Enhanced Degradation of Phenol in Aqueous Solution via Persulfate Activation by Sulfur-Doped Biochar: Insights into Catalytic Mechanisms and Structural Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Material Characterizations
3.2. Catalytic Degradation of Phenol Using the Biochar
3.2.1. Effect of Biochar Composition on Phenol’s Catalytic Degradation
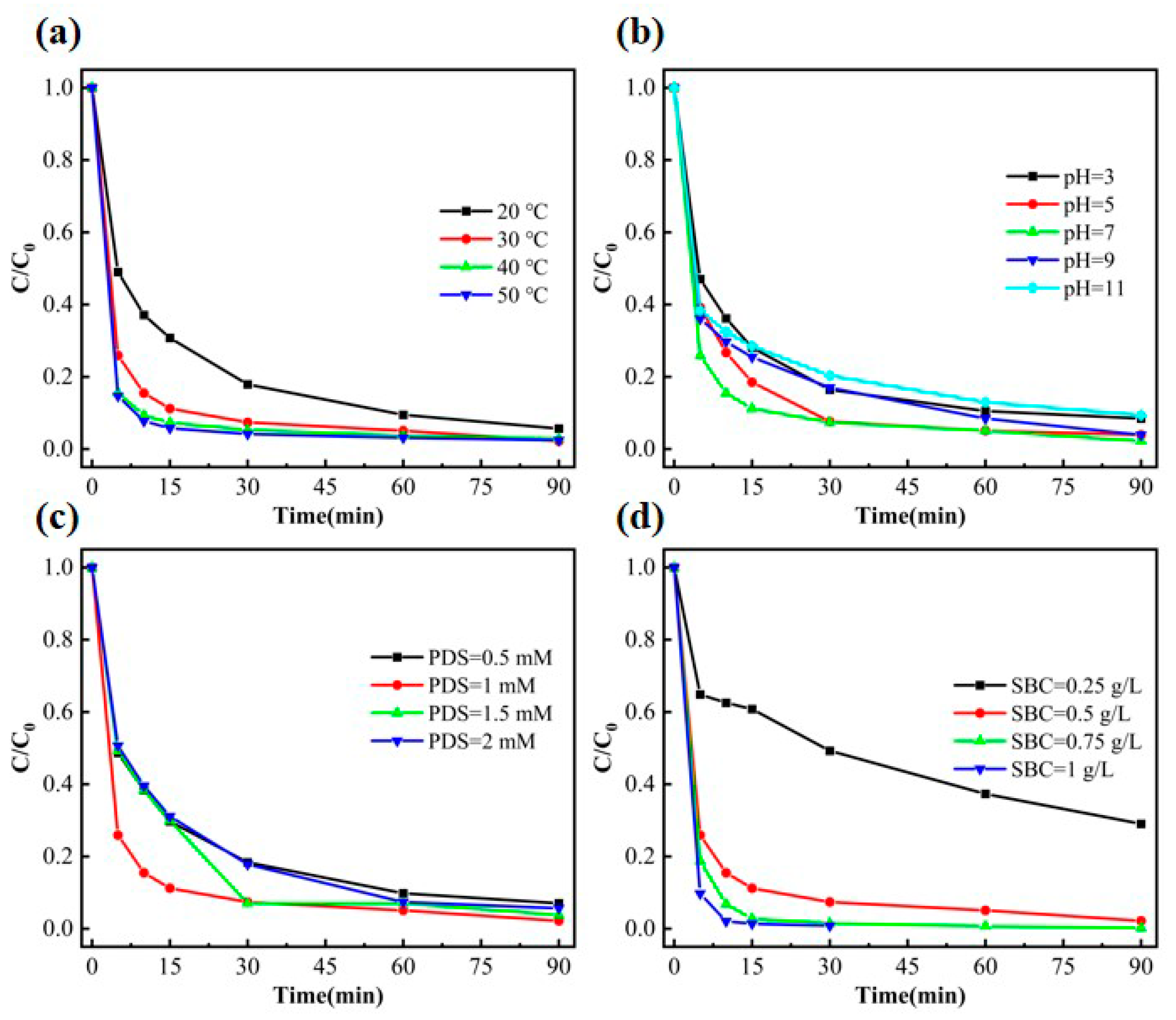
3.2.2. Effect of Varied Reaction Conditions in the PDS System on Phenol Degradation
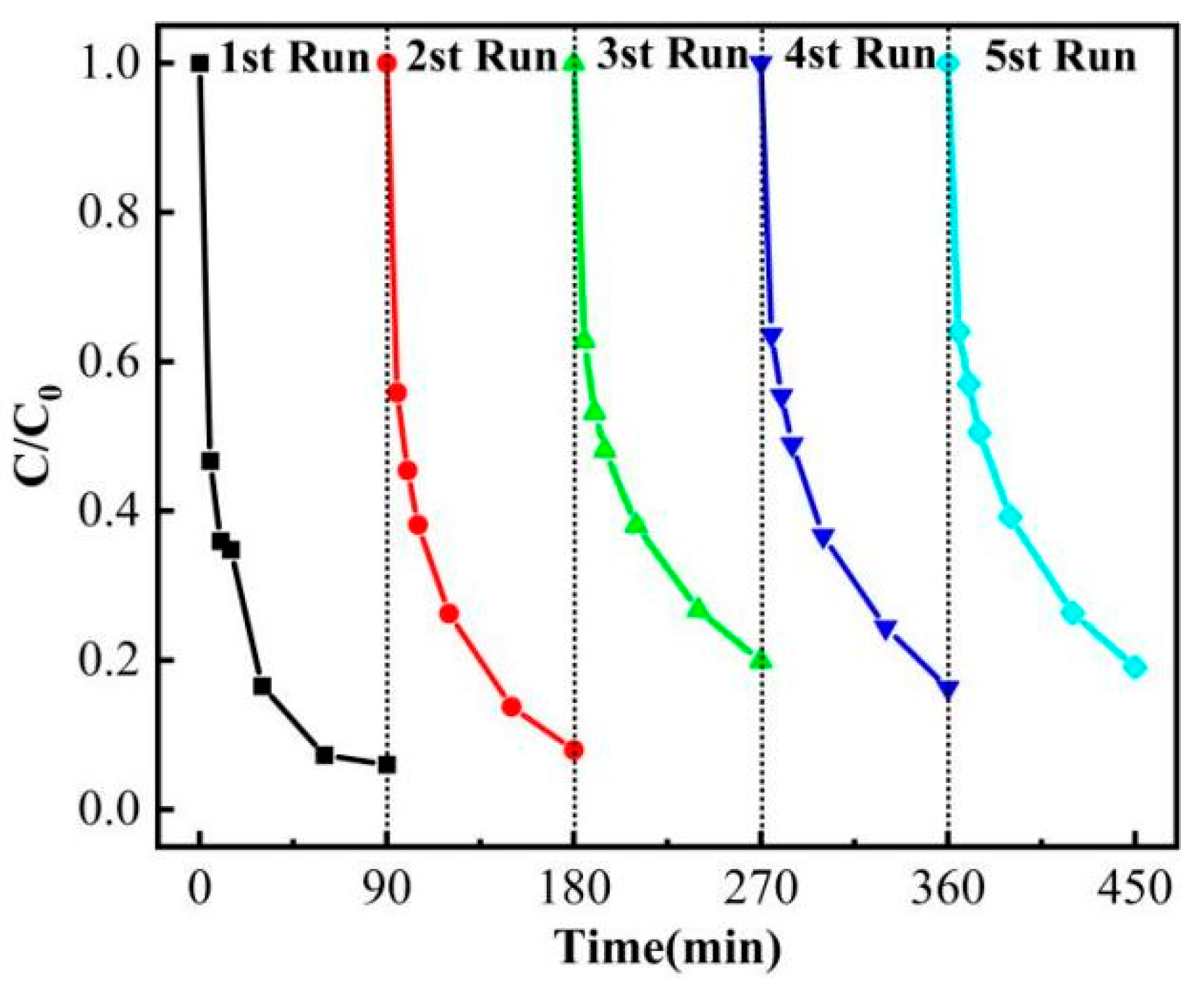
3.3. Circulation Experiment
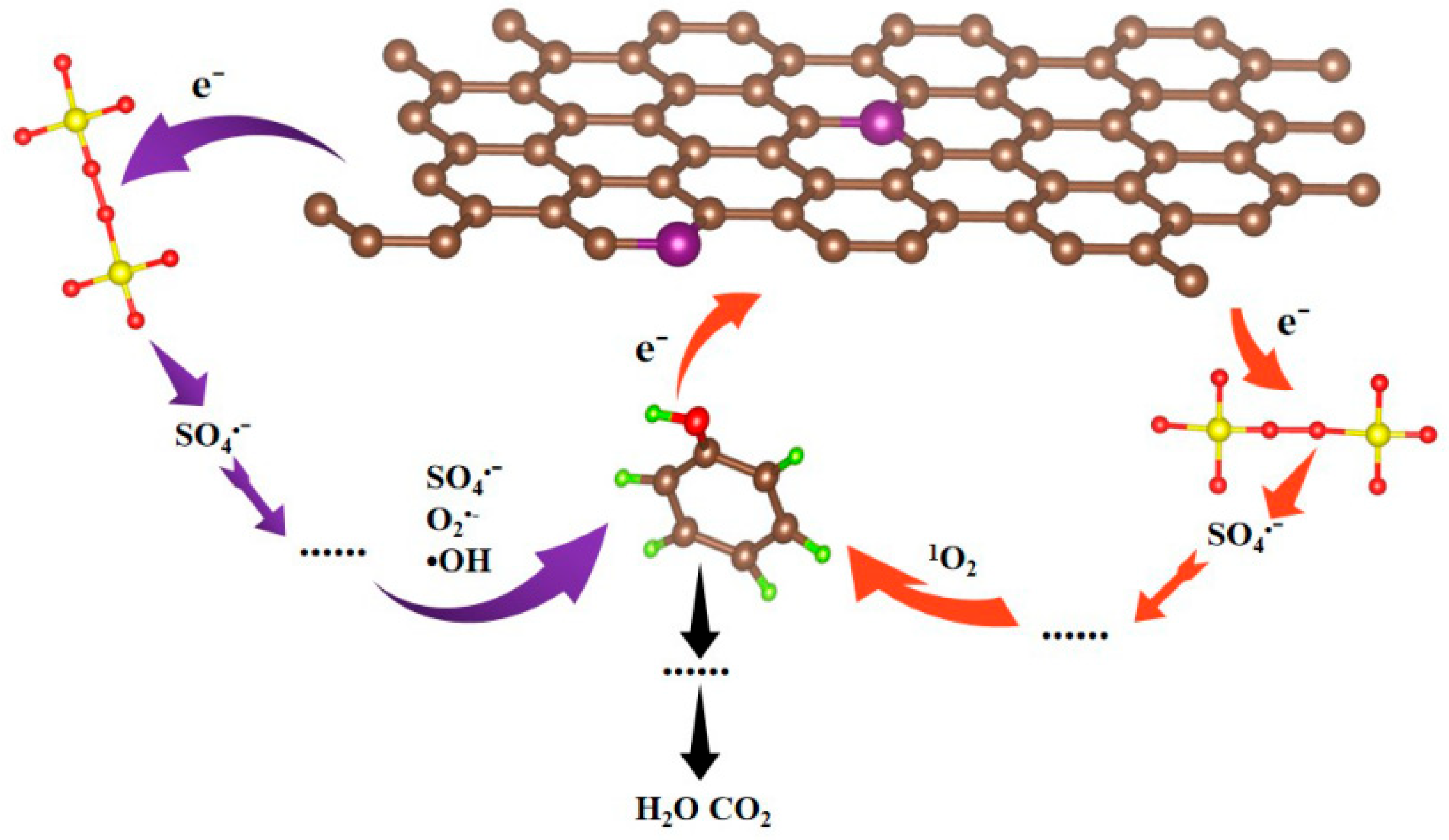
3.4. Degradation Mechanism Analysis
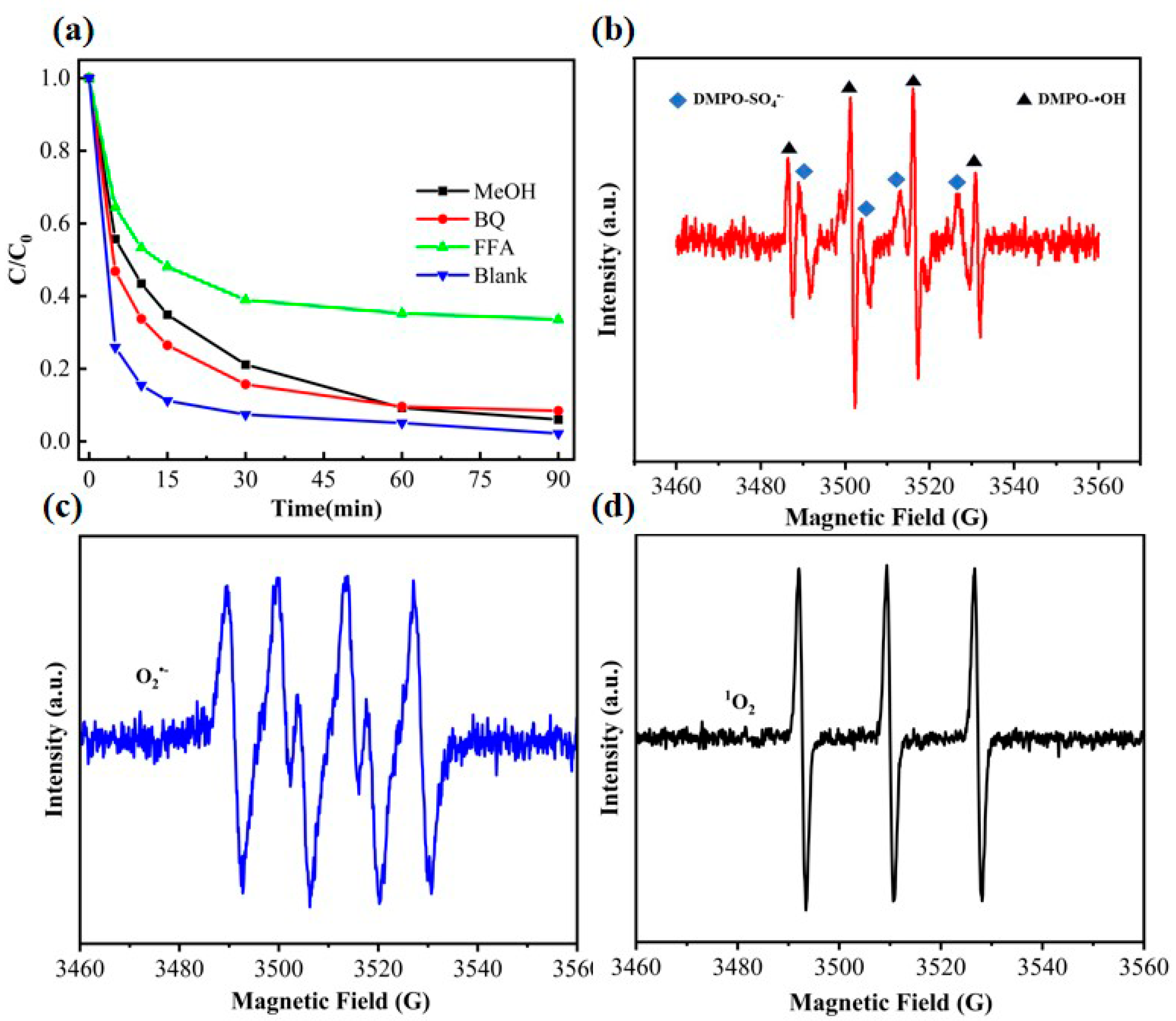
3.4.1. Radical Trapping Experiments and Electron Spin Resonance (ESR) Analysis
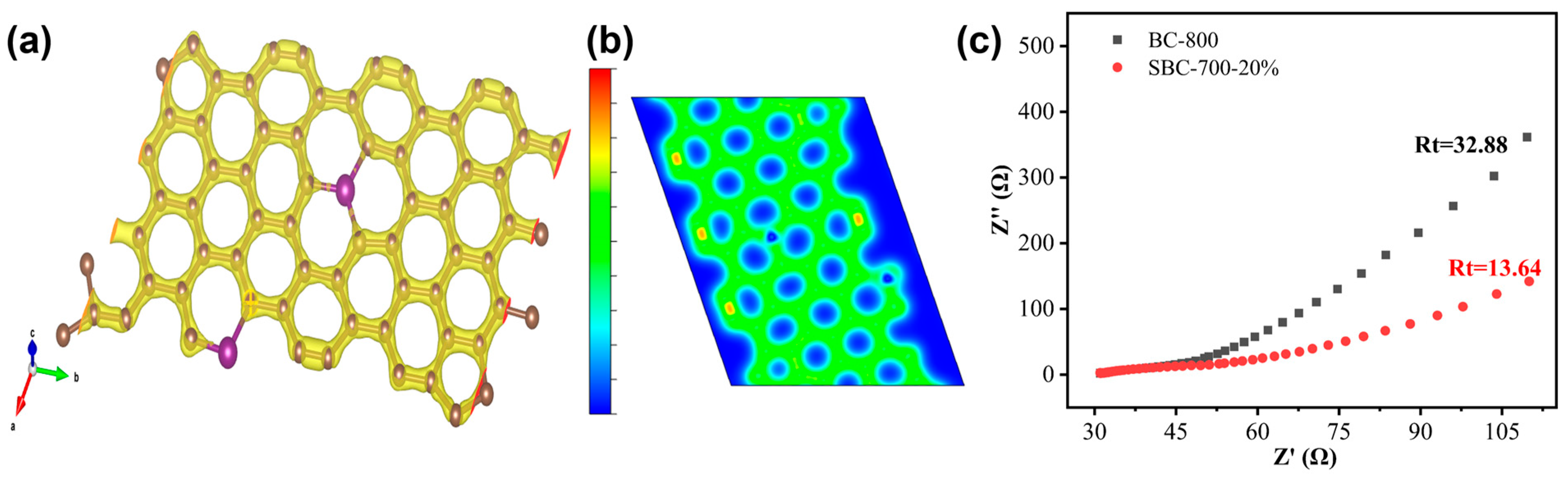
3.4.2. Electrochemical Impedance Spectroscopy (EIS) and Density Functional Theory (DFT) Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dargahi, A.; Hasani, K.; Mokhtari, S.A.; Vosoughi, M.; Moradi, M.; Vaziri, Y. Highly effective degradation of 2,4-Dichlorophenoxyacetic acid herbicide in a three-dimensional sono-electro-Fenton (3D/SEF) system using powder activated carbon (PAC)/Fe3O4 as magnetic particle electrode. J. Environ. Chem. Eng. 2021, 9, 105889. [Google Scholar] [CrossRef]
- Ma, J.; Wei, W.; Qin, G.; Jiang, L.; Wong, N.H.; Sunarso, J.; Liu, S. Electrochemical oxidation of phenol in a PtRu/NbC membrane-based catalytic nanoreactor. J. Environ. Chem. Eng. 2023, 11, 111128. [Google Scholar] [CrossRef]
- Pang, B.; Lam, S.S.; Shen, X.J.; Cao, X.F.; Liu, S.J.; Yuan, T.Q.; Sun, R.C. Valorization of technical lignin for the production of desirable resins with high substitution rate and controllable viscosity. ChemSusChem 2020, 13, 4446–4454. [Google Scholar] [CrossRef]
