Sustainable Sugarcane Bagasse-Derived Activated Carbon for High-Performance Symmetric Supercapacitor Devices Applications
Abstract
1. Introduction
2. Experimental Method
Electrode Preparation and Characterization
3. Result and Discussion
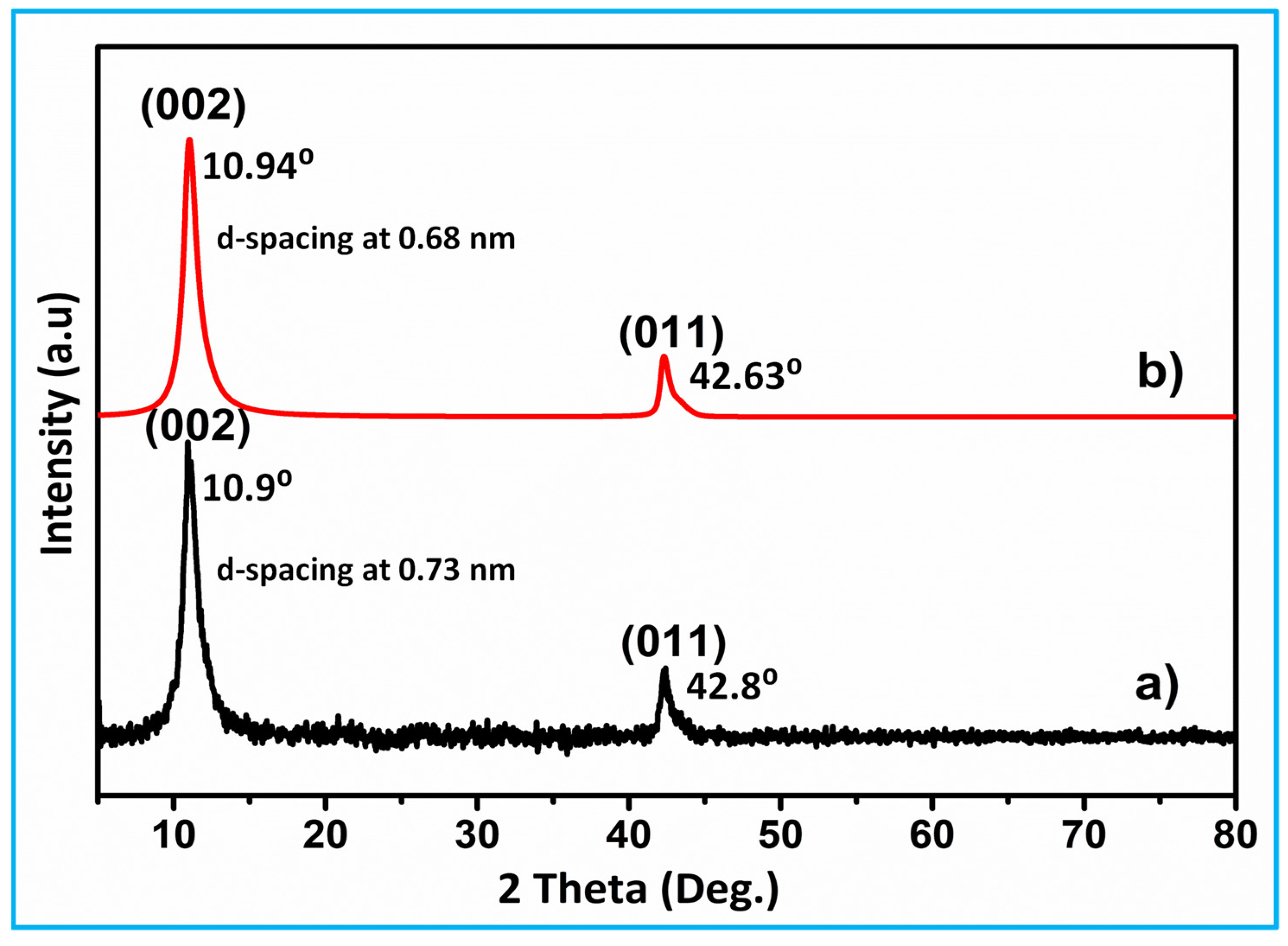
3.1. XRD Analysis
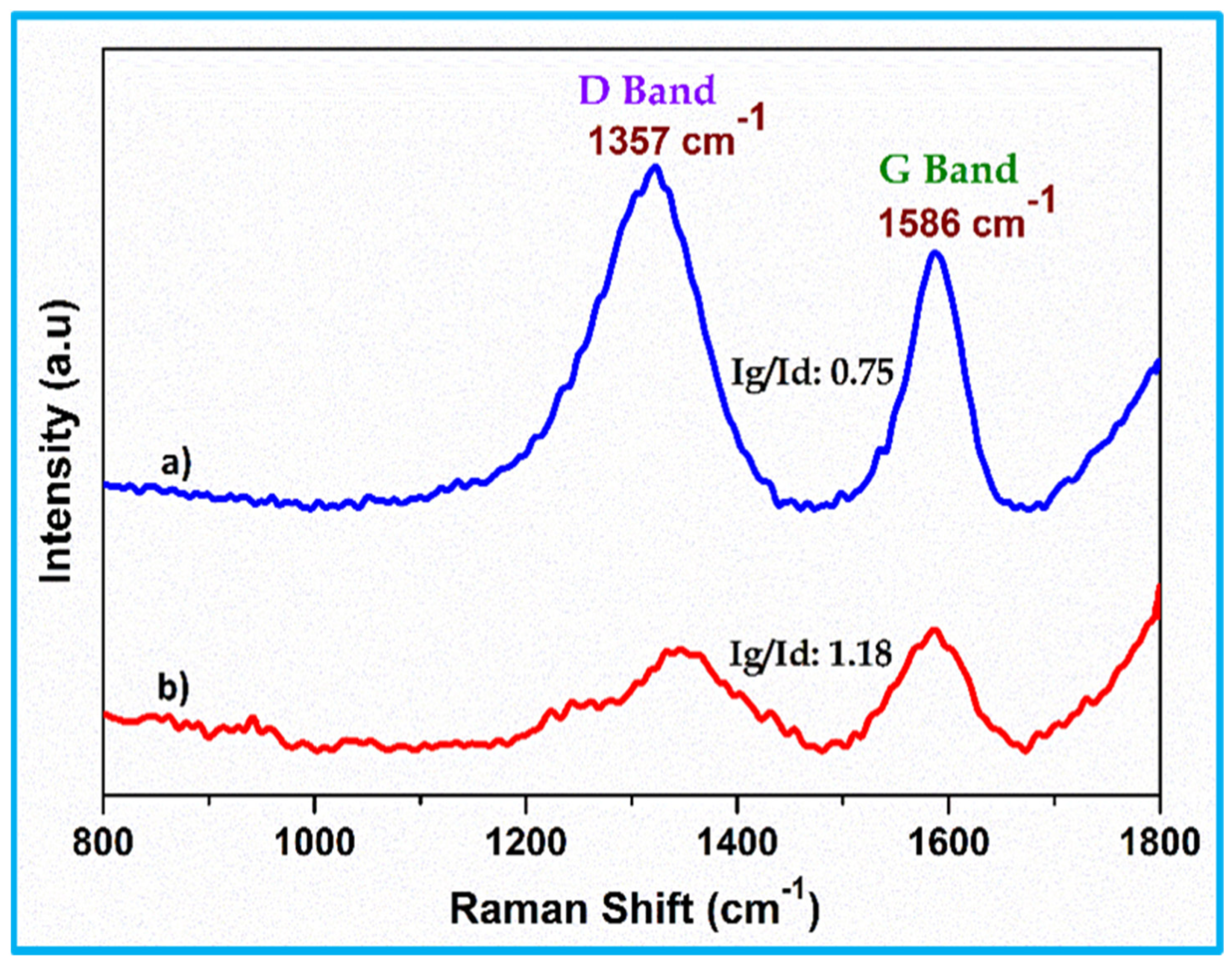
3.2. FT-Raman Analysis
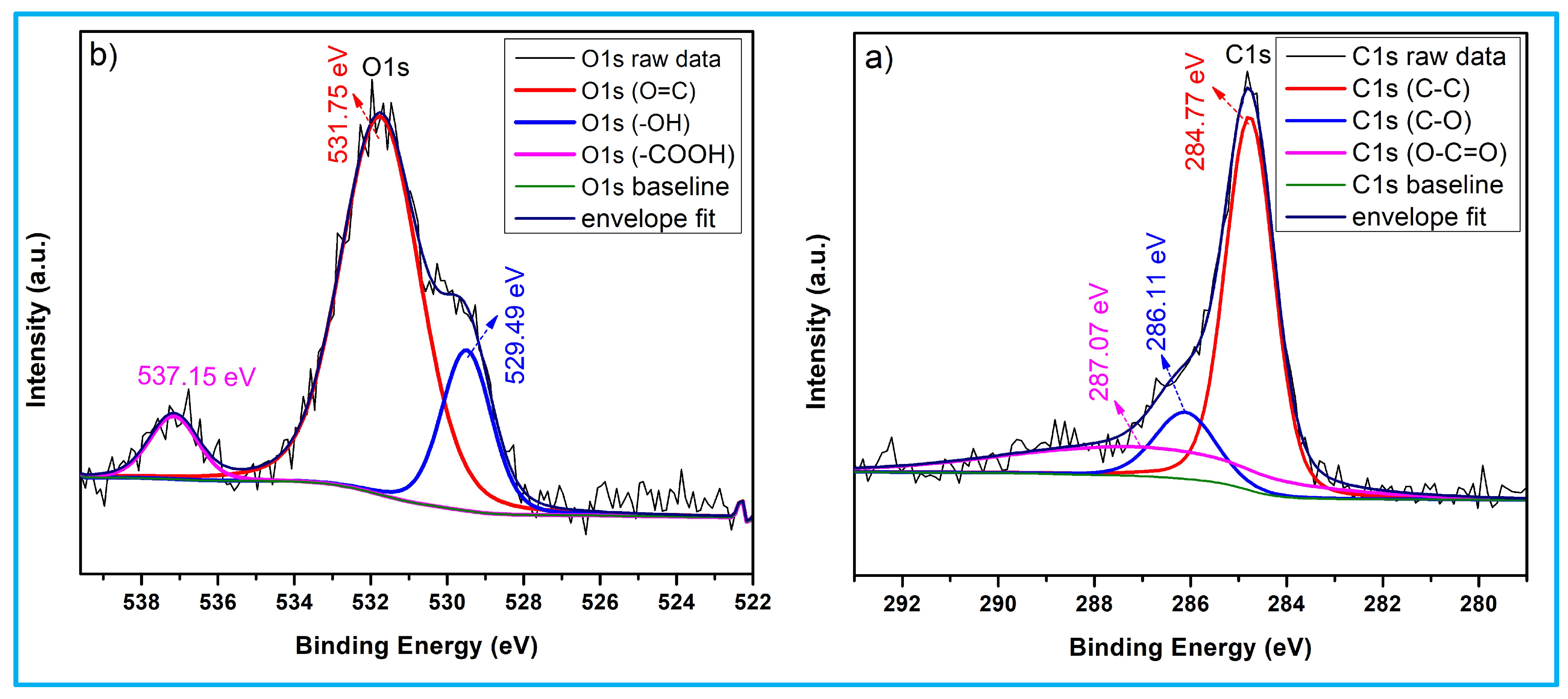
3.3. X-Ray Photoelectron Spectroscopy Analysis
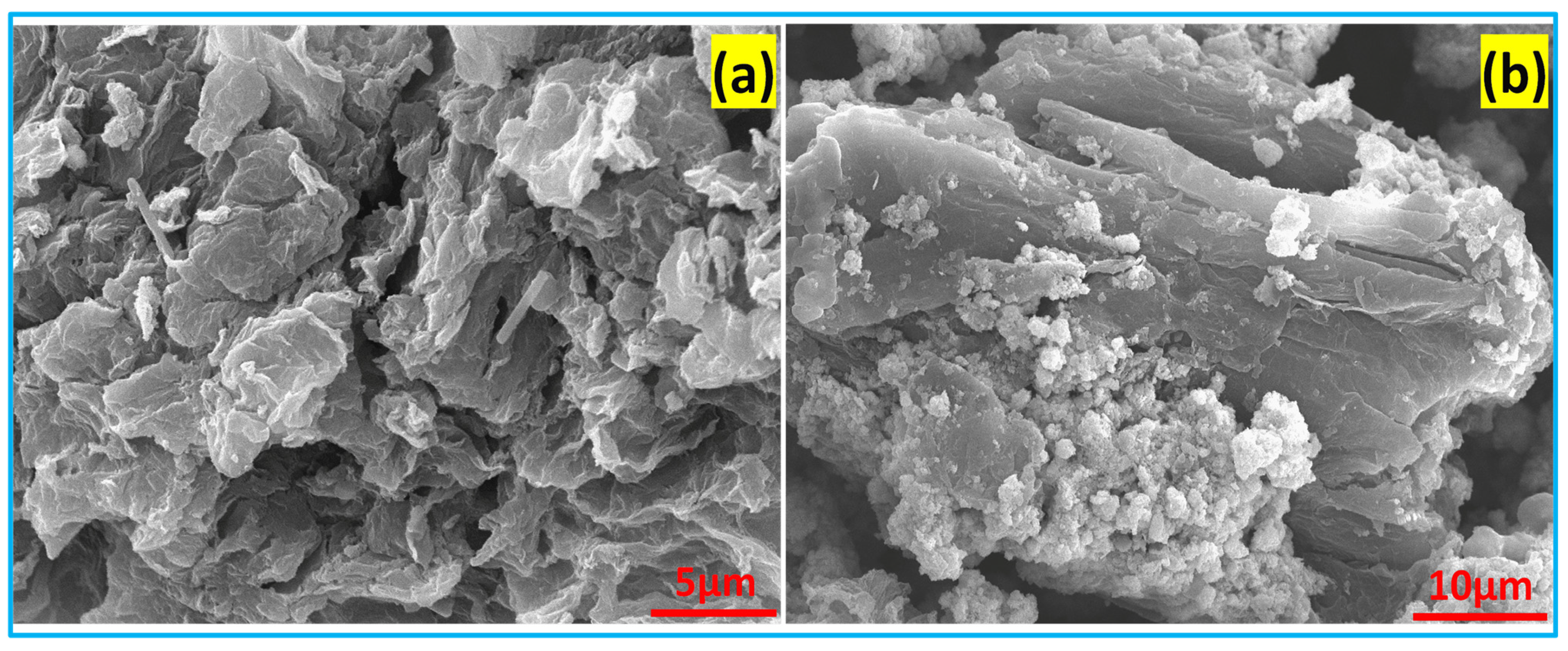
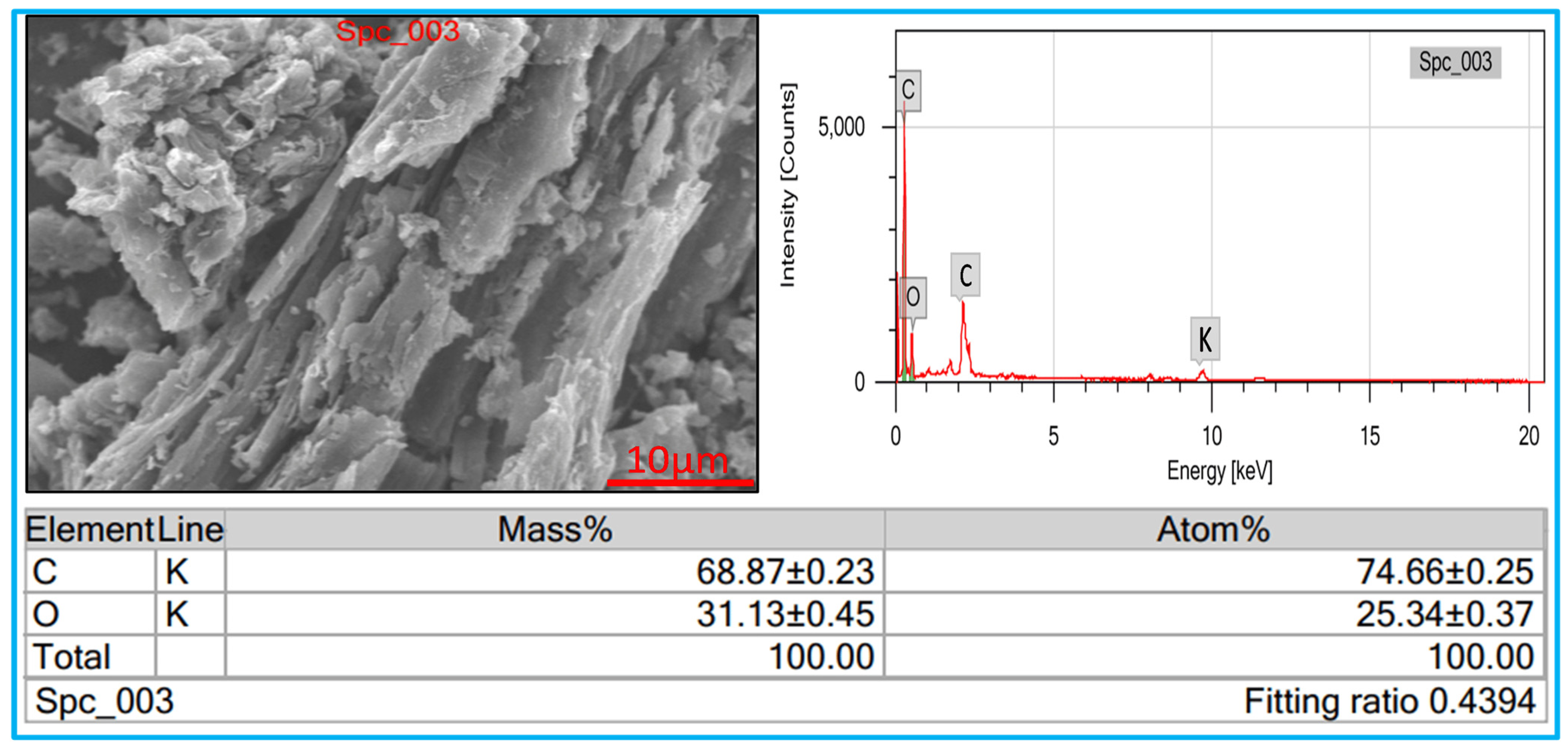
3.4. Morphology Analysis
3.5. Elemental Analysis
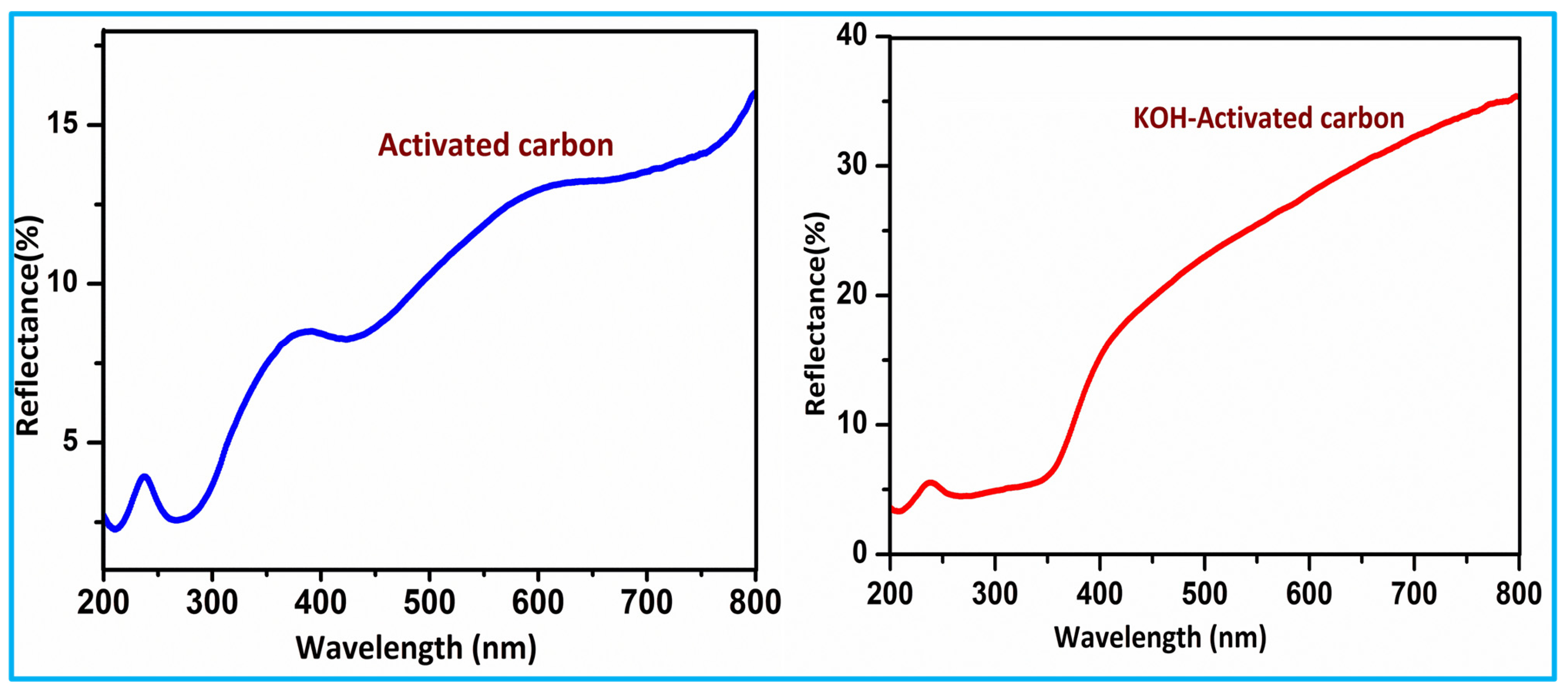
3.6. UV-DRS
4. Electrochemical Measurements
4.1. Three-Electrode Cell System of CV, GCD, and EIS
4.2. Electrochemical Performance of Two-Electrode Symmetric Device Configuration
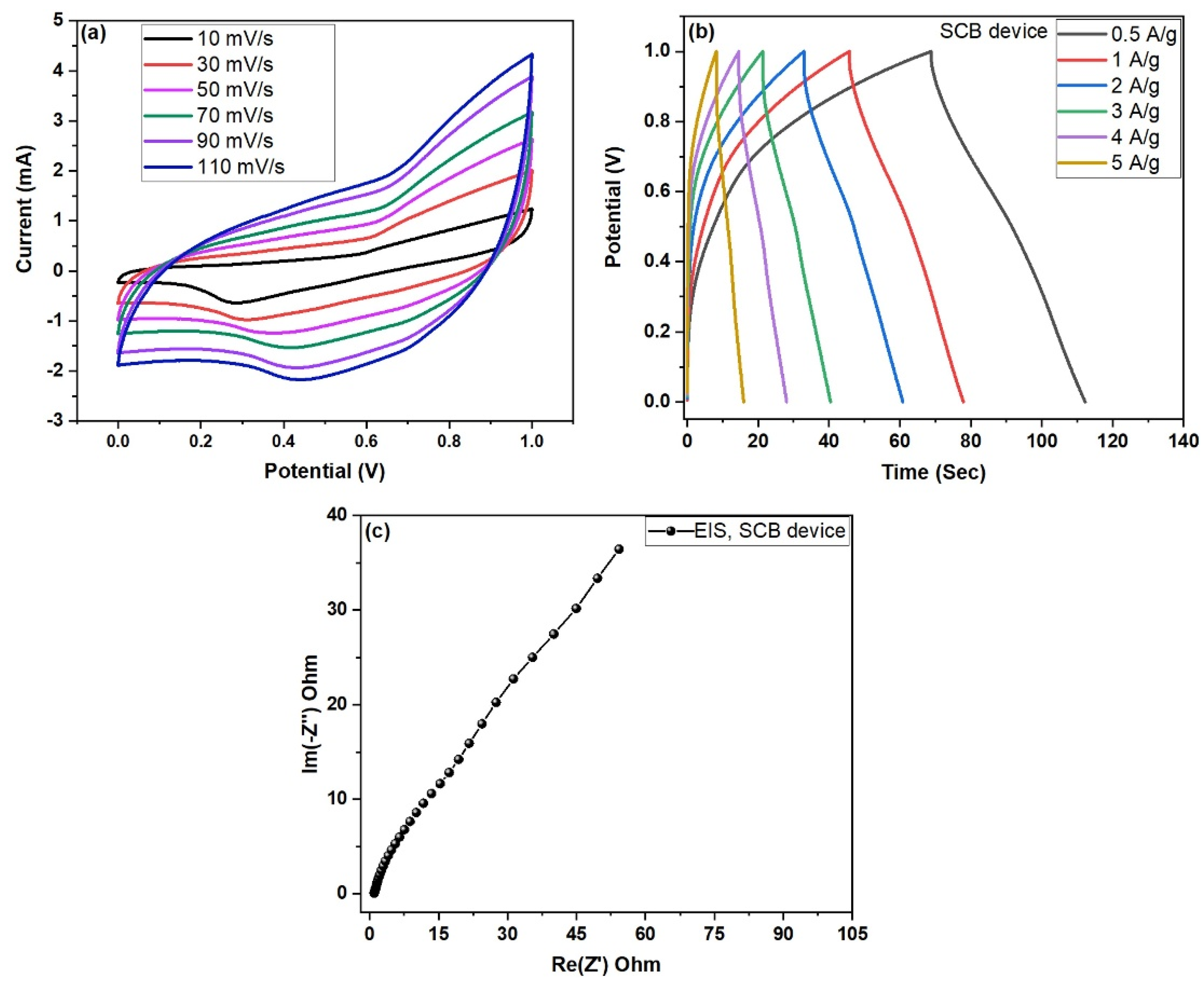
4.3. Electrochemical Performance of SCB//SCB Symmetric Device
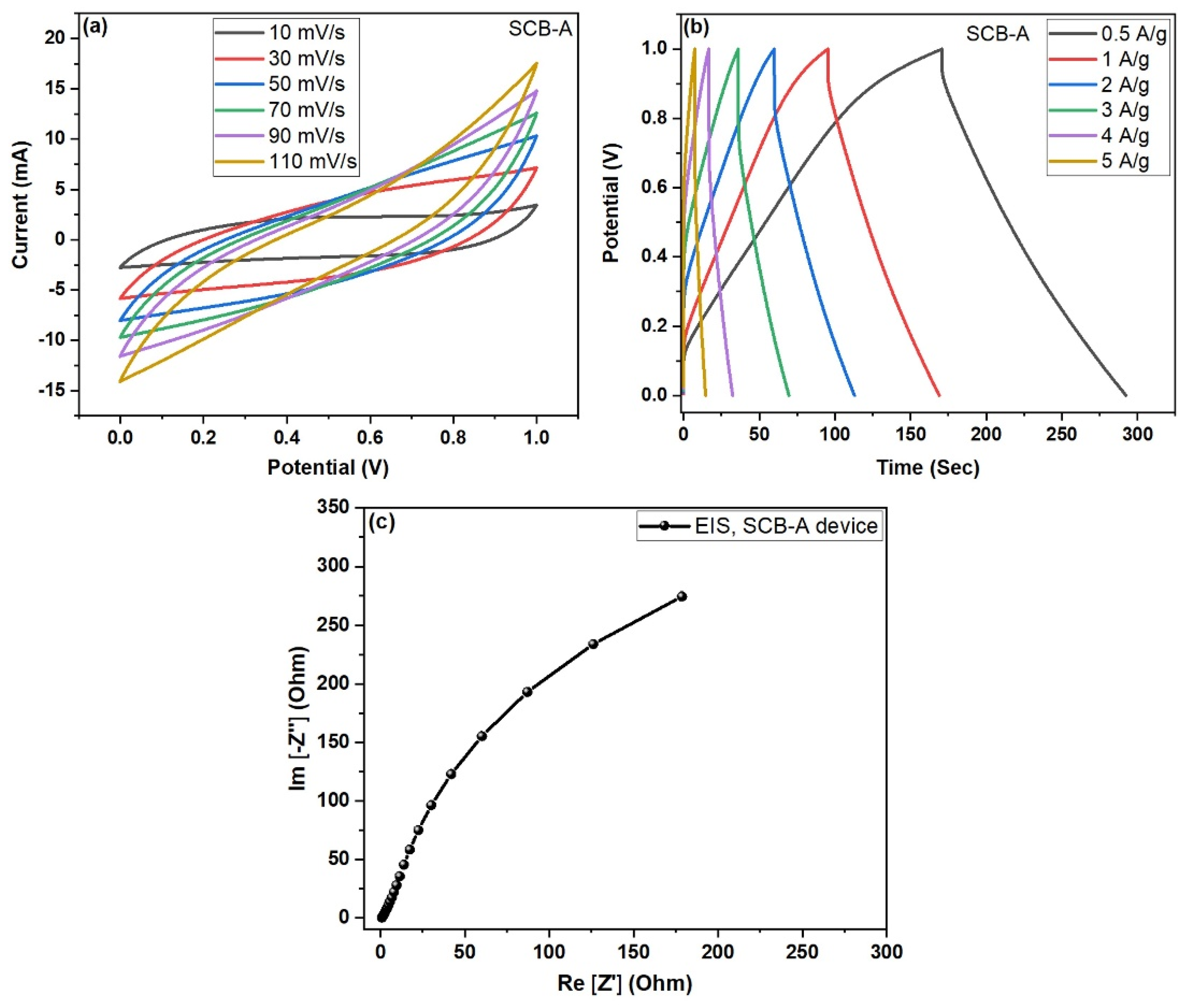
4.4. Electrochemical Performance of Activated SCB-A//SCB-A Symmetric Device
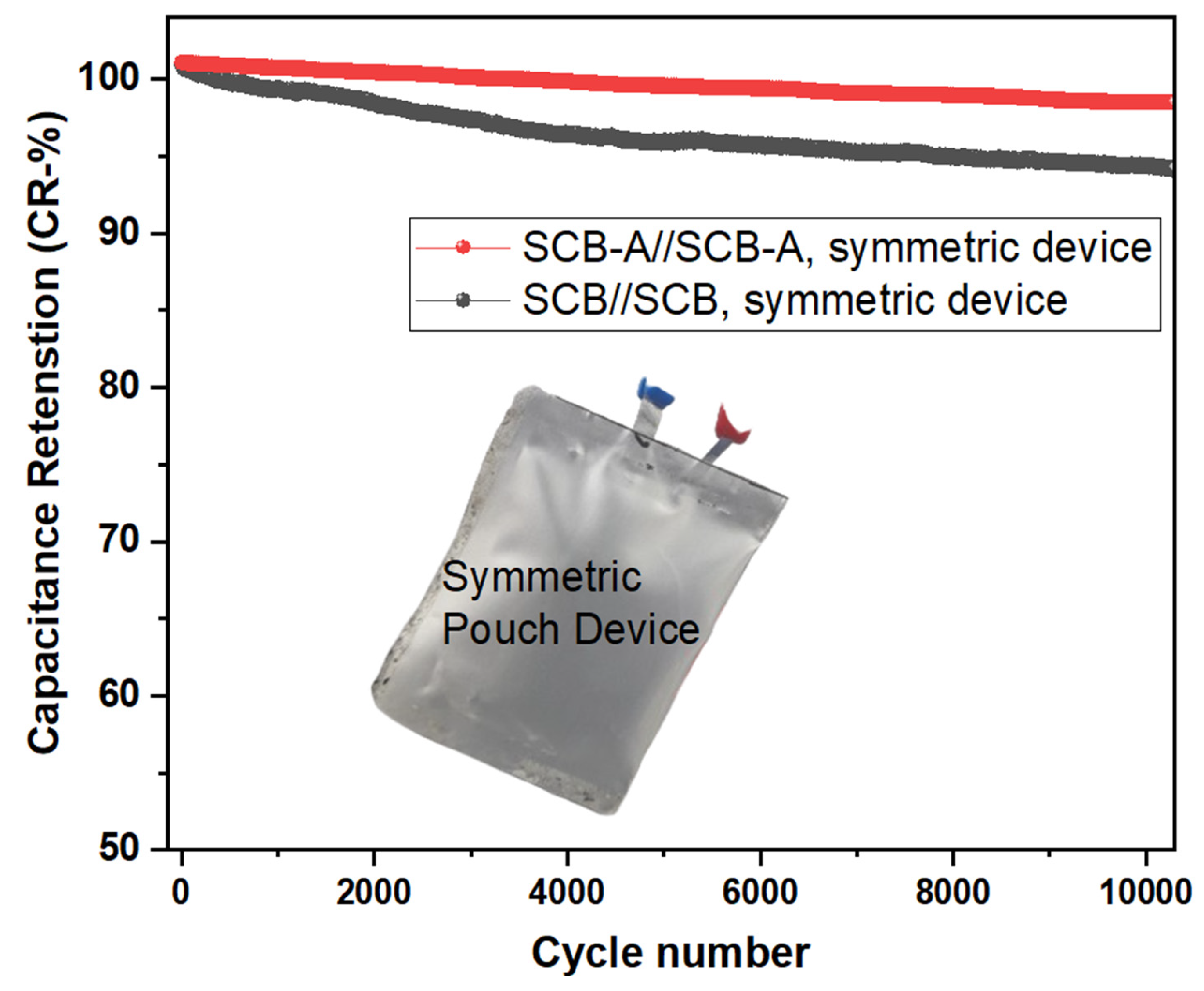
4.5. Cycling Stability and Capacity Retention (CR%)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, L.; Yang, M.; Chen, G. Continuous synthesis of reduced graphene oxide-supported bimetallic NPs in liquid–liquid segmented flow. Ind. Eng. Chem. Res. 2020, 59, 8456–8468. [Google Scholar] [CrossRef]
