Multifunctional Upconversion Nanoparticles Transforming Photoacoustic Imaging: A Review
Abstract
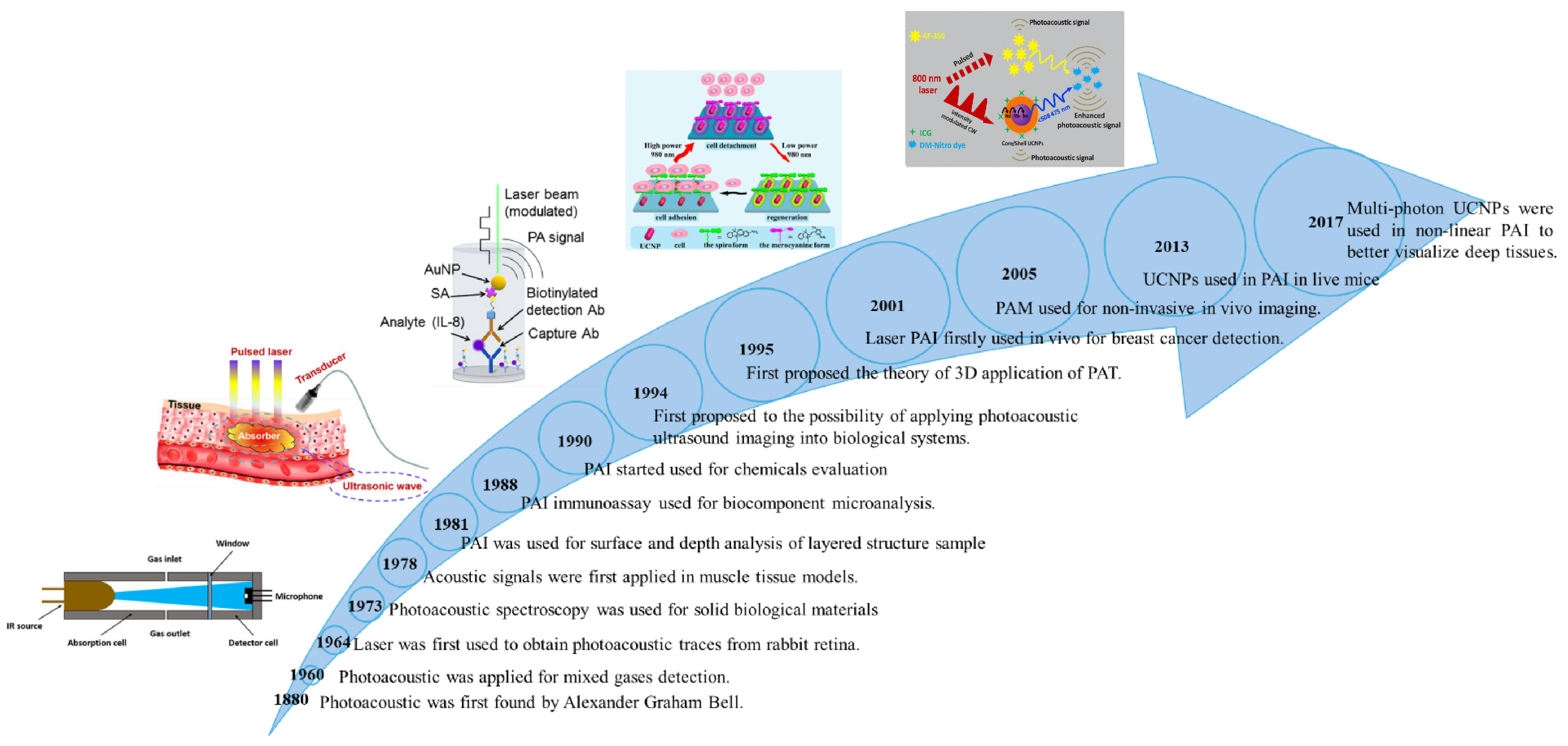
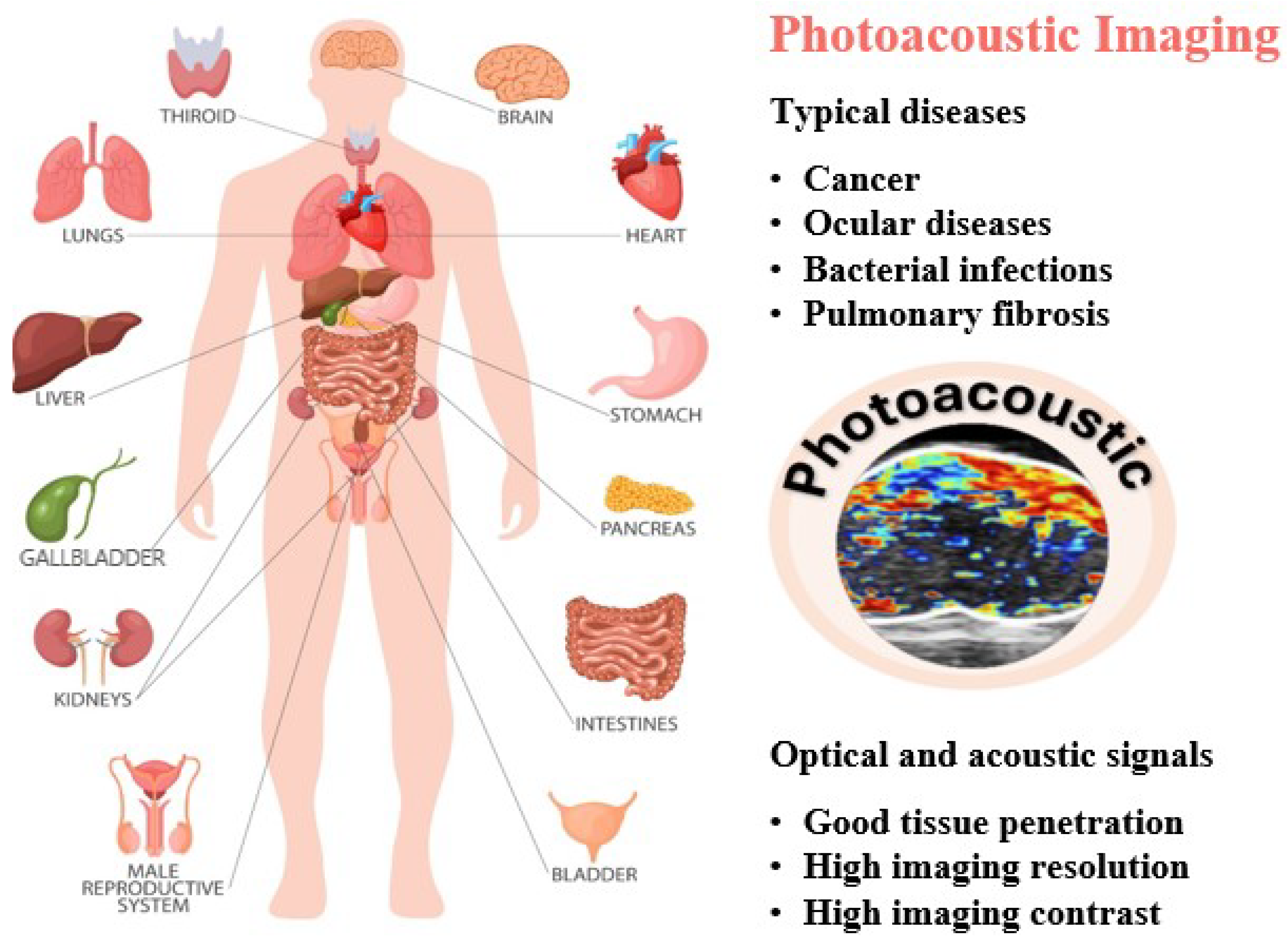
1. Introduction
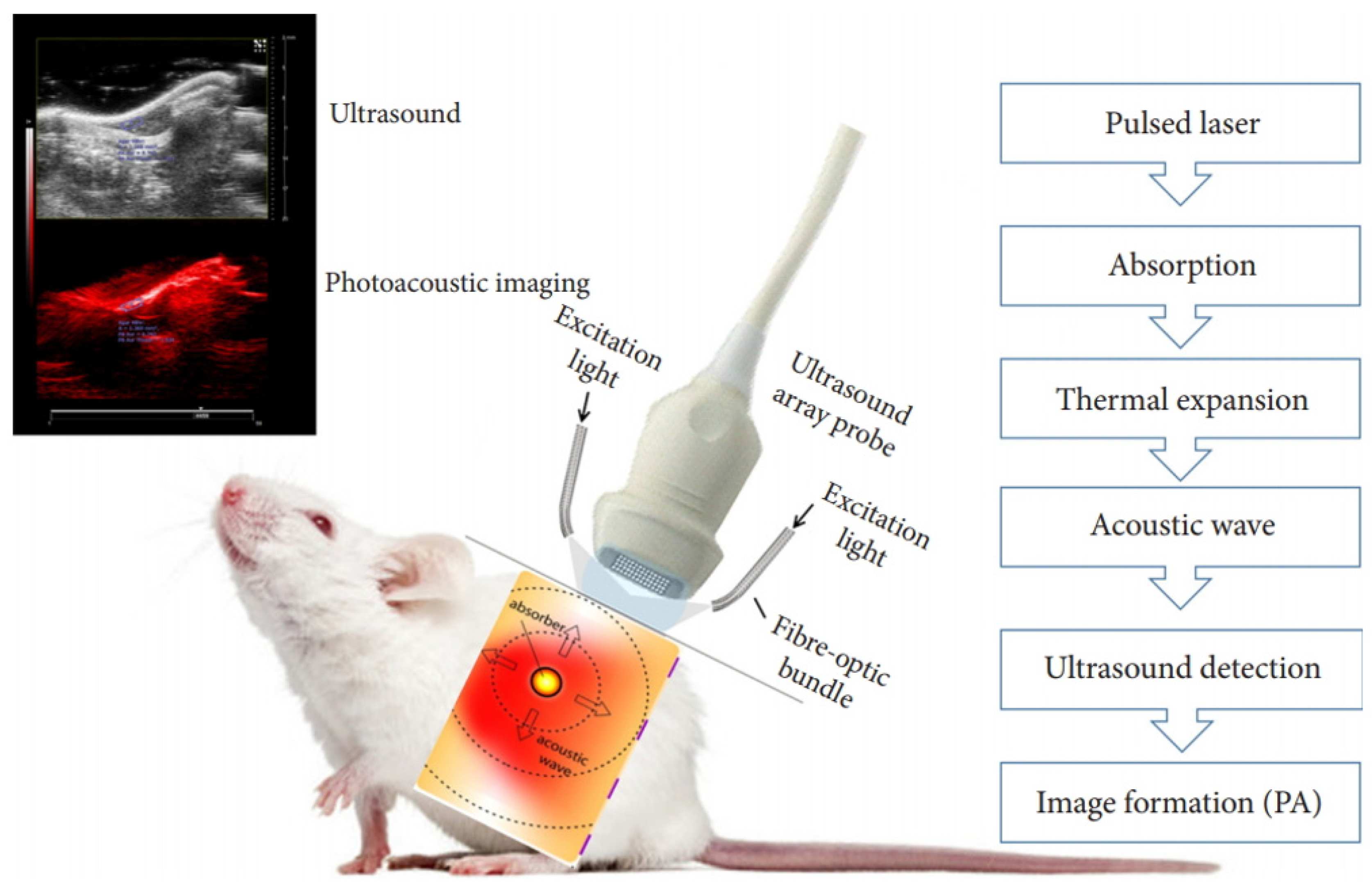
2. Working Principle of PAI
2.1. Physical Underpinnings of PAI
2.2. PAI Implementation: Tomography and Microscopy

3. Contrast Agent for PAI
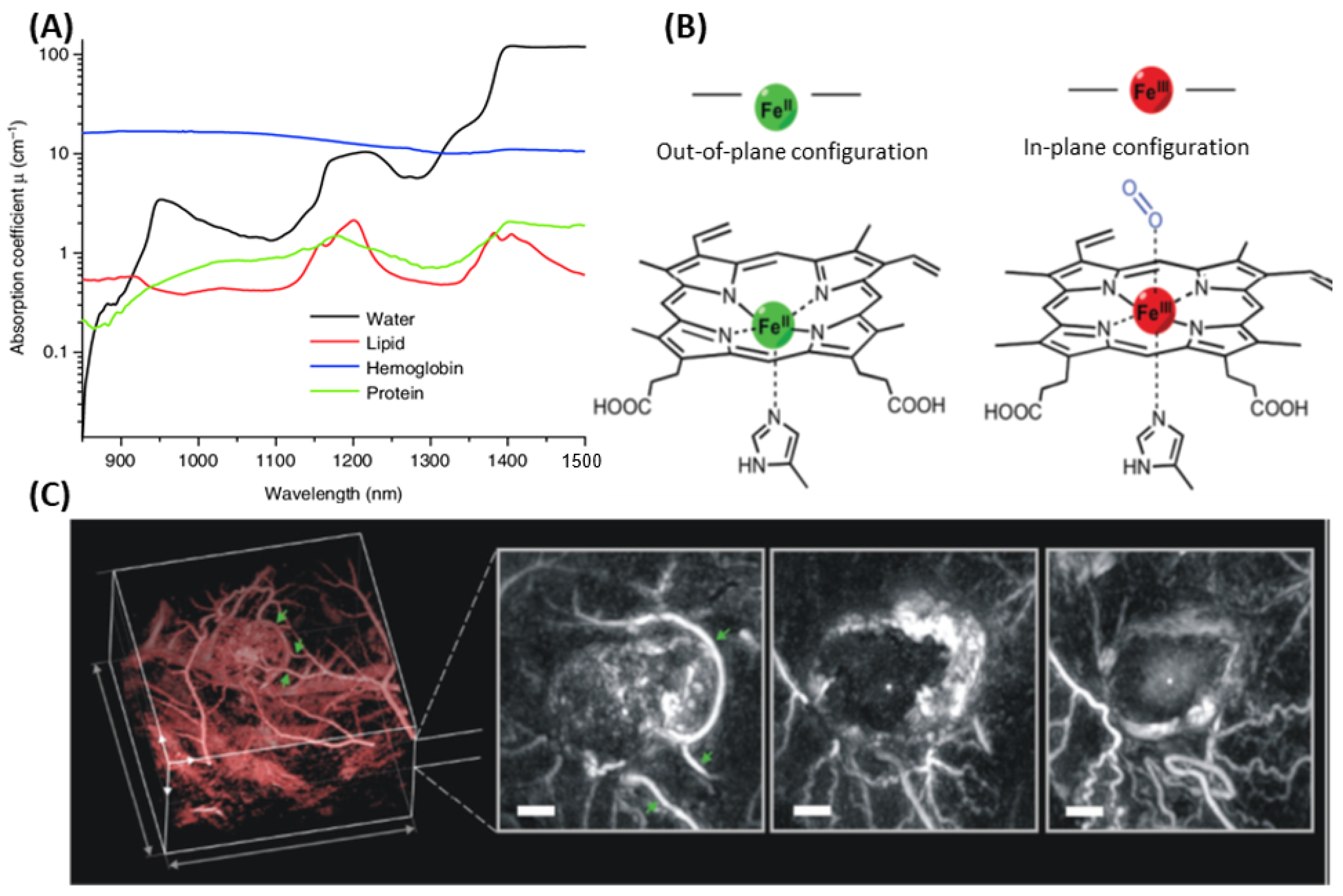
3.1. Endogenous PAI—Intrinsic Chromophores
3.2. Exogenous PAI—Key Factors Governing Contrast Agent Design
- (i)
- Photophysical properties
- (ii)
- Biological properties
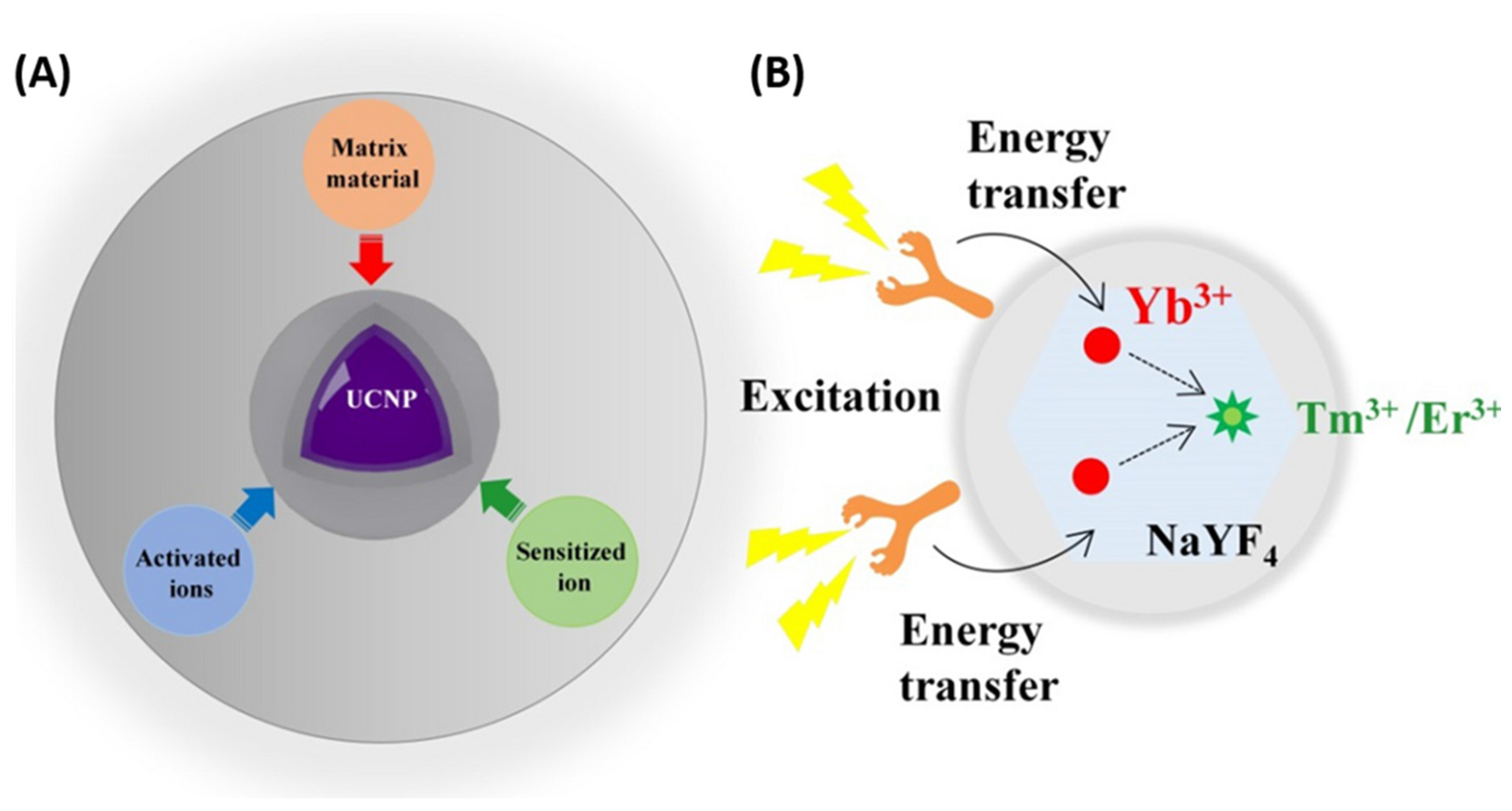
3.3. UCNPs as Contrast Agent for PAI
- (i)
- Synthesis and Functionalization of UCNPs
- (ii)
- Dopant/Host Selection Criteria
- (iii)
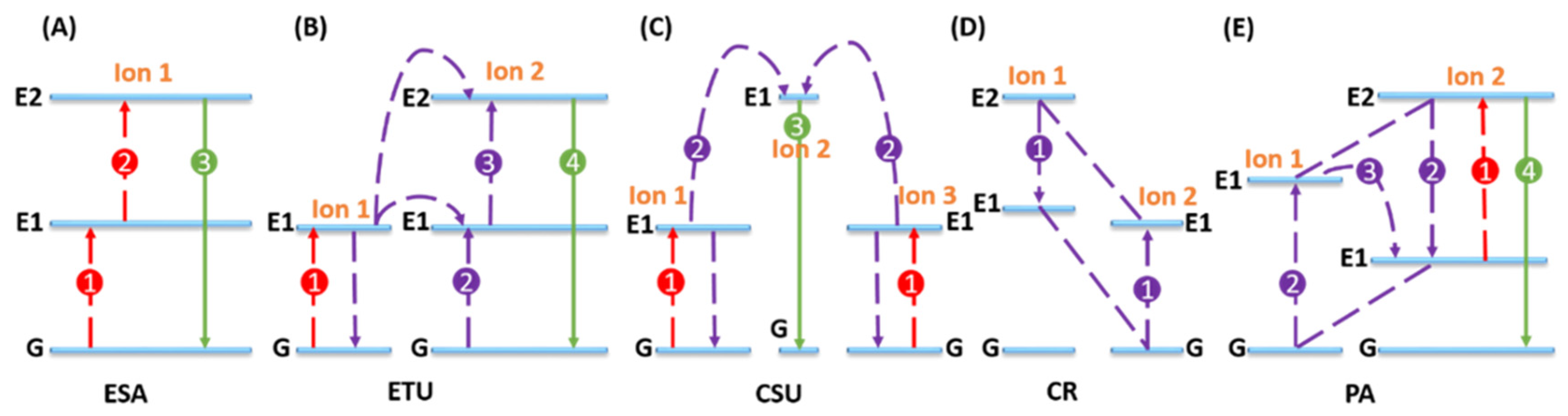
- Mechanism of Upconversion—Optical Imaging Agent
- (iv) UCNPs works as contrast agent for PAI
4. Photoacoustic Imaging—A Perfect Imaging Modality of UCNPs
4.1. Coated UCNPs for PAI
4.2. UCNP Nanocomposites for PAI
4.3. Outcomes of Combining UCNPs for PAI with Other Imaging Modalities
5. Outlook and Future Developments
Author Contributions
Funding
Conflicts of Interest
References
- Miao, Q.; Xie, C.; Zhen, X.; Lyu, Y.; Duan, H.; Liu, X.; Jokerst, J.V.; Pu, K. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 2017, 35, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Ishigaki, Y.; Hu, Y.; Sugimoto, K.; Zeng, W.; Harimoto, T.; Sun, Y.; He, J.; Suzuki, T.; Jiang, X.; et al. H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo. Nat. Commun. 2020, 11, 446. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, H.; Tang, L.; Wang, Y.; Li, Y.; Liu, N.; Zeng, X.; Yan, Y.; Wu, J.; Chen, S.; et al. Mn-Loaded apolactoferrin dots for in vivo MRI and NIR-II cancer imaging. J. Mater. Chem. C 2019, 7, 9448–9454. [Google Scholar] [CrossRef]
- Tu, L.; Xu, Y.; Ouyang, Q.; Li, X.; Sun, Y. Recent advances on small-molecule fluorophores with emission beyond 1000 nm for better molecular imaging in vivo. Chin. Chem. Lett. 2019, 30, 1731–1737. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, M.; Agyekum, A.A.; Wu, J.; Chen, Q.; Zuo, M.; El-Seedi, H.R.; Tao, F.; Shi, J.; Ouyang, Q.; et al. Quantitative detection of apple watercore and soluble solids content by near infrared transmittance spectroscopy. J. Food Eng. 2020, 279, 109955. [Google Scholar] [CrossRef]
- Li, C.; Niu, Z.; Zuo, M.; Wang, T.; Zou, X.; Sun, Z. Detection and identification of foreign bodies in conditioned steak based on ultrasound imaging. Food Sci. Technol. Res. 2024, 30, 269–280. [Google Scholar] [CrossRef]
- Ong, S.Y.; Zhang, C.; Dong, X.; Yao, S.Q. Recent Advances in Polymeric Nanoparticles for Enhanced Fluorescence and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2021, 60, 17797–17809. [Google Scholar] [CrossRef]
- Chen, X.; Song, J.; Chen, X.; Yang, H. X-ray-activated nanosystems for theranostic applications. Chem. Soc. Rev. 2019, 48, 3073–3101. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Photoacoustic imaging in the second near-infrared window: A review. J. Biomed. Opt. 2019, 24, 040901. [Google Scholar] [CrossRef]
- Nie, L.; Chen, X. Structural and functional photoacoustic molecular tomography aided by emerging contrast agents. Chem. Soc. Rev. 2014, 43, 7132–7170. [Google Scholar] [CrossRef]
- Li, W.; Chen, X. Gold nanoparticles for photoacoustic imaging. Nanomedicine 2015, 10, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Pu, K. Activatable Molecular Probes for Second Near-Infrared Fluorescence, Chemiluminescence, and Photoacoustic Imaging. Angew. Chem. Int. Ed. 2020, 59, 11717–11731. [Google Scholar] [CrossRef]
- Bell, A.G. On the production and reproduction of sound by light. Am. J. Sci. 1880, 3, 305–324. [Google Scholar] [CrossRef]
- Wang, L.V. Tutorial on photoacoustic microscopy and computed tomography. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 171–179. [Google Scholar] [CrossRef]
- Khulal, U.; Zhao, J.; Hu, W.; Chen, Q. Nondestructive quantifying total volatile basic nitrogen (TVB-N) content in chicken using hyperspectral imaging (HSI) technique combined with different data dimension reduction algorithms. Food Chem. 2016, 197, 1191–1199. [Google Scholar] [CrossRef]
- Li, H.; Kutsanedzie, F.; Zhao, J.; Chen, Q. Quantifying Total Viable Count in Pork Meat Using Combined Hyperspectral Imaging and Artificial Olfaction Techniques. Food Anal. Methods 2016, 9, 3015–3024. [Google Scholar] [CrossRef]
- Chitra, S.; Muhammad Mudassir Arif, C.; Jitendra, P. Classification of pulse flours using near-infrared hyperspectral imaging. LWT 2022, 154, 112799. [Google Scholar]
- Zhu, Y.; Zou, X.; Shen, T.; Shi, J.; Zhao, J.; Holmes, M.; Li, G. Determination of total acid content and moisture content during solid-state fermentation processes using hyperspectral imaging. J. Food Eng. 2016, 174, 75–84. [Google Scholar] [CrossRef]
- Yuan, L.-M.; Cai, J.-R.; Sun, L.; Ye, C. A Preliminary Discrimination of Cluster Disqualified Shape for Table Grape by Mono-Camera Multi-Perspective Simultaneously Imaging Approach. Food Anal. Methods 2016, 9, 758–767. [Google Scholar] [CrossRef]
- Weng, S.; Tang, P.; Yuan, H.; Guo, B.; Yu, S.; Huang, L.; Xu, C. Hyperspectral imaging for accurate determination of rice variety using a deep learning network with multi-feature fusion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118237. [Google Scholar] [CrossRef]
- Sun, J.; Lu, X.; Mao, H.; Wu, X.; Gao, H. Quantitative Determination of Rice Moisture Based on Hyperspectral Imaging Technology and BCC-LS-SVR Algorithm. J. Food Process Eng. 2017, 40, e12446. [Google Scholar] [CrossRef]
- Jiang, S.; Sun, J.; Xin, Z.; Mao, H.; Wu, X.; Li, Q. Visualizing distribution of pesticide residues in mulberry leaves using NIR hyperspectral imaging. J. Food Process Eng. 2017, 40, e12510. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, J.; Mao, H.; Wu, X.; Zhang, X.; Yang, N. Visualization research of moisture content in leaf lettuce leaves based on WT-PLSR and hyperspectral imaging technology. J. Food Process Eng. 2018, 41, e12647. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Q.; Liu, Y.; Sun, X.; Huang, Q.; Ouyang, Q.; Zhao, J. A Novel Hyperspectral Microscopic Imaging System for Evaluating Fresh Degree of Pork. Korean J. Food Sci. Anim. Resour. 2018, 38, 362–375. [Google Scholar]
- Chen, X.; Ding, H.; Yuan, L.-M.; Cai, J.-R.; Chen, X.; Lin, Y. New approach of simultaneous, multi-perspective imaging for quantitative assessment of the compactness of grape bunches. Aust. J. Grape Wine Res. 2018, 24, 413–420. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Wu, X.; Chen, Q.; Lu, B.; Dai, C. Detection of viability of soybean seed based on fluorescence hyperspectra and CARS-SVM-AdaBoost model. J. Food Process. Preserv. 2019, 43, e14238. [Google Scholar] [CrossRef]
- Ge, X.; Sun, J.; Lu, B.; Chen, Q.; Xun, W.; Jin, Y. Classification of oolong tea varieties based on hyperspectral imaging technology and BOSS-LightGBM model. J. Food Process Eng. 2019, 42, e13289. [Google Scholar] [CrossRef]
- Maturi, M.; Locatelli, E.; Monaco, I.; Comes Franchini, M. Current concepts in nanostructured contrast media development for in vivo photoacoustic imaging. Biomater. Sci. 2019, 7, 1746–1775. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, T.; Cheng, Q.; Wang, X.; Du, S.; Sato, N.; Yuan, J.; Kuniyil Ajith Singh, M. Towards Clinical Translation of LED-Based Photoacoustic Imaging: A Review. Sensors 2020, 20, 2484. [Google Scholar] [CrossRef]
- Mao, W.; Tang, J.; Dai, L.; He, X.; Li, J.; Cai, L.; Liao, P.; Jiang, R.; Zhou, J.; Wu, H. A General Strategy to Design Highly Fluorogenic Far-Red and Near-Infrared Tetrazine Bioorthogonal Probes. Angew. Chem. Int. Ed. 2021, 60, 2393–2397. [Google Scholar] [CrossRef]
- Gargiulo, S.; Albanese, S.; Mancini, M. State-of-the-Art Preclinical Photoacoustic Imaging in Oncology: Recent Advances in Cancer Theranostics. Contrast Media Mol. Imaging 2019, 2019, 5080267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, F.; Zhang, W.; Xiong, K.; Yang, S. Towards in vivo photoacoustic human imaging: Shining a new light on clinical diagnostics. Fundam. Res. 2024, 4, 1314–1330. [Google Scholar] [CrossRef] [PubMed]
