Preparation of Superhydrophobic P-TiO2-SiO2/HDTMS Self-Cleaning Coatings with UV-Aging Resistance by Acid Precipitation Method
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials and Reagents
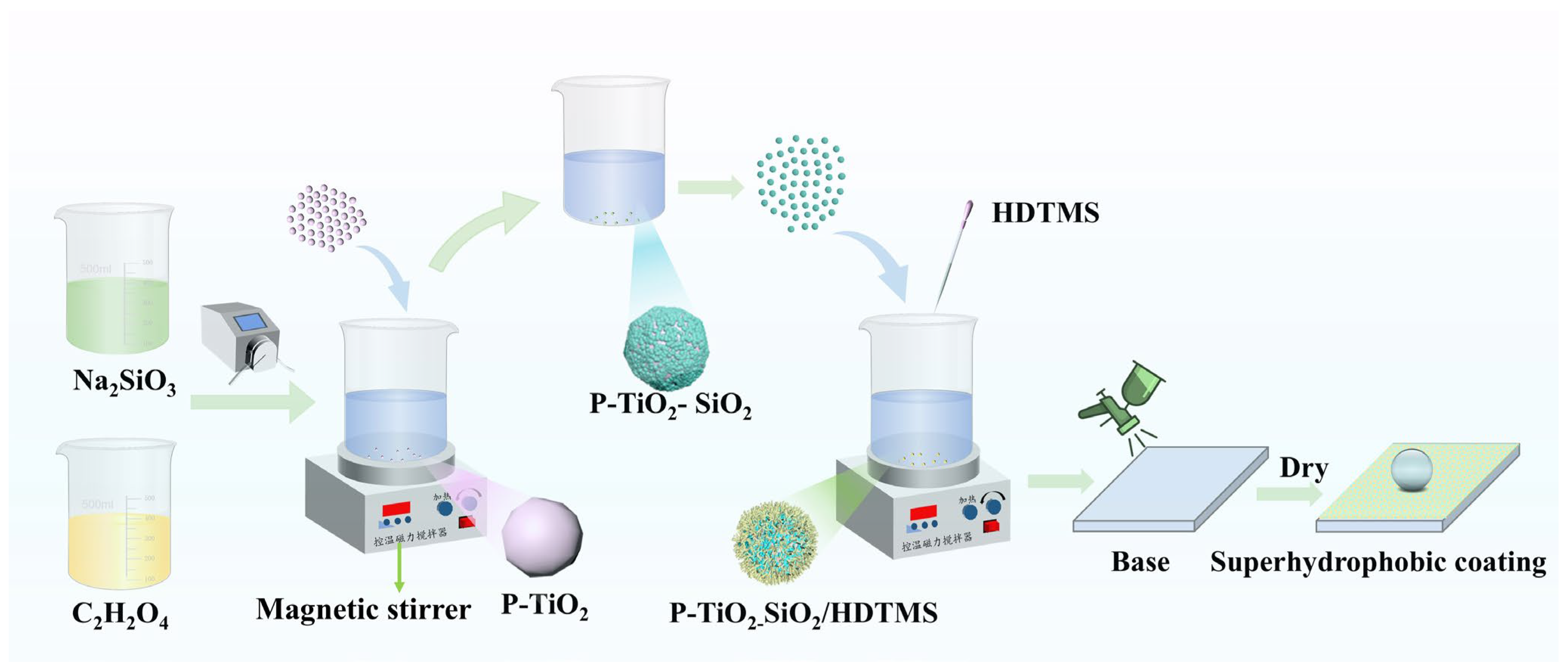
2.2. Preparation of P-TiO2-SiO2 Composite Particles
- A total of 20 g P-TiO2 was added to 100 mL of distilled water, and it was ultrasonically dispersed. Then, it was heated to 85 °C in a thermostat water bath.
- The pH value was adjusted to 10 by a 10% NaOH solution, and 0.03 g of sodium silicate was added. Then, it was stirred and dispersed by a magnetic stirrer for 20 min.
- A total of 8 g sodium silicate was added to 100 mL of water. Then, it was ultrasonically dispersed for 20 min. The prepared sodium silicate solution and 10% oxalic acid solution were added to a peristaltic pump. The silicon source was slowly dripped, and the pH of the suspension was maintained at 9.
- After all the silicon sources were added dropwise, the suspension reacted for 90 min. Then, it was matured at 25 °C for 120 min.
- The suspension was centrifuged and washed. Then, the precipitate was dried at 60 °C and grinded to obtained the P-TiO2-SiO2 composite material.
2.3. Preparation of the P-TiO2-SiO2/HDTMS Coating
2.4. Experimental Methods and Characterization
3. Results and Discussion
3.1. Properties and Surface Structure of the P-TiO2-SiO2/HDTMS Coating
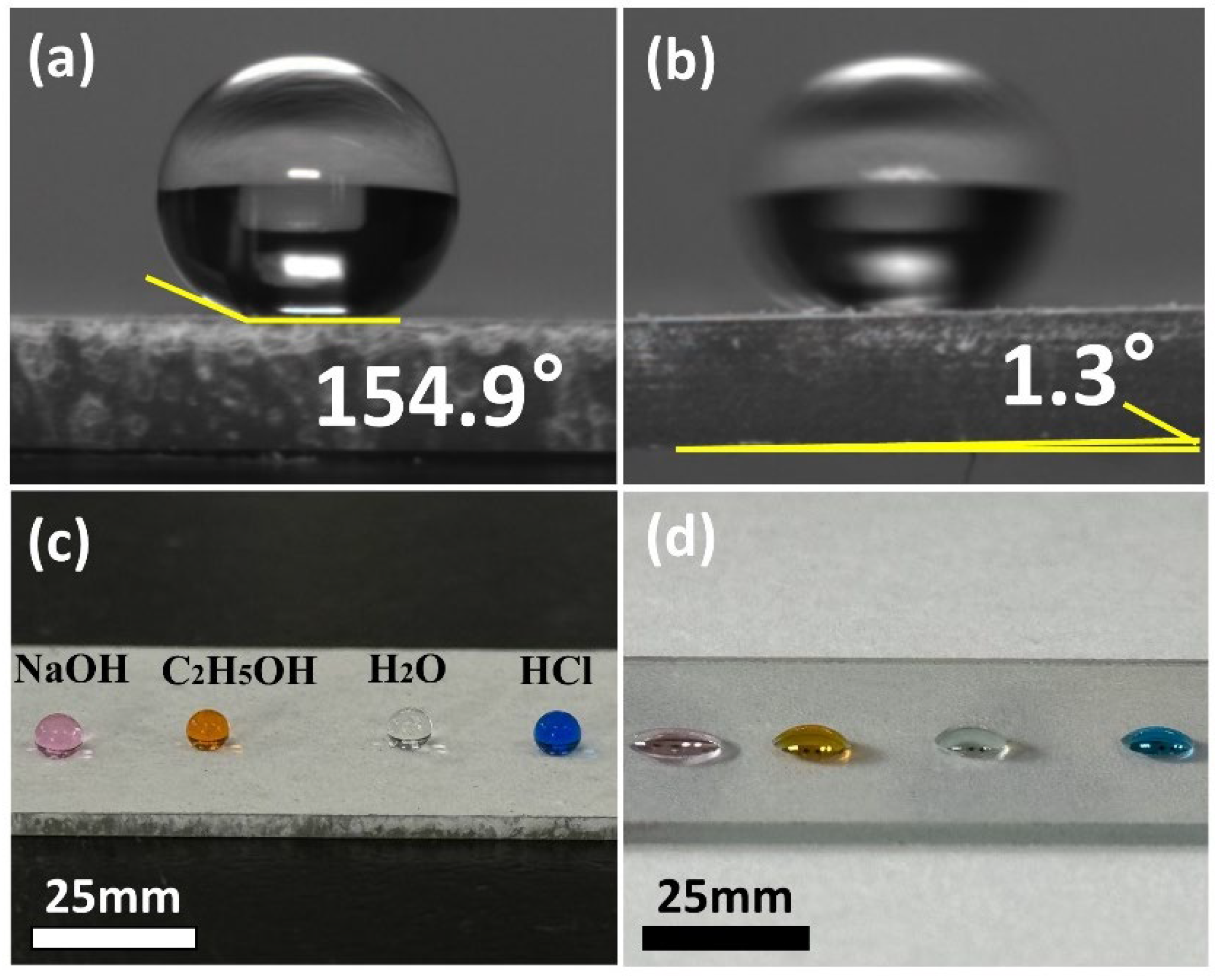
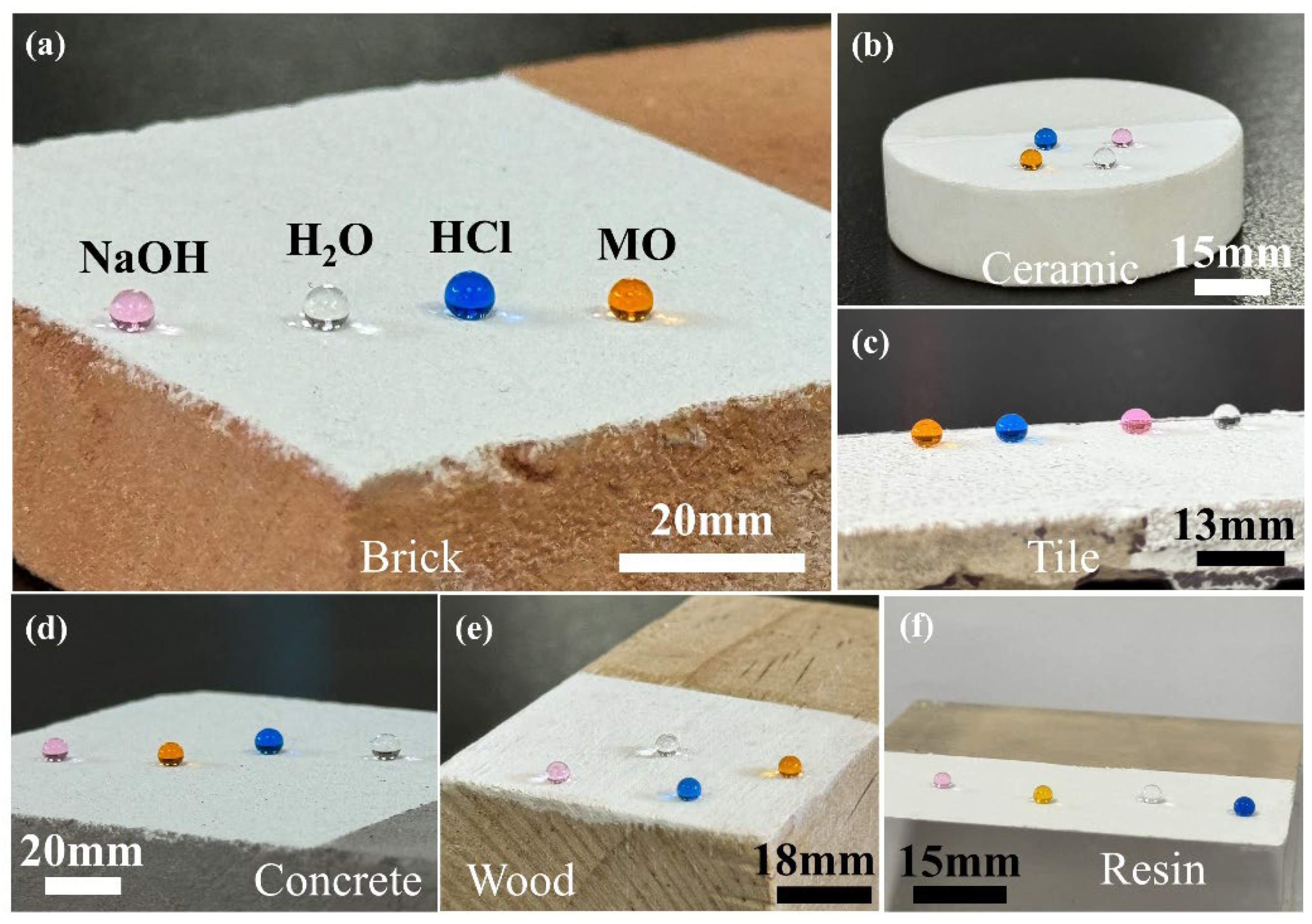
3.1.1. Wettability and Adaptability of the P-TiO2-SiO2/HDTMS Coating Surface
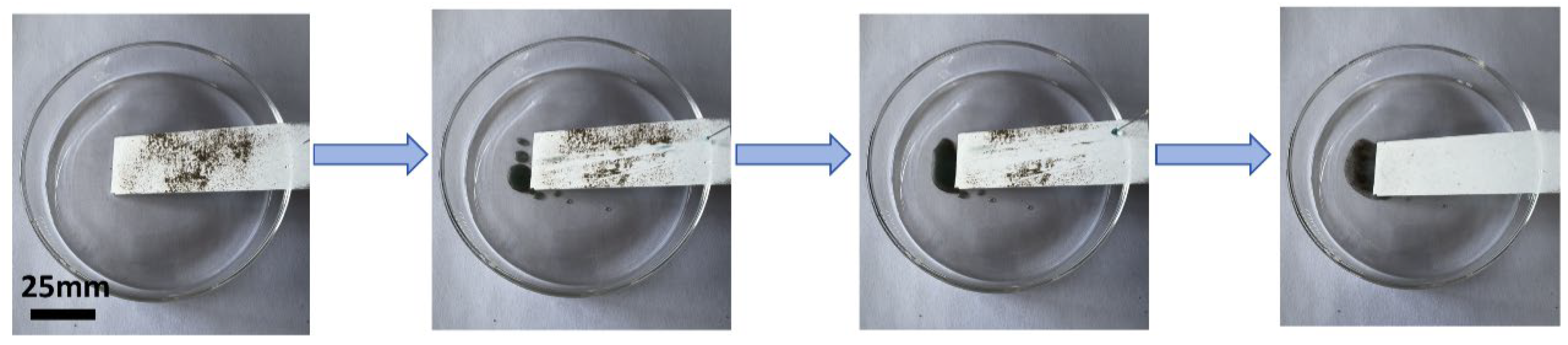
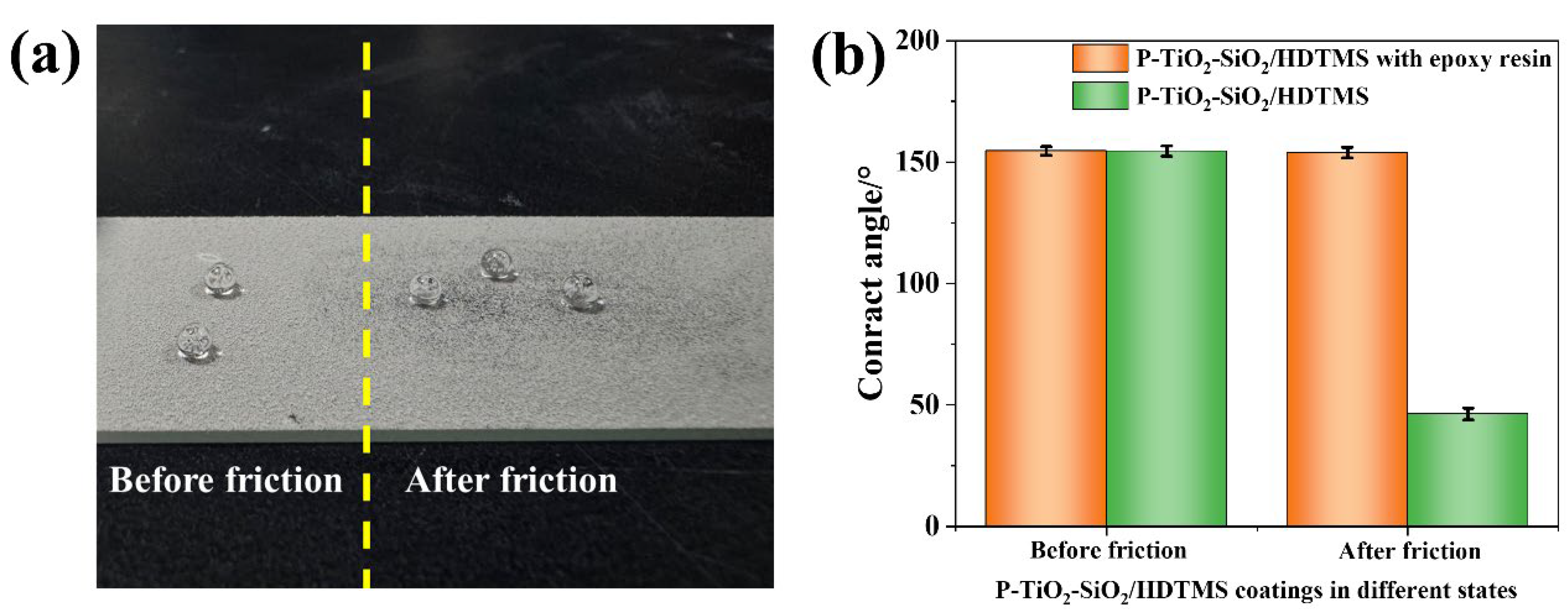
3.1.2. Self-Cleaning Property of the P-TiO2-SiO2/HDTMS Coating
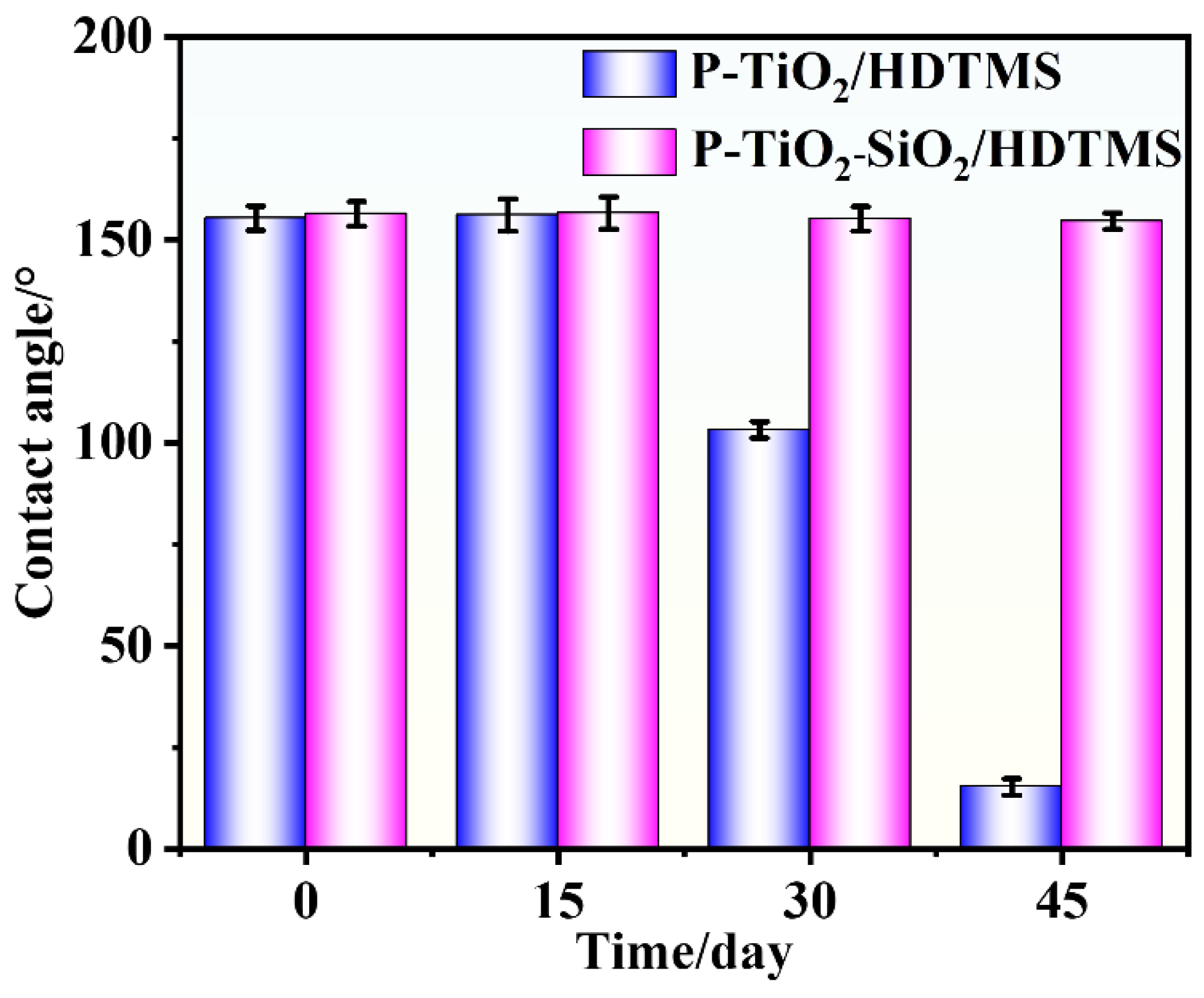
3.1.3. UV-Aging Resistance of the As-Prepared Coating
3.1.4. Mechanical Strength of the As-Prepared Coating
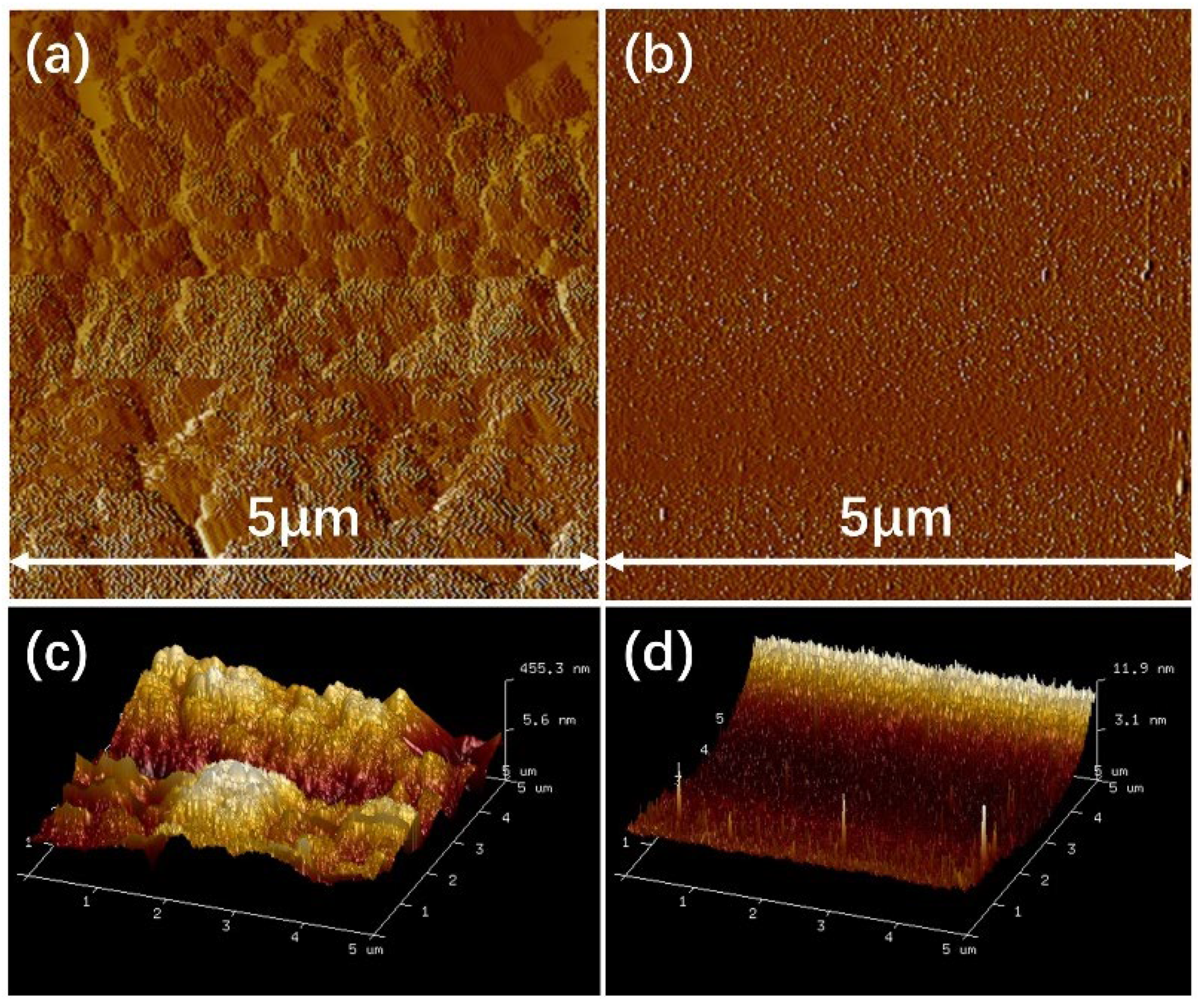
3.1.5. Roughness of the Surfaces
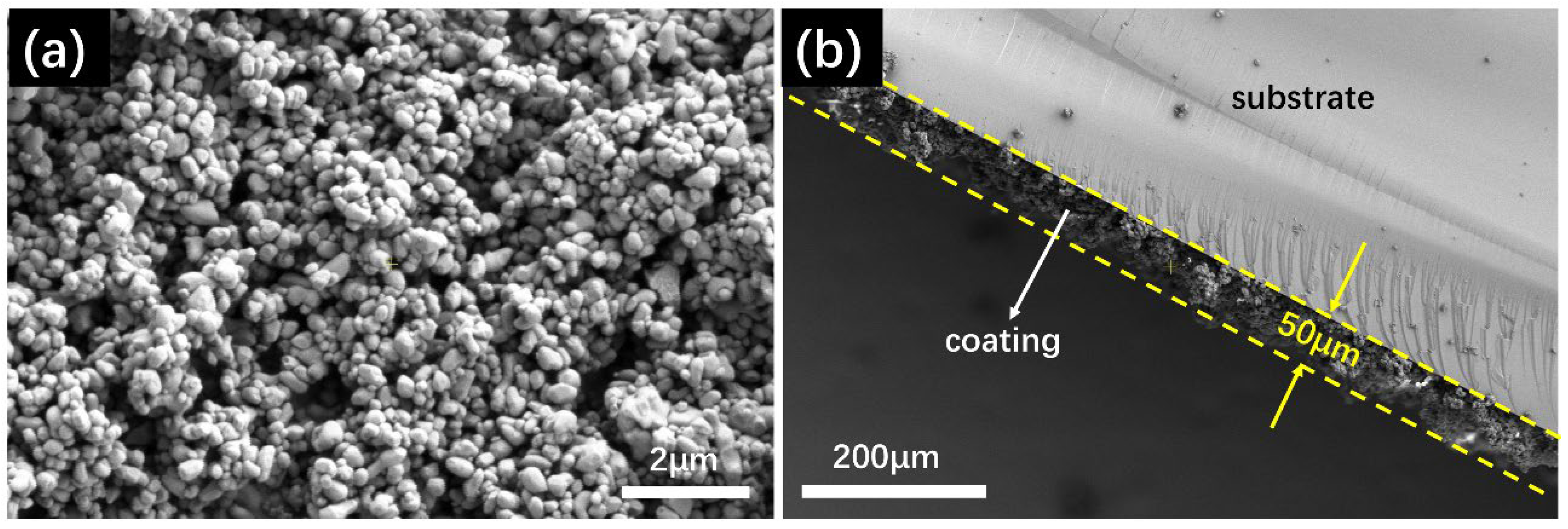
