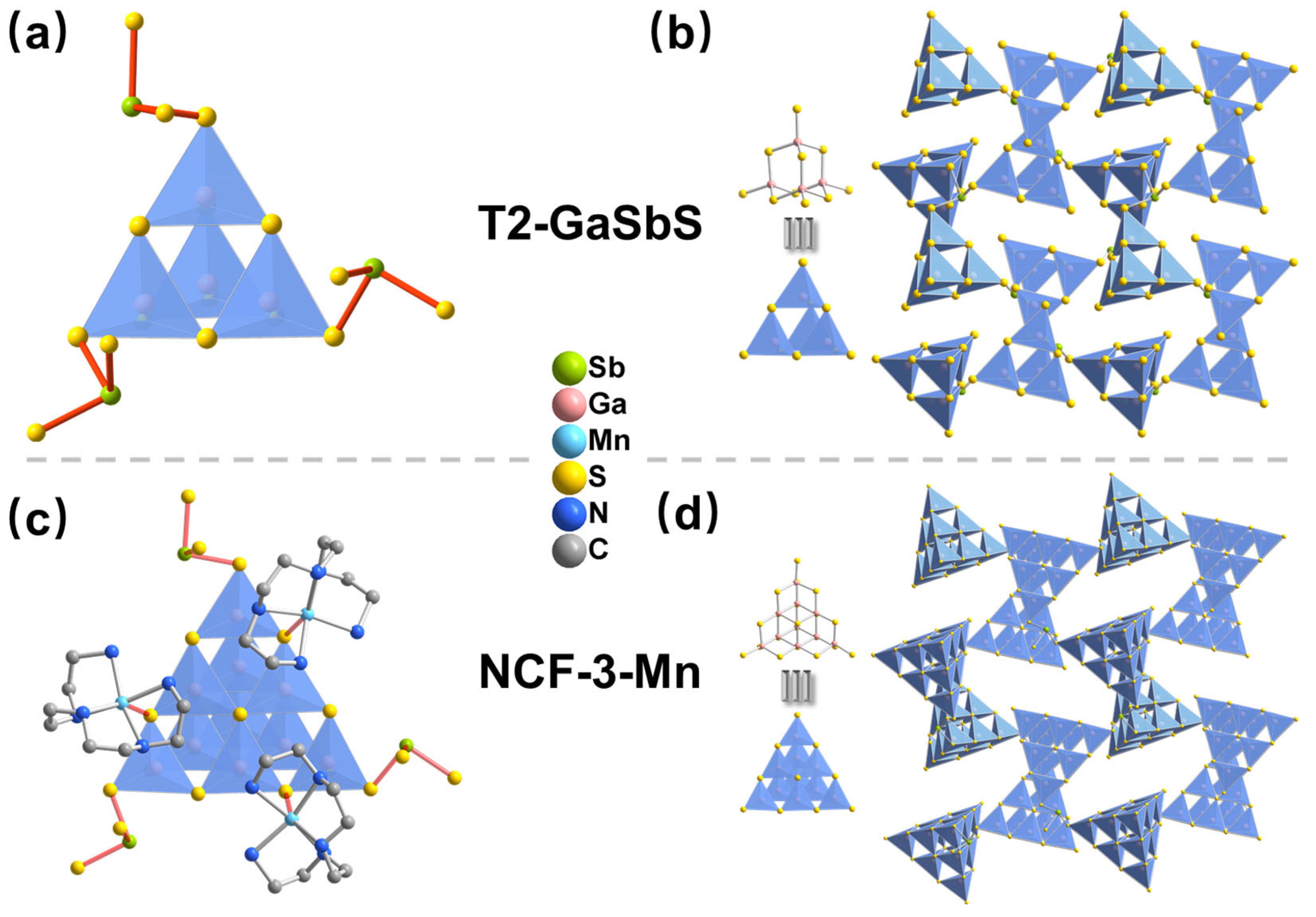
3.1. Structure Characterization
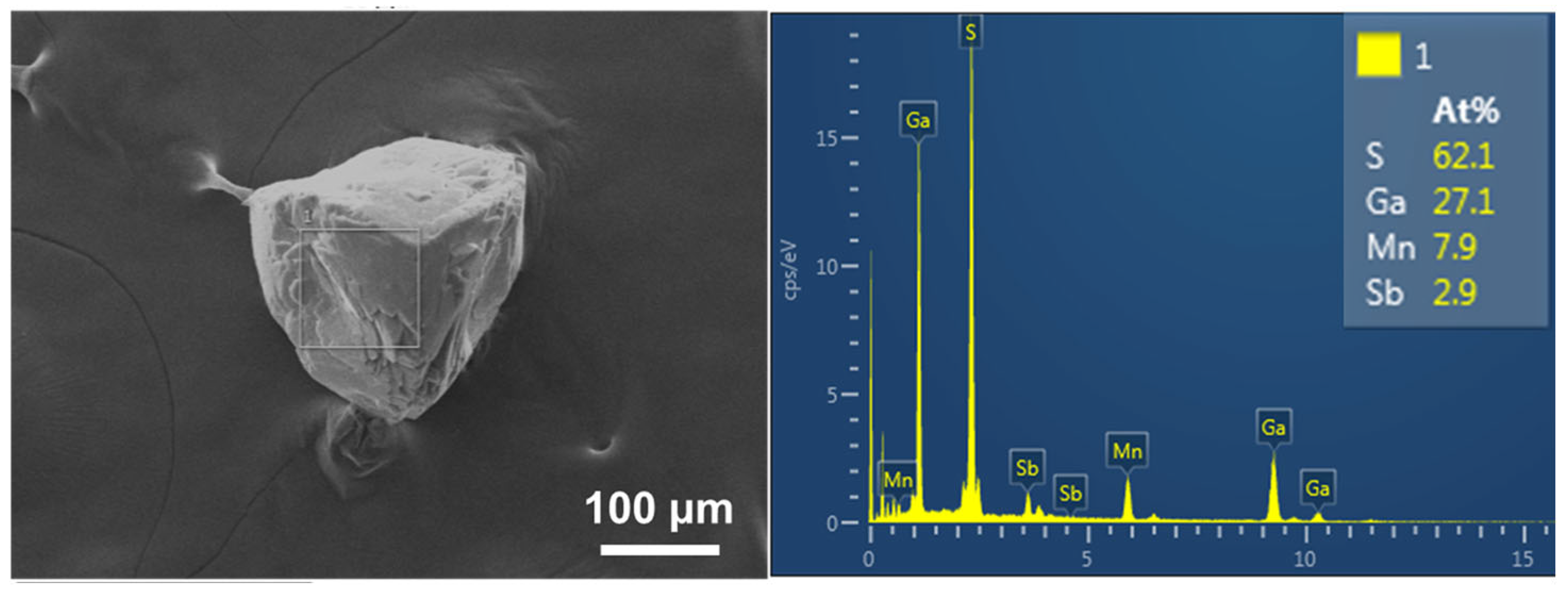
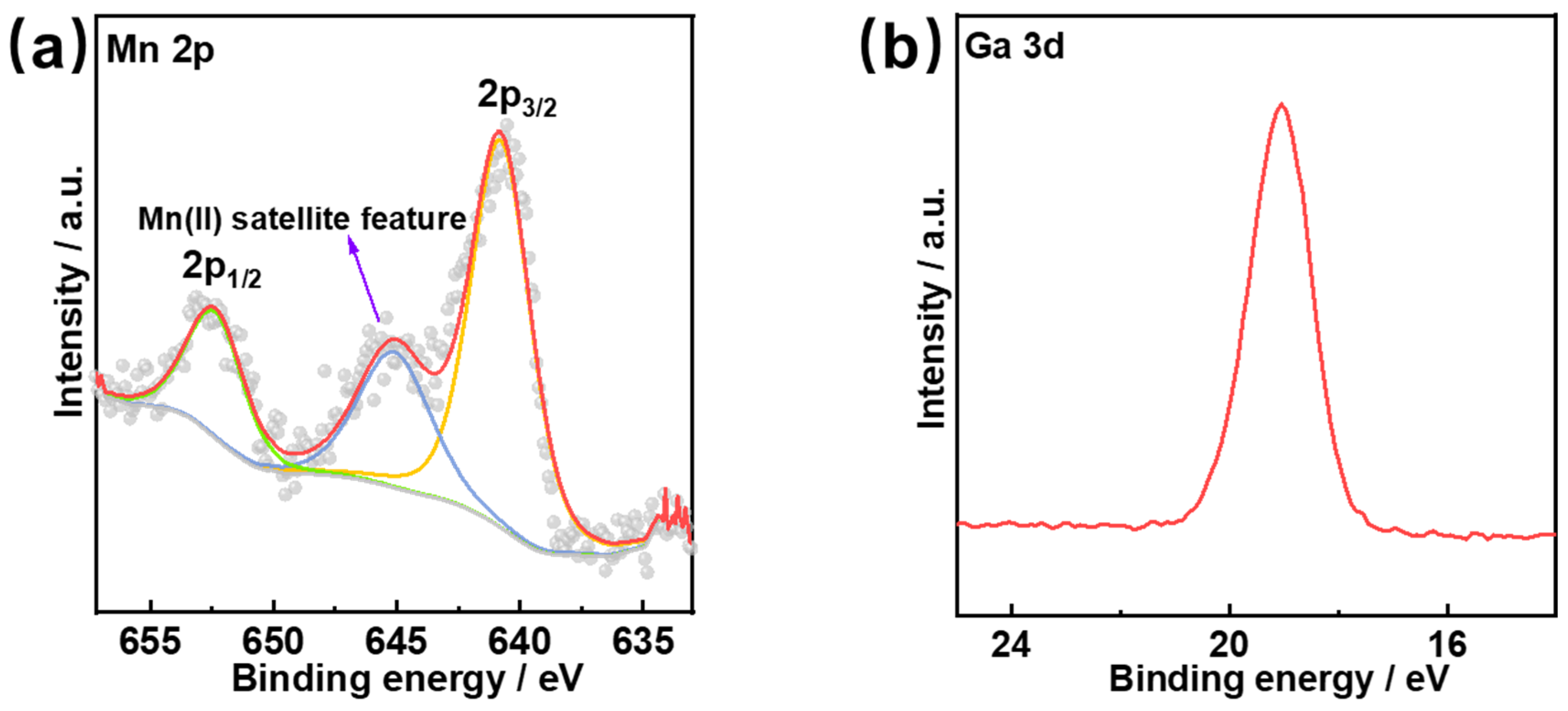
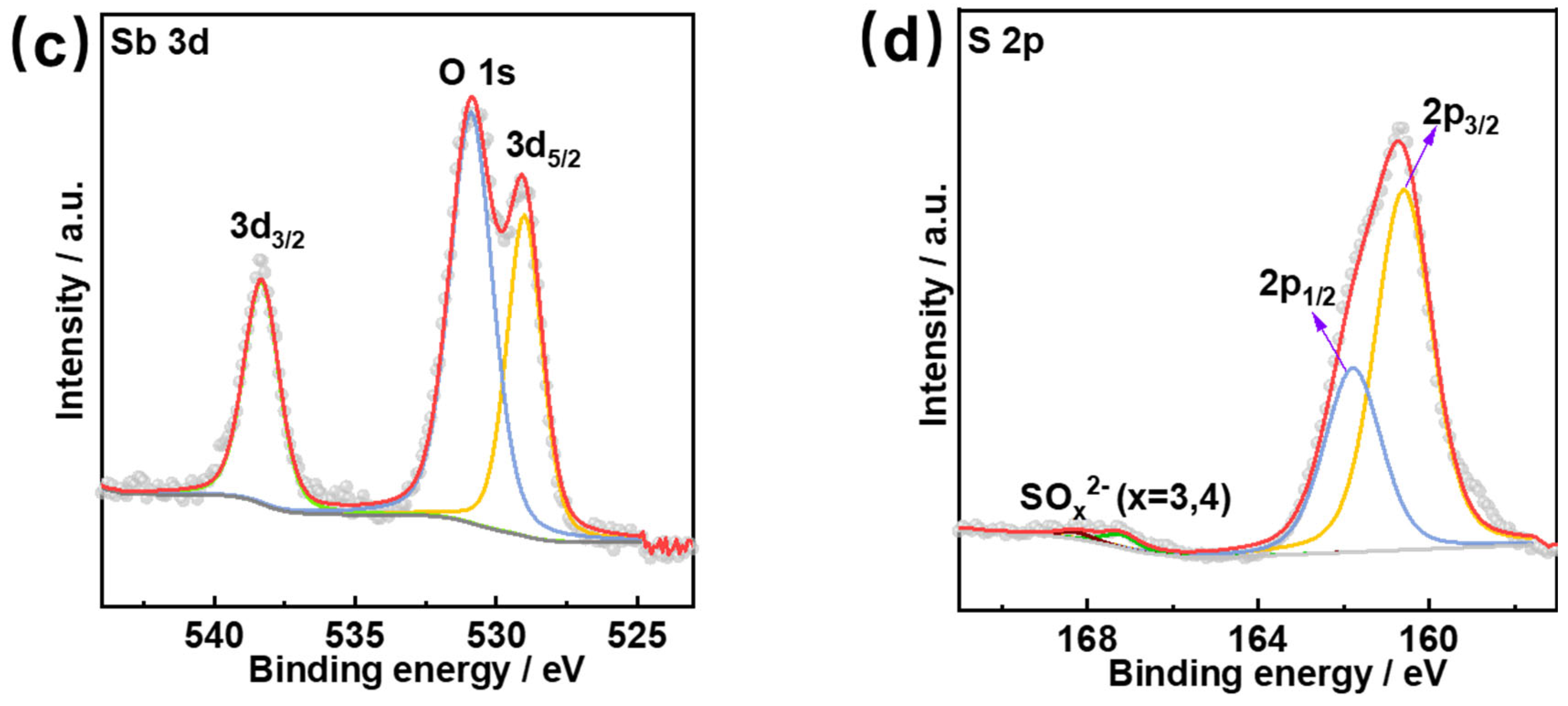
3.2. Surface Morphology and Chemical State Characterization
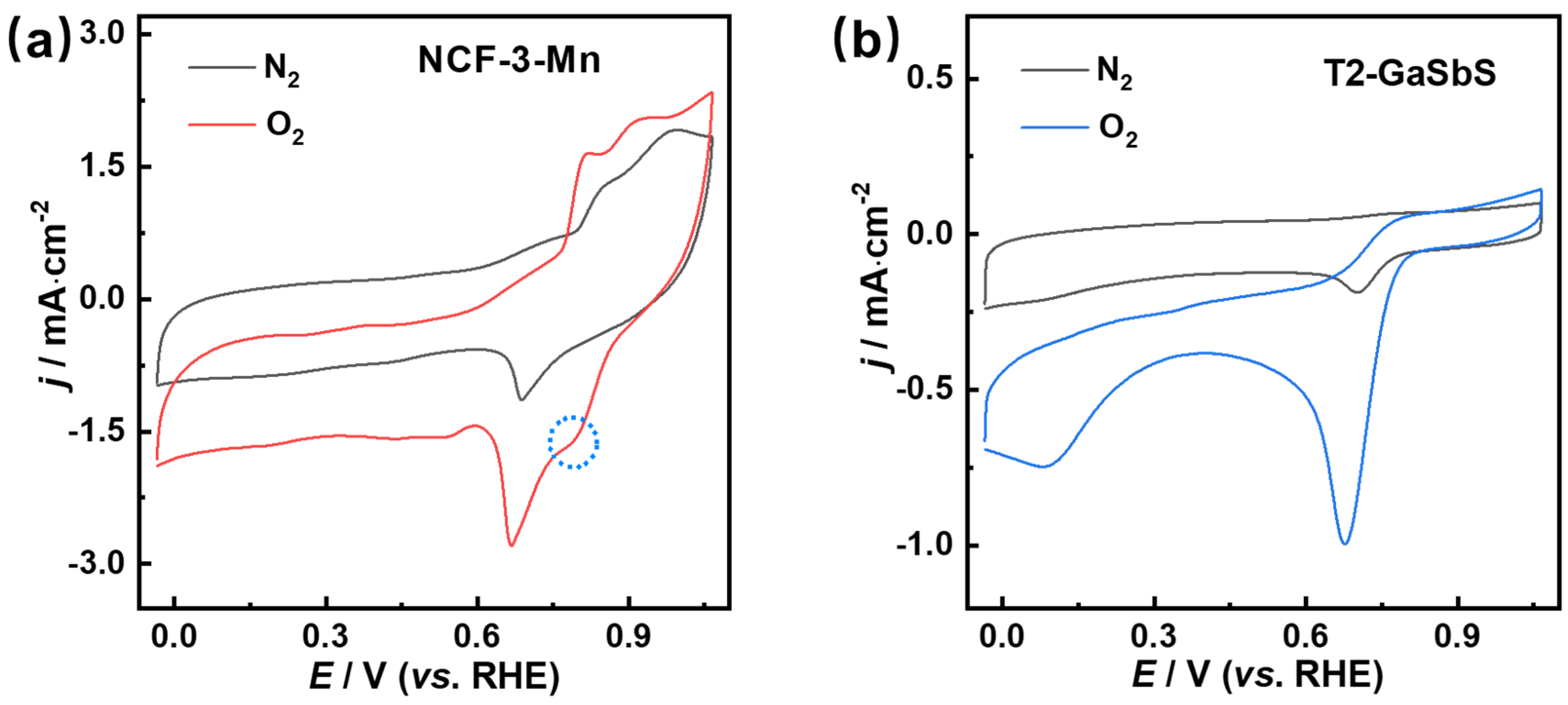
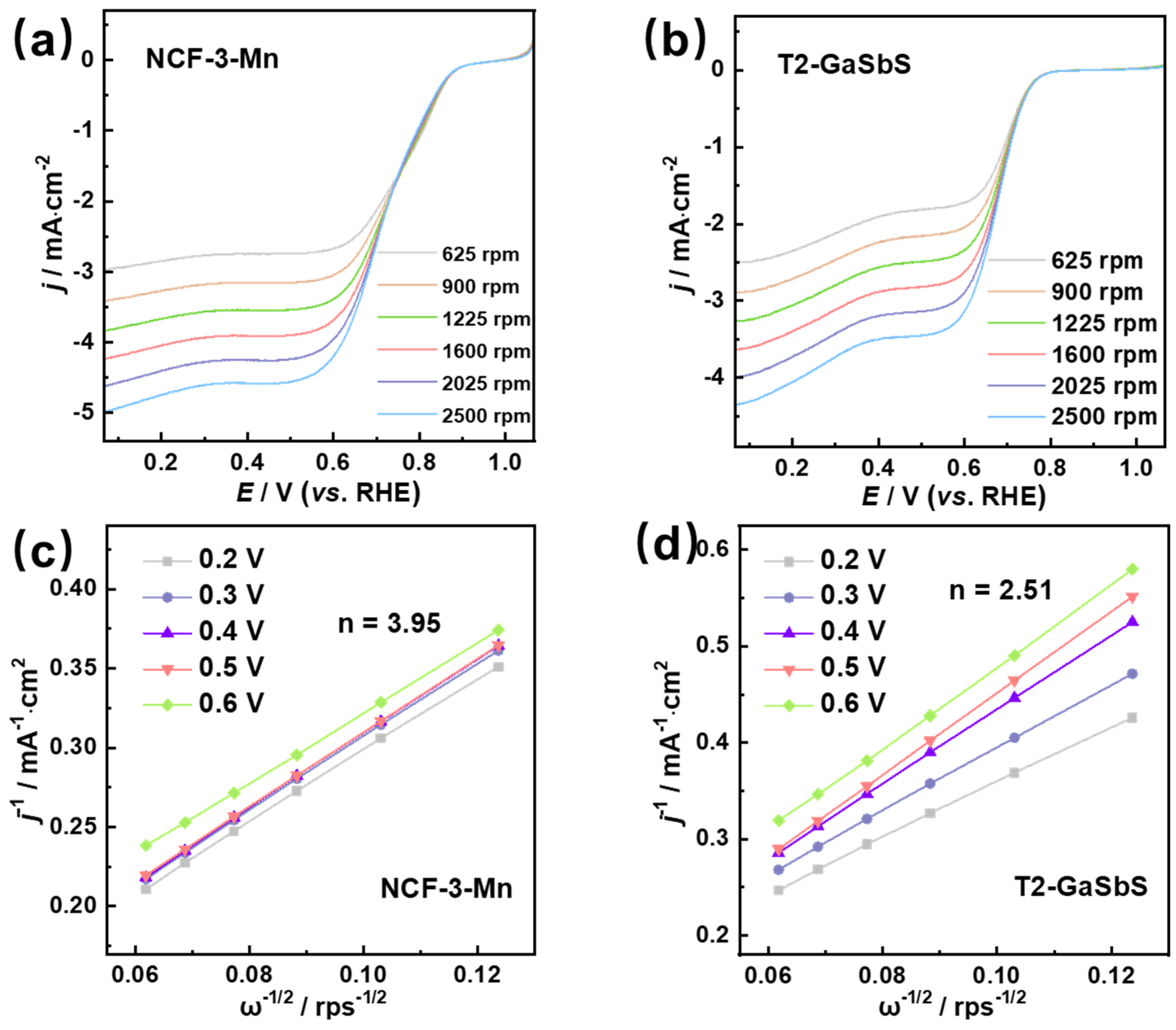
3.3. Electrocatalytic Performance of ORRs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Shi, L.; Guo, Y.; Wang, J.; Liu, D.; Zhao, S. Selective oxygen reduction reaction: Mechanism understanding, catalyst design and practical application. Chem. Sci. 2024, 15, 11188–11228. [Google Scholar] [CrossRef]
- Zhai, Q.; Huang, H.; Lawson, T.; Xia, Z.; Giusto, P.; Antonietti, M.; Jaroniec, M.; Chhowalla, M.; Baek, J.-B.; Liu, Y.; et al. Recent Advances on Carbon-Based Metal-Free Electrocatalysts for Energy and Chemical Conversions. Adv. Mater. 2024, 36, 2405664. [Google Scholar] [CrossRef] [PubMed]
- Kment, Š.; Bakandritsos, A.; Tantis, I.; Kmentová, H.; Zuo, Y.; Henrotte, O.; Naldoni, A.; Otyepka, M.; Varma, R.S.; Zbořil, R. Single Atom Catalysts Based on Earth-Abundant Metals for Energy-Related Applications. Chem. Rev. 2024, 124, 11767–11847. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Peltier, C.R.; Zeng, R.; Schimmenti, R.; Li, Q.; Huang, X.; Yan, Z.; Potsi, G.; Selhorst, R.; Lu, X.; et al. Electrocatalysis in Alkaline Media and Alkaline Membrane-Based Energy Technologies. Chem. Rev. 2022, 122, 6117–6321. [Google Scholar] [CrossRef]