- Al-Sakkaf, M.K.; Basfer, I.; Iddrisu, M.; Bahadi, S.A.; Nasser, M.S.; Abussaud, B.; Drmosh, Q.A.; Onaizi, S.A. An Up-to-Date Review on the Remediation of Dyes and Phenolic Compounds from Wastewaters Using Enzymes Immobilized on Emerging and Nanostructured Materials: Promises and Challenges. Nanomaterials 2023, 13, 2152. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, D.; Yang, J.; Wang, C. Metal-organic frameworks-derived catalysts for contaminant degradation in persulfate-based advanced oxidation processes. J. Clean. Prod. 2022, 375, 134118. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Cloud point extraction of phenolic compounds from pretreated olive mill wastewater. J. Environ. Chem. Eng. 2014, 2, 1480–1486. [Google Scholar] [CrossRef]
- Sabio, E.; Gonzalez-Martin, M.L.; Ramiro, A.; Gonzalez, J.F.; Bruque, J.M.; Labajos-Broncano, L.; Encinar, J.M. Influence of the regeneration temperature on the phenols adsorption on activated carbon. J. Colloid Interface Sci. 2001, 242, 31–35. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, C.G.; Park, S.J. Adsorption of phenol on kenaf-derived biochar: Studies on physicochemical and adsorption characteristics and mechanism. Biomass Convers. Biorefin. 2024, 14, 9621–9638. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Hussain, A.; Dubey, S.K.; Kumar, V. Kinetic study for aerobic treatment of phenolic wastewater. Water Resour. Ind. 2015, 11, 81–90. [Google Scholar] [CrossRef]
- Nazos, T.T.; Ghanotakis, D.F. Biodegradation of phenol by alginate immobilized Chlamydomonas reinhardtii cells. Arch. Microbiol. 2021, 203, 5805–5816. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Kasaeian, A.; Pourfayaz, F.; Sheikhpour, M.; Wen, D. Novel ZnO-Ag/MWCNT nanocomposite for the photocatalytic degradation of phenol. Mater. Sci. Semicond. Process. 2018, 83, 175–185. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, X.R.; Liu, C.X.; Qian, X.X.; Wen, Y.R.; Yang, Q.; Sun, T.; Chang, W.Y.; Liu, X.; Chen, Z. Facile Construction of All-Solid-State Z-Scheme g-C3N4/TiO2 Thin Film for the Efficient Visible-Light Degradation of Organic Pollutant. Nanomaterials 2020, 10, 600. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.C.; Nanda, B.; Parida, K.M.; Rao, G.R. Fabrication of the mesoporous Fe@ MnO2 NPs–MCM-41 nanocomposite: An efficient photocatalyst for rapid degradation of phenolic compounds. J. Phys. Chem. C 2015, 119, 14145–14159. [Google Scholar] [CrossRef]
- Wu, Y.; Yin, Y.; Su, X.; Yi, G.; Shi, S.; Oderinde, O.; Xiao, G.; Zhang, C.; Zhang, Y. Efficiently enhanced degradation for the organic pollutants over GO/BiOI/NiWO4 with Z-scheme heterojunction and DFT studies. J. Environ. Chem. Eng. 2024, 12, 113609. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Lu, X.; Yan, B.; Li, N.; Wang, S. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Li, M.; Huang, H.; Low, J.; Gao, C.; Long, R.; Xiong, Y. Recent progress on electrocatalyst and photocatalyst design for nitrogen reduction. Small Methods 2019, 3, 1800388. [Google Scholar] [CrossRef]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of sulfate and hydroxyl radical based advanced oxidation of phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Jin, Z.; Li, Y.; Dong, H.; Xiao, S.; Xiao, J.; Chu, D.; Hou, X.; Xiang, S.; Dong, Q.; Li, L. A comparative study on the activation of persulfate by mackinawite@biochar and pyrite@biochar for sulfamethazine degradation: The role of different natural iron-sulfur minerals doping. Chem. Eng. J. 2022, 436, 129183. [Google Scholar] [CrossRef]
- Chu, D.; Dong, H.; Li, Y.; Xiao, J.; Hou, X.; Xiang, S.; Dong, Q. Sulfur or nitrogen-doped rGO supported Fe-Mn bimetal-organic frameworks composite as an efficient heterogeneous catalyst for degradation of sulfamethazine via peroxydisulfate activation. J. Hazard. Mater. 2022, 436, 129183. [Google Scholar] [CrossRef]