3.1.6. Surface Morphology and Thickness of the Coating
3.2. Mechanism for Improving the UV-Aging Resistance of the As-Prepared Coating
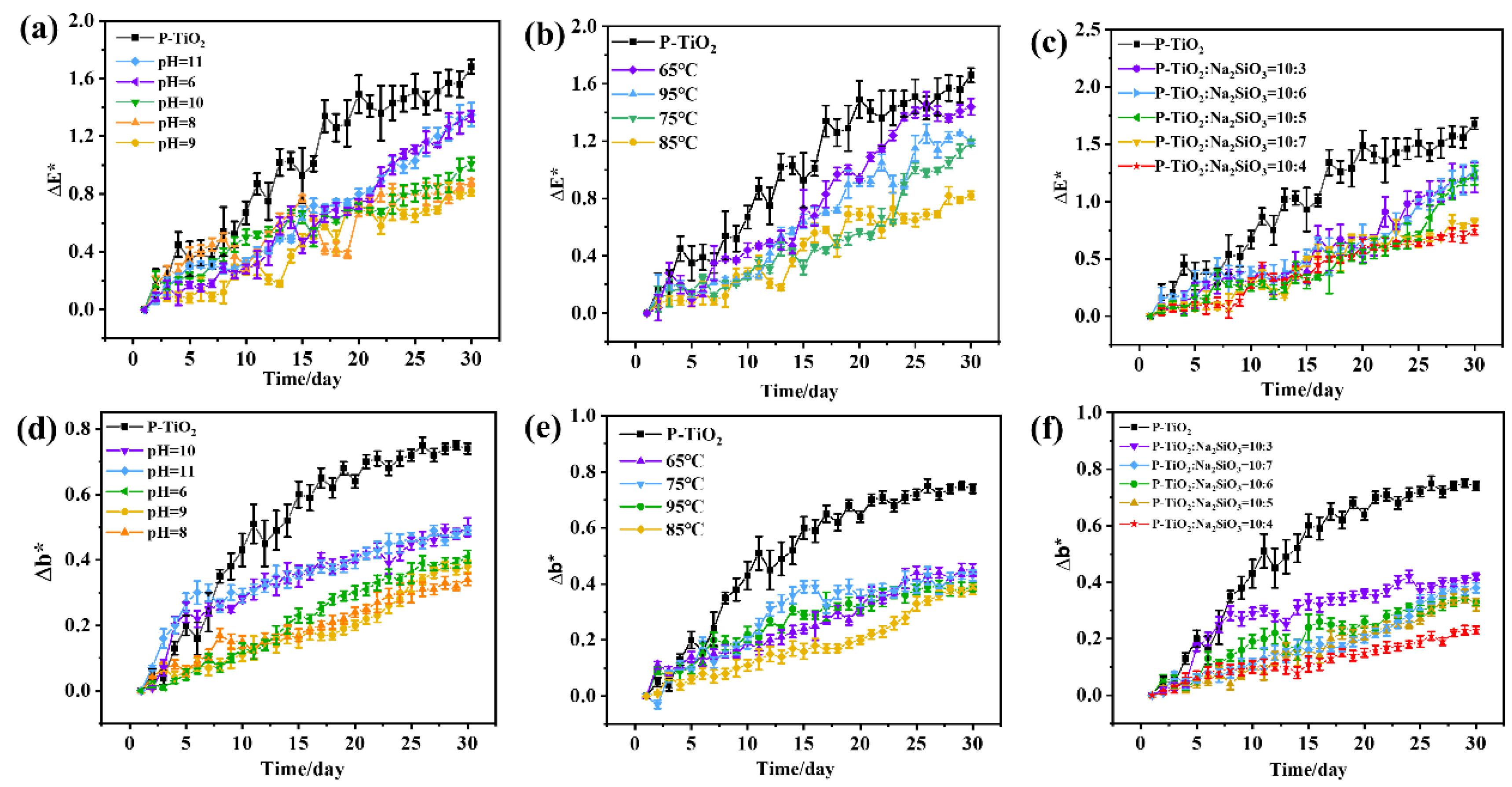
3.2.1. UV-Aging Resistance of P-TiO2-SiO2 Composite Particles
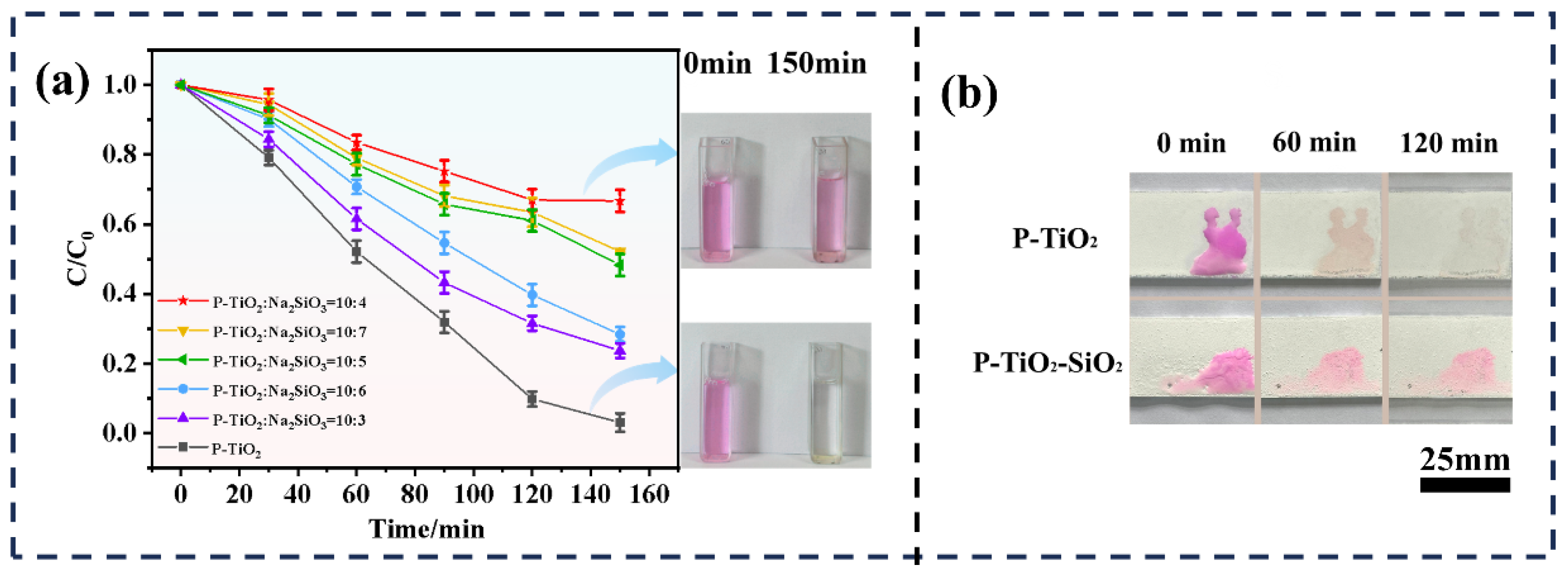
3.2.2. Photocatalytic Degradation Performance of P-TiO2-SiO2
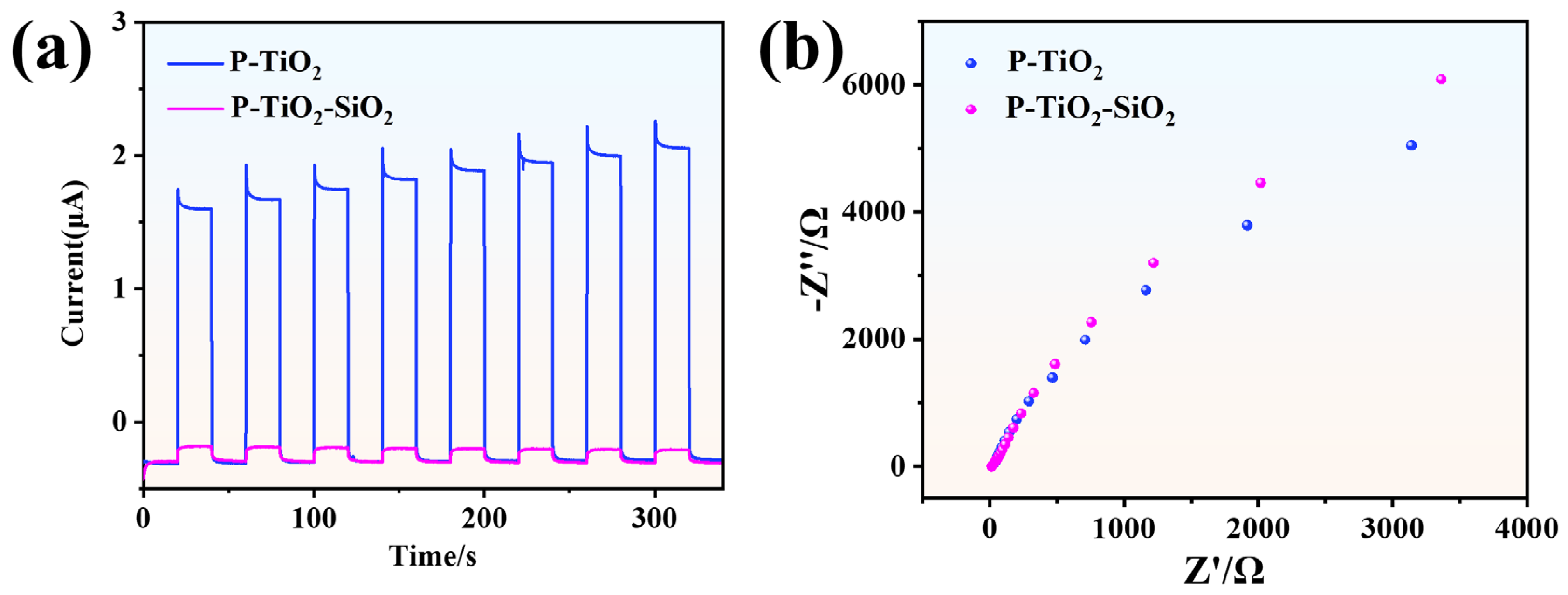
3.2.3. Photoelectrochemical Performance of P-TiO2-SiO2
3.3. Structural Basis of the P-TiO2-SiO2/HDTMS Coating
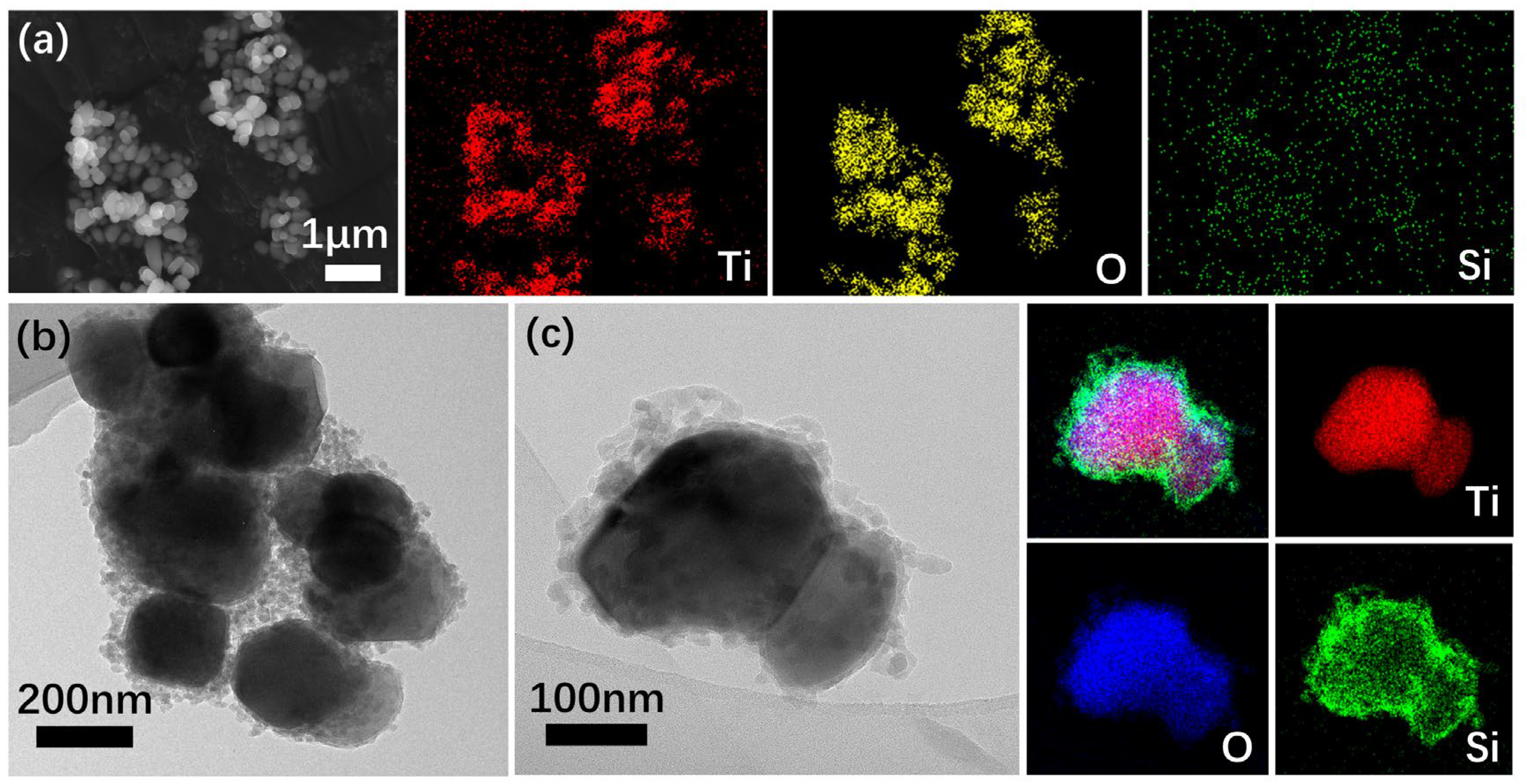
3.3.1. Morphology of P-TiO2-SiO2
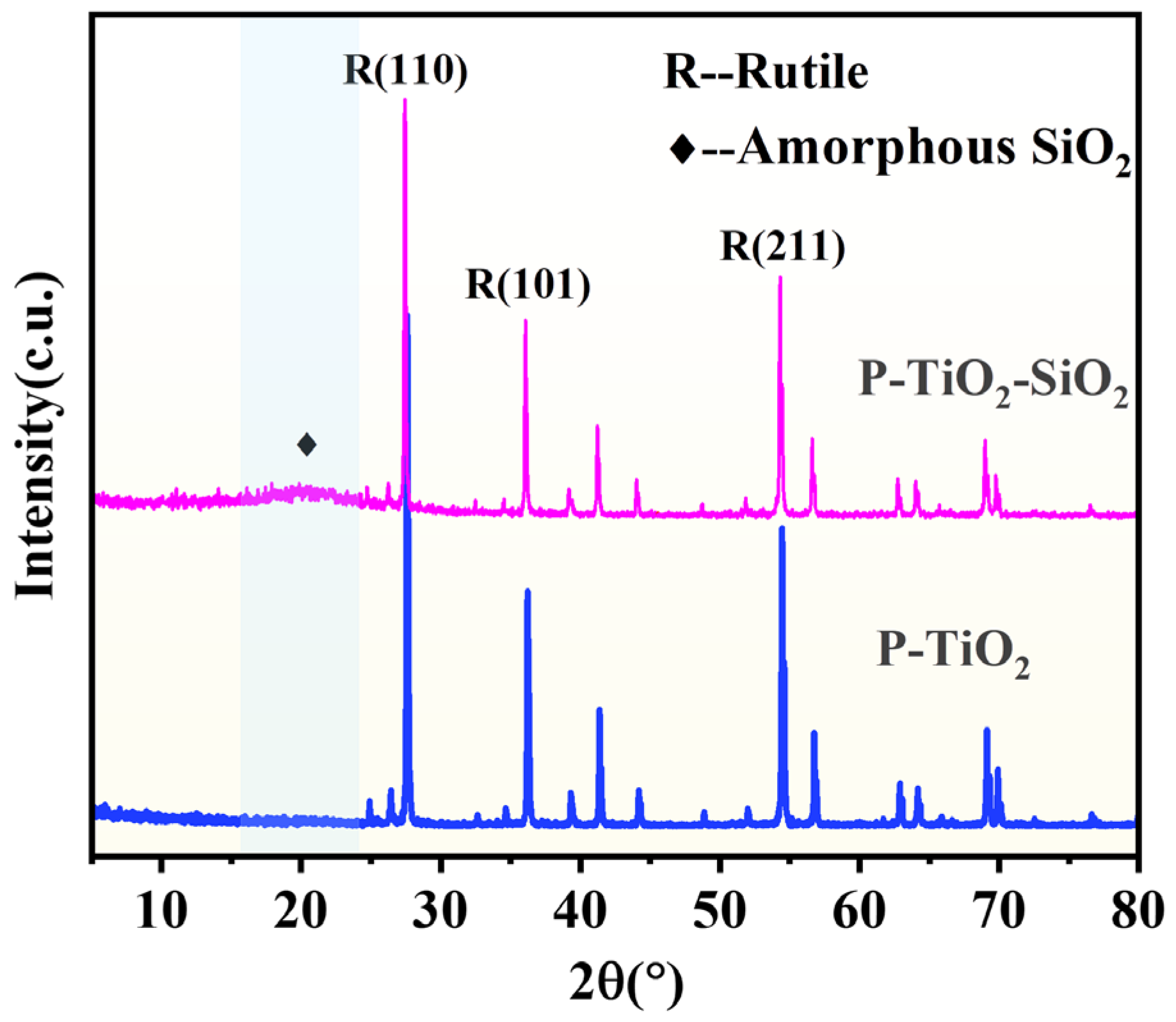
3.3.2. Phase Component of P-TiO2-SiO2
3.3.3. Chemical Composition of P-TiO2-SiO2
3.4. Combination of Raw Materials
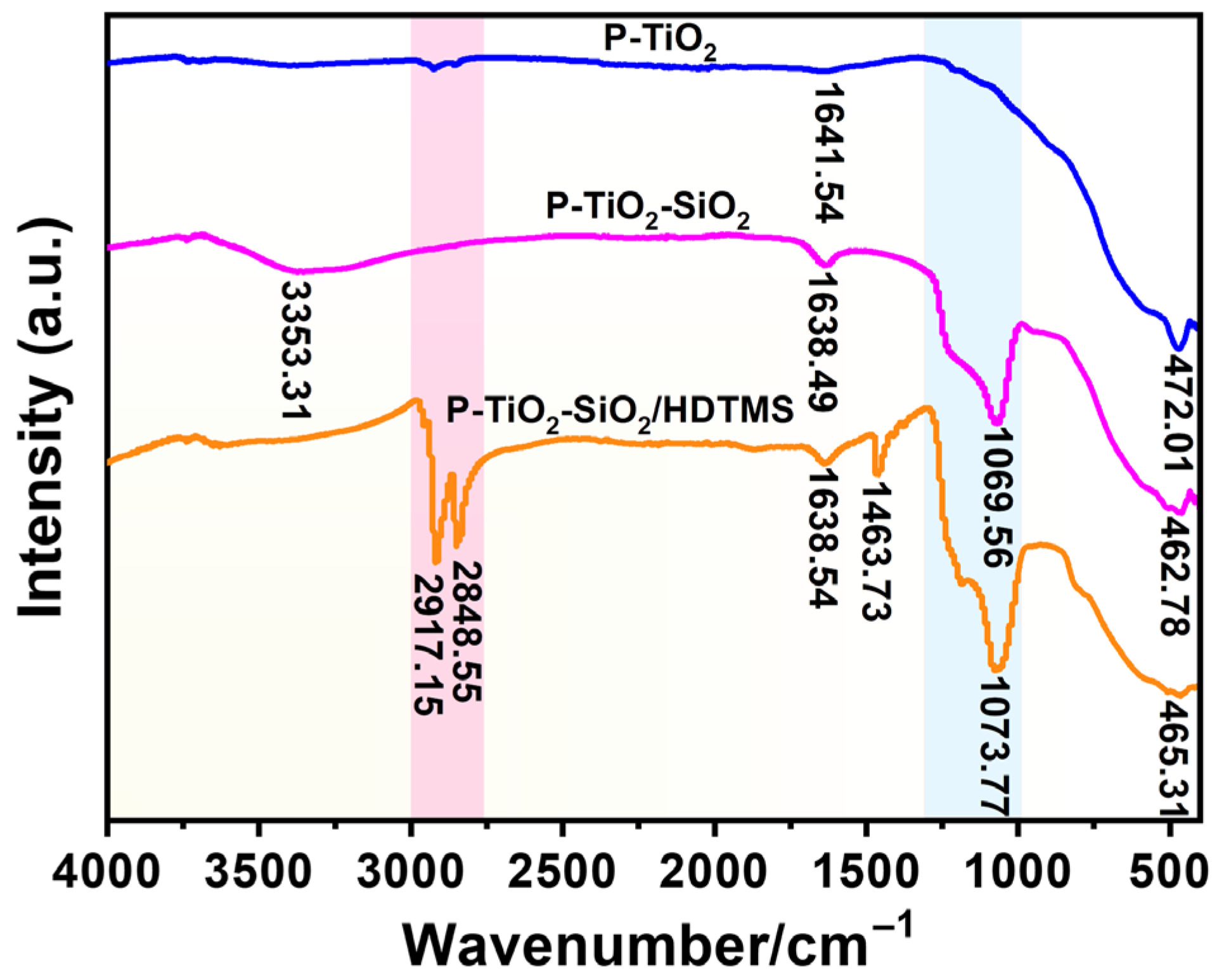
3.4.1. FT-IR Analysis
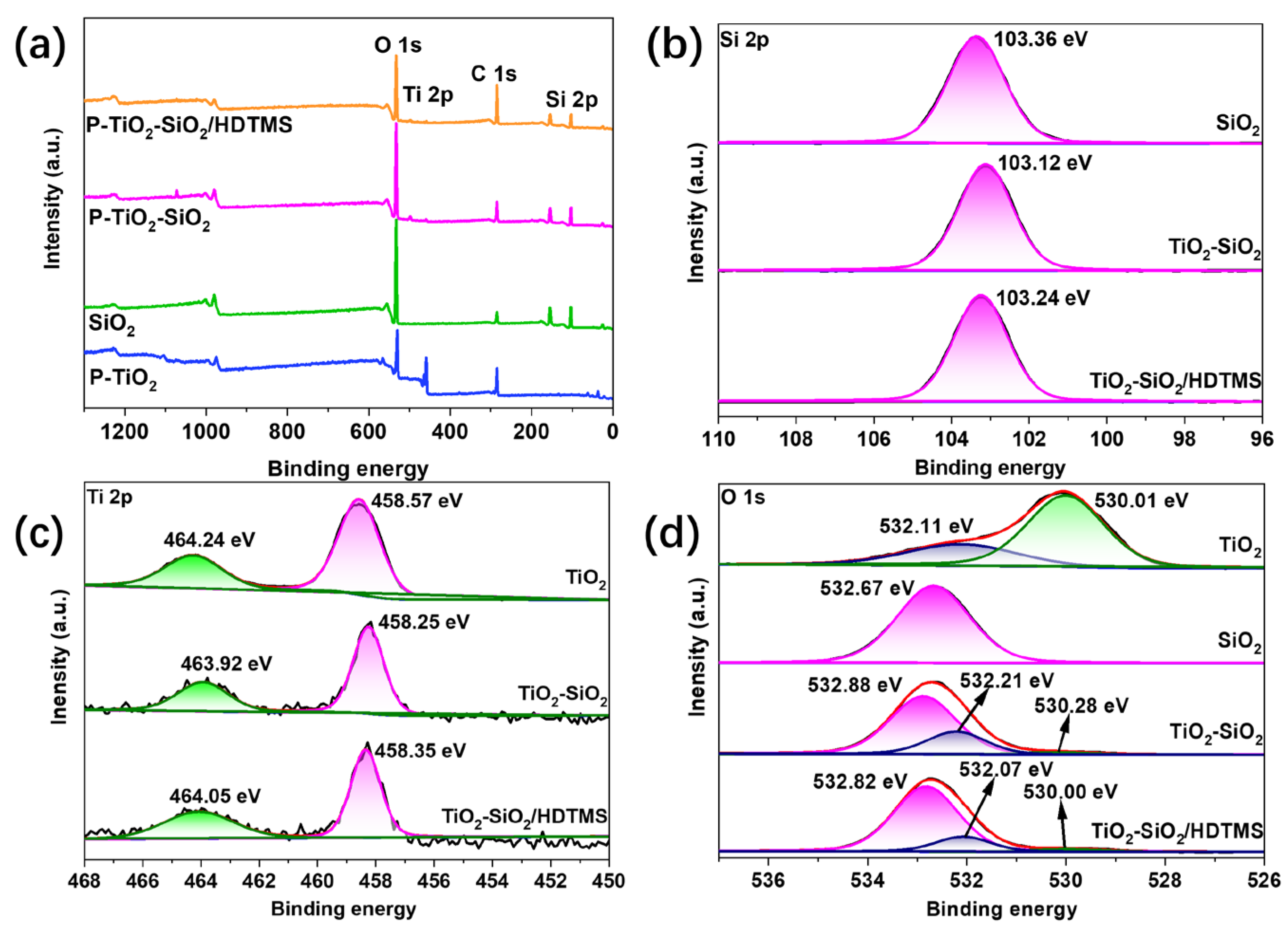
3.4.2. XPS Analysis
3.5. Properties of the Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Liu, Y.; Wang, X.; Chen, D.; Ding, H. Fabrication of sericite-TiO2/HDTMS superhydrophobic self-cleaning coatings by hydrothermal method. J. Alloys Compd. 2025, 1014, 178677. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Y.; Tang, S. Self-Adaptive Smart Thermochromic Film with Quick Response for All-Year Radiative Cooling and Solar Heating. ACS Appl. Mater. Interfaces 2024, 16, 68407–68415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhu, Z.; She, W. A superhydrophobic mortar with ultra-robustness for self-cleaning, anti-icing, and anti-corrosion. Chem. Eng. J. 2024, 495, 153488. [Google Scholar] [CrossRef]
- Wang, X.; Jia, X.; Ren, H.; Yang, J.; Song, H. Breathable, self-cleaning superhydrophobic DDA-PDA@BNNS/silicon resin coating. Prog. Org. Coat. 2024, 192, 108511. [Google Scholar] [CrossRef]
