Cost-Effective Bimetallic Catalysts for Green H2 Production in Anion Exchange Membrane Water Electrolyzers
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of OER Electrocatalysts
2.2. Synthesis of HER Electrocatalysts
2.3. Electrocatalysts Characterization
2.4. Anionic Electrolyte
2.5. Membrane Electrode Assembly and Electrochemical Studies
2.6. Electrochemical Studies
3. Results and Discussion
3.1. OER Electrocatalysts
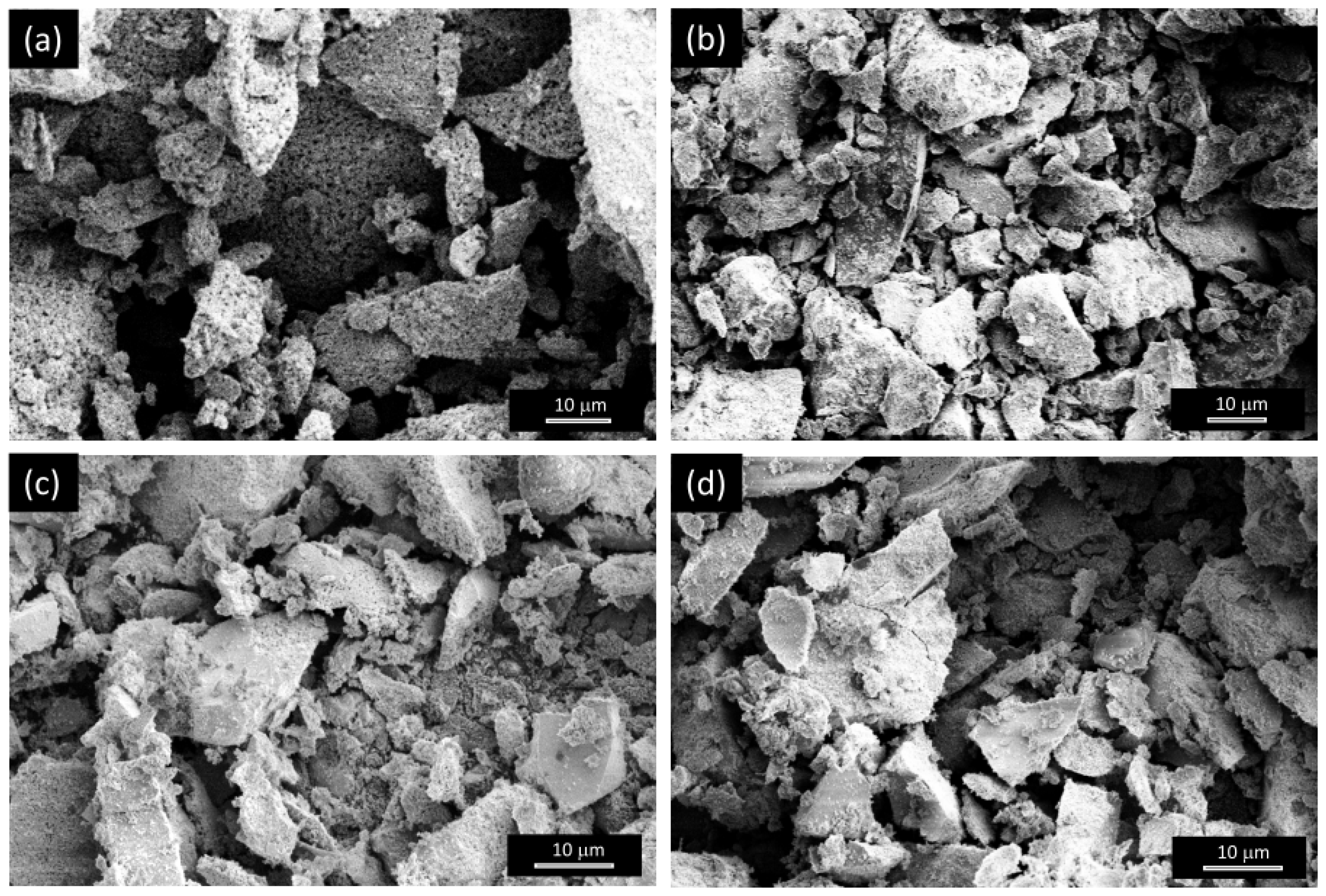
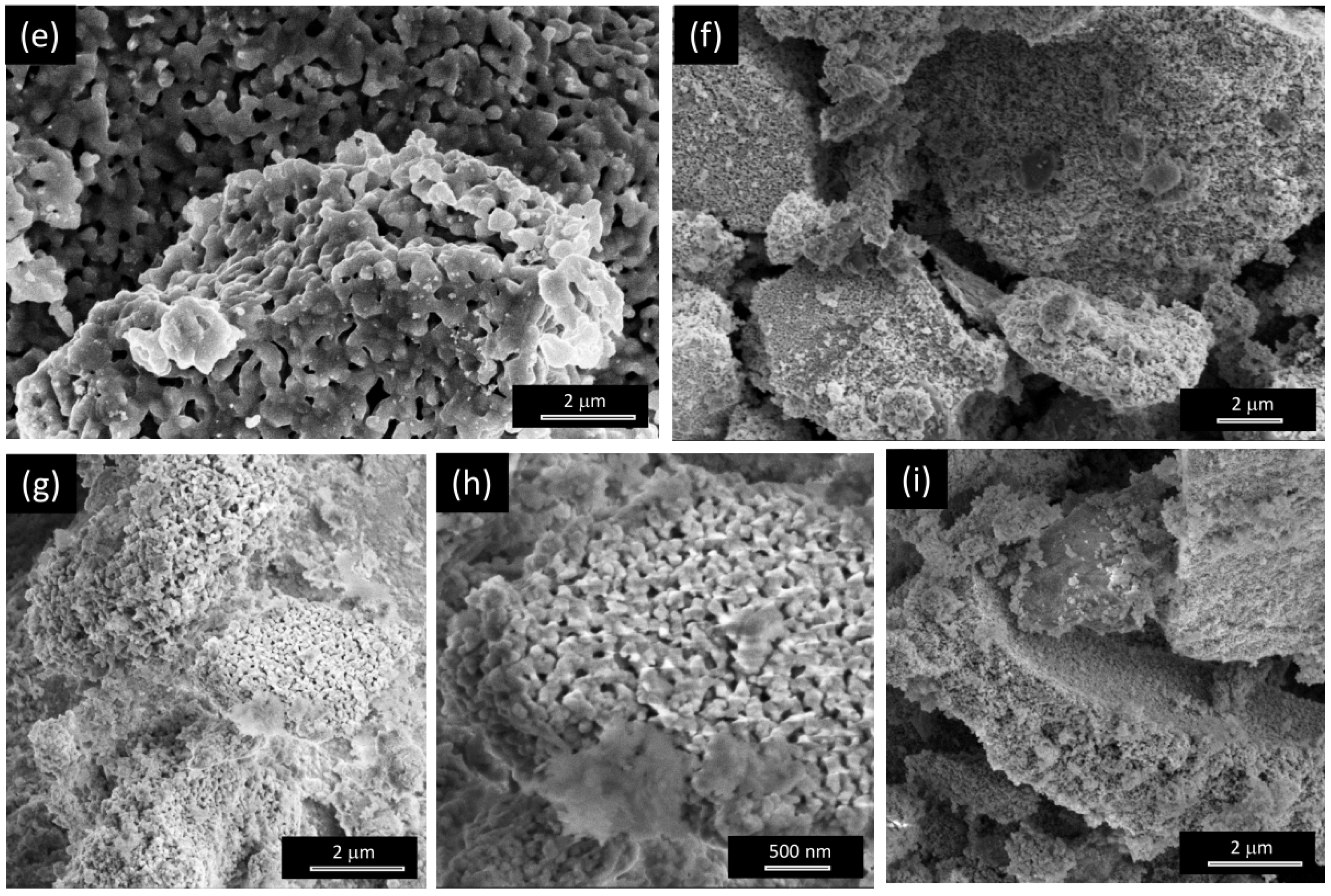
3.1.1. Morphology
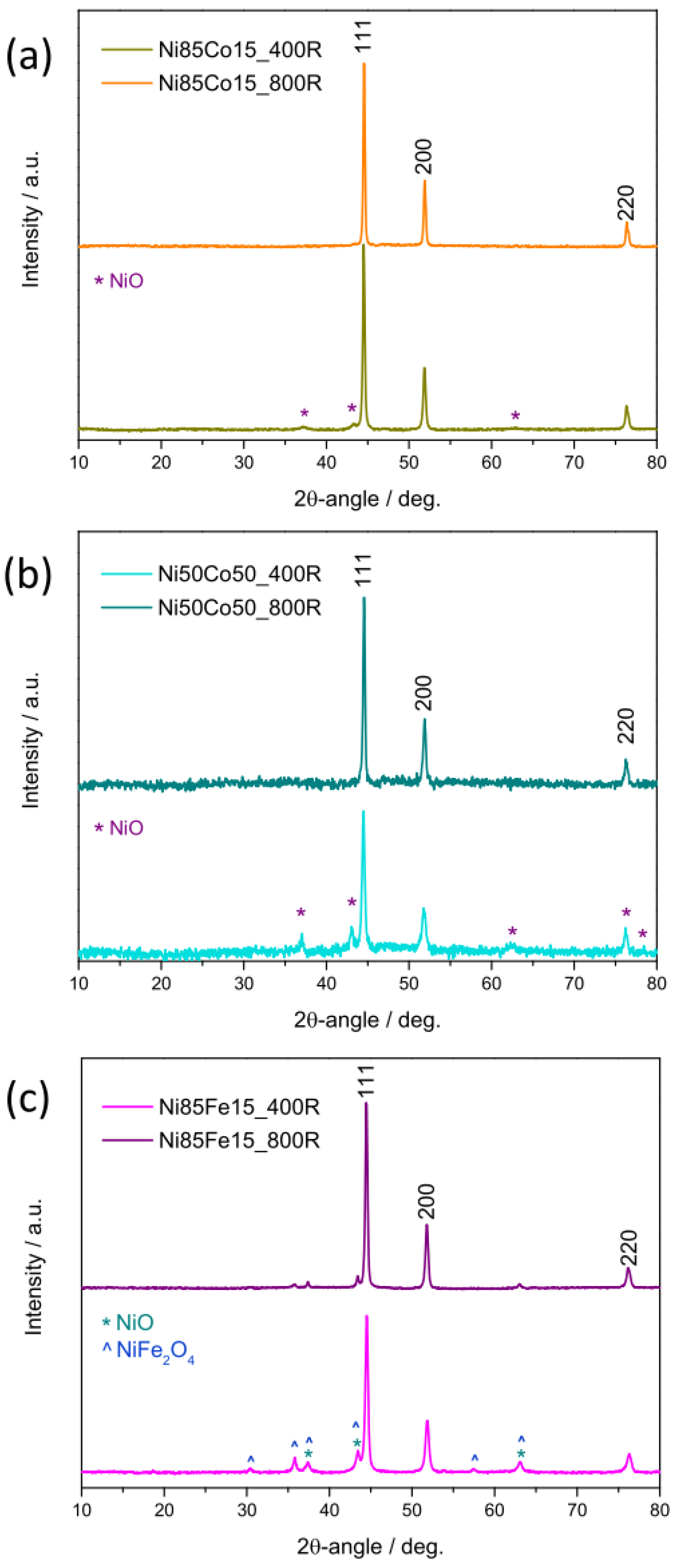
3.1.2. Phase(s) and Average Crystallite Size of the Oxides
3.1.3. Spatial Uniformity and Crystallization Degree of the Oxides
3.2. HER Electrocatalysts
3.2.1. Morphology
3.2.2. Crystalline Phase(s) and Average Crystallite Size
3.2.3. Nanomaterial Selection
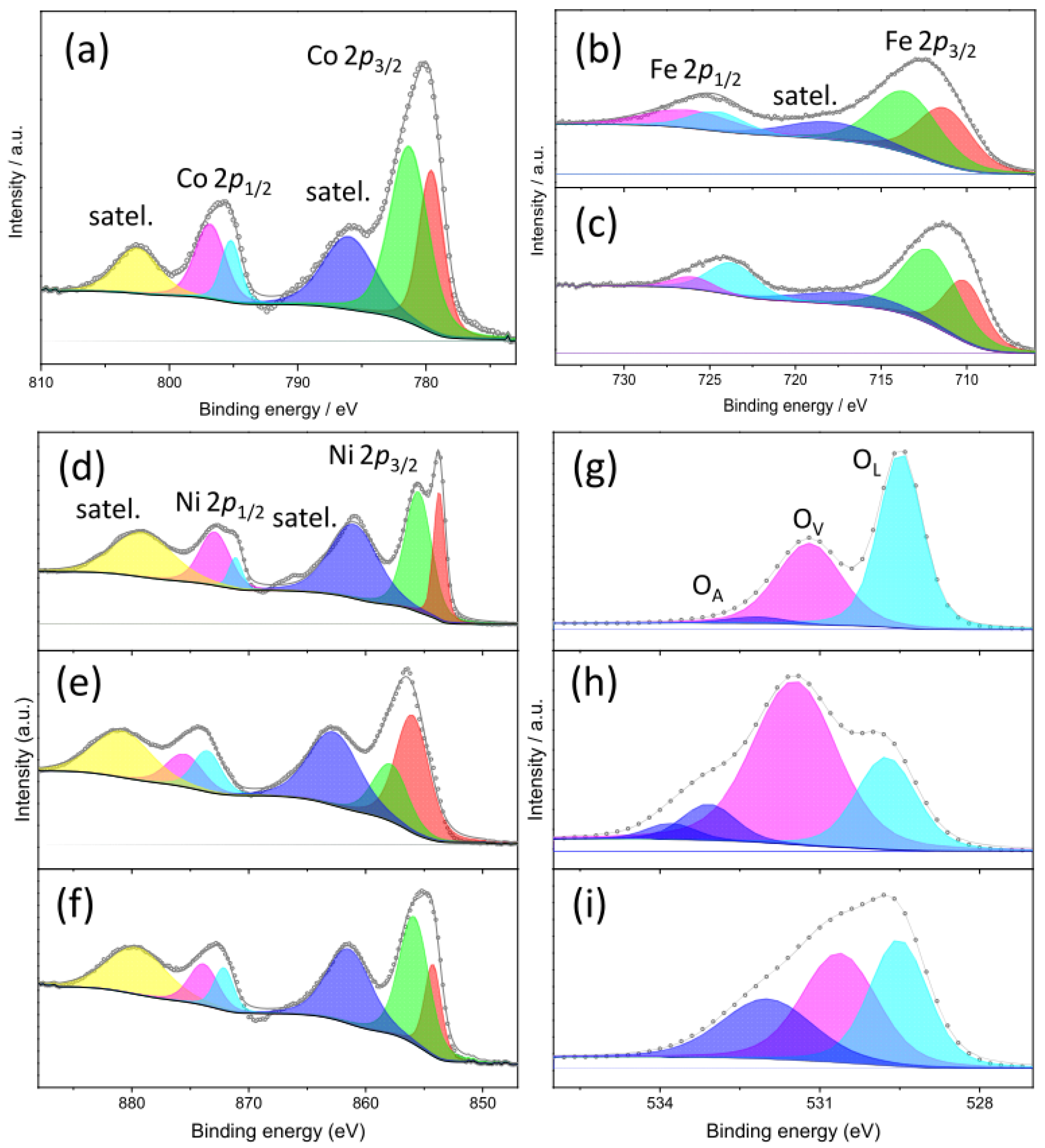
3.2.4. Species on the Surface of OER Electrocatalysts
3.2.5. Species on the Surface of HER Electrocatalysts
3.3. Electrochemical Performance of the Catalysts
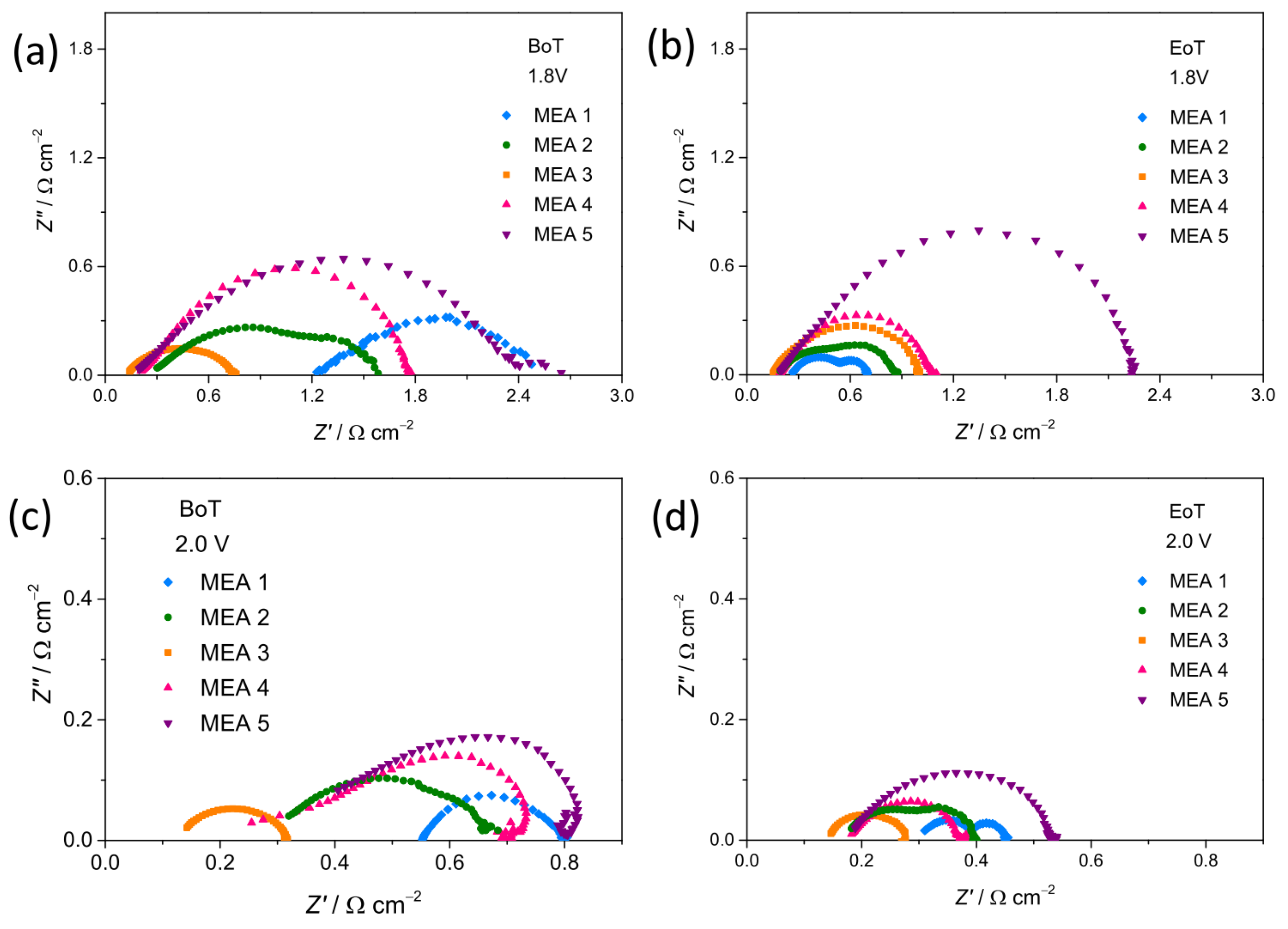
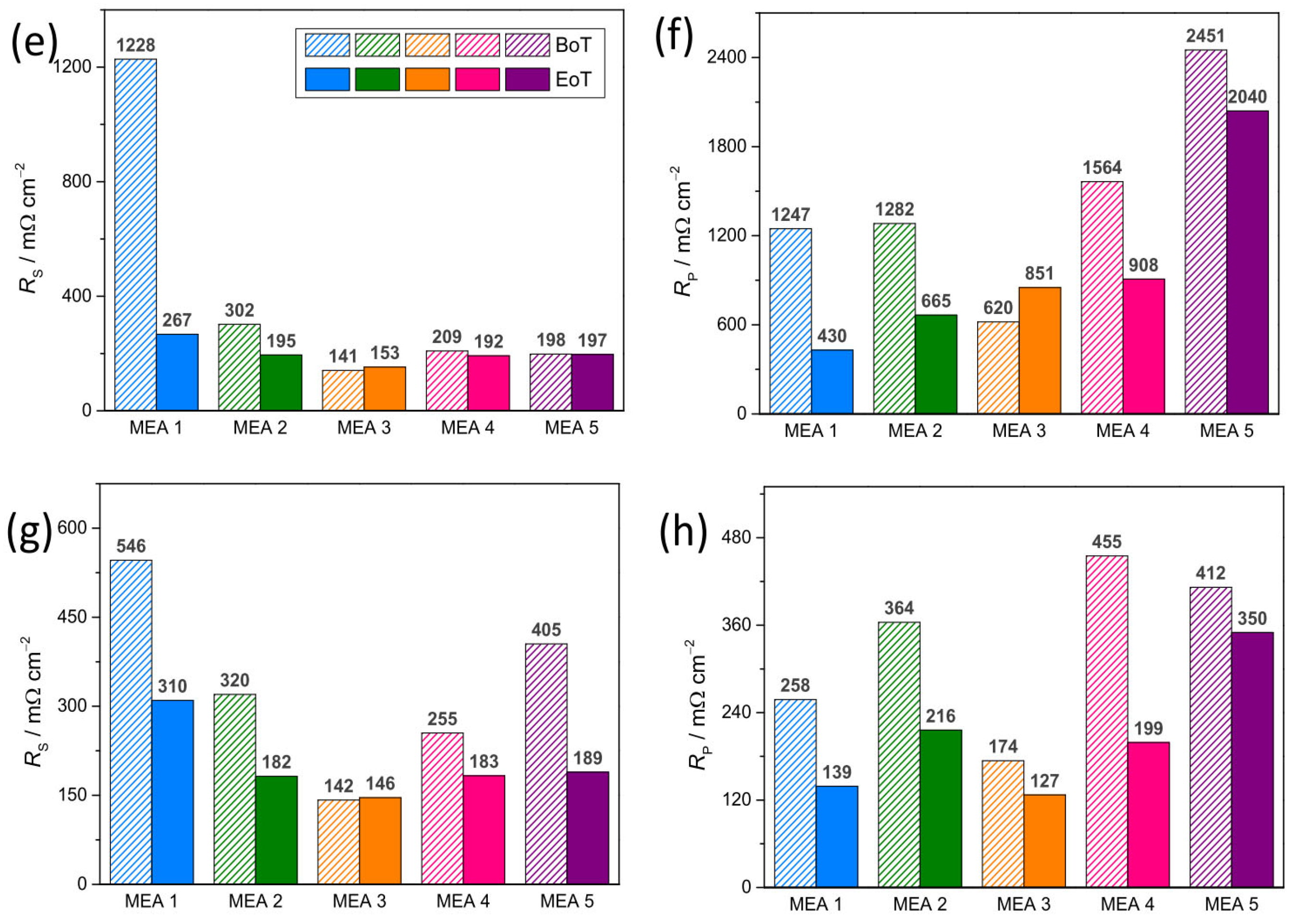
3.4. Structural Stability of the Electrocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IEA. World Energy Outlook 2024; IEA: Paris, France, 2024; Available online: https://www.iea.org/reports/world-energy-outlook-2024 (accessed on 11 June 2025).
- Hassan, Q.; Viktor, P.; Al-Musawi, T.J.; Ali, B.M.; Algburi, S.; Alzoubi, H.M.; Al-Jiboory, A.K.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. The Renewable Energy Role in the Global Energy Transformations. Renew. Energy Focus 2024, 48, 100545. [Google Scholar] [CrossRef]
- Algburi, S.; Al-Dulaimi, O.; Fakhruldeen, H.F.; Khalaf, D.H.; Hanoon, R.N.; Jabbar, F.I.; Hassan, Q.; Al-Jiboory, A.K.; Kiconco, S. The Green Hydrogen Role in the Global Energy Transformations. Renew. Sustain. Energy Transit. 2025, 8, 100118. [Google Scholar] [CrossRef]
- Le, P.-A.; Trung, V.D.; Nguyen, P.L.; Bac Phung, T.V.; Natsuki, J.; Natsuki, T. The Current Status of Hydrogen Energy: An Overview. RSC Adv. 2023, 13, 28262–28287. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.; Mahajan, N.; Singh, S.A.; Sreedhar, I. Green Hydrogen Production Pathways for Sustainable Future with Net Zero Emissions. Fuel 2024, 359, 130131. [Google Scholar] [CrossRef]
- Bhuiyan, M.M.H.; Siddique, Z. Hydrogen as an Alternative Fuel: A Comprehensive Review of Challenges and Opportunities in Production, Storage, and Transportation. Int. J. Hydrogen Energy 2025, 102, 1026–1044. [Google Scholar] [CrossRef]
- Kumar, S.S.; Lim, H. An Overview of Water Electrolysis Technologies for Green Hydrogen Production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Mulk, W.U.; Aziz, A.R.A.; Ismael, M.A.; Ghoto, A.A.; Ali, S.A.; Younas, M.; Gallucci, F. Electrochemical Hydrogen Production through Anion Exchange Membrane Water Electrolysis (AEMWE): Recent Progress and Associated Challenges in Hydrogen Production. Int. J. Hydrogen Energy 2024, 94, 1174–1211. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Kalauni, K.; Pahwa, R. A Review of Water Electrolysis Technologies with Insights into Optimization and Numerical Simulations. Int. J. Hydrogen Energy 2025, 140, 694–727. [Google Scholar] [CrossRef]
