Advancements in Nanotechnology for Spinal Surgery: Innovations in Spinal Fixation Devices for Enhanced Biomechanical Performance and Osteointegration
Abstract
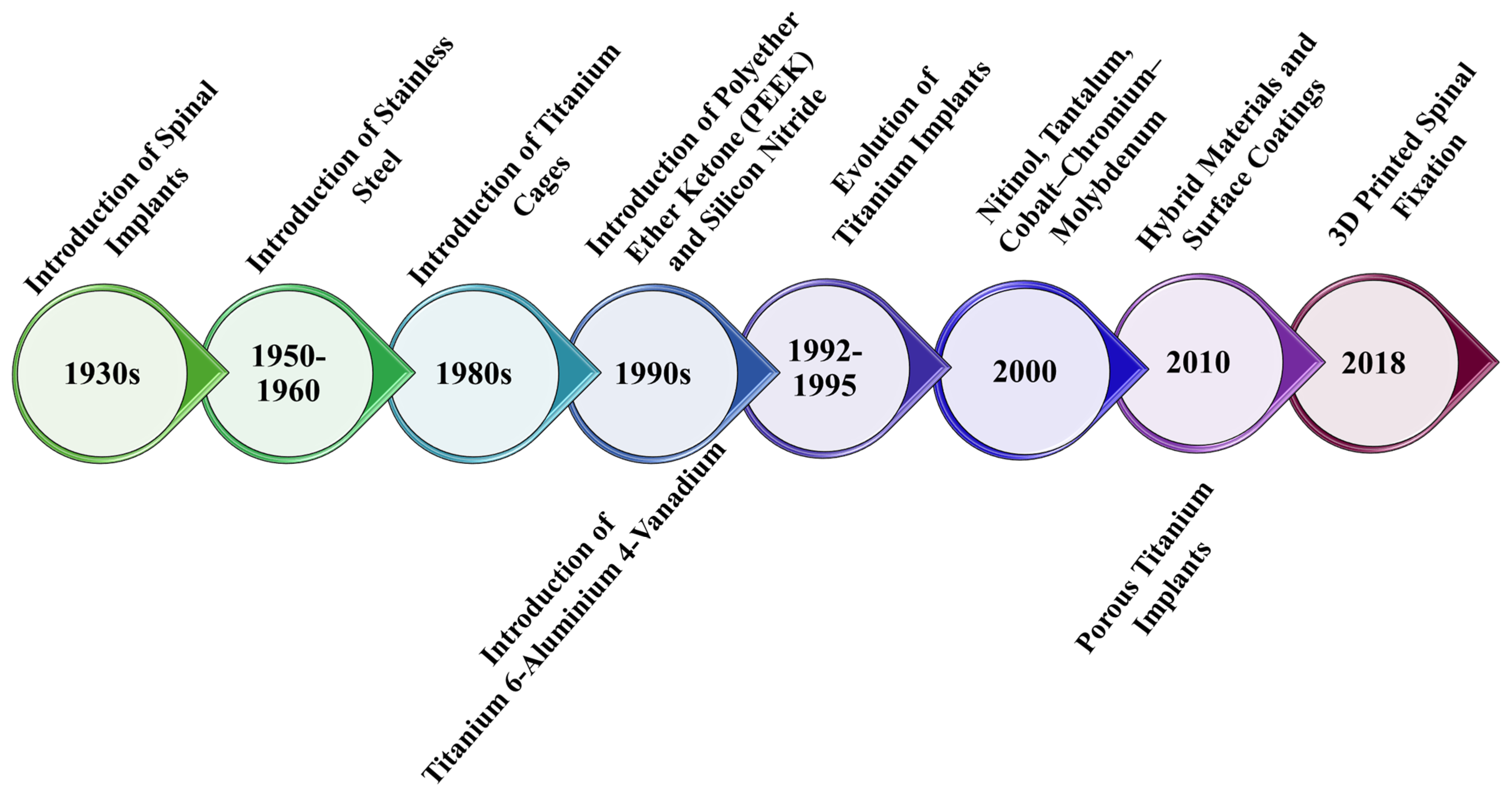
1. Introduction
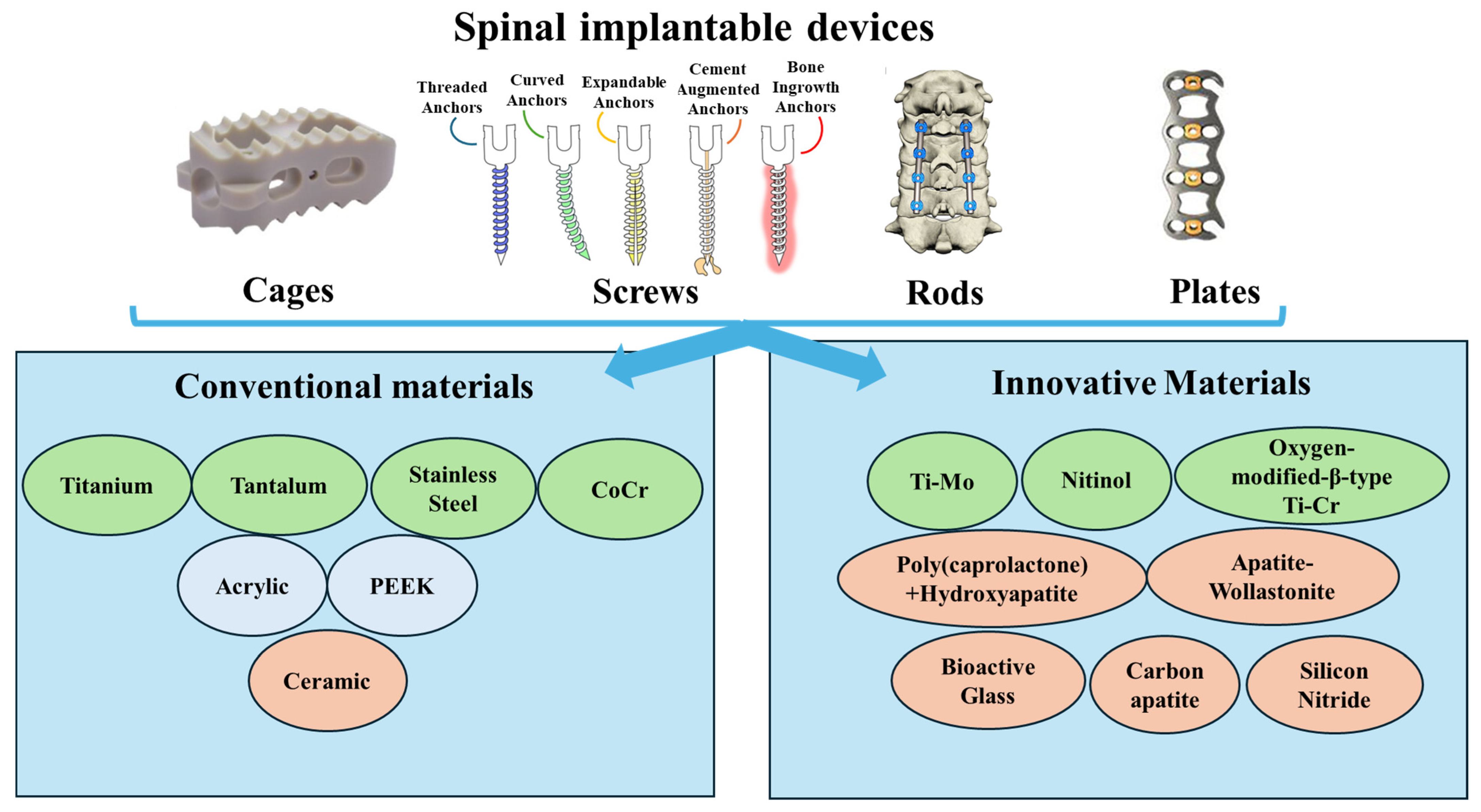
2. Biomechanical and Biological Requirements for Spinal Implants
| Category | Class | Material | Applications | Properties | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|---|---|---|
| Conventional materials | Metals | Titanium and its alloys | Screws, rods, cages | Elastic modulus: 110 GPa Yield strength: 789–1013 MPa Fatigue limit: 500–600 MPa | High strength MRI-compatible Corrosion-resistant Promote osseointegration | Too stiff -> stress shielding Poor multilevel fusion Produces imaging artifacts | [7,38,39,44,52,53,54] |
| Stainless steel and its alloys | Rods, screws | Elastic modulus: 200 GPa Yield strength: 690 MPa Fatigue limit 350–500 MPa | High strength Low-cost fabrication | Poor biocompatibility Low corrosion resistance Risk of cracking | [7,39,54,55,56,57,58] | ||
| CoCr and its alloys | Rods, screws | Elastic modulus: 200–300 GPa Yield strength: 800–950 MPa Fatigue limit: >600 MPa | High hardness Wear resistance, fatigue strength Relatively expensive High ductility | Stiffness Allergic potential Produces imaging artifacts | [7,39,40,54,55,57,58,59] | ||
| Tantalum | Screws, rods, cages | Elastic modulus: 3 GPa Yield strength: 789–1013 MPa Fatigue limit: 500–600 MPa | Biocompatible Porous Promotes fusion | Limited availability High-cost production High melting point High chances of infections post-implantation | [7,54,60,61,62,63] | ||
| Polymers | Polyetheretherketone (PEEK) | Cages, rods | Elastic modulus: 3.6 GPa Yield strength: 165 MPa Fatigue limit: 99.4–107.4 MPa | High stability High strength Good wear resistance and fatigue properties Non-toxic Elastic modulus similar to cortical bone tissue Reduce the extent of stress shielding | Poor osteointegration Risk of loosening/migration | [7,64,65,66,67,68] | |
| Polylactic acid (PLA) | Cages | Elastic modulus: 3500 GPa Yield strength: 60 MPa Fatigue limit: 13.7 MPa | Biocompatible and bioresorbable Does not require surgery to remove the implant | Low bioactivity Low strength | [69,70,71,72,73] | ||
| Poly (vinyl alcohol) (PVA) | Replacement in the intervertebral disk herniation | Elastic modulus: 0.0012–0.85 MPa Tensile strength: 1.73 GPa | Flexible Biocompatible | Low mechanical stability | [74,75] | ||
| Innovative Materials | Ceramics | Bioglass | Cages | Elastic modulus: 13 ± 2 GPa Yield strength: 253.34 ± 9.31 MPa Fatigue limit: 30 MPa | Promotes bone integration Good radiological outcomes | Less effective than autograft Limited long-term data | [7,76,77,78,79] |
| Silicon Nitride | Cages | Elastic modulus: 236 ± 10 GPa Yield strength: 65.3–127 GPa Flexural strength: 0.750 GPa | Osteoinductive Non-toxic Has antimicrobial properties High strenth | Large-scale clinical trials are limited High production costs | [7,80,81,82,83,84] | ||
| Apatite Wollastonite | Cages | Elastic modulus: 32 GPa Maximum compressive strength: 121 MPa | Bioactive Biocompatible Biodegradable Induces osseointegration Reduce stress on the implant | Large-scale studies are limited | [7,85,86] | ||
| Metals | Nitinol | Cages, rods, screws, supporter bands | Elastic modulus: 48 GPa Yield strength: 1050 MPa Tensile strength: 1521 MPa | High mechanical resistance Corrosion resistante Low corrosion rate Induce osseointegration Osteoinductive | Nickel toxicity concern Large-scale clinical trials are limited | [7,87,88,89,90] | |
| Composite | Carbon-fiber-reinforced (CFR)-PEEK | Pedicle screw, cages, vertebral body replacements, rods | Elastic modulus: 18 GPa Compressive strength: 301.00 ± 1.27 MPa Flexural strength: 728.25 ± 22.5 MPa | Customizable stiffness Artifact-free imaging Enhance artifact-free imaging to evaluate therapeutic success Their mechanical properties depend on the carbon fibers’ amount resulting in tunable mechanical properties Roods can provide effective primary stability Screws have higher pullout strength Carbon-fiber cage has shown safety and durability | Torsional stiffness and yield torque are lower Increased chance of bacterial adhesion High risk of screw loosening | [91,92,93,94,95] |
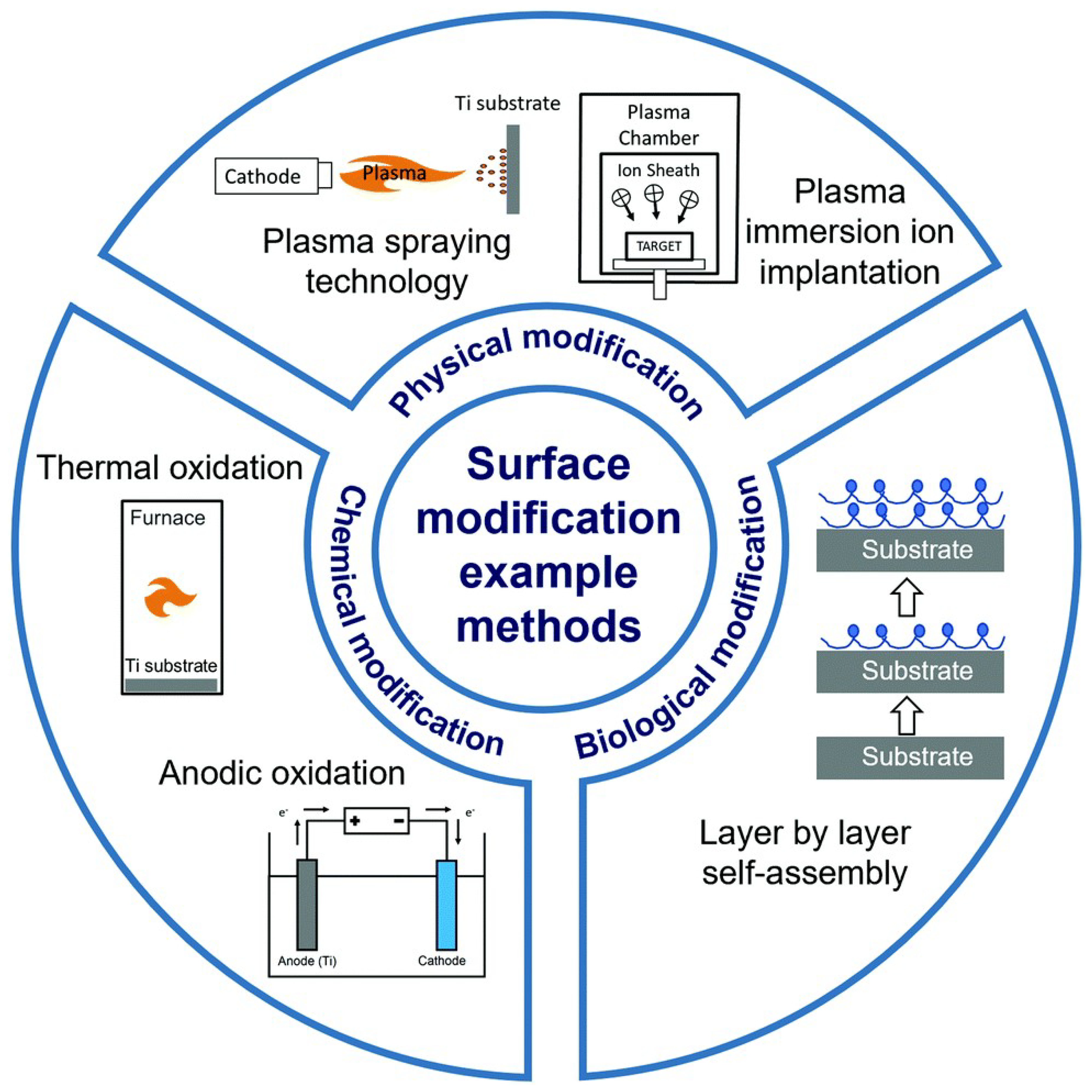
3. Nanotechnology in Spinal Implant Materials: Innovations and Applications
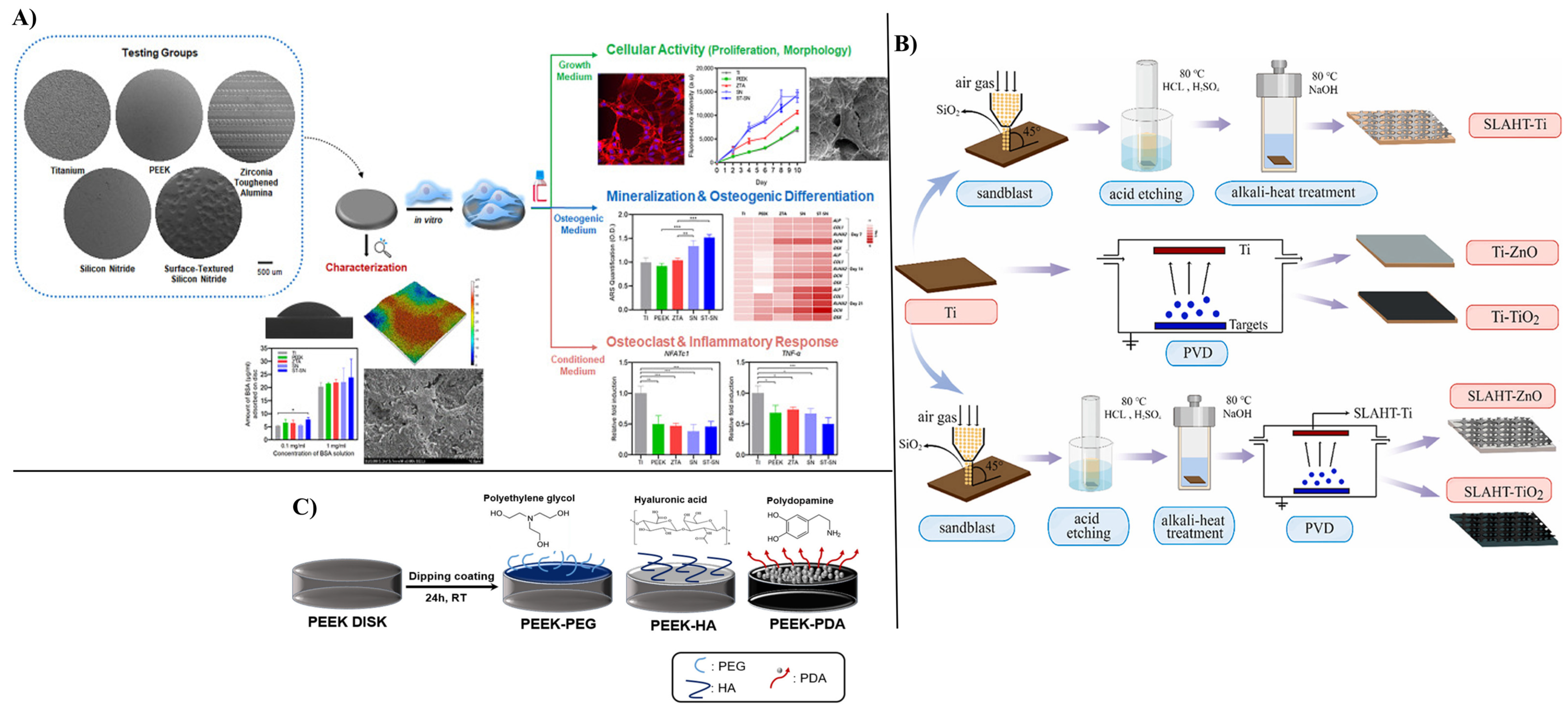
| Material Type | Implant Surface | Surface Modification | Surface Modification Methods | Biological Activity | Limitations | Refs. |
|---|---|---|---|---|---|---|
| Titanium | Titanium Alloy (Ti6Al4V) Disks | Roughness | Sandblast, large grit Acid-etching | Ti has one of the highest rough surfaces Ti showed a proinflammatory response | Inflammatory markers increased on Ti This surface has a low apatite formation | [124] |
| Pure Titanium Disks | Hybrid coating Antimicrobial peptides GL13K+ silver nanoparticles (AgNPs) | Acid-etching and immersion | Antimicrobial efficacy against S. gordonii, MRSA, and P. aeruginosa No cytotoxic effect on hBMSCs cells In vivo tests showed reduced inflammation | Long-term stability, integration, and toxicity must still be evaluated in future work | [127] | |
| Titanium Substrate | Rough, porous surface Coating with ZnO and TiO2 nanoparticles | Sandblast, Acid-etching, and Hydrothermal Treatment (SLAHT) | Enhanced roughness Coating promoted cell viability in L929 cells No cytotoxic effects observed A high antimicrobial effect was provided | Long-term stability, integration, and toxicity must still be evaluated in future work | [125] | |