- Zhao, Y.; Adiyeri Saseendran, D.P.; Huang, C.; Triana, C.A.; Marks, W.R.; Chen, H.; Zhao, H.; Patzke, G.R. Oxygen Evolution/Reduction Reaction Catalysts: From In Situ Monitoring and Reaction Mechanisms to Rational Design. Chem. Rev. 2023, 123, 6257–6358. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, T.; Ali, M.; Meng, Y.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable Batteries for Grid Scale Energy Storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Fu, H. Research progress on the construction of synergistic electrocatalytic ORR/OER self-supporting cathodes for zinc–air batteries. J. Mater. Chem. A 2023, 11, 4400–4427. [Google Scholar] [CrossRef]
- Li, J. Oxygen Evolution Reaction in Energy Conversion and Storage: Design Strategies Under and Beyond the Energy Scaling Relationship. Nano-Micro Lett. 2022, 14, 112. [Google Scholar] [CrossRef]
- Kumar, Y.; Mooste, M.; Tammeveski, K. Recent progress of transition metal-based bifunctional electrocatalysts for rechargeable zinc–air battery application. Curr. Opin. Electrochem. 2023, 38, 101229. [Google Scholar] [CrossRef]
- Sarapuu, A.; Lilloja, J.; Akula, S.; Zagal, J.H.; Specchia, S.; Tammeveski, K. Recent Advances in Non-Precious Metal Single-Atom Electrocatalysts for Oxygen Reduction Reaction in Low-Temperature Polymer-Electrolyte Fuel Cells. ChemCatChem 2023, 15, e202300849. [Google Scholar] [CrossRef]
- Li, X.; Lei, H.; Xie, L.; Wang, N.; Zhang, W.; Cao, R. Metalloporphyrins as Catalytic Models for Studying Hydrogen and Oxygen Evolution and Oxygen Reduction Reactions. Acc. Chem. Res. 2022, 55, 878–892. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Xie, J.; Cheng, J.; Wei, F.; Lyu, S.; Zhu, J.; Shi, X.; Yang, X.; Wu, B.; Xu, Z.J. Spin-State Manipulation of Atomic Manganese Center by Phosphide-Support Interactions for Enhanced Oxygen Reduction. Adv. Mater. 