- Qin, H.; Lu, P.; Xu, J.; Jiang, K.; Zhao, D.; Jiang, Z.; Zhao, J.; Zhang, Y. Robust superhydrophobic PEEK surface with stable antifouling and self-cleaning performances. Appl. Surf. Sci. 2025, 688, 162326. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, S.; Wang, Y.; Zhang, X.; Chu, H.; Huang, H.; Huang, F.; Wu, H.; Xiong, X. A novel metal-free fluorine-free self-cleaning coating with photocatalytic activity and superhydrophobicity based on RP/HTCC@PDMS for hydraulic concrete. Sep. Purif. Technol. 2025, 363, 132142. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-Hydrophobic Surfaces: From Natural to Artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Chen, L.; Guo, Z.; Liu, W. Biomimetic Multi-functional Superamphiphobic FOTS-TiO2 Particles beyond Lotus Leaf. ACS Appl. Mater. Interfaces 2016, 8, 27188–27198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Hao, L.; Song, L.; Tian, L.; Fan, Y.; Zhao, J.; Liu, C.; Ming, W.; Ren, L. Lotus-leaf-inspired hierarchical structured surface with non-fouling and mechanical bactericidal performances. Chem. Eng. J. 2020, 398, 125609. [Google Scholar] [CrossRef]
- Chu, Z.; Jiao, W.; Huang, Y.; Wang, R.; Ding, G.; Zhong, X.; Yan, M.; Zheng, Y.; Wang, R. FDTS-Modified SiO2/rGO Wrinkled Films with a Micro-Nanoscale Hierarchical Structure and Anti-Icing/Deicing Properties under Condensation Condition. Adv. Mater. Interfaces 2019, 7, 1901446. [Google Scholar] [CrossRef]
- Lei, Y.; Jiang, B.; Liu, H.; Zhang, F.; An, Y.; Zhang, Y.; Yuan, Y.; Xu, J.; Li, X.; Liu, T. Mechanically robust superhydrophobic polyurethane coating for anti-icing application. Prog. Org. Coat. 2023, 183, 107795. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Wang, M.; Li, Y.; Jiang, Z.; Zhang, W.; Yang, H.; Wang, C.; Ho, S.-H. Facile designing a superhydrophobic anti-icing surface applied for reliable long-term deicing. Chin. Chem. Lett. 2023, 34, 108353. [Google Scholar] [CrossRef]
- Varshney, P.; Lomga, J.; Gupta, P.K.; Mohapatra, S.S.; Kumar, A. Durable and regenerable superhydrophobic coatings for aluminium surfaces with excellent self-cleaning and anti-fogging properties. Tribol. Int. 2018, 119, 38–44. [Google Scholar] [CrossRef]