- Rich, L.J.; Seshadri, M. Photoacoustic monitoring of tumor and normal tissue response to radiation. Sci. Rep. 2016, 6, 21237. [Google Scholar] [CrossRef]
- Lovelock, J. A photoionization detector for gases and vapours. Nature 1960, 188, 401. [Google Scholar] [CrossRef] [PubMed]
- Rosencwaig, A. Photoacoustic spectroscopy of solids. Opt. Commun. 1973, 7, 305–308. [Google Scholar] [CrossRef]
- Vinson, F.S.; Eggleton, R.; Meiss, R. Variations in acoustic velocity in skeletal muscle determined by acoustic microscopy. In Ultrasound in Medicine; Springer: Berlin/Heidelberg, Germany, 1978; Volume 4, pp. 519–534. [Google Scholar]
- Helander, P.; Lundström, I.; McQueen, D. Photoacoustic study of layered samples. J. Appl. Phys. 1981, 52, 1146–1151. [Google Scholar] [CrossRef]
- Masujima, T.; Munekane, Y.; Kawai, C.; Yoshida, H.; Imai, H.; Juing-Yi, L.; Sato, Y. Photoacoustic imaging immunoassay for biological component microanalysis. In Proceedings of the Photoacoustic and Photothermal Phenomena: Proceedings of the 5th International Topical Meeting, Heidelberg, Germany, 27–30 July 1987; pp. 558–560. [Google Scholar]
- Masujima, T. X-ray photoacoustics for characterization and non-destructive evaluation. In Photoacoustic and Photothermal Phenomena II, Proceedings of the 6th International Topical Meeting, Baltimore, MD, USA, 31 July –3 August 1989; Springer: Berlin/Heidelberg, Germany, 1990; pp. 222–234. [Google Scholar]
- Kruger, R.A. Photoacoustic ultrasound. Med. Phys. 1994, 21, 127–131. [Google Scholar] [CrossRef]
- Kruger, R.A.; Liu, P.; Fang, Y.R.; Appledorn, C.R. Photoacoustic ultrasound (PAUS)—Reconstruction tomography. Med. Phys. 1995, 22, 1605–1609. [Google Scholar] [CrossRef]
- Oraevsky, A.A.; Karabutov, A.A.; Solomatin, S.V.; Savateeva, E.V.; Andreev, V.A.; Gatalica, Z.; Singh, H.; Fleming, R.D. Laser optoacoustic imaging of breast cancer in vivo. In Proceedings of the Biomedical Optoacoustics II, San Jose, CA, USA, 20–26 January 2001; pp. 6–15. [Google Scholar]
- Consalvey, M.; Perkins, R.G.; Paterson, D.M.; Underwood, G.J. PAM fluorescence: A beginners guide for benthic diatomists. Diatom Res. 2005, 20, 1–22. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, X.; Peng, J.; Li, F. Core–shell lanthanide upconversion nanophosphors as four-modal probes for tumor angiogenesis imaging. Acs. Nano 2013, 7, 11290–11300. [Google Scholar] [CrossRef]
- Gulzar, A.; Xu, J.; Yang, P.; He, F.; Xu, L. Upconversion processes: Versatile biological applications and biosafety. Nanoscale 2017, 9, 12248–12282. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Hu, S.-H.; Fan, C.-T.; Li, M.-L.; Chen, Y.-Y.; Chen, S.-Y.; Liu, D.-M. A novel multifunctional nano-platform with enhanced anti-cancer and photoacoustic imaging modalities using gold-nanorod-filled silica nanobeads. Chem. Commun. 2013, 49, 892–894. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Fan, Y.; Ma, X.; Zhan, Y.; Lu, X.; Sun, Y. Recent development of near-infrared photoacoustic probes based on small-molecule organic dye. RSC Chem. Biol. 2021, 2, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Campbell, R.E.; Ting, A.Y.; Tsien, R.Y. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 2002, 3, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Schröck, E.; Du Manoir, S.; Veldman, T.; Schoell, B.; Wienberg, J.; Ferguson-Smith, M.; Ning, Y.; Ledbetter, D.; Bar-Am, I.; Soenksen, D. Multicolor spectral karyotyping of human chromosomes. Science 1996, 273, 494–497. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Khan, G.M.; Abeer, M.M.; Pujara, N.; Wan-Chi Tse, B.; McGuckin, M.A.; Popat, A.; Kumeria, T. Efficient photoacoustic imaging using indocyanine green (ICG) loaded functionalized mesoporous silica nanoparticles. Biomater. Sci. 2019, 7, 5002–5015. [Google Scholar] [CrossRef]
- de la Zerda, A.; Liu, Z.; Bodapati, S.; Teed, R.; Vaithilingam, S.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Ultrahigh sensitivity carbon nanotube agents for photoacoustic molecular imaging in living mice. Nano Lett. 2010, 10, 2168–2172. [Google Scholar] [CrossRef]
- de la Zerda, A.; Bodapati, S.; Teed, R.; May, S.Y.; Tabakman, S.M.; Liu, Z.; Khuri-Yakub, B.T.; Chen, X.; Dai, H.; Gambhir, S.S. Family of enhanced photoacoustic imaging agents for high-sensitivity and multiplexing studies in living mice. ACS Nano 2012, 6, 4694–4701. [Google Scholar] [CrossRef]
- Pramanik, M.; Song, K.H.; Swierczewska, M.; Green, D.; Sitharaman, B.; Wang, L.V. In vivo carbon nanotube-enhanced non-invasive photoacoustic mapping of the sentinel lymph node. Phys. Med. Biol. 2009, 54, 3291. [Google Scholar] [CrossRef]
- Wu, X.; Liu, H.; Liu, J.; Haley, K.N.; Treadway, J.A.; Larson, J.P.; Ge, N.; Peale, F.; Bruchez, M.P. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 2003, 21, 41–46. [Google Scholar] [CrossRef]
- Kim, S.; Lim, Y.T.; Soltesz, E.G.; De Grand, A.M.; Lee, J.; Nakayama, A.; Parker, J.A.; Mihaljevic, T.; Laurence, R.G.; Dor, D.M. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat. Biotechnol. 2004, 22, 93–97. [Google Scholar] [CrossRef]
- Jaiswal, J.K.; Mattoussi, H.; Mauro, J.M.; Simon, S.M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 2003, 21, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, F.; Huang, C. Phosphorescent chemosensors based on heavy-metal complexes. Chem. Soc. Rev. 2010, 39, 3007–3030. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Huang, C.; Li, F. Phosphorescent heavy-metal complexes for bioimaging. Chem. Soc. Rev. 2011, 40, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Shaner, N.C.; Steinbach, P.A.; Tsien, R.Y. A guide to choosing fluorescent proteins. Nat. Methods 2005, 2, 905–909. [Google Scholar] [CrossRef]
- Nagai, T.; Ibata, K.; Park, E.S.; Kubota, M.; Mikoshiba, K.; Miyawaki, A. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 2002, 20, 87–90. [Google Scholar] [CrossRef]
- Zhang, Y.; Jeon, M.; Rich, L.J.; Hong, H.; Geng, J.; Zhang, Y.; Shi, S.; Barnhart, T.E.; Alexandridis, P.; Huizinga, J.D. Non-invasive multimodal functional imaging of the intestine with frozen micellar naphthalocyanines. Nat. Nanotechnol. 2014, 9, 631–638. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.; Jeon, M.Y.; Kim, J.; Kim, C. In vitro photoacoustic measurement of hemoglobin oxygen saturation using a single pulsed broadband supercontinuum laser source. Appl. Opt. 2014, 53, 3884–3889. [Google Scholar] [CrossRef]
- Koo, J.; Jeon, M.; Oh, Y.; Kang, H.W.; Kim, J.; Kim, C.; Oh, J. In vivo non-ionizing photoacoustic mapping of sentinel lymph nodes and bladders with ICG-enhanced carbon nanotubes. Phys. Med. Biol. 2012, 57, 7853. [Google Scholar] [CrossRef]
- Akers, W.J.; Kim, C.; Berezin, M.; Guo, K.; Fuhrhop, R.; Lanza, G.M.; Fischer, G.M.; Daltrozzo, E.; Zumbusch, A.; Cai, X. Noninvasive photoacoustic and fluorescence sentinel lymph node identification using dye-loaded perfluorocarbon nanoparticles. ACS Nano 2011, 5, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Song, H.-M.; Cai, X.; Yao, J.; Wei, A.; Wang, L.V. In vivo photoacoustic mapping of lymphatic systems with plasmon-resonant nanostars. J. Mater. Chem. 2011, 21, 2841–2844. [Google Scholar] [CrossRef]
- Kim, C.; Jeon, M.; Wang, L.V. Nonionizing photoacoustic cystography in vivo. Opt. Lett. 2011, 36, 3599–3601. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Zhang, M.; Yang, Y.; Din, Z.U.; Chen, Q. Mesoporous silica-modified upconversion biosensor coupled with real-time ion release properties for ultrasensitive detection of Staphylococcus aureus in meat. Food Control 2023, 145, 109444. [Google Scholar] [CrossRef]
- Yin, L.; Hu, X.; Hao, M.; Shi, J.; Zou, X.; Dusabe, K.D. Upconversion nanoparticles-based background-free selective fluorescence sensor developed for immunoassay of fipronil pesticide. J. Food Meas. Charact. 2023, 17, 3125–3133. [Google Scholar] [CrossRef]
- Zhang, B.; Li, H.; Pan, W.; Chen, Q.; Ouyang, Q.; Zhao, J. Dual-Color Upconversion Nanoparticles (UCNPs)-Based Fluorescent Immunoassay Probes for Sensitive Sensing Foodborne Pathogens. Food Anal. Methods 2017, 10, 2036–2045. [Google Scholar] [CrossRef]
