Biological Impact of True-to-Life PET and Titanium-Doped PET Nanoplastics on Human-Derived Monocyte (THP-1) Cells
Abstract
1. Introduction
2. Material and Methods
2.1. Particle Source and Characteristics
2.2. Cell Culture
2.3. Cell Viability
2.4. Internalization Studies
2.4.1. Flow Cytometry Fluorescent Particle Detection
2.4.2. Internalization Kinetics and Cell Complexity Analysis
2.4.3. Confocal Microscopic Imaging
2.4.4. Scanning and Transmission Electron Microscopy
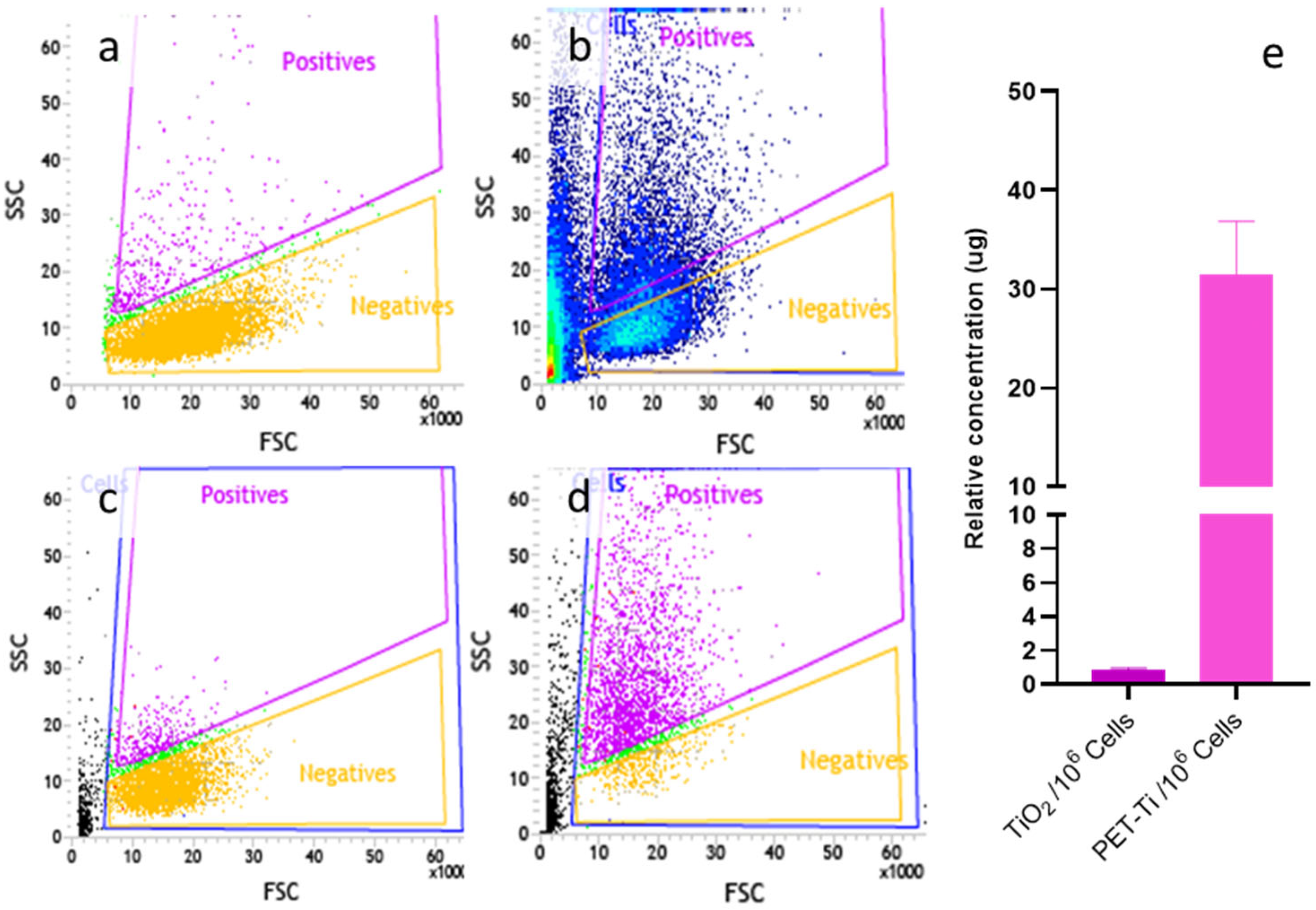
2.5. Cell Sorting and Titanium Quantification
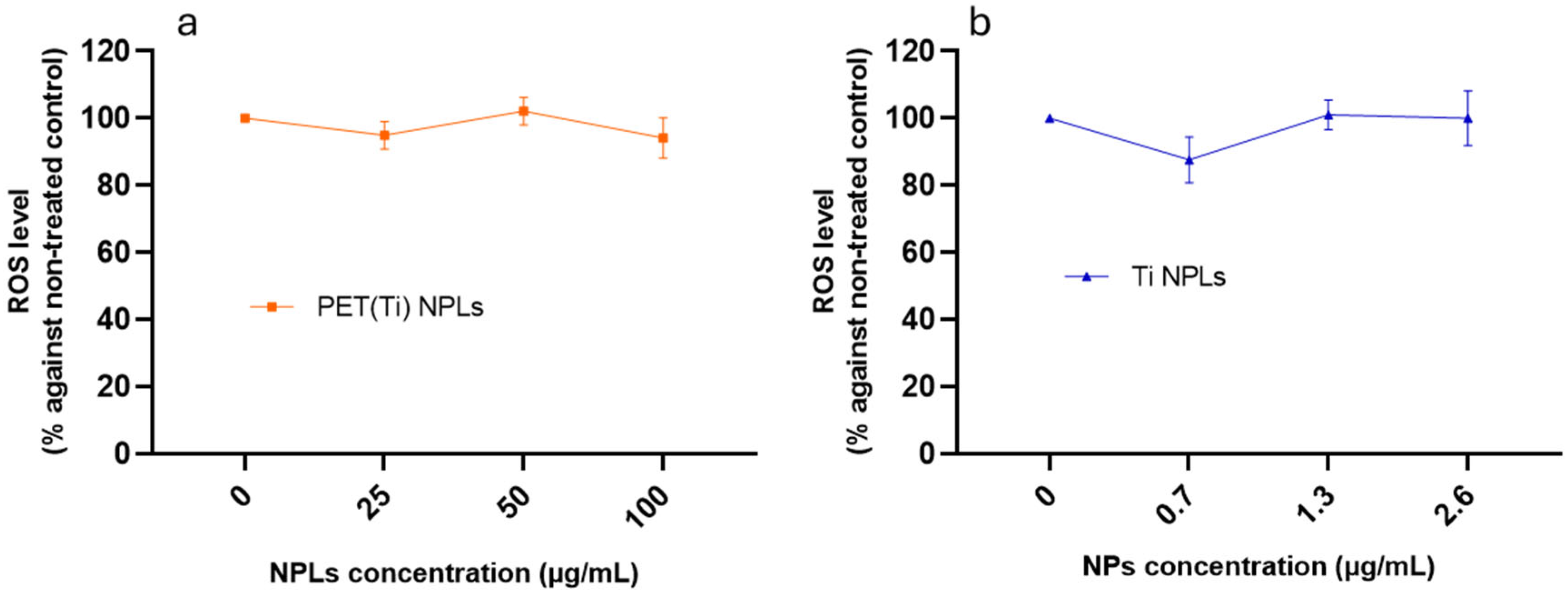