- Miller, H.A.; Bouzek, K.; Hnat, J.; Loos, S.; Bernäcker, C.I.; Weißgärber, T.; Röntzsch, L.; Meier-Haack, J. Green Hydrogen from Anion Exchange Membrane Water Electrolysis: A Review of Recent Developments in Critical Materials and Operating Conditions. Sustain. Energy Fuels 2020, 4, 2114–2133. [Google Scholar] [CrossRef]
- Santos, D.M.F.; Sequeira, C.A.C.; Figueiredo, J.L. Hydrogen Production by Alkaline Water Electrolysis. Quím. Nova 2013, 36, 1176–1193. [Google Scholar] [CrossRef]
- In Lee, H.; Dung, D.T.; Kim, J.; Pak, J.H.; Kim, S.K.; Cho, H.S.; Cho, W.C.; Kim, C.H. The Synthesis of a Zirfon-type Porous Separator with Reduced Gas Crossover for Alkaline Electrolyzer. Int. J. Energy Res. 2020, 44, 1875–1885. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A Comprehensive Review on PEM Water Electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen Production by PEM Water Electrolysis—A Review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Zhang, K.; Liang, X.; Wang, L.; Sun, K.; Wang, Y.; Xie, Z.; Wu, Q.; Bai, X.; Hamdy, M.S.; Chen, H.; et al. Status and Perspectives of Key Materials for PEM Electrolyzer. Nano Res. Energy 2022, 1, e9120032. [Google Scholar] [CrossRef]
- Park, J.E.; Kang, S.Y.; Oh, S.-H.; Kim, J.K.; Lim, M.S.; Ahn, C.-Y.; Cho, Y.-H.; Sung, Y.-E. High-Performance Anion-Exchange Membrane Water Electrolysis. Electrochim. Acta 2019, 295, 99–106. [Google Scholar] [CrossRef]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Chem. Rev. 2022, 122, 11830–11895. [Google Scholar] [CrossRef]
- Hua, D.; Huang, J.; Fabbri, E.; Rafique, M.; Song, B. Development of Anion Exchange Membrane Water Electrolysis and the Associated Challenges: A Review. ChemElectroChem 2023, 10, e202200999. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis: A Review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Lee, S.A.; Kim, J.; Kwon, K.C.; Park, S.H.; Jang, H.W. Anion Exchange Membrane Water Electrolysis for Sustainable Large-scale Hydrogen Production. Carbon Neutralization 2022, 1, 26–48. [Google Scholar] [CrossRef]
- Volk, E.K.; Kreider, M.E.; Kwon, S.; Alia, S.M. Recent Progress in Understanding the Catalyst Layer in Anion Exchange Membrane Electrolyzers—Durability, Utilization, and Integration. EES Catal. 2024, 2, 109–137. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Catalysts for Electrochemical Water Splitting: Current State and Prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Faid, A.Y.; Barnett, A.O.; Seland, F.; Sunde, S. Highly Active Nickel-Based Catalyst for Hydrogen Evolution in Anion Exchange Membrane Electrolysis. Catalysts 2018, 8, 614. [Google Scholar] [CrossRef]
- Darband, G.B.; Aliofkhazraei, M.; Rouhaghdam, A.S. Facile Electrodeposition of Ternary Ni-Fe-Co Alloy Nanostructure as a Binder Free, Cost-Effective and Durable Electrocatalyst for High-Performance Overall Water Splitting. J. Colloid Interface Sci. 2019, 547, 407–420. [Google Scholar] [CrossRef]