| Titanium Alloy Cages | Ag-HA coating | Not reported | Improved osseointegration Prevented infection No Ag-related complications | There were no control groups Short time of trial Small sample size Long-term, large-scale trials are needed | [128] | |
| Pure Titanium Pieces | Roughness Coating based on Ag-HA | Sandblast Thermal spray technique | Ag-HA provided enhanced osteoconductivity and improved bone contact -> improved spinal fusion No neurotoxic effect was noticed Ag-HA coating represents a potential biologically safe strategy | Short-term study (8 weeks) Silver accumulation in other organs still needs evaluation | [129] | |
| Titanium Implants | Coating based on HA substituted with silver (Ag+) and strontium (Sr2+) | CoBlast | Sr-HA promoted MG-63 cell metabolic activity, compared with the other coatings Ag-HA showed significant antimicrobial efficacy and inhibited biofilm formation | Ag-Sr-HA needs optimization Long-term stability, integration, and toxicity must still be evaluated in future work | [130] | |
| PEEK | PEEK Disks | None | As Machined | Low inflammation | Poor cellular adhesion due to high hydrophobicity Showed low mineralization and osteogenic gene expression | [124] |
| PEEK Substrate | Bioactive coating with strontium-modified Eucommia ulmoides polysaccharides (EUP-Sr) Porous structure | Not reported | Enhanced MC3T3-F1 proliferation, adhesion, RUNX2 and Col1-α1 expression, ostegenic and anti-inflammatory | Higher concentration of UP-Sr can produce cytotoxicity | [131] | |
| PEEK Disks | Nanocoating with osteogenic and antimicrobial properties Graphene oxide (GO) nanosheets, Polydopamine (PDA) nanofilm, and bone-forming peptide (BFP) | Immersion coating | Promoted osteoblast proliferation Promoted apatite formation High antimicrobial efficacy | Long-term stability, integration, and toxicity must still be evaluated in future work | [132] | |
| PEEK Disks and Intervertebral Cages | Coating based on PEG, HA, PDA | Immersion coating | All the coatings promoted osteoblast proliferation | Long-term stability, integration, and toxicity must still be evaluated in future work. No significant differences between mechanical performances | [126] | |
| Silicon nitride | Silicon Nitride Disks | None | As-Fired | Silicon nitride presented the best apatite formation and promoted cell proliferation High protein adsorption Biomimetic aspect | Long-term stability, integration, and toxicity must still be evaluated in future work. No significant differences between mechanical performances | [124] |
| Surface-Textured Silicon Nitride Disks | Roughness | Laser-patterned surface | ||||
| Stainless Steel | Stainless Steel Plates | Niosomes—nonionic vesicular nanocarriers Vancomycin-loaded niosomes | Layer-by-layer technique Dip-coating | Sustained antibiotic release (28 h) Reduced bacterial adhesion and colony formation No cytotoxic effect was observed on L929 cells | Long-term stability, integration, and toxicity must still be evaluated in future work | [133] |
4. Limitations, Future Perspectives, and Emerging Trends
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, Y.; Fang, M.; Li, Z.; Xue, Y.; Wang, K.; Lin, F.; Zhang, N. Embracing the future: The application of regenerative biomaterials in the spinal disorders. Biomed. Technol. 2025, 9, 100068. [Google Scholar] [CrossRef]
- Raciborski, F.; Gasik, R.; Kłak, A. Disorders of the spine. A major health and social problem. Reumatologia 2016, 54, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Khan, M.R. Selection of suitable pedicle screw for degenerated cortical and cancellous bone of human lumbar spine: A finite element study. Int. J. Artif. Organs 2020, 44, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Zimmerman, Z.E.; Mass, H.; Makhni, M.C. Diagnosis and Management of Lumbar Spinal Stenosis: A Review. JAMA 2022, 327, 1688–1699. [Google Scholar] [CrossRef]
- Brumat, P.; Kunšič, O.; Novak, S.; Slokar, U.; Pšenica, J.; Topolovec, M.; Mihalič, R.; Trebše, R. The Surgical Treatment of Osteoarthritis. Life 2022, 12, 982. [Google Scholar] [CrossRef]
- Costăchescu, B.; Niculescu, A.-G.; Grumezescu, A.M.; Teleanu, D.M. Screw Osteointegration—Increasing Biomechanical Resistance to Pull-Out Effect. Materials 2023, 16, 5582. [Google Scholar] [CrossRef]
- Warburton, A.; Girdler, S.J.; Mikhail, C.M.; Ahn, A.; Cho, S.K. Biomaterials in Spinal Implants: A Review. Neurospine 2020, 17, 101–110. [Google Scholar] [CrossRef]
- Martz, E.O.; Goel, V.K.; Pope, M.H.; Park, J.B. Materials and design of spinal implants—A review. J. Biomed. Mater. Res. 1997, 38, 267–288. [Google Scholar] [CrossRef]
- Tomar, S. Spinal Surgery and Implants: A Systematic Review and Update. Indian J. Appl. Res. 2025, XV, 1–2. [Google Scholar]
- Yao, Y.-C.; Chou, P.-H.; Lin, H.-H.; Wang, S.-T.; Chang, M.-C. Outcome of Ti/PEEK Versus PEEK Cages in Minimally Invasive Transforaminal Lumbar Interbody Fusion. Glob. Spine J. 2021, 13, 219256822110003. [Google Scholar] [CrossRef]
- de Kater, E.P.; Sakes, A.; Edström, E.; Elmi-Terander, A.; Kraan, G.; Breedveld, P. Beyond the pedicle screw–a patent review. Eur. Spine J. 2022, 31, 1553–1565. [Google Scholar] [CrossRef]
- Kiapour, A.; Khandha, A.; Massaad, E.; Connolly, I.D.; Hadzipasic, M.; Shankar, G.M.; Goel, V.; Shin, J.H. Effects of rod diameter on kinematics of posterior cervical spine instrumented constructs: An ex vivo study. J. Neurosurg. Spine 2022, 37, 749–757. [Google Scholar] [CrossRef]
- Zhao, X.f.; Zhao, Y.b.; Lu, X.d.; Wang, W.x.; Qi, D.t.; Yang, X.; Wang, X.n.; Zhou, R.t.; Jin, Y.z.; Zhao, B. Development and Biomechanical Study of a New Open Dynamic Anterior Cervical Nail Plate System: Anterior Cervical Nail Plate System. Orthop. Surg. 2020, 12, 254–261. [Google Scholar] [CrossRef]
