Comparative In-Depth Investigation of Benchmark Graphene Oxides in the Perspective of Their Integration into Industrial Production Processes
Abstract
1. Introduction
2. Materials and Methods
3. Characterization Techniques
4. Results
4.1. Characterization of Commercial GO Samples
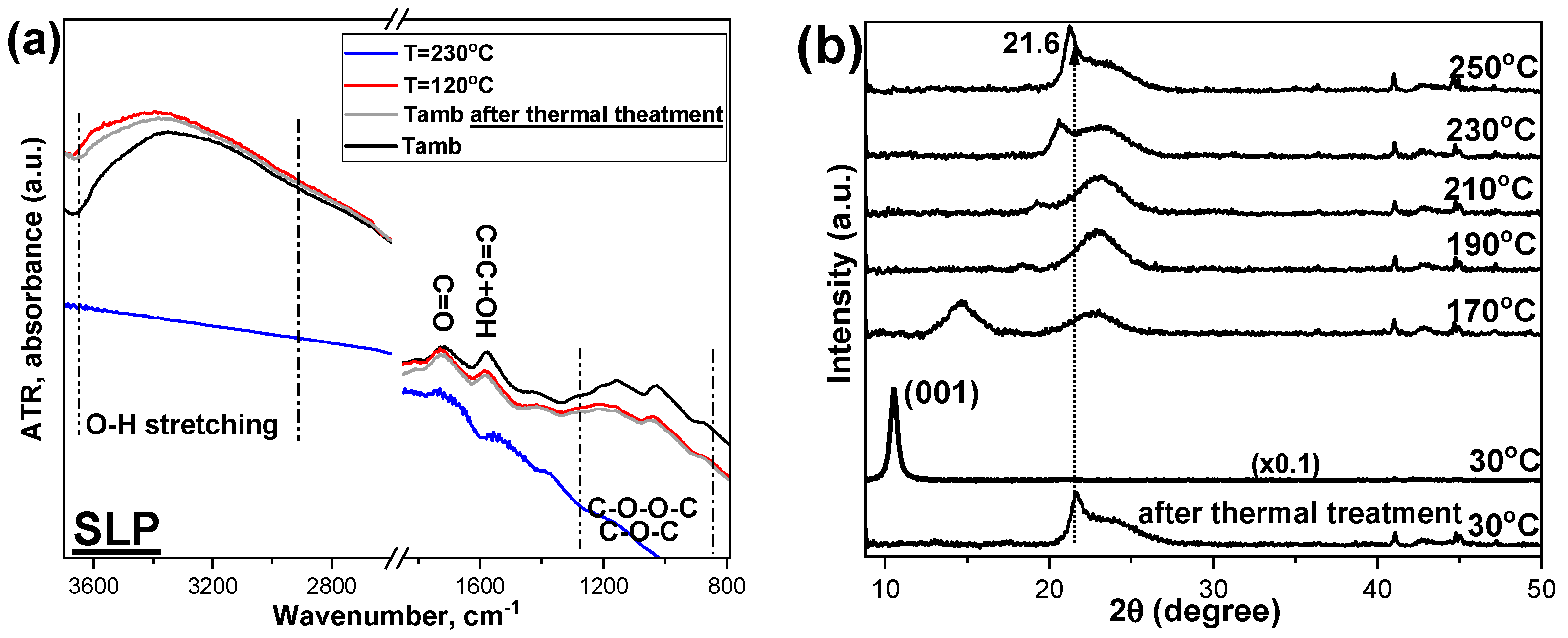
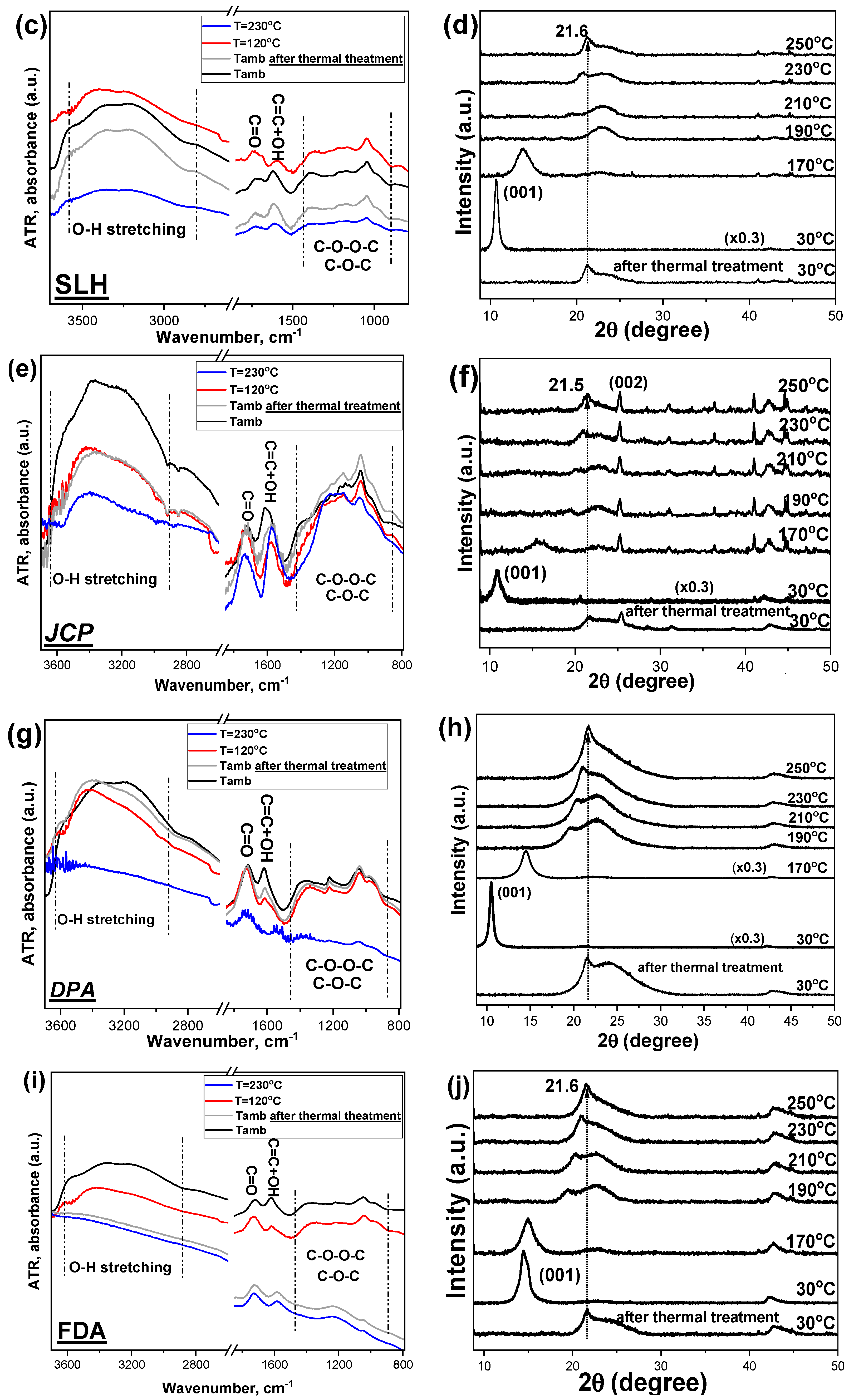
4.2. The Temperature Dependent Structural Differentiation in GO Morphology Using XRD and ATR-IR Measurements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ray, S.C. (Ed.) Chapter 2—Application and Uses of Graphene Oxide and Reduced Graphene Oxide. In Applications of Graphene and Graphene-Oxide Based Nanomaterials; William Andrew Publishing: Oxford, UK, 2015; pp. 39–55. [Google Scholar]
- Perrozzi, F.; Prezioso, S.; Ottaviano, L. Graphene oxide: From fundamentals to applications. J. Phys. Condens. Matter 2015, 27, 013002. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Cheng, H.-M.; Enoki, T.; Gogotsi, Y.; Hurt, R.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Mirzapour, M.; Robert, M.; Benmokrane, B. In Situ Processing to Achieve High-Performance Epoxy Nanocomposites with Low Graphene Oxide Loading. C 2024, 10, 52. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Wu, J.; Lin, H.; Moss, D.J.; Loh, K.P.; Jia, B. Graphene oxide for photonics, electronics and optoelectronics. Nat. Rev. Chem. 2023, 7, 162–183. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.; Zhao, J.; Zhang, S. Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 2015, 16, 488–515. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R. Graphene Utilization for Efficient Energy Storage and Potential Applications: Challenges and Future Implementations. Energies 2023, 16, 2927. [Google Scholar] [CrossRef]