- Park, Y.S.; Jeong, J.; Noh, Y.; Jang, M.J.; Lee, J.; Lee, K.H.; Lim, D.C.; Seo, M.H.; Kim, W.B.; Yang, J.; et al. Commercial Anion Exchange Membrane Water Electrolyzer Stack through Non-Precious Metal Electrocatalysts. Appl. Catal. B Environ. 2021, 292, 120170. [Google Scholar] [CrossRef]
- Faid, A.Y.; Barnett, A.O.; Seland, F.; Sunde, S. NiCu Mixed Metal Oxide Catalyst for Alkaline Hydrogen Evolution in Anion Exchange Membrane Water Electrolysis. Electrochim. Acta 2021, 371, 137837. [Google Scholar] [CrossRef]
- Campagna Zignani, S.; Lo Faro, M.; Trocino, S.; Aricò, A.S. Investigation of NiFe-Based Catalysts for Oxygen Evolution in Anion-Exchange Membrane Electrolysis. Energies 2020, 13, 1720. [Google Scholar] [CrossRef]
- Sun, H.; Yan, Z.; Liu, F.; Xu, W.; Cheng, F.; Chen, J. Self-Supported Transition-Metal-Based Electrocatalysts for Hydrogen and Oxygen Evolution. Adv. Mater. 2020, 32, 1806326. [Google Scholar] [CrossRef]
- Campagna Zignani, S.; Faro, M.L.; Carbone, A.; Italiano, C.; Trocino, S.; Monforte, G.; Aricò, A.S. Performance and Stability of a Critical Raw Materials-Free Anion Exchange Membrane Electrolysis Cell. Electrochim. Acta 2022, 413, 140078. [Google Scholar] [CrossRef]
- Bhat, K.S.; Nagaraja, H.S. Recent Trends and Insights in Nickel Chalcogenide Nanostructures for Water-Splitting Reactions. Mater. Res. Innov. 2021, 25, 29–52. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, M.; Qin, Y.; Zhang, Y.; Zhang, X.; Fang, J.; Zhang, X.; Zhu, W.; Zhuang, Z. Ultrathin NiFeS Nanosheets as Highly Active Electrocatalysts for Oxygen Evolution Reaction. Chin. Chem. Lett. 2022, 33, 3916–3920. [Google Scholar] [CrossRef]
- Kandel, M.R.; Pan, U.N.; Paudel, D.R.; Dhakal, P.P.; Kim, N.H.; Lee, J.H. Hybridized Bimetallic Phosphides of Ni–Mo, Co–Mo, and Co–Ni in a Single Ultrathin-3D-Nanosheets for Efficient HER and OER in Alkaline Media. Compos. Part B Eng. 2022, 239, 109992. [Google Scholar] [CrossRef]
- Park, D.-H.; Kim, M.-H.; Kim, M.; Byeon, J.-H.; Jang, J.-S.; Kim, J.-H.; Lim, D.-M.; Park, S.-H.; Gu, Y.-H.; Kim, J.; et al. Spherical Nickel Doped Cobalt Phosphide as an Anode Catalyst for Oxygen Evolution Reaction in Alkaline Media: From Catalysis to System. Appl. Catal. B Environ. 2023, 327, 122444. [Google Scholar] [CrossRef]
- Batool, M.; Hameed, A.; Nadeem, M.A. Recent Developments on Iron and Nickel-Based Transition Metal Nitrides for Overall Water Splitting: A Critical Review. Coord. Chem. Rev. 2023, 480, 215029. [Google Scholar] [CrossRef]
- Moschkowitsch, W.; Zion, N.; Honig, H.C.; Levy, N.; Cullen, D.A.; Elbaz, L. Mixed-Metal Nickel–Iron Oxide Aerogels for Oxygen Evolution Reaction. ACS Catal. 2022, 12, 12162–12169. [Google Scholar] [CrossRef]
- Van Limpt, R.T.M.; Lao, M.; Tsampas, M.N.; Creatore, M. Unraveling the Role of the Stoichiometry of Atomic Layer Deposited Nickel Cobalt Oxides on the Oxygen Evolution Reaction. Adv. Sci. 2024, 11, 2405188. [Google Scholar] [CrossRef]
- Patriarchi, A.; Triolo, C.; Minnetti, L.; Muñoz-Márquez, M.Á.; Nobili, F.; Santangelo, S. Evaluation of High-Entropy (Cr, Mn, Fe, Co, Ni)-Oxide Nanofibers and Nanoparticles as Passive Fillers for Solid Composite Electrolytes. Electrochim. Acta 2025, 512, 145425. [Google Scholar] [CrossRef]
