Synthesized Nano-Titanium Dioxide (Nano-TiO2) via Ammonium Fluorotitanate ((NH4)2TiF6) Precipitation with Ammonia Solution
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Characterization
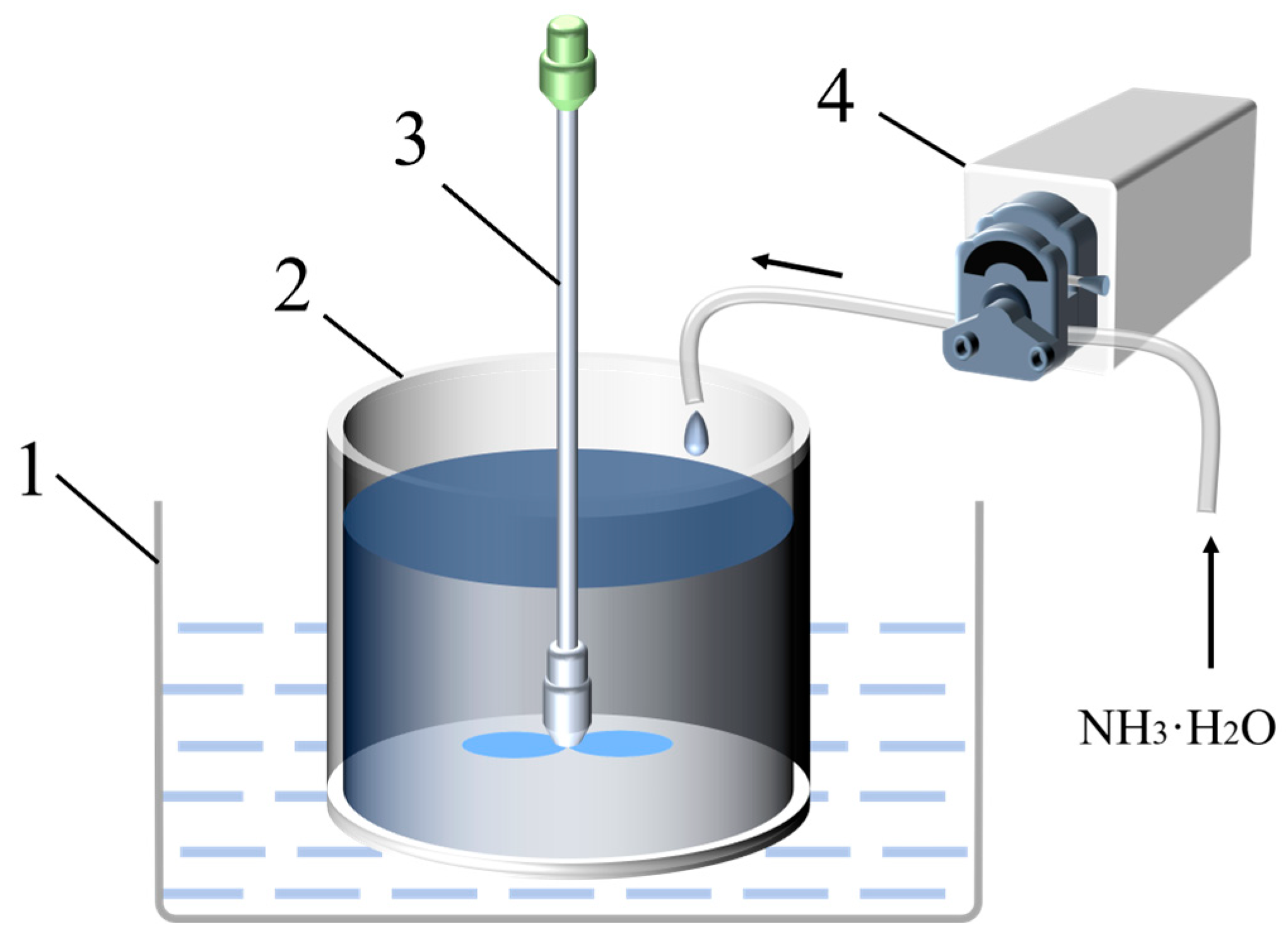
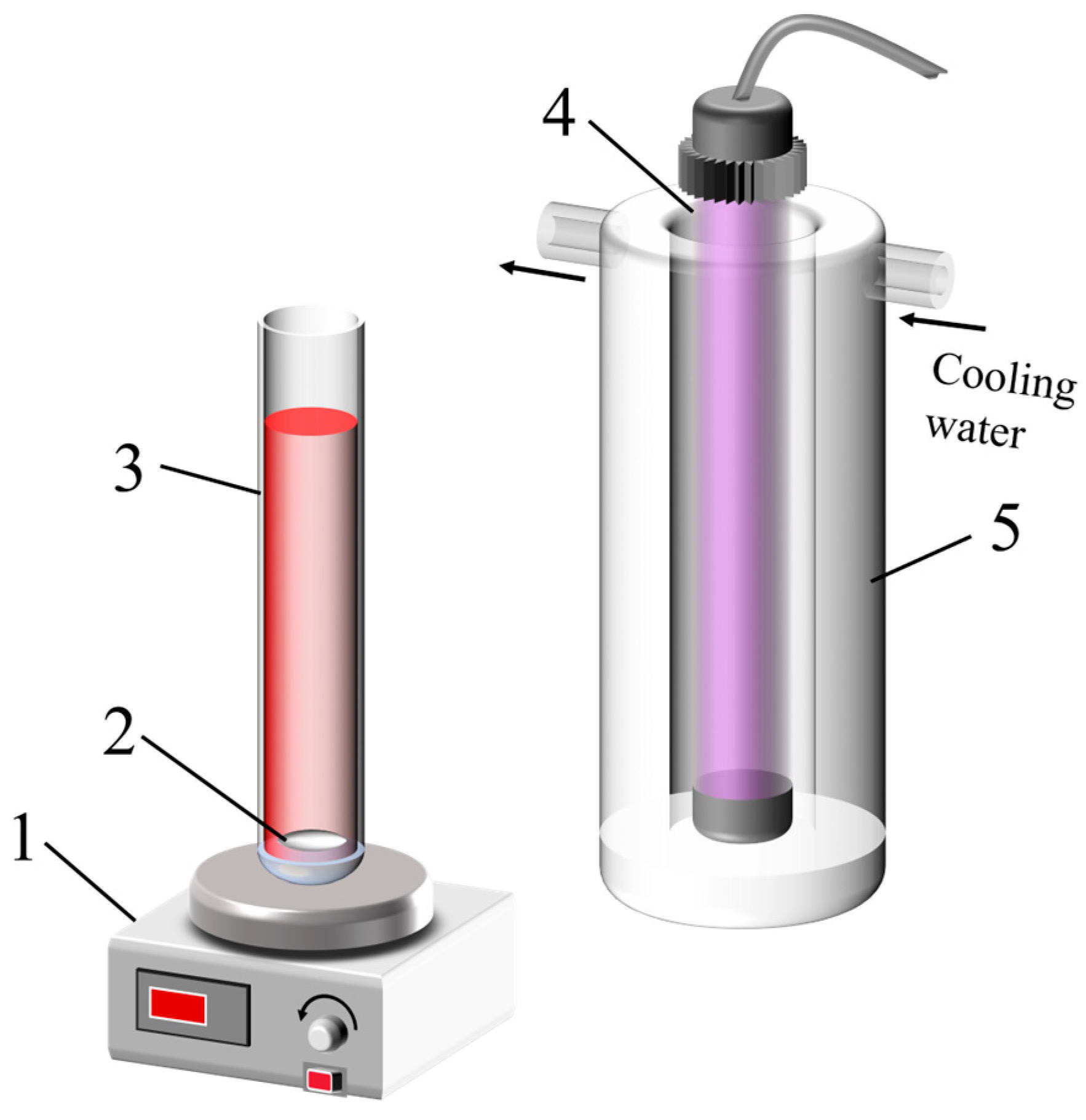
2.2. Preparation of Nano-TiO2
2.3. Catalytic Experiments
2.4. Experimental Design Method
3. Results and Discussion
3.1. Characterization
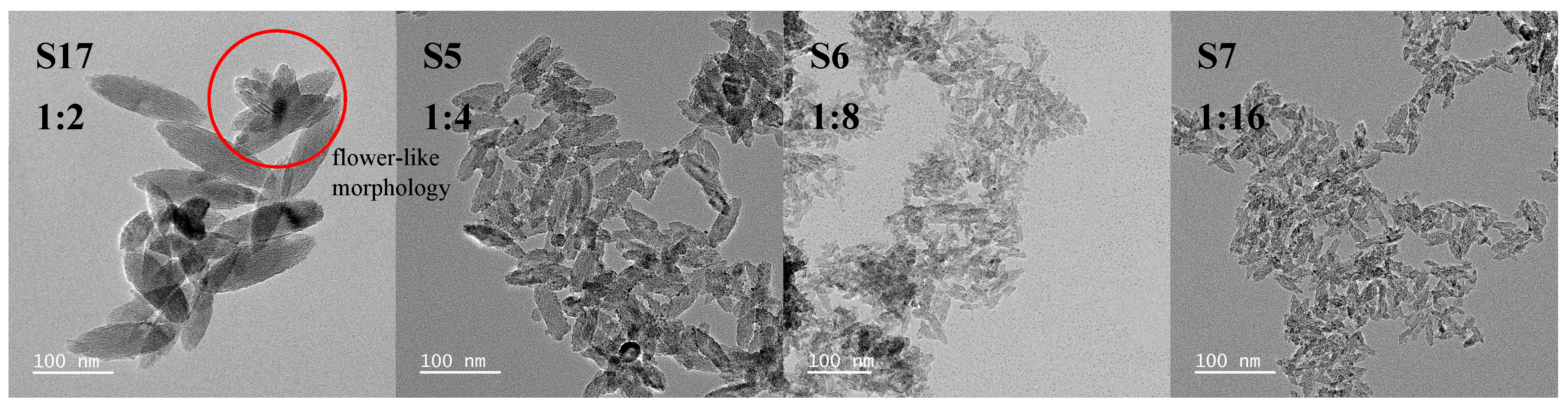
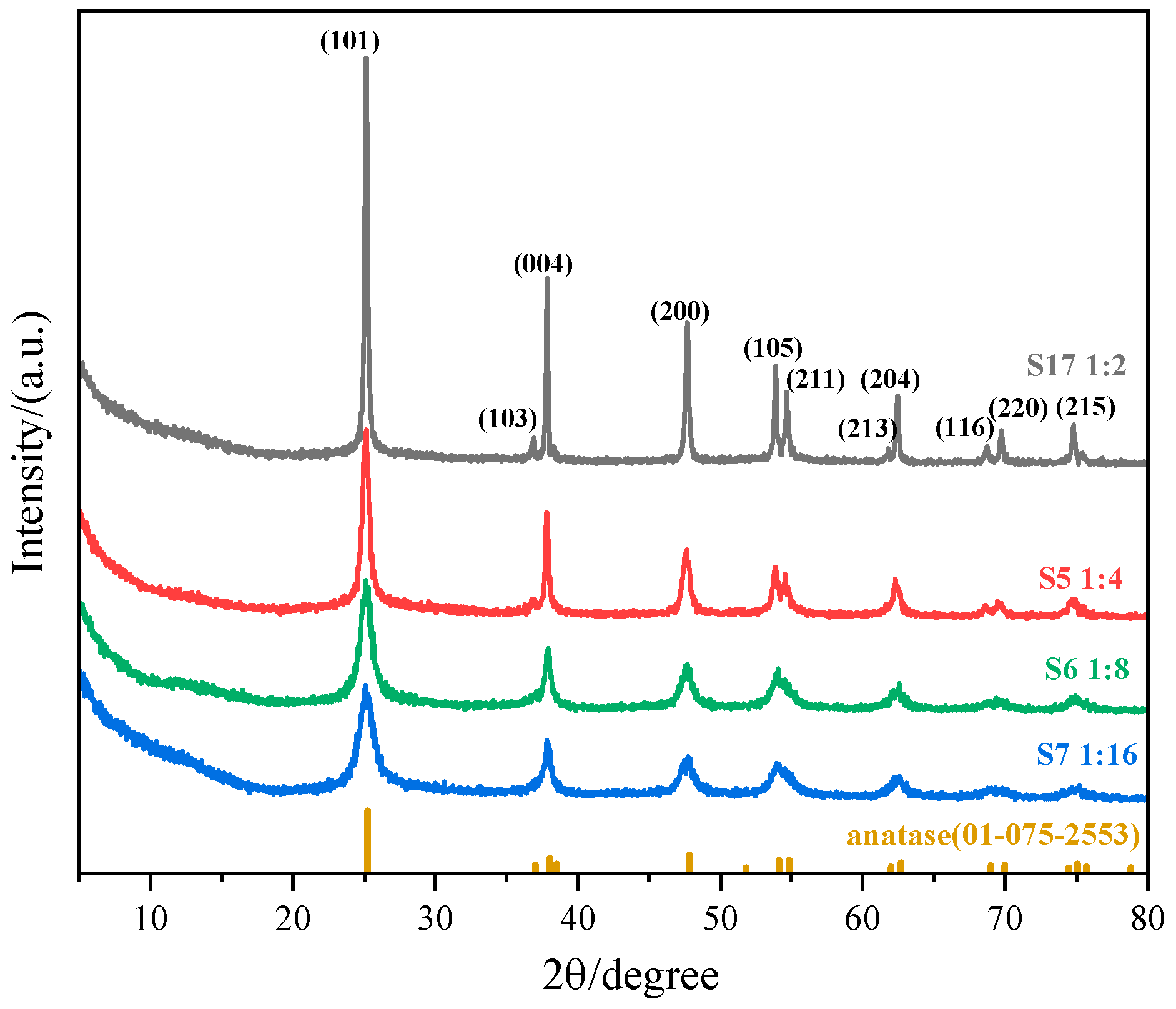
3.1.1. The Effect of the Molar Ratio of (NH4)2TiF6 to Ammonia
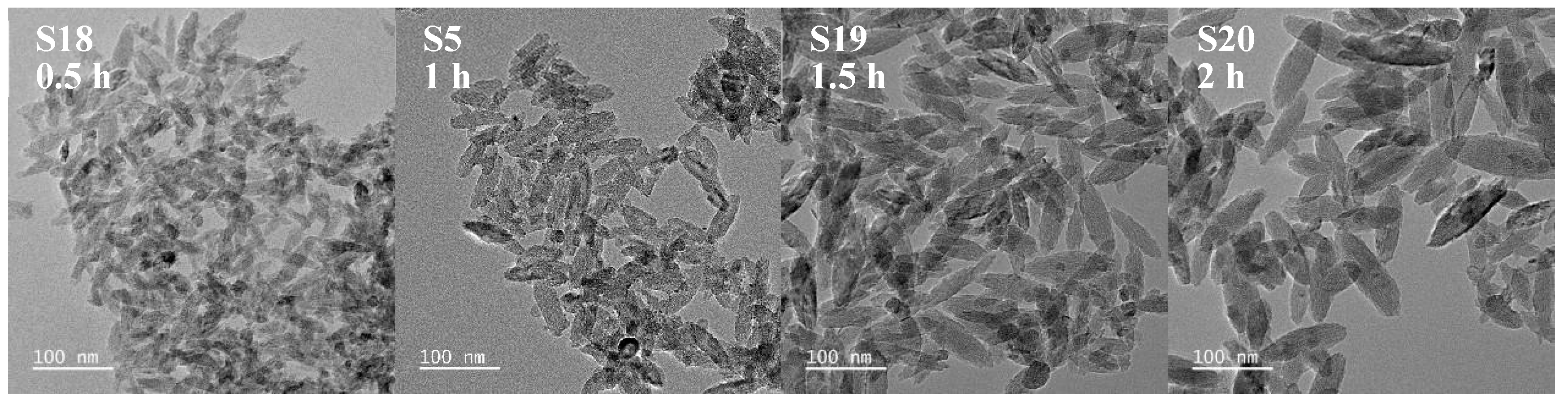
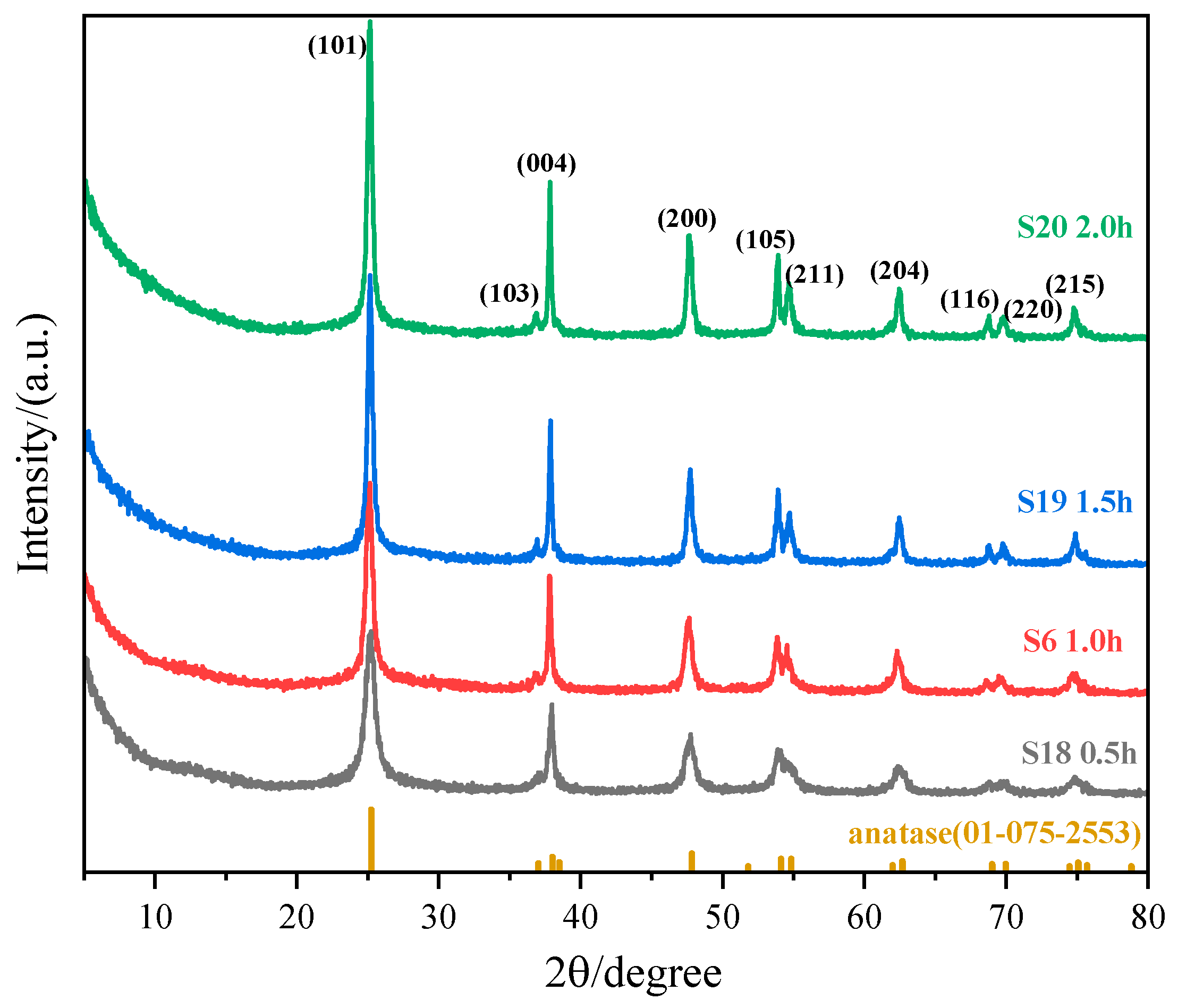
3.1.2. Impact of Reaction Time
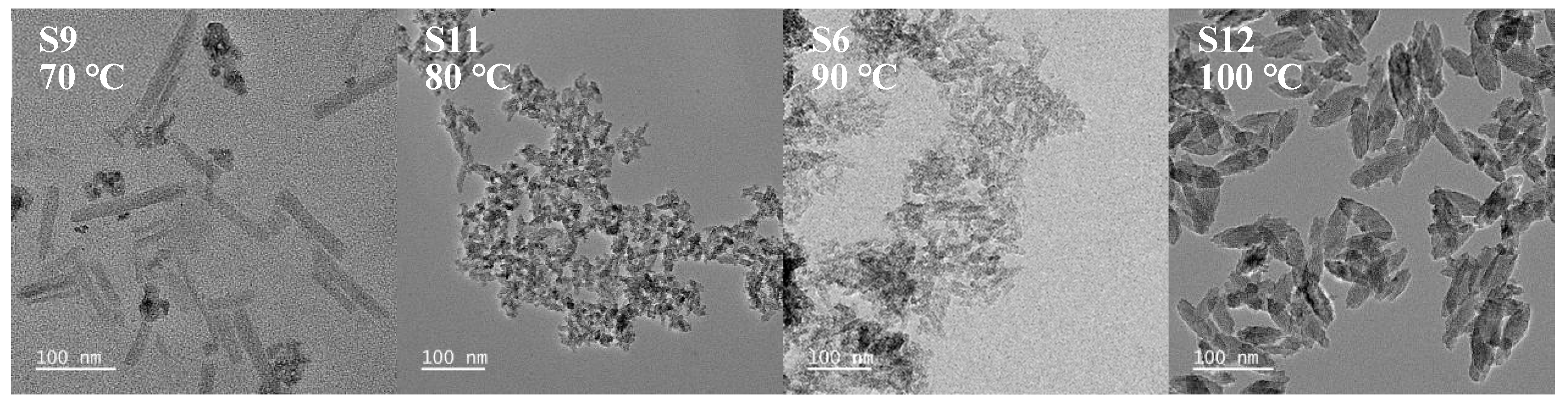
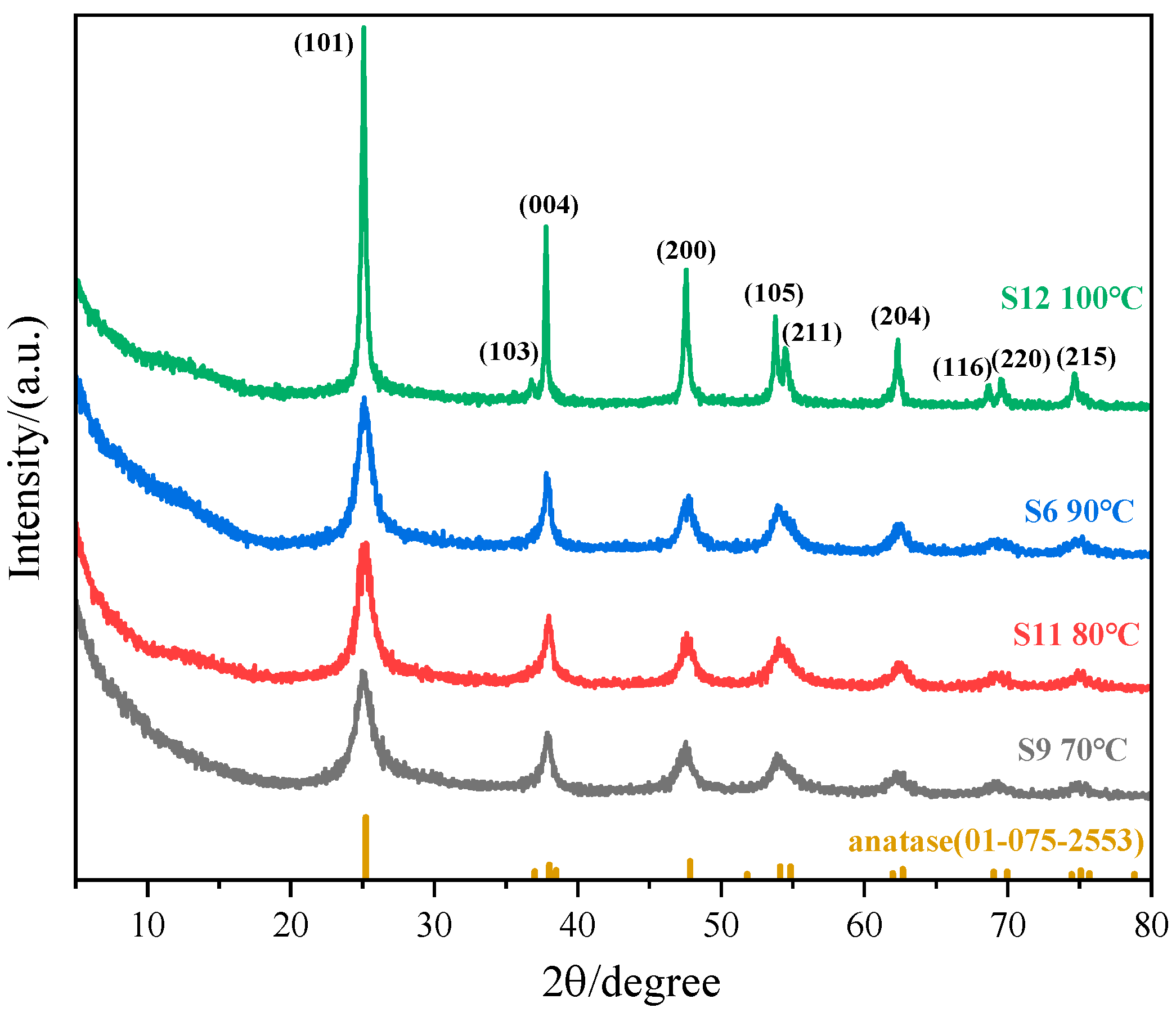
3.1.3. Effect of Reaction Temperature
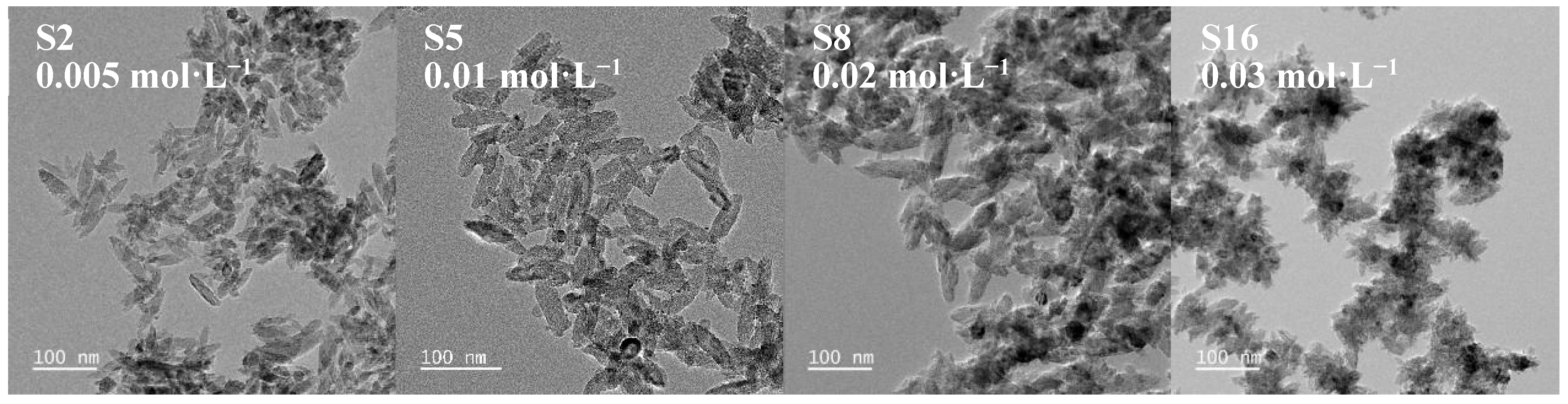
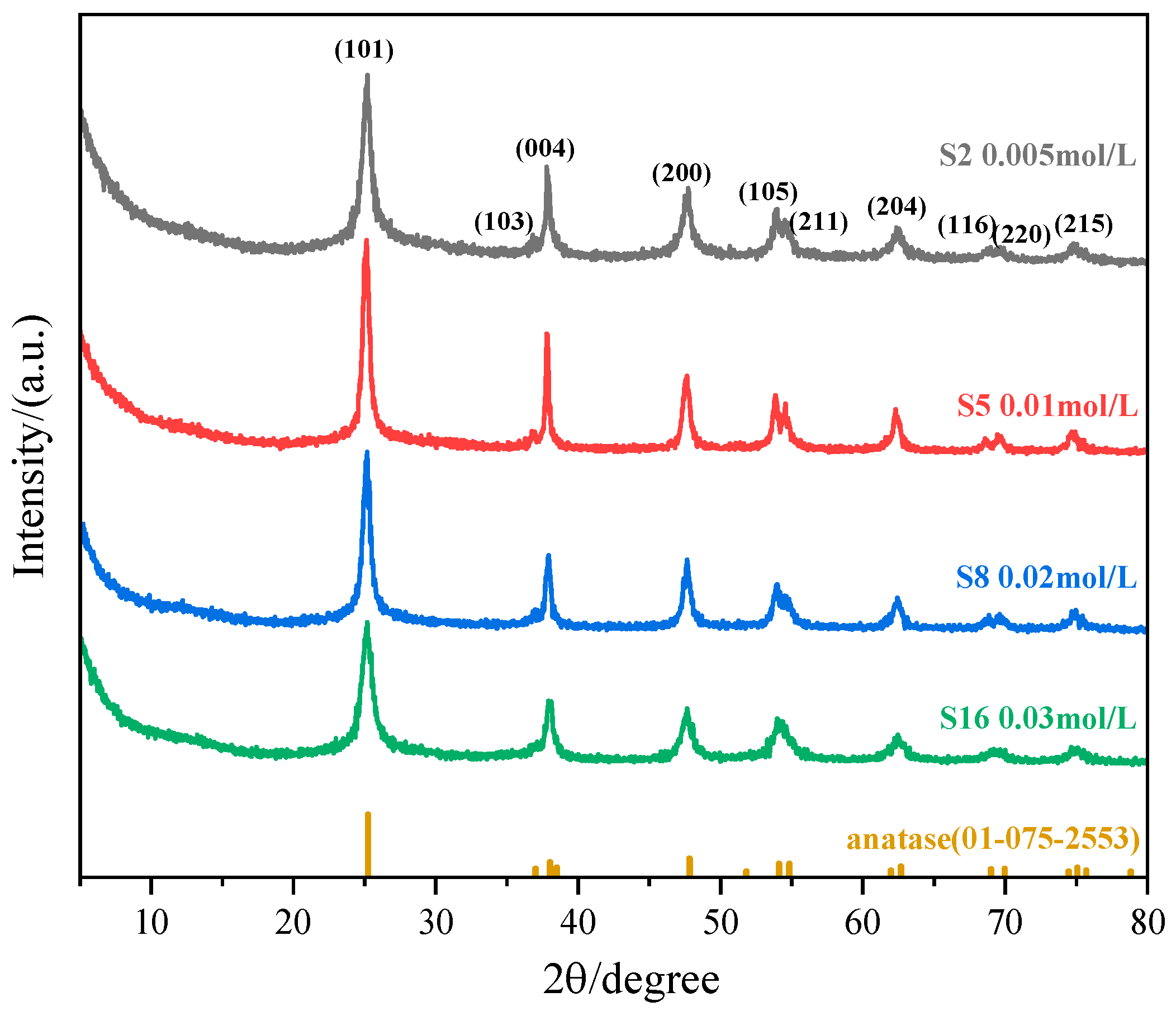
3.1.4. Effect of (NH4)2TiF6 Concentration
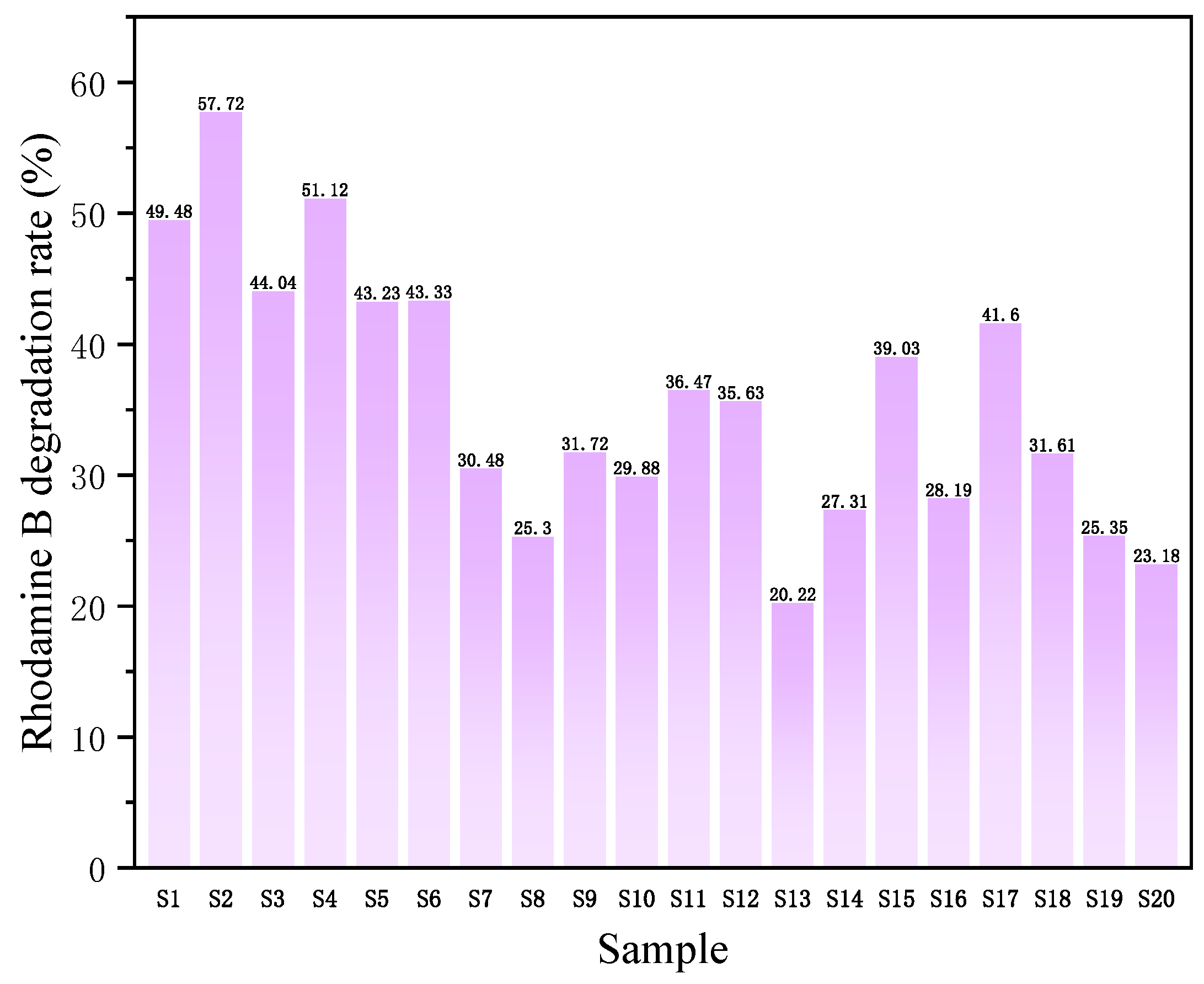
3.2. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Artifon, W.; Cesca, K.; de Andrade, C.J.; Ulson de Souza, A.A.; de Oliveira, D. Dyestuffs from textile industry wastewaters: Trends and gaps in the use of bioflocculants. Process Biochem. 2021, 111, 181–190. [Google Scholar] [CrossRef]
- Lallali, H.; Bentouami, A.; Cherief, M.; Benzaidi, O.; Henni, L.; Tighilt, F.Z.; Belhousse, S.; Manseri, A.; Bouchama, A.; Sam, S. Innovative multi-Z-scheme Bi2MoO6/BiOBr0.75/BiOI0.25/g-C3N4 quaternary composite with superior visible-light photocatalytic performance for degrading rhodamine B and furfural. Sustain. Mater. Technol. 2025, 44, e01391. [Google Scholar] [CrossRef]