2025, 37, 2504585. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, X.; Liu, S.; Cui, Y.; Sun, Y.; Zhang, Y.; Liu, Y.; Liu, G.; Kim, Y.; Xing, W.; et al. Mn–N–C with High-Density Atomically Dispersed Mn Active Sites for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2025, 64, e202503934. [Google Scholar]
- Zhong, G.; Zou, L.; Chi, X.; Meng, Z.; Chen, Z.; Li, T.; Huang, Y.; Fu, X.; Liao, W.; Zheng, S.; et al. Atomically dispersed Mn–Nx catalysts derived from Mn-hexamine coordination frameworks for oxygen reduction reaction. Carbon Energy 2024, 6, e484. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Li, L.; Shi, Y.; Zhang, Y.; Wei, L.; Dong, C.-L.; Lin, Z.; Su, J. Concurrently Boosting Activity and Stability of Oxygen Reduction Reaction Catalysts via Judiciously Crafting Fe–Mn Dual Atoms for Fuel Cells. Nano-Micro Lett. 2024, 17, 88. [Google Scholar] [CrossRef]
- Pei, J.; Shang, H.; Mao, J.; Chen, Z.; Sui, R.; Zhang, X.; Zhou, D.; Wang, Y.; Zhang, F.; Zhu, W.; et al. A replacement strategy for regulating local environment of single-atom Co-SxN4−x catalysts to facilitate CO2 electroreduction. Nat. Commun. 2024, 15, 416. [Google Scholar] [CrossRef]
- Lu, X.; Yang, P.; Wan, Y.; Zhang, H.; Xu, H.; Xiao, L.; Li, R.; Li, Y.; Zhang, J.; An, M. Active site engineering toward atomically dispersed M−N−C catalysts for oxygen reduction reaction. Coord. Chem. Rev. 2023, 495, 215400. [Google Scholar] [CrossRef]
- Dong, F.; Wu, M.; Chen, Z.; Liu, X.; Zhang, G.; Qiao, J.; Sun, S. Atomically Dispersed Transition Metal-Nitrogen-Carbon Bifunctional Oxygen Electrocatalysts for Zinc-Air Batteries: Recent Advances and Future Perspectives. Nano-Micro Lett. 2021, 14, 36. [Google Scholar] [CrossRef]
