Abstract
Bismuth ferrite (BiFeO3, BFO) is a promising multiferroic material, but its optoelectronic potential is limited by a wide bandgap and charge recombination. Here, we report the sol–gel synthesis of Co-doped BiFeO3/Bi25FeO40 heterostructured nanopowders (x = 0.07, 0.15) alongside pristine BFO to explore Co doping and phase engineering as strategies to enhance their functional properties. Using X-ray diffraction (XRD) with Rietveld refinement, Fourier-transform infrared spectroscopy (FTIR), field-emission scanning electron microscopy (FE-SEM), UV-Vis spectroscopy, and dielectric analysis, we reveal a biphasic structure (rhombohedral R3c and cubic I23 phases) with tuned phase ratios (~73:27 for x = 0.07; ~76:24 for x = 0.15). Co doping induces lattice strain and oxygen vacancies, reducing the bandgap from 1.78 eV in BFO to 1.31 eV in BFO0.15 and boosting visible light absorption. Dielectric measurements show reduced permittivity and altered conduction, driven by [Co2+-V0••] defect dipoles. These synergistic modifications, including phase segregation, defect chemistry, and nanoscale morphology, significantly enhance optoelectronic performance, making these heterostructures compelling for photocatalytic and photovoltaic applications.
1. Introduction
Bandgap engineering is essential for optimizing the photocatalytic efficiency of semiconductor materials. Bismuth ferrite (BiFeO3, BFO), a room-temperature multiferroic perovskite, combines robust ferroelectricity (TC~1103 K) and G-type antiferromagnetism (TN~643 K), making it a compelling candidate for applications ranging from spintronics to photocatalysis [,]. Its narrow bandgap (~2.2–2.7 eV) and ability to absorb visible light further position it as a promising photocatalyst. However, its practical application is hindered by intrinsic limitations such as high leakage currents from oxygen vacancies, suppressed macroscopic magnetization due to spiral spin structures, and rapid electron–hole recombination which hinder its practical utility [,]. These defects degrade its dielectric stability, suppress magnetization, and impair charge carrier dynamics, necessitating strategies to enhance BFO’s functional properties.
Transition metal doping has emerged as an effective approach to overcoming these limitations. Cobalt (Co), in particular, is a compelling substitute due to its compatible ionic radius (Co2+: 0.745 Å vs. Fe3+: 0.645 Å) and multivalent nature, which facilitate lattice substitution at Fe3+ sites. It stands out for its unique ability to simultaneously tune electronic, magnetic, and structural properties [,]. Introducing Co2+/Co3+ ions into the BFO lattice induces critical modifications. The larger ionic radius of Co2+ generates lattice strain, reducing crystallite size and disrupting the antiferromagnetic cycloidal spin order to enhance weak ferromagnetism []. Additionally, Co doping hybridizes its three d orbitals with O 2p states, creating intermediate energy levels that narrow the bandgap and significantly improve its ability to absorb visible light [,]. Furthermore, aliovalent Co2+ doping promotes charge compensation via oxygen vacancy formation, which optimizes electrical conductivity while stabilizing the perovskite structure []. However, isolated doping often fails to effectively suppress photogenerated charge recombination, limiting catalytic performance.
To further enhance charge separation, heterostructure engineering, particularly at the BiFeO3/Bi25FeO40 interfaces, offers a promising solution by facilitating charge separation and improving electron mobility. These heterojunctions leverage interfacial charge transfer between phases to suppress electron–hole recombination, extend carrier lifetimes, and enhance redox activity [,]. The built-in electric field at the BiFeO3/Bi25FeO40 interface facilitates the efficient separation of photogenerated charges, while the synergistic alignment of band structures broadens light absorption and improves photocatalytic efficiency []. Additionally, the heterostructure’s increased surface-to-volume ratio exposes more active sites for catalytic reactions, further amplifying performance []. Conventional methods like solid-state synthesis lack the precision to achieve nanoscale homogeneity, underscoring the need for advanced fabrication techniques.
In achieving precise stoichiometric control and minimizing secondary phases, the sol–gel method is uniquely advantageous []. Unlike solid-state synthesis, sol–gel processing enables atomic-level homogeneity and low-temperature crystallization (~550 °C), mitigating bismuth volatilization and ensuring uniform Co incorporation [,]. This precision is crucial for constructing heterostructured systems, such as BiFeO3/Bi25FeO40 []. While Co substitution and heterostructure engineering have been studied individually, their combined effects on band structure modulation, defect passivation, and multiferroic enhancement remain unexplored. Specifically, the interplay between Co-induced lattice distortions, heterophase stability, and interfacial charge transfer in BiFeO3/Bi25FeO40 systems is lacking further investigation.
In this work, we synthesize Co-doped BiFeO3/Bi25FeO40 heterostructured nanopowders (x = 0.07, 0.15) via sol–gel processing, alongside pristine BFO for comparison. We systematically tune the phase composition, crystallite size, and optical properties to achieve a bandgap reduction that surpasses those of individual BFO and sillenite. Through systematic structural, vibrational, optical and dielectric analyses, we demonstrate how Co doping and heterojunction formation synergistically enhance electrical conductivity and promote band gap tuning. This study provides new insights into the correlation between Co doping, Co-doped BiFeO3/Bi25FeO40 heterostructure formation and band gap reduction, which might provide a framework for designing advanced multiferroic nanocomposites that are promising for environmental applications.
2. Materials and Methods
2.1. Synthesis Procedure
BiFeO3 and Co-doped BiFeO3/Bi25FeO40 heterostructured nanopowders with cobalt substitution levels of x = 0.07 and 0.15, labelled as BFO, BFCO0.07, and BFCO0.15, were synthesized using a citrate-modified sol–gel approach []. Stoichiometric quantities of bismuth (III) nitrate pentahydrate (Bi (NO3)3·5H2O), iron (III) nitrate nonahydrate (Fe (NO3)3·9H2O), and cobalt (II) nitrate hexahydrate (Co (NO3)2·6H2O) were dissolved separately in deionized water under continuous magnetic stirring. Nitric acid (HNO3) was added dropwise to each solution to ensure complete dissolution. The individual solutions were combined and homogenized at 25 °C for 30 min. Citric acid (C6H8O7) and ethylene glycol (C2H6O2) were introduced into the mixture in stoichiometric proportions as complexing and cross-linking agents, respectively. The solution was heated at 70 °C to induce gelation. The resulting gel was dehydrated at 100 °C for 4 h to form a xerogel, which was then ground into a fine powder and calcined at 600 °C in air for 1 h to yield crystallized nanoparticles.
2.2. Characterization Techniques
Crystalline phase identification and structural analysis were performed using X-ray diffraction (XRD) with a Bruker D8 Advance XR01 diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) equipped with Cu Kα radiation (λ = 1.5406 Å), which includes both Kα1 (λ = 1.54056 Å) and Kα2 (λ = 1.54439 Å) components. Data were collected over a 2θ range of 20–80° with an angular resolution of 0.02°. Phase identification and lattice parameters were quantified through Rietveld refinement using the FullProf suite (version: April 2023). Moreover, to analyze bonding configurations and detect organic residuals, Fourier-transform infrared spectroscopy (FTIR) was employed. FTIR measurements were carried out using a Perkin Elmer FTIR-100 spectrometer (PerkinElmer, Waltham, MA, USA) over the spectral range of 400–4000 cm−1. Powder morphology was analyzed via scanning electron microscopy (SEM) using a TESCAN VEGA3 SBH microscope (TESCAN, Brno, Czech Republic) equipped with a Bruker XFlash 410M energy-dispersive spectroscopy (EDS) (Bruker Corporation, Billerica, MA, USA) detector for elemental analysis. Optical properties and bandgap energies were determined using UV-Vis spectroscopy with an Evolution 220 spectrometer (Thermo Scientific Co., Ltd., Waltham, MA, USA). For dielectric measurements, cylindrical disc-shaped pellets were fabricated, and a uniform layer of conductive silver paste was applied to both surfaces to ensure optimal electrical contact. Dielectric properties were characterized using a Wayne Kerr 6425 component analyzer (Wayne Kerr Electronics Ltd., Bognor Regis, West Sussex, UK).