- Lomga, J.; Varshney, P.; Nanda, D.; Satapathy, M.; Mohapatra, S.S.; Kumar, A. Fabrication of durable and regenerable superhydrophobic coatings with excellent self-cleaning and anti-fogging properties for aluminium surfaces. J. Alloys Compd. 2017, 702, 161–170. [Google Scholar] [CrossRef]
- Xiang, T.; Chen, D.; Lv, Z.; Yang, Z.; Yang, L.; Li, C. Robust superhydrophobic coating with superior corrosion resistance. J. Alloys Compd. 2019, 798, 320–325. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, Z.; Wu, H.; Li, L.; Fu, X. Study on Preparation of Superhydrophobic Ni-Co Coating and Corrosion Resistance by Sandblasting-Electrodeposition. Coatings 2020, 10, 1164. [Google Scholar] [CrossRef]
- Byeon, H.; Sunil, J. Exploring the novel environmentally friendly highly hydrophobic TiO2 coating for enhanced anti-corrosion performance of steel in potential industrial applications. Results Chem. 2025, 14, 102087. [Google Scholar] [CrossRef]
- Xia, S.; Yu, Z.; Pang, Y.; Chen, Z.; Chen, Y.; Zhang, X.; Guo, S. Advances in the application of superhydroobicph fabric surfaces for oil-water separation and extension of functionalization. J. Environ. Chem. Eng. 2024, 12, 114156. [Google Scholar] [CrossRef]
- Du, B.; Chen, F.; Luo, R.; Li, H.; Zhou, S.; Liu, S.; Hu, J. Superhydrophobic Surfaces with pH-Induced Switchable Wettability for Oil–Water Separation. ACS Omega 2019, 4, 14994–15002. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Cheng, D. Restoration of the distorted color to detect the discoloration status of a steel bridge coating using digital image measurements. Adv. Eng. Inform. 2017, 33, 96–111. [Google Scholar] [CrossRef]
- Chen, X.; Hu, D.; Zhang, Z.; Ma, W. In situ assembly of halloysite nanotubes@cerium oxide nanohybrid for highly UV-shielding and superhydrophobic coating. J. Alloys Comp. 2019, 798, 151986. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, Z.; Liu, M.; Lin, J.; Chen, X.; Ma, W. Multifunctional UV-shielding nanocellulose films modified with halloysite nanotubes-zinc oxide nanohybrid. Cellulose 2019, 26, 6749–6762. [Google Scholar] [CrossRef]