- Zhang, Y.; Hassan, M.M.; Rong, Y.; Liu, R.; Li, H.; Ouyang, Q.; Chen, Q. An upconversion nanosensor for rapid and sensitive detection of tetracycline in food based on magnetic-field-assisted separation. Food Chem. 2022, 373, 131497. [Google Scholar] [CrossRef]
- Yahui, L.; Yanxiao, L.; Di, Z.; Weilong, T.; Jiyong, S.; Zhihua, L.; Hanyu, L.; Yinyin, Y.; Liu, Y.; Xin, W.; et al. A fluorescence resonance energy transfer probe based on functionalized graphene oxide and upconversion nanoparticles for sensitive and rapid detection of zearalenone. LWT 2021, 147, 111541. [Google Scholar]
- Huang, Y.; Du, Z.; Bao, G.; Fang, G.; Cappadona, M.; McClements, L.; Tuch, B.E.; Lu, H.; Xu, X. Smart Drug-Delivery System of Upconversion Nanoparticles Coated with Mesoporous Silica for Controlled Release. Pharmaceutics 2023, 15, 89. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, M.; Sun, Y.; Zhang, X.; Zhu, X.; Wu, Z.; Wu, D.; Li, F. Fluorine-18-labeled Gd3+/Yb3+/Er3+ co-doped NaYF4 nanophosphors for multimodality PET/MR/UCL imaging. Biomaterials 2011, 32, 1148–1156. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pu, F.; Huang, S.; Yuan, Q.; Ren, J.; Qu, X. Long-circulating Gd2O3: Yb3+, Er3+ up-conversion nanoprobes as high-performance contrast agents for multi-modality imaging. Biomaterials 2013, 34, 1712–1721. [Google Scholar] [CrossRef]
- Fan, W.; Shen, B.; Bu, W.; Chen, F.; Zhao, K.; Zhang, S.; Zhou, L.; Peng, W.; Xiao, Q.; Xing, H. Rattle-structured multifunctional nanotheranostics for synergetic chemo-/radiotherapy and simultaneous magnetic/luminescent dual-mode imaging. J. Am. Chem. Soc. 2013, 135, 6494–6503. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Liu, Z. Upconversion nanoparticles and their composite nanostructures for biomedical imaging and cancer therapy. Nanoscale 2013, 5, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Zhang, Q. Recent advances on functionalized upconversion nanoparticles for detection of small molecules and ions in biosystems. Adv. Sci. 2018, 5, 1700609. [Google Scholar] [CrossRef]
- Rong, Y.; Ali, S.; Ouyang, Q.; Wang, L.; Li, H.; Chen, Q. Development of a bimodal sensor based on upconversion nanoparticles and surface-enhanced Raman for the sensitive determination of dibutyl phthalate in food. J. Food Compos. Anal. 2021, 100, 103929. [Google Scholar] [CrossRef]
- Rennie, C.; Huang, Y.; Siwakoti, P.; Du, Z.; Padula, M.; Bao, G.; Tuch, B.E.; Xu, X.; McClements, L. In Vitro Evaluation of A Hybrid Drug-Delivery Nanosystem for Fibrosis Prevention in Cell Therapy for Type 1 Diabetes. Nanomedicine 2023, 18, 53–66. [Google Scholar] [CrossRef]
- Vetrone, F.; Naccache, R.; de la Fuente, A.J.; Sanz-Rodríguez, F.; Blazquez-Castro, A.; Rodriguez, E.M.; Jaque, D.; Solé, J.G.; Capobianco, J.A. Intracellular imaging of HeLa cells by non-functionalized NaYF4: Er3+, Yb3+ upconverting nanoparticles. Nanoscale 2010, 2, 495–498. [Google Scholar] [CrossRef]
- Krämer, K.W.; Biner, D.; Frei, G.; Güdel, H.U.; Hehlen, M.P.; Lüthi, S.R. Hexagonal sodium yttrium fluoride based green and blue emitting upconversion phosphors. Chem. Mater. 2004, 16, 1244–1251. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Jiang, S. Multicolor core/shell-structured upconversion fluorescent nanoparticles. Adv. Mater. 2008, 20, 4765–4769. [Google Scholar] [CrossRef]
- Lin, H.; Duan, Y.; Man, Z.; Zareef, M.; Wang, Z.; Chen, Q. Quantitation of volatile aldehydes using chemoselective response dyes combined with multivariable data analysis. Food Chem. 2021, 353, 129485. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-W.; Zou, X.-B.; Shi, J.-Y.; Li, Z.-H.; Zhao, J.-W. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar]
- Lin, H.; Man, Z.X.; Kang, W.C.; Guan, B.B.; Chen, Q.S.; Xue, Z.L. A novel colorimetric sensor array based on boron-dipyrromethene dyes for monitoring the storage time of rice. Food Chem. 2018, 268, 300–306. [Google Scholar] [CrossRef]
- Lin, H.; Kang, W.; Kutsanedzie, F.Y.H.; Chen, Q. A Novel Nanoscaled Chemo Dye–Based Sensor for the Identification of Volatile Organic Compounds During the Mildewing Process of Stored Wheat. Food Anal. Methods 2019, 12, 2895–2907. [Google Scholar] [CrossRef]
- Ningqiu, T.; Jun, S.; Min, X.; Kunshan, Y.; Yan, C.; Dengjie, L. Identification of fumigated and dyed Lycium barbarum by hyperspectral imaging technology. J. Food Process Eng. 2022, 45, e13950. [Google Scholar] [CrossRef]
- Wang, F.; Banerjee, D.; Liu, Y.; Chen, X.; Liu, X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, Y.; Wang, T.; Ji, S.; Zhang, X.; Shen, T.; Huang, X.; Xiao, J.; Farag, M.A.; Shi, J.; et al. Quantum dots as advanced nanomaterials for food quality and safety applications: A comprehensive review and future perspectives. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13339. [Google Scholar] [CrossRef]
- Yosri, N.; Gao, S.; Zhou, R.; Wang, C.; Zou, X.; El-Seedi, H.R.; Guo, Z. Innovative quantum dots-based SERS for ultrasensitive reporting of contaminants in food: Fundamental concepts and practical implementations. Food Chem. 2025, 467, 142395. [Google Scholar] [CrossRef]
- Zhang, C.; Han, Y.; Lin, L.; Deng, N.; Chen, B.; Liu, Y. Development of Quantum Dots-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine. J. Agric. Food Chem. 2017, 65, 1290–1295. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Wang, J.; Wang, Q.; Meng, H.; Wang, Z. One-Step Core/Multishell Quantum Dots-Based Fluoroimmunoassay for Screening of Deoxynivalenol in Maize. Food Anal. Methods 2018, 11, 2569–2578. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Sun, L.; Kong, D.; Sheng, J.; Wang, K.; Dong, C. A Green, Simple, and Rapid Detection for Amaranth in Candy Samples Based on the Fluorescence Quenching of Nitrogen-Doped Graphene Quantum Dots. Food Anal. Methods 2019, 12, 1658–1665. [Google Scholar] [CrossRef]
- Bi, X.; Li, L.; Luo, L.; Liu, X.; Li, J.; You, T. A ratiometric fluorescence aptasensor based on photoinduced electron transfer from CdTe QDs to WS2 NTs for the sensitive detection of zearalenone in cereal crops. Food Chem. 2022, 385, 132657. [Google Scholar] [CrossRef]
- Liang, N.; Hu, X.; Li, W.; Wang, Y.; Guo, Z.; Huang, X.; Li, Z.; Zhang, X.; Zhang, J.; Xiao, J.; et al. A dual-signal fluorescent sensor based on MoS2 and CdTe quantum dots for tetracycline detection in milk. Food Chem. 2022, 378, 132076. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shi, J.; Shi, Y.; Zou, X.; Arslan, M.; Zhang, W.; Huang, X.; Li, Z.; Xu, Y. Use of a smartphone for visual detection of melamine in milk based on Au@Carbon quantum dots nanocomposites. Food Chem. 2019, 272, 58–65. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Li, F. Upconversion nanophosphors for small-animal imaging. Chem. Soc. Rev. 2012, 41, 1323–1349. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion luminescent materials: Advances and applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, P.; Tu, D.; Ma, E.; Zhu, H.; Chen, X. Lanthanide-doped upconversion nano-bioprobes: Electronic structures, optical properties, and biodetection. Chem. Soc. Rev. 2015, 44, 1379–1415. [Google Scholar] [CrossRef]
- Sun, L.-D.; Wang, Y.-F.; Yan, C.-H. Paradigms and challenges for bioapplication of rare earth upconversion luminescent nanoparticles: Small size and tunable emission/excitation spectra. Acc. Chem. Res. 2014, 47, 1001–1009. [Google Scholar] [CrossRef]
- Sedlmeier, A.; Gorris, H.H. Surface modification and characterization of photon-upconverting nanoparticles for bioanalytical applications. Chem. Soc. Rev. 2015, 44, 1526–1560. [Google Scholar] [CrossRef] [PubMed]
- Prodi, L.; Rampazzo, E.; Rastrelli, F.; Speghini, A.; Zaccheroni, N. Imaging agents based on lanthanide doped nanoparticles. Chem. Soc. Rev. 2015, 44, 4922–4952. [Google Scholar] [CrossRef]
- Park, Y.I.; Lee, K.T.; Suh, Y.D.; Hyeon, T. Upconverting nanoparticles: A versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging. Chem. Soc. Rev. 2015, 44, 1302–1317. [Google Scholar] [CrossRef]