- Gao, C.; Mu, S.; Yan, R.; Chen, F.; Ma, T.; Cao, S.; Li, S.; Ma, L.; Wang, Y.; Cheng, C. Recent Advances in ZIF-Derived Atomic Metal–N–C Electrocatalysts for Oxygen Reduction Reaction: Synthetic Strategies, Active Centers, and Stabilities. Small 2022, 18, 2105409. [Google Scholar] [CrossRef]
- Yu, S.; Levell, Z.; Jiang, Z.; Zhao, X.; Liu, Y. What Is the Rate-Limiting Step of Oxygen Reduction Reaction on Fe–N–C Catalysts? J. Am. Chem. Soc. 2023, 145, 25352–25356. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, C.; Li, B.; Liang, J.; Zachman, M.J.; Cullen, D.A.; Hermann, R.P.; Alp, E.E.; Lavina, B.; Karakalos, S.; et al. Tuning the thermal activation atmosphere breaks the activity–stability trade-off of Fe–N–C oxygen reduction fuel cell catalysts. Nat. Catal. 2023, 6, 1215–1227. [Google Scholar] [CrossRef]
- Charalampopoulos, G.; Daletou, M.K. Comparative development and evaluation of Fe–N–C electrocatalysts for the oxygen reduction reaction: The effect of pyrolysis and iron-bipyridine structures. Mater. Rep. Energy 2025, 5, 100328. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y. Origin of Selective Production of Hydrogen Peroxide by Electrochemical Oxygen Reduction. J. Am. Chem. Soc. 2021, 143, 9423–9428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, P.; Bu, X.; Wu, T. Atomically precise metal chalcogenide supertetrahedral clusters: Frameworks to molecules, and structure to function. Natl. Sci. Rev. 2021, 9, nwab076. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, X.; Feng, P.; Wu, T. Metal Chalcogenide Supertetrahedral Clusters: Synthetic Control over Assembly, Dispersibility, and Their Functional Applications. Acc. Chem. Res. 2020, 53, 2261–2272. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.-X.; Ma, H.; Xu, Y.-L.; Zhou, R.; Li, D.-S.; Yuan, S.-F.; Wu, T. Atomic- and molecular-level modulation of Mn2+-related emission using atomically-precise metal chalcogenide semiconductor nanoclusters. Coord. Chem. Rev. 2024, 510, 215844. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.-X.; Su, F. Synthesis, structure, and electrocatalytic oxygen reduction reaction properties of metal chalcogenide non-supertetrahedral In-Sn-S cluster materials. Chin. J. Inorg. Chem 2023, 39, 1369–1378. [Google Scholar]
- Wang, X.X.; Guo, Y.N.; Su, F.; Han, C.; Sun, L. Synthesis, structure, and electrocatalytic oxygen reduction reaction properties of metal antimony based chalcogenide clusters. Chin. J. Inorg. Chem 2024, 40, 1201–1208. [Google Scholar]
- Zhang, Y.; Hu, D.; Xue, C.; Yang, H.; Wang, X.; Wu, T. A 3D neutral chalcogenide framework built from a supertetrahedral T3 cluster and a metal complex for the electrocatalytic oxygen reduction reaction. Dalton Trans. 2018, 47, 3227–3230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Ding, Z.; Zhang, H.; Ma, X.; Zhou, X.; Ma, Y.; Mu, Y.; Yu, J.; Huang, T. The structure and catalytic performance for oxygen reduction reaction of worm-like CNTs with Fe3P and Fe0.8Mn0.2 alloy encapsulated. J. Electroanal. Chem. 2025, 990, 119175. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Wang, X.; Ding, Y.; Wu, Z.; Sun, P.; Tang, J.; Zhang, J.; Li, D.-S.; Chen, N.; et al. Stable 3D neutral gallium thioantimonate frameworks decorated with transition metal complexes for a tunable photocatalytic hydrogen evolution. Dalton Trans. 