3. Results and Discussion
3.1. XRD Analysis
The crystal structures of the BiFeO3 and Co-doped BiFeO3/Bi25FeO40 heterostructured nanopowders (BFCO0.07, BFCO0.15) were systematically investigated using X-ray diffraction coupled with Rietveld refinement. Figure 1a displays the XRD patterns of all samples, where the sharp, high-intensity peaks with narrow full-width-at-half-maximum (FWHM) values confirm the high crystallinity of the synthesized materials. For BFO, the diffraction peaks align with the rhombohedral perovskite structure (space group R3c, JCPDS No. 20-0169) []. In contrast, the BFCO0.07 and BFCO0.15 samples exhibit additional peaks primarily indexed to the cubic iron–sillenite-phase Bi25FeO40 (space group I23, JCPDS No. 46-0416) [], confirming the coexistence of perovskite (R3c) and sillenite (I23) phases. This phase segregation, modulated by cobalt doping, arises from the controlled Bi3+/Fe3+ molar ratio during synthesis, as previously reported by Wang et al. []. Specifically, excess Bi3+ promotes the formation of Bi25FeO40 alongside BiFeO3, while excess Fe3+ favours Bi2Fe4O9, highlighting the critical role of stoichiometric control in phase engineering.
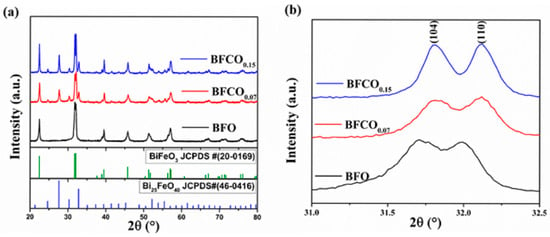
Figure 1.
(a) X-ray diffraction patterns of BFO, BFCO0.07, and BFCO0.15 along with the standard JCPDS data of BiFeO3 and Bi25FeO40. (b) Partial enlargement of the XRD patterns at 31–32.5°.
A magnified view of the (104) and (110) Bragg reflections in Figure 1b reveal distinct peak shifts that broaden with the cobalt substitution. For BFCO0.07, a systematic shift toward higher angles indicates lattice contraction due to the doping of Fe3+ (ionic radius: 0.645 Å) by smaller Co3+ ions (0.545 Å). Conversely, BFCO0.15 shows negligible peak shifting, suggesting competing effects from larger Co2+ (0.745 Å) and/or oxygen vacancy formation at higher doping levels. The partial oxidation of Co2+ to Co3+ during synthesis is facilitated by nitric acid and ethylene glycol []. This oxidation process introduces a dual effect: initial lattice contraction (Co3+ doping) followed by potential expansion due to residual Co2+ or oxygen vacancies at higher doping levels. These observations underscore the doping-dependent interplay between ionic radius mismatch and defect chemistry.
Rietveld refinement quantitatively validated the phase composition and structural parameters of all samples, as shown in Figure 2. BFO crystallizes purely in the rhombohedral R3c phase, whereas BFCO0.07 and BFCO0.15 exhibit biphasic structures (R3c + I23), with phase ratios of ~73:27 and ~76:24, respectively. The lattice parameters and unit cell volumes decrease progressively with cobalt doping (Table 1). Although the sillenite phase was primarily identified in the study as Bi25FeO40, the progressive lattice contraction (a = 10.203 Å for BFCO0.07 and 10.194 Å for BFCO0.15) suggests that the sillenite phase corresponds to Co-doped Bi25FeO40 i.e., (Bi25(Fe,Co)O40) []. This aligns with partial doping of smaller Co3+ ions into Fe3+ sites, rather than the formation of distinct Bi25CoO40, which would require a stoichiometric Co:Fe ratio (1:0) and exhibit a smaller lattice parameter of (~10.13 Å) [].
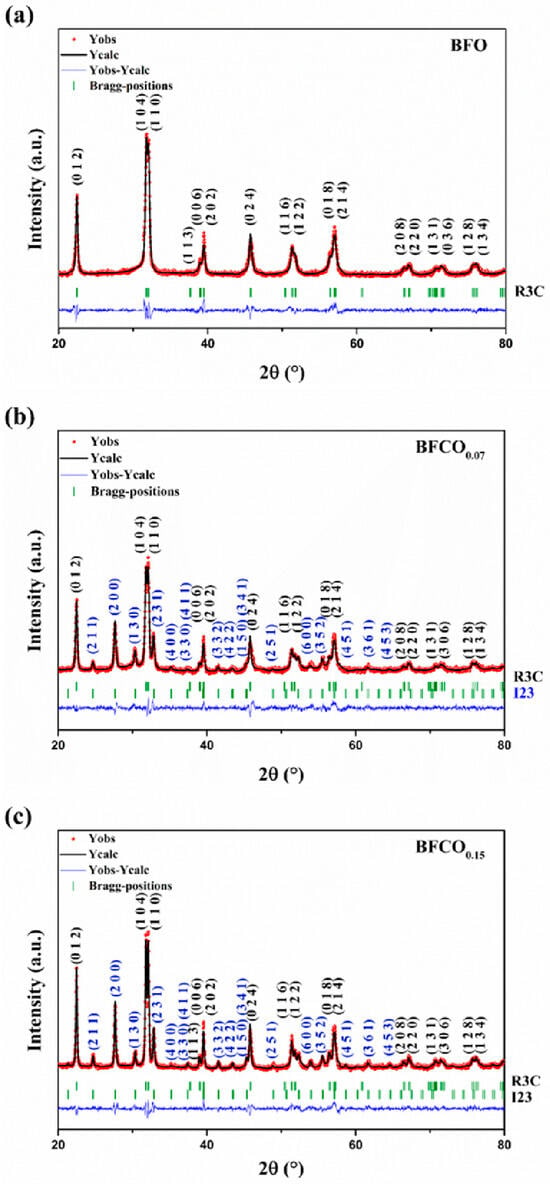
Figure 2.
Rietveld refinement of the X-ray diffraction patterns of (a) BFO, (b) BFCO0.07, and (c) BFCO0.15 nanopowders.

Table 1.
Rietveld refinement parameters for BFO, BFCO0.07, and BFCO0.15: lattice constants, space group, and agreement factors.
Further structural insights were derived from bond lengths and angles (Table 2). In BFCO0.07, the shortened Fe–O bond (1.994 Å vs. 2.013 Å in BFO) and near-linear Fe–O–Fe angle (166.09°) reflect reduced octahedral tilting and compressive strain within the dominant R3c phase. Coherent interfaces with the cubic I23 phase (27% fraction) further alleviate interfacial strain. In contrast, BFCO0.15 exhibits elongated Fe–O bonds (2.009 Å) and a distorted Fe–O–Fe angle (160.23°), indicative of tensile strain and enhanced octahedral tilting. While the overall lattice parameters indicate compressive strain in the perovskite phase due to lattice mismatch at the R3c/I23 heterointerface, local atomic distortions within the perovskite phase, particularly in BFCO0.15, show evidence of tensile strain. This local tensile strain, evidenced by elongated Fe–O bonds (2.009 Å) and distorted Fe–O–Fe angles (160.23°), arises from the incorporation of larger Co2+ ions (0.745 Å) and potential oxygen vacancies, which cause local lattice expansion despite the overall compressive trend []. A schematic presentation of bond lengths and angles in all samples using Vesta software is depicted in Figure 3.

Table 2.
Comparison of Fe–O bond lengths (Å) and Fe–O–Fe angles (°) in BFO, BFCO0.07, and BFCO0.15.
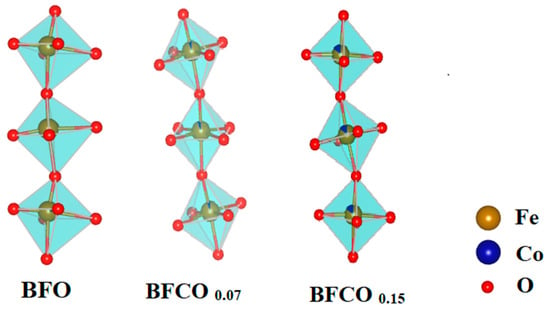
Figure 3.
A schematic representation of bond lengths and angles in BFO, BFCO0.07, and BFCO0.15 nanopowders using Vesta software (version: 4.3.0).
