TiO2 Nanorod Array for Betavoltaic Cells: Performance Validation and Enhancement with Electron Beam and 63Ni Irradiations
Abstract
1. Introduction
2. Simulation and Preparation of Devices
2.1. Monte Carlo Modeling and Simulation
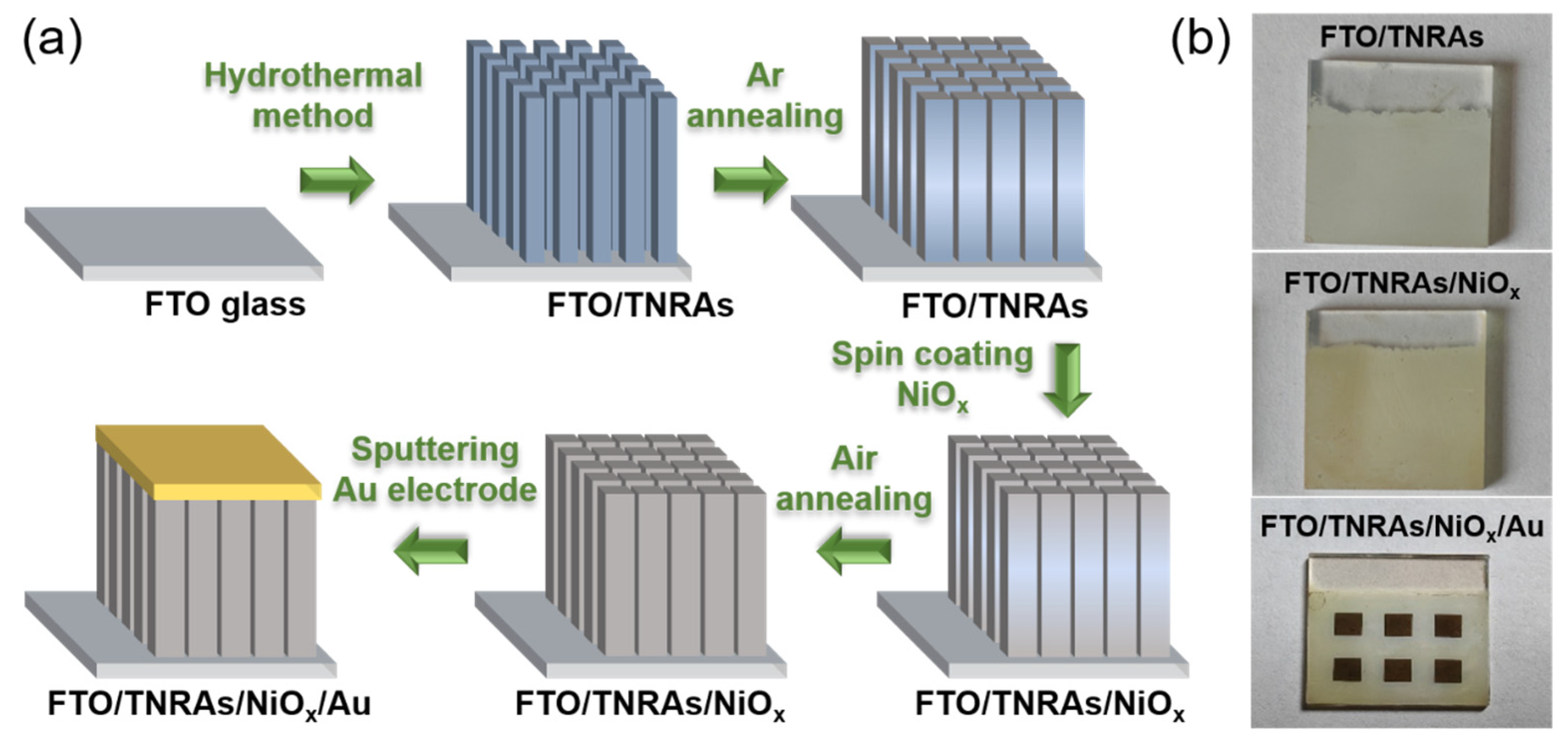
2.2. Preparation of BV Cells
2.2.1. Growth of FTO/TNRAs
2.2.2. Coating of NiOx Film on FTO/TNRAs
2.2.3. Sputtering of Au Electrodes on FTO/TNRAs/NiOx
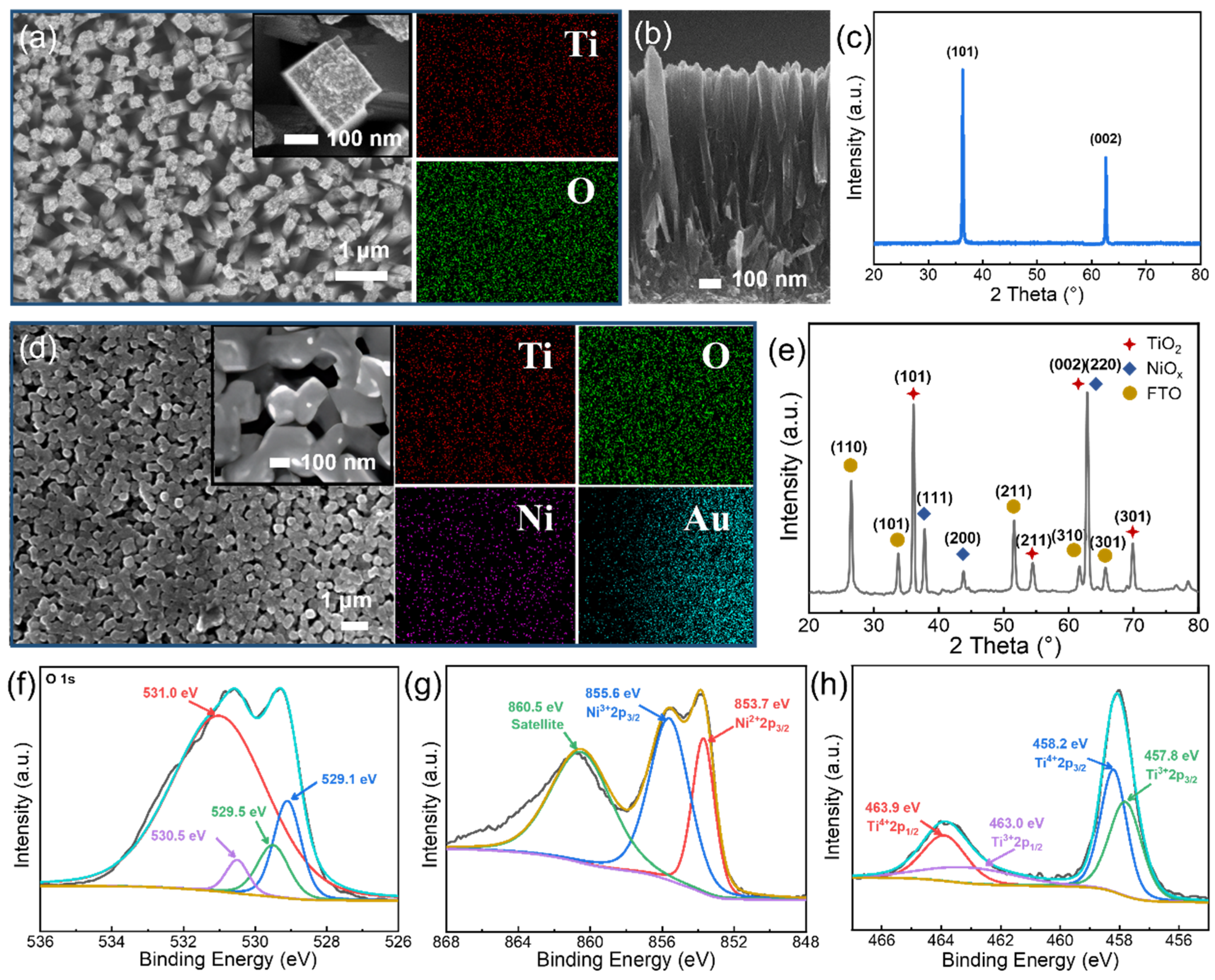
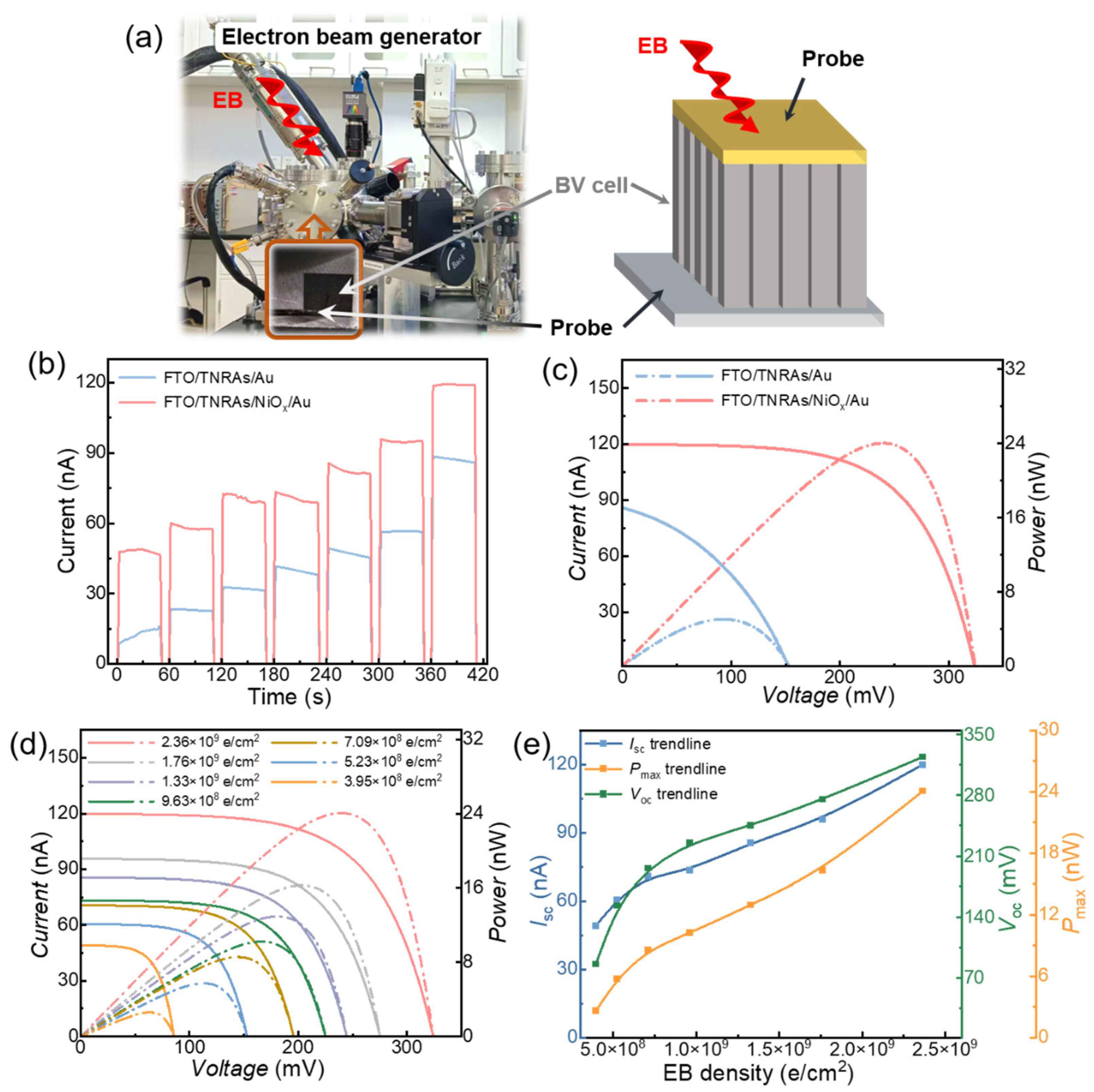
2.3. Material Characterization and Device Measurements
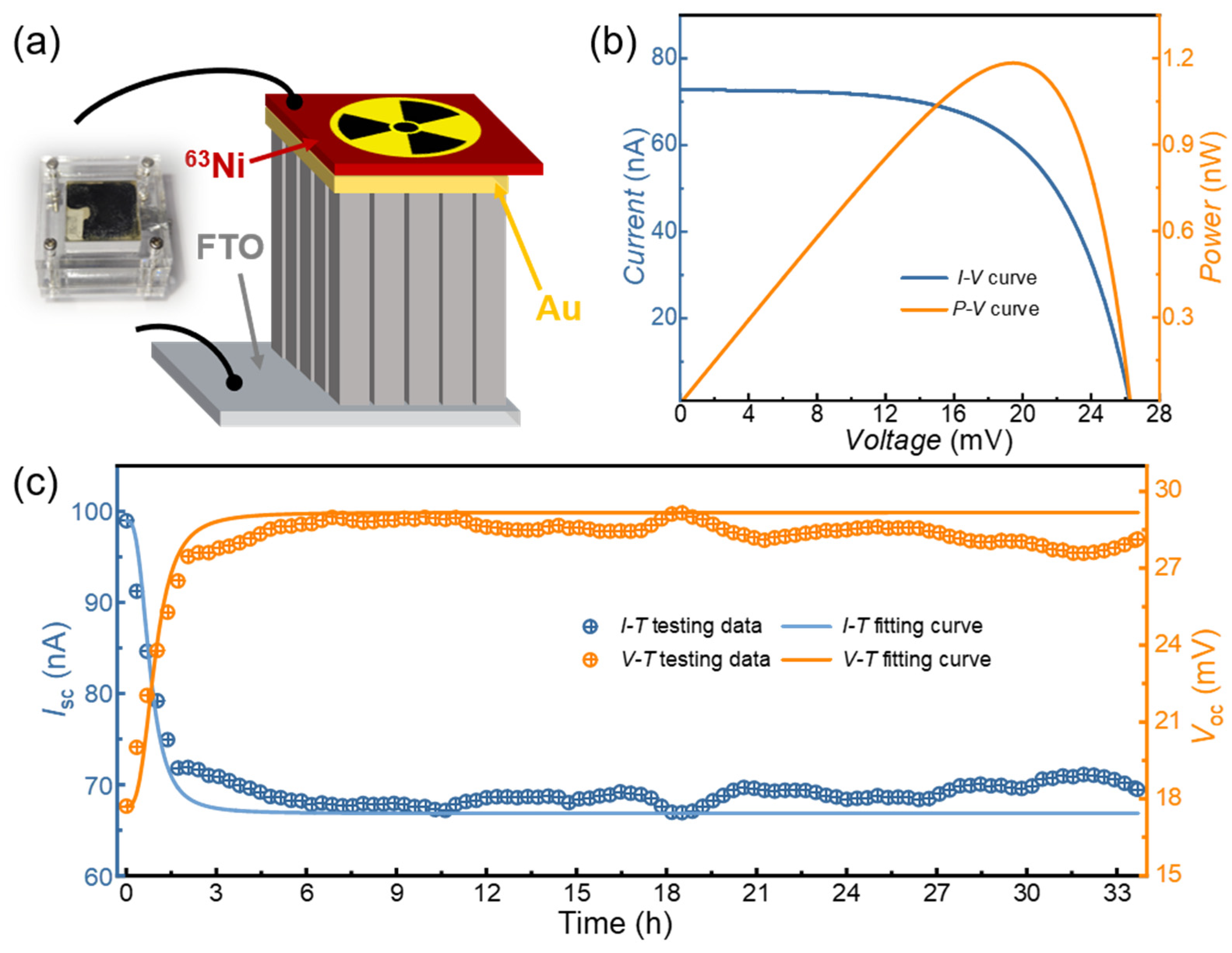
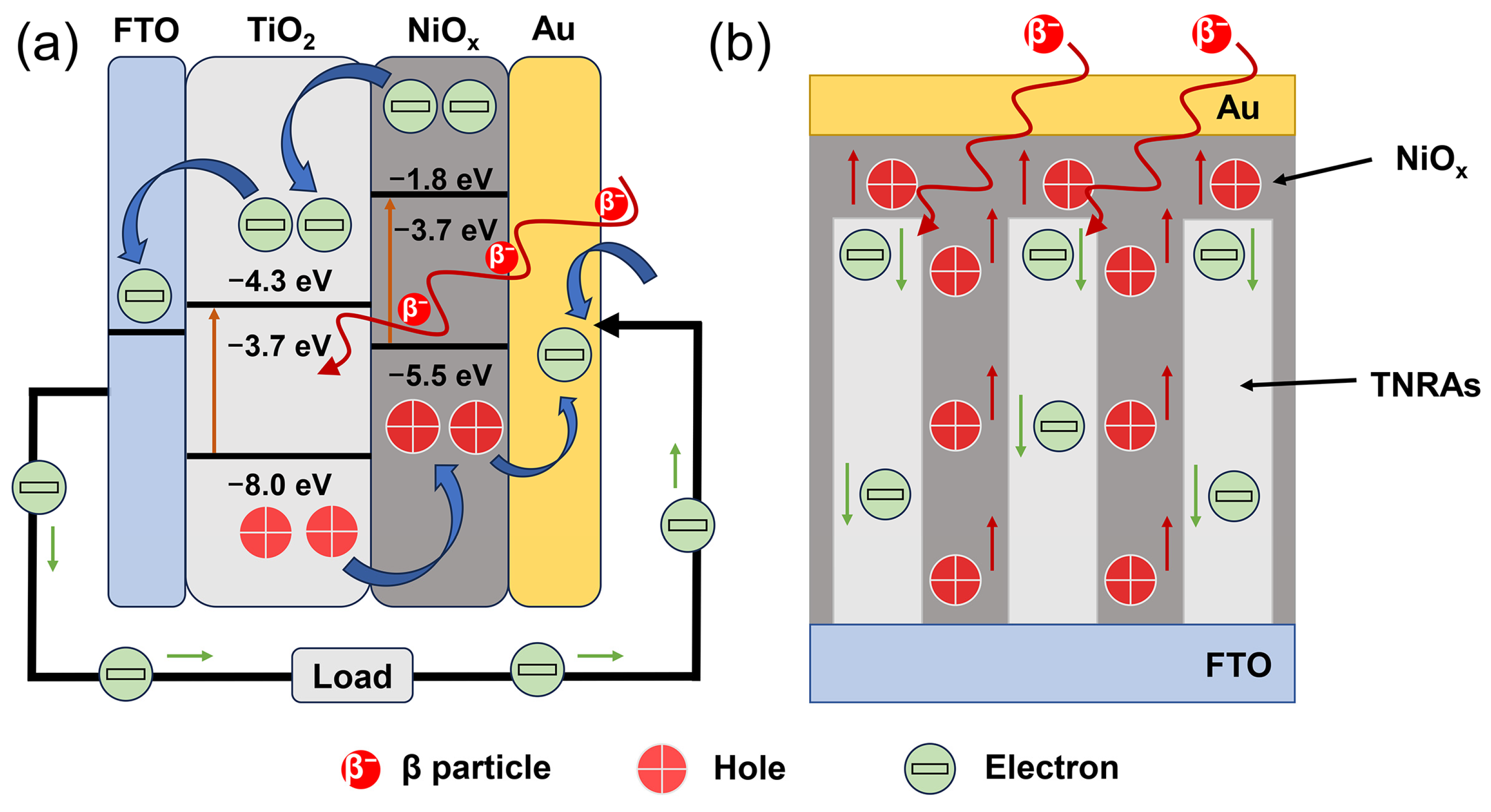
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BV | betavoltaic |
| TNRA | TiO2 nanorod array |
| HTL | hole transport layer |
| ECE | energy conversion efficiency |
| Voc | open-circuit voltage |
| 1-D | one-dimensional |
| MC | Monte Carlo |
| EB | electron beam |
| ED | energy deposition |
| ECS | energy conversion structure |
| OV | oxygen vacancy |
| I-V | current–voltage |
| P-V | power–voltage |
| Isc | short-circuit current |
| Pmax | maximum output power |
| FF | fill factor |
| EDD | energy deposition density |
References
- Lange, R.G.; Carroll, W.P. Review of recent advances of radioisotope power systems. Energy Convers. Manag. 2008, 49, 393–401. [Google Scholar] [CrossRef]
- El-Genk, M.S. Special Issue on Space Nuclear Power and Propulsion. Energy Convers. Manag. 2008, 49, 381. [Google Scholar] [CrossRef]