- Alzahrani, F.M.A.; Aldhafeeri, T.R.; Boukhris, I.; Al-Buriahi, M.S.; Shahid, M.; Chaudhary, K.; Warsi, M.F. Unveiling the potential of Ce-In2S3 microflowers encased in graphene: Enhancing synergistic effects with Ce as an electron mediator for photocatalytic annihilation of organic dyes. J. Water Process Eng. 2025, 73, 107651. [Google Scholar] [CrossRef]
- Ismail, A.; Djerad, S.; Haddad, A.; Alleg, S.; Ebrahimi, M. Synthesis of manganese oxides with defects for MO degradation. Solid State Sci. 2025, 164, 107919. [Google Scholar] [CrossRef]
- Wang, Y.G.; Liu, X.M. Application Status and Development Trends of Red Mud-Based Photocatalytic Materials in Degrading Organic Pollutants in Water. Chin. J. Eng. 2021, 43, 22–32. [Google Scholar] [CrossRef]
- Akuma, D.A.; Lund, H.; Hoa Duong, T.T.; Fufa, F.; Strunk, J.; Steinfeldt, N. Optimization of anatase TiO2 photocatalyst for diclofenac degradation by using response surface methodology. Appl. Sci. 2025, 15, 1401. [Google Scholar] [CrossRef]
- Javaid, A.; Imran, M.; Kanwal, F.; Latif, S. Doping-induced enhancement of dual functionality of cerium tungstate for dielectric and photocatalytic applications. Inorg. Chem. Commun. 2025, 178, 114462. [Google Scholar] [CrossRef]
- Silva, V.; Fernandes, J.F.A.; Tomás, M.C.; Silva, C.P.; Calisto, V.; Otero, M.; Lima, D.L.D. Enhanced solar driven photocatalytic removal of antibiotics from aquaculture effluents by TiO2/carbon quantum dot composites. Catal. Today 2023, 419, 114150. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Tian, M.; Ahmad, N.; Jia, K.; Luo, Z.; Qiao, B.; Cheng, J.; Zhao, C. Porphyrinic based hydrogen-bonded organic framework/TiO2 nanocomposites for efficient photocatalytic degradation of sulfadiazine. J. Environ. Sci. 2025, 152, 287–301. [Google Scholar] [CrossRef]
- Qiao, X.; Xu, Y.; Liu, X.; Chen, S.; Zhong, Z.; Li, Y.; Lü, J. Nitrogen–doped titanium dioxide/schwertmannite nanocomposites as heterogeneous photo–fenton catalysts with enhanced efficiency for the degradation of bisphenol a. J. Environ. Sci. 2024, 143, 1–11. [Google Scholar] [CrossRef]
- Hidayat, R.; Fadillah, G.; Ohira, S.-I.; Fajarwati, F.I.; Setyorini, D.A.; Saputra, A. Facile green synthesis of Ag doped TiO2 nanoparticles using maple leaf for bisphenol-a degradation and its antibacterial properties. Mater. Today Sustain. 2024, 26, 100752. [Google Scholar] [CrossRef]
- Venu Sreekala, S.; Sreeja Pramod, A.; Parola, A.; Kazhuthuttil Kochu, J.; Thoppil Ramakrishnan, R. Modified bentonite loaded with nonmetal doped titanium dioxide for the removal of heavy metal ions and dyes from wastewater. J. Sol-Gel Sci. Technol. 2025, 114, 349–364. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Bian, Z.-F.; Ren, J.; Liu, Y.-M.; Cao, Y.; Li, H.-X.; He, H.-Y.; Fan, K.-N. Nanocrystalline anatase TiO2 photocatalysts prepared via a facile low temperature nonhydrolytic sol–gel reaction of TiCl4 and benzyl alcohol. Appl. Catal. B Environ. 2007, 76, 82–91. [Google Scholar] [CrossRef]