To deconvolute the contributions of crystallite size and lattice strain to X-ray diffraction peak broadening, a Williamson–Hall (W-H) analysis was employed. The W-H method assumes that these two factors independently contribute to the full-width-at-half-maximum (FWHM, β) of Bragg reflections, with their combined effects expressed as:
By incorporating Bragg’s law (nλ = 2d sinθ) and approximating the strain as a function of sinθ, the relationship is linearized into []:
Here, β (in radians) is the measured FWHM, θ is the Bragg angle, k is a dimensionless shape factor (~0.9 for spherical crystallites), λ is the X-ray wavelength (1.5406 Å for Cu-Kα), D is the volume-weighted crystallite size, and represents the lattice microstrain.
To quantify D and , a W-H plot was constructed by graphing β cosθ (y-axis) against 4sinθ (x-axis). The slope of the linear fit corresponds to , while the y-intercept yields D via D = kλ/(intercept). To ensure the accuracy of the Williamson–Hall (W-H) analysis, the pseudo-Voigt function was employed to extract FWHM values. While the W-H plot (Figure 4) might appear scattered, which is inherent to polycrystalline systems with strain anisotropy, the linear regression yielded a goodness-of-fit value which confirms dominant isotropic contributions. The extracted D and ε values for BFO, BFCO0.07, and BFCO0.15 are compiled in Table 3.
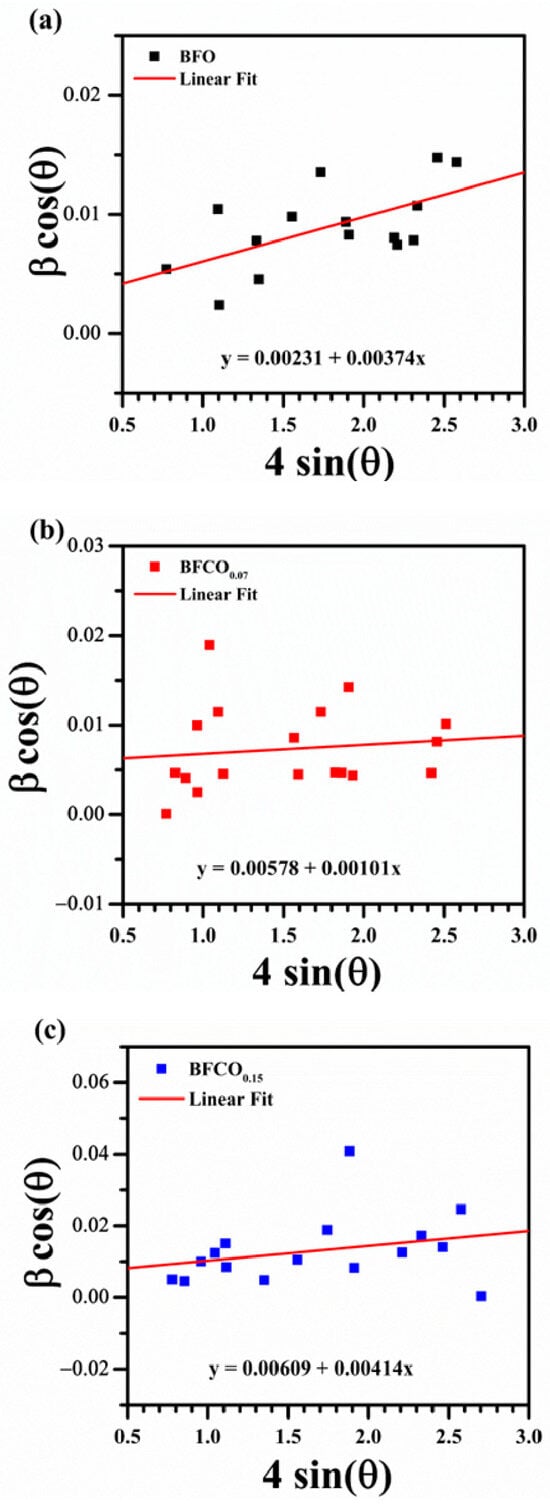
Figure 4.
Williamson–Hall plots for (a) BFO, (b) BFCO0.07, and (c) BFCO0.15.

Table 3.
Comparison of the lattice strain and crystallite size of BFO, BFCO0.07, and BFCO0.15.
The Williamson–Hall (W-H) analysis quantified the crystallite size and microstrain evolution. BFO exhibits moderate tensile strain (3.74 × 10−3), intrinsic to its rhombohedral structure []. BFCO0.07 shows a significantly reduced strain (1.01 × 10−3), attributed to Co3+ induced lattice contraction and probably interfacial strain buffering by the I23 phase []. However, BFCO0.15 exhibits heightened strain (4.14 × 10−3), surpassing even undoped BFO, due to Co2+-mediated lattice expansion, oxygen vacancies, and possibly to the diminished I23 phase contribution [,]. The excellent fit of the Rietveld refinement for BFCO0.07 and BFCO0.15 in addition to the reduced microstrain in BFCO0.07 suggest well-integrated BiFeO3 and sillenite phases, potentially forming coherent interfaces. For BFCO0.15, the higher microstrain and lower sillenite content (23.68%) may indicate less coherence, but the heterostructure persists. A heterointerface is presumed to form between low-index planes based on similar d-spacings consistent with general principles in ceramic heterostructures [,].
The crystal structure of Co-doped BiFeO3/Bi25FeO40 heterostructured nanopowders is influenced by strain from two sources: (1) interfacial lattice mismatch between the perovskite (R3c) and sillenite (I23) phases, and (2) Co doping. The mismatch between the R3c (a ≈ 5.58 Å) and I23 (a ≈ 10.2 Å) phases induces compressive strain in the perovskite and tensile strain in the sillenite at their interface, as evidenced by lattice contraction (Table 1: R3c a-axis from 5.583 Å in BFO to 5.572 Å in BFCO0.15; I23 a-axis from 10.203 Å in BFCO0.07 to 10.194 Å in BFCO0.15). In BFCO0.07, Co3+ substitution (ionic radius 0.545 Å) enhances this compression, reducing microstrain (1.01 × 10−3 vs. 3.74 × 10−3 in BFO) and shortening Fe–O bonds (1.994 Å vs. 2.013 Å in BFO), with the I23 phase (27%) buffering interfacial stress. In contrast, BFCO0.15 shows higher microstrain (4.14 × 10−3) due to lower I23 content (24%) and local tensile strain within the perovskite phase, caused by larger Co2+ ions (0.745 Å) and oxygen vacancies, leading to elongated Fe–O bonds (2.009 Å) and distorted Fe–O–Fe angles (160.23°). These strain effects, supported by similar BiFeO3-based heterostructures [,], enhance bandgap tuning and electrical properties.
These findings underscore the delicate balance required to optimize multifunctional performance in perovskite–sillenite heterostructured nanopowders.