- Elhousseini, M.H.; Isık, T.; Kap, Ö.; Verpoort, F.; Horzum, N. Dual Remediation of Waste Waters from Methylene Blue and Chromium (VI) Using Thermally Induced ZnO Nanofibers. Appl. Surf. Sci. 2020, 514, 145939. [Google Scholar] [CrossRef]
- Pantò, F.; Dahrouch, Z.; Saha, A.; Patanè, S.; Santangelo, S.; Triolo, C. Photocatalytic Degradation of Methylene Blue Dye by Porous Zinc Oxide Nanofibers Prepared via Electrospinning: When Defects Become Merits. Appl. Surf. Sci. 2021, 557, 149830. [Google Scholar] [CrossRef]
- Vezzù, K.; Triolo, C.; Moulaee, K.; Pagot, G.; Ponti, A.; Pinna, N.; Neri, G.; Santangelo, S.; Di Noto, V. Interplay Between Calcination Temperature and Alkaline Oxygen Evolution of Electrospun High-Entropy (Cr1/5Mn1/5Fe1/5Co1/5Ni1/5)3O4 Nanofibers. Small 2025, 21, 2408319. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O 3−δ Nanocomposite: A New High-Performance Cobalt-Free Triple-Conducting Cathode for Protonic Ceramic Fuel Cells Operating at Reduced Temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Popa, N.C. The (Hkl) Dependence of Diffraction-Line Broadening Caused by Strain and Size for All Laue Groups in Rietveld Refinement. J. Appl. Crystallogr. 1998, 31, 176–180. [Google Scholar] [CrossRef]
- Casas-Cabanas, M.; Palacín, M.R.; Rodríguez-Carvajal, J. Microstructural Analysis of Nickel Hydroxide: Anisotropic Size versus Stacking Faults. Powder Diffr. 2005, 20, 334–344. [Google Scholar] [CrossRef]
- Li, T.; Dang, N.; Zhang, W.; Liang, W.; Yang, F. Determining the Degree of [001] Preferred Growth of Ni(OH)2 Nanoplates. Nanomaterials 2018, 8, 991. [Google Scholar] [CrossRef]
- Jayashree, R.S.; Kamath, P.V.; Subbanna, G.N. The Effect of Crystallinity on the Reversible Discharge Capacity of Nickel Hydroxide. J. Electrochem. Soc. 2000, 147, 2029. [Google Scholar] [CrossRef]
- Tientong, J.; Garcia, S.; Thurber, C.R.; Golden, T.D. Synthesis of Nickel and Nickel Hydroxide Nanopowders by Simplified Chemical Reduction. J. Nanotechnol. 2014, 2014, 193162. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Prabu, P.; Nagarajan, S.; Kim, C.H.; Park, J.H.; Kim, H.Y. Synthesis of Nickel Oxide Nanoparticles Using Nickel Acetate and Poly (Vinyl Acetate) Precursor. Mater. Sci. Eng. B 2006, 128, 111–114. [Google Scholar] [CrossRef]
- Ahmad, T.; Ramanujachary, K.V.; Lofland, S.E.; Ganguli, A.K. Magnetic and Electrochemical Properties of Nickel Oxide Nanoparticles Obtained by the Reverse-Micellar Route. Solid State Sci. 2006, 8, 425–430. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Fereshteh, Z. Synthesis of Nickel and Nickel Oxide Nanoparticles via Heat-Treatment of Simple Octanoate Precursor. J. Alloys Compd. 2010, 494, 410–414. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Cuong, N.D.; Tran, T.D.; Nguyen, Q.T.; Van Minh Hai, H.; Hoa, T.T.; Quang, D.T.; Klysubun, W.; Tran, P.D. Highly Porous Co-Doped NiO Nanorods: Facile Hydrothermal Synthesis and Electrocatalytic Oxygen Evolution Properties. R. Soc. Open sci. 2021, 8, 202352. [Google Scholar] [CrossRef] [PubMed]
