Nanoporous Copper Films via Dynamic Hydrogen Bubbling: A Promising SERS Substrate for Sensitive Detection of Methylene Blue
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Fabrication of NPC
2.3. Characterization of NPC
2.4. SERS Measurements
3. Results and Discussions
3.1. Morphological Characterization
3.2. Structural Characterization
3.3. SERS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, P.; Wu, Z.; Zhao, Y. Hierarchical porous Cu with high surface area and fluid permeability. Scr. Mater. 2019, 172, 119–124. [Google Scholar] [CrossRef]
- Cao, C.; Cheng, J. Fabrication of robust surfaces with special wettability on porous copper substrates for various oil/water separations. Chem. Eng. J. 2018, 347, 585–594. [Google Scholar] [CrossRef]
- Vassileva, E.; Mihaylov, L.; Lyubenova, L.; Spassov, T.; Scaglione, F.; Rizzi, P. Porous metallic structures by dealloying amorphous alloys. J. Alloys Compd. 2023, 969, 172417. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L.; Denis, P.; Fecht, H.-J. Electrodeposited platinum on de-alloyed nanoporous gold with enhanced electro-catalytic performance. Appl. Surf. Sci. 2019, 476, 412–417. [Google Scholar] [CrossRef]
- Scaglione, F.; Xue, Y.; Celegato, F.; Rizzi, P.; Battezzati, L. Amorphous molybdenum sulphide @ nanoporous gold as catalyst for hydrogen evolution reaction in acidic environment. J. Mater. Sci. 2018, 53, 12388–12398. [Google Scholar] [CrossRef]
- Paschalidou, E.M.; Scaglione, F.; Gebert, A.; Oswald, S.; Rizzi, P.; Battezzati, L. Partially and fully de-alloyed glassy ribbons based on Au: Application in methanol electro-oxidation studies. J. Alloys Compd. 2016, 667, 302–309. [Google Scholar] [CrossRef]