- Hoa, N.T.Q.; Lee, Z.; Kim, E.-T. Nanographitic layer-mediated synthesis of carbon-TiO2 hybrid nanobelts by metalorganic chemical vapor deposition. Mater. Lett. 2012, 81, 20–22. [Google Scholar] [CrossRef]
- Cai, P.; Zhang, Q.; Xie, Y.; Zhang, X. Design of Multi-Jet H2/Air Flame Combustion Reactor and Morphology Control of TiO2 Nanoparticles. In 2023 9th International Conference on Machinery, Materials and Computing Technology; Francis Academic Press: London, UK, 2023; Available online: https://webofproceedings.org/proceedings_series/article/artId/22420.html#abstract (accessed on 26 September 2024).
- Bao, R.; Liu, B.; Zhang, T.; Wu, B.; Dong, E.; Yuwen, C. Rutile TiO2 Production: Optimization of Microwave Calcination of Metatitanic Acid Using Response Surface Methodology. Chem. Eng. Technol. 2022, 45, 1826–1834. [Google Scholar] [CrossRef]
- Zharvan, V.; Yudoyono, G.; Darminto, D. Structure identification of nanopowder TiO2 synthesized by coprecipitation method. J. Fis. Dan Apl. 2023, 19, 25. [Google Scholar] [CrossRef]
- Schalk, N.; Tkadletz, M.; Mitterer, C. Hard coatings for cutting applications: Physical vs. chemical vapor deposition and future challenges for the coatings community. Surf. Coat. Technol. 2022, 429, 127949. [Google Scholar] [CrossRef]
- Maekawa, T.; Kurosaki, K.; Tanaka, T.; Yamanaka, S. Thermal conductivity of titanium dioxide films grown by metal-organic chemical vapor deposition. Surf. Coat. Technol. 2008, 202, 3067–3071. [Google Scholar] [CrossRef]