- Tharayil, N.J.; Sagar, S.; Raveendran, R.; Vaidyan, A.V. Dielectric Studies of Nanocrystalline Nickel–Cobalt Oxide. Phys. B Condens. Matter 2007, 399, 1–8. [Google Scholar] [CrossRef]
- Iacomi, F.; Calin, G.; Scarlat, C.; Irimia, M.; Doroftei, C.; Dobromir, M.; Rusu, G.G.; Iftimie, N.; Sandu, A.V. Functional Properties of Nickel Cobalt Oxide Thin Films. Thin Solid Film. 2011, 520, 651–655. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, X.-X.; Ding, X.-L.; Kong, H.-C.; Sha, H.-D.; Lin, H.; Wen, W.; Shen, G.; Guo, Z.; Ma, Z.-F.; et al. Effects of Cobalt Precursor on Pyrolyzed Carbon-Supported Cobalt-Polypyrrole as Electrocatalyst toward Oxygen Reduction Reaction. Nanoscale Res. Lett. 2013, 8, 478. [Google Scholar] [CrossRef]
- Liu, G.; Gao, X.; Wang, K.; He, D.; Li, J. Mesoporous Nickel–Iron Binary Oxide Nanorods for Efficient Electrocatalytic Water Oxidation. Nano Res. 2017, 10, 2096–2105. [Google Scholar] [CrossRef]
- Ravikumar, P.; Kisan, B.; Perumal, A. Enhanced Room Temperature Ferromagnetism in Antiferromagnetic NiO Nanoparticles. AIP Adv. 2015, 5, 087116. [Google Scholar] [CrossRef]
- Liu, D.; Li, D.; Yang, D. Size-Dependent Magnetic Properties of Branchlike Nickel Oxide Nanocrystals. AIP Adv. 2017, 7, 015028. [Google Scholar] [CrossRef]
- Usharani, N.J.; Shringi, R.; Sanghavi, H.; Subramanian, S.; Bhattacharya, S.S. Role of Size, Alio-/Multi-Valency and Non-Stoichiometry in the Synthesis of Phase-Pure High Entropy Oxide (Co, Cu, Mg, Na, Ni, Zn)O. Dalton Trans. 2020, 49, 7123–7132. [Google Scholar] [CrossRef]
- D’Ippolito, V.; Andreozzi, G.B.; Bersani, D.; Lottici, P.P. Raman Fingerprint of Chromate, Aluminate and Ferrite Spinels. J. Raman Spectrosc. 2015, 46, 1255–1264. [Google Scholar] [CrossRef]
- Pantò, F.; Fan, Y.; Frontera, P.; Stelitano, S.; Fazio, E.; Patanè, S.; Marelli, M.; Antonucci, P.; Neri, F.; Pinna, N.; et al. Are Electrospun Carbon/Metal Oxide Composite Fibers Relevant Electrode Materials for Li-Ion Batteries? J. Electrochem. Soc. 2016, 163, A2930–A2937. [Google Scholar] [CrossRef]
- Ercolino, G.; Grodzka, A.; Grzybek, G.; Stelmachowski, P.; Specchia, S.; Kotarba, A. The Effect of the Preparation Method of Pd-Doped Cobalt Spinel on the Catalytic Activity in Methane Oxidation Under Lean Fuel Conditions. Top. Catal. 2017, 60, 333–341. [Google Scholar] [CrossRef]
- Farhadi, S.; Javanmard, M.; Nadri, G. Characterization of Cobalt Oxide Nanoparticles Prepared by the Thermal Decomposition of [Co(NH3)5(H2O)](NO3)3 Complex and Study of Their Photocatalytic Activity. Acta Chim. Slov. 2016, 63, 335–343. [Google Scholar] [CrossRef]
- Santangelo, S.; Fiore, M.; Pantò, F.; Stelitano, S.; Marelli, M.; Frontera, P.; Antonucci, P.; Longoni, G.; Ruffo, R. Electro-Spun Co3O4 Anode Material for Na-Ion Rechargeable Batteries. Solid State Ion. 2017, 309, 41–47. [Google Scholar] [CrossRef]
- Venkatachalam, V.; Alsalme, A.; Alswieleh, A.; Jayavel, R. Shape Controlled Synthesis of Rod-like Co3O4 Nanostructures as High-Performance Electrodes for Supercapacitor Applications. J. Mater. Sci. Mater. Electron. 2018, 29, 6059–6067. [Google Scholar] [CrossRef]