- Baisnab, D.K.; Mukherjee, S.; Das, S. Chapter 7—A short review on inorganic thin films from device perspective. In Chemical Solution Synthesis for Materials Design and Thin Film Device Applications; Das, S., Dhara, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 231–275. [Google Scholar]
- Anand, A.; Unnikrishnan, B.; Wei, S.-C.; Chou, C.P.; Zhang, L.-Z.; Huang, C.-C. Graphene oxide and carbon dots as broad-spectrum antimicrobial agents—A minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef]
- Ghulam, A.N.; dos Santos, O.A.L.; Hazeem, L.; Pizzorno Backx, B.; Bououdina, M.; Bellucci, S. Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment. J. Funct. Biomater. 2022, 13, 77. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, Z.; Yang, J.; Kim, J.-K. Graphene oxide-based transparent conductive films. Prog. Mater. Sci. 2014, 64, 200–247. [Google Scholar] [CrossRef]
- Chumakova, N.; Kokorin, A. Graphene Oxide Membranes—Synthesis, Properties, and Applications. Membranes 2023, 13, 771. [Google Scholar] [CrossRef]
- Tiwary, S.K.; Singh, M.; Chavan, S.V.; Karim, A. Graphene oxide-based membranes for water desalination and purification. npj 2D Mater. Appl. 2024, 8, 27. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Hafusa, A.; Yaragalla, S.; Thomas, S. Chapter Five—Analytical applications of graphene oxide for membrane processes as separation and concentration methods. In Comprehensive Analytical Chemistry; Penkova, A.V.; Dmitrenko, M.E.; Hafusa, A.; Yaragalla, S.; Thomas, S. Elsevier: Amsterdam, The Netherlands, 2020; Volume 91, pp. 99–124. [Google Scholar]