3.2. FTIR Analysis
The Fourier-transform infrared (FTIR) spectra of pristine BFO and Co-doped BiFeO3/Bi25FeO40 heterostructured nanopowders, shown in Figure 5, reveal critical insights into their structural and compositional characteristics. Initial observations of the FTIR spectra reveal distinctive features present for all samples, which are a broad absorption band spanning 450–600 cm−1 that confirms the perovskite framework, with distinct vibrational modes attributed to metal–oxygen bonding []. For undoped BFO, the prominent peak at 474 cm−1 corresponds to Fe-O stretching vibrations in octahedralFeO6, while a secondary peak at 538 cm−1 aligns with symmetric O-Fe-O bending modes [,]. Notably, the presence of a distinct peak at 810 cm−1 in BFO materials reflects their structural crystallinity []. The detection of a consistent peak near 845 cm−1 in all our samples confirms their high crystallinity, which aligns closely with the XRD results. Moreover, the Bi-O stretching vibrations are typically observed near 1000 cm−1 in BFO samples [,]. In our work, Bi-O stretching vibrations are detected at about 1045 cm−1 in the BFO sample, while they were obscured in Co-doped BiFeO3/Bi25FeO40 samples due to Co3+ doping-induced lattice distortions. Residual nitrate ions (NO3−), identified between 1325 and 1390 cm−1 [], and traces of ethylene glycol (C-H bending at 1476 cm−1), as reported in analogous studies [,]. The weak absorption band from 1500 cm−1 to 1646 cm−1 is indicative of the presence of water molecules [,]. In BFCO0.07, Co3+ doping (ionic radius: 0.545 Å) induces lattice contraction (Fe–O: 1.994 Å) and compressive strain (1.01 × 10−3), manifesting as a slight redshift in the O–Fe–O bending mode (from 538 to 535 cm−1) and peak broadening due to interfacial strain buffering in the cubic sillenite phase (I23, 27% phase fraction) [,]. Conversely, BFCO0.15 exhibits a relatively pronounced redshift compared to BFO (from 538 to 530 cm−1) and a diminished Fe–O intensity, aligning with XRD-derived bond elongation (Fe–O: 2.009 Å), tensile strain (4.14 × 10−3), and octahedral distortion (Fe–O–Fe angle: 160.23°) caused by partial Co2+ (0.745 Å) incorporation and oxygen vacancies []. Interfacial strain is evidenced by correlated R3c/I23 lattice contractions (Table 1) and IR spectral shifts. The redshift in O–Fe–O bending modes from 538 to 530 cm−1 reflects compressive strain in the perovskite phase due to lattice mismatch with the cubic sillenite phase. This strain is buffered by the sillenite phase (24% fraction), which reduces interfacial tension, as observed in analogous composites []. Such strain transfer might reflect the dielectric/optical trends of the material which is consistent with prior studies on strained BiFeO3 heterostructures []. The cubic sillenite phase, while prominent in XRD (~24–27%), lacks distinct IR signatures (e.g., 578 cm−1 Fe–O stretch) due to spectral overlap with perovskite modes or weak IR activity, as observed in analogous Bi25FeO40/Bi2Fe4O9 composites [].
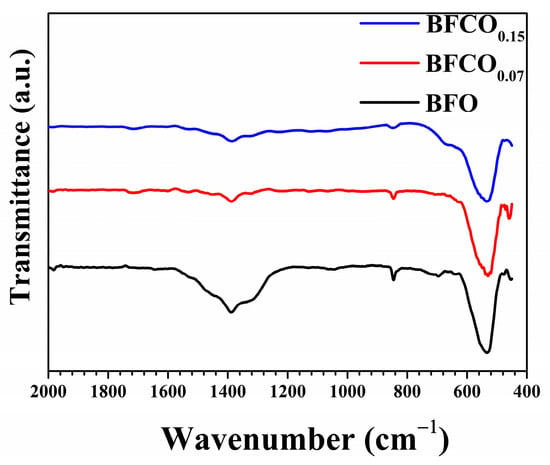
Figure 5.
FTIR spectra of BFO, BFCO0.07, and BFCO0.15 nanopowders.
3.3. Microstructural Analysis
Field emission scanning electron microscopy (FE-SEM) reveals significant morphological and microstructural evolution in BFO, BFCO0.07, and BFCO0.15 nanopowders as a function of cobalt doping, as shown in Figure 6a. BFO consists of homogeneous, spherical particles averaging 75 nm in diameter, as shown in Figure 6a. With cobalt doping, BFCO0.07 and BFCO0.15 exhibit a monotonic reduction in particle size to ~28 nm, as shown in Figure 6b,c, along with increased agglomeration and a narrower size distribution as depicted in the insets in the figures. This size reduction aligns with the XRD-derived crystallite dimensions estimated from the Williamson–Hall analysis (~23–24 nm). The biphasic heterostructures (phase ratios R3c-I23 of 73:27 and 76:24) of BFCO0.07 and BFCO0.15, respectively, promote interphase agglomeration, as coherent R3c-I23 interfaces was found to reduce interfacial strain.
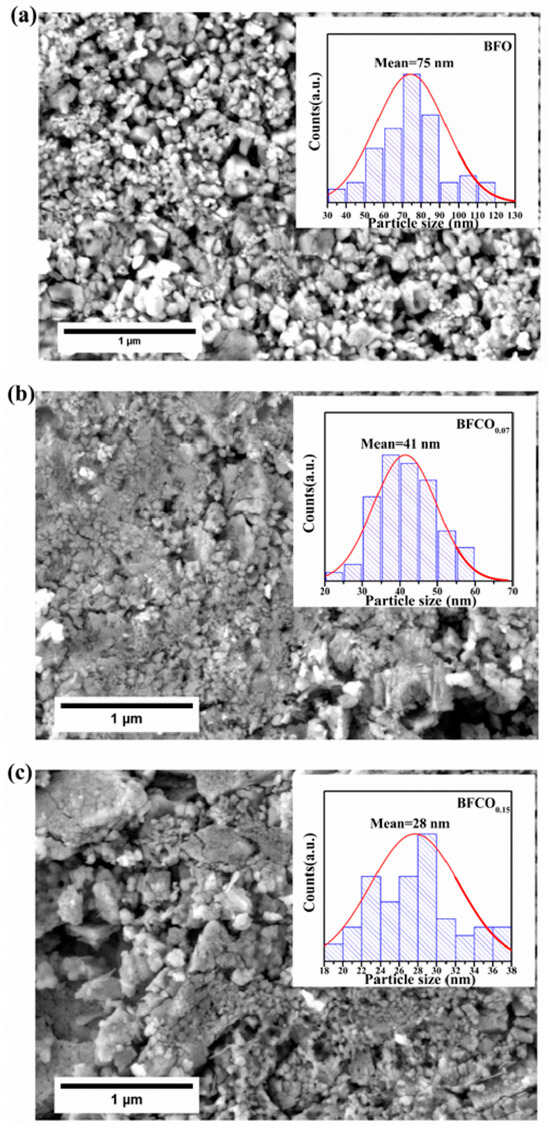
Figure 6.
FESEM micrographs of (a) BFO, (b) BFCO0.07, and (c) BFCO0.15 nanopowders with the corresponding average grain size distribution shown in the inset.
The size reduction follows doping-dependent mechanisms: In BFCO0.07, the substitution of Fe3+ (0.645 Å) by smaller Co3+ (0.545 Å) induces lattice contraction, while the 27% I23 phase buffers interfacial strain, collectively limiting crystallite growth []. In BFCO0.15, a reduced I23 phase content (24%) leads to diminished strain accommodation, allowing tensile strain from partial Co2+ substitution (0.745 Å) and oxygen vacancies to dominate. This strain distorts the lattice, despite the smaller crystallites. Agglomeration intensifies in doped samples due to the increased nanoscale surface energy [].
Energy-dispersive X-ray spectroscopy (EDX), depicted in Figure 7, confirmed the elemental composition and phase purity of the nanoparticles. The spectra show Bi, Fe, Co, and O across all samples, with characteristic peaks at O (Kα, 0.52 keV), Fe (Lα, Kα, Kβ at 0.70, 6.40, and 7.05 keV), Bi (Mα, Lα, Lβ at 2.49, 10.38, and 13.02 keV), and Co (Kα, Lα at 6.93 and 0.77 keV). No extraneous peaks were detected, confirming phase purity.
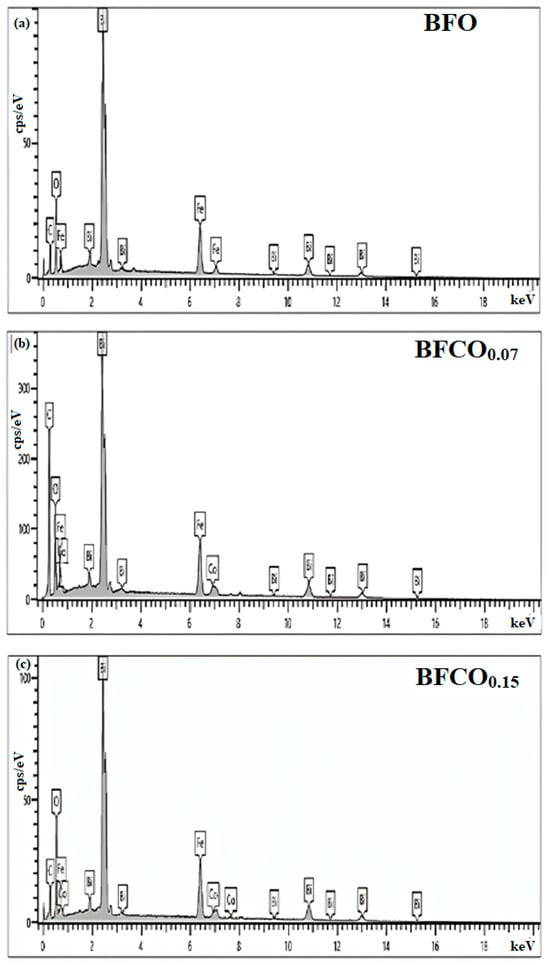
Figure 7.