- Scaglione, F.; Alladio, E.; Damin, A.; Turci, F.; Baggiani, C.; Giovannoli, C.; Bordiga, S.; Battezzati, L.; Rizzi, P. Functionalized nanoporous gold as a new biosensor platform for ultra-low quantitative detection of human serum albumin. Sens. Actuators B Chem. 2019, 288, 460–468. [Google Scholar] [CrossRef]
- Fujita, T. Hierarchical nanoporous metals as a path toward the ultimate three-dimensional functionality. Sci. Technol. Adv. Mater. 2017, 18, 724–740. [Google Scholar] [CrossRef]
- Raj, D.; Scaglione, F.; Rizzi, P. Rapid Fabrication of Fe and Pd Thin Films as SERS-Active Substrates via Dynamic Hydrogen Bubble Template Method. Nanomaterials 2022, 13, 135. [Google Scholar] [CrossRef]
- Cialone, M.; Celegato, F.; Scaglione, F.; Barrera, G.; Raj, D.; Coïsson, M.; Tiberto, P.; Rizzi, P. Nanoporous FePd alloy as multifunctional ferromagnetic SERS-active substrate. Appl. Surf. Sci. 2021, 543, 148759. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G. Nanoporous Gold-Based Sensing. Coatings 2020, 10, 899. [Google Scholar] [CrossRef]
- Jiang, J.; Lim, Y.S.; Park, S.; Kim, S.-H.; Yoon, S.; Piao, L. Hollow porous Cu particles from silica-encapsulated Cu2O nanoparticle aggregates effectively catalyze 4-nitrophenol reduction. Nanoscale 2017, 9, 3873–3880. [Google Scholar] [CrossRef]
- Lv, J.; Jouny, M.; Luc, W.; Zhu, W.; Zhu, J.; Jiao, F. A Highly Porous Copper Electrocatalyst for Carbon Dioxide Reduction. Adv. Mater. 2018, 30, e1803111. [Google Scholar] [CrossRef]
- Rehman, T.-U.; Ali, H.M. Experimental investigation on paraffin wax integrated with copper foam based heat sinks for electronic components thermal cooling. Int. Commun. Heat Mass Transf. 2018, 98, 155–162. [Google Scholar] [CrossRef]