- Muhr, V.; Wilhelm, S.; Hirsch, T.; Wolfbeis, O.S. Upconversion nanoparticles: From hydrophobic to hydrophilic surfaces. Acc. Chem. Res. 2014, 47, 3481–3493. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, C.-H.; Capobianco, J.A. Photon upconversion nanomaterials. Chem. Soc. Rev. 2015, 44, 1299–1301. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Zhao, D. Lab on upconversion nanoparticles: Optical properties and applications engineering via designed nanostructure. Chem. Soc. Rev. 2015, 44, 1346–1378. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, S.; Zhang, S.; Zhang, H. Stimuli-responsive nanotheranostics based on lanthanide-doped upconversion nanoparticles for cancer imaging and therapy: Current advances and future challenges. Nano Today 2019, 25, 38–67. [Google Scholar] [CrossRef]
- Dong, H.; Du, S.-R.; Zheng, X.-Y.; Lyu, G.-M.; Sun, L.-D.; Li, L.-D.; Zhang, P.-Z.; Zhang, C.; Yan, C.-H. Lanthanide nanoparticles: From design toward bioimaging and therapy. Chem. Rev. 2015, 115, 10725–10815. [Google Scholar] [CrossRef]
- Chen, X.; Peng, D.; Ju, Q.; Wang, F. Photon upconversion in core–shell nanoparticles. Chem. Soc. Rev. 2015, 44, 1318–1330. [Google Scholar] [CrossRef]
- Chen, G.; Ågren, H.; Ohulchanskyy, T.Y.; Prasad, P.N. Light upconverting core–shell nanostructures: Nanophotonic control for emerging applications. Chem. Soc. Rev. 2015, 44, 1680–1713. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Zhao, D. Highly efficient lanthanide upconverting nanomaterials: Progresses and challenges. Nano Today 2013, 8, 643–676. [Google Scholar] [CrossRef]
- Xu, W.; Chen, X.; Song, H. Upconversion manipulation by local electromagnetic field. Nano Today 2017, 17, 54–78. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.; Kim, C. Photoacoustic imaging in nanomedicine. In Applications of Nanoscience in Photomedicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 31–47. [Google Scholar]
- Kim, C.; Favazza, C.; Wang, L.V. In vivo photoacoustic tomography of chemicals: High-resolution functional and molecular optical imaging at new depths. Chem. Rev. 2010, 110, 2756–2782. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.A.; Pu, Y.; Pratavieira, S.; Budansky, Y.; Alfano, R.R. Deep optical imaging of tissue using the second and third near-infrared spectral windows. J. Biomed. Opt. 2014, 19, 056004. [Google Scholar] [CrossRef]
- Cox, B.T.; Laufer, J.G.; Beard, P.C.; Arridge, S.R. Quantitative spectroscopic photoacoustic imaging: A review. J. Biomed. Opt. 2012, 17, 061202. [Google Scholar] [CrossRef]
- Maslov, K.I.; Wang, L.V. Photoacoustic imaging of biological tissue with intensity-modulated continuous-wave laser. J. Biomed. Opt. 2008, 13, 024006. [Google Scholar] [CrossRef]
- Wang, X.; Pang, Y.; Ku, G.; Xie, X.; Stoica, G.; Wang, L.V. Noninvasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol. 2003, 21, 803–806. [Google Scholar] [CrossRef]
- Lashkari, B.; Mandelis, A. Comparison between pulsed laser and frequency-domain photoacoustic modalities: Signal-to-noise ratio, contrast, resolution, and maximum depth detectivity. Rev. Sci. Instrum. 2011, 82, 094903. [Google Scholar] [CrossRef]
- Xia, J.; Yao, J.; Wang, L.V. Photoacoustic tomography: Principles and advances. Electromagn. Waves 2014, 147, 1–22. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 041101. [Google Scholar] [CrossRef]
- Han Seung, H. Review of Photoacoustic Imaging for Imaging-Guided Spinal Surgery. Neurospine 2018, 15, 306–322. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Kim, C.; Lovell, J.F. Opportunities for photoacoustic-guided drug delivery. Curr. Drug Targets 2015, 16, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, M.; Wang, L.V. Thermoacoustic and photoacoustic sensing of temperature. J. Biomed. Opt. 2009, 14, 054024. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Jung, Y.; Chang, S.; Park, J.; Zhang, Y.; Lovell, J.F.; Kim, C. Programmable real-time clinical photoacoustic and ultrasound imaging system. Sci. Rep. 2016, 6, 35137. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.V. Photoacoustic microscopy. Laser Photonics Rev. 2013, 7, 758–778. [Google Scholar] [CrossRef]
- Wang, L.V.; Xia, J.; Yao, J. Photoacoustic Neuroimaging. In Neurophotonics and Brain Mapping; CRC Press: Boca Raton, FL, USA, 2017; pp. 235–256. [Google Scholar]
- Zhang, C.; Wang, Y. Comparison of various imaging modes for photoacoustic tomography. In Proceedings of the 13th International Conference on Biomedical Engineering, Valletta, Malta, 24–26 February 2020; pp. 121–124. [Google Scholar]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Danielli, A.; Maslov, K.I.; Garcia-Uribe, A.; Winkler, A.M.; Li, C.; Wang, L.; Chen, Y.; Dorn, G.W., II; Wang, L.V. Label-free photoacoustic nanoscopy. J. Biomed. Opt. 2014, 19, 086006. [Google Scholar] [CrossRef]
- Jeon, M.; Kim, J.; Kim, C. Multiplane spectroscopic whole-body photoacoustic imaging of small animals in vivo. Med. Biol. Eng. Comput. 2016, 54, 283–294. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. Fast optical-resolution photoacoustic microscopy using a 2-axis water-proofing MEMS scanner. Sci. Rep. 2015, 5, 7932. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. A PDMS-based 2-axis waterproof scanner for photoacoustic microscopy. Sensors 2015, 15, 9815–9826. [Google Scholar] [CrossRef]
- Harrison, T.; Ranasinghesagara, J.; Lu, H.; Zemp, R.J. Fast-scanning ultrasonic-photoacoustic biomicroscope: In vivo performance. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2010, San Francisco, CA, USA, 24–26 January 2010; p. 75641X. [Google Scholar]
- Xie, Z.; Jiao, S.; Zhang, H.F.; Puliafito, C.A. Laser-scanning optical-resolution photoacoustic microscopy. Opt. Lett. 2009, 34, 1771–1773. [Google Scholar] [CrossRef]
- Rao, B.; Li, L.; Maslov, K.; Wang, L. Hybrid-scanning optical-resolution photoacoustic microscopy for in vivo vasculature imaging. Opt. Lett. 2010, 35, 1521–1523. [Google Scholar] [CrossRef]
- Heijblom, M.; Steenbergen, W.; Manohar, S. Clinical photoacoustic breast imaging: The twente experience. IEEE Pulse 2015, 6, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.G.; Lee, S.; Park, S.; Kang, H.; Lee, S.G.; Jeong, C.; Lee, Y.-S.; Kim, C.; Jeong, D.H. A dual modal silver bumpy nanoprobe for photoacoustic imaging and SERS multiplexed identification of in vivo lymph nodes. Nanoscale 2017, 9, 12556–12564. [Google Scholar] [CrossRef] [PubMed]
- Yajing, L.; Liming, N.; Xiaoyuan, C. Photoacoustic Molecular Imaging: From Multiscale Biomedical Applications Towards Early-Stage Theranostics. Trends Biotechnol. 2016, 34, 420–433. [Google Scholar]
- Jung, D.; Park, S.; Lee, C.; Kim, H. Recent Progress on Near-Infrared Photoacoustic Imaging: Imaging Modality and Organic Semiconducting Agents. Polymers 2019, 11, 1693. [Google Scholar] [CrossRef]
- Laufer, J.G.; Zhang, E.Z.; Treeby, B.E.; Cox, B.T.; Beard, P.C.; Johnson, P.; Pedley, B. In vivo preclinical photoacoustic imaging of tumor vasculature development and therapy. J. Biomed. Opt. 2012, 17, 056016. [Google Scholar] [CrossRef]
- Bohndiek, S.E.; Sasportas, L.S.; Machtaler, S.; Jokerst, J.V.; Hori, S.; Gambhir, S.S. Photoacoustic tomography detects early vessel regression and normalization during ovarian tumor response to the antiangiogenic therapy trebananib. J. Nucl. Med. 2015, 56, 1942–1947. [Google Scholar] [CrossRef]
- Guggenheim, J.A.; Allen, T.J.; Plumb, A.; Zhang, E.Z.; Rodriguez-Justo, M.; Punwani, S.; Beard, P.C. Photoacoustic imaging of human lymph nodes with endogenous lipid and hemoglobin contrast. J. Biomed. Opt. 2015, 20, 050504. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, L.V.; Zhu, Q. In vivo photoacoustic tomography of mouse cerebral edema induced by cold injury. J. Biomed. Opt. 2011, 16, 066020. [Google Scholar] [CrossRef]
- Sarpong, F.; Yu, X.; Zhou, C.; Hongpeng, Y.; Uzoejinwa, B.B.; Bai, J.; Wu, B.; Ma, H. Influence of anti-browning agent pretreatment on drying kinetics, enzymes inactivation and other qualities of dried banana (Musa ssp.) under relative humidity-convective air dryer. J. Food Meas. Charact. 2018, 12, 1229–1241. [Google Scholar] [CrossRef]
- Zhang, H.; Mahunu, G.K.; Castoria, R.; Apaliya, M.T.; Yang, Q. Augmentation of biocontrol agents with physical methods against postharvest diseases of fruits and vegetables. Trends Food Sci. Technol. 2017, 69, 36–45. [Google Scholar] [CrossRef]