- Cassady, R.J.; Frisbee, R.H.; Gilland, J.H.; Houts, M.G.; LaPointe, M.R.; Maresse-Reading, C.M.; Oleson, S.R.; Polk, J.E.; Russell, D.; Sengupta, A. Recent advances in nuclear powered electric propulsion for space exploration. Energy Convers. Manag. 2008, 49, 412–435. [Google Scholar] [CrossRef]
- Spencer, M.G.; Alam, T. High power direct energy conversion by nuclear batteries. Appl. Phys. Rev. 2019, 6, 031305. [Google Scholar] [CrossRef]
- Prelas, M.; Boraas, M.; Aguilar, F.; Seelig, J.; Tchouaso, M.; Wisniewski, D. Nuclear Batteries and Radioisotopes; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Naseem, M.; Kim, H.; Lee, J.; Kim, C.; In, S. Betavoltaic Nuclear Battery: A Review of Recent Progress and Challenges as an Alternative Energy Source. J. Phys. Chem. C 2023, 127, 7565–7579. [Google Scholar] [CrossRef]
- Prelas, M.; Weaver, C.; Watermann, M.; Lukosi, E.; Schott, R.; Wisniewski, D. A review of nuclear batteries. Prog. Nucl. Energy 2014, 75, 117–148. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, J.; Wang, X.; Yang, Y.; Xu, P.; Li, P.; Zhang, L.; Chen, Z.; Feng, H.; Wu, W. Review--Betavoltaic Cell: The Past, Present, and Future. ECS J. Solid State Sci. Technol. 2021, 10, 027005. [Google Scholar] [CrossRef]
- Ding, Z.; Jiang, T.; Zheng, R.; Wang, N.; Zhang, L.; Liu, S.; Li, X.; San, H. Quantitative modeling, optimization, and verification of 63Ni-powered betavoltaic cells based on three-dimensional ZnO nanorod arrays. Nucl. Sci. Technol. 2022, 33, 144. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, R.; Chi, T.; Jiang, T.; Ding, Z.; Li, X.; Liu, S.; Zhang, L.; San, H. Betavoltaic-powered electrochemical cells using TiO2 nanotube arrays incorporated with carbon nanotubes. Compos. Part B-Eng. 2022, 239, 109952. [Google Scholar] [CrossRef]
- Zheng, R.; Ding, Z.; Wang, W.; Wang, N.; Wang, Z.; Jiang, T.; Li, X.; Liu, S.; Zhang, L.; San, H. Electrochemical enhanced betavoltaic cells based on ZrO2@TiO2 nanorod arrays with type-I band alignment. Appl. Surf. Sci. 2023, 611, 155757. [Google Scholar] [CrossRef]
- Sun, W.; Kherani, N.P.; Hirschman, K.D.; Gadeken, L.L.; Fauchet, P.M. A three-dimensional porous silicon p-n diode for betavoltaics and photovoltaics. Adv. Mater. 2005, 17, 1230–1233. [Google Scholar] [CrossRef]
- Murphy, J.W.; Voss, L.F.; Frye, C.D.; Shao, Q.; Kazkaz, K.; Stoyer, M.A.; Henderson, R.A.; Nikolic, R.J. Design considerations for three-dimensional betavoltaics. Aip. Adv. 2019, 9, 065208. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, A.; Bai, S.; Zhao, Y. Understanding efficiency differences of betavoltaic batteries measured by electron gun mimicked source and radioactive β source. Appl. Phys. Lett. 2020, 117, 193901. [Google Scholar] [CrossRef]
- Zhao, C.; Lei, L.; Liao, F.; Yuan, D.; Zhao, Y. Efficiency prediction of planar betavoltaic batteries basing on precise modeling of semiconductor units. Appl. Phys. Lett. 2020, 117, 263901. [Google Scholar] [CrossRef]