- Sun, H.; Xi, Y.; Tao, Y.; Zhang, J. Facile fabrication of multifunctional transparent glass with superhydrophobic, self-cleaning and ultraviolet-shielding properties via polymer coatings. Prog. Org. Coat. 2021, 154, 106360. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Politano, G.G. Optical Properties of Thick TiO2-P25 Films. Nanomaterials 2025, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Gu, H.; Xu, M.; Sun, R.; Li, H.; Sun, Y.; Zhang, Y.; Song, L. Ultra-flexible inorganic coating realized by scale-like nanosheets. Surf. Coat. Technol. 2024, 483, 130757. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Y.; Li, Y.; Liu, W.; Wu, J.; Liu, C.; Yang, B.; Pang, Z. The enhanced surface properties of geopolymer inorganic coatings by adding with MgO. J. Coat. Technol. Res. 2022, 19, 947–957. [Google Scholar] [CrossRef]
- Victor, B.; Kim, D.; Sarah, M.; Søren, K. Acid-resistant organic coatings for the chemical industry: A review. J. Coat. Technol. Res. 2017, 14, 279–306. [Google Scholar]
- Esin, A.; Ramazan, U. Light-activated hybrid organic/inorganic antimicrobial coatings. J. Sol-Gel Sci. Techn. 2018, 87, 183–194. [Google Scholar]
- He, Z.; Li, X.; Soucek, M.; Castaneda, H. Inhibition of acid undercutting of inorganic/organic hybrid polyurethane coatings. Prog. Org. Coat. 2019, 134, 169–176. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Jiang, L.; Li, C.; Zheng, S. Investigation on the film-coating mechanism of alumina-coated rutile TiO2 and its dispersion stability. Adv. Powder Technol. 2017, 28, 1982–1988. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, H.; Wang, A.; Liu, C.; Yu, L.; Jiang, T.; Hang, Y. Evolution of zirconia coating layer on rutile TiO2 surface and the pigmentary property. J. Phys. Chem. Solids 2010, 71, 1458–1466. [Google Scholar] [CrossRef]