- Visvini, G.A.; Mathioudakis, G.N.; Soto Beobide, A.; Voyiatzis, G.A. Tuning of Water Vapor Permeability in 2D Nanocarbon-Based Polypropylene Composite Membranes. Nanomaterials 2025, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, W.; Wang, L.; Xue, J.; Hou, S. Large-scale preparation of graphene oxide film and its application for electromagnetic interference shielding. RSC Adv. 2021, 11, 33302–33308. [Google Scholar] [CrossRef]
- Politano, G.G. Optimizing Graphene Oxide Film Quality: The Role of Solvent and Deposition Technique. C 2024, 10, 90. [Google Scholar] [CrossRef]
- Valencia, A.M.; Valencia, C.H.; Zuluaga, F.; Grande-Tovar, C.D. Synthesis and fabrication of films including graphene oxide functionalized with chitosan for regenerative medicine applications. Heliyon 2021, 7, e07058. [Google Scholar] [CrossRef]
- Hidalgo, R.S.; López-Díaz, D.; Velázquez, M.M. Graphene Oxide Thin Films: Influence of Chemical Structure and Deposition Methodology. Langmuir 2015, 31, 2697–2705. [Google Scholar] [CrossRef]
- Trikkaliotis, D.G.; Christoforidis, A.K.; Mitropoulos, A.C.; Kyzas, G.Z. Graphene Oxide Synthesis, Properties and Characterization Techniques: A Comprehensive Review. ChemEngineering 2021, 5, 64. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Soares, Y.C.F.; Cargnin, E.; Naccache, M.F.; Andrade, R.J.E. Influence of Oxidation Degree of Graphene Oxide on the Shear Rheology of Poly(ethylene glycol) Suspensions. Fluids 2020, 5, 41. [Google Scholar] [CrossRef]
- Méndez-Reséndiz, A.; Vega-Becerra, O.E.; Pérez-García, S.A.; Licea-Jiménez, L. Controlling oxidation degree and crystallinity to achieve high Young’s modulus of graphene derivatives. Diam. Relat. Mater. 2025, 151, 111847. [Google Scholar] [CrossRef]
