Binary Solvent Engineering Modulates the Microstructure of Stretchable Organic Field-Effect Transistors for Highly Sensitive NO2 Sensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
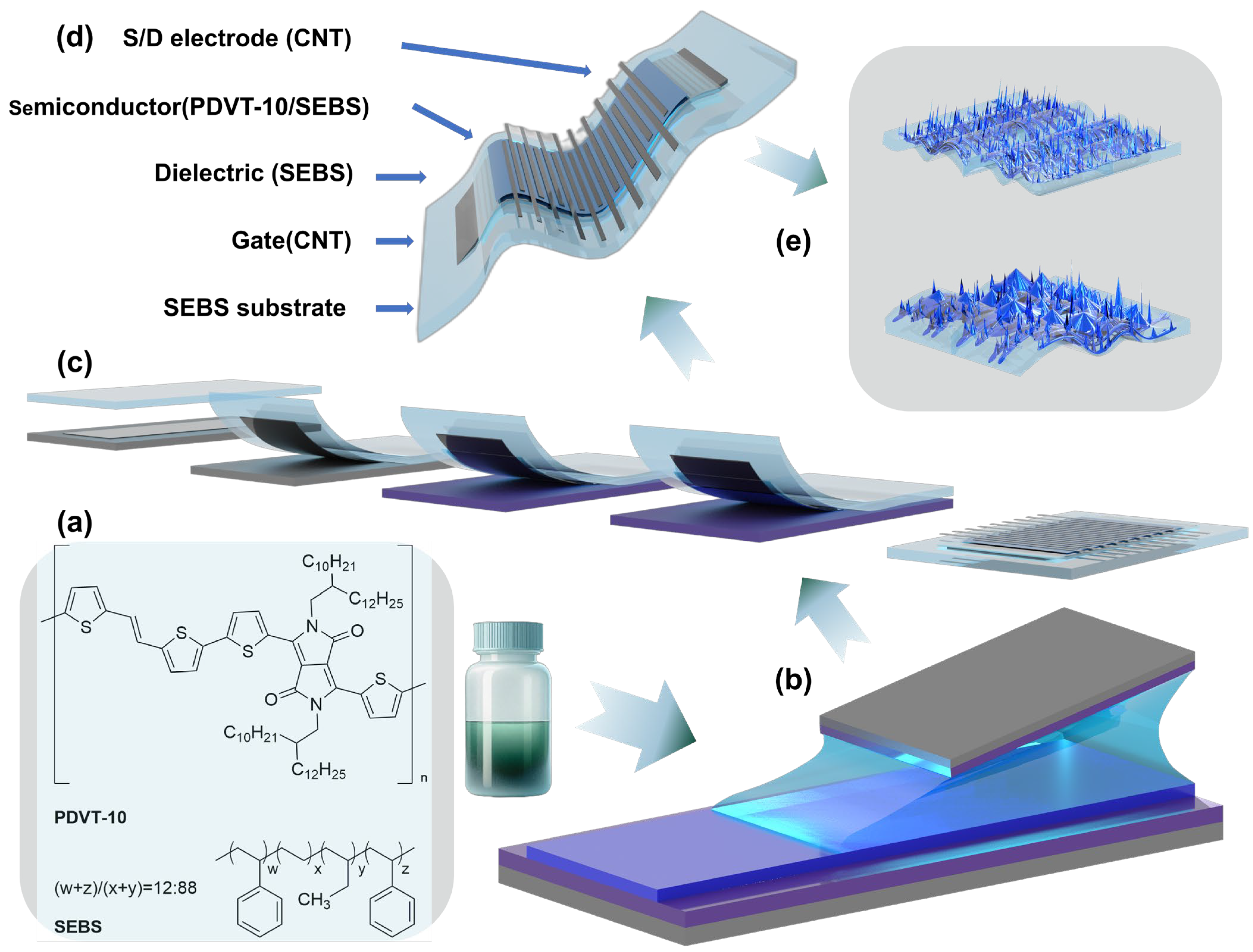
2.2. Preparation of Organic Semiconductor Polymer Films
2.3. Preparation of Organic Field-Effect Transistor Devices
2.4. Structural Characterization of Organic Semiconductor Polymer Films
2.5. Performance Testing of Organic Field-Effect Transistor Devices
3. Results and Discussion
3.1. Device Design of Stretchable Organic Semiconductors Based on Multicomponent Systems
3.2. Structural Characterization of Stretchable Organic Semiconductors Based on Multicomponent Systems
3.3. Force–Electric Property Balance of Stretchable Organic Field-Effect Transistors
3.4. Stretchable Organic Field-Effect Transistors for Gas-Sensing Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kashtan, Y.; Nicholson, M.; Finnegan, C.J.; Ouyang, Z.; Garg, A.; Lebel, E.D.; Rowland, S.T.; Michanowicz, D.R.; Herrera, J.; Nadeau, K.C.; et al. Nitrogen dioxide exposure, health outcomes, and associated demographic disparities due to gas and propane combustion by U.S. stoves. Sci. Adv. 2024, 10, eadm8680. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, G.B.B.M.; Penney, W.G. Shape of the NO2 Molecule. Nature 1935, 136, 146–147. [Google Scholar] [CrossRef]
- Blomberg, A.; Krishna, M.T.; Bocchino, V.; Biscione, G.L.; Shute, J.K.; Kelly, F.J.; Frew, A.J.; Holgate, S.T.; Sandström, T. The Inflammatory Effects of 2 ppm NO2 on the Airways of Healthy Subjects. Am. J. Respir. Crit. Care Med. 1997, 156, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Banzon, T.M.; Jung, Y.S.; Greco, K.F.; Li, L.; Nadeau, K.; Permaul, P.; Koutrakis, P.; Gaffin, J.M.; Phipatanakul, W. Biomarkers of inflammation associated with radon exposure in the School Inner-City Asthma Study (SICAS). J. Allergy Clin. Immunol. 2025, 155, 1866–1872. [Google Scholar] [CrossRef]
- Lu, C.; Wang, F.; Liu, Q.; Deng, M.; Yang, X.; Ma, P. Effect of NO2 exposure on airway inflammation and oxidative stress in asthmatic mice. J. Hazard. Mater. 2023, 457, 131787. [Google Scholar] [CrossRef]
- Ma, J.; Yao, Y.; Xie, Y.; Yin, H.; Yang, S.; Shang, B.; You, X.; Guo, Y.; Chen, W. Omega-3 Modify the Adverse Effects of Long-Term Exposure to Ambient Air Pollution on the Incidence of Chronic Obstructive Pulmonary Disease: Evidence from a Nationwide Prospective Cohort Study. Environ. Health 2025. [Google Scholar] [CrossRef]
- Chauhan, A.J.; Inskip, H.M.; Linaker, C.H.; Smith, S.; Schreiber, J.; Johnston, S.L.; Holgate, S.T. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet 2003, 361, 1939–1944. [Google Scholar] [CrossRef]
- Pilotto, L.S.; Douglas, R.M. Measuring NO2 exposure in respiratory disease. Nat. Med. 1995, 1, 517–518. [Google Scholar] [CrossRef]