- Zeng, X.; Tang, L.; Zhang, W.; Hong, X.; Xiao, Y. Shape and size effects of gold nanoparticles for tumor photoacoustic imaging and photothermal therapy within the NIR-I and NIR-II Biowindows. Small 2025, 21, 2412296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, M.; Wu, F.; Liu, L.; Ren, X.; Hai, Z. Intracellular Formation of Hemicyanine Nanoparticle Enhances Tumor-Targeting Photoacoustic Imaging and Photothermal Therapy. Adv. Healthc. Mater. 2023, 12, 2202676. [Google Scholar] [CrossRef]
- Wang, F.; Men, X.; Chen, H.; Mi, F.; Xu, M.; Men, X.; Yuan, Z.; Lo, P.K. Second near-infrared photoactivatable biocompatible polymer nanoparticles for effective in vitro and in vivo cancer theranostics. Nanoscale 2021, 13, 13410–13420. [Google Scholar] [CrossRef]
- Sridharan, B.; Lim, H.G. Advances in photoacoustic imaging aided by nano contrast agents: Special focus on role of lymphatic system imaging for cancer theranostics. J. Nanobiotechnol. 2023, 21, 437. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Tu, L.; Zhang, Y.; Huang, D.; Liu, X.; Zhang, X.; Du, J.; Fan, R.; Yang, C.; Krämer, K.W. Size-dependent lanthanide energy transfer amplifies upconversion luminescence quantum yields. Nat. Photonics 2024, 18, 440–449. [Google Scholar] [CrossRef]
- Fardian-Melamed, N.; Skripka, A.; Ursprung, B.; Lee, C.; Darlington, T.P.; Teitelboim, A.; Qi, X.; Wang, M.; Gerton, J.M.; Cohen, B.E. Infrared nanosensors of piconewton to micronewton forces. Nature 2025, 637, 70–75. [Google Scholar] [CrossRef]
- Casar, J.R.; McLellan, C.A.; Shi, C.; Stiber, A.; Lay, A.; Siefe, C.; Parakh, A.; Gaerlan, M.; Gu, X.W.; Goodman, M.B. Upconverting microgauges reveal intraluminal force dynamics in vivo. Nature 2025, 637, 76–83. [Google Scholar] [CrossRef]
- Chen, T.; Su, L.; Lin, L.; Ge, X.; Bai, F.; Niu, M.; Wang, C.; Song, J.; Guo, S.; Yang, H. Mesoporous radiosensitized nanoprobe for enhanced NIR-II photoacoustic imaging-guided accurate radio-chemotherapy. Nano Res. 2022, 15, 4154–4163. [Google Scholar] [CrossRef]
- Al-Salihi, M.; Ghellab, S.E.; Li, Y.; Luo, C.; Kalsoom, U.-E.; Liu, L. Effective Rapid Fluorescence Lifetime Imaging of the Brain: A Novel Approach Using Upconversion Photoluminescence Lifetime Based on Gate-Width Acquisition. Nano Lett. 2024, 24, 14973–14982. [Google Scholar] [CrossRef] [PubMed]
- Dukhno, O.; Ghosh, S.; Greiner, V.; Bou, S.; Godet, J.; Muhr, V.; Buchner, M.; Hirsch, T.; Mély, Y.; Przybilla, F. Targeted single particle tracking with upconverting nanoparticles. ACS Appl. Mater. Interfaces 2024, 16, 11217–11227. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Chen, C.; Shan, X.; Liu, B.; Wang, D.; Du, Z.; Zhao, G.; Su, Q.P.; Yang, Y.; Halkon, B. Optical nonlinearity enabled super-resolved multiplexing microscopy. Adv. Mater. 2024, 36, 2308844. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Wei, Y.; Liang, Y.; An, R.; Liu, S.; Lei, P.; Zhang, H. Near-Infrared-Responsive Rare Earth Nanoparticles for Optical Imaging and Wireless Phototherapy. Adv. Sci. 2024, 11, 2305308. [Google Scholar] [CrossRef]
- Liu, X.-M.; Zhu, Z.-Z.; He, X.-R.; Zou, Y.-H.; Chen, Q.; Wang, X.-Y.; Liu, H.-M.; Qiao, X.; Wang, X.; Xu, J.-Y. NIR light and GSH dual-responsive upconversion nanoparticles loaded with multifunctional platinum (IV) prodrug and RGD peptide for precise cancer therapy. ACS Appl. Mater. Interfaces 2024, 16, 40753–40766. [Google Scholar] [CrossRef]
- Das, B. Transition Metal Complex-Loaded Nanosystems: Advances in Stimuli-Responsive Cancer Therapies. Small 2025, 21, 2410338. [Google Scholar] [CrossRef]
- Hale, G.M.; Querry, M.R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 1973, 12, 555–563. [Google Scholar] [CrossRef]
- Van Veen, R.; Sterenborg, H.J.; Pifferi, A.; Torricelli, A.; Chikoidze, E.; Cubeddu, R. Determination of visible near-IR absorption coefficients of mammalian fat using time-and spatially resolved diffuse reflectance and transmission spectroscopy. J. Biomed. Opt. 2005, 10, 054004. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Chen, J.-C.; Wang, W.-J. Near-infrared absorption property of biological soft tissue constituents. J. Med. Biol. Eng. 2001, 21, 7–14. [Google Scholar]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Lynch, I.; Dawson, K.A. Protein-nanoparticle interactions. Nano Today 2008, 3, 40–47. [Google Scholar] [CrossRef]
- Zhou, M.; Huang, H.; Wang, D.; Lu, H.; Chen, J.; Chai, Z.; Yao, S.Q.; Hu, Y. Light-Triggered PEGylation/dePEGylation of the Nanocarriers for Enhanced Tumor Penetration. Nano Lett. 2019, 19, 3671–3675. [Google Scholar] [CrossRef]
- Lundqvist, M. Nanoparticles: Tracking protein corona over time. Nat. Nanotechnol. 2013, 8, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Wang, S.; Wang, X.; Rong, P.; Ma, Y.; Liu, G.; Huang, P.; Lu, G.; Chen, X. In vivo volumetric photoacoustic molecular angiography and therapeutic monitoring with targeted plasmonic nanostars. Small 2014, 10, 1585–1593. [Google Scholar] [CrossRef]
- Chatni, M.R.; Xia, J.; Maslov, K.I.; Guo, Z.; Wang, K.; Anastasio, M.A.; Wang, L.V.; Sohn, R.; Arbeit, J.M.; Zhang, Y. Tumor glucose metabolism imaged in vivo in small animals with whole-body photoacoustic computed tomography. J. Biomed. Opt. 2012, 17, 076012. [Google Scholar] [CrossRef]
- Dragulescu-Andrasi, A.; Kothapalli, S.-R.; Tikhomirov, G.A.; Rao, J.; Gambhir, S.S. Activatable oligomerizable imaging agents for photoacoustic imaging of furin-like activity in living subjects. J. Am. Chem. Soc. 2013, 135, 11015–11022. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, J.; Zheng, K.; Bednarkiewicz, A.; Liu, X.; Jin, D. Advances in highly doped upconversion nanoparticles. Nat. Commun. 2018, 9, 2415. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Deng, R.; Tian, J.; Zong, Y.; Jin, D.; Liu, X. Multicolor barcoding in a single upconversion crystal. J. Am. Chem. Soc. 2014, 136, 4893–4896. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Gao, N.; Deng, R.; Sun, Q.; Xu, Q.-H.; Liu, X. Mechanistic investigation of photon upconversion in Nd3+-sensitized core–shell nanoparticles. J. Am. Chem. Soc. 2013, 135, 12608–12611. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Johnson, N.J.; Nguyen Huu, V.A.; Huang, Y.; Almutairi, A. Leveraging spectral matching between photosensitizers and upconversion nanoparticles for 808 nm-activated photodynamic therapy. Chem. Mater. 2018, 30, 3991–4000. [Google Scholar] [CrossRef]
- Campos-Goncalves, I.; Costa, B.F.; Santos, R.F.; Duraes, L. Superparamagnetic core-shell nanocomplexes doped with Yb3+: Er3+/Ho3+ rare-earths for upconversion fluorescence. Mater. Des. 2017, 130, 263–274. [Google Scholar] [CrossRef]
- Rinkel, T.; Raj, A.N.; Dühnen, S.; Haase, M. Synthesis of 10 nm β-NaYF4: Yb, Er/NaYF4 core/shell upconversion nanocrystals with 5 nm particle cores. Angew. Chem. Int. Ed. 2016, 55, 1164–1167. [Google Scholar] [CrossRef]
- Xu, Z.; Kang, X.; Li, C.; Hou, Z.; Zhang, C.; Yang, D.; Li, G.; Lin, J. Ln3+ (Ln = Eu, Dy, Sm, and Er) ion-doped YVO4 nano/microcrystals with multiform morphologies: Hydrothermal synthesis, growing mechanism, and luminescent properties. Inorg. Chem. 2010, 49, 6706–6715. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.-L.; Zhang, Y.-X.; Hou, W.; Wu, X.-L.; Xu, S.-K. Two-phase solvothermal synthesis of rare-earth doped NaYF4 upconversion fluorescent nanocrystals. Mater. Lett. 2009, 63, 325–327. [Google Scholar] [CrossRef]
- Tang, S.; Huang, M.; Wang, J.; Yu, F.; Shang, G.; Wu, J. Hydrothermal synthesis and luminescence properties of GdVO4: Ln3+ (Ln = Eu, Sm, Dy) phosphors. J. Alloys Compd. 2012, 513, 474–480. [Google Scholar] [CrossRef]
- Min, Y.; Li, J.; Liu, F.; Padmanabhan, P.; Yeow, E.K.; Xing, B. Recent advance of biological molecular imaging based on lanthanide-doped upconversion-luminescent nanomaterials. Nanomaterials 2014, 4, 129–154. [Google Scholar] [CrossRef]
- Mao, L.; Lu, Z.; He, N.; Zhang, L.; Deng, Y.; Duan, D. A new method for improving the accuracy of miRNA detection with NaYF 4: Yb, Er upconversion nanoparticles. Sci. China Chem. 2017, 60, 157–162. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Xing, B.; Yang, P.; Lin, J. Multifunctional UCNPs@ PDA-ICG nanocomposites for upconversion imaging and combined photothermal/photodynamic therapy with enhanced antitumor efficacy. J. Mater. Chem. B 2016, 4, 4884–4894. [Google Scholar] [CrossRef]