EDX spectra of BFO, BFCO0.07, and BFCO0.15 nanopowders.
The reduced particle size and increased surface area in BiFe1−xCoxO3/Bi25(Fe,Co)O40 heterostructured nanopowders may enhance light absorption and active sites for redox reactions. Coupled with the R3c-I23 heterojunction, which facilitates charge separation at phase interfaces, this could improve photocatalytic efficiency, as reported for similar biphasic oxides [].
3.4. UV–Visible Analysis
The optical absorption properties of BFO, BFCO0.07, and BFCO0.15 heterostructured nanopowders were examined to investigate the synergistic impact of phase segregation and cobalt doping on bandgap modulation. Figure 8a presents the UV–Vis absorption spectra of BFO, BFCO0.07, and BFCO0.15. An initial examination of Figure 8a clearly indicates that BFCO0.07 and BFCO0.15 exhibit modified optoelectronic responses relative to BFO. These changes correlate with the biphasic perovskite–sillenite structure, lattice strain, and morphology tuned by substitution. For clarity, UV-Vis measurements were performed in direct transmission mode on consolidated pellets (1 mm thickness and 8 mm diameter) to ensure reproducibility. Absorption coefficients (α) were calculated using the Beer–Lambert law (α = 2.303 A/t, where t = 1 mm) [,]. BFO nanoparticles show moderate absorption in the 200–770 nm range with a peak of approximately 80%. This behaviour reflects its rhombohedral perovskite structure and inherent bandgap. In contrast, cobalt-doped samples display a redshift in the absorption edge and enhanced absorption in the visible range. For BFCO0.15, the absorption exceeds 90% at 400–770 nm. This enhancement arises from Co doping and interfacial strain between the perovskite (R3c) and sillenite (I23) phases. Co3+ substitution (ionic radius 0.545 Å) and partial Co2+ incorporation in BFCO0.15 introduce new electronic states, while lattice mismatch (R3c: a ≈ 5.58 Å; I23: a ≈ 10.2 Å) induces compressive strain in the perovskite phase, narrowing the bandgap from 1.78 eV in BFO to 1.31 eV in BFCO0.15 [,]. Critically, XRD refinement (Figure 2) confirms the absence of Bi25CoO40, ensuring the optical response originates from the R3c perovskite and sillenite phases.
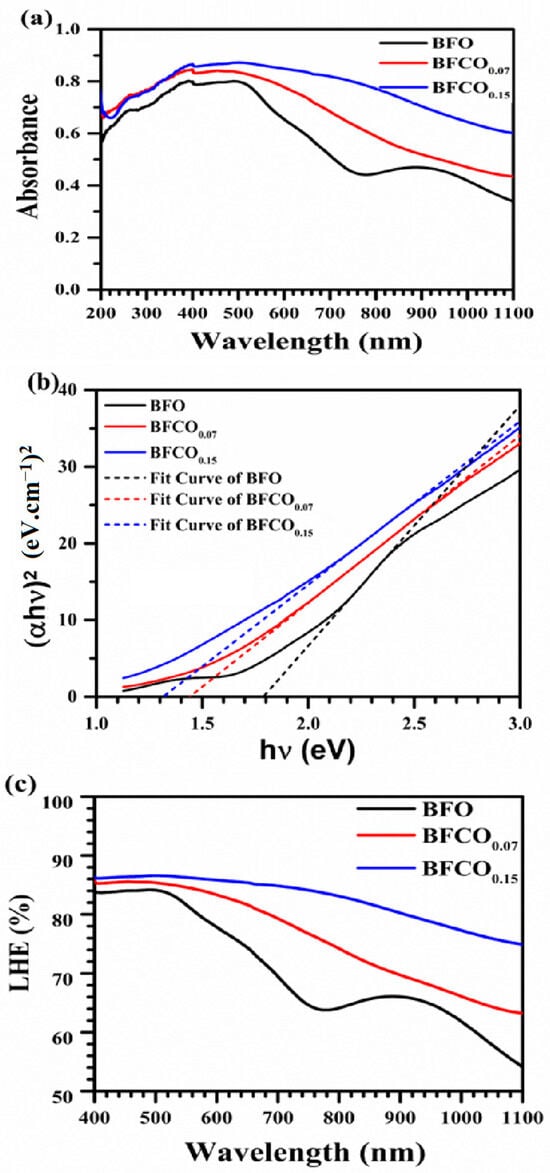
Figure 8.
(a) Absorbance spectra of composition. (b) Tauc’s plots to extract the optical bandgap. (c) Light-harvesting efficiency of BFO, BFCO0.07, and BFCO0.15 nanopowders.
The optical bandgap (Eg) was derived from the Tauc relation for direct transitions:
where is the photon energy [].
Bandgap values were determined exclusively via Tauc plots [(αhν)2 vs. hν], as consolidated pellets were analyzed in absorption mode. This method directly correlates the absorption edge to the dominant R3c phase, even in biphasic systems [].
BiFeO3 shows a direct bandgap of 1.78 eV due to Fe3+-O 3d–p charge transfer in its R3c phase [,]. Cobalt incorporation on the other side shifts the absorption edge toward a lower energy. The bandgap decreases to 1.42 eV for BFCO0.07 and to 1.31 eV for BFCO0.15, as confirmed by the Tauc plots in Figure 8b. This reduction reflects Co3+ 3d6 mid-gap states, tensile strain (evidenced by XRD lattice contraction), and heterojunction-driven charge separation between the R3c and I23 phases.
The reduction in bandgap could be attributed to correlated factors, namely, Co3+ substitution at the Fe3+ site might introduce intermediate 3d6 states below the conduction band []. In addition, tensile lattice strain and oxygen vacancies at higher doping levels might further perturb the Fe/Co–O hybridization []. Furthermore, particle size reduction (from 75 nm in BiFeO3 to 28 nm in BFCO0.15) could increase the surface-to-volume ratio, which might enhance the defect-mediated absorption [].
Taking the above into account, the low band gap energy of BFCO0.15 could be attributed to disorder-induced bandgap narrowing due to the tensile strain []. In addition, oxygen vacancies and Co defect states might introduce mid-gap levels that counteract strain-induced shifts [,]. Notably, the heterointerface between the R3c and sillenite phases enhances light trapping and charge transfer, as evidenced by dielectric enhancement (Figure 6a) and prior studies on similar systems [].
In the same context, Figure 8c reveals that the light-harvesting efficiency can be estimated as []:
where A is the material’s absorbance, which was found to improve accordingly. As show in Figure 8c, BFCO0.15 attains an LHE greater than 90% for wavelengths above 600 nm, compared with 72% for BiFeO3 nanoparticles. This improvement is probably linked to enhanced visible-to-NIR photon capture through interfacial exciton dissociation []. The observed 26% reduction in bandgap and the high LHE in BFCO0.15 is indicative of its promising photocatalytic performance [,].
Bandgap engineering in these systems results from phase segregation, defect chemistry, and morphological evolution. In BFCO0.07, compressive strain and shortened Fe–O bonds promote delocalized charge carriers, while the I23 phase (27% fraction) reduces interfacial recombination []. In BFCO0.15, tensile strain and elongated Fe–O bonds enhance defect-assisted absorption despite increased charge trapping [].
This work establishes Co-doped BiFeO3/Bi25FeO40 heterostructures as tunable optoelectronic platforms. Strain-engineered band alignment, biphasic synergy, and nanoscale agglomeration improve light absorption and charge separation, which are crucial for photocatalysis and photovoltaic applications [].