2022, 51, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, W.-A.; Liao, Y.-Y.; Zhang, C.; Feng, M.-L.; Huang, X.-Y. [CH3NH3]4Ga4SbS9S0.28O0.72H: A Three-Dimensionally Open-Framework Heterometallic Chalcogenidoantimonate Exhibiting Ni2+ Ion-Exchange Property. Chem-Asian J. 2018, 13, 672–678. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Hu, D.; Xue, C.; Wang, W.; Yang, H.; Li, D.; Wu, T. Monodisperse Ultrasmall Manganese-Doped Multimetallic Oxysulfide Nanoparticles as Highly Efficient Oxygen Reduction Electrocatalyst. ACS Appl. Mater. Interfaces 2018, 10, 13413–13424. [Google Scholar] [CrossRef]
- Sun, P.; Wu, J.; Wang, Z.; Wang, X.; Chen, N.; Wu, T. A pillar-layered chalcogenide framework assembled by [Mn5S12N12]n layers and [Sb2S5] inorganic pillars. Dalton Trans. 2021, 50, 16473–16477. [Google Scholar] [CrossRef]
- Wang, X.-L.; Wu, Z.; Wang, X.; Xue, C.; Liu, C.; Zhang, J.; Zhou, R.; Li, D.-S.; Wu, T. Bifunctional electrocatalysts derived from cluster-based ternary sulfides for oxygen electrode reactions. Electrochim. Acta 2021, 376, 138048. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Carré, B.; Caucheteux, J.; Compère, P.; Léonard, A.F.; Job, N. Development of In Situ Methods for Preparing La-Mn-Co-Based Compounds over Carbon Xerogel for Oxygen Reduction Reaction in an Alkaline Medium. Nanomaterials 2024, 14, 1362. [Google Scholar] [CrossRef]
- Pan, H.; Wang, X.-L.; Li, F.; Xu, Q. A one-stone-two-birds strategy to construct metal–organic framework-derived cobalt phosphide as an efficient bifunctional electrocatalyst for oxygen electrode reactions. J. Mater. Chem. A 2023, 11, 15006–15013. [Google Scholar] [CrossRef]