- Ahlawat, A.; Sathe, V.G. Raman Study of NiFe2O4 Nanoparticles, Bulk and Films: Effect of Laser Power. J. Raman Spectrosc. 2011, 42, 1087–1094. [Google Scholar] [CrossRef]
- Silva, M.M.S.; Raimundo, R.A.; Ferreira, L.S.; Macedo, D.A.; Morales, M.A.; Souza, C.P.; Santos, A.G.; Lopes-Moriyama, A.L. Effects of Morphology on the Electrochemical Performance of NiFe2 O4 Nanoparticles with Battery-type Behavior. Int. J. Appl. Ceram. Tech. 2022, 19, 2016–2028. [Google Scholar] [CrossRef]
- Ali, B.; Tasirin, S.; Aminayi, P.; Yaakob, Z.; Ali, N.; Noori, W. Non-Supported Nickel-Based Coral Sponge-Like Porous Magnetic Alloys for Catalytic Production of Syngas and Carbon Bio-Nanofilaments via a Biogas Decomposition Approach. Nanomaterials 2018, 8, 1053. [Google Scholar] [CrossRef]
- Jacob, G.A.; Sellaiyan, S.; Uedono, A.; Joseyphus, R.J. Magnetic Properties of Metastable Bcc Phase in Fe64Ni36 Alloy Synthesized through Polyol Process. Appl. Phys. A 2020, 126, 120. [Google Scholar] [CrossRef]
- Senthamizhan, A.; Balusamy, B.; Aytac, Z.; Uyar, T. Grain Boundary Engineering in Electrospun ZnO Nanostructures as Promising Photocatalysts. CrystEngComm 2016, 18, 6341–6351. [Google Scholar] [CrossRef]
- Montalto, M.; Freitas, W.D.S.; Mastronardo, E.; Ficca, V.C.A.; Placidi, E.; Baglio, V.; Mosca, E.; Vecchio, C.L.; Gatto, I.; Mecheri, B.; et al. Spinel-Type High-Entropy Oxides for Enhanced Oxygen Evolution Reaction Activity in Anion Exchange Membrane Water Electrolyzers. Chem. Eng. J. 2025, 507, 160641. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Wang, Z.; Wei, W.; Tang, W.; Shi, J.; Xiong, R. Electronic Structure Studies of the Spinel CoFe2O4 by X-Ray Photoelectron Spectroscopy. Appl. Surf. Sci. 2008, 254, 6972–6975. [Google Scholar] [CrossRef]
- Chuang, T.J.; Brundle, C.R.; Rice, D.W. Interpretation of the X-Ray Photoemission Spectra of Cobalt Oxides and Cobalt Oxide Surfaces. Surf. Sci. 1976, 59, 413–429. [Google Scholar] [CrossRef]
- Triolo, C.; Moulaee, K.; Bellato, F.; Pagot, G.; Ponti, A.; Maida, S.; Pinna, N.; Neri, G.; Di Noto, V.; Santangelo, S. Interplay between Alkaline Water Oxidation Temperature, Composition and Performance of Electrospun High-Entropy Non-Equimolar (Cr, Mn, Fe, Co, Ni) Oxide Electrocatalysts. J. Power Sources 2025. submitted.
- Zhang, W.; Jiang, C.; Guan, H.; Wang, Y.; Hu, Y.; Wang, W.; Tian, W.; Hao, L. Unlocking OER Catalytic Potential and Chiral Fe3 O4 Film as a Game-Changer for Electrochemical Water Oxidation Pathway and by-Product Control. Mater. Adv. 2024, 5, 1340–1347. [Google Scholar] [CrossRef]
- Sulemana, H.; Yi, C.; Nawaz, M.I.; Zhang, B.; Yi, R.; Zhang, J.; Nkudede, E. Synthesis and Characterization of Nickel Ferrite (NiFe2O4) Nano-Catalyst Films for Ciprofloxacin Degradation. Ceram. Int. 2025, 51, 8376–8387. [Google Scholar] [CrossRef]
- Tomellini, M. X-Ray Photoelectron Spectra of Defective Nickel Oxide. J. Chem. Soc. Faraday Trans. 1 1988, 84, 3501. [Google Scholar] [CrossRef]
- Kim, K.S.; Winograd, N. X-Ray Photoelectron Spectroscopic Studies of Nickel-Oxygen Surfaces Using Oxygen and Argon Ion-Bombardment. Surf. Sci. 1974, 43, 625–643. [Google Scholar] [CrossRef]