- Schlücker, S. Surface-Enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem. Int. Ed. Engl. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Plowman, B.J.; Jones, L.A.; Bhargava, S.K. Building with bubbles: The formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition. Chem. Commun. 2015, 51, 4331–4346. [Google Scholar] [CrossRef]
- Nam, S.; Jo, H.; Choe, H.; Ahn, D.; Choi, H. Development of Nanoporous Copper Foams by Chemical Dealloying of Mechanically Alloyed Al–Cu Compounds. Mater. Trans. 2014, 55, 1414–1418. [Google Scholar] [CrossRef]
- Asnavandi, M.; Zhao, C. Hydrogen Bubble-Assisted Electrodeposition of Metal Nanoparticles from Protic Ionic Liquids for Electrocatalysis. ACS Sustain. Chem. Eng. 2016, 5, 85–89. [Google Scholar] [CrossRef]
- Pang, K.; Hou, Y.; Wu, W.; Tian, S.; Sun, N. Control of the morphology of electrodeposited three-dimensional copper foam by tuning the pressure. Sci. China Chem. 2012, 55, 1325–1329. [Google Scholar] [CrossRef]
- Darayen, J.; Chailapakul, O.; Praserthdam, P.; Panpranot, J.; Tungasmita, D.N.; Boonyongmaneerat, Y. Porous Electrodeposited Cu as a Potential Electrode for Electrochemical Reduction Reactions of CO2. Appl. Sci. 2021, 11, 11104. [Google Scholar] [CrossRef]
- Vivegnis, S.; Delhalle, J.; Mekhalif, Z.; Renner, F. Copper–zinc alloy electrodeposition mediated by triethanolamine as a complexing additive and chemical dealloying. Electrochim. Acta 2019, 319, 400–409. [Google Scholar] [CrossRef]
- Shin, H.; Dong, J.; Liu, M. Nanoporous Structures Prepared by an Electrochemical Deposition Process. Adv. Mater. 2003, 15, 1610–1614. [Google Scholar] [CrossRef]
- Shin, H.-C.; Liu, M. Copper Foam Structures with Highly Porous Nanostructured Walls. Chem. Mater. 2004, 16, 5460–5464. [Google Scholar] [CrossRef]
- Li, Y.; Jia, W.-Z.; Song, Y.-Y.; Xia, X.-H. Superhydrophobicity of 3D Porous Copper Films Prepared Using the Hydrogen Bubble Dynamic Template. Chem. Mater. 2007, 19, 5758–5764. [Google Scholar] [CrossRef]