2.6. Effects on ROS Levels
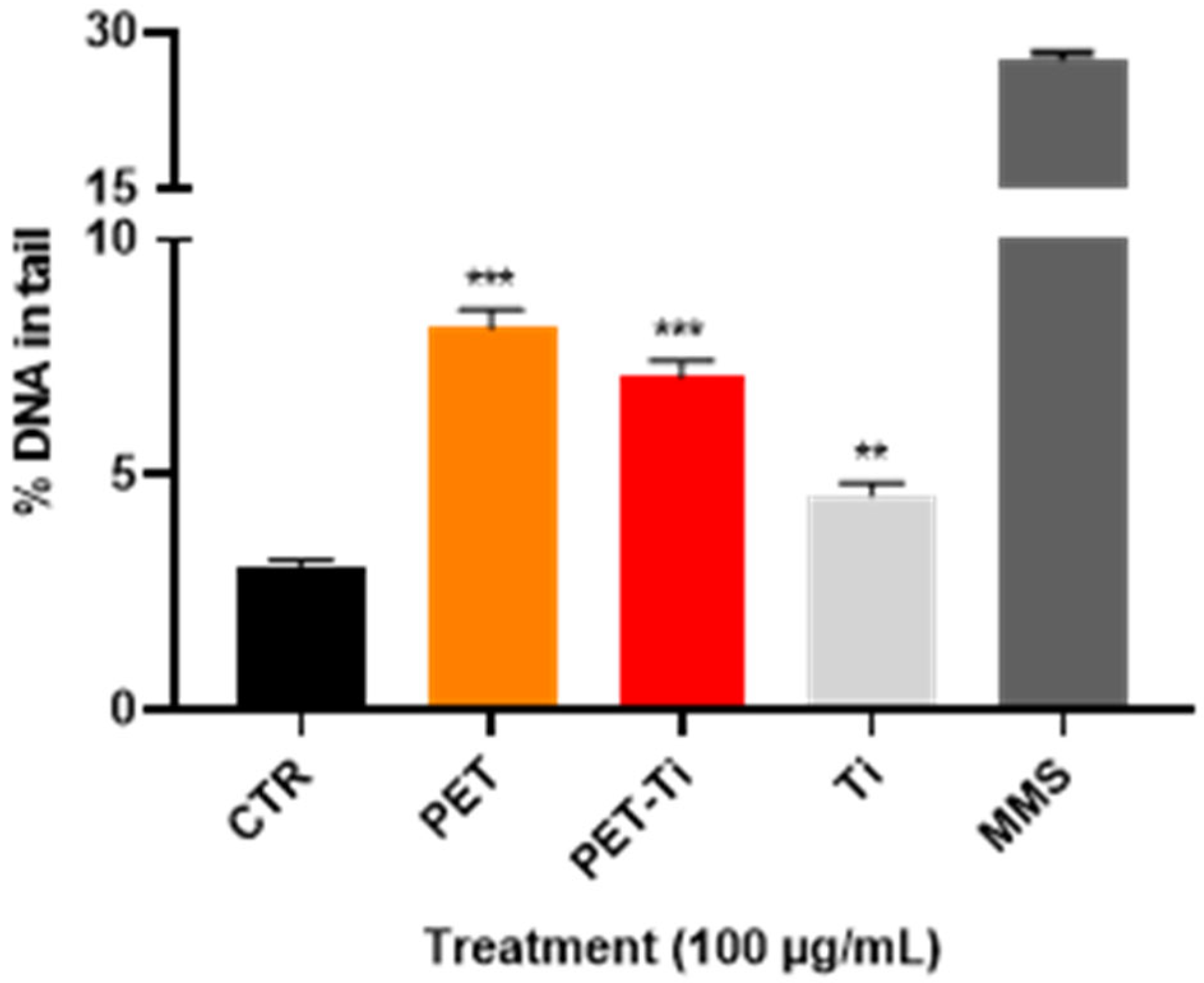
2.7. Genotoxicity
3. Results and Discussion
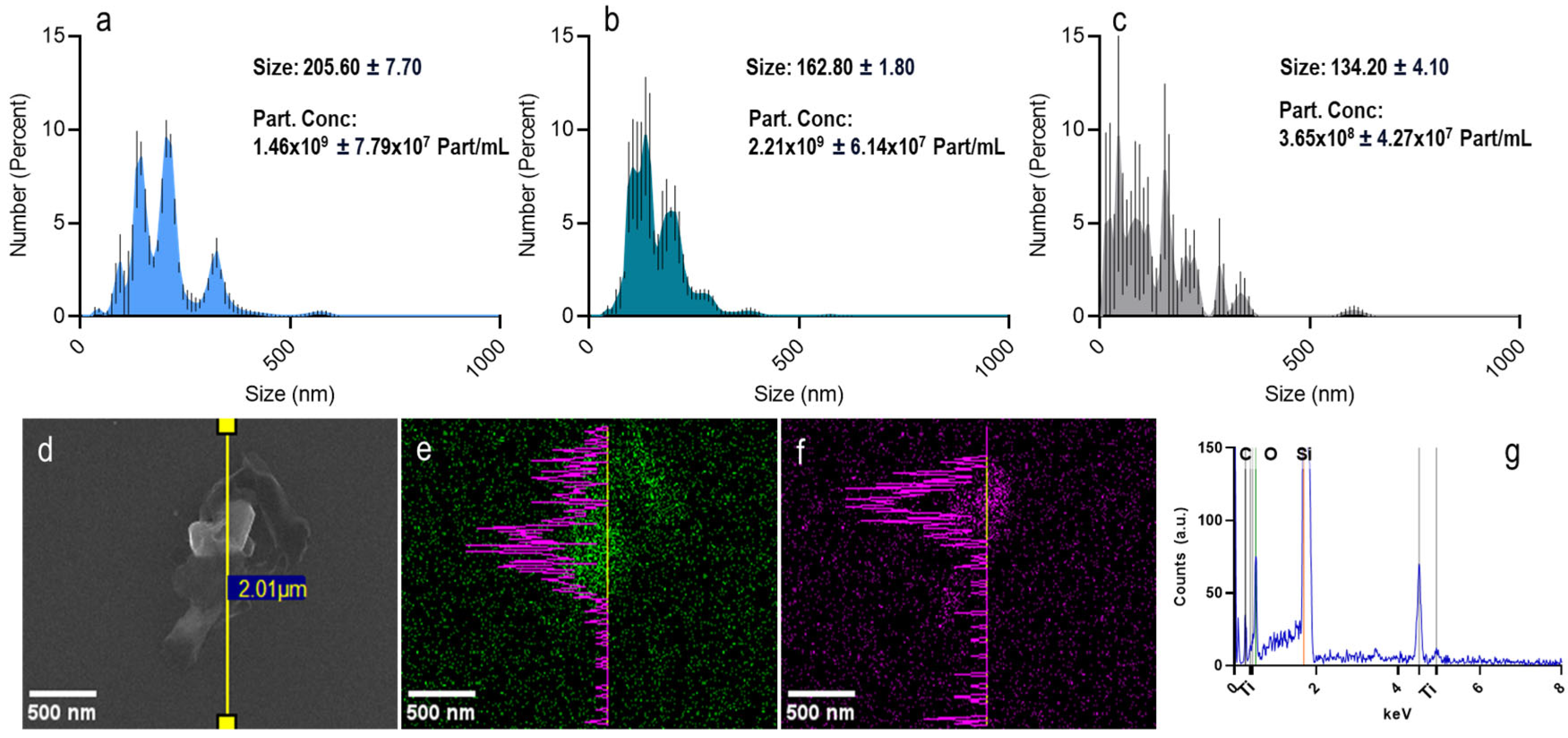
3.1. Nanoparticle Characteristics
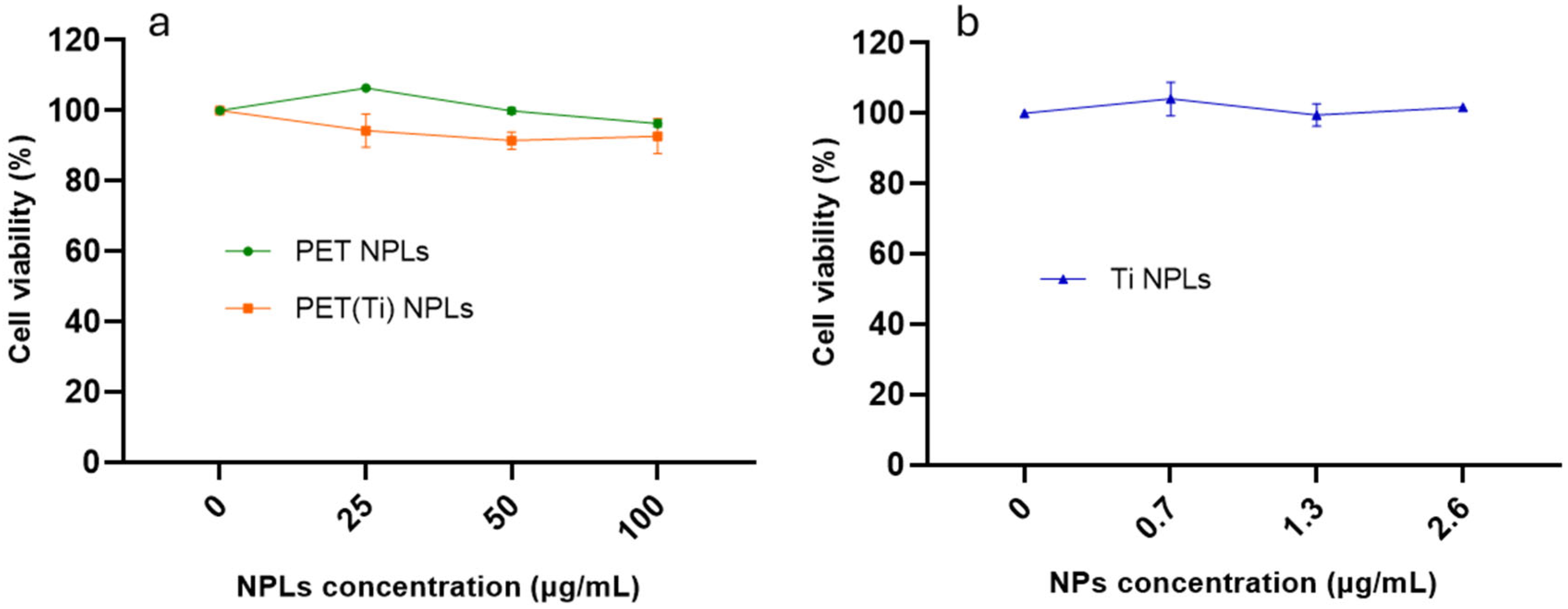
3.2. NPL Cytotoxicity
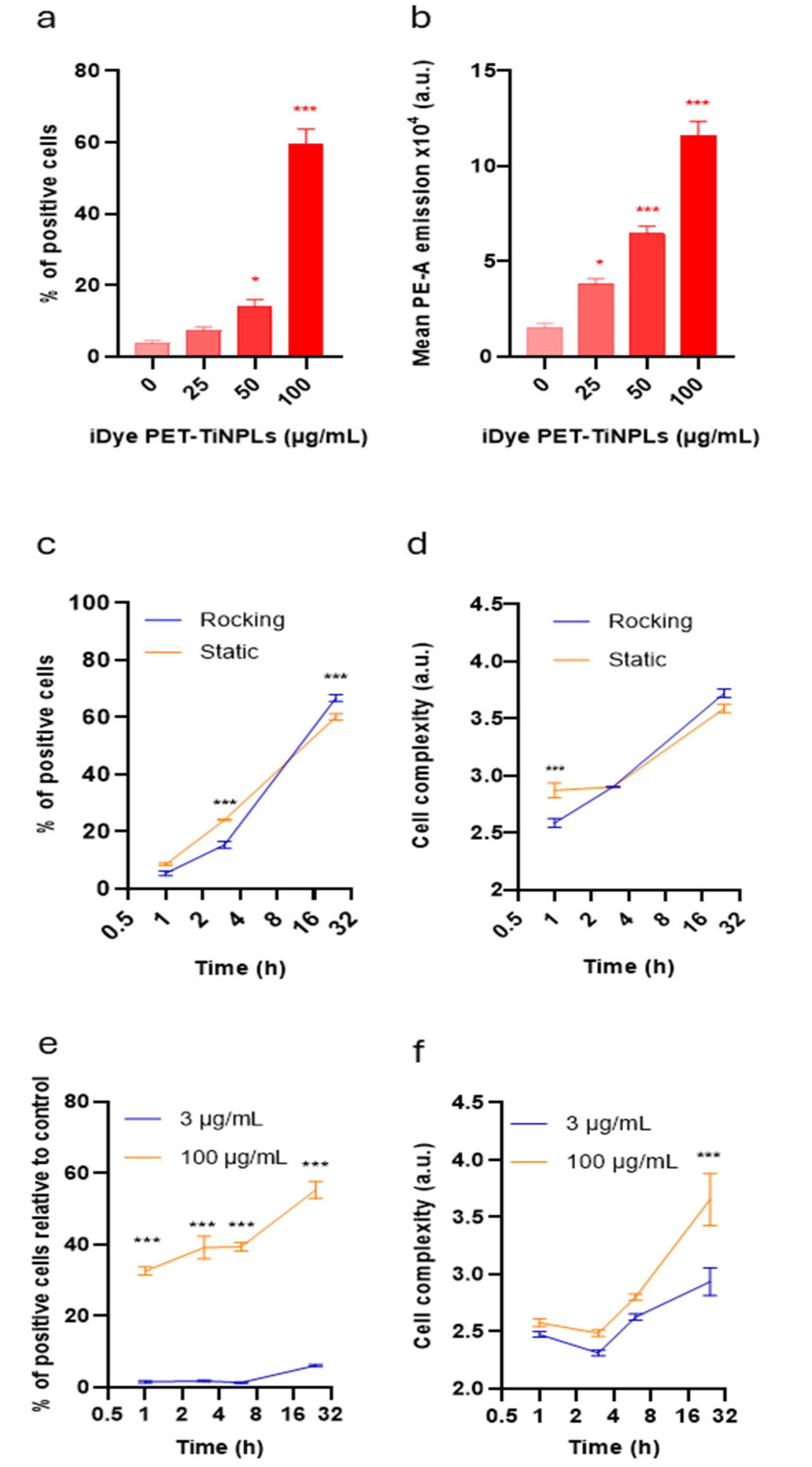
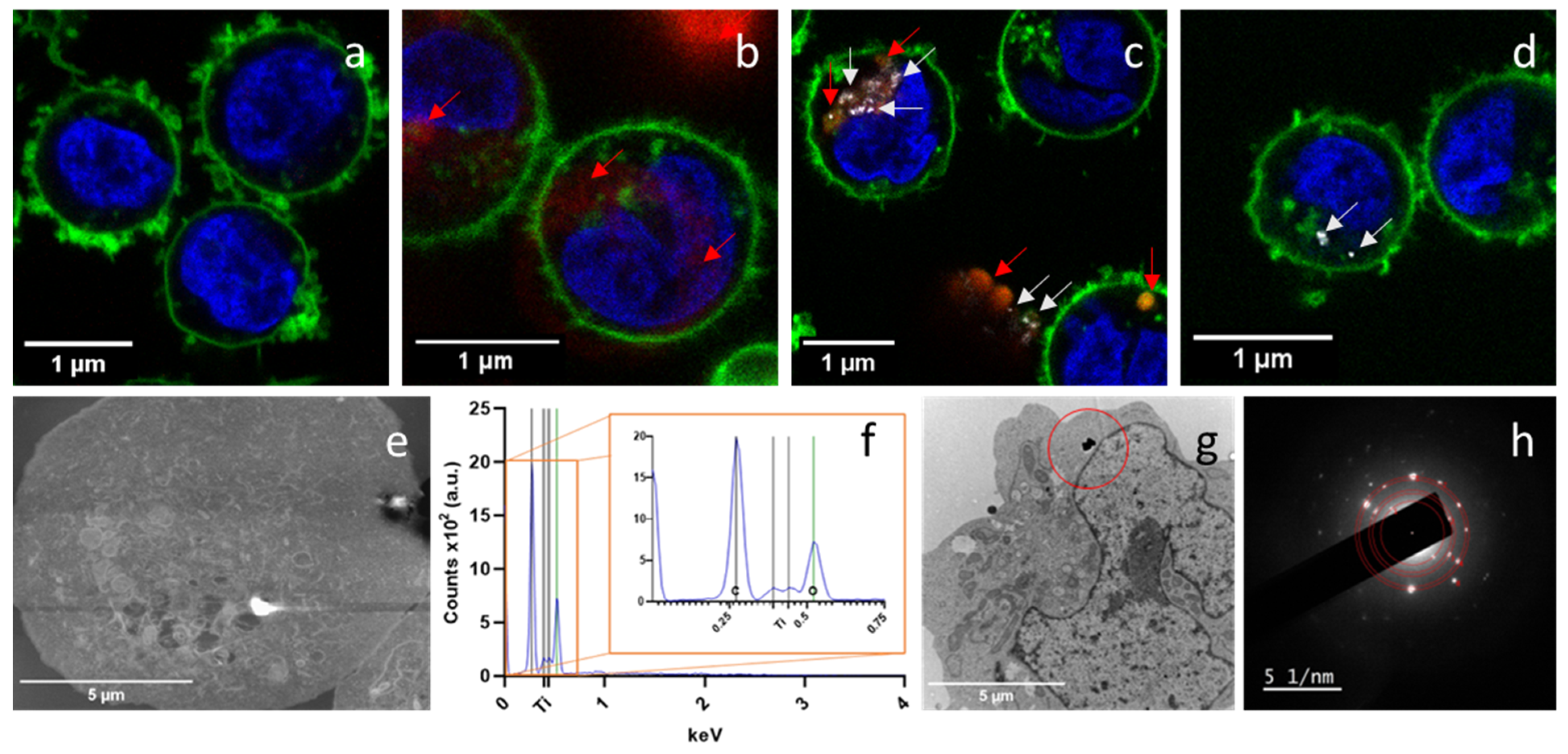
3.3. Internalization of PET(Ti)-NPLs
3.4. Cell Sorting and Mass Spectroscopy
3.5. Reactive Oxygen Species Related to PET(Ti)-NPL and TiO2-NP Exposure
3.6. DNA Damage Induction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, T.; Wang, J. Exposure sources and