- Kim, H.; Roh, D.; Chang, J.; Kim, D. SiO2 coated platy TiO2 designed for noble UV/IR-shielding materials. Ceram. Int. 2019, 45, 16880–16885. [Google Scholar] [CrossRef]
- Xie, S.; Zhao, J.; Zhang, B.; Wang, Z.; Ma, H.; Yu, C.; Yu, M.; Li, L.; Li, J. Graphene Oxide Transparent Hybrid Film and Its Ultraviolet Shielding Property. ACS Appl. Mater. Interfaces 2015, 7, 17558–17564. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Lin, Y.; Zhang, S.; Tian, R.; Liang, R.; Wei, M.; Evans, D.G.; Duan, X. Study on UV-shielding mechanism of layered double hydroxide materials. Phys. Chem. Chem. Phys. 2013, 15, 18217–18222. [Google Scholar] [CrossRef] [PubMed]
- Kollias, N.; Ruvolo, E.; Sayre, R.M. The value of the ratio of UVA to UVB in sunlight. Photochem. Photobiol. 2011, 87, 1474–1475. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, R.; Luo, J. Improvement of anti-aging property of UV-curable coatings with silica-coated TiO2. Prog. Org. Coat. 2023, 179, 107479. [Google Scholar] [CrossRef]
- Amaral, S.P.; Grosche, L.C.; Sousa, J.P.S. Synergistic UV protection: Hybrid TiO2@SiO2/UVabs nanoparticles with augmented UV-shielding efficiency for the development of durable protective coatings. Prog. Org. Coat. 2025, 205, 109286. [Google Scholar] [CrossRef]
- Nakhaei, O.; Shahtahmassebi, N.; Roknabadi, M.R.; Behdani, M. Fabrication and study of UV-shielding and photocatalytic performance of uniform TiO2/SiO2 core-shell nanofibers via single-nozzle co-electrospinning and interface sol–gel reaction. Sci. Iran. 2016, 23, 4018. [Google Scholar]
- Fakin, D.; Kleinschek, K.S.; Kurečič, M.; Ojstršek, A. Effects of nano TiO2–SiO2 on the hydrophilicity/dyeability of polyester fabric and photostability of disperse dyes under UV irradiation. Surf. Coat. Technol. 2014, 247, 18–24. [Google Scholar]