- Napruszewska, B.D.; Walczyk, A.; Duraczyńska, D.; Kryściak-Czerwenka, J.; Karcz, R.; Gaweł, A.; Nowak, P.; Serwicka, E.M. TiO2 Nanoparticles with Adjustable Phase Composition Prepared by an Inverse Microemulsion Method: Physicochemical Characterization and Photocatalytic Properties. Nanomaterials 2024, 14, 1130. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, R.; Dutta, S.; Kohli, H.P.; Sen, R.M. Hybrid manufacturing process for the synthesis of doped TiO2 nanoparticles and optimization to determine an efficient photocatalyst. J. Indian Chem. Soc. 2024, 101, 101304. [Google Scholar] [CrossRef]
- Nain, P.; Pawar, M.; Rani, S.; Sharma, B.; Kumar, S.; Majeed Khan, M.A. (Ce, Nd) co-doped TiO2 NPs via hydrothermal route:Structural, optical, photocatalytic and thermal behavior. Mater. Sci. Eng. B 2024, 309, 117648. [Google Scholar] [CrossRef]
- Pan, S.; Xin, Y.; Miao, C.; Nie, S.; Xiao, W. Electrospun three dimensional TiO2@N/P co-doped carbon nanofibers framework as high-performance anode materials for lithium-ion batteries. Electrochim. Acta 2024, 503, 144916. [Google Scholar] [CrossRef]
- Khoiriah, K.; Putri, R.A. Biosynthesis of titanium dioxide nanoparticles using peel extract of parkia speciosa for methyl orange degradation. S. Afr. J. Bot. 2024, 170, 120–129. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, P.V.; Vijay, M.; Thiyagarajan, T.K.; Sreekumar, K.P.; Selvarajan, V.; Yu, J.; Liu, S. In-flight formation of nano-crystalline titanium dioxide powder in a plasma jet and its characterization. Plasma Sci. Technol. 2010, 12, 426. [Google Scholar] [CrossRef]