- Liu, P.; Sun, S.; Huang, S.; Wu, Y.; Li, X.; Wei, X.; Wu, S. KOH Activation Mechanism in the Preparation of Brewer’s Spent Grain-Based Activated Carbons. Catalysts 2024, 14, 814. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Q.; Wang, J.; Zhang, S.; Wei, C.; Cao, L.; Zhang, S. In Situ, Nitrogen-Doped Porous Carbon Derived from Mixed Biomass as Ultra-High-Performance Supercapacitor. Nanomaterials 2024, 14, 1368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Du, H.; Wei, Z.; Zhang, X.; Wang, R. Ultrafast microwave synthesis of nickel–cobalt sulfide/graphene hybrid electrodes for high-performance asymmetrical supercapacitors. ACS Appl. Energy Mater. 2021, 4, 8262–8274. [Google Scholar] [CrossRef]
- Zhang, M.; Nautiyal, A.; Du, H.; Wei, Z.; Zhang, X.; Wang, R. Electropolymerization of polyaniline as high-performance binder free electrodes for flexible supercapacitor. Electrochim. Acta 2021, 376, 138037. [Google Scholar] [CrossRef]
- Dideikin, A.T.; Vul’, A.Y. Graphene oxide and derivatives: The place in graphene family. Front. Phys. 2019, 6, 149. [Google Scholar] [CrossRef]