- Wang, S.; Chi, H.; Chen, L.; Li, W.; Li, Y.; Li, G.; Ge, X. Surface Functionalization of Graphene Oxide with Polymer Brushes for Improving Thermal Properties of the Polymer Matrix. Adv. Polym. Technol. 2021, 2021, 5591420. [Google Scholar] [CrossRef]
- Xiang, X.; He, Q.; Xia, S.; Deng, Z.; Zhang, H.; Li, H. Study of capacitance type flexible electronic devices based on polyacrylamide and reduced graphene oxide composite hydrogel. Eur. Polym. J. 2022, 171, 111200. [Google Scholar] [CrossRef]
- Hong, X.; Lee, J.C.; Ng, J.L.; Md Yusof, Z.; He, Q.; Li, Q. Effect of Graphene Oxide on the Mechanical Properties and Durability of High-Strength Lightweight Concrete Containing Shale Ceramsite. Materials 2023, 16, 2756. [Google Scholar] [CrossRef]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Aujara, K.M.; Chieng, B.W.; Ibrahim, N.A.; Zainuddin, N.; Thevy Ratnam, C. Gamma-Irradiation Induced Functionalization of Graphene Oxide with Organosilanes. Int. J. Mol. Sci. 2019, 20, 1910. [Google Scholar] [CrossRef]
- Yu, H.; He, Y.; Xiao, G.; Fan, Y.; Ma, J.; Gao, Y.; Hou, R.; Yin, X.; Wang, Y.; Mei, X. The roles of oxygen-containing functional groups in modulating water purification performance of graphene oxide-based membrane. Chem. Eng. J. 2020, 389, 124375. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Donato, K.Z.; Tan, H.L.; Marangoni, V.S.; Martins, M.V.S.; Ng, P.R.; Costa, M.C.F.; Jain, P.; Lee, S.J.; Koon, G.K.W.; Donato, R.K.; et al. Graphene oxide classification and standardization. Sci. Rep. 2023, 13, 6064. [Google Scholar] [CrossRef]
- Melo de Lima, L.R.; Martins, F.P.; Lagarinhos, J.N.; Santos, L.; Lima, P.; Torcato, R.; Marques, P.A.A.P.; Rodrigues, D.L.; Melo, S.; Grilo, J.; et al. Characterization of commercial graphene-based materials for application in thermoplastic nanocomposites. Mater. Today Proc. 2020, 20, 383–390. [Google Scholar] [CrossRef]