pathways of micro- and nanoplastics in the environment, with emphasis on potential effects in humans: A systematic review. Integr. Environ. Assess. Manag. 2023, 19, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; Mcgonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef]
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef]
- Villacorta, A.; Rubio, L.; Alaraby, M.; López-Mesas, M.; Fuentes-Cebrian, V.; Moriones, O.H.; Marcos, R.; Hernández, A. A new source of representative secondary PET nanoplastics. Obtention, characterization, and hazard evaluation. J. Hazard. Mater. 2022, 439, 129593. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Barguilla, I.; Domenech, J.; Marcos, R.; Hernández, A. Biological effects, including oxidative stress and genotoxic damage, of polystyrene nanoparticles in different human hematopoietic cell lines. J. Hazard. Mater. 2020, 398, 122900. [Google Scholar] [CrossRef]
- Landrigan, P.J. Plastics, fossil carbon, and the heart. N. Engl. J. Med. 2024, 390, 948–950. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.L.; Savanagouder, J.; de Almeida Monteiro Melo Ferraz, M.; Noonan, M.J. The need for environmentally realistic studies on the health effects of terrestrial microplastics. Microplast. Nanoplast. 2023, 3, 11. [Google Scholar] [CrossRef]
- Marchesi, C.; Rani, M.; Federici, S.; Alessandri, I.; Vassalini, I.; Ducoli, S.; Borgese, L.; Zacco, A.; Núñez-Delgado, A.; Bontempi, E.; et al. Quantification of ternary microplastic mixtures through an ultra-compact near-infrared spectrometer coupled with chemometric tools. Environ. Res. 2023, 216 Pt 3, 114632. [Google Scholar] [CrossRef]
- Villacorta, A.; Vela, L.; Morataya-Reyes, M.; Llorens-Chiralt, R.; Rubio, L.; Alaraby, M.; Marcos, R.; Hernández, A. Titanium-doped PET nanoplastics of environmental origin as a true-to-life model of nanoplastic. Sci. Total Environ. 2023, 880, 163151. [Google Scholar] [CrossRef]
- Alaraby, M.; Villacorta, A.; Abass, D.; Hernández, A.; Marcos, R. Titanium-doped PET nanoplastics, from opaque milk bottle degradation, as a model of environmental true-to-life nanoplastics. Hazardous effects on Drosophila. Environ. Poll. 2024, 341, 122968. [Google Scholar] [CrossRef]
- Nanogenotox. 2011. Available online: http://www.nanogenotox.eu/files/PDF/Deliverables/nanogenotox%20 deliverable%203_ wp4_%20dispersion%20protocol.pdf (accessed on 4 March 2025).