- Rahmati, F.; Sabouhanian, N.; Lipkowski, J.; Chen, A. Synthesis of 3D Porous Cu Nanostructures on Ag Thin Film Using Dynamic Hydrogen Bubble Template for Electrochemical Conversion of CO2 to Ethanol. Nanomaterials 2023, 13, 778. [Google Scholar] [CrossRef]
- Du, D.; Lan, R.; Humphreys, J.; Sengodan, S.; Xie, K.; Wang, H.; Tao, S. Achieving Both High Selectivity and Current Density for CO2 Reduction to Formate on Nanoporous Tin Foam Electrocatalysts. ChemistrySelect 2016, 1, 1711–1715. [Google Scholar] [CrossRef]
- Hui, S.; Shaigan, N.; Neburchilov, V.; Zhang, L.; Malek, K.; Eikerling, M.; De Luna, P. Three-Dimensional Cathodes for Electrochemical Reduction of CO2: From Macro- to Nano-Engineering. Nanomaterials 2020, 10, 1884. [Google Scholar] [CrossRef] [PubMed]
- Bommireddy, N.; Palathedath, S.K. Surfactant mediated electrodeposition of copper nanostructures for environmental electrochemistry: Influence of morphology on electrochemical nitrate reduction reaction. J. Solid State Electrochem. 2022, 26, 2733–2742. [Google Scholar] [CrossRef]
- Das, M.; Biswas, A.; Purkait, T.; Boruah, T.; Bhardwaj, S.; Das, S.K.; Dey, R.S. The versatility of the dynamic hydrogen bubble template derived copper foam on the emerging energy applications: Progress and future prospects. J. Mater. Chem. A 2022, 10, 13589–13624. [Google Scholar] [CrossRef]
- Shao, Q.; Que, R.; Shao, M.; Cheng, L.; Lee, S. Copper Nanoparticles Grafted on a Silicon Wafer and Their Excellent Surface-Enhanced Raman Scattering. Adv. Funct. Mater. 2012, 22, 2067–2070. [Google Scholar] [CrossRef]
- Zhu, T.; Sun, Y.; Lu, W.; Wang, G.; Zhang, X.; Chen, S.; Zhang, C.; Li, Z.; Man, B.; Yang, C. Theoretical and experimental investigation of the flexible Ag nano-tree@Cu mesh SERS substrate. J. Alloys Compd. 2022, 908, 164622. [Google Scholar] [CrossRef]