- Albers, P.W.; Leich, V.; Ramirez-Cuesta, A.J.; Cheng, Y.; Hönig, J.; Parker, S.F. The characterisation of commercial 2D carbons: Graphene, graphene oxide and reduced graphene oxide. Mater. Adv. 2022, 3, 2810–2826. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene Oxide Synthesized by using Modified Hummers Approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Gupta, V.; Sharma, N.; Singh, U.; Arif, M.; Singh, A. Higher oxidation level in graphene oxide. Optik 2017, 143, 115–124. [Google Scholar] [CrossRef]
- Amir Faiz, M.S.; Che Azurahanim, C.A.; Raba’ah, S.A.; Ruzniza, M.Z. Low cost and green approach in the reduction of graphene oxide (GO) using palm oil leaves extract for potential in industrial applications. Results Phys. 2020, 16, 102954. [Google Scholar] [CrossRef]
- Whitby, R.L.D.; Gun’ko, V.M.; Korobeinyk, A.; Busquets, R.; Cundy, A.B.; László, K.; Skubiszewska-Zięba, J.; Leboda, R.; Tombácz, E.; Toth, I.Y.; et al. Driving Forces of Conformational Changes in Single-Layer Graphene Oxide. ACS Nano 2012, 6, 3967–3973. [Google Scholar] [CrossRef] [PubMed]
- Ban, F.Y.; Majid, S.R.; Huang, N.M.; Lim, H.N. Graphene Oxide and Its Electrochemical Performance. Int. J. Electrochem. Sci. 2012, 7, 4345–4351. [Google Scholar] [CrossRef]
- Andrikopoulos, K.S.; Bounos, G.; Tasis, D.; Sygellou, L.; Drakopoulos, V.; Voyiatzis, G.A. The Effect of Thermal Reduction on the Water Vapor Permeation in Graphene Oxide Membranes. Adv. Mater. Interfaces 2014, 1, 1400250. [Google Scholar] [CrossRef]
- Chammingkwan, P.; Matsushita, K.; Taniike, T.; Terano, M. Enhancement in Mechanical and Electrical Properties of Polypropylene Using Graphene Oxide Grafted with End-Functionalized Polypropylene. Materials 2016, 9, 240. [Google Scholar] [CrossRef]