- Meramo-Hurtado, S.; Moreno-Sader, K.; González-Delgado, Á.D. Computer-aided simulation and exergy analysis of TiO2 nanoparticles production via green chemistry. PeerJ 2019, 7, e8113. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Chen, Y.Q.; Cao, Y.; Lin, S.W. Technical and structure study of coprecipitation method for nano-titanium dioxide. Adv. Mater. Res. 2012, 418–420, 827–830. [Google Scholar] [CrossRef]
- Sanchez-Martinez, A.; Ceballos-Sanchez, O.; Koop-Santa, C.; López-Mena, E.R.; Orozco-Guareño, E.; García-Guaderrama, M. N-doped TiO2 nanoparticles obtained by a facile coprecipitation method at low temperature. Ceram. Int. 2018, 44, 5273–5283. [Google Scholar] [CrossRef]
- Yeh, S.-W.; Ko, H.-H.; Chiang, H.-M.; Chen, Y.-L.; Lee, J.-H.; Wen, C.-M.; Wang, M.-C. Characteristics and properties of a novel in situ method of synthesizing mesoporous TiO2 nanopowders by a simple coprecipitation process without adding surfactant. J. Alloys Compd. 2014, 613, 107–116. [Google Scholar] [CrossRef]
- Liu, R.; Wu, H.S.; Yeh, R.; Lee, C.Y.; Hung, Y. Synthesis and bactericidal ability of TiO2 and Ag-TiO2 prepared by coprecipitation method. Int. J. Photoenergy 2012, 2012, 640487. [Google Scholar] [CrossRef][Green Version]
- Song, T.Y.; Xu, J.N.; Cheng, G.Z.; Shi, S.H. Inorganic Chemistry; Higher Education Press: Beijing, China, 2010. [Google Scholar]