- Chen, L.; Yu, J.; Fujita, T.; Chen, M. Nanoporous Copper with Tunable Nanoporosity for SERS Applications. Adv. Funct. Mater. 2009, 19, 1221–1226. [Google Scholar] [CrossRef]
- Tan, Y.; Gu, J.; Xu, L.; Zang, X.; Liu, D.; Zhang, W.; Liu, Q.; Zhu, S.; Su, H.; Feng, C.; et al. High-Density Hotspots Engineered by Naturally Piled-Up Subwavelength Structures in Three-Dimensional Copper Butterfly Wing Scales for Surface-Enhanced Raman Scattering Detection. Adv. Funct. Mater. 2012, 22, 1578–1585. [Google Scholar] [CrossRef]
- Thetford, D. Triphenylmethane and Related Dyes. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons: Hoboken, NJ, USA, 2000; pp. 1–19. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Pradel, J.S.; Tong, W.G. Determination of malachite green, crystal violet, brilliant green and methylene blue by micro-cloud-point extraction and nonlinear laser wave-mixing detection interfaced to micellar capillary electrophoresis. Anal. Methods 2017, 9, 6411–6419. [Google Scholar] [CrossRef]
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Sarwar, T.; Khan, S. Textile Industry: Pollution Health Risks and Toxicity. In Textile Wastewater Treatment: Sustainable Bio-nano Materials and Macromolecules, Volume 1; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–28. [Google Scholar]
- Plakas, S.M.; Doerge, D.R.; Turnipseed, S.B. Disposition and Metabolism of Malachite Green and Other Thera-peutic Dyes in Fish. In Xenobiotics in Fish; Springer: Berlin/Heidelberg, Germany, 1999; pp. 149–166. [Google Scholar]