- Jiao, K.; Zhu, T.; Li, X.; Shan, M.; Xu, Z.; Jiao, Y. Assembly of graphene oxide on nonconductive nonwovens by the synergistic effect of interception and electrophoresis. J. Nanoparticle Res. 2015, 17, 373. [Google Scholar] [CrossRef]
- Haubner, K.; Murawski, J.; Olk, P.; Eng, L.M.; Ziegler, C.; Adolphi, B.; Jaehne, E. The route to functional graphene oxide. Chemphyschem 2010, 11, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Dash, T.; Rout, T.K.; Sahu, A.K.; Biswal, S.K.; Mishra, B.K. Preparation of graphene oxide by dry planetary ball milling process from natural graphite. RSC Adv. 2016, 6, 12657–12668. [Google Scholar] [CrossRef]
- Li, H.; Zhu, G.; Liu, Z.-H.; Yang, Z.; Wang, Z. Fabrication of a hybrid graphene/layered double hydroxide material. Carbon 2010, 48, 4391–4396. [Google Scholar] [CrossRef]
- Sygellou, L.; Paterakis, G.; Galiotis, C.; Tasis, D. Work Function Tuning of Reduced Graphene Oxide Thin Films. J. Phys. Chem. C 2016, 120, 281–290. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, X.; Bao, H.; Yang, Z.; Ma, F. The effects of hydroxide and epoxide functional groups on the mechanical properties of graphene oxide and its failure mechanism by molecular dynamics simulations. RSC Adv. 2020, 10, 29610–29617. [Google Scholar] [CrossRef]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural Characterization of Graphene Oxide: Surface Functional Groups and Fractionated Oxidative Debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef]
| Code | Commercial GOs | Company | Particle Diameter | Thickness |
|---|---|---|---|---|
| SLH | Single Layer-H | ACS Material | 1–5 μm | 0.8–1.2 nm |