- Zhang, L.; Ling, B.; Wang, L.; Chen, H. A near-infrared luminescent Mn2+-doped NaYF4: Yb, Tm/Fe3+ upconversion nanoparticles redox reaction system for the detection of GSH/Cys/AA. Talanta 2017, 172, 95–101. [Google Scholar] [CrossRef]
- Chen, G.; Qiu, H.; Prasad, P.N.; Chen, X. Upconversion nanoparticles: Design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. [Google Scholar] [CrossRef]
- Tian, Y.; Tian, Y.; Huang, P.; Wang, L.; Shi, Q.; Cui, C.E. Effect of Yb3+ concentration on upconversion luminescence and temperature sensing behavior in Yb3+/Er3+ co-doped YNbO4 nanoparticles prepared via molten salt route. Chem. Eng. J. 2016, 297, 26–34. [Google Scholar] [CrossRef]
- Cheng, X.; Pan, Y.; Yuan, Z.; Wang, X.; Su, W.; Yin, L.; Xie, X.; Huang, L. Er3+ sensitized photon upconversion nanocrystals. Adv. Funct. Mater. 2018, 28, 1800208. [Google Scholar] [CrossRef]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. J. Nanobiotechnol. 2020, 18, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Passuello, T.; Pedroni, M.; Piccinelli, F.; Polizzi, S.; Marzola, P.; Tambalo, S.; Conti, G.; Benati, D.; Vetrone, F.; Bettinelli, M. PEG-capped, lanthanide doped GdF3 nanoparticles: Luminescent and T2 contrast agents for optical and MRI multimodal imaging. Nanoscale 2012, 4, 7682–7689. [Google Scholar] [CrossRef]
- Schäfer, H.; Ptacek, P.; Zerzouf, O.; Haase, M. Synthesis and optical properties of KYF4/Yb, Er nanocrystals, and their surface modification with undoped KYF4. Adv. Funct. Mater. 2008, 18, 2913–2918. [Google Scholar] [CrossRef]
- Chen, C.; Sun, L.-D.; Li, Z.-X.; Li, L.-L.; Zhang, J.; Zhang, Y.-W.; Yan, C.-H. Ionic liquid-based route to spherical NaYF4 nanoclusters with the assistance of microwave radiation and their multicolor upconversion luminescence. Langmuir 2010, 26, 8797–8803. [Google Scholar] [CrossRef]
- Schäfer, H.; Ptacek, P.; Eickmeier, H.; Haase, M. Synthesis of Hexagonal Yb3+, Er3+-Doped NaYF4 Nanocrystals at Low Temperature. Adv. Funct. Mater. 2009, 19, 3091–3097. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Y.; Huang, F.; Wang, Y. Phase transition from hexagonal LnF3 (Ln = La, Ce, Pr) to cubic Ln0.8M0.2F2.8 (M = Ca, Sr, Ba) nanocrystals with enhanced upconversion induced by alkaline-earth doping. Chem. Commun. 2011, 47, 2601–2603. [Google Scholar] [CrossRef]
- Yin, W.; Zhao, L.; Zhou, L.; Gu, Z.; Liu, X.; Tian, G.; Jin, S.; Yan, L.; Ren, W.; Xing, G. Enhanced red emission from GdF3: Yb3+, Er3+ upconversion nanocrystals by Li+ doping and their application for bioimaging. Chem.–A Eur. J. 2012, 18, 9239–9245. [Google Scholar] [CrossRef]
- Li, F.; Li, C.; Liu, X.; Bai, T.; Dong, W.; Zhang, X.; Shi, Z.; Feng, S. Microwave-assisted synthesis and up–down conversion luminescent properties of multicolor hydrophilic LaF3: Ln3+ nanocrystals. Dalton Trans. 2013, 42, 2015–2022. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Upconversion multicolor fine-tuning: Visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticles. J. Am. Chem. Soc. 2008, 130, 5642–5643. [Google Scholar] [CrossRef] [PubMed]
- Gnach, A.; Bednarkiewicz, A. Lanthanide-doped up-converting nanoparticles: Merits and challenges. Nano Today 2012, 7, 532–563. [Google Scholar] [CrossRef]
- Rafique, R.; Baek, S.H.; Chang, S.-J.; Gul, A.R.; Park, T.J. A facile hydrothermal synthesis of highly luminescent NaYF4: Yb3+/Er3+ upconversion nanoparticles and their biomonitoring capability. Mater. Sci. Eng. C 2019, 99, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.H.; Chan, K.K.; Yap, S.H.K.; Yong, K.T. NIR-responsive nanomaterials and their applications; upconversion nanoparticles and carbon dots: A perspective. J. Chem. Technol. Biotechnol. 2018, 93, 1519–1528. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, H.; Yuan, W.; Su, Q.; Li, F. Upconversion nanoprobes for biodetections. Coord. Chem. Rev. 2018, 354, 155–168. [Google Scholar] [CrossRef]
- Tong, R.; Lin, H.; Chen, Y.; An, N.; Wang, G.; Pan, X.; Qu, F. Near-infrared mediated chemo/photodynamic synergistic therapy with DOX-UCNPs@ mSiO2/TiO2-TC nanocomposite. Mater. Sci. Eng. C 2017, 78, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Abbineni, G.; Clevenger, A.; Mao, C.; Xu, S. Upconversion nanoparticles: Synthesis, surface modification and biological applications. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 710–729. [Google Scholar] [CrossRef]
- Shangguan, M.; Xia, H.; Wang, C.; Qiu, J.; Shentu, G.; Zhang, Q.; Dou, X.; Pan, J.-W. All-fiber upconversion high spectral resolution wind lidar using a Fabry-Perot interferometer. Opt. Express 2016, 24, 19322–19336. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yu, Y.; Huang, P.; Wang, Y. Nanocrystallization of lanthanide trifluoride in an aluminosilicate glass matrix: Dimorphism and rare earth partition. CrystEngComm 2009, 11, 1686–1690. [Google Scholar] [CrossRef]
- He, M.; Pang, X.; Liu, X.; Jiang, B.; He, Y.; Snaith, H.; Lin, Z. Monodisperse dual-functional upconversion nanoparticles enabled near-infrared organolead halide perovskite solar cells. Angew. Chem. Int. Ed. 2016, 55, 4280–4284. [Google Scholar] [CrossRef]
- Wang, M.; Mi, C.; Zhang, Y.; Liu, J.; Li, F.; Mao, C.; Xu, S. NIR-responsive silica-coated NaYbF4: Er/Tm/Ho upconversion fluorescent nanoparticles with tunable emission colors and their applications in immunolabeling and fluorescent imaging of cancer cells. J. Phys. Chem. C 2009, 113, 19021–19027. [Google Scholar] [CrossRef]
- Gu, Z.; Yan, L.; Tian, G.; Li, S.; Chai, Z.; Zhao, Y. Recent advances in design and fabrication of upconversion nanoparticles and their safe theranostic applications. Adv. Mater. 2013, 25, 3758–3779. [Google Scholar] [CrossRef]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Wang, S.; Lin, M.; Jin, Y.; Zhang, S.; Cui, X.; Gong, Y.; Li, A.; Xu, F.; Lu, T.J. Upconversion nanoparticles based FRET aptasensor for rapid and ultrasenstive bacteria detection. Biosens. Bioelectron. 2017, 90, 525–533. [Google Scholar] [CrossRef]
- Kumar, R.; Nyk, M.; Ohulchanskyy, T.Y.; Flask, C.A.; Prasad, P.N. Combined optical and MR bioimaging using rare earth ion doped NaYF4 nanocrystals. Adv. Funct. Mater. 2009, 19, 853–859. [Google Scholar] [CrossRef]
- Xing, H.; Bu, W.; Ren, Q.; Zheng, X.; Li, M.; Zhang, S.; Qu, H.; Wang, Z.; Hua, Y.; Zhao, K. A NaYbF4: Tm3+ nanoprobe for CT and NIR-to-NIR fluorescent bimodal imaging. Biomaterials 2012, 33, 5384–5393. [Google Scholar] [CrossRef]
- Ni, D.; Bu, W.; Zhang, S.; Zheng, X.; Li, M.; Xing, H.; Xiao, Q.; Liu, Y.; Hua, Y.; Zhou, L. Single Ho3+-doped upconversion nanoparticles for high-performance T2-weighted brain tumor diagnosis and MR/UCL/CT multimodal imaging. Adv. Funct. Mater. 2014, 24, 6613–6620. [Google Scholar] [CrossRef]
- Teng, X.; Zhu, Y.; Wei, W.; Wang, S.; Huang, J.; Naccache, R.; Hu, W.; Tok, A.I.Y.; Han, Y.; Zhang, Q. Lanthanide-doped NaxScF3+x nanocrystals: Crystal structure evolution and multicolor tuning. J. Am. Chem. Soc. 2012, 134, 8340–8343. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ohulchanskyy, T.Y.; Kumar, R.; Ågren, H.; Prasad, P.N. Ultrasmall monodisperse NaYF4: Yb3+/Tm3+ nanocrystals with enhanced near-infrared to near-infrared upconversion photoluminescence. ACS Nano 2010, 4, 3163–3168. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.-T.; Vetrone, F.; Naccache, R.; Chan, H.L.W.; Hao, J.; Capobianco, J.A. Water dispersible ultra-small multifunctional KGdF4: Tm3+, Yb3+ nanoparticles with near-infrared to near-infrared upconversion. J. Mater. Chem. 2011, 21, 16589–16596. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Q.; Li, Y. Upconversion luminescence of monodisperse CaF2: Yb3+/Er3+ nanocrystals. J. Am. Chem. Soc. 2009, 131, 14200–14201. [Google Scholar] [CrossRef]
- Dong, N.-N.; Pedroni, M.; Piccinelli, F.; Conti, G.; Sbarbati, A.; Ramírez-Hernández, J.E.; Maestro, L.M.; Iglesias-de la Cruz, M.C.; Sanz-Rodriguez, F.; Juarranz, A. NIR-to-NIR two-photon excited CaF2:Tm3+, Yb3+ nanoparticles: Multifunctional nanoprobes for highly penetrating fluorescence bio-imaging. ACS Nano 2011, 5, 8665–8671. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Yang, T.; Feng, W.; Li, C.; Li, F. Sub-10 nm hexagonal lanthanide-doped NaLuF4 upconversion nanocrystals for sensitive bioimaging in vivo. J. Am. Chem. Soc. 2011, 133, 17122–17125. [Google Scholar] [CrossRef]