- Villacorta, A.; Cazorla-Ares, C.; Fuentes-Cebrian, V.; Valido, I.H.; Vela, L.; Carrillo-Navarrete, F.; Morataya-Reyes, M.; Mejia-Carmona, K.; Pastor, S.; Velázquez, A.; et al. Fluorescent labeling of micro/nanoplastics for biological applications with a focus on “true-to-life” tracking. J. Hazard. Mater. 2024, 476, 135134. [Google Scholar] [CrossRef] [PubMed]
- Andanson, J.M.; Kazarian, S.G. In situ ATR-FTIR spectroscopy of poly(ethylene terephthalate) subjected to high-temperature methanol. Macromol. Symp. 2008, 265, 195–204. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. The thermal analysis of poly(ethylene terephthalate) by FTIR spectroscopy. Thermochim. Acta 2013, 552, 123–130. [Google Scholar] [CrossRef]
- Dos Santos Pereira, A.P.; Da Silva, M.H.P.; Lima, É.P.; Dos Santos Paula, A.; Tommasini, F.J. Processing and characterization of PET composites reinforced with geopolymer concrete waste. Mater. Res. 2017, 20, 411–420. [Google Scholar] [CrossRef]
- Johnson, L.M.; Mecham, J.B.; Krovi, S.A.; Moreno Caffaro, M.M.; Aravamudhan, S.; Kovach, A.L.; Fennell, T.R.; Mortensen, N.P. Fabrication of polyethylene terephthalate (PET) nanoparticles with fluorescent tracers for studies in mammalian cells. Nanoscale Adv. 2021, 3, 339–346. [Google Scholar] [CrossRef]
- Alaraby, M.; Villacorta, A.; Abass, D.; Hernández, A.; Marcos, R. The hazardous impact of true-to-life PET nanoplastics in Drosophila. Sci. Total Environ. 2023, 863, 160954. [Google Scholar] [CrossRef]
- Annangi, B.; Villacorta, A.; Vela, L.; Tavakolpournegari, A.; Marcos, R.; Hernández, A. Effects of true-to-life PET nanoplastics using primary human nasal epithelial cells. Environ. Toxicol. Pharmacol. 2023, 100, 104140. [Google Scholar] [CrossRef]
- Arribas Arranz, J.; Villacorta, A.; Rubio, L.; García-Rodríguez, A.; Sánchez, G.; Llorca, M.; Farre, M.; Ferrer, J.F.; Marcos, R.; Hernández, A. Kinetics and toxicity of nanoplastics in ex vivo exposed human whole blood as a model to understand their impact on human health. Sci. Total Environ. 2024, 948, 174725. [Google Scholar] [CrossRef]
- Domenech, J.; Villacorta, A.; Ferrer, J.F.; Llorens-Chiralt, R.; Marcos, R.; Hernández, A.; Catalán, J. In vitro cell-transforming potential of secondary polyethylene terephthalate and polylactic acid nanoplastics. J. Hazard. Mater. 2024, 469, 134030. [Google Scholar] [CrossRef]
- Tavakolpournegari, A.; Villacorta, A.; Morataya-Reyes, M.; Arribas Arranz, J.; Banaei, G.; Pastor, S.; Velázquez, A.; Marcos, R.; Hernández, A.; Annangi, B. Harmful effects of true-to-life nanoplastics derived from PET water bottles in human alveolar macrophages. Environ. Pollut. 2024, 348, 123823. [Google Scholar] [CrossRef]
- Gutiérrez-García, J.; Egea, R.; Barguilla, I.; Nymark, P.; García-Rodríguez, A.; Guyot, B.; Maguer-Satta, V.; Marcos, R.; Rubio, L.; Hernández, A. Long-term exposure to real-life polyethylene terephthalate nanoplastics induces carcinogenesis in vitro. Environ. Sci. Technol. 2025, 59, 10891–10904. [Google Scholar] [CrossRef] [PubMed]
- Tavakolpournegari, A.; Annangi, B.; Villacorta, A.; Banaei, G.; Martin, J.; Pastor, S.; Marcos, R.; Hernández, A. Hazard assessment of different-sized polystyrene nanoplastics in hematopoietic human cell lines. Chemosphere 2023, 325, 138360. [Google Scholar] [CrossRef] [PubMed]
- Martín-Pérez, J.; Villacorta, A.; Banaei, G.; Morataya-Reyes, M.; Tavakolpournegari, A.; Marcos, R.; Hernández, A.; García-Rodriguez, A. Hazard assessment of nanoplastics is driven by their surface-functionalization. Effects in human-derived primary endothelial cells. Sci. Total Environ. 2024, 934, 173236. [Google Scholar] [CrossRef]