- Liu, R.; Young, M.T.; Chen, J.-C.; Kaufman, J.D.; Chen, H. Ambient Air Pollution Exposures and Risk of Parkinson Disease. Environ. Health Perspect. 2016, 124, 1759–1765. [Google Scholar] [CrossRef]
- Antonsen, S.; Mok, P.L.H.; Webb, R.T.; Mortensen, P.B.; McGrath, J.J.; Agerbo, E.; Brandt, J.; Geels, C.; Christensen, J.H.; Pedersen, C.B. Exposure to air pollution during childhood and risk of developing schizophrenia: A national cohort study. Lancet Planet. Health 2020, 4, e64–e73. [Google Scholar] [CrossRef]
- Li, H.; Chen, L.; Guo, Z.; Sang, N.; Li, G. In vivo screening to determine neurological hazards of nitrogen dioxide (NO2) using Wistar rats. J. Hazard. Mater. 2012, 225–226, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.; Hassan, N.; Wilson, S.; Ardle, R.M.; Su, L.; Slight, S. Outdoor air pollution as a risk factor for Alzheimer’s disease: A systematic review. Alzheimer’s Dement. 2024, 20, e089538. [Google Scholar] [CrossRef]
- Ye, S.; Wei, L.; Jiang, Y.; Yuan, Y.; Zeng, Y.; Zhu, L.; Xiao, F. Mechanism of NO2-induced migraine in rats: The exploration of the role of miR-653-3p/IGF1 axis. J. Hazard. Mater. 2024, 465, 133362. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Liu, C.; Chen, R.; Sera, F.; Vicedo-Cabrera, A.M.; Milojevic, A.; Guo, Y.; Tong, S.; Coelho, M.d.S.Z.S.; Saldiva, P.H.N.; et al. Short term associations of ambient nitrogen dioxide with daily total, cardiovascular, and respiratory mortality: Multilocation analysis in 398 cities. BMJ 2021, 372, n534. [Google Scholar] [CrossRef]
- Kephart, J.L.; Fandiño-Del-Rio, M.; Williams, K.N.; Malpartida, G.; Lee, A.; Steenland, K.; Naeher, L.P.; Gonzales, G.F.; Chiang, M.; Checkley, W.; et al. Nitrogen dioxide exposures from LPG stoves in a cleaner-cooking intervention trial. Environ. Int. 2021, 146, 106196. [Google Scholar] [CrossRef]
- Gamonal, A.; Sun, C.; Mariano, A.L.; Fernandez-Bartolome, E.; Guerrero-SanVicente, E.; Vlaisavljevich, B.; Castells-Gil, J.; Marti-Gastaldo, C.; Poloni, R.; Wannemacher, R.; et al. Divergent Adsorption-Dependent Luminescence of Amino-Functionalized Lanthanide Metal–Organic Frameworks for Highly Sensitive NO2 Sensors. J. Phys. Chem. Lett. 2020, 11, 3362–3368. [Google Scholar] [CrossRef]
- Pandey, D.; Samarth, T.; Verma, V.K.; Patel, C.; Ponvijayakanthan, L.; Jaiswal, N.K.; Mukherjee, S.; Raghuvanshi, A. Two-dimensional Cu(i)-MOF with mesoporous architecture towards chemiresistive NO2 sensing. J. Mater. Chem. A 2025, 13, 11416–11424. [Google Scholar] [CrossRef]
- Feng, S.; Huang, L.; Lu, Q.; Wang, T.; Han, R.; Sun, X.; Liang, X.; Liu, F.; Liu, F.; Lu, G. Ppb-level fuel cell type NO2 sensor based on Nafion proton membrane and Au-Ni/CF sensitive electrode. Sens. Actuators B Chem. 2025, 433, 137558. [Google Scholar] [CrossRef]