- Olsen, L.C.; Cabauy, P.; Elkind, B.J. Betavoltaic power sources. Phys. Today 2012, 65, 12. [Google Scholar] [CrossRef]
- Zhao, C.; Liao, F.; Liu, K.; Zhao, Y. Breaking the myth: Wide-bandgap semiconductors not always the best for betavoltaic batteries. Appl. Phys. Lett. 2021, 119, 153904. [Google Scholar] [CrossRef]
- Chen, C.S.; Chen, J.; Wang, Z.; Zhang, J.; San, H.; Liu, S.; Wu, C.; Hofmann, W. Free-standing ZnO nanorod arrays modified with single-walled carbon nanotubes for betavoltaics and photovoltaics. J. Mater. Sci. Technol. 2020, 19, 48–57. [Google Scholar] [CrossRef]
- Zheng, R.R.; Wang, Z.; Zhang, C.Q.; Chen, B.; San, H.; Yu, H. Photocatalytic enhancement using defect-engineered black mesoporous TiO2/CeO2 nanocomposite aerogel. Compos. Part B-Eng. 2021, 222, 109037. [Google Scholar] [CrossRef]
- Gao, R.; Liu, L.; Li, Y.; Shen, L.; Wan, P.; Ouyang, X.; Zhang, H.; Ruan, J.; Zhou, L.; Chen, L.; et al. High-performance alpha-voltaic cell based on a 4H-SiC PIN junction diode. Energy Convers. Manag. 2022, 252, 115090. [Google Scholar] [CrossRef]
- San, H.; Yao, S.; Wang, X.; Chen, Z.; Chen, X. Design and simulation of GaN based Schottky betavoltaic nuclear micro-battery. Appl. Radiat. Isotopes 2013, 80, 17–22. [Google Scholar] [CrossRef]
- Spencer, M.G. High efficiency 4H-SiC betavoltaic power sources using tritium radioisotopes. Appl. Phys. Lett. 2016, 108, 013505. [Google Scholar] [CrossRef]