- Collin-Faure, V.; Vitipon, M.; Torres, A.; Tanyeres, O.; Dalzon, B.; Rabilloud, T. The internal dose makes the poison: Higher internalization of polystyrene particles induce increased perturbation of macrophages. Front. Immunol. 2023, 14, 1092743. [Google Scholar] [CrossRef]
- Pironti, C.; Notarstefano, V.; Ricciardi, M.; Motta, O.; Giorgini, E.; Montano, L. First evidence of microplastics in human urine, a preliminary study of intake in the human body. Toxics 2023, 11, 40. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Ragusa, A.; Notarstefano, V.; Svelato, A.; Belloni, A.; Gioacchini, G.; Blondeel, C.; Zucchelli, E.; De Luca, C.; D’avino, S.; Gulotta, A.; et al. Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers 2022, 14, 2700. [Google Scholar] [CrossRef]
- Mogha, N.K.; Shin, D. Nanoplastic detection with surface enhanced Raman spectroscopy: Present and future. TrAC-Trend. Anal. Chem. 2023, 158, 116885. [Google Scholar] [CrossRef]
- Ballesteros, S.; Domenech, J.; Barguilla, I.; Cortés, C.; Marcos, R.; Hernández, A. Genotoxic and immunomodulatory effects in human white blood cells after ex vivo exposure to polystyrene nanoplastics. Environ. Sci. Nano 2020, 7, 3431–3446. [Google Scholar] [CrossRef]
- Hendriks, L.; Kissling, V.M.; Buerki-Thurnherr, T.; Mitrano, D.M. Development of single-cell ICP-TOFMS to measure nanoplastics association with human cells. Environ. Sci. Nano 2023, 10, 3439–3449. [Google Scholar] [CrossRef]
- Mitrano, D.M.; Beltzung, A.; Frehland, S.; Schmiedgruber, M.; Cingolani, A.; Schmidt, F. Synthesis of metal-doped nanoplastics and their utility to investigate fate and behaviour in complex environmental systems. Nat. Nanotech. 2019, 14, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aquilina, G.; Castle, L.; Engel, K.H.; Fowler, P.; Frutos Fernandez, M.J.; Fürst, P.; Gundert-Remy, U.; Gürtler, R.; Husøy, T.; et al. Safety assessment of titanium dioxide (E171) as a food additive. EFSA J. 2021, 19, e06585. [Google Scholar] [CrossRef] [PubMed]
- European Union. COMMISSION REGULATION (EU) 2022/63. Amending Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as Regards the Food Additive Titanium Dioxide (E 171). 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32022R0063 (accessed on 4 March 2025).
- Carbone, M.; Arron, S.T.; Beutler, B.; Bononi, A.; Cavenee, W.; Cleaver, J.E.; Croce, C.M., Jr.; D’aNdrea, A.; Foulkes, W.D.; Gaudino, G.; et al. Tumour predisposition and cancer syndromes as models to study gene-environment interactions. Nat. Rev. Cancer 2020, 20, 533–549. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villacorta, A.; Morataya-Reyes, M.; Vela, L.; Arribas Arranz, J.; Martín-Perez, J.; Barguilla, I.; Marcos, R.; Hernández, A. Biological Impact of True-to-Life PET and Titanium-Doped PET Nanoplastics on Human-Derived Monocyte (THP-1) Cells. Nanomaterials 2025, 15, 1040. https://doi.org/10.3390/nano15131040
Villacorta A, Morataya-Reyes M, Vela L, Arribas Arranz J, Martín-Perez J, Barguilla I, Marcos R, Hernández A. Biological Impact of True-to-Life PET and Titanium-Doped PET Nanoplastics on Human-Derived Monocyte (THP-1) Cells. Nanomaterials. 2025; 15(13):1040. https://doi.org/10.3390/nano15131040
Chicago/Turabian StyleVillacorta, Aliro, Michelle Morataya-Reyes, Lourdes Vela, Jéssica Arribas Arranz, Joan Martín-Perez, Irene Barguilla, Ricard Marcos, and Alba Hernández. 2025. "Biological Impact of True-to-Life PET and Titanium-Doped PET Nanoplastics on Human-Derived Monocyte (THP-1) Cells" Nanomaterials 15, no. 13: 1040. https://doi.org/10.3390/nano15131040
APA StyleVillacorta, A., Morataya-Reyes, M., Vela, L., Arribas Arranz, J., Martín-Perez, J., Barguilla, I., Marcos, R., & Hernández, A. (2025). Biological Impact of True-to-Life PET and Titanium-Doped PET Nanoplastics on Human-Derived Monocyte (THP-1) Cells. Nanomaterials, 15(13), 1040. https://doi.org/10.3390/nano15131040