- Byrne, T.N.; Benzel, E.C.; Waxman, S.G. Chapter 11 Spine Trauma, Spinal Cord Injury, and Spinal Stability. In Diseases of the Spine and Spinal Cord; Oxford University Press: Oxford, UK, 2000; pp. 359–376. [Google Scholar]
- Bîrcă, A.C.; Minculescu, M.A.; Niculescu, A.-G.; Hudiță, A.; Holban, A.M.; Alberts, A.; Grumezescu, A.M. Nanoparticle-Enhanced Collagen Hydrogels for Chronic Wound Management. J. Funct. Biomater. 2025, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of nanotechnology in medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, properties, and environmental toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Ahmeda, M.H.S.; Ahmida, N.H.S.; Ahmeida, A. Introduction to nanotechnology: Definition, terms, occurrence and applications in environment. Libyan Int. Med. Univ. J. 2022, 2, 12–26. [Google Scholar] [CrossRef]
- Ioniță-Radu, F.; Nicolau, I.-N.; Petrache, O.-G.; Groșeanu, M.-L.; Bojincă, V.-C.; Negru, M.-M.; Bucurică, S.; Anghel, D. Correlation between Trabecular Bone Score and Homocysteine Level in Rheumatoid Arthritis Patients on Anti-TNF Inhibitors. Life 2024, 14, 463. [Google Scholar] [CrossRef]
- Mercan, D.-A.; Tudorache, D.-I.; Niculescu, A.-G.; Mogoantă, L.; Mogoşanu, G.D.; Bîrcă, A.C.; Vasile, B.Ș.; Hudiță, A.; Voinea, I.C.; Stan, M.S.; et al. Antimicrobial Coatings Based on Hybrid Iron Oxide Nanoparticles. Nanomaterials 2025, 15, 637. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Mitra, I.; Goodman, S.B.; Kumar, M.; Bose, S. Improving biocompatibility for next generation of metallic implants. Prog. Mater. Sci. 2023, 133, 101053. [Google Scholar] [CrossRef] [PubMed]
- Vaiani, L.; Boccaccio, A.; Uva, A.E.; Palumbo, G.; Piccininni, A.; Guglielmi, P.; Cantore, S.; Santacroce, L.; Charitos, I.A.; Ballini, A. Ceramic Materials for Biomedical Applications: An Overview on Properties and Fabrication Processes. J. Funct. Biomater. 2023, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Kalirajan, C.; Dukle, A.; Nathanael, A.J.; Oh, T.H.; Manivasagam, G. A Critical Review on Polymeric Biomaterials for Biomedical Applications. Polymers 2021, 13, 3015. [Google Scholar] [CrossRef]
- Rehner, A.M.; Moldoveanu, E.-T.; Niculescu, A.-G.; Bîclesanu, F.C.; Pangică, A.M.; Grumezescu, A.M.; Croitoru, G.-A. Advances in Dental Implants: A Review of In Vitro and In Vivo Testing with Nanoparticle Coatings. J. Compos. Sci. 2025, 9, 140. [Google Scholar] [CrossRef]
- Taymour, N.; Hassan, M.G.; AlGhamdi, M.A.; Omara, W.S. From Detection to Treatment: Nanomaterial-Based Biosensors Transforming Prosthetic Dentistry and Oral Health Care: A Scoping Review. Prosthesis 2025, 7, 51. [Google Scholar] [CrossRef]
- Chen, M.Q. Recent Advances and Perspective of Nanotechnology-Based Implants for Orthopedic Applications. Front. Bioeng. Biotechnol. 2022, 10, 878257. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Lopez, K.G.; Alathur Ramakrishnan, S.; Hallinan, J.T.P.D.; Fuh, J.Y.H.; Pandita, N.; Madhu, S.; Kumar, A.; Benneker, L.M.; Vellayappan, B.A. Evolution of materials for implants in metastatic spine disease till date—Have we found an ideal material? Radiother. Oncol. 2021, 163, 93–104. [Google Scholar] [CrossRef]
- Muthiah, N.; Yolcu, Y.U.; Alan, N.; Agarwal, N.; Hamilton, D.K.; Ozpinar, A. Evolution of polyetheretherketone (PEEK) and titanium interbody devices for spinal procedures: A comprehensive review of the literature. Eur. Spine J. 2022, 31, 2547–2556. [Google Scholar] [CrossRef]
- Rao, P.J.; Pelletier, M.H.; Walsh, W.R.; Mobbs, R.J. Spine Interbody Implants: Material Selection and Modification, Functionalization and Bioactivation of Surfaces to Improve Osseointegration. Orthop. Surg. 2014, 6, 81–89. [Google Scholar] [CrossRef]
- Iorio, J.A.; Jakoi, A.M.; Singla, A. Biomechanics of Degenerative Spinal Disorders. Asian Spine J. 2016, 10, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Galbusera, F. Chapter 14—Biomechanics of the spine. In Human Orthopaedic Biomechanics; Innocenti, B., Galbusera, F., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 265–283. [Google Scholar]
- Barrett, J.M.; McKinnon, C.; Callaghan, J.P. Cervical spine joint loading with neck flexion. Ergonomics 2020, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Costi, J.J.; Ledet, E.H.; O’Connell, G.D. Spine biomechanical testing methodologies: The controversy of consensus vs scientific evidence. JOR Spine 2021, 4, e1138. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Christiansen, B.A.; Roberts, B.J.; Valentine, M.J.; Manoharan, R.K.; Bouxsein, M.L. A biomechanical model for estimating loads on thoracic and lumbar vertebrae. Clin. Biomech. 2010, 25, 853–858. [Google Scholar] [CrossRef]
- Schafer, R.; Trompeter, K.; Fett, D.; Heinrich, K.; Funken, J.; Willwacher, S.; Bruggemann, G.P.; Platen, P. The mechanical loading of the spine in physical activities. Eur. Spine J. 2023, 32, 2991–3001. [Google Scholar] [CrossRef]
- Moghadasi, K.; Mohd Isa, M.S.; Ariffin, M.A.; Mohd Jamil, M.Z.; Raja, S.; Wu, B.; Yamani, M.; Bin Muhamad, M.R.; Yusof, F.; Jamaludin, M.F.; et al. A review on biomedical implant materials and the effect of friction stir based techniques on their mechanical and tribological properties. J. Mater. Res. Technol. 2022, 17, 1054–1121. [Google Scholar] [CrossRef]
- Hosseini, S.; Limooei, M.B. Fatigue of Ti-6Al-4V. In Biomedical Engineering—Technical Applications in Medicine; Hudak, R., Penhaker, M., Majernik, J., Eds.; IntechOpen: Rijeka, Croatia, 2012. [Google Scholar][Green Version]
- Al-Shalawi, F.D.; Mohamed Ariff, A.H.; Jung, D.-W.; Mohd Ariffin, M.K.; Seng Kim, C.L.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as Implants in the Orthopedic Field for Regenerative Medicine: Metal versus Synthetic Polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef]