- Lim, S.-H.; Mah, D.-G.; Cho, W.-J. Enhancing the Stability and Mobility of TFTs via Indium–Tungsten Oxide and Zinc Oxide Engineered Heterojunction Channels Annealed in Oxygen Ambient. Nanomaterials 2024, 14, 1252. [Google Scholar] [CrossRef]
- Vijjapu, M.T.; Surya, S.; Zalte, M.; Yuvaraja, S.; Baghini, M.S.; Salama, K.N. Towards a low cost fully integrated IGZO TFT NO2 detection and quantification: A solution-processed approach. Sens. Actuators B Chem. 2021, 331, 129450. [Google Scholar] [CrossRef]
- Ren, X.; Yang, F.; Gao, X.; Cheng, S.; Zhang, X.; Dong, H.; Hu, W. Organic Field-Effect Transistor for Energy-Related Applications: Low-Power-Consumption Devices, Near-Infrared Phototransistors, and Organic Thermoelectric Devices. Adv. Energy Mater. 2018, 8, 1801003. [Google Scholar] [CrossRef]
- Jang, S.C.; Lee, G.; Park, I.; Noh, B.; Park, J.-M.; Lee, J.; Lee, K.J.; Kim, H.-S. Tunable dielectric properties of a parylene dielectric layer through surface-modulation by click chemistry. J. Mater. Chem. C 2025, 13, 6614–6623. [Google Scholar] [CrossRef]
- Peng, B.; Cao, K.; Lau, A.H.Y.; Chen, M.; Lu, Y.; Chan, P.K.L. Crystallized Monolayer Semiconductor for Ohmic Contact Resistance, High Intrinsic Gain, and High Current Density. Adv. Mater. 2020, 32, 2002281. [Google Scholar] [CrossRef]
- Waldrip, M.; Jurchescu, O.D.; Gundlach, D.J.; Bittle, E.G. Contact Resistance in Organic Field-Effect Transistors: Conquering the Barrier. Adv. Funct. Mater. 2020, 30, 1904576. [Google Scholar] [CrossRef]
- Jeong, G.; Shin, S.Y.; Kyokunzire, P.; Cheon, H.J.; Wi, E.; Woo, M.; Chang, M. High-Performance Nitric Oxide Gas Sensors Based on an Ultrathin Nanoporous Poly(3-hexylthiophene) Film. Biosensors 2023, 13, 132. [Google Scholar] [CrossRef]
- Johnson, M.; Peakall, J.; Fairweather, M.; Barnes, M.; Davison, S.; Jia, X.; Clare, M.A.; Harbottle, D.; Hunter, T.N. Sediment Microstructure and the Establishment of Gas Migration Pathways during Bubble Growth. Environ. Sci. Technol. 2019, 53, 12882–12892. [Google Scholar] [CrossRef]
- Lyu, B.; Kim, M.; Jing, H.; Kang, J.; Qian, C.; Lee, S.; Cho, J.H. Large-Area MXene Electrode Array for Flexible Electronics. ACS Nano 2019, 13, 11392–11400. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, P.; Hu, W. Organic field-effect transistor-based gas sensors. Chem. Soc. Rev. 2015, 44, 2087–2107. [Google Scholar] [CrossRef]
- Ko, I.H.; Park, Y.D. Recent Research Trends for Developing Highly Sensitive, Flexible Organic Field-Effect Transistor-Based Gas Sensors. ACS Appl. Polym. Mater. 2025, 7, 2749–2760. [Google Scholar] [CrossRef]
- Yuvaraja, S.; Nawaz, A.; Liu, Q.; Dubal, D.; Surya, S.G.; Salama, K.N.; Sonar, P. Organic field-effect transistor-based flexible sensors. Chem. Soc. Rev. 2020, 49, 3423–3460. [Google Scholar] [CrossRef]