- Yang, Y.; Tao, Q.; Srinivasan, G.; Takoudis, C.G. Cyclic Chemical Vapor Deposition of Nickel Ferrite Thin Films Using Organometallic Precursor Combination. ECS J. Solid State Sci. Technol. 2014, 3, P345–P352. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, J.; Liang, Z.; Xie, W.; Zhao, B.; He, L. Anodic Product-Derived Bi-MOF as Pre-catalyst for Cathodic CO2 Reduction: A Novel Strategy for Paired Electrolysis. ChemCatChem 2023, 15, e202201321. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Liu, Y. Interpretation of Oxygen 1s X-Ray Photoelectron Spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The Roles of Oxygen Vacancies in Electrocatalytic Oxygen Evolution Reaction. Nano Energy 2020, 73, 104761. [Google Scholar] [CrossRef]
- He, L.; Kang, H.; Hou, G.; Qiao, X.; Jia, X.; Qin, W.; Wu, X. Low-Temperature Synthesis of Nano-Porous High Entropy Spinel Oxides with High Grain Boundary Density for Oxygen Evolution Reaction. Chem. Eng. J. 2023, 460, 141675. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Johnston, D.D.; Coatsworth, L.L.; Davidson, R.D.; Brown, J.R. X-ray Photoelectron Spectroscopic Studies of Thin Film Oxides of Cobalt and Molybdenum. Surf. Interface Anal. 1990, 15, 265–272. [Google Scholar] [CrossRef]
- Furstenau, R.P.; McDougall, G.; Langell, M.A. Initial Stages of Hydrogen Reduction of NiO(100). Surf. Sci. 1985, 150, 55–79. [Google Scholar] [CrossRef]
- Huang, K.; Xia, J.; Lu, Y.; Zhang, B.; Shi, W.; Cao, X.; Zhang, X.; Woods, L.M.; Han, C.; Chen, C.; et al. Self-Reconstructed Spinel Surface Structure Enabling the Long-Term Stable Hydrogen Evolution Reaction/Oxygen Evolution Reaction Efficiency of FeCoNiRu High-Entropy Alloyed Electrocatalyst. Adv. Sci. 2023, 10, 2300094. [Google Scholar] [CrossRef]
- Triolo, C.; Moulaee, K.; Ponti, A.; Pagot, G.; Di Noto, V.; Pinna, N.; Neri, G.; Santangelo, S. Spinel-Structured High-Entropy Oxide Nanofibers as Electrocatalysts for Oxygen Evolution in Alkaline Solution: Effect of Metal Combination and Calcination Temperature. Adv. Funct. Mater. 2024, 34, 2306375. [Google Scholar] [CrossRef]
- Trocino, S.; Lo Vecchio, C.; Campagna Zignani, S.; Carbone, A.; Saccà, A.; Baglio, V.; Gómez, R.; Aricò, A.S. Dry Hydrogen Production in a Tandem Critical Raw Material-Free Water Photoelectrolysis Cell Using a Hydrophobic Gas-Diffusion Backing Layer. Catalysts 2020, 10, 1319. [Google Scholar] [CrossRef]
- Vincent, I.; Kruger, A.; Bessarabov, D. Development of Efficient Membrane Electrode Assembly for Low Cost Hydrogen Production by Anion Exchange Membrane Electrolysis. Int. J. Hydrogen Energy 2017, 42, 10752–10761. [Google Scholar] [CrossRef]
- Liu, Z.; Sajjad, S.D.; Gao, Y.; Yang, H.; Kaczur, J.J.; Masel, R.I. The Effect of Membrane on an Alkaline Water Electrolyzer. Int. J. Hydrogen Energy 2017, 42, 29661–29665. [Google Scholar] [CrossRef]
- Vincent, I.; Lee, E.-C.; Kim, H.-M. Highly Cost-Effective Platinum-Free Anion Exchange Membrane Electrolysis for Large Scale Energy Storage and Hydrogen Production. RSC Adv. 2020, 10, 37429–37438. [Google Scholar] [CrossRef]