- Kumar, N.M.S.; Suresh, G.; Dumpala, R.; Bradagunta, R.S. In Vitro Degradation Studies on Mg-Zn-CeO2 Composites for Biodegradable Implant Applications. Biointerface Res. Appl. Chem. 2024, 14, 86. [Google Scholar]
- Shaikh, M.; Kahwash, F.; Lu, Z.; Alkhreisat, M.; Mohammad, A.; Shyha, I. Revolutionising orthopaedic implants—A comprehensive review on metal 3D printing with materials, design strategies, manufacturing technologies, and post-process machining advancements. Int. J. Adv. Manuf. Technol. 2024, 134, 1043–1076. [Google Scholar] [CrossRef]
- Gloria, A.; Russo, T.; De Santis, R.; Ambrosio, L. 7—Composite materials for spinal implants. In Biomedical Composites, 2nd ed.; Ambrosio, L., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 139–161. [Google Scholar]
- Che Lah, N.A.; Hussin, M.H. Titanium and Titanium Based Alloys as Metallic Biomaterials in Medical Applications-Spine Implant Case Study. Pertanika J. Sci. Technol. 2019, 27, 459–472. [Google Scholar]
- Abd-Elaziem, W.; Darwish, M.A.; Hamada, A.; Daoush, W.M. Titanium-Based alloys and composites for orthopedic implants Applications: A comprehensive review. Mater. Des. 2024, 241, 112850. [Google Scholar] [CrossRef]
- Satyanarayana, C.P.; Raju, L.S.; Raju, L.R.; Dondapati, S.; Dumpala, R.; Buradagunta, R.S. Comparative investigations on the bioactivity of surface grain refined titanium and surface oxidized titanium for biomedical implant applications. Biointerface Res. App. 2023, 13, 1–10. [Google Scholar]
- Ude, C.C.; Dzidotor, G.K.; Iloeje, K.; Nair, L.S.; Laurencin, C.T. Corrosion of Metals During Use in Arthroplasty. ACS Appl. Bio Mater. 2023, 6, 2029–2042. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wei, C.; Deng, L.; Wang, P.; Zhong, W.; Huang, W. A review of non-biodegradable alloys implantation induced inflammatory and immune cell responses. J. Alloys Compd. 2024, 977, 173086. [Google Scholar] [CrossRef]
- Mobarak, M.H.; Islam, M.A.; Hossain, N.; Al Mahmud, M.Z.; Rayhan, M.T.; Nishi, N.J.; Chowdhury, M.A. Recent advances of additive manufacturing in implant fabrication—A review. Appl. Surf. Sci. Adv. 2023, 18, 100462. [Google Scholar] [CrossRef]
- Meena, V.K.; Kumar, P.; Kalra, P.; Sinha, R.K. Additive manufacturing for metallic spinal implants: A systematic review. Ann. 3D Print. Med. 2021, 3, 100021. [Google Scholar] [CrossRef]
- Katsuura, Y.; Wright-Chisem, J.; Wright-Chisem, A.; Virk, S.; McAnany, S. The Importance of Surface Technology in Spinal Fusion. HSS J. Musculoskelet. J. Hosp. Spec. Surg. 2020, 16, 113–116. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A. Biomedical Applications of Titanium Alloys: A Comprehensive Review. Materials 2024, 17, 114. [Google Scholar] [CrossRef]
- Hekimoglu, M.; Ozer, H.; Kiraz, K.; Onursal, C.; Siyahcan, F.; Ozer, A.F. Surface hardening of Ti-Al-V superalloy spinal implant by using the boronization method. Biomed. Mater. Eng. 2024, 35, 39–52. [Google Scholar] [CrossRef]
- Tahal, D.; Madhavan, K.; Chieng, L.O.; Ghobrial, G.M.; Wang, M.Y. Metals in Spine. World Neurosurg. 2017, 100, 619–627. [Google Scholar] [CrossRef]
- Litak, J.; Szymoniuk, M.; Czyżewski, W.; Hoffman, Z.; Litak, J.; Sakwa, L.; Kamieniak, P. Metallic Implants Used in Lumbar Interbody Fusion. Materials 2022, 15, 3650. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.C.; Burnham, M.; Ojameruaye, O.; McKee, M.D. A systematic review of the use of titanium versus stainless steel implants for fracture fixation. OTA Int. 2021, 4, e138. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Rezvani Ghomi, E.; Alipour, S.; Ramakrishna, S.; Liana Sukiman, N. A state-of-the-art review of the fabrication and characteristics of titanium and its alloys for biomedical applications. Bio-Des. Manuf. 2022, 5, 371–395. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, E.; Montazerian, H.; Mirhakimi, A.S.; Zhianmanesh, M.; Ibhadode, O.; Shahabad, S.I.; Esmaeilizadeh, R.; Sarikhani, E.; Toorandaz, S.; Sarabi, S.A.; et al. Additively manufactured metallic biomaterials. Bioact. Mater. 2022, 15, 214–249. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Traxel, K.D.; Avila, J.D.; Mitra, I.; Bose, S. 1.3.3C—CoCr Alloys. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 257–269. [Google Scholar]
- Lebhar, J.; Kriegel, P.; Chatellier, P.; Breton, Y.; Ropars, M.; Huten, D. Tantalum implants for posterior lumbar interbody fusion: A safe method at medium-term follow-up? Orthop. Traumatol. Surg. Res. 2020, 106, 269–274. [Google Scholar] [CrossRef]
- Huang, G.; Pan, S.-T.; Qiu, J.-X. The Clinical Application of Porous Tantalum and Its New Development for Bone Tissue Engineering. Materials 2021, 14, 2647. [Google Scholar] [CrossRef]
- Hanc, M.; Fokter, S.K.; Vogrin, M.; Molicnik, A.; Recnik, G. Porous tantalum in spinal surgery: An overview. Eur. J. Orthop. Surg. Traumatol. 2016, 26, 1–7. [Google Scholar] [CrossRef]
- Putrantyo, I.; Anilbhai, N.; Vanjani, R.; De Vega, B. Tantalum as a Novel Biomaterial for Bone Implant: A Literature Review. J. Biomim. Biomater. Biomed. Eng. 2021, 52, 55–65. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Z.; Zhu, W.; Weng, X. Polyetheretherketone development in bone tissue engineering and orthopedic surgery. Front. Bioeng. Biotechnol. 2023, 11, 1207277. [Google Scholar] [CrossRef]
- Shahbazi, A.; Sepehrinezhad, A.; Vahdani, E.; Jamali, R.; Ghasempour, M.; Massoudian, S.; Sahab Negah, S.; Larsen, F.S. Gut Dysbiosis and Blood-Brain Barrier Alteration in Hepatic Encephalopathy: From Gut to Brain. Biomedicines 2023, 11, 1272. [Google Scholar] [CrossRef]