- Lutterotti, L.; Wenk, H.; Matthies, S. MAUD (Material Analysis Using Diffraction): A User Friendly Java Program for Rietveld Texture Analysis and More. In Proceedings of the Twelfth International Conference on Textures of Materials (ICOTOM-12), Montreal, QC, Canada, 9–13 August 1999; Volume 2, pp. 1599–1604. [Google Scholar]
- Nikolić, N.; Popov, K.; Pavlović, L.; Pavlović, M. The effect of hydrogen codeposition on the morphology of copper electrodeposits. I. The concept of effective overpotential. J. Electroanal. Chem. 2006, 588, 88–98. [Google Scholar] [CrossRef]
- Giura, A.; Zucco, M.; Ribotta, L. SurfILE: An Open-Source Python Package for Surface Topography Analysis. Metrology 2024, 4, 695–717. [Google Scholar] [CrossRef]
- Song, Y.; Hu, J.; Tang, J.; Gu, W.; He, L.; Ji, X. Real-Time X-ray Imaging Reveals Interfacial Growth, Suppression, and Dissolution of Zinc Dendrites Dependent on Anions of Ionic Liquid Additives for Rechargeable Battery Applications. ACS Appl. Mater. Interfaces 2016, 8, 32031–32040. [Google Scholar] [CrossRef]
- Naujok, R.R.; Duevel, R.V.; Corn, R.M. Fluorescence and Fourier Transform surface-enhanced Raman scattering measurements of methylene blue adsorbed onto a sulfur-modified gold electrode. Langmuir 1993, 9, 1771–1774. [Google Scholar] [CrossRef]
- Raj, D.; Palumbo, M.; Fiore, G.; Celegato, F.; Scaglione, F.; Rizzi, P. Sustainable nanoporous gold with excellent SERS performances. Mater. Chem. Phys. 2022, 293, 126883. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, J.; Li, R.; Xu, C.; Liu, X.; Wang, Y.; Fu, E.; Lu, Z. Ion Irradiation-Enhanced Raman Scattering on Nanoporous Copper. Langmuir 2018, 34, 13041–13046. [Google Scholar] [CrossRef] [PubMed]
- Diao, F.; Xiao, X.; Luo, B.; Sun, H.; Ding, F.; Ci, L.; Si, P. Two-step fabrication of nanoporous copper films with tunable morphology for SERS application. Appl. Surf. Sci. 2018, 427, 1271–1279. [Google Scholar] [CrossRef]