3.5. Dielectric Analysis
The dielectric properties of Co-doped BiFeO3/Bi25FeO40 heterostructures exhibit frequency-dependent behaviour governed by interfacial polarization, defect dynamics, and structural evolution. Figure 9a illustrates the variation in permittivity as a function of frequency at room temperature for pure BFO and cobalt-doped samples BFCO0.07 and BFCO0.15.
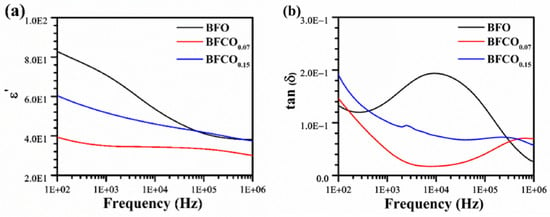
Figure 9.
Variation in dielectric properties of BFO, BFCO0.07, and BFCO0.15 as a function of frequency at room temperature: (a) real dielectric constant and (b) the loss factor tan δ.
It can be seen that, for all samples, the overall dielectric permittivity decreases with increasing frequency, a typical behaviour of ferroelectric materials consistent with Maxwell–Wagner–Sillars (MWS) polarization [,].
At low frequencies, all samples experience a peak in their real permittivity (ε′) values; this observation is attributed to the collective contribution of different polarization mechanisms including space charge, dipolar, and ionic polarization. However, as the frequency increases, the ability of charge carriers to follow the rapid variations in the electric field diminishes, leading to a gradual decrease in permittivity until a plateau is reached at high frequency, where only electronic and dipolar contributions remain.
Pure BFO exhibits the highest permittivity at low frequencies, which arises from oxygen vacancy (VO••) migration. The doping of 7 mol% Co to BiFeO3/Bi25FeO40 leads to a significant reduction in permittivity. This reduction stems from two synergistic factors:
- [Co2+-VO••] defect dipole formation in the perovskite phase, which traps charges, restricts charge carrier mobility, and reduces space charge polarization [,].
- The intrinsic dielectric behaviour of the (I23) sillenite phase (~27% in BFCO0.07, Table 1), which exhibits a markedly lower permittivity (~60–100 at 1 kHz) compared to pristine BFO (~200–300) due to its cubic symmetry and reduced ionic/polarization activity []. The sillenite phase dilutes the overall ε′ and suppresses oxygen vacancy migration, aligning with the diminished low-frequency permittivity.
In contrast, at 15 mol% doping, a partial increase in permittivity is observed, reflecting competing effects:
- Reduced VO•• density due to Co3+ substitution and new defect dipoles from residual Co2+ [].
- Tensile strain from the sillenite phase enhances dipolar polarization in the perovskite lattice [], and the slight increase in perovskite content (73% (BFCO0.07) vs. 76.32% (BFCO0.15)) amplifies its contribution to ε′.
Figure 9b presents the evolution of dielectric losses (tan δ) as a function of frequency for pure BiFeO3 (BFO) and the cobalt-doped samples BFCO0.07 and BFCO0.15. At low frequencies (100 Hz–1 kHz), the dielectric loss peak is due to ionic/space-charge conduction. As the frequency increases, tan δ gradually decreases until it reaches the minimum value. This can be explained by the reduced contribution of interfacial polarization and space charge conduction, as charge carriers can no longer follow the rapidly alternating field. Beyond 100 kHz, tan δ slightly increases again, indicating the activation of electronic mechanisms such as dipolar relaxation and transitions between localized electronic states.
Regarding the effect of cobalt substitution, pure BFO exhibits the highest dielectric losses, especially at low frequencies, due to the high concentration of oxygen vacancies and mobile charge carriers []. With Co doping, dielectric losses are significantly reduced, not only due to decreased charged defects ([Co2+-VO••] trapping) but also because the low-loss sillenite phase (~27–28%) suppresses bulk ionic conduction.
Figure 10 illustrates the evolution of AC conductivity as a function of frequency and the cobalt doping rate, highlighting the modifications in conduction mechanisms induced by these parameters.
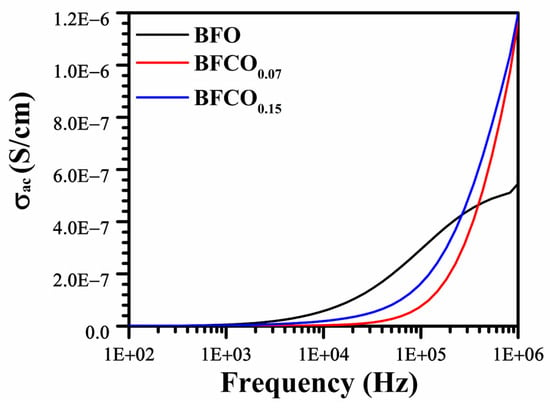
Figure 10.
AC conductivity vs. frequency curves for pure BFO, BFCO0.07, and BFCO0.15 at room temperature.
The AC conductivity (σAC) behaviour follows Jonscher’s power law (σAC∝ωn), where frequency exponent, n, values of 0.42 (BFO), 1.05 (BFCO0.07), and 0.81 (BFCO0.15) reveal distinct conduction mechanisms.
- Perovskite-dominated conduction: Low n = 0.42 in BFO indicates small-polaron hopping (Fe2+/Fe3+ pairs) [].
- Defect dipole and sillenite effects: n = 1.05 in BFCO0.07 reflects suppressed polaron hopping (Co3+-mediated VO•• reduction) and the sillenite phase’s low ionic conductivity, which limits bulk conduction [].
- Mixed mechanisms in BFCO0.15: Intermediate n = 0.81 signifies tensile strain-enhanced delocalization in the perovskite phase and residual Co2+-induced localized hopping [].
These results highlight the interplay between defect chemistry, phase boundaries, and hopping mechanisms as a result of cobalt doping.
3.6. Impedance Analysis
An analysis of Figure 11a,b illustrates the evolution of impedance as a function of frequency for the BFO, BFCO0.07, and BFCO0.15 samples. The impedance analysis reveals a progressive decrease in the real impedance (Z′) with increasing frequency for all compositions. Additionally, the curves for BFCO0.07 and BFCO0.15 exhibit a higher low-frequency Z′ for BFCO0.07 due to [Co2+-VO••] trapping, while BFCO0.15′s lower Z′ reflects Co3+-mediated VO•• reduction [].
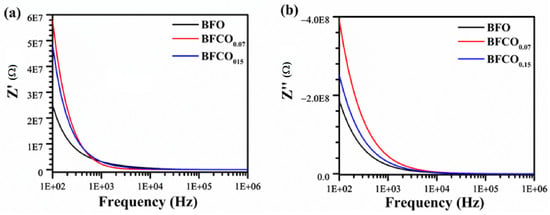
Figure 11.
Frequency dependence of (a) real and (b) imaginary impedance of pure BFO, BFCO0.07, and BFCO0.15 at room temperature.
Additionally, Figure 12 presents the Nyquist plot evolution as a function of the substitution rate.
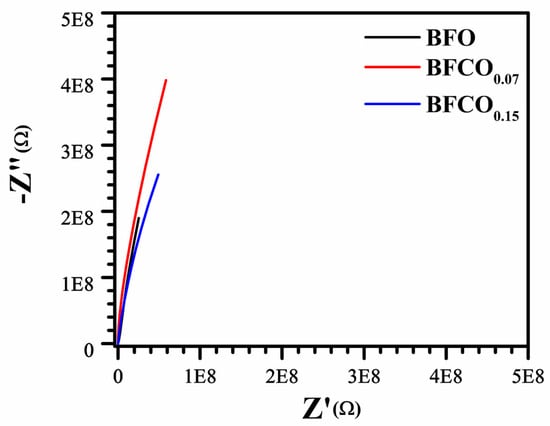
Figure 12.
Nyquist plots of real (Z’) versus imaginary (Z’’) impedance of BFO, BFCO0.07, and BFCO0.15.