- Kersten, R.F.M.R.; Gaalen, S.; Gast, A.; Oner, F. Polyetheretherketone (PEEK) cages in cervical applications: A systematic review. Spine J. Off. J. North Am. Spine Soc. 2013, 15, 1446–1460. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Liu, S.-J. Polyetheretherketone for orthopedic applications: A review. Curr. Opin. Chem. Eng. 2021, 32, 100687. [Google Scholar] [CrossRef]
- Xin, H.; Shepherd, D.E.T.; Dearn, K.D. Strength of poly-ether-ether-ketone: Effects of sterilisation and thermal ageing. Polym. Test. 2013, 32, 1001–1005. [Google Scholar] [CrossRef]
- Wuisman, P.I.; Smit, T.H. Bioresorbable polymers: Heading for a new generation of spinal cages. Eur. Spine J. 2006, 15, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-S.; Lee, S.; Lee, D.; Jho, J.Y. Mechanical Properties and Bioactivity of Poly(Lactic Acid) Composites Containing Poly(Glycolic Acid) Fiber and Hydroxyapatite Particles. Nanomaterials 2021, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Travieso-Rodriguez, J.A.; Jerez-Mesa, R.; Llumà, J.; Traver-Ramos, O.; Gomez-Gras, G.; Roa Rovira, J.J. Mechanical Properties of 3D-Printing Polylactic Acid Parts subjected to Bending Stress and Fatigue Testing. Materials 2019, 12, 3859. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Bermudo Gamboa, C.; Martín-Béjar, S.; Bañón García, F.; Sevilla Hurtado, L. Enhancing Fatigue Resistance of Polylactic Acid through Natural Reinforcement in Material Extrusion. Polymers 2024, 16, 2422. [Google Scholar] [CrossRef]
- Subagio, E.A.; Permana, G.I.; Bajamal, A.H.; Faris, M.; Suroto, N.S.; Rasyida, A.; Utomo, B. Biomechanical properties of polyvinyl alcohol hydrogel as a nucleus pulposus replacement in intervertebral disc herniation: A systematic review. World Acad. Sci. J. 2023, 5, 37. [Google Scholar] [CrossRef]
- Jazayeri, H.E.; Tahriri, M.; Razavi, M.; Khoshroo, K.; Fahimipour, F.; Dashtimoghadam, E.; Almeida, L.; Tayebi, L. A current overview of materials and strategies for potential use in maxillofacial tissue regeneration. Mater. Sci. Eng. C 2017, 70, 913–929. [Google Scholar] [CrossRef]
- Kwon, J.-W.; Lee, Y.H.; Lee, B.H.; Kim, J.H.; Suk, K.S. Clinical and radiological outcomes of non-window-type bioactive glass–ceramic cage in single-level ACDF versus PEEK cage filled with autologous bone. Sci. Rep. 2024, 14, 4035. [Google Scholar] [CrossRef] [PubMed]
- Cottrill, E.; Pennington, Z.; Lankipalle, N.; Ehresman, J.; Valencia, C.; Schilling, A.; Feghali, J.; Perdomo-Pantoja, A.; Theodore, N.; Sciubba, D.M.; et al. The effect of bioactive glasses on spinal fusion: A cross-disciplinary systematic review and meta-analysis of the preclinical and clinical data. J. Clin. Neurosci. 2020, 78, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109895. [Google Scholar] [CrossRef] [PubMed]
- Motavallian, P.; Rabiee, S.M.; Jamshidi Aval, H. The microstructure and mechanical and corrosion properties of Mg matrix composites reinforced with bioactive glass: The combined effect of bioactive glass content and extrusion speed. Mater. Chem. Phys. 2023, 307, 128135. [Google Scholar] [CrossRef]
- Ament, J.D.; Vokshoor, A.; Yee, R.; Johnson, J.P. A Systematic Review and Meta-Analysis of Silicon Nitride and Biomaterial Modulus as it Relates to Subsidence Risk in Spinal Fusion Surgery. North Am. Spine Soc. J. (NASSJ) 2022, 12, 100168. [Google Scholar] [CrossRef]
- Calvert, G.C.; VanBuren Huffmon, G., 3rd; Rambo, W.M., Jr.; Smith, M.W.; McEntire, B.J.; Bal, B.S. Clinical outcomes for lumbar fusion using silicon nitride versus other biomaterials. J. Spine Surg. 2020, 6, 33–48. [Google Scholar] [CrossRef]
- Du, X.; Lee, S.S.; Blugan, G.; Ferguson, S.J. Silicon Nitride as a Biomedical Material: An Overview. Int. J. Mol. Sci. 2022, 23, 6551. [Google Scholar] [CrossRef]
- Zou, R.; Bi, L.; Huang, Y.; Wang, Y.; Wang, Y.; Li, L.; Liu, J.; Feng, L.; Jiang, X.; Deng, B. A biocompatible silicon nitride dental implant material prepared by digital light processing technology. J. Mech. Behav. Biomed. Mater. 2023, 141, 105756. [Google Scholar] [CrossRef]
- Andraskar, N.D.; Tiwari, G.; Goel, M.D. Impact response of ceramic structures—A review. Ceram. Int. 2022, 48, 27262–27279. [Google Scholar] [CrossRef]
- Bozkurt, C.; Şenköylü, A.; Aktaş, E.; Sarıkaya, B.; Sipahioğlu, S.; Gürbüz, R.; Timuçin, M. Biomechanical Evaluation of a Novel Apatite-Wollastonite Ceramic Cage Design for Lumbar Interbody Fusion: A Finite Element Model Study. BioMed Res. Int. 2018, 2018, 4152543. [Google Scholar] [CrossRef]
- Ahmetoglu, C.; Park, J.; Korkusuz, F.; Ozturk, A.; Timuçin, M. Production and Properties of Apatite-Wollastonite Ceramics for Biomedical Applications. InterCeram Int. Ceram. Rev. 2009, 58, 86–90. [Google Scholar]
- Parsafar, M.; Sadrnezhaad, S.K.; Nemati, N.H. Design and preparation of nickel-titanium implant for lumbar vertebra. J. Alloys Compd. 2023, 936, 167969. [Google Scholar] [CrossRef]
- Sadrnezhaad, S.K.; Parsafar, M.; Rashtiani, Y.; Jadidi, M. Jadidi Nitinol Spinal Vertebrae: A Favorable New Substitute. Int. J. Eng. 2019, 32, 842–851. [Google Scholar]
- Zhang, Y.; Attarilar, S.; Wang, L.; Lu, W.; Yang, J.; Fu, Y. A Review on Design and Mechanical Properties of Additively Manufactured NiTi Implants for Orthopedic Applications. Int. J. Bioprinting 2021, 7, 340. [Google Scholar] [CrossRef]
- Vojtěch, D.; Michalcová, A.; Čapek, J.; Marek, I.; Dragounová, L. Structural and mechanical stability of the nano-crystalline Ni–Ti (50.9 at.% Ni) shape memory alloy during short-term heat treatments. Intermetallics 2014, 49, 7–13. [Google Scholar] [CrossRef]
- Joerger, A.-K.; Shiban, E.; Krieg, S.M.; Meyer, B. Carbon-fiber reinforced PEEK instrumentation for spondylodiscitis: A single center experience on safety and efficacy. Sci. Rep. 2021, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Ghermandi, R.; Tosini, G.; Lorenzi, A.; Griffoni, C.; La Barbera, L.; Girolami, M.; Pipola, V.; Barbanti Brodano, G.; Bandiera, S.; Terzi, S.; et al. Carbon Fiber-Reinforced PolyEtherEtherKetone (CFR-PEEK) Instrumentation in Degenerative Disease of Lumbar Spine: A Pilot Study. Bioengineering 2023, 10, 872. [Google Scholar] [CrossRef]