| JCP | JC05 58 powder | Williamblythe | variable | |
| SLP | Single Layer powder | Nanografi | 2 μm | 1 nm |
| FDA | Freeze dried beads | Abalonyx | 4–5 mm | |
| DPA | Dry Powder < 100 mesh | Abalonyx |
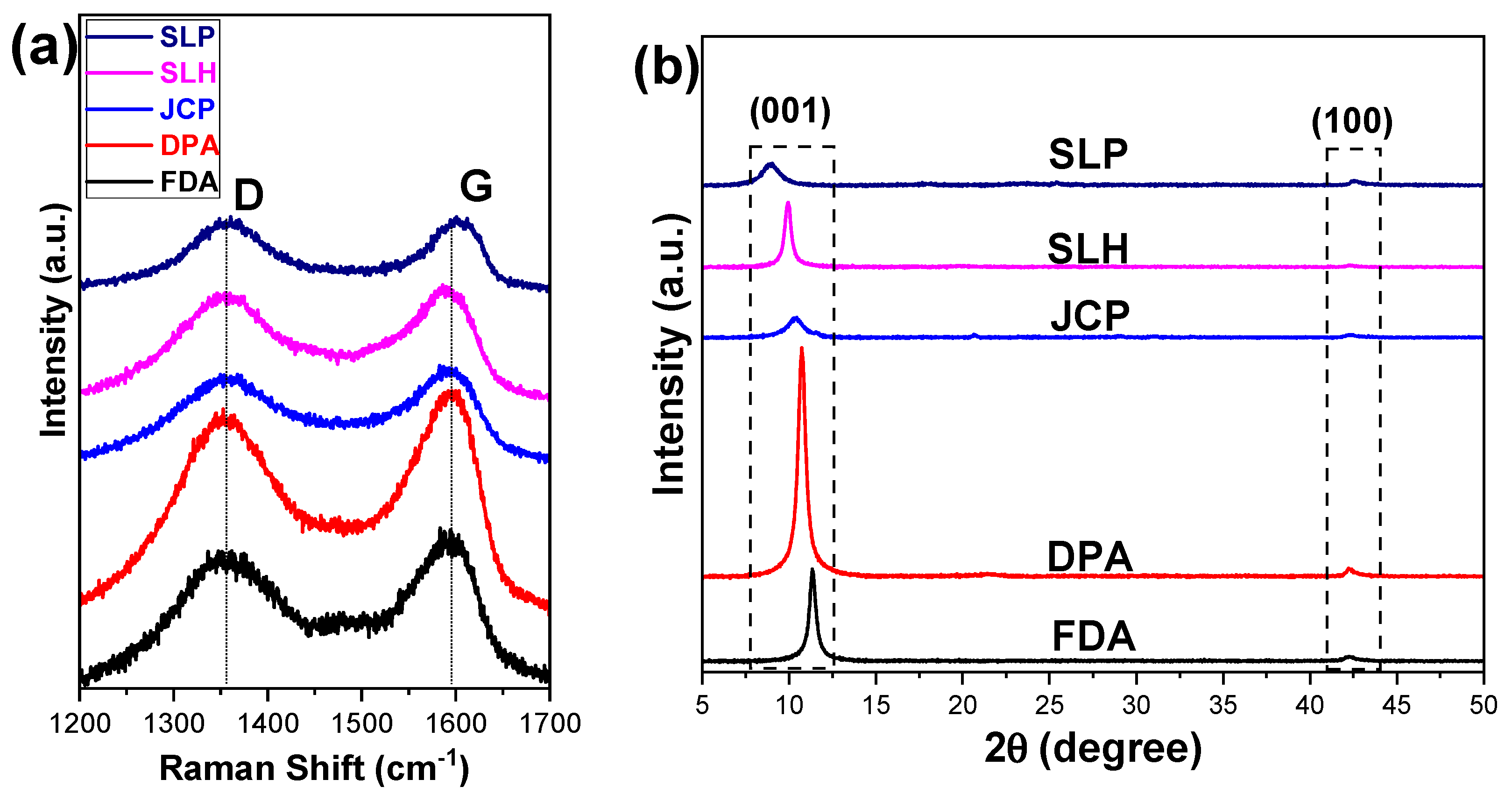
| Commercial GOs | Raman | XRD | |
|---|---|---|---|
| 2θ (°) | d (Å) | ||
| SLP | 0.97 ± 0.02 | 8.9 | 9.9 |
| SLH | 0.95 ± 0.03 | 9.9 | 8.9 |
| JCP | 0.91 ± 0.02 | 10.4 | 8.5 |
| DPA | 0.89 ± 0.02 | 10.8 | 8.2 |
| FDA | 0.86 ± 0.04 | 11.3 | 7.8 |
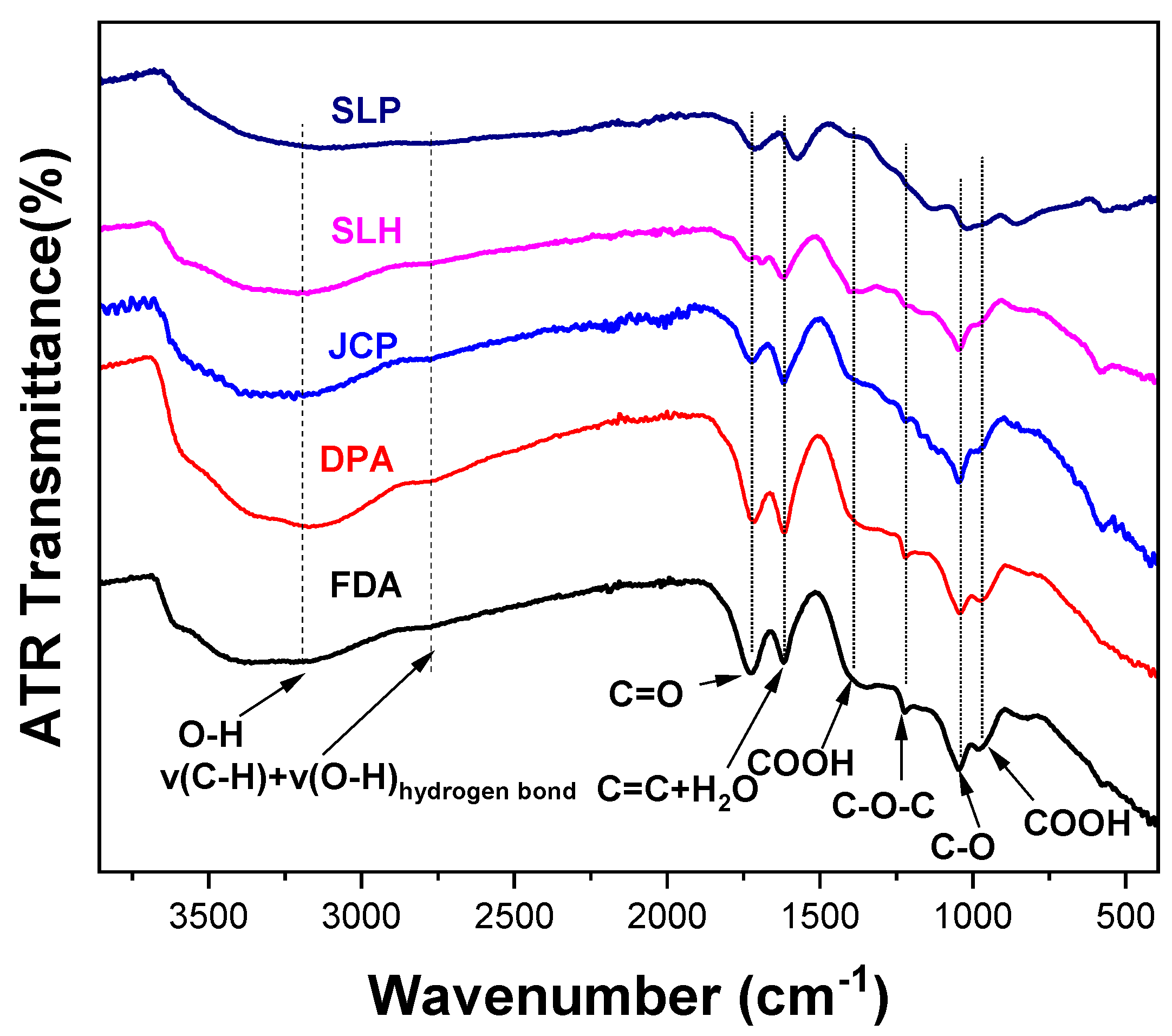
| Wavenumber (cm−1) | Assignment | Oxygen-Containing Groups |
|---|---|---|
| 3000–3500 (broad) | O-H | hydroxyls |
| 2773 | v(C-H) + v(O-H) hydrogen bond | |
| 1720 | C=O | carbonyls |
| ~1600 | H2O (1616 cm−1) | water |
| C=C (1580 cm−1) | “graphene layers” | |
| 1380 | C-OOH | carboxyls |
| 1220 | C-O-C | ethers |
| ~1170 | C-O | epoxides |
| 1040 | C-O | epoxides |
| 970 | COOH | carboxyls |
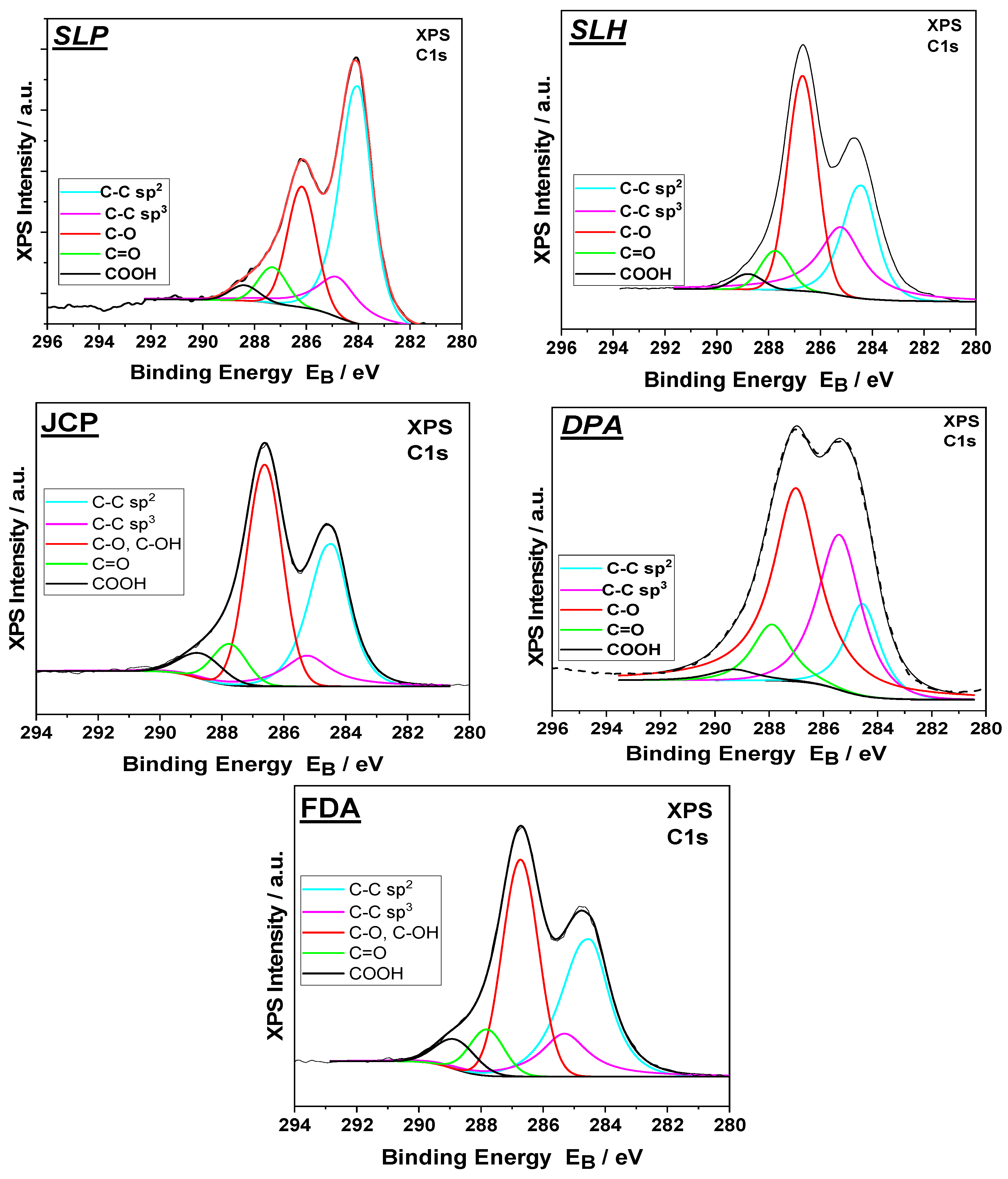
| C1s Components | Atomic Concentration | |||||||
|---|---|---|---|---|---|---|---|---|
| C-C sp2 | C-C sp3 | C-O/ C-OH | C=O Carbonyl | COOH Carboxyl | O | C | S | |
| Binding Energy (eV) | 284.4 | 285.2 | 286.7 | 287.8 | 288.8 | 532.4 | - | 168.3 |
| SLP (% Conc.) * | 52.5 | 15.8 | 22.2 | 6.7 | 2.8 | 34.0 | 56.5 | 5.8 |
| SLH (% Conc.) | 25.3 | 26.7 | 38.3 | 7.0 | 2.7 | 33.1 | 65.5 | 1.4 |
| JCP (% Conc.) | 33.3 | 11.2 | 42.2 | 7.6 | 5.8 | 32.7 | 66.0 | 1.3 |
| DPA (% Conc.) | 13.1 | 27.6 | 48.4 | 8.9 | 2.0 | 30.1 | 69.9 | - |
| FDA (% Conc.) | 32.4 | 16.9 | 36.6 | 8.5 | 5.5 | 33.2 | 69.8 | 1.0 |
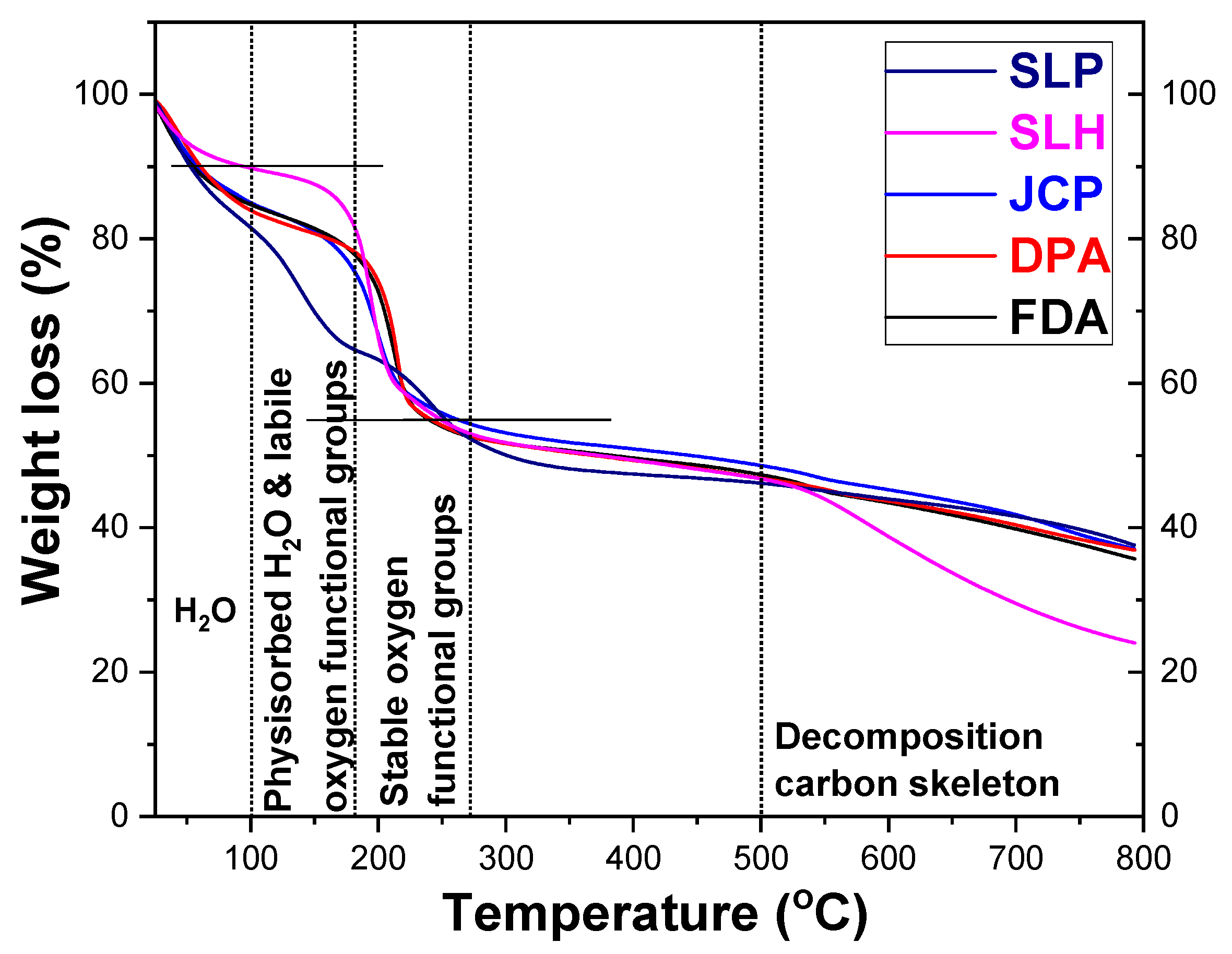
| Benchmark GOs | Raman and XRD | TGA | XPS | T-Dependent ATR | T-Dependent XRD |
|---|---|---|---|---|---|