- Shimaoka, T.; Umezawa, H.; Ichikawa, K.; Pernot, J.; Koizumi, S. Ultrahigh conversion efficiency of betavoltaic cell using diamond pn junction. Appl. Phys. Lett. 2020, 117, 103902. [Google Scholar] [CrossRef]
- Munson, C.; Gaimard, Q.; Merghem, K.; Sundaram, S.; Rogers, D.J.; Sanoit, J.; Voss, P.L.; Ramdane, A.; Salvestrini, J.P.; Ougazzaden, A. Modeling, Design, Fabrication and Experimentation of a GaN-based, 63Ni Betavoltaic Battery. J. Phys. D Appl. Phys. 2017, 51, 035101. [Google Scholar] [CrossRef]
- Dixon, J.; Rajan, A.; Bohlemann, S.; Coso, D.; Upadhyaya, A.; Rohatgi, A.; Chu, S.; Majumdar, A.; Yee, S. Evaluation of a silicon 90Sr betavoltaic power source. Sci. Rep. 2016, 6, 38182. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, H.; Ling, Y.; Tang, Y.; Yang, X.; Fitzmorris, R.C.; Wang, C.C.; Zhang, J.; Li, Y. Hydrogen-treated TNRAs nanowire arrays for photo-electrochemical water splitting. Nano Lett. 2011, 11, 7. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Majima, T. Superstructure of TiO2 crystalline nanoparticles yields effective conduction pathways for photogenerated charges. J. Phys. Chem. Lett. 2012, 3, 1422–1427. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Jiang, Y.; Yu, X.; Sun, W. General strategy to construct hierarchical TNRAs nanorod arrays coupling with plasmonic resonance for dye-sensitized solar cells. Electrochim. Acta 2015, 173, 483–489. [Google Scholar] [CrossRef]
- Park, J.Y.; Choi, S.W.; Lee, J.W.; Lee, C.; Kim, S.S. Synthesis and gas sensing properties of TiO2-ZnO core-shell nanofibers. J. Am. Ceram. Soc. 2009, 92, 2551–2554. [Google Scholar] [CrossRef]