- Cawley, D.T.; Alzakri, A.; Fujishiro, T.; Kieser, D.C.; Tavalaro, C.; Boissiere, L.; Obeid, I.; Pointillart, V.; Vital, J.M.; Gille, O. Carbon-fibre cage reconstruction in anterior cervical corpectomy for multilevel cervical spondylosis: Mid-term outcomes. J. Spine Surg. 2019, 5, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Yao, S.; Zhao, J.; Zhou, C.; Oates, T.; Weir, M.; Wu, J.; Xu, H. Review on Development and Dental Applications of Polyetheretherketone-Based Biomaterials and Restorations. Materials 2021, 14, 408. [Google Scholar] [CrossRef]
- Qin, W.; Xing, T.; Tang, B.; Chen, W. Mechanical properties and osteogenesis of CFR-PEEK composite with interface strengthening by graphene oxide for implant application. J. Mech. Behav. Biomed. Mater. 2023, 148, 106222. [Google Scholar] [CrossRef]
- Ramezani, M.; Ripin, Z.M. An Overview of Enhancing the Performance of Medical Implants with Nanocomposites. J. Compos. Sci. 2023, 7, 199. [Google Scholar] [CrossRef]
- Oladele, I.O.; Onuh, L.N.; Agbeboh, N.I.; Alewi, D.D.; Lephuthing, S.S. The relationship and functional links between human age, growth, and biomedical implants: A review on the application of bulk and nanomaterials. Nano Sel. 2023, 4, 419–441. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, C.; Bai, J.; Zhang, H.; Long, H.; Jiang, B.; Dai, H.; Wang, J.; Zhang, H.; Zhao, J. Current developments and future perspectives of nanotechnology in orthopedic implants: An updated review. Front. Bioeng. Biotechnol. 2024, 12, 1342340. [Google Scholar] [CrossRef] [PubMed]
- Harawaza, K.; Cousins, B.; Roach, P.; Fernandez, A. Modification of the surface nanotopography of implant devices: A translational perspective. Mater. Today Bio 2021, 12, 100152. [Google Scholar] [CrossRef]
- Zhang, G.; Zhen, C.; Yang, J.; Wang, J.; Wang, S.; Shang, P. Recent advances of nanoparticles on bone cells. Nanoscale Adv. 2024, 6, 1957–1973. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, G.; Li, J.J. Advances in implant surface modifications to improve osseointegration. Mater. Adv. 2021, 2, 6901–6927. [Google Scholar] [CrossRef]
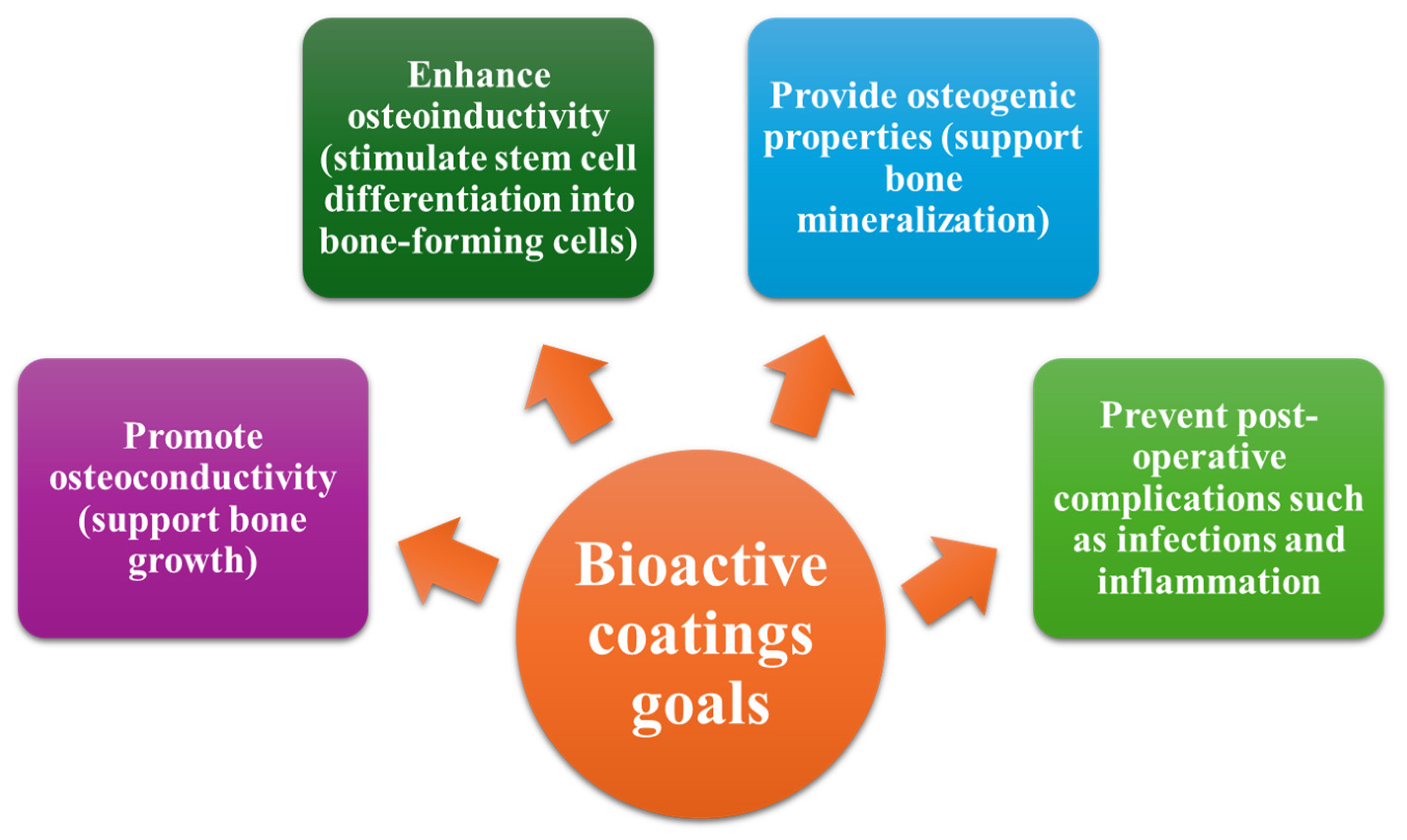
- Nikolova, M.P.; Apostolova, M.D. Advances in Multifunctional Bioactive Coatings for Metallic Bone Implants. Materials 2022, 16, 183. [Google Scholar] [CrossRef]
- Butler, J.; Handy, R.D.; Upton, M.; Besinis, A. Review of Antimicrobial Nanocoatings in Medicine and Dentistry: Mechanisms of Action, Biocompatibility Performance, Safety, and Benefits Compared to Antibiotics. ACS Nano 2023, 17, 7064–7092. [Google Scholar] [CrossRef]
- Joshi, M.; Adak, B. 5.10—Advances in Nanotechnology Based Functional, Smart and Intelligent Textiles: A Review. In Comprehensive Nanoscience and Nanotechnology, 2nd ed.; Andrews, D.L., Lipson, R.H., Nann, T., Eds.; Academic Press: Oxford, UK, 2019; pp. 253–290. [Google Scholar]
- Panaitescu, T.G.; Niculescu, A.-G.; Grumezescu, V.; Costăchescu, B.; Bircă, A.C.; Balaure, P.C.; Oprea, O.C.; Voinea, I.C.; Stan, M.S.; Holban, A.M.; et al. Nanostructured Coatings for Spinal Fixation Screws: A Dual-Function Approach Against Biofilm Formation and Implant Failure. Coatings 2025, 15, 584. [Google Scholar] [CrossRef]
- Alberts, A.; Bratu, A.G.; Niculescu, A.-G.; Grumezescu, A.M. Collagen-Based Wound Dressings: Innovations, Mechanisms, and Clinical Applications. Gels 2025, 11, 271. [Google Scholar] [CrossRef]
- Grămadă, A.M.; Stoica, A.-E.; Niculescu, A.-G.; Bîrcă, A.C.; Vasile, B.Ș.; Holban, A.M.; Mihaiescu, T.; Șerban, A.I.; Ciceu, A.; Balta, C. Zinc Oxide-Loaded Recycled PET Nanofibers for Applications in Healthcare and Biomedical Devices. Polymers 2024, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Iosub, G.; Niculescu, A.-G.; Grumezescu, V.; Dorcioman, G.; Gherasim, O.; Crăciun, V.; Rădulescu, D.M.; Grumezescu, A.M.; Stan, M.S.; Constantinescu, S. Graphene Oxide–Antibiotic Coatings with Improved Resistance to Microbial Colonization for Arthroplasty Implants. J. Compos. Sci. 2025, 9, 82. [Google Scholar] [CrossRef]
- Levy, H.A.; Karamian, B.A.; Yalla, G.R.; Canseco, J.A.; Vaccaro, A.R.; Kepler, C.K. Impact of surface roughness and bulk porosity on spinal interbody implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rath, B.; Tingart, M.; Eschweiler, J. Role of implants surface modification in osseointegration: A systematic review. J Biomed. Mater. Res. A 2020, 108, 470–484. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, X.; Xu, A.; Wang, L.; Luo, Z.; Zheng, Y.; Deng, F.; Wei, J.; Tang, Z.; Wei, S. Effect of surface roughness on osteogenesis in vitro and osseointegration in vivo of carbon fiber-reinforced polyetheretherketone-nanohydroxyapatite composite. Int. J. Nanomed. 2015, 10, 1425–1447. [Google Scholar] [CrossRef]
- Scariano, G.; Meade, S.; Sultan, A.; Shost, M.; Benzel, E.C.; Krishnaney, A.; Mroz, T.; Steinmetz, M.P.; Habboub, G. Exploring tribology and material contact science in spine surgery: Implications for implant design. J. Neurosurg. Spine 2024, 40, 801–810. [Google Scholar] [CrossRef]
- Litak, J.; Czyzewski, W.; Szymoniuk, M.; Pastuszak, B.; Litak, J.; Litak, G.; Grochowski, C.; Rahnama-Hezavah, M.; Kamieniak, P. Hydroxyapatite Use in Spine Surgery-Molecular and Clinical Aspect. Materials 2022, 15, 2906. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, R.; Kumar, S. Coatings on orthopedic implants to overcome present problems and challenges: A focused review. Mater. Today Proc. 2021, 45, 5269–5276. [Google Scholar] [CrossRef]