- Barakat, Z.M.; Dehua, Z.; Ulf-Peter, A.; Takahiro, N.; Refaie, S.E.; Kenawy, S.; Jianjian, L. Dual-heteroatom-doped reduced graphene oxide sheets conjoined Co–Ni-based carbide and sulfide nanoparticles for efficient oxygen evolution reaction. ACS Catal. 2020, 12. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, J.; Du, F. Synthesis of highly reduced graphene oxide for supercapacitor. J. Nanomater. 2016, 1, 4840301. [Google Scholar] [CrossRef]
- Posudievsky, O.Y.; Khazieieva, O.A.; Koshechko, V.G.; Pokhodenko, V.D. Preparation of graphene oxide by solvent-free mechanochemical oxidation of graphite. J. Mater. Chem. 2012, 22, 12465–12467. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Q.; Wan, M.; Yao, Z.; Hu, M. Heteroatom-Doped Carbon-Based Catalysts Synthesized through a “Cook-Off” Process for Oxygen Reduction Reaction. Processes 2024, 12, 264. [Google Scholar] [CrossRef]
- Suárez-Iglesias, O.; Collado, S.; Oulego, P. Graphene-family nanomaterials in wastewater treatment plants. Chem. Eng. J. 2017, 313, 121–135. [Google Scholar] [CrossRef]
- Ndagijimana, P.; Rong, H.; Ndokoye, P.; Mwizerwa, J.P.; Nkinahamira, F.; Luo, S.; Guo, D.; Cui, B. A review on activated carbon/graphene composite-based materials: Synthesis and applications. J. Clean. Prod. 2023, 417, 138006. [Google Scholar] [CrossRef]
- Sharma, K.; Kadyan, P.; Sharma, R.K.; Grover, S. Heteroatom doping in bio-waste derived activated carbon for enhanced supercapacitor performance: A review. J. Energy Storage 2024, 100, 113679. [Google Scholar] [CrossRef]