- Manders, J.R.; Tsang, S.W.; Hartel, M.J.; Lai, T.H.; Chen, S.; Amb, C.M.; Reynolds, J.R.; So, F. Solution-processed nickel oxide hole transport layers in high efficiency polymer photovoltaic cells. Adv. Funct. Mater. 2013, 23, 2993–3001. [Google Scholar] [CrossRef]
- Cao, J.; Yu, H.; Zhou, S.; Qin, M.; Ki, L.T.; Lu, X.; Zhao, N.; Wong, C.P. Low temperature solution-processed NiOx films for air-stable perovskite solar cells. J. Mater. Chem. A 2017, 5, 22. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Y.; Chen, J.; Chen, C.S.; San, H.S.; Chen, J.G.; Cheng, Z.D. Defectinduced betavoltaic enhancement in black titania nanotube arrays. Nanoscale 2018, 10, 13028–13036. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.J.; Liu, K.; Ralchenko, V.; Dzmitrovich, D.; Yang, L.; Yang, Y.N.; Zhang, X.Y.; Su, Z.H.; Zhao, J.W.; Shu, G.Y.; et al. Effect of americium-241 source activity on total conversion efficiency of diamond alpha-voltaic battery. Int. J. Energy Res. 2019, 43, 6038–6044. [Google Scholar] [CrossRef]
- Frye, C.D.; Murphy, J.W.; Shao, Q.H.; Voss, L.F.; Harrison, S.E.; Edgar, J.H.; Nikolic, R.J. Hall effect characterization of α-irradiated p-Type 4H-SiC. Phys. Status Solidi B 2021, 258, 13036. [Google Scholar] [CrossRef]
- Xi, S.X.; Li, L.X.; Zhou, C.Z.; Li, H.J.; Huang, G.W.; Wu, K.; Wang, Z.G.; Zhang, Y.Y. Researches on the performance of GaN-PIN betavoltaic nuclear battery. Radiat. Eff. Defects Solids 2022, 177, 213–229. [Google Scholar] [CrossRef]