- Shi, F.; Wang, J.; Zhai, X.; Zhao, D.; Qin, W. Facile synthesis of β-NaLuF4: Yb/Tm hexagonal nanoplates with intense ultraviolet upconversion luminescence. CrystEngComm 2011, 13, 3782–3787. [Google Scholar] [CrossRef]
- Yang, D.; Dai, Y.; Ma, P.; Kang, X.; Cheng, Z.; Li, C.; Lin, J. One-step synthesis of small-sized and water-solule NaREF4 upconversion nanoparticles for in vitro cell imaging and drug delivery. Chem.–A Eur. J. 2013, 19, 2685–2694. [Google Scholar] [CrossRef]
- Sarkar, S.; Meesaragandla, B.; Hazra, C.; Mahalingam, V. Sub-5 nm Ln3+-doped BaLuF5 Nanocrystals: A Platform to Realize Upconversion via Interparticle Energy Transfer (IPET). Adv. Mater. 2013, 25, 856–860. [Google Scholar] [CrossRef]
- Yersin, H.; Donges, D. Low-lying electronic states and photophysical properties of organometallic Pd (II) and Pt (II) compounds. Modern research trends presented in detailed case studies. In Transition Metal and Rare Earth Compounds: Excited States, Transitions, Interactions II; Springer: Berlin/Heidelberg, Germany, 2001; pp. 81–186. [Google Scholar]
- Gamelin, D.R.; Güdel, H.U. Design of luminescent inorganic materials: New photophysical processes studied by optical spectroscopy. Acc. Chem. Res. 2000, 33, 235–242. [Google Scholar] [CrossRef]
- Qiu, H.; Chen, G.; Sun, L.; Hao, S.; Han, G.; Yang, C. Ethylenediaminetetraacetic acid (EDTA)-controlled synthesis of multicolor lanthanide doped BaYF5 upconversion nanocrystals. J. Mater. Chem. 2011, 21, 17202–17208. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Pan, W.; Wang, H.; Li, N.; Tang, B. Fluorescence and photoacoustic dual-mode imaging of tumor-related mRNA with a covalent linkage-based DNA nanoprobe. Chem. Commun. 2018, 54, 3656–3659. [Google Scholar] [CrossRef]
- Ye, Z.; Srivastava, P.K.; Xu, Y.; Wang, W.; Jing, L.; Chen, S.-L.; Tu, C.-C. Surface-Functionalized Silicon Nanoparticles as Contrast Agents for Photoacoustic Microscopy Imaging. ACS Appl. Nano Mater. 2019, 2, 7577–7584. [Google Scholar] [CrossRef]
- Mantri, Y.; Jokerst, J.V. Engineering Plasmonic Nanoparticles for Enhanced Photoacoustic Imaging. ACS Nano 2020, 14, 9408–9422. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Seeding growth for size control of 5–40 nm diameter gold nanoparticles. Langmuir 2001, 17, 6782–6786. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Gobin, A.M.; Lowery, A.R.; Tam, F.; Drezek, R.A.; Halas, N.J.; West, J.L. Metal nanoshells. Ann. Biomed. Eng. 2006, 34, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kutsanedzie, F.Y.H.; Ali, S.; Wang, P.; Li, C.; Ouyang, Q.; Li, H.; Chen, Q. Cysteamine-mediated upconversion sensor for lead ion detection in food. J. Food Meas. Charact. 2021, 15, 4849–4857. [Google Scholar] [CrossRef]
- Wu, W.; Ahmad, W.; Hassan, M.M.; Wu, J.; Ouyang, Q.; Chen, Q. An upconversion biosensor based on inner filter effect for dual-role recognition of sulfadimethoxine in aquatic samples. Food Chem. 2024, 437, 137832. [Google Scholar] [CrossRef]
- Ouyang, Q.; Rong, Y.; Wang, B.; Ahmad, W.; Liu, S.; Chen, Q. An innovative solid-phase biosensor for rapid on-site detection of N-nitrosodimethylamine incorporating zein film and upconversion nanoparticles. Food Chem. 2024, 430, 136981. [Google Scholar] [CrossRef]
- Li, S.; Wu, J.; Zhang, S.; Jiao, T.; Wei, J.; Chen, X.; Chen, Q.; Chen, Q. Inner filter effect-based upconversion nanosensor for rapid detection of thiram pesticides using upconversion nanoparticles and dithizone–cadmium complexes. Food Chem. 2024, 434, 137438. [Google Scholar] [CrossRef]
- Li, H.; Bei, Q.; Hassan, M.M.; Marimuthu, M.; Adade, S.Y.S.S.; Chen, Q.; Zareef, M. A Cu2+-modulated UCNPs@RBD sensor for sensitive detection of tetracyclines in food based on the spirolactam open-loop reaction. J. Food Compos. Anal. 2024, 133, 106374. [Google Scholar] [CrossRef]
- Liu, Y.; Ouyang, Q.; Li, H.; Chen, M.; Zhang, Z.; Chen, Q. Turn-On Fluoresence Sensor for Hg2+ in Food Based on FRET between Aptamers-Functionalized Upconversion Nanoparticles and Gold Nanoparticles. J. Agric. Food Chem. 2018, 66, 6188–6195. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Liao, L.-D.; Thakor, N.; Tan, M.C. Rare-earth doped particles as dual-modality contrast agent for minimally-invasive luminescence and dual-wavelength photoacoustic imaging. Sci. Rep. 2014, 4, 6562. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, N.; Lv, J.; Zhou, Z.; Zhao, Q.; Ma, L.; Chen, Z.; Ren, L.; Nie, L. Deep photoacoustic/luminescence/magnetic resonance multimodal imaging in living subjects using high-efficiency upconversion nanocomposites. Adv. Mater. 2016, 28, 6411–6419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ai, X.; Zhang, Z.; Wang, Y.; Wu, X.; Haindl, R.; Yeow, E.K.; Drexler, W.; Gao, M.; Xing, B. NIR nanoprobe-facilitated cross-referencing manifestation of local disease biology for dynamic therapeutic response assessment. Chem. Sci. 2020, 11, 803–811. [Google Scholar] [CrossRef]
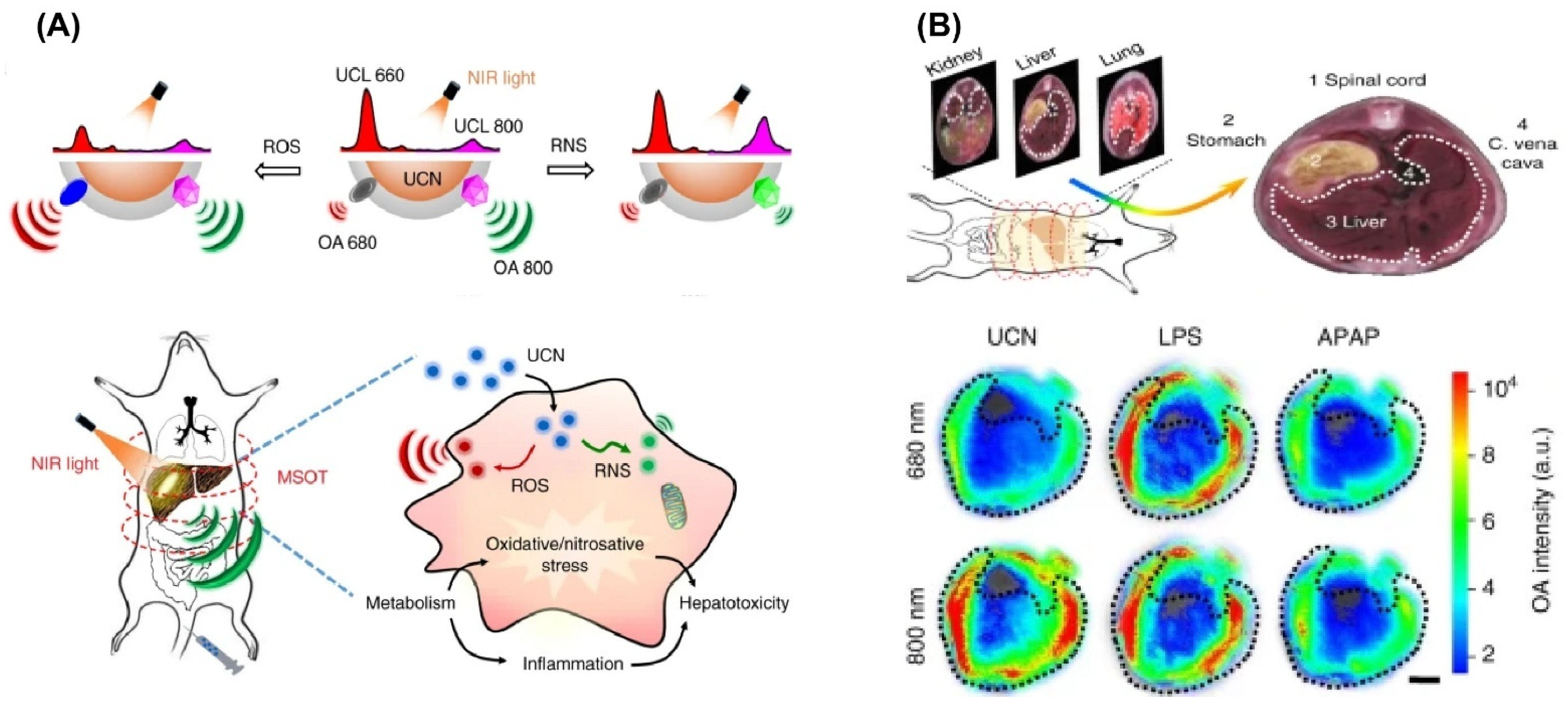
- Zhuang, H.; Li, B.; Zhao, M.; Wei, P.; Yuan, W.; Zhang, M.; Han, X.; Chen, Y.; Yi, T. Real-time monitoring and accurate diagnosis of drug-induced hepatotoxicity in vivo by ratio-fluorescence and photoacoustic imaging of peroxynitrite. Nanoscale 2020, 12, 10216–10225. [Google Scholar] [CrossRef]
- Lv, R.; Wang, D.; Xiao, L.; Chen, G.; Xia, J.; Prasad, P.N. Stable ICG-loaded upconversion nanoparticles: Silica core/shell theranostic nanoplatform for dual-modal upconversion and photoacoustic imaging together with photothermal therapy. Sci. Rep. 2017, 7, 15753. [Google Scholar] [CrossRef]
- He, F.; Yang, G.; Yang, P.; Yu, Y.; Lv, R.; Li, C.; Dai, Y.; Gai, S.; Lin, J. A new single 808 nm NIR light-induced imaging-guided multifunctional cancer therapy platform. Adv. Funct. Mater. 2015, 25, 3966–3976. [Google Scholar] [CrossRef]
- Hou, Z.; Deng, K.; Wang, M.; Liu, Y.; Chang, M.; Huang, S.; Li, C.; Wei, Y.; Cheng, Z.; Han, G. Hydrogenated titanium oxide decorated upconversion nanoparticles: Facile laser modified synthesis and 808 nm near-infrared light triggered phototherapy. Chem. Mater. 2019, 31, 774–784. [Google Scholar] [CrossRef]
- He, S.; Song, J.; Liu, J.; Liu, L.; Qu, J.; Cheng, Z. Enhancing Photoacoustic Intensity of Upconversion Nanoparticles by Photoswitchable Azobenzene-Containing Polymers for Dual NIR-II and Photoacoustic Imaging In Vivo. Adv. Opt. Mater. 2019, 7, 1900045. [Google Scholar] [CrossRef]
- Du, K.; Lei, P.; Dong, L.; Zhang, M.; Gao, X.; Yao, S.; Feng, J.; Zhang, H. In situ decorating of ultrasmall Ag2Se on upconversion nanoparticles as novel nanotheranostic agent for multimodal imaging-guided cancer photothermal therapy. Appl. Mater. Today 2020, 18, 100497. [Google Scholar] [CrossRef]
- He, F.; Feng, L.; Yang, P.; Liu, B.; Gai, S.; Yang, G.; Dai, Y.; Lin, J. Enhanced up/down-conversion luminescence and heat: Simultaneously achieving in one single core-shell structure for multimodal imaging guided therapy. Biomaterials 2016, 105, 77–88. [Google Scholar] [CrossRef]
- Sun, M.; Xu, L.; Ma, W.; Wu, X.; Kuang, H.; Wang, L.; Xu, C. Hierarchical plasmonic nanorods and upconversion core–satellite nanoassemblies for multimodal imaging-guided combination phototherapy. Adv. Mater. 2016, 28, 898–904. [Google Scholar] [CrossRef]
- Sheng, Y.; Liao, L.-D.; Bandla, A.; Liu, Y.-H.; Thakor, N.; Tan, M.C. Size and shell effects on the photoacoustic and luminescence properties of dual modal rare-earth-doped nanoparticles for infrared photoacoustic imaging. ACS Biomater. Sci. Eng. 2016, 2, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.K.; Sreejith, S.; Joseph, J.; Lin, M.; He, T.; Tong, Y.; Sun, H.; Yu, S.W.-K.; Zhao, Y. Upconversion Nanoparticles as a Contrast Agent for Photoacoustic Imaging in Live Mice. Adv. Mater. 2014, 26, 5633–5638. [Google Scholar] [CrossRef] [PubMed]
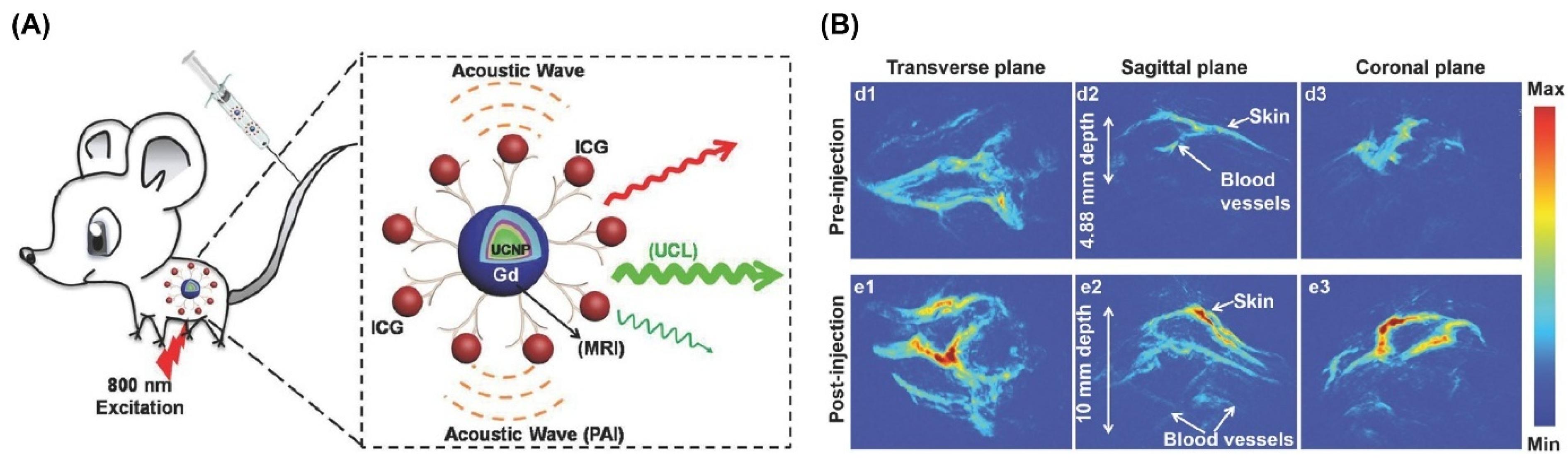