- Han, F.; Li, H.; Huang, L.; Zhou, X.; Su, R.; Yu, H.; Tian, Q.; Zhao, H.; Li, Q.; Sun, J.; et al. Hybrid microstructure-based stretchable biosensors for multi-physiological signal sensing. eScience 2025, 5, 100327. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Tong, W.; Li, X.; Zhang, Y. Microstructure designed flexoelectric materials and tip force for multifunctional applications. Nano Energy 2025, 133, 110442. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Li, Z.; Zhang, W.; Meng, Q. Flexible tactile sensors with biomimetic microstructures: Mechanisms, fabrication, and applications. Adv. Colloid Interface Sci. 2023, 320, 102988. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Hosneolfat, Z.; Nekoonam, N.; Bhagwat, S.; Helmer, D.; Rapp, B.E. Fabricating Microstructures on Freeform Surfaces via Flexible Hydrogel Micromolds. Small 2025, 21, 2411751. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Z.; Song, Q.; Sun, X.; Liu, H.; Fang, R.; Jiang, X. Microstructures Formation through Liquid-Assisted Assembly of Functional Materials for High-Performance Electronics. Adv. Funct. Mater. 2024, 34, 2315162. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, C.; Xiao, C.; Zhao, T.; Luo, T.; Zhou, W. Fabrication of Hierarchical Microstructures via Laser-Induced Shrinkage of Shape Memory Polymers for Flexible Pressure Sensing. ACS Appl. Mater. Interfaces 2024, 16, 45732–45744. [Google Scholar] [CrossRef]
- Qin, J.; Yin, L.-J.; Hao, Y.-N.; Zhong, S.-L.; Zhang, D.-L.; Bi, K.; Zhang, Y.-X.; Zhao, Y.; Dang, Z.-M. Flexible and Stretchable Capacitive Sensors with Different Microstructures. Adv. Mater. 2021, 33, 2008267. [Google Scholar] [CrossRef]
- Park, D.; Tarsoly, G.; Kwon, D.; Shin, T.J.; Pyo, S. Photo-response modulation of organic transistors for multi-level light sensing using active layer microstructure control. J. Mater. Chem. C 2023, 11, 4365–4374. [Google Scholar] [CrossRef]
- Tsui, T.-H.; Ekama, G.A.; Chen, G.-H. Quantitative characterization and analysis of granule transformations: Role of intermittent gas sparging in a super high-rate anaerobic system. Water Res. 2018, 139, 177–186. [Google Scholar] [CrossRef]
- Xu, L.; Liu, C.; Ma, X.; Xu, Y.; Zhou, W.; Guan, W.; Qiang, Q.; Lang, T.; Peng, L.; Zhong, Y.; et al. Two-birds-one-stone: Flexible PANI film with bionic microstructures for multifunctional sensing of physical and chemical stimuli. Chem. Eng. J. 2023, 451, 138820. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Y.; Huang, H.; Liu, C.; Wu, D.; Xia, J. Direct laser patterning of organic semiconductors for high performance OFET-based gas sensors. J. Mater. Chem. C 2023, 11, 7088–7097. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, B.; Yang, J.; Gao, H.; Li, S.; Wang, L.; Wu, P.; Su, Y.; Zhao, Y.; Feng, J.; et al. Wafer-scale integration of stretchable semiconducting polymer microstructures via capillary gradient. Nat. Commun. 2021, 12, 7038. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.-H.; Kim, W.-J.; Jung, C.-H.; Hwang, I.-T.; Khim, D.; Kim, D.-Y.; Lee, J.-S.; Ku, B.-C.; Choi, J.-H. A simple PAN-based fabrication method for microstructured carbon electrodes for organic field-effect transistors. Carbon 2015, 87, 257–268. [Google Scholar] [CrossRef]