- Li, L.; Wen, J.; Liang, J.; Cheng, X.; Yao, J.; Gao, Y.; Hu, S.; Wu, H.; Zheng, J.; Li, G. Facile Synthesis of N-Doped Porous Carbon Materials Derived from Bombyx mori Silk for High-Performance Symmetric Supercapacitors. Chem. Eur. J. 2025, 31, e202404569. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Sharma, K.; Shafi, P.M. An overview, methods of synthesis and modification of carbon-based electrodes for supercapacitor. J. Energy Storage 2022, 55, 105727. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, W.; Alhebshi, N.A.; Salah, N.; Alshareef, H.N. Synthesis strategies of porous carbon for supercapacitor applications. Small Methods 2020, 4, 1900853. [Google Scholar] [CrossRef]
- Okonkwo, C.A.; Menkiti, M.C.; Obiora-Okafo, I.A.; Ezenwa, O.N. Controlled pyrolysis of sugarcane bagasse enhanced mesoporous carbon for improving capacitance of supercapacitor electrode. Biomass Bioenergy 2021, 146, 105996. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Mishra, A.K.; Rajasekar, R.; Kim, N.H.; Lee, J.H. Carbon-based nanostructured materials and their composites as supercapacitor electrodes. J. Mater. Chem. 2012, 22, 767–784. [Google Scholar] [CrossRef]