- Najibah, M.; Hnát, J.; Plevová, M.; Dayan, A.; Stojadinovic, J.; Žitka, J.; Bae, C.; Bouzek, K.; Henkensmeier, D. PPS-Reinforced Poly(Terphenylene) Anion-Exchange Membranes with Different Quaternary Ammonium Groups for Use in Water Electrolysers. J. Membr. Sci. 2025, 713, 123335. [Google Scholar] [CrossRef]





| Code | Metal Molar Concentrations | TC/°C | ||
|---|---|---|---|---|
| Ni | Co | Fe | ||
| Ni100 | 1.00 | 400 | ||
| Ni85Co15_400 | 0.85 | 0.15 | 400 | |
| Ni85Co15_800 | 0.85 | 0.15 | 800 | |
| Ni50Co50_400 | 0.50 | 0.50 | 400 | |
| Ni50Co50_800 | 0.50 | 0.50 | 800 | |
| Ni85Fe15_400 | 0.85 | 0.15 | 400 | |
| Ni85Fe15_800 | 0.85 | 0.15 | 800 | |
| Code | Phase(s) Abundance/wt% | d/nm | |||
|---|---|---|---|---|---|
| β-Ni(OH)2 | RS | CM | SP | ||
| NiH100 | 100.0 | 24 | |||
| Ni100 | 100.0 | 21 | |||
| Ni85Co15_400 | 100.0 | 36 | |||
| Ni85Co15_800 | 100.0 | 105 | |||
| Ni50Co50_400 | 46.8 | 30.6 | 22.6 | 42 | |
| Ni50Co50_800 | 72.2 | 27.8 | 105 | ||
| Ni85Fe15_400 | 77.2 | 12.4 | 10.4 | 28 | |
| Ni85Fe15_800 | 81.4 | 18.6 | 55 | ||
| Sample | Phase(s) Abundance/wt% | d/nm | ||
|---|---|---|---|---|
| CM | RS | SP | ||
| NiH100R | 100.0 | 61.3 | ||
| Ni100R | 100.0 | 61.0 | ||
| Ni85Co15_400R | 93.3 | 6.7 | 55.9 | |
| Ni85Co15_800R | 100.0 | 139.4 | ||
| Ni50Co50_400R | 81.8 | 18.2 | 39.4 | |
| Ni50Co50_800R | 100.0 | 88.6 | ||
| Ni85Fe15_400R | 71.7 | 18.5 | 9.8 | 38.5 |
| Ni85Fe15_800R | 91.1 | 2.9 | 6.0 | 59.5 |
| MEA Code and Composition | Cathode | Anode | VC/V | J/A cm−2 | |
|---|---|---|---|---|---|
| 1 | NiH100-based | NiH100R | NiH100 | 2.13 | 1.08 |
| 2 | Ni100-based | Ni100R | Ni100 | 2.14 | 1.09 |
| 3 | Ni85Co15_400-based | Ni85Co15_400R | Ni85Co15_400 | 2.03 | 1.78 |
| 4 | Ni85Fe15_400-based | Ni85Fe15_400R | Ni85Fe15_400 | 2.20 | 0.90 |
| 5 | Ni85Fe15_800-based | Ni85Fe15_800R | Ni85Fe15_800 | 2.20 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campagna Zignani, S.; Fazio, M.; Pascale, M.; Alessandrello, C.; Triolo, C.; Musolino, M.G.; Santangelo, S. Cost-Effective Bimetallic Catalysts for Green H2 Production in Anion Exchange Membrane Water Electrolyzers. Nanomaterials 2025, 15, 1042. https://doi.org/10.3390/nano15131042
Campagna Zignani S, Fazio M, Pascale M, Alessandrello C, Triolo C, Musolino MG, Santangelo S. Cost-Effective Bimetallic Catalysts for Green H2 Production in Anion Exchange Membrane Water Electrolyzers. Nanomaterials. 2025; 15(13):1042. https://doi.org/10.3390/nano15131042
Chicago/Turabian StyleCampagna Zignani, Sabrina, Marta Fazio, Mariarosaria Pascale, Chiara Alessandrello, Claudia Triolo, Maria Grazia Musolino, and Saveria Santangelo. 2025. "Cost-Effective Bimetallic Catalysts for Green H2 Production in Anion Exchange Membrane Water Electrolyzers" Nanomaterials 15, no. 13: 1042. https://doi.org/10.3390/nano15131042
APA StyleCampagna Zignani, S., Fazio, M., Pascale, M., Alessandrello, C., Triolo, C., Musolino, M. G., & Santangelo, S. (2025). Cost-Effective Bimetallic Catalysts for Green H2 Production in Anion Exchange Membrane Water Electrolyzers. Nanomaterials, 15(13), 1042. https://doi.org/10.3390/nano15131042