- Lei, C.X.; Zhou, H.; Feng, Z.D. Effect of liquid-phase-deposited parameters on the photogenerated cathodic protection properties of TiO2 films. J. Alloys Compd. 2012, 542, 164–169. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, L.; Zhu, W.; Kanda, H. Direct synthesis of hydrogen fluoride-free multilayered Ti3C2/TiO2 composite and its applications in photocatalysis. Heliyon 2023, 9, e18718. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-N.; Wang, J.-Y.; Huang, J.-J. Titanium oxide hollow structure layer for dye sensitized solar cell by liquid phase deposition. SN Appl. Sci. 2019, 1, 1306. [Google Scholar] [CrossRef]
- Xu, C.; Zhong, Y.; Zheng, Y.; Huang, W.; Jin, C.; Xu, B.; Yao, R.; Feng, Z. Micromixing-assisted preparation of TiO2 films from ammonium hexafluorotitanate and urea by liquid phase deposition based on simulation of mixing process in T-shaped micromixer. Ceram. Int. 2019, 45, 11325–11334. [Google Scholar] [CrossRef]
- Lin, X.X. Practical Technology of the Taguchi Method; Haitian Press: Shenzhen, China, 2004; Available online: https://book.douban.com/subject/1188277/ (accessed on 3 April 2025).
- Hot, J.; Frayret, J.; Sonois-Mazars, V.; Ringot, E. From hexafluorotitanate waste to TiO2 powder: Characterization and evaluation of the influence of synthesis parameters by the experimental design method. Adv. Powder Technol. 2022, 33, 103472. [Google Scholar] [CrossRef]
| Sample | IF | Degradation Rate Ranking | OF1 | OF2 |
|---|---|---|---|---|
| S2 | 3 | 1 | 1 | 1 |
| S4 | 1 | 2 | 1 | 1 |
| S1 | 4 | 3 | 1 | 1 |
| S3 | 1 | 4 | 1 | 1 |
| S6 | 2 | 5 | 1 | 1 |
| S5 | 1 | 6 | 1 | 0 |
| S17 | 4 | 7 | 1 | 0 |
| S15 | 0 | 8 | 1 | 0 |
| S11 | 1 | 9 | 1 | 0 |
| S12 | 3 | 10 | 1 | 0 |
| S9 | 0 | 11 | 0 | 0 |
| S18 | 1 | 12 | 0 | 0 |