- Saini, S.; Chand, P.; Joshi, A. Biomass derived carbon for supercapacitor applications: Review. J. Energy Storage 2021, 39, 102646. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Egbedina, A.O.; Bolade, O.P.; Ewuzie, U.; Lima, E.C. Emerging trends in the application of carbon-based materials: A review. J. Environ. Chem. Eng. 2022, 10, 107260. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Sankar, K.V.; Melo, J.S.; Gedanken, A.; Selvan, R.K. Carbon-based hybrid composites as advanced electrodes for supercapacitors. Adv. Funct. Mater. 2015, 25, 399–431. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abbas, Q.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Sayed, E.T. Carbon-based materials for supercapacitors: Recent progress, challenges and barriers. Batteries 2022, 9, 19. [Google Scholar] [CrossRef]
- Lamba, P.; Singh, P.; Singh, P.; Singh, P.; Kumar, A.; Gupta, M.; Kumar, Y. Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance. J. Energy Storage 2022, 48, 103871. [Google Scholar] [CrossRef]
- Luo, L.; Zhou, Y.; Yan, W.; Wu, X.; Wang, S.; Zhao, W. Two-step synthesis of B and N co-doped porous carbon composites by microwave-assisted hydrothermal and pyrolysis process for supercapacitor application. Electrochim. Acta 2020, 360, 137010. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, D.; Xie, Z.; Kang, Y.; Tang, Z.; Dai, Y.; Liang, F. Activated carbon prepared from waste tire pyrolysis carbon black via CO2/KOH activation used as supercapacitor electrode. Sci. China Technol. Sci. 2022, 65, 2337–2347. [Google Scholar] [CrossRef]