- Wang, M.; Wei, N.; Fu, W.; Yan, M.; Long, L.; Yao, Y.; Yin, G.; Liao, X.; Huang, Z.; Chen, X. An Efficient and Recyclable Urchin-Like Yolk–Shell Fe3O4@SiO2@Co3O4 Catalyst for Photocatalytic Water Oxidation. Catal. Lett. 2015, 145, 1067–1071. [Google Scholar] [CrossRef]
- Lemaire, J.; Buès, M.; Kabeche, T.; Hanna, K.; Simonnot, M.O. Oxidant selection to treat an aged PAH contaminated soil by in situ chemical oxidation. J. Environ. Chem. Eng. 2013, 1, 1261–1268. [Google Scholar] [CrossRef]
- Luo, H.; Fu, H.; Yin, H.; Lin, Q. Carbon materials in persulfate-based advanced oxidation processes: The roles and construction of active sites. J. Hazard. Mater. 2021, 426, 128044. [Google Scholar] [CrossRef] [PubMed]
- Han, M.Y.; Liu, Z.Y.; Huang, S.Y.; Zhang, H.X.; Yang, H.L.; Liu, Y.; Zhang, K.; Zeng, Y.S. Application of Biochar-Based Materials for Effective Pollutant Removal in Wastewater Treatment. Nanomaterials 2024, 14, 1933. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Jiang, Z.; Hu, X.; Li, X.; Wang, H.; Xiao, R. Enhanced activation of persulfate by nitric acid/annealing modified multi-walled carbon nanotubes via non-radical process. Chemosphere 2019, 220, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lin, Q.; Zhang, X.; Huang, Z.; Liu, S.; Jiang, J.; Xiao, R.; Liao, X. New insights into the formation and transformation of active species in nZVI/BC activated persulfate in alkaline solutions. Chem. Eng. J. 2018, 359, 1215–1223. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, Z.; Zeng, G.; Lai, C.; Xiao, R.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. Insight into the mechanism of persulfate activated by bone char: Unraveling the role of functional structure of biochar. Chem. Eng. J. 2020, 401, 126127. [Google Scholar] [CrossRef]