- Yousefi, F.; Mousavi, S.B.; Heris, S.Z.; Naghash-Hamed, S. UV-shielding properties of a cost-effective hybrid PMMA-based thin film coatings using TiO2 and ZnO nanoparticles: A comprehensive evaluation. Sci. Rep. 2023, 13, 34120. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zou, G.; Chang, L.; Guo, W.; Tian, K.; Li, X.; Wang, H. UV-stimulated self-healing SiO2/CeO2 microcapsule with excellent UV-blocking capability in epoxy coating. Bull. Mater. Sci. 2023, 46, 2991. [Google Scholar] [CrossRef]
- Godnjavec, J.; Zabret, J.; Znoj, B.; Skale, S.; Veronovski, N.; Venturini, P. Investigation of surface modification of rutile TiO2 nanoparticles with SiO2/Al2O3 on the properties of polyacrylic composite coating. Prog. Org. Coat. 2014, 77, 47–52. [Google Scholar] [CrossRef]
- Park, O.K.; Kang, Y.S. Preparation and characterization of silica-coated TiO2 nanoparticle. Colloids Surf. A 2005, 257, 261–265. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Wang, C.; Zhou, R.; Li, Y.; Li, W.; Ao, W. Self-healing superhydrophobic A-SiO2/N-TiO2@HDTMS coating with self-cleaning property. Appl. Surf. Sci. 2021, 559, 150808. [Google Scholar] [CrossRef]
- Sun, L.; Fang, K.; Chen, W.; Liu, K.; Zhu, J.; Zhang, C. Fabrication of a novel superhydrophobic cotton by HDTMS with TiO2-supported activated carbon nanocomposites for photocatalysis and oil/water separation. Ind. Crops Prod. 2022, 186, 115836. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Xu, Z.; Zhang, J.; Yao, Y. Preparation of a UV anti-aging and superhydrophobic self-cleaning coating by loading nano-rutile on sericite and being modified with HDTMS. Appl. Clay Sci. 2024, 247, 107212. [Google Scholar] [CrossRef]
- Amarasinghe, P.M.; Katti, K.S.; Katti, D.R. Molecular hydraulic properties of montmorillonite: A polarized Fourier transform infrared spectroscopic study. Appl. Spectrosc. 2008, 62, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Yeom, C.; Kim, Y. Purification of oily seawater/wastewater using superhydrophobic nano-silica coated mesh and sponge. J. Ind. Eng. Chem. 2016, 40, 47–53. [Google Scholar] [CrossRef]