- Xu, Z.; Erinomo, A.M.; Dan, Z.; Qin, F.; Chang, H. Flexible SERS substrates with gradient porous Cu structure dealloying from the thermal diffusion couples of Al/Cu stacking foils. Chem. Eng. J. 2024, 490, 151871. [Google Scholar] [CrossRef]
- Li, M.; Su, Y.; Zhao, J.; Geng, H.; Zhang, J.; Zhang, L.; Yang, C.; Zhang, Y. One-pot preparation of thin nanoporous copper foils with enhanced light absorption and SERS properties. CrystEngComm 2014, 17, 1296–1304. [Google Scholar] [CrossRef]
- Yang, H.; Hao, X.; Tang, J.; Jin, W.; Liu, C.; Hou, H.; Ji, X.; Hu, J. Dual-functional porous copper films modulated via dynamic hydrogen bubble template for in situ SERS monitoring electrocatalytic reaction. Appl. Surf. Sci. 2019, 494, 731–739. [Google Scholar] [CrossRef]
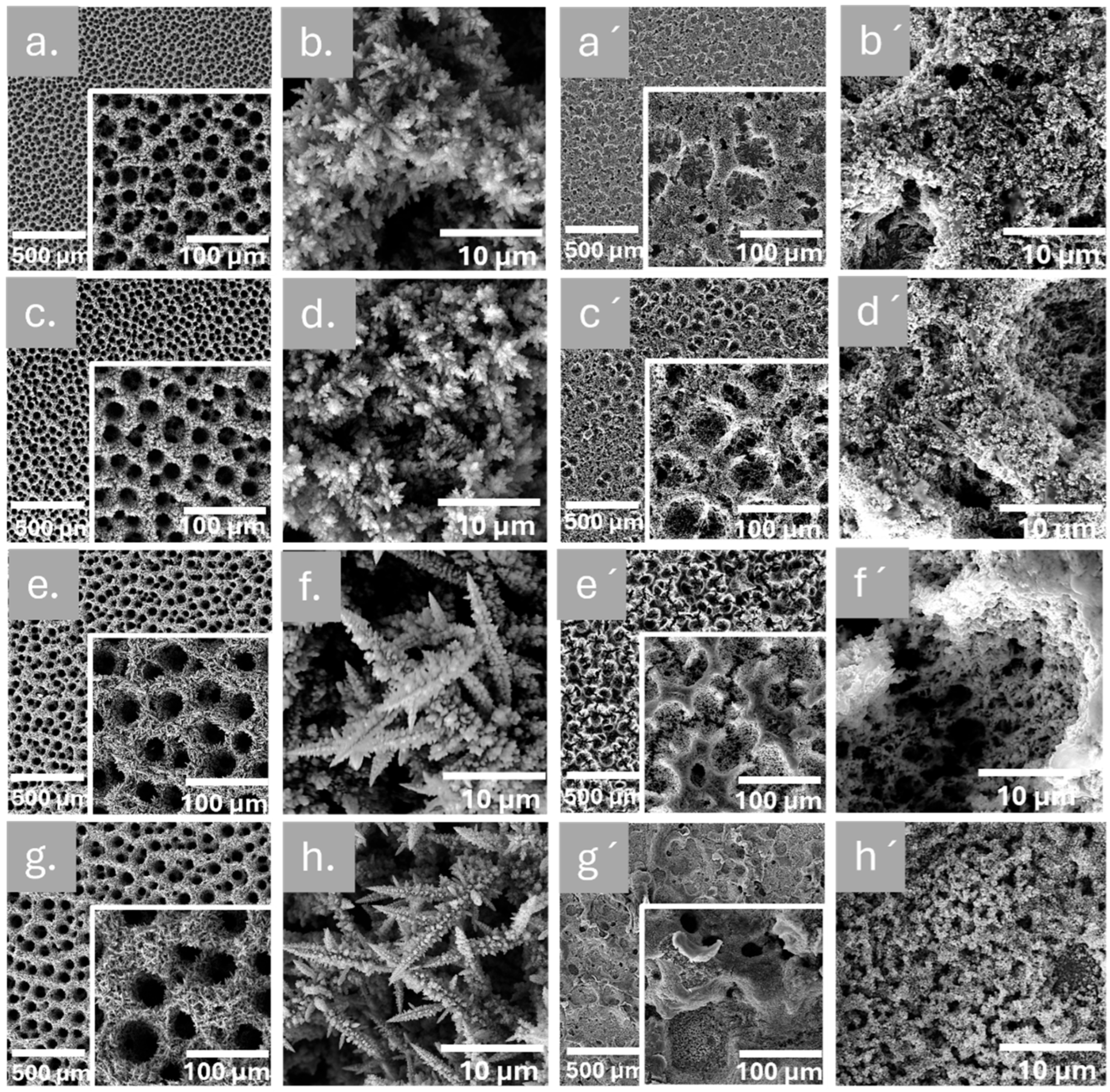
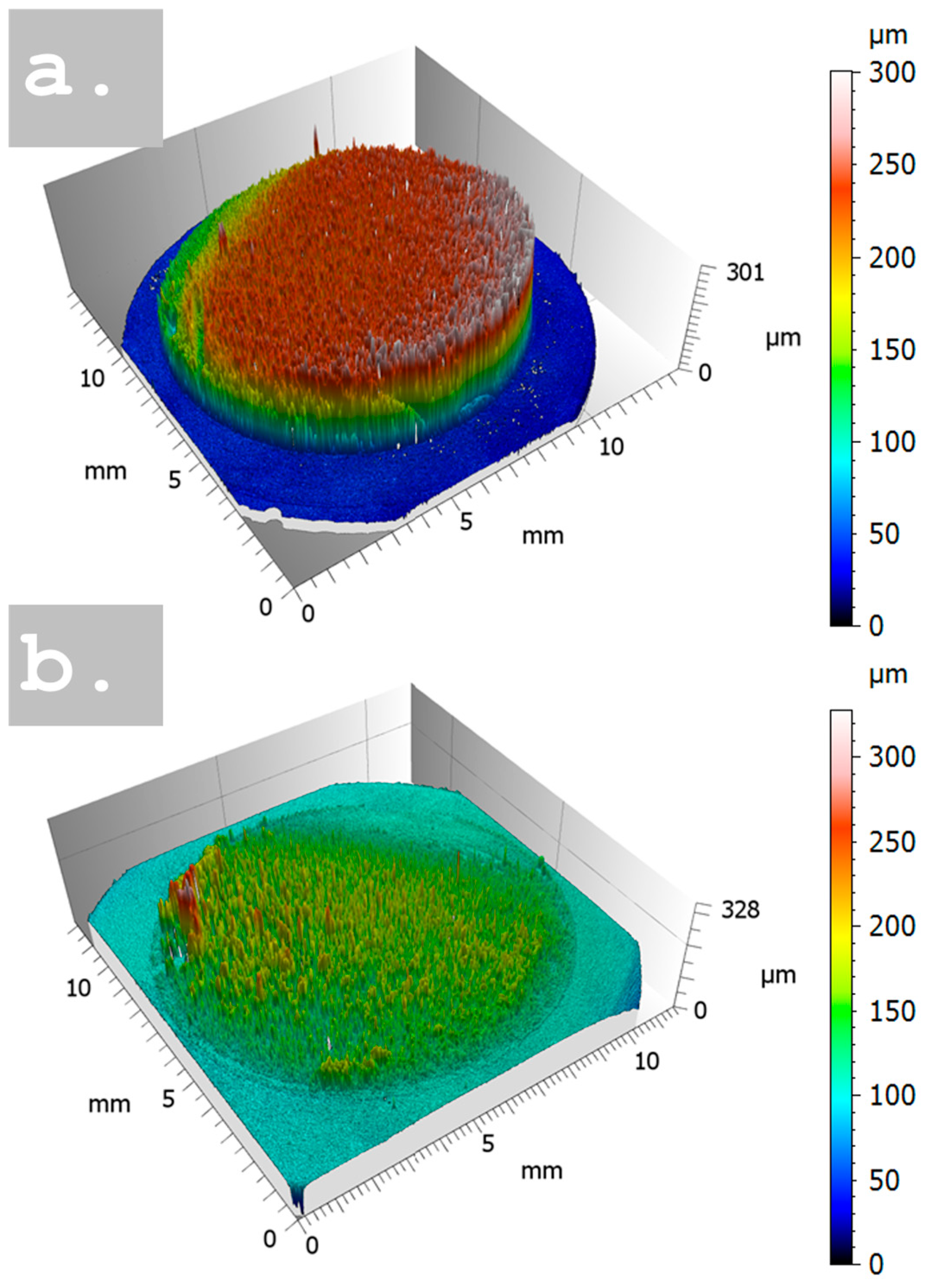
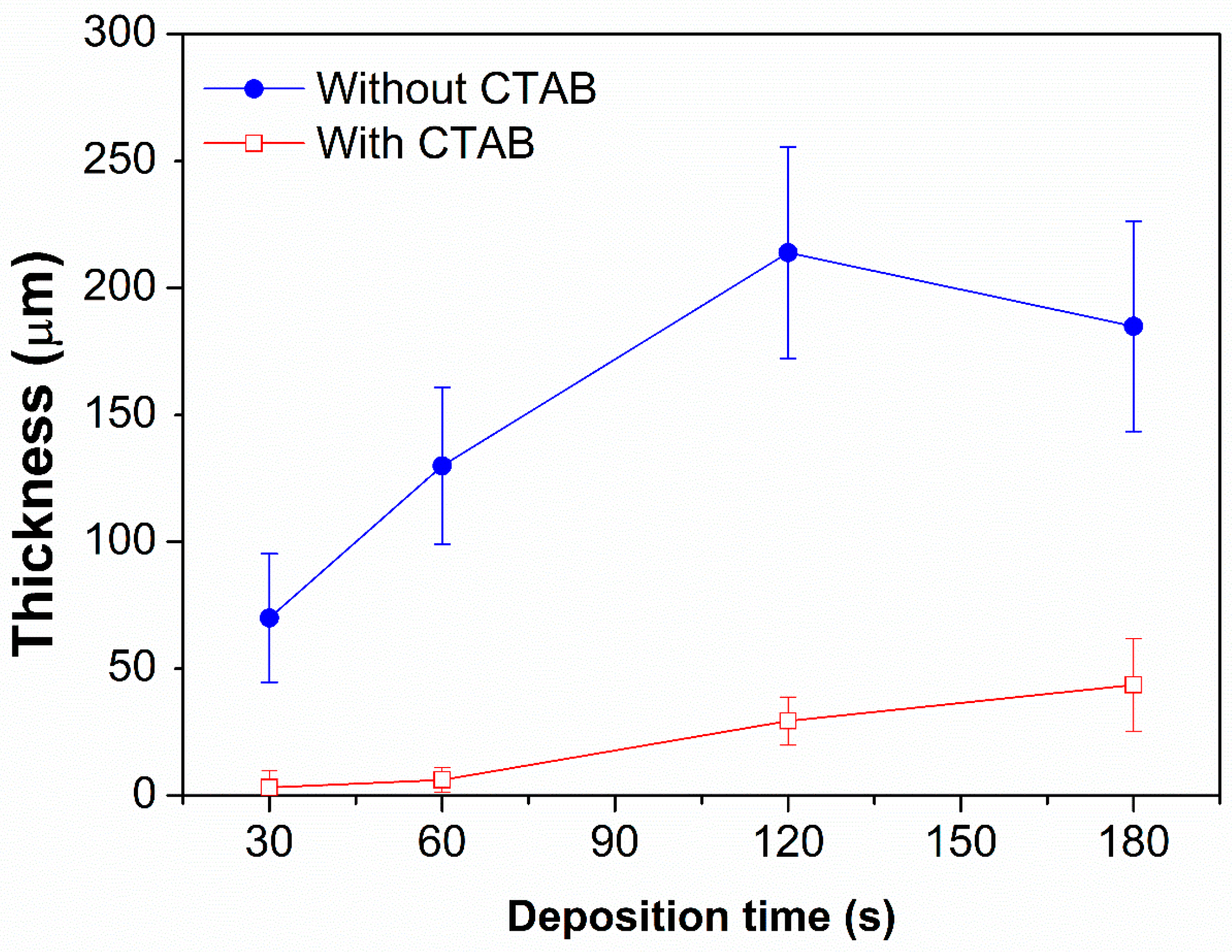
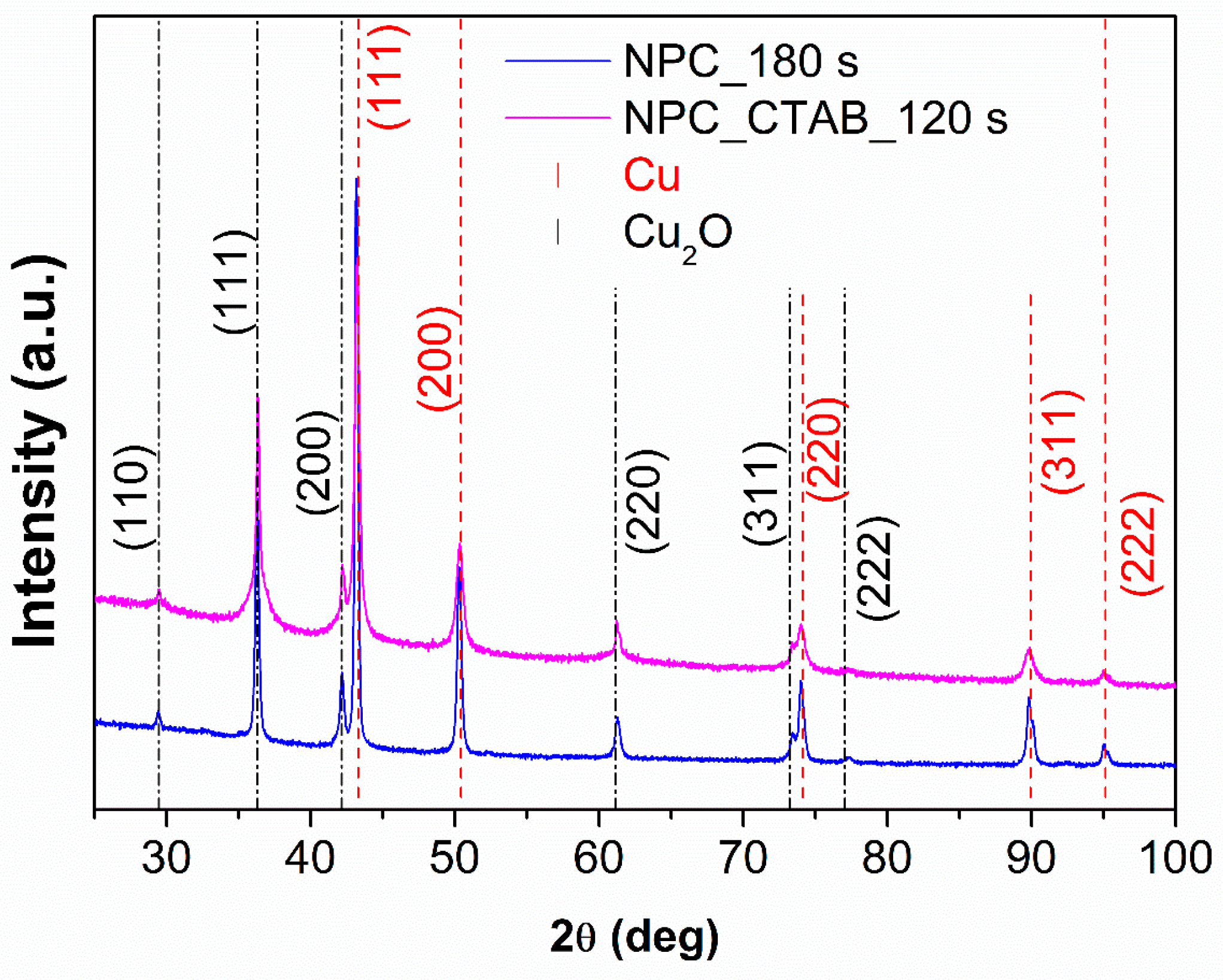
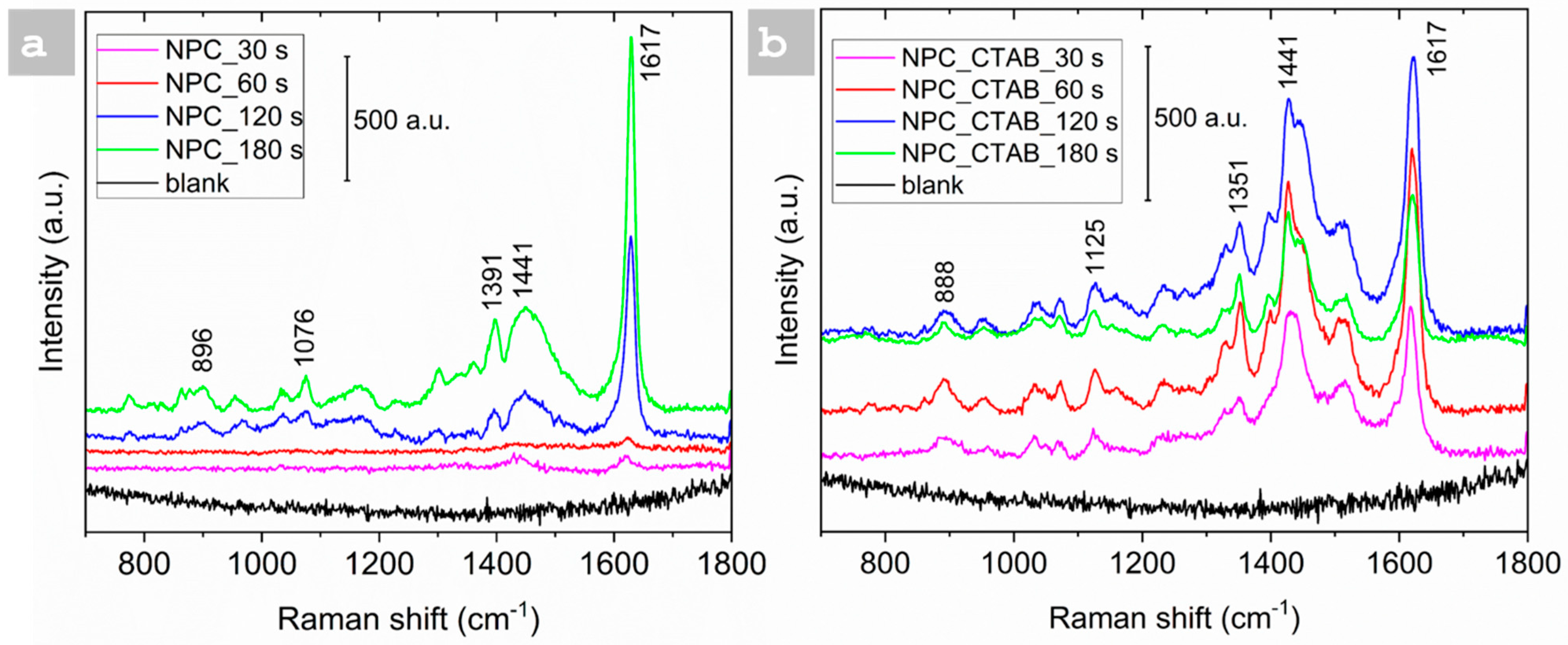
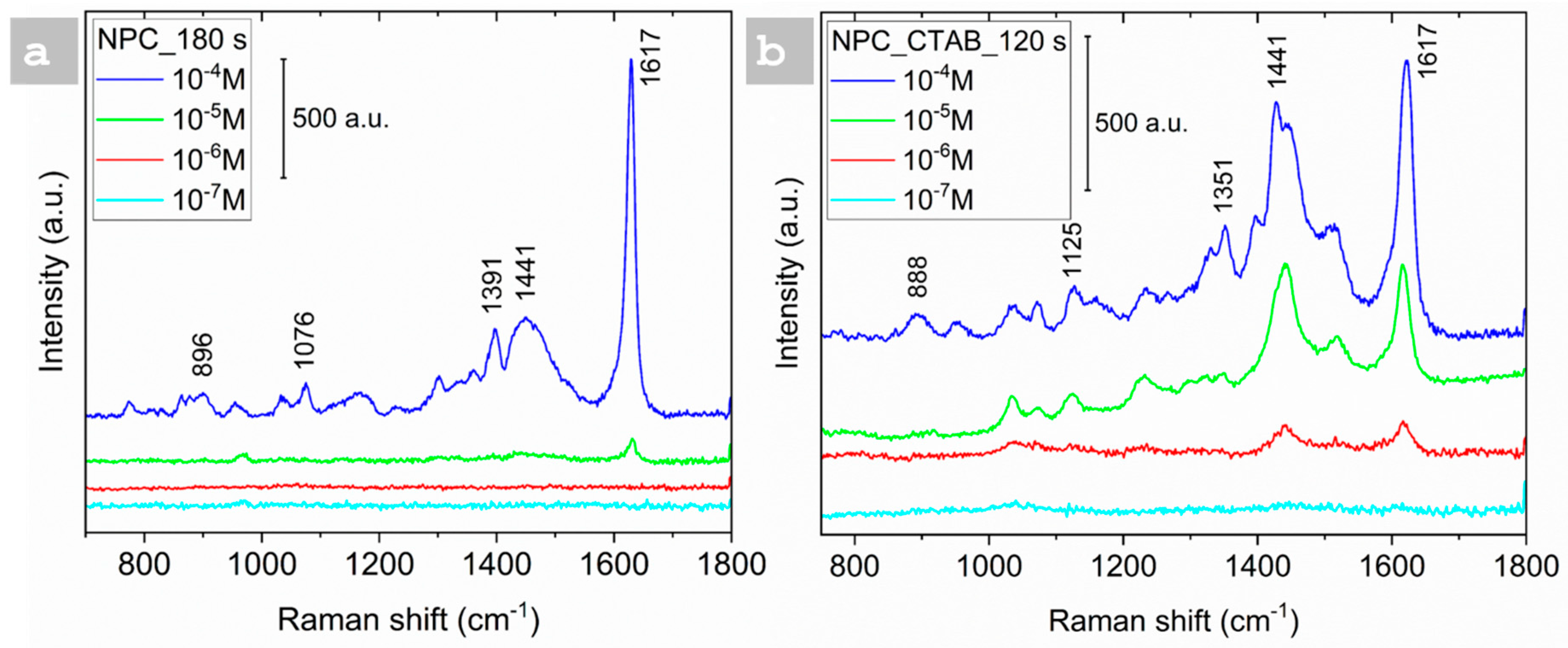
| Sample | Time of Deposition (s) | Pores Size (µm) | Dendrite Length (µm) |
|---|---|---|---|
| NPC_30 s | 30 | 26.8 ± 9.4 | 2.9 ± 0.5 |
| NPC_60 s | 60 | 31.5 ± 12.7 | 4.3 ± 0.4 |
| NPC_120 s | 120 | 54.8 ± 17.2 | 18.4 ± 5.4 |
| NPC_180 s | 180 | 73.3 ± 32.2 | 28.2 ± 5.9 |
| NPC_CTAB_30 s | 30 | 12.8 ± 4.8 | / |
| NPC_CTAB_60 s | 60 | 23.5 ± 3.1 | / |
| NPC_CTAB_120 s | 120 | 24.4 ± 4.8 | / |
| NPC_CTAB_180 s | 180 | less than 1.0 | / |
| Sample | Lattice Parameter of Phases (Å) | Weight Fraction of Phases (wt.%) | ||
|---|---|---|---|---|
| Cu | Cu2O | Cu | Cu2O | |
| NPC_180 s | 3.62 ± 0.01 | 4.28 ± 0.01 | 74 ± 1 | 26 ± 1 |
| NPC_CTAB_120 s | 3.62 ± 0.01 | 4.24 ± 0.01 | 61 ± 1 | 39 ± 1 |