- Yu, W.; Lian, F.; Cui, G.; Liu, Z. N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 2018, 193, 8–16. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, G.; Zhong, J.; Shi, Z.; Zhu, Y.; Su, D.S.; Wang, J.; Bao, X.; Ma, D. Nitrogen-Doped sp2-Hybridized Carbon as a Superior Catalyst for Selective Oxidation. Angew. Chem. Int. Ed. 2013, 52, 2109–2113. [Google Scholar] [CrossRef]
- Ding, D.; Yang, S.; Qian, X.; Chen, L.; Cai, T. Nitrogen-doping positively whilst sulfur-doping negatively affect the catalytic activity of biochar for the degradation of organic contaminant. Appl. Catal. B-Environ. 2020, 263, 118348. [Google Scholar] [CrossRef]
- Yang, M.T.; Du, Y.; Tong, W.C.; Yip, A.C.K.; Lin, K.Y.A. Cobalt-impregnated biochar produced from CO2-mediated pyrolysis of Co/lignin as an enhanced catalyst for activating peroxymonosulfate to degrade acetaminophen. Chemosphere 2019, 226, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Gao, M.; Guo, X.; Ai, F.; Wang, Z. Enhanced degradation performance of bisphenol M using peroxymonosulfate activated by zero-valent iron in aqueous solution: Kinetic study and product identification. Chemosphere 2019, 221, 314–323. [Google Scholar] [CrossRef]
- Liu, S.; Lai, C.; Li, B.; Zhang, C.; Zhang, M.; Huang, D.; Qin, L.; Yi, H.; Liu, X.; Huang, F.; et al. Role of radical and non-radical pathway in activating persulfate for degradation of p-nitrophenol by sulfur-doped ordered mesoporous carbon. Chem. Eng. J. 2020, 384, 123304. [Google Scholar] [CrossRef]
- Restivo, J.; Rocha, R.P.; Silva, A.M.T.; Órfão, J.J.M.; Pereira, M.F.R.; Figueiredo, J.L. Catalytic performance of heteroatom-modified carbon nanotubes in advanced oxidation processes, Chin. J. Catal. 2014, 35, 896–905. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Tade, M.O.; Ang, H.M.; Wang, S. Nitrogen- and Sulfur-Codoped Hierarchically Porous Carbon for Adsorptive and Oxidative Removal of Pharmaceutical Contaminants. ACS Appl. Mater. Interfaces 2016, 8, 7184–7193. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Sun, H.; Indrawirawan, S.; Wang, Y.; Kang, J.; Liang, F.; Zhu, Z.H.; Wang, S. Nitrogen-doped graphene for generation and evolution of reactive radicals by metal-free catalysis. ACS Appl. Mater. Interfaces 2015, 7, 4169–4178. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tan, Y.; Bai, R.; Li, Y.; Zhao, L.; Zhuang, X.; Wang, Y.; Chen, Z.; Li, P.; You, X.; et al. Effect of Melt Superheat Treatment on Solidification Behavior and Microstructure of New Ni-Co Based Superalloy. J. Mater. Res. Technol. 2021, 15, 4970–4980. [Google Scholar] [CrossRef]
- Yun, E.T.; Lee, J.H.; Kim, J.; Park, H.D.; Lee, J. Identifying the Nonradical Mechanism in the Peroxymonosulfate Activation Process: Singlet Oxygenation Versus Mediated Electron Transfer. Environ. Sci. Technol. 2018, 52, 7032–7042. [Google Scholar] [CrossRef]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon. 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Ionothermal synthesis of sulfur-doped porous carbons hybridized with graphene as superior anode materials for lithium-ion batteries. Chem. Commun. 2012, 4886, 10663–10665. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.A.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction. ACS Nano 2011, 6, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Poh, H.L.; Šimek, P.; Sofer, Z.; Pumera, M. Sulfur-Doped Graphene via Thermal Exfoliation of Graphite Oxide in H2S, SO2, or CS2 Gas. ACS Nano 2013, 7, 5262–5272. [Google Scholar] [CrossRef]