- Cai, Y.; Zhao, Q.; Quan, X.; Feng, W.; Wang, Q. Fluorine-free and hydrophobic hexadecyltrimethoxysilane-TiO2 coated mesh for gravity-driven oil/water separation. Colloid Surface A 2019, 586, 124189. [Google Scholar] [CrossRef]
| Sample | TiO2 (%) | SiO2 (%) | K2O (%) | Al2O3 (%) | Na2O (%) | Others (%) |
|---|---|---|---|---|---|---|
| P-TiO2 | 99.50 | 0.01 | 0.16 | 0.08 | 0.01 | 0.24 |
| P-TiO2-SiO2 | 86.59 | 12.54 | 0.03 | 0.11 | 0.52 | 0.21 |
| UV-Aging Resistance | Superhydrophobicity | Mechanical Strength | |
|---|---|---|---|
| P-TiO2-SiO2 composite particles | √ | × | × |
| P-TiO2-SiO2/HDTMS coating | √ | √ | × |
| P-TiO2-SiO2/HDTMS coating with epoxy resin | √ | √ | √ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, Y.; Bai, X.; Ding, H.; Wang, X.; Chen, D.; Zhang, Y. Preparation of Superhydrophobic P-TiO2-SiO2/HDTMS Self-Cleaning Coatings with UV-Aging Resistance by Acid Precipitation Method. Nanomaterials 2025, 15, 1127. https://doi.org/10.3390/nano15141127
Zhang L, Liu Y, Bai X, Ding H, Wang X, Chen D, Zhang Y. Preparation of Superhydrophobic P-TiO2-SiO2/HDTMS Self-Cleaning Coatings with UV-Aging Resistance by Acid Precipitation Method. Nanomaterials. 2025; 15(14):1127. https://doi.org/10.3390/nano15141127
Chicago/Turabian StyleZhang, Le, Ying Liu, Xuefeng Bai, Hao Ding, Xuan Wang, Daimei Chen, and Yihe Zhang. 2025. "Preparation of Superhydrophobic P-TiO2-SiO2/HDTMS Self-Cleaning Coatings with UV-Aging Resistance by Acid Precipitation Method" Nanomaterials 15, no. 14: 1127. https://doi.org/10.3390/nano15141127
APA StyleZhang, L., Liu, Y., Bai, X., Ding, H., Wang, X., Chen, D., & Zhang, Y. (2025). Preparation of Superhydrophobic P-TiO2-SiO2/HDTMS Self-Cleaning Coatings with UV-Aging Resistance by Acid Precipitation Method. Nanomaterials, 15(14), 1127. https://doi.org/10.3390/nano15141127