- Haghighi, P.L.; Berktas, I.; Ali, H.Q.; Saner Okan, B.; Yıldız, M. Toward next-generation carbon-based materials derived from waste and biomass for high-performance energy applications. Energy Technol. 2020, 8, 2000714. [Google Scholar] [CrossRef]
- Mehare, M.D.; Deshmukh, A.D.; Dhoble, S.J. Bio-waste lemon peel derived carbon-based electrode in perspective of supercapacitor. J. Mater. Sci. Mater. Electron. 2021, 32, 14057–14071. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.S. Biomass-derived carbon electrode materials for supercapacitors. Sustain. Energy Fuels 2017, 1, 1265–1281. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Cai, Y.; Sang, X.; Zhang, Q.; Ma, J.; Chen, X. Nitrogen-Doped Carbon Materials As Supercapacitor Electrodes: A Mini Review. Energy Fuels 2024, 38, 10542–10559. [Google Scholar] [CrossRef]
- Wang, N.; Wu, C.; Li, J.; Dong, G.; Guan, L. Binder-free manganese oxide/carbon nanomaterials thin film electrode for supercapacitors. ACS Appl. Mater. Interfaces 2011, 3, 4185–4189. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.I.; Khan, A.H.; Ahmad, R.; Khan, I.; Mehmood, K.; Khan, F.; Shah, S.M.; Shah, A.; Bibi, S.; Khan, S. Enhanced Electrochemical Performance of Supercapacitors Using ZnO/N-Doped Graphene Composite Electrodes. Mater. Sci. Eng. B 2023, 294, 117151. [Google Scholar] [CrossRef]