- Pu, M.; Ma, Y.; Wan, J.; Wang, Y.; Huang, M.; Chen, Y. Fe/S doped granular activated carbon as a highly active heterogeneous persulfate catalyst toward the degradation of Orange G and diethyl phthalate. J. Colloid Interface Sci. 2013, 418, 330–337. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, H.; Zhang, Y.; Korshin, G.V.; Yang, B. Insights into the mechanism of nonradical reactions of persulfate activated by carbon nanotubes: Activation performance and structure-function relationship. Water Res. 2019, 157, 406–414. [Google Scholar] [CrossRef]
- Enoki; Fujii, S.; Takai, K. Zigzag and armchair edges in graphene. Carbon 2012, 50, 3141–3145. [Google Scholar] [CrossRef]
- Frank, B.; Zhang, J.; Blume, R.; Schlögl, R.; Su, D.S. Heteroatoms Increase the Selectivity in Oxidative Dehydrogenation Reactions on Nanocarbons. Angew. Chem. Int. Ed. 2009, 48, 6913–6917. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wu, J.; Zhao, Z.; Wang, X.; Dai, H.; Li, Y.; Wei, Y.; Xu, G.; Hu, F. High efficiency degradation of tetracycline by peroxymonosulfate activated with Fe/NC catalysts: Performance, intermediates, stability and mechanism. Environ. Res. 2021, 205, 112538. [Google Scholar] [CrossRef]
- Gong, Y.S.; Wang, Y.; Lin, N.P.; Wang, R.T.; Wang, M.D.; Zhang, X.D. Iron-based materials for simultaneous removal of heavy metal(loid)s and emerging organic contaminants from the aquatic environment: Recent advances and perspectives. Environ. Pollut. 2022, 299, 118871. [Google Scholar] [CrossRef]
- Xu, Y.S.; Li, S.T.; He, H.W.; Song, Y.X.; Xia, Q.; Peng, W.C. Aminated N-doped Graphene Hydrogel for Long-term Catalytic Oxidation in Strong Acidic Environment. J. Hazard. Mater. 2020, 401, 123742. [Google Scholar] [CrossRef]
- Li, N.; Sheng, G.P.; Lu, Y.Z.; Zeng, R.J.; Yu, H.Q. Removal of antibiotic resistance genes from wastewater treatment plant effluent by coagulation. Water Res. 2017, 111, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Q.; Ji, G.Z.; Li, A.M. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2021, 429, 132387. [Google Scholar] [CrossRef]