| S7 | 2 | 13 | 0 | 0 |
| S10 | 1 | 14 | 0 | 0 |
| S16 | 0 | 15 | 0 | 0 |
| S14 | 0 | 16 | 0 | 0 |
| S19 | 3 | 17 | 0 | 0 |
| S8 | 4 | 18 | 0 | 0 |
| S20 | 4 | 19 | 0 | 0 |
| S13 | 1 | 20 | 0 | 0 |
| OF1 Top 50% | 0 | 1 | 2 | 3 | 4 |
| Factor/Level | |||||
| IF | 0.25 | 0.57 | 0.50 | 0.67 | 0.00 |
| OF2 Top 25% | |||||
| Factor/Level | 0 | 1 | 2 | 3 | 4 |
| IF | 0.00 | 0.29 | 0.50 | 0.33 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Zhou, C.; Wang, S.; Chen, F.; Xie, Y.; Zhang, J.; Yang, L. Synthesized Nano-Titanium Dioxide (Nano-TiO2) via Ammonium Fluorotitanate ((NH4)2TiF6) Precipitation with Ammonia Solution. Nanomaterials 2025, 15, 930. https://doi.org/10.3390/nano15120930
Guo Y, Zhou C, Wang S, Chen F, Xie Y, Zhang J, Yang L. Synthesized Nano-Titanium Dioxide (Nano-TiO2) via Ammonium Fluorotitanate ((NH4)2TiF6) Precipitation with Ammonia Solution. Nanomaterials. 2025; 15(12):930. https://doi.org/10.3390/nano15120930
Chicago/Turabian StyleGuo, Yufeng, Cong Zhou, Shuai Wang, Feng Chen, Yanqin Xie, Jinlai Zhang, and Lingzhi Yang. 2025. "Synthesized Nano-Titanium Dioxide (Nano-TiO2) via Ammonium Fluorotitanate ((NH4)2TiF6) Precipitation with Ammonia Solution" Nanomaterials 15, no. 12: 930. https://doi.org/10.3390/nano15120930
APA StyleGuo, Y., Zhou, C., Wang, S., Chen, F., Xie, Y., Zhang, J., & Yang, L. (2025). Synthesized Nano-Titanium Dioxide (Nano-TiO2) via Ammonium Fluorotitanate ((NH4)2TiF6) Precipitation with Ammonia Solution. Nanomaterials, 15(12), 930. https://doi.org/10.3390/nano15120930