- Huarote-Garcia, E.; Cardenas-Riojas, A.A.; Monje, I.E.; López, E.O.; Arias-Pinedo, O.M.; Planes, G.A.; Baena-Moncada, A.M. Activated Carbon Electrodes for Supercapacitors from Purple Corncob (Zea mays L.). ACS Environ. Au 2024, 4, 80–88. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, X.; Zhang, Y.; Qian, X. Fe2O3/Reduced Graphene Oxide/Fe3O4 Composite in Situ Grown on Fe Foil for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30133–30142. [Google Scholar] [CrossRef]
- Yaqoob, L.; Noor, T.; Iqbal, N. Conversion of plastic waste to carbon-based compounds and application in energy storage devices. ACS Omega 2022, 7, 13403–13435. [Google Scholar] [CrossRef]
- Enock, T.K.; King’ondu, C.K.; Pogrebnoi, A.; Jande, Y.A.C. Status of biomass derived carbon materials for supercapacitor application. Int. J. Electrochem. 2017, 2017, 6453420. [Google Scholar] [CrossRef]




| Current Density A/g | Specific Capacitance (F/g) | |
|---|---|---|
| (SCB) Activated Carbon | KOH-Activated Carbon (SCB-A) | |
| 0.5 | 132.20 | 253.41 |
| 1 | 93.26 | 173.88 |
| 2 | 61.95 | 131.30 |
| 3 | 38.34 | 85.11 |
| 4 | 21.93 | 41.73 |
| 5 | 14.91 | 20.85 |