- Lin, J.; Dong, Y.; Zhang, Q.; Hu, D.; Li, N.; Wang, L.; Liu, Y.; Wu, T. Interrupted Chalcogenide-Based Zeolite-Analogue Semiconductor: Atomically Precise Doping for Tunable Electro-/Photoelectrochemical Properties. Angew. Chem. Int. Ed. 2015, 54, 5103–5107. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, J.; Feng, L.; Wang, Q.; Guan, W.; Dong, L.-Z.; Zhang, L.; Yan, L.-K.; Lan, Y.-Q.; Zhou, H.-C. Multielectron transportation of polyoxometalate-grafted metalloporphyrin coordination frameworks for selective CO2-to-CH4 photoconversion. Natl. Sci. Rev. 2019, 7, 53–63. [Google Scholar] [CrossRef]
- Wang, Y.-R.; Huang, Q.; He, C.-T.; Chen, Y.; Liu, J.; Shen, F.-C.; Lan, Y.-Q. Oriented electron transmission in polyoxometalate-metalloporphyrin organic framework for highly selective electroreduction of CO2. Nat. Commun. 2018, 9, 4466. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Su, C.; Xu, X.; Zhou, W.; Ran, R.; Shao, Z. A Universal and Facile Way for the Development of Superior Bifunctional Electrocatalysts for Oxygen Reduction and Evolution Reactions Utilizing the Synergistic Effect. Chem. Eur. J. 2014, 20, 15533–15542. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, X.-L.; Hu, D.; Wu, S.; Liu, C.; Wang, X.; Zhou, R.; Li, D.-S.; Wu, T. A new cluster-based chalcogenide zeolite analogue with a large inter-cluster bridging angle. Inorg. Chem. Front. 2019, 6, 3063–3069. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhang, J.; Yang, H.; Luo, M.; Xue, C.; Lin, Z.; Wu, T. Three-Dimensional Superlattices Based on Unusual Chalcogenide Supertetrahedral In–Sn–S Nanoclusters. Inorg. Chem. 2019, 58, 31–34. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Hu, D.; Yang, H.; Xue, C.; Lin, Z.; Wu, T. An Unusual Metal Chalcogenide Zeolitic Framework Built from the Extended Spiro-5 Units with Supertetrahedral Clusters as Nodes. Inorg. Chem. 2018, 57, 921–925. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, J.; Xue, C.; Hu, D.; Wang, X.; Li, D.-S.; Wu, T. Two Penta-Supertetrahedral Cluster-Based Chalcogenide Open Frameworks: Effect of the Cluster Spatial Connectivity on the Electron-Transport Efficiency. Inorg. Chem. 2019, 58, 3582–3585. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, J.; Wu, T. A Stable Metal Chalcogenide Cluster-Based Framework Decorated with Transition Metal Complexes for an Efficient Electrocatalytic O2 Reduction Reaction. Nanomaterials 2025, 15, 1186. https://doi.org/10.3390/nano15151186
Wang X, Li J, Wu T. A Stable Metal Chalcogenide Cluster-Based Framework Decorated with Transition Metal Complexes for an Efficient Electrocatalytic O2 Reduction Reaction. Nanomaterials. 2025; 15(15):1186. https://doi.org/10.3390/nano15151186
Chicago/Turabian StyleWang, Xiang, Juan Li, and Tao Wu. 2025. "A Stable Metal Chalcogenide Cluster-Based Framework Decorated with Transition Metal Complexes for an Efficient Electrocatalytic O2 Reduction Reaction" Nanomaterials 15, no. 15: 1186. https://doi.org/10.3390/nano15151186
APA StyleWang, X., Li, J., & Wu, T. (2025). A Stable Metal Chalcogenide Cluster-Based Framework Decorated with Transition Metal Complexes for an Efficient Electrocatalytic O2 Reduction Reaction. Nanomaterials, 15(15), 1186. https://doi.org/10.3390/nano15151186