| SLP | Higher defects and interlayer distance | Higher H2O physiosorbed | Lowest C=O content | Low -OH content | |
| SLH | Lower H2O physiosorbed | Lowest COOH content | |||
| JPC | Highest COOH content | Quite stable at high T | Workable till ~200 °C | ||
| DPA | Highest C=O content | Low -OH content | Workable till ~200 °C | ||
| FDA | Lower defects and interlayer distance | High C=O content | Stable till 170 °C and workable till ~200 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathioudakis, G.N.; Visvini, G.A.; Sygellou, L.; Soto Beobide, A.; Voyiatzis, G.A. Comparative In-Depth Investigation of Benchmark Graphene Oxides in the Perspective of Their Integration into Industrial Production Processes. Nanomaterials 2025, 15, 980. https://doi.org/10.3390/nano15130980
Mathioudakis GN, Visvini GA, Sygellou L, Soto Beobide A, Voyiatzis GA. Comparative In-Depth Investigation of Benchmark Graphene Oxides in the Perspective of Their Integration into Industrial Production Processes. Nanomaterials. 2025; 15(13):980. https://doi.org/10.3390/nano15130980
Chicago/Turabian StyleMathioudakis, Georgios N., Glykeria A. Visvini, Labrini Sygellou, Amaia Soto Beobide, and George A. Voyiatzis. 2025. "Comparative In-Depth Investigation of Benchmark Graphene Oxides in the Perspective of Their Integration into Industrial Production Processes" Nanomaterials 15, no. 13: 980. https://doi.org/10.3390/nano15130980
APA StyleMathioudakis, G. N., Visvini, G. A., Sygellou, L., Soto Beobide, A., & Voyiatzis, G. A. (2025). Comparative In-Depth Investigation of Benchmark Graphene Oxides in the Perspective of Their Integration into Industrial Production Processes. Nanomaterials, 15(13), 980. https://doi.org/10.3390/nano15130980