| Current Density A/g | Specific Capacitance (F/g) | |
|---|---|---|
| SCB//SCB Symmetric Device | SCB-A//SCB-A Symmetric Device | |
| 0.5 | 84.64 | 145.21 |
| 1 | 61.06 | 103.06 |
| 2 | 47.65 | 81.44 |
| 3 | 32.10 | 68.01 |
| 4 | 19.73 | 51.80 |
| 5 | 9.81 | 37.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajivgandhi, P.; Thirumal, V.; Sekar, A.; Kim, J. Sustainable Sugarcane Bagasse-Derived Activated Carbon for High-Performance Symmetric Supercapacitor Devices Applications. Nanomaterials 2025, 15, 1028. https://doi.org/10.3390/nano15131028
Rajivgandhi P, Thirumal V, Sekar A, Kim J. Sustainable Sugarcane Bagasse-Derived Activated Carbon for High-Performance Symmetric Supercapacitor Devices Applications. Nanomaterials. 2025; 15(13):1028. https://doi.org/10.3390/nano15131028
Chicago/Turabian StyleRajivgandhi, Perumal, Vediyappan Thirumal, Alagan Sekar, and Jinho Kim. 2025. "Sustainable Sugarcane Bagasse-Derived Activated Carbon for High-Performance Symmetric Supercapacitor Devices Applications" Nanomaterials 15, no. 13: 1028. https://doi.org/10.3390/nano15131028
APA StyleRajivgandhi, P., Thirumal, V., Sekar, A., & Kim, J. (2025). Sustainable Sugarcane Bagasse-Derived Activated Carbon for High-Performance Symmetric Supercapacitor Devices Applications. Nanomaterials, 15(13), 1028. https://doi.org/10.3390/nano15131028