- Gao, R.L.; Liu, L.Y.; Xia, X.C.; Wan, P.Y.; Ouyang, X.; Ma, W.Y.; Geng, X.L.; Wang, H.Y.; Xu, R.L.; Zhang, K.X.; et al. Isoelectronic aluminum-doped gallium nitride alpha-voltaic cell with efficiency exceeding 4.5%. Commun. Mater. 2023, 4, 50. [Google Scholar] [CrossRef]
- Shilpa, A.; Narasimha Murty, N.V.L. Alphavoltaic Performance of 4H-SiC Schottky Barrier Diodes. IEEE Trans. Nucl. Sci. 2024, 71, 2507–2514. [Google Scholar] [CrossRef]
- He, H.J.; Han, Y.C.; Wang, X.Y.; Ren, L.; Meng, X.D.; Zheng, M.J. Maximizing Output Power in p–n Junction Betavoltaic Batteries via Monte Carlo and Physics-Based Compact Model Cosimulation. IEEE Trans. Nucl. Sci. 2024, 71, 2515–2529. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.X.; Lu, J.B.; Li, X.Y.; Zheng, R.Z.; Cui, Q.M.; Zhang, Y.; Li, H.L.; Liu, X.R.; Zhang, K.; et al. Theoretical investigation of parallel 63NiO/GaP heterojunction nuclear battery with graphene layer and its time-related performance. Sci. Rep. 2025, 15, 7630. [Google Scholar] [CrossRef]
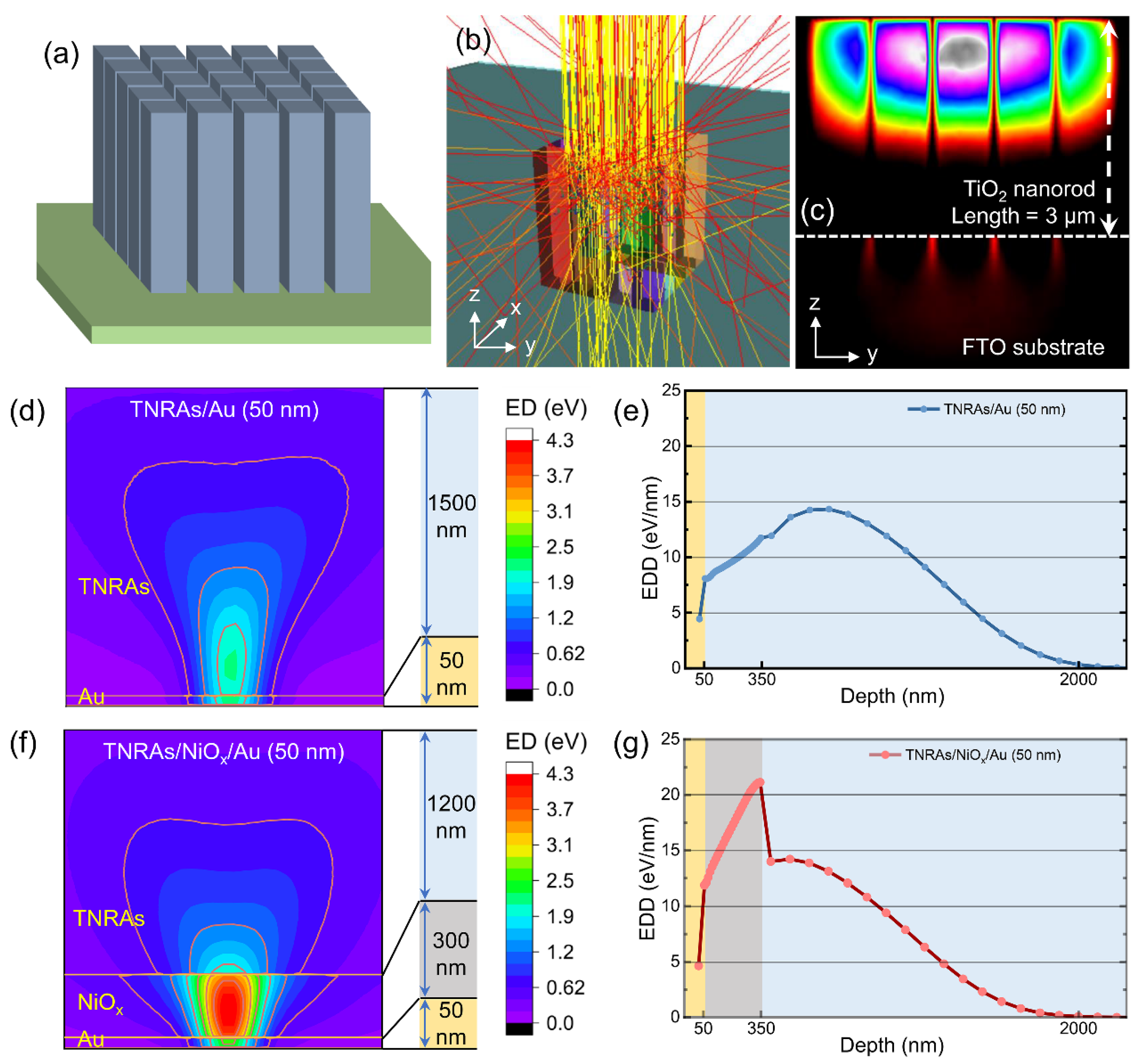
| Au (keV) | NiOx (keV) | TNRAs (keV) | ED Efficiency | |
|---|---|---|---|---|
| TNRAs/Au | 0.22 | — | 16.74 | 96.2% |
| TNRAs/NiOx/Au | 0.23 | 5.11 | 11.52 | 95.6% |
| Radiation Source | Conversion Device | Voc (V) | η (a.u.) | Ref. | |
|---|---|---|---|---|---|
| 1 | 241Am | Diamond | 1.06 | 1.41% | [33] |
| 2 | 63Ni | ZnO | 0.50 | 3.58% | [18] |