The Nyquist plots for BFO, BFCO0.07, and BFCO0.15 reveal a doping-dependent evolution in grain boundary resistance and conduction mechanisms, intricately linked to structural heterogeneity and defect chemistry. For BFCO0.07, the enlarged semicircle radius signifies elevated grain boundary resistance, attributed to the formation of [Co2+-VO••] defect dipoles that localize charges at R3c-I23 heterointerfaces [,]. This trapping effect is amplified by Co3+ doping-induced lattice contraction and agglomerated nanoparticles []. In contrast, BFCO0.15 exhibits a diminished semicircle radius despite higher Co doping, reflecting reduced grain boundary resistance due to the Co3+-mediated suppression of oxygen vacancies [] and tensile strain from elongated Fe–O bonds and distorted Fe–O–Fe angles, which promote delocalized hopping []. The depressed, non-ideal semicircles across all samples arise from distributed relaxation times, a hallmark of structural disorder in biphasic R3c-I23 systems [] and mixed Co2+/Co3+ valence states []. The convergence of the impedance curves at high frequencies depicted in Figure 11 underscores intrinsic lattice conduction, minimally perturbed by doping, while the low-frequency intercepts correlate with total resistance trends. These findings collectively demonstrate that cobalt doping in BFO heterostructures modulates interfacial polarization and defect dynamics, enabling tailored control over grain boundary-dominated conduction pathways.
4. Conclusions
This study demonstrates the transformative potential of Co-doped BiFeO3/Bi25FeO40 heterostructured nanopowders (x = 0.07, 0.15), synthesized via sol–gel processing, in advancing multiferroic nanomaterials. Co doping and biphasic engineering (R3c perovskite and I23 sillenite phases) synergistically reduce the bandgap by 26%, from 1.78 eV in pristine BiFeO3 to 1.31 eV at x = 0.15, while enhancing visible light absorption. Structural analyses reveal that Co2+/Co3+ doping and oxygen vacancies drive lattice strain and defect-mediated charge transport, as evidenced by frequency-dependent dielectric suppression. These tailored heterojunctions, with optimized interfacial strain, significantly boost optoelectronic performance, unlocking new possibilities for efficient photocatalysis and photovoltaics. Our findings highlight the critical interplay of doping, phase segregation, and nanoscale morphology, offering a robust framework for designing advanced multiferroic nanocomposites. Future work could explore higher doping levels or alternative transition metals to further tune nanocomposites’ functionality for energy and environmental applications.
Author Contributions
Conceptualization, N.A.; methodology, D.B., A.D., N.A. and Z.B.; software, D.B. and A.D.; validation, D.B., A.D., N.A., M.T. and Z.B.; formal analysis, D.B. and A.D.; investigation, D.B., A.D., N.A., M.T. and Z.B.; resources, N.A. and M.T.; data curation, D.B. and A.D.; writing—original draft preparation, D.B. and A.D.; writing—review and editing, D.B., A.D., N.A., M.T. and Z.B.; visualization, D.B., A.D., N.A., M.T. and Z.B.; supervision, N.A. and Z.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Zohra Benzarti gratefully acknowledges support from project no. 7225-ILLIANCE High Performing Energy, Project supported by the RRP-Recovery and Resilience Plan and the European NextGeneration EU Funds, following the AVISON.°02/C05-i01/2022, Component 5, Capitalization and Business Innovation, Mobilizing Agendas for Business Innovation. She also acknowledges support from national funds through FCT-Foundation for Science and Technology, under project UID/00285, Centre for Mechanical Engineering, Materials and Processes, and LA/P/0112/2020.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ramesh, R.; Spaldin, N.A. Multiferroics: Progress and Prospects in Thin Films. Nat. Mater. 2007, 6, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Catalan, G.; Scott, J.F. Physics and Applications of Bismuth Ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Dutta, D.P.; Mandal, B.P.; Naik, R.; Lawes, G.; Tyagi, A.K. Magnetic, Ferroelectric, and Magnetocapacitive Properties of Sonochemically Synthesized Sc-Doped BiFeO3 Nanoparticles. J. Phys. Chem. C 2013, 117, 2382–2389. [Google Scholar] [CrossRef]
- Wani, W.A.; Kundu, S.; Ramaswamy, K.; Venkataraman, H. Structural, Morphological, Optical and Dielectric Investigations in Cobalt Doped Bismuth Ferrite Nanoceramics Prepared Using the Sol-Gel Citrate Precursor Method. J. Alloys Compd. 2020, 846, 156334. [Google Scholar] [CrossRef]
- Rhaman, M.M.; Matin, M.A.; Hossain, M.N.; Mozahid, F.A.; Hakim, M.A.; Islam, M.F. Bandgap Engineering of Cobalt-Doped Bismuth Ferrite Nanoparticles for Photovoltaic Applications. Bull. Mater. Sci. 2019, 42, 190. [Google Scholar] [CrossRef]
- Khajonrit, J.; Wongpratat, U.; Kidkhunthod, P.; Pinitsoontorn, S.; Maensiri, S. Effects of Co Doping on Magnetic and Electrochemical Properties of BiFeO3 Nanoparticles. J. Magn. Magn. Mater. 2018, 449, 423–434. [Google Scholar] [CrossRef]
- Xiao, X.H.; Zhu, J.; Li, Y.R.; Luo, W.B.; Yu, B.F.; Fan, L.X.; Ren, F.; Liu, C.; Jiang, C.Z. Greatly Reduced Leakage Current in BiFeO3 Thin Film by Oxygen Ion Implantation. J. Phys. D Appl. Phys. 2007, 40, 5775. [Google Scholar] [CrossRef]
- Yang, C.; Lv, P.; Qian, J.; Han, Y.; Ouyang, J.; Lin, X.; Huang, S.; Cheng, Z. Fatigue-Free and Bending-Endurable Flexible Mn-Doped Na0.5Bi0.5TiO3-BaTiO3-BiFeO3 Film Capacitor with an Ultrahigh Energy Storage Performance. Adv. Energy Mater. 2019, 9, 1803949. [Google Scholar] [CrossRef]
- Rashad, M.M. Effect of Synthesis Conditions on the Preparation of BiFeO3 Nanopowders Using Two Different Methods. J. Mater. Sci. Mater. Electron. 2012, 23, 882–888. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Wang, Q.; Li, J.; Yan, L. Phase Transformation and Heterojunction Nanostructures of Bismuth Iron Oxide. J. Mater. Sci. Mater. Electron. 2023, 34, 2236. [Google Scholar] [CrossRef]
- Hu, B.; Wang, J.-F.; Zhang, J.; Gu, Z.-B.; Zhang, S.-T. Synthesis, Structures and Properties of Single Phase BiFeO3 and Bi2Fe4O9 Powders by Hydrothermal Method. J. Mater. Sci. Mater. Electron. 2015, 26, 6887–6891. [Google Scholar] [CrossRef]
- Wang, X.; Mao, W.; Wang, Q.; Zhu, Y.; Min, Y.; Zhang, J.; Yang, T.; Yang, J.; Li, X.; Huang, W. Low-Temperature Fabrication of Bi25FeO40/rGO Nanocomposites with Efficient Photocatalytic Performance under Visible Light Irradiation. RSC Adv. 2017, 7, 10064–10069. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Yan, L.; Li, J. Synthesis of BiFeO3/Bi25FeO40 Heterojunction Structure and Precise Adjustment of Forbidden Band Width. Mater. Chem. Phys. 2023, 305, 127935. [Google Scholar] [CrossRef]
- Zhu, J.C.; Hu, X.; Mao, X.Y.; Wang, W.; Chen, X.B. Properties of Co Substituted Bismuth Ferrite System by Rapid Liquid Phase Sintering. Mater. Sci. Forum 2010, 636, 356–360. [Google Scholar] [CrossRef]