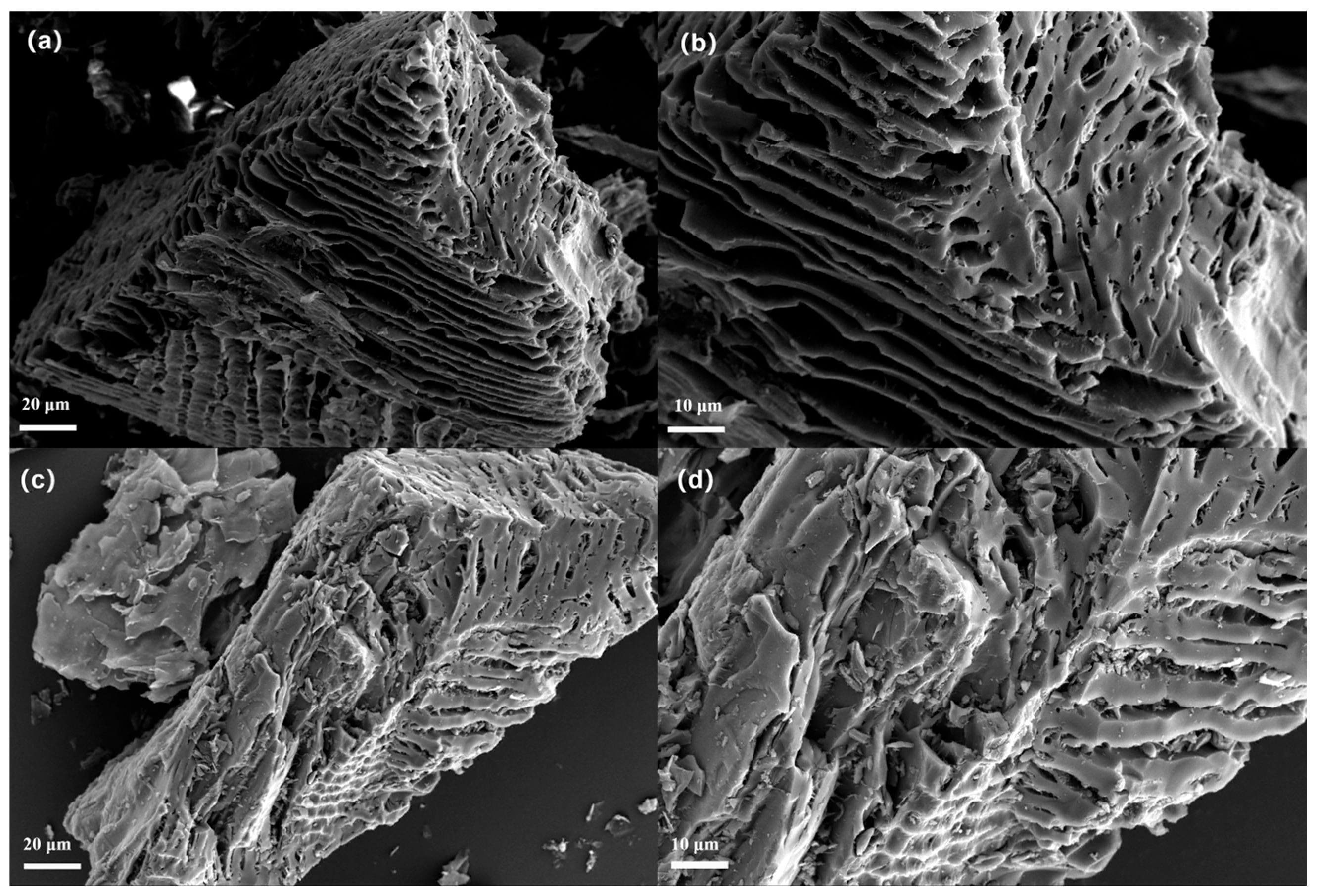
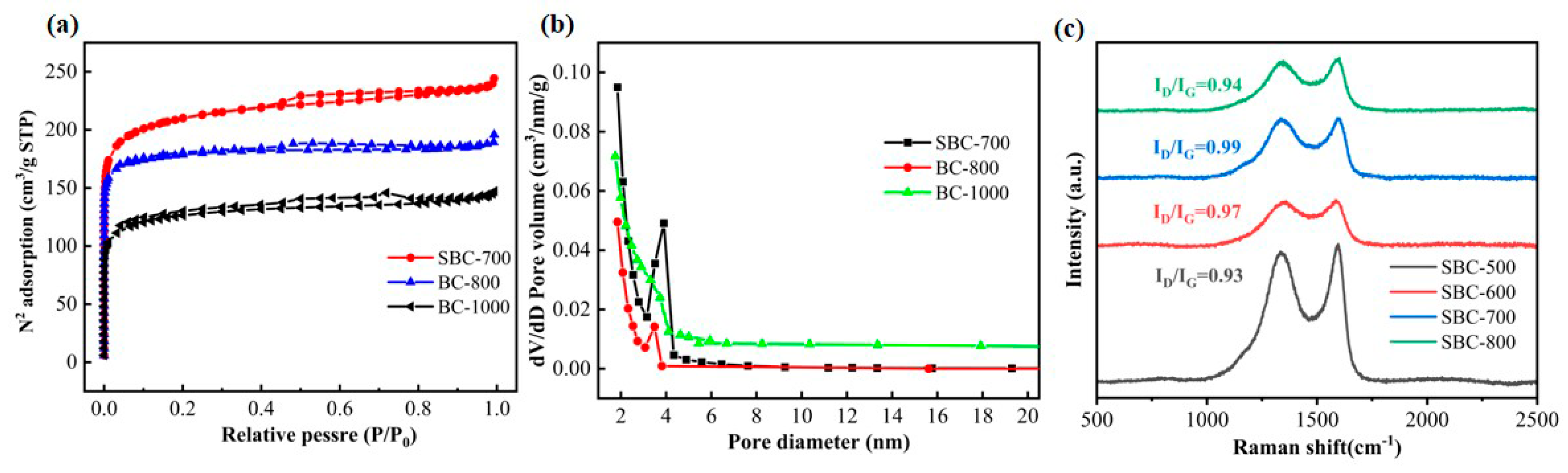
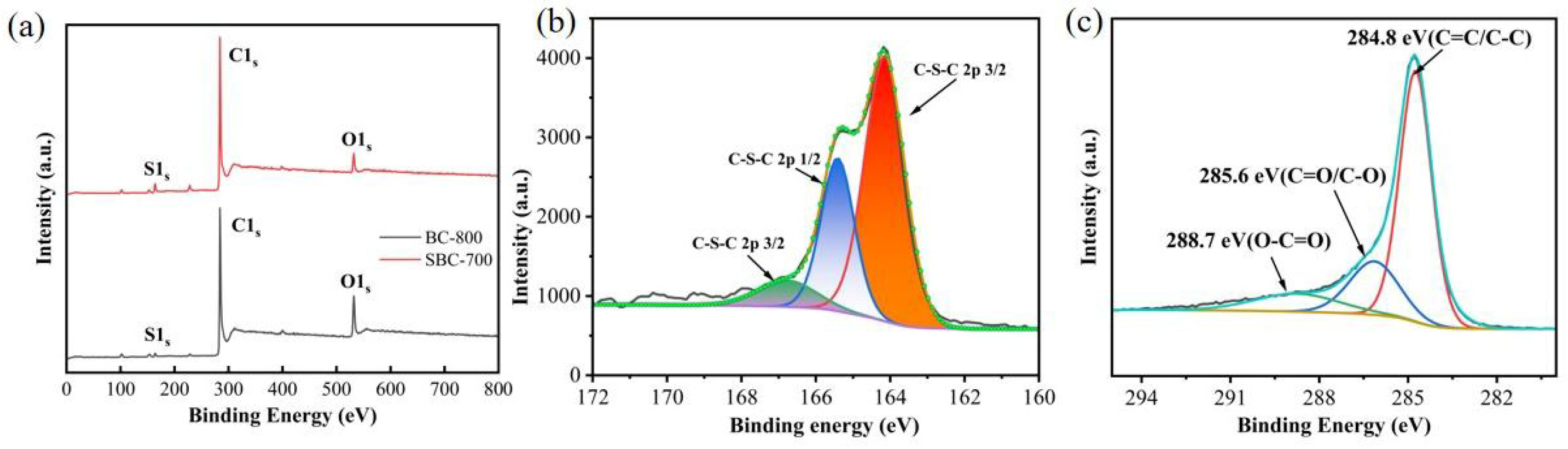
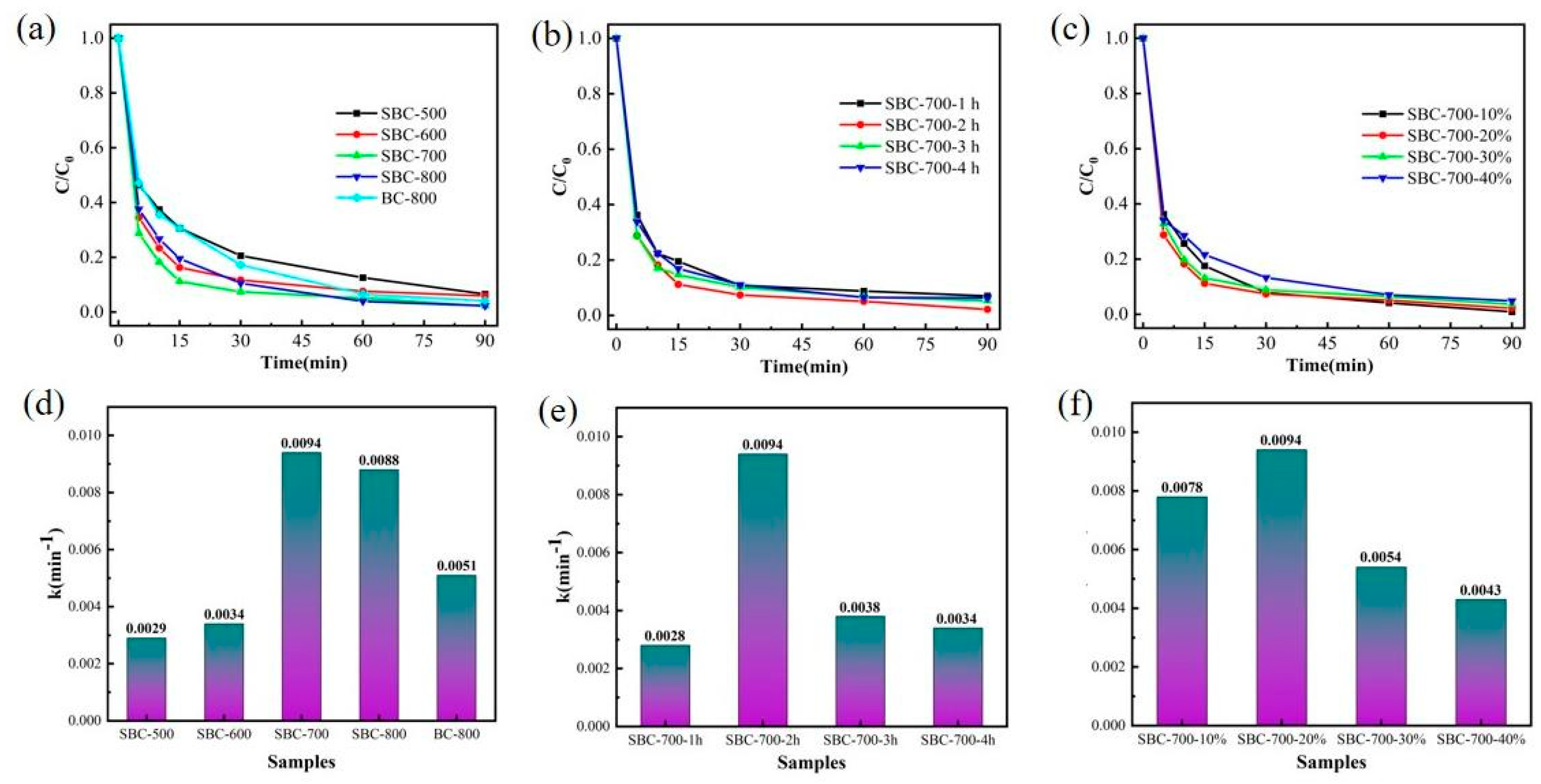
| Sample | Specific Surface Area (m2/g) | Mesopore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| SBC-500 | 711 | 0.25 | 1.79 |
| SBC-600 | 718 | 0.26 | 1.74 |
| SBC-700 | 756 | 0.16 | 1.86 |
| SBC-800 | 730 | 0.33 | 2.91 |
| BC-800 | 706 | 0.30 | 3.10 |
| Element. | SBC-700 | BC-800 |
|---|---|---|
| C (at%) | 88.15 | 86.37 |
| O (at%) | 5.2 | 9.83 |
| S (at%) | 3.15 | 1.04 |
| N (at%) | 2.25 | 1.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Kou, L.; Li, C.; Xu, B.; Wu, Y. Enhanced Degradation of Phenol in Aqueous Solution via Persulfate Activation by Sulfur-Doped Biochar: Insights into Catalytic Mechanisms and Structural Properties. Nanomaterials 2025, 15, 979. https://doi.org/10.3390/nano15130979
Wang G, Kou L, Li C, Xu B, Wu Y. Enhanced Degradation of Phenol in Aqueous Solution via Persulfate Activation by Sulfur-Doped Biochar: Insights into Catalytic Mechanisms and Structural Properties. Nanomaterials. 2025; 15(13):979. https://doi.org/10.3390/nano15130979
Chicago/Turabian StyleWang, Guanyu, Lihong Kou, Chenghao Li, Bing Xu, and Yuanfeng Wu. 2025. "Enhanced Degradation of Phenol in Aqueous Solution via Persulfate Activation by Sulfur-Doped Biochar: Insights into Catalytic Mechanisms and Structural Properties" Nanomaterials 15, no. 13: 979. https://doi.org/10.3390/nano15130979
APA StyleWang, G., Kou, L., Li, C., Xu, B., & Wu, Y. (2025). Enhanced Degradation of Phenol in Aqueous Solution via Persulfate Activation by Sulfur-Doped Biochar: Insights into Catalytic Mechanisms and Structural Properties. Nanomaterials, 15(13), 979. https://doi.org/10.3390/nano15130979