| Substrate | Shape/Structure | Analyte | LS/PS/Diameter | LOD (mol/L) | EF | Ref. |
|---|---|---|---|---|---|---|
| Cu | butterfly wing scales | R6G | / | 10−8 | / | [33] |
| NP-Cu | Ribbon | R6G | ~1 µm | 10−4 | 4.4 × 104 | [46] |
| NPC | Film | R6G | 41.6 ± 9.2 nm | 10−9 | 4.7 × 107 | [47] |
| GP-Cu | Laminated | R6G | ~24 nm | 10−17 | 6.6 × 1012 | [48] |
| NPCu | Ribbon | R6G/CV | ~34 nm | 10−5 | 1.85 × 105 | [32] |
| NPCFs | Foil | R6G | ~200 nm | 10−6 | 2.52 × 105 | [49] |
| PCu | Copper sheet | MB | 43.1 µm | 10−9 | / | [50] |
| NPCs | Cu Disks | MB | 12–73 µm | 10−6 | 2.5 × 103 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tayyaba, N.; Zago, S.; Giura, A.; Fiore, G.; Ribotta, L.; Scaglione, F.; Rizzi, P. Nanoporous Copper Films via Dynamic Hydrogen Bubbling: A Promising SERS Substrate for Sensitive Detection of Methylene Blue. Nanomaterials 2025, 15, 945. https://doi.org/10.3390/nano15120945
Tayyaba N, Zago S, Giura A, Fiore G, Ribotta L, Scaglione F, Rizzi P. Nanoporous Copper Films via Dynamic Hydrogen Bubbling: A Promising SERS Substrate for Sensitive Detection of Methylene Blue. Nanomaterials. 2025; 15(12):945. https://doi.org/10.3390/nano15120945
Chicago/Turabian StyleTayyaba, Noor, Stefano Zago, Andrea Giura, Gianluca Fiore, Luigi Ribotta, Federico Scaglione, and Paola Rizzi. 2025. "Nanoporous Copper Films via Dynamic Hydrogen Bubbling: A Promising SERS Substrate for Sensitive Detection of Methylene Blue" Nanomaterials 15, no. 12: 945. https://doi.org/10.3390/nano15120945
APA StyleTayyaba, N., Zago, S., Giura, A., Fiore, G., Ribotta, L., Scaglione, F., & Rizzi, P. (2025). Nanoporous Copper Films via Dynamic Hydrogen Bubbling: A Promising SERS Substrate for Sensitive Detection of Methylene Blue. Nanomaterials, 15(12), 945. https://doi.org/10.3390/nano15120945