- Dudek, K.; Strach, A.; Wasilkowski, D.; Łosiewicz, B.; Kubisztal, J.; Mrozek-Wilczkiewicz, A.; Zioła, P.; Barylski, A. Comparison of Key Properties of Ag-TiO2 and Hydroxyapatite-Ag-TiO2 Coatings on NiTi SMA. J. Funct. Biomater. 2024, 15, 264. [Google Scholar] [CrossRef]
- Sobolev, A.; Valkov, A.; Kossenko, A.; Wolicki, I.; Zinigrad, M.; Borodianskiy, K. Bioactive Coating on Ti Alloy with High Osseointegration and Antibacterial Ag Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39534–39544. [Google Scholar] [CrossRef] [PubMed]
- Enders, J.J.; Coughlin, D.; Mroz, T.E.; Vira, S. Surface Technologies in Spinal Fusion. Neurosurg. Clin. North Am. 2020, 31, 57–64. [Google Scholar] [CrossRef]
- Costa Filho, P.M.D.; Marcantonio, C.C.; Oliveira, D.P.; Lopes, M.E.S.; Puetate, J.C.S.; Faria, L.V.; Carvalho, L.F.; Molon, R.S.; Garcia Junior, I.R.; Nogueira, A.V.B.; et al. Titanium micro-nano textured surface with strontium incorporation improves osseointegration: An in vivo and in vitro study. J. Appl. Oral Sci. Rev. FOB 2024, 32, e20240144. [Google Scholar] [CrossRef]
- Toop, N.; Dhaliwal, J.; Gifford, C.S.; Gibbs, D.; Keister, A.; Miracle, S.; Forghani, R.; Grossbach, A.J.; Farhadi, H.F. Promotion of higher rates of early fusion using activated titanium versus polyetheretherketone cages in adults undergoing 1- and 2-level transforaminal lumbar interbody fusion procedures: A randomized controlled trial. J. Neurosurg. Spine 2023, 39, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Pesce, A.; Palmieri, M.; Armocida, D.; Frati, A.; Santoro, A. Letter: Neurosurgery and Coronavirus (COVID-19) Epidemic: Doing our Part. Neurosurgery 2020, 87, E48–E49. [Google Scholar] [CrossRef]
- Shiban, E.; Joerger, A.K.; Janssen, I.; Issa, M.; Lange, N.; Wagner, A.; Feihl, S.; Ringel, F.; Meyer, B. Low-Grade Infection and Implant Failure Following Spinal Instrumentation: A Prospective Comparative Study. Neurosurgery 2020, 87, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Bratschitsch, G.; Puchwein, P.; Zollner-Schwetz, I.; Sadoghi, P.; Radl, R.; Leithner, A.; Leitner, L. Spinal surgery site infection leading to implant loosening is influenced by the number of prior operations. Glob. Spine J. 2022, 12, 458–463. [Google Scholar] [CrossRef]
- Lee, S.S.; Huber, S.; Ferguson, S.J. Comprehensive in vitro comparison of cellular and osteogenic response to alternative biomaterials for spinal implants. Mater. Sci. Eng. C 2021, 127, 112251. [Google Scholar] [CrossRef]
- Ma, X.; Ji, Z.; Li, T.; Liu, P.; Wang, J.; Ma, F.; Zhang, K.; Chen, X.; Liu, J.; Li, W. Influence of surface topographic morphologies and nanoparticle incorporation on surface properties of pure Ti implants for oral applications. Smart Mater. Manuf. 2024, 2, 100049. [Google Scholar] [CrossRef]
- Park, S.; Jung, T.G. Surface Modification of Polyetheretherketone (PEEK) Intervertebral Fusion Implant Using Polydopamine Coating for Improved Bioactivity. Bioengineering 2024, 11, 343. [Google Scholar] [CrossRef]
- Ye, Z.; Sang, T.; Li, K.; Fischer, N.G.; Mutreja, I.; Echeverria, C.; Kumar, D.; Tang, Z.; Aparicio, C. Hybrid nanocoatings of self-assembled organic-inorganic amphiphiles for prevention of implant infections. Acta Biomater. 2022, 140, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Tsukamoto, M.; Aita, K.; Fujita, N.; Mawatari, M. First clinical experience with posterior lumbar interbody fusion using a thermal-sprayed silver-containing hydroxyapatite-coated cage. J. Orthop. Surg. Res. 2023, 18, 392. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Morimoto, T.; Hashimoto, A.; Kii, S.; Tsukamoto, M.; Miyamoto, H.; Todo, M.; Sonohata, M.; Mawatari, M. Osteoconductivity and neurotoxicity of silver-containing hydroxyapatite coating cage for spinal interbody fusion in rats. JOR Spine 2023, 6, e1236. [Google Scholar] [CrossRef] [PubMed]
- O’ Sullivan, C.; O’ Neill, L.; O’ Leary, N.D.; O’ Gara, J.P.; Crean, A.M.; Ryan, K.B. Osteointegration, antimicrobial and antibiofilm activity of orthopaedic titanium surfaces coated with silver and strontium-doped hydroxyapatite using a novel blasting process. Drug Deliv. Transl. Res. 2021, 11, 702–716. [Google Scholar] [CrossRef]
- Mengdi, Z.; Jiayi, L.; Canfeng, L.; Guofeng, W.; Yutong, W.; Pengzhou, H.; Yikun, Z.; Xintao, Z.; Bin, T. Surface modification of polyetheretherketone (PEEK) to enhance osteointegration by grafting strontium Eucommia ulmoides polysaccharides. Int. J. Biol. Macromol. 2022, 211, 230–237. [Google Scholar] [CrossRef]
- Wang, S.; Duan, C.; Yang, W.; Gao, X.; Shi, J.; Kang, J.; Deng, Y.; Shi, X.L.; Chen, Z.G. Two-dimensional nanocoating-enabled orthopedic implants for bimodal therapeutic applications. Nanoscale 2020, 12, 11936–11946. [Google Scholar] [CrossRef]
- Dwivedi, A.; Mazumder, A.; Nasongkla, N. Layer-by-layer nanocoating of antibacterial niosome on orthopedic implant. Int. J. Pharm. 2018, 547, 235–243. [Google Scholar] [CrossRef]
- Yang, J.; Cai, H.; Lv, J.; Zhang, K.; Leng, H.; Wang, Z.; Liu, Z. Biomechanical and histological evaluation of roughened surface titanium screws fabricated by electron beam melting. PLoS ONE 2014, 9, e96179. [Google Scholar] [CrossRef] [PubMed]
- Alkhodary, M.A. Effect of controlled surface roughness and biomimetic coating on titanium implants adhesion to the bone: An experiment animal study. Saudi Dent. J. 2023, 35, 819–826. [Google Scholar] [CrossRef]
- Croft, A.J.; Chanbour, H.; Chen, J.W.; Young, M.W.; Stephens, B.F. Implant Surface Technologies to Promote Spinal Fusion: A Narrative Review. Int. J. Spine Surg. 2023, 17, S35–S43. [Google Scholar] [CrossRef]