| 3 | 147Pm | Si | 0.17 | 2.30% | [34] |
| 4 | 147Pm | GaN | 1.25 | 2.78% | [35] |
| 5 | 237Np | SiC | 1.99 | 0.88% | [20] |
| 6 | He2+ | GaN | 1.13 | 4.51% | [36] |
| 7 | 63Ni | SiC | 0.40 | 0.28% | [37] |
| 8 | 63Ni | Si | 0.26 | 2.21% | [38] |
| 9 | 63Ni | GaP | 1.48 | 2.68% | [39] |
| 10 | 63Ni/EB | TNRAs | 0.026/0.32 | 3.74%/4.84% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Jiang, T.; Cao, Y.; Zhao, W.; San, H.; Li, X.; Zhang, L.; Li, X. TiO2 Nanorod Array for Betavoltaic Cells: Performance Validation and Enhancement with Electron Beam and 63Ni Irradiations. Nanomaterials 2025, 15, 923. https://doi.org/10.3390/nano15120923
Li S, Jiang T, Cao Y, Zhao W, San H, Li X, Zhang L, Li X. TiO2 Nanorod Array for Betavoltaic Cells: Performance Validation and Enhancement with Electron Beam and 63Ni Irradiations. Nanomaterials. 2025; 15(12):923. https://doi.org/10.3390/nano15120923
Chicago/Turabian StyleLi, Sijie, Tongxin Jiang, Yu Cao, Wendi Zhao, Haisheng San, Xue Li, Lifeng Zhang, and Xin Li. 2025. "TiO2 Nanorod Array for Betavoltaic Cells: Performance Validation and Enhancement with Electron Beam and 63Ni Irradiations" Nanomaterials 15, no. 12: 923. https://doi.org/10.3390/nano15120923
APA StyleLi, S., Jiang, T., Cao, Y., Zhao, W., San, H., Li, X., Zhang, L., & Li, X. (2025). TiO2 Nanorod Array for Betavoltaic Cells: Performance Validation and Enhancement with Electron Beam and 63Ni Irradiations. Nanomaterials, 15(12), 923. https://doi.org/10.3390/nano15120923