- Li, X.; Tang, Y.; Cheng, W.; Liu, Y.; Dong, H.; Li, X.; Shao, C.; Fu, S.; Liu, Y. Highly flexible all-inorganic nanofiber networks with stress-accommodating microstructure for light-activated wearable chemiresistive sensor. Chem. Eng. J. 2023, 455, 140768. [Google Scholar] [CrossRef]
- Xu, J.; Wang, S.; Wang, G.-J.N.; Zhu, C.; Luo, S.; Jin, L.; Gu, X.; Chen, S.; Feig, V.R.; To, J.W.F.; et al. Highly stretchable polymer semiconductor films through the nanoconfinement effect. Science 2017, 355, 59–64. [Google Scholar] [CrossRef]
- Michels, J.J.; Zhang, K.; Wucher, P.; Beaujuge, P.M.; Pisula, W.; Marszalek, T. Predictive modelling of structure formation in semiconductor films produced by meniscus-guided coating. Nat. Mater. 2021, 20, 68–75. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Kosbar, L.L.; Murray, C.E.; Copel, M.; Afzali, A. High-mobility ultrathin semiconducting films prepared by spin coating. Nature 2004, 428, 299–303. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Y.; Zhang, J.; Li, X.; Yang, H.; Hu, W. Highly stretchable and high-mobility simiconducting nanofibrous blend films for fully stretchable organic transistors. Sci. China Mater. 2023, 66, 1891–1898. [Google Scholar] [CrossRef]
- Root, S.E.; Savagatrup, S.; Printz, A.D.; Rodriquez, D.; Lipomi, D.J. Mechanical Properties of Organic Semiconductors for Stretchable, Highly Flexible, and Mechanically Robust Electronics. Chem. Rev. 2017, 117, 6467–6499. [Google Scholar] [CrossRef]
- Pan, Y.; Dong, L.; Yin, X.; Wu, H. Compact and Highly Sensitive NO2 Photoacoustic Sensor for Environmental Monitoring. Molecules 2020, 25, 1201. [Google Scholar] [CrossRef]
- Song, Z.; Tong, Y.; Zhao, X.; Ren, H.; Tang, Q.; Liu, Y. A flexible conformable artificial organ-damage memory system towards hazardous gas leakage based on a single organic transistor. Mater. Horiz. 2019, 6, 717–726. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, B.; Ji, X.; Pei, K.; Chan, P.K.L. Marangoni-Effect-Assisted Bar-Coating Method for High-Quality Organic Crystals with Compressive and Tensile Strains. Adv. Funct. Mater. 2017, 27, 1703443. [Google Scholar] [CrossRef]
- van Honschoten, J.W.; Brunets, N.; Tas, N.R. Capillarity at the nanoscale. Chem. Soc. Rev. 2010, 39, 1096–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, C.; Liu, Q. Experimental characterization of elastocapillary and osmocapillary effects on multi-scale gel surface topography. Soft Matter 2023, 19, 8698–8705. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, W.; Wang, J.; Luo, D.; Qiao, X.; Qin, X.; Wang, T. Ultrasensitive Surface-Enhanced Raman Scattering Sensor of Gaseous Aldehydes as Biomarkers of Lung Cancer on Dendritic Ag Nanocrystals. Anal. Chem. 2017, 89, 1416–1420. [Google Scholar] [CrossRef]