- Ganko, R.; Madhavan, A.; Hamouda, W.; Muthu, S.; Jain, A.; Yoon, S.T.; El-Rozz, H.; Cyril, D.; Pabbruwe, M.; Tipper, J.L.; et al. Spinal implant wear particles: Generation, characterization, biological impacts, and future considerations. iScience 2025, 28, 112193. [Google Scholar] [CrossRef] [PubMed]
- Montazerian, M.; Hosseinzadeh, F.; Migneco, C.; Fook, M.V.L.; Baino, F. Bioceramic coatings on metallic implants: An overview. Ceram. Int. 2022, 48, 8987–9005. [Google Scholar] [CrossRef]
- Corrêa, C.B.; Ribeiro, A.L.R.; Moretti, L.A.C.; Consolaro, A.; Junior, E.M. Treatment of implant surfaces: Insights, outcomes and limitations. Adv. Med. Biol. 2015, 82, 115–134. [Google Scholar]
- Kaura, S.; Sharma, S.; Garima; Verma, D.; Sethi, N. Pros and Cons of Nanotechnology. Int. J. Sci. Inf. 2023, 1, 7–26. [Google Scholar]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in healthcare, and its safety and environmental risks. J. Nanobiotechnol. 2024, 22, 715. [Google Scholar] [CrossRef]
- Wasti, S.; Lee, I.H.; Kim, S.; Lee, J.H.; Kim, H. Ethical and legal challenges in nanomedical innovations: A scoping review. Front. Genet. 2023, 14, 1163392. [Google Scholar] [CrossRef]
- Laubach, M.; Kobbe, P.; Hutmacher, D.W. Biodegradable interbody cages for lumbar spine fusion: Current concepts and future directions. Biomaterials 2022, 288, 121699. [Google Scholar] [CrossRef]
- Karavasili, C.; Boyce, H.; Blanco, J.; Young, T.; Connolly, I.D.; Park, S.; Bernstock, J.D.; Jimenez, M.; Kang, Z.; Muller, B.; et al. 3D-printed antibiotic-eluting pedicle screws for antimicrobial prophylaxis in instrumented spinal fusion. Cell Rep. Phys. Sci. 2024, 5, 102320. [Google Scholar] [CrossRef]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef]
- Laratta, J.L.; Vivace, B.J.; López-Peña, M.; Guzón, F.M.; Gonzalez-Cantalpeidra, A.; Jorge-Mora, A.; Villar-Liste, R.M.; Pino-Lopez, L.; Lukyanchuk, A.; Taghizadeh, E.A.; et al. 3D-printed titanium cages without bone graft outperform PEEK cages with autograft in an animal model. Spine J. 2022, 22, 1016–1027. [Google Scholar] [CrossRef]
| Spinal Region | Loading Type | Activity | Typical Magnitude | Observation | Refs. |
|---|---|---|---|---|---|
| Cervical | Compression | Flexion | 550 N | Maximum for C4–C5 and C7–T1 | [34] |
| Bending Moments | Flexion Extension | 1.6–3.5 (Nm) | These estimation times were evaluated in vitro | [35] | |
| Shear | Flexion | 100 N | These forces increase in flexion for C0–C3 and decrease at C6–C7 and C7–T1 | ||
| Thoracic | Compression | Relaxed | 283 (N) | - | [36] |
| Flexion | 752 (N) | ||||
| Lateral Bending | 438 (N) | ||||
| Lumbar | Compression | Standing | 596 (N) | Axial load during regular standing | [37] |
| Bending Forward (30°) | 1271 (N) | It was observed that compression increases with forward bending | |||
| Bending Forward (90°) | 2195 (N) | A peak load was observed during the bending | |||
| Walking | 966 (N) | Moderate load | |||
| Climbing Stairs | 1206 (N) | Increased load | |||
| Getting up | 2384 (N) | Highest load | |||
| Bending Moments | Flexion Extension | 2.6–10 (Nm) | This estimation was evaluated in vitro | [35,36] |
| Implant Type | Implantable Material | Surface Modification Technique | Observations |
|---|---|---|---|
| Interbody | Titanium | Surface roughening | Improve initial fixation Stimulates osteoblast differentiation Leads to better bone formation |
| Porous surface | Reduce stress shielding Promote bone ingrowth Increased porosity leads to wear debris | ||
| Chemical Modification | Enhances osseointegration Mimic bone’s chemical composition | ||
| PEEK | Coating with composite materials | Improve osseointegration Enhances bone growth | |
| Porous surface | Mimic the structure of the trabecular bone Improves cell attachment and bone ingrowth | ||
| Pedicle Screws | Titanium | Roughened Titanium | Increase pull-out strength Promote osteoblast activity Reduce the risk of loosening |
| Titanium Stainless steel | Hydroxyapatite coating | Enhance bone deposition along the screw surface Improve osseointegration Reduce loosening rates | |
| Titanium alloy | Carbon Fiber-Reinforced PEEK (CF/PEEK) | Reduce imaging artifacts Improve the postoperative assessment They are costly and not widely adopted | |
| Titanium | Gold nanoparticles | Enhance osseointegration Promote osteogenic differentiation | |
| Silver nanoparticles | Provide antibacterial properties Reduce the risk of infections |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costăchescu, B.; Moldoveanu, E.-T.; Niculescu, A.-G.; Grumezescu, A.M.; Teleanu, D.M. Advancements in Nanotechnology for Spinal Surgery: Innovations in Spinal Fixation Devices for Enhanced Biomechanical Performance and Osteointegration. Nanomaterials 2025, 15, 1073. https://doi.org/10.3390/nano15141073
Costăchescu B, Moldoveanu E-T, Niculescu A-G, Grumezescu AM, Teleanu DM. Advancements in Nanotechnology for Spinal Surgery: Innovations in Spinal Fixation Devices for Enhanced Biomechanical Performance and Osteointegration. Nanomaterials. 2025; 15(14):1073. https://doi.org/10.3390/nano15141073
Chicago/Turabian StyleCostăchescu, Bogdan, Elena-Theodora Moldoveanu, Adelina-Gabriela Niculescu, Alexandru Mihai Grumezescu, and Daniel Mihai Teleanu. 2025. "Advancements in Nanotechnology for Spinal Surgery: Innovations in Spinal Fixation Devices for Enhanced Biomechanical Performance and Osteointegration" Nanomaterials 15, no. 14: 1073. https://doi.org/10.3390/nano15141073
APA StyleCostăchescu, B., Moldoveanu, E.-T., Niculescu, A.-G., Grumezescu, A. M., & Teleanu, D. M. (2025). Advancements in Nanotechnology for Spinal Surgery: Innovations in Spinal Fixation Devices for Enhanced Biomechanical Performance and Osteointegration. Nanomaterials, 15(14), 1073. https://doi.org/10.3390/nano15141073