- Rieffel, J.; Chen, F.; Kim, J.; Chen, G.; Shao, W.; Shao, S.; Chitgupi, U.; Hernandez, R.; Graves, S.A.; Nickles, R.J. Hexamodal imaging with porphyrin-phospholipid-coated upconversion nanoparticles. Adv. Mater. 2015, 27, 1785–1790. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, H.; Zeng, Q.; He, F.; Liu, C.; Gai, S.; Ding, H.; Yang, P. A bubble-enhanced lanthanide-doped up/down-conversion platform with tumor microenvironment response for dual-modal photoacoustic and near-infrared-II fluorescence imaging. J. Colloid Interface Sci. 2024, 659, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Bastos, V.; Oskoei, P.; Andresen, E.; Saleh, M.I.; Rühle, B.; Resch-Genger, U.; Oliveira, H. Stability, dissolution, and cytotoxicity of NaYF4-upconversion nanoparticles with different coatings. Sci. Rep. 2022, 12, 3770. [Google Scholar] [CrossRef]
- Saleh, M.I.; Rühle, B.; Wang, S.; Radnik, J.; You, Y.; Resch-Genger, U. Assessing the protective effects of different surface coatings on NaYF4:Yb3+, Er3+ upconverting nanoparticles in buffer and DMEM. Sci. Rep. 2020, 10, 19318. [Google Scholar] [CrossRef]
- Liu, C.; Gao, Z.; Zeng, J.; Hou, Y.; Fang, F.; Li, Y.; Qiao, R.; Shen, L.; Lei, H.; Yang, W.; et al. Magnetic/Upconversion Fluorescent NaGdF4:Yb,Er Nanoparticle-Based Dual-Modal Molecular Probes for Imaging Tiny Tumors In Vivo. ACS Nano 2013, 7, 7227–7240. [Google Scholar] [CrossRef]
- Sun, Y.; Feng, W.; Yang, P.; Huang, C.; Li, F. The biosafety of lanthanide upconversion nanomaterials. Chem. Soc. Rev. 2015, 44, 1509–1525. [Google Scholar] [CrossRef] [PubMed]
- Abdul Jalil, R.; Zhang, Y. Biocompatibility of silica coated NaYF4 upconversion fluorescent nanocrystals. Biomaterials 2008, 29, 4122–4128. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lin, J.; Wang, T.; Chen, X.; Huang, P. Recent Advances in Photoacoustic Imaging for Deep-Tissue Biomedical Applications. Theranostics 2016, 6, 2394–2413. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Wong, K.-L.; All, A.H. Lanthanide-doped upconversion nanoparticles as nanoprobes for bioimaging. Biomater. Sci. 2024, 12, 4650–4663. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Butt, H.J. Near-infrared-sensitive materials based on upconverting nanoparticles. Adv. Mater. 2016, 28, 1208–1226. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Hassan, M.M.; Guo, Z.; Zhang, Z.-Z.; Chen, Q. Fabricating an Acetylcholinesterase Modulated UCNPs-Cu2+ Fluorescence Biosensor for Ultrasensitive Detection of Organophosphorus Pesticides-Diazinon in Food. J. Agric. Food Chem. 2019, 67, 4071–4079. [Google Scholar] [CrossRef]
- Lee, T.; Baac, H.W.; Li, Q.; Guo, L.J. Efficient photoacoustic conversion in optical nanomaterials and composites. Adv. Opt. Mater. 2018, 6, 1800491. [Google Scholar] [CrossRef]
- Ai, X.; Wang, Z.; Cheong, H.; Wang, Y.; Zhang, R.; Lin, J.; Zheng, Y.; Gao, M.; Xing, B. Multispectral optoacoustic imaging of dynamic redox correlation and pathophysiological progression utilizing upconversion nanoprobes. Nat. Commun. 2019, 10, 1087. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, J.; Wei, W.; Zeng, Q.; Li, X.; Xing, D.; Zhou, B.; Zhang, T. Switching the NIR upconversion of nanoparticles for the orthogonal activation of photoacoustic imaging and phototherapy. Nat. Commun. 2022, 13, 3149. [Google Scholar] [CrossRef]
- Kim, D.W.; Wrede, P.; Rodríguez-Camargo, A.; Chen, Y.; Dogan, N.O.; Glück, C.; Lotsch, B.V.; Razansky, D.; Sitti, M. Upconversion Nanoparticle-Covalent Organic Framework Core–shell Particles as Therapeutic Microrobots Trackable with Optoacoustic Imaging. Adv. Mater. 2025, 2418425. [Google Scholar] [CrossRef]
- Rizvi, S.F.A.; Zhang, H.; Fang, Q. Engineering peptide drug therapeutics through chemical conjugation and implication in clinics. Med. Res. Rev. 2024, 44, 2420–2471. [Google Scholar] [CrossRef] [PubMed]



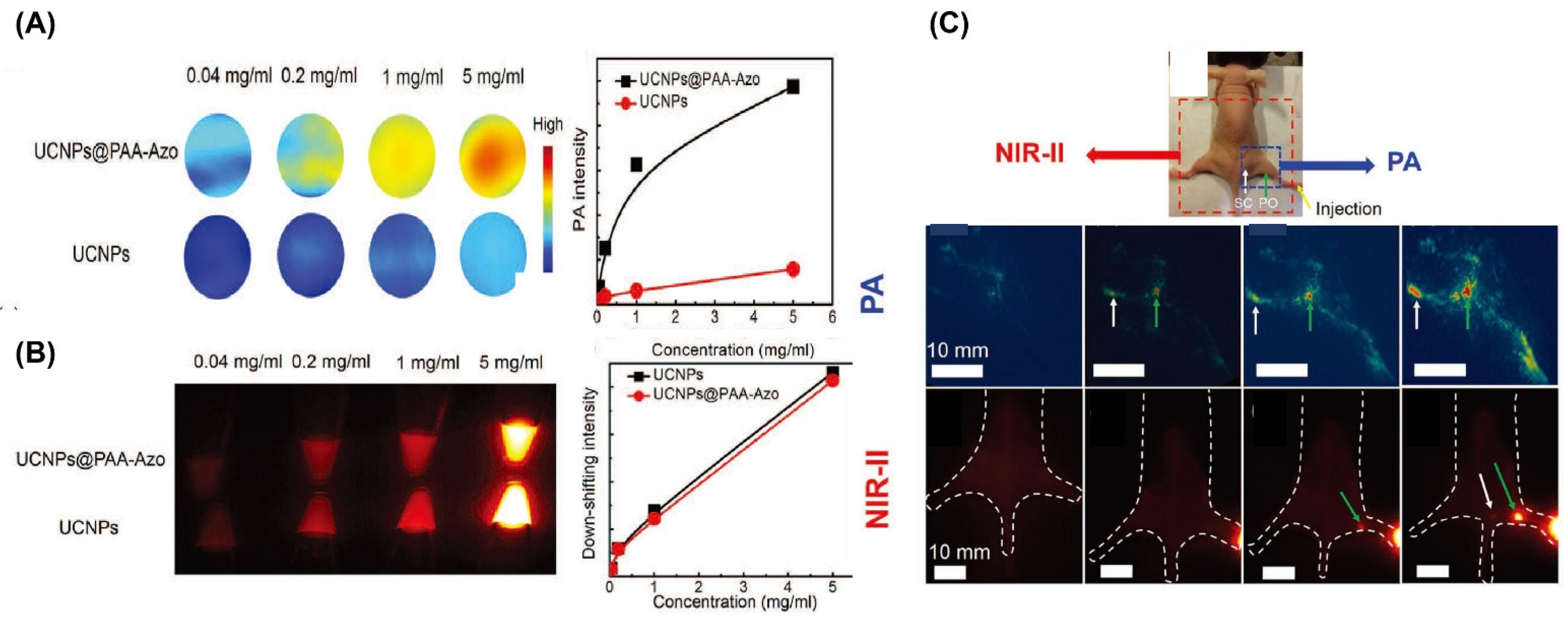
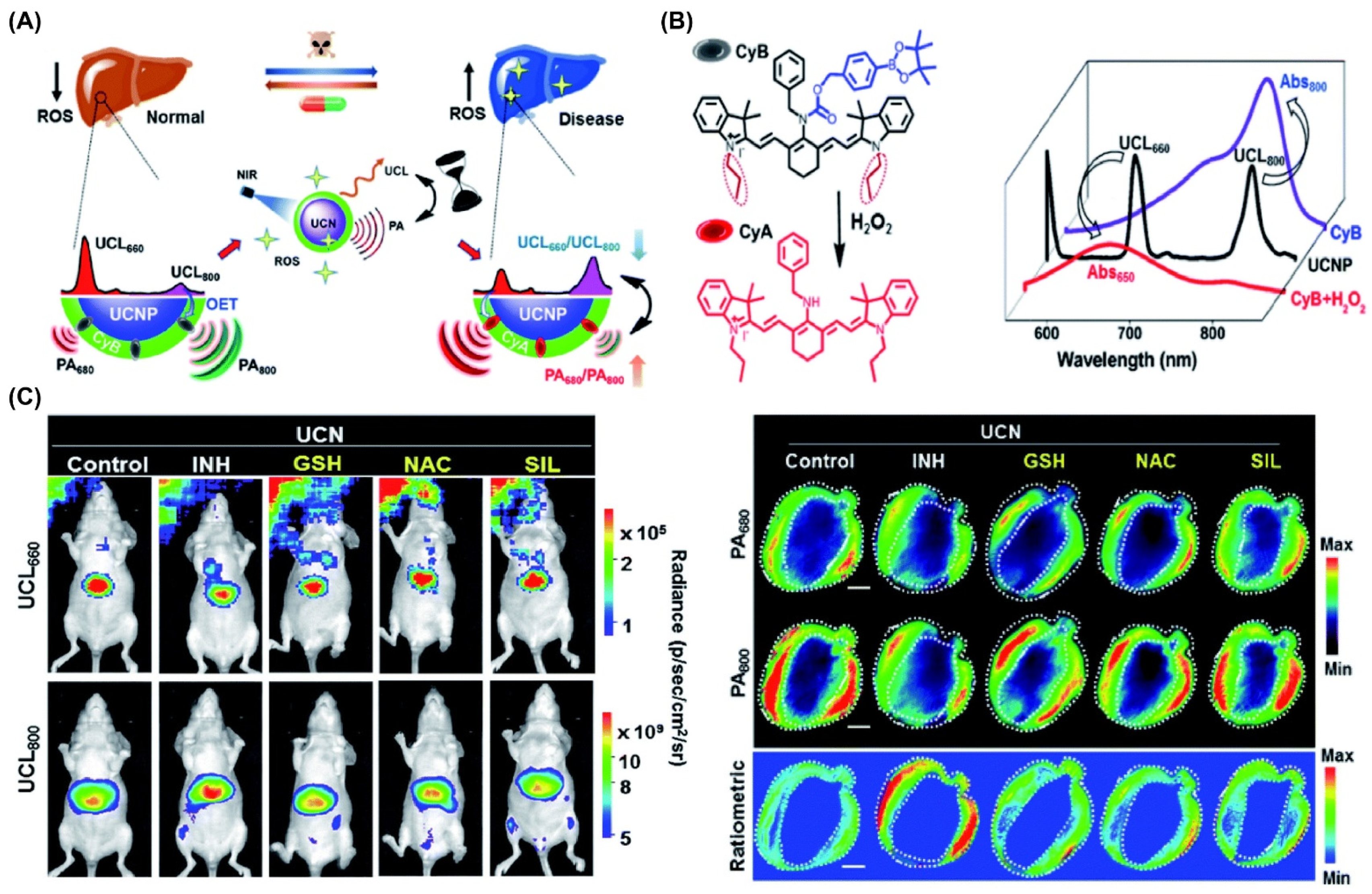
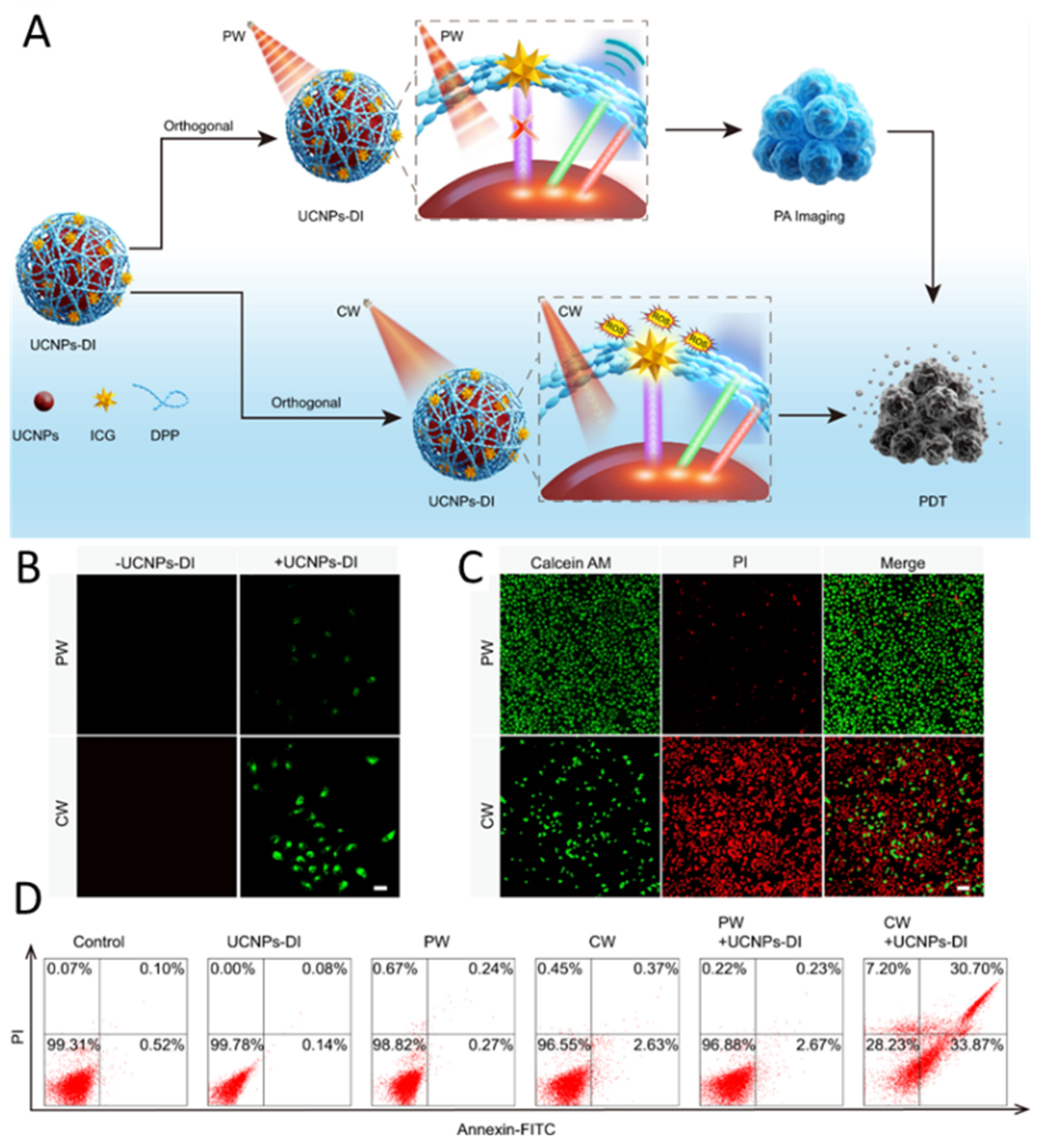
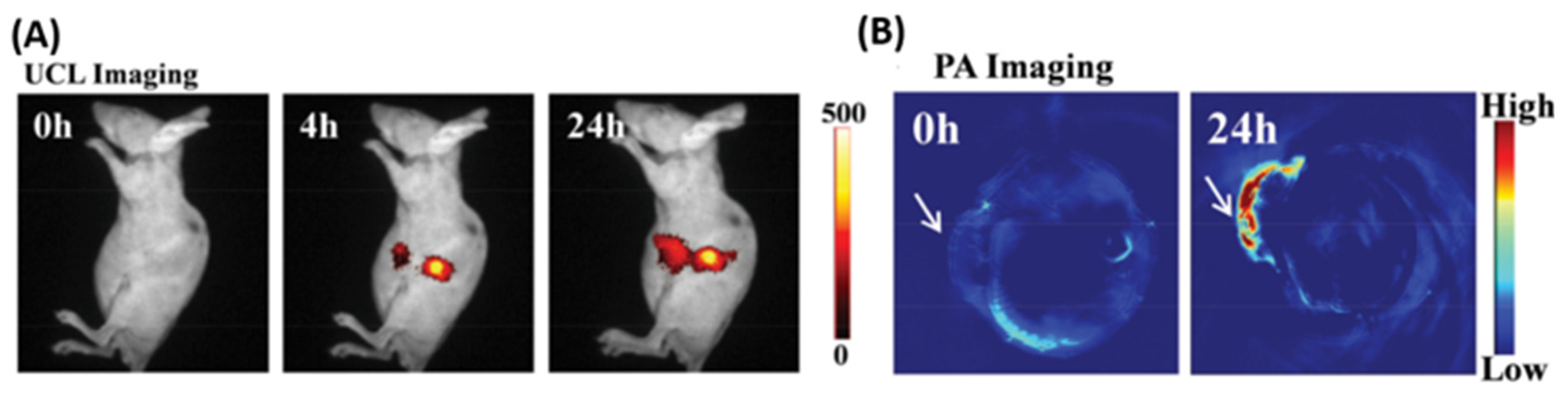

| Main Synthetic Method | Material, Refs | Size Range (nm) | Advantages | Disadvantages |
|---|---|---|---|---|
| Thermal decomposition | NaNdF4 [180] β-NaErF4 [181] | 50–500 | High-quality, uniform size | Intermediate toxicity, high cost |
| Microemulsion | NaYF4 [182] LaF3 [183] | 4–500 | Easy to operate, narrow size distribution, high stability | Calcination or annealing usually required |
| Phase transfer hydrothermal synthesis | (La-Dy)VO4 [184] YVO4 [184] NaYF4 [185] | 10–1000 | Good dispersion, simple procedures, tunable size | Specialized reaction vessels are needed |
| Sol-gel processing | GdVO4 [186] | 30–600 | Cheap raw materials, simple procedures | Broad particle size and unsuitable for bioapplication |
| Dopant Ions and Composition | Major λ Emissions (nm) | Reference | |||||
|---|---|---|---|---|---|---|---|
| Host Lattice | Sensitizer | Activator | Shell | Blue | Green | Red | |
| β-NaYF4 | 20%Yb3+ | 2%Er3+ | 450, 476 | 520 | 654 | Ref. [208] | |
| 20%Yb3+ | 0.2%Tm3+ | 540 | Ref. [209] | ||||
| 20%Yb3+ | 2%Ho3+ | 541 | Ref. [209] | ||||
| β-NaYF4 | 20%Yb3+ | 0.3%Tm3+ | 20%Yb3+, 2%Er3+ | 450 | 520 | 653 | Ref. [210] |
| 475 | 540 | ||||||
| Li+ doped β-NaYF4 | 20%Yb3+ | 0.5%Tm3+ | 452, 479 | 650 | Ref. [211] | ||
| Mn+ doped β-NaYF4 | 20%Yb3+ | 2%Er3+ | 657 | Ref. [212] | |||
| β-NaYF4 | 18.6%Yb3+ | 2.2%Er3+ | TRITC-SiO2 | 407 | Ref. [213] | ||
| 25%Yb3+ | 0.3%Tm3+ | SiO2 | 450, 479 | 521, 539 | 651 | Ref. [85] | |
| 25%Yb3+ | 0.3%Tm3+ | FITC-SiO2 | 450, 479 | 521, 539, 580 | 651 | Ref. [214] | |
| α-NaYF4 | 25%Yb3+ | 0.3%Tm3+ | QD-SiO2 | 450, 479 | 540 | 651 | Ref. [214] |
| 20%Yb3+ | 2%Er3+ | 411 | 660 | Ref. [202] | |||
| 20%Yb3+ | 0.2%Tm3+ | 450 | 540 | 644 | Ref. [215] | ||
| 20%Yb3+ | 0.2%Er3+ | 475 | 525 | 693 | Ref. [215] | ||
| Photoacoustic Contrast Agent | Excitation λ (nm) | PA Sensitivity | Size (nm) | Multimodal Imaging Capability | In Vivo/In Vitro | Reference |
|---|---|---|---|---|---|---|
| PAA-NaYF4:Yb,Er | 520, 975 | - | 40–60 | PAI | In vivo | Ref. [242] |
| NaYF4:Yb:Er@NaYF4:Yb@NaNdF4:Yb@NaYF4 @NaGdF4-HAD-G2 ICG (CS2-ICG) | 808/540, 650 | ICG | 54.3 ± 4.1 | MRI, PA, UCL | both | Ref. [243] |
| CyB/NaGdF4:Yb/Tm/Er@NaGdF4 | 980/650, 800 | Isoniazid (INH) | ~78 | PAI, UCL | In vivo | Ref. [244] |
| UCY7 | 680 | ONOO− | ~35 | PAI, RFLI | In vivo | Ref. [245] |
| UCNP@mSiO2-ICG | 800 | ICG | ~60 | PA, UCL | both | Ref. [246] |
| UCNPs@MS-Au25 -PEG | 808 | 28–46 | PA, MRI, CT, PDT, PTT | both | Ref. [247] | |
| Nd:UCNPs@H-TiO2 | 808 | H-TiO2 | ~60 | PA, PDT, PTT, UCL | both | Ref. [248] |
| UCNP@PAA-AzoNaYF4:Yb30%, Tm0.5%, Nd5% | 808 | Azobenzene-containing poly | ~21 | PA, NIR-II | both | Ref. [249] |
| UCNPs@CS#Ag2Se | 808 | Ag+ | 141.8 | UCL, CT, PAI, PTT | both | Ref. [250] |
| LDNPs-Au25-PEG | 808 | ~40 | PTT, PA, PTI, PDT, MRI | both | Ref. [251] | |
| Nanorod (NR) dimer-UCNP-Ce6 | 980, 808 | NR-dimer | ~70 | PA, UCL, CT, MRI | In vivo | Ref. [252] |
| Core/Shell β-NaYF4:Yb,Er | 975 | 50.3 ± 5.6 | PAT | both | Ref. [253] | |
| NaYF4:Yb/Er@ α-cyclodextrin | 980 | α-CD | PAI | In vivo | Ref. [254] | |
| PoP-UCNP | 980 | PoP combined with detergent | 74 ± 3.6 | PA, FL, UC, PET, CL, CT | In vivo | Ref. [255] |
| LDNP@DMSN-Au@CaCO3 | 980 | Au-NPs | ~174 | PA, NIR-II, FL | both | Ref. [256] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Li, Z.; Du, Z.; Pan, J.; Huang, Y. Multifunctional Upconversion Nanoparticles Transforming Photoacoustic Imaging: A Review. Nanomaterials 2025, 15, 1074. https://doi.org/10.3390/nano15141074
Zhang Y, Li Z, Du Z, Pan J, Huang Y. Multifunctional Upconversion Nanoparticles Transforming Photoacoustic Imaging: A Review. Nanomaterials. 2025; 15(14):1074. https://doi.org/10.3390/nano15141074
Chicago/Turabian StyleZhang, Yuqian, Zerui Li, Ziqing Du, Jianming Pan, and Yanan Huang. 2025. "Multifunctional Upconversion Nanoparticles Transforming Photoacoustic Imaging: A Review" Nanomaterials 15, no. 14: 1074. https://doi.org/10.3390/nano15141074
APA StyleZhang, Y., Li, Z., Du, Z., Pan, J., & Huang, Y. (2025). Multifunctional Upconversion Nanoparticles Transforming Photoacoustic Imaging: A Review. Nanomaterials, 15(14), 1074. https://doi.org/10.3390/nano15141074