- Simões, E.W.; de Souza, S.G.; da Silva, M.L.P.; Furlan, R.; Maldonado Peres, H.E. Study of preconcentration of non-polar compounds in microchannels with constrictions. Sens. Actuators B Chem. 2006, 115, 232–239. [Google Scholar] [CrossRef]
- Huang, W.; Zhuang, X.; Melkonyan, F.S.; Wang, B.; Zeng, L.; Wang, G.; Han, S.; Bedzyk, M.J.; Yu, J.; Marks, T.J.; et al. UV–Ozone Interfacial Modification in Organic Transistors for High-Sensitivity NO2 Detection. Adv. Mater. 2017, 29, 1701706. [Google Scholar] [CrossRef]
- Han, S.; Cheng, J.; Fan, H.; Yu, J.; Li, L. Achievement of High-Response Organic Field-Effect Transistor NO2 Sensor by Using the Synergistic Effect of ZnO/PMMA Hybrid Dielectric and CuPc/Pentacene Heterojunction. Sensors 2016, 16, 1763. [Google Scholar] [CrossRef]
- Zeng, Y.; Huang, W.; Shi, W.; Yu, J. Enhanced sensing performance of nitrogen dioxide sensor based on organic field-effect transistor with mechanically rubbed pentacene active layer. Appl. Phys. A 2015, 118, 1279–1285. [Google Scholar] [CrossRef]
- Song, R.; Zhou, X.; Wang, Z.; Zhu, L.; Lu, J.; Xue, D.; Wang, Z.; Huang, L.; Chi, L. High selective gas sensors based on surface modified polymer transistor. Org. Electron. 2021, 91, 106083. [Google Scholar] [CrossRef]
- Zhu, Y.; Dong, J.; Li, G.; Liu, C.; Xie, Q.; Wang, L.; Wang, L.J.; You, M. Bilayer polymer dielectric systems for high response NO2 gas sensors. Mater. Lett. 2021, 288, 129370. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Zeng, J.; Zhang, L.; Zhang, Z.; Zhu, R. Binary Solvent Engineering Modulates the Microstructure of Stretchable Organic Field-Effect Transistors for Highly Sensitive NO2 Sensing. Nanomaterials 2025, 15, 922. https://doi.org/10.3390/nano15120922
Jiang X, Zeng J, Zhang L, Zhang Z, Zhu R. Binary Solvent Engineering Modulates the Microstructure of Stretchable Organic Field-Effect Transistors for Highly Sensitive NO2 Sensing. Nanomaterials. 2025; 15(12):922. https://doi.org/10.3390/nano15120922
Chicago/Turabian StyleJiang, Xiao, Jiaqi Zeng, Linxuan Zhang, Zhen Zhang, and Rongjiao Zhu. 2025. "Binary Solvent Engineering Modulates the Microstructure of Stretchable Organic Field-Effect Transistors for Highly Sensitive NO2 Sensing" Nanomaterials 15, no. 12: 922. https://doi.org/10.3390/nano15120922
APA StyleJiang, X., Zeng, J., Zhang, L., Zhang, Z., & Zhu, R. (2025). Binary Solvent Engineering Modulates the Microstructure of Stretchable Organic Field-Effect Transistors for Highly Sensitive NO2 Sensing. Nanomaterials, 15(12), 922. https://doi.org/10.3390/nano15120922