- Pan, C.; Wang, Z.; Lou, Y.; Zhang, Y.; Dong, Y.; Zhu, Y. The Construction of a Wide-Spectrum-Responsive and High-Activity Photocatalyst, Bi25CoO40, via the Creation of Large External Dipoles. J. Mater. Chem. A 2021, 9, 3616–3627. [Google Scholar] [CrossRef]
- Zhu, C.; Niu, X.; Fu, Y.; Li, N.; Hu, C.; Chen, Y.; He, X.; Na, G.; Liu, P.; Zai, H.; et al. Strain Engineering in Perovskite Solar Cells and Its Impacts on Carrier Dynamics. Nat. Commun. 2019, 10, 815. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.A.; Abrishami, M.E.; Yousefi, R. X-Ray Analysis of ZnO Nanoparticles by Williamson–Hall and Size–Strain Plot Methods. Solid State Sci. 2011, 13, 251–256. [Google Scholar] [CrossRef]
- Liu, T.; Yang, C.; Si, J.; Sun, W.; Su, D.; Li, C.; Yuan, X.; Huang, S.; Cheng, X.; Cheng, Z. Self-Poled Bismuth Ferrite Thin Film Micromachined for Piezoelectric Ultrasound Transducers. Adv. Mater. 2025, 37, 2414711. [Google Scholar] [CrossRef]
- Bai, L.; Sun, M.; Ma, W.; Yang, J.; Zhang, J.; Liu, Y. Enhanced Magnetic Properties of Co-Doped BiFeO3 Thin Films via Structural Progression. Nanomaterials 2020, 10, 1798. [Google Scholar] [CrossRef]
- Baiyee, Z.M.; Chen, C.; Ciucci, F. A DFT+U Study of A-Site and B-Site Substitution in BaFeO3−δ. Phys. Chem. Chem. Phys. 2015, 17, 23511–23520. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-J.; Bi, X.-Y.; Xia, P.; Sun, F.; Chen, Z.-X.; Zhang, X.-Y.; Zhang, T. A Simple One-Pot Method for the Synthesis of BiFeO3/Bi25FeO40 Heterojunction for High-Performance Photocatalytic Degradation Applications. Int. J. Mol. Sci. 2025, 26, 196. [Google Scholar] [CrossRef]
- Yao, R.; Cao, C.; Zheng, C.; Lei, Q. Lattice Mismatch Induced Strained Phase for Magnetization, Exchange Bias and Polarization in Multiferroic BiFeO3. RSC Adv. 2013, 3, 24231–24236. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, J.; Yu, S.; Che, L.; Meng, Z. Hydrothermal Synthesis of Perovskite Bismuth Ferrite Crystallites. J. Cryst. Growth 2006, 291, 135–139. [Google Scholar] [CrossRef]
- Hemanta Singh, H.; Basantakumar Sharma, H. Investigation on Electrical, Magnetic and Magneto-Dielectric Properties of Yttrium and Cobalt Co-Doped Bismuth Ferrite Nanoparticles. Indian J. Phys. 2020, 94, 1561–1572. [Google Scholar] [CrossRef]
- Verma, R.; Chauhan, A.; Batoo, K.M.; Kumar, R.; Hadhi, M.; Raslan, E.H. Effect of Calcination Temperature on Structural and Morphological Properties of Bismuth Ferrite Nanoparticles. Ceram. Int. 2021, 47, 3680–3691. [Google Scholar] [CrossRef]
- Dhanya, S.R.; Nair, S.G.; Satapathy, J.; Kumar, N.P. Structural and Spectroscopic Characterization of Bismuth-Ferrites. AIP Conf. Proc. 2019, 2166, 020017. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Sarkar, I.; Shirolkar, M.M.; Jeng, U.-S.; Yeh, Y.-Q.; Rajendra, R.; Ballav, N.; Kulkarni, S. Probing Bismuth Ferrite Nanoparticles by Hard X-Ray Photoemission: Anomalous Occurrence of Metallic Bismuth. Appl. Phys. Lett. 2014, 105, 102910. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Wu, Y.; Hu, J. Hydrothermal Synthesis and Mechanism and Property Study of La-Doped BiFeO3 Crystallites. J. Mater. Sci. Mater. Electron. 2012, 23, 1402–1408. [Google Scholar] [CrossRef]
- Lotey, G.S.; Verma, N.K. Multiferroic Properties of Tb-Doped BiFeO3 Nanowires. J. Nanopart. Res. 2013, 15, 1553. [Google Scholar] [CrossRef]
- Safari-Naderi, M.-H.; Shakouri, M.; Ghasemi-Ghalebahman, A. A Bond-Based Peridynamics Model Based on Variable Material Properties for Modeling Elastoplastic Behavior. Mater. Today Commun. 2023, 35, 105890. [Google Scholar] [CrossRef]
- Sando, D.; Agbelele, A.; Daumont, C.; Rahmedov, D.; Ren, W.; Infante, I.C.; Lisenkov, S.; Prosandeev, S.; Fusil, S.; Jacquet, E.; et al. Control of Ferroelectricity and Magnetism in Multi-Ferroic BiFeO3 by Epitaxial Strain. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2014, 372, 20120438. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Cheng, D.; He, T.; Hu, Y.; Deng, Q.; Mao, Y.; Wang, S. Enhanced Visible-Light Responsive Photocatalytic Activity of Bi25FeO40/Bi2Fe4O9 Composites and Mechanism Investigation. J. Mater. Sci. Mater. Electron. 2019, 30, 10923–10933. [Google Scholar] [CrossRef]
- Gupta, S.; Tomar, M.; James, A.R.; Gupta, V. Ce-Doped Bismuth Ferrite Thin Films with Improved Electrical and Functional Properties. J. Mater. Sci. 2014, 49, 5355–5364. [Google Scholar] [CrossRef]
- Sinha, A.K.; Bhushan, B.; Gupta, N.; Sen, S.; Prajapat, C.L.; Nuwad, J.; Bhatt, P.; Mishra, S.K.; Meena, S.S.; Priyam, A. Effect of Cobalt-Doping on Dielectric, Magnetic and Optical Properties of BiFeO3 Nanocrystals Synthesized by Sol–Gel Technique. Solid State Sci. 2020, 102, 106168. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phy. Status Solidi b 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Sahoo, P.; Dixit, A. Interband Electronic Transitions and Optical Phonon Modes in Size-Dependent Multiferroic BiFeO3 Nanoparticles. Phys. Chem. Chem. Phys. 2024, 26, 9675–9686. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, H.; King, S.W.; Afanas’ev, V.V.; Baklanov, M.R.; de Marneffe, J.-F.; Nishi, Y.; Shohet, J.L. Publisher’s Note: “Defect-Induced Bandgap Narrowing in Low-k Dielectrics” [Appl. Phys. Lett. 107, 082903 (2015)]. Appl. Phys. Lett. 2015, 107, 139903. [Google Scholar] [CrossRef]
- Yang, W.; Yu, Y.; Starr, M.B.; Yin, X.; Li, Z.; Kvit, A.; Wang, S.; Zhao, P.; Wang, X. Ferroelectric Polarization-Enhanced Photoelectrochemical Water Splitting in TiO2–BaTiO3 Core–Shell Nanowire Photoanodes. Nano Lett. 2015, 15, 7574–7580. [Google Scholar] [CrossRef]
- Liu, S.; Luo, Z.; Li, L.; Li, H.; Chen, M.; Wang, T.; Gong, J. Multifunctional TiO2 Overlayer for P-Si/n-CdS Heterojunction Photocathode with Improved Efficiency and Stability. Nano Energy 2018, 53, 125–129. [Google Scholar] [CrossRef]
- Cody, G.D.; Tiedje, T.; Abeles, B.; Brooks, B.; Goldstein, Y. Disorder and the Optical-Absorption Edge of Hydrogenated Amorphous Silicon. Phys. Rev. Lett. 1981, 47, 1480–1483. [Google Scholar] [CrossRef]
- Kharandiuk, T.; Hussien, E.J.; Cameron, J.; Petrina, R.; Findlay, N.J.; Naumov, R.; Klooster, W.T.; Coles, S.J.; Ai, Q.; Goodlett, S.; et al. Noncovalent Close Contacts in Fluorinated Thiophene–Phenylene–Thiophene Conjugated Units: Understanding the Nature and Dominance of O···H versus S···F and O···F Interactions with Respect to the Control of Polymer Conformation. Chem. Mater. 2019, 31, 7070–7079. [Google Scholar] [CrossRef]
- Sharmila, M.; Abdul Kader, S.M.; Jain Ruth, D.E.; Veera Gajendra Babu, M.; Bagyalakshmi, B.; Ananth Kumar, R.T.; Pathinettam Padiyan, D.; Sundarakannan, B. Effect of Cobalt Substitution on the Optical Properties of Bismuth Ferrite Thin Films. Mater. Sci. Semicond. Process. 2015, 34, 109–113. [Google Scholar] [CrossRef]
- Li, Y.; Adamo, C.; Rowland, C.E.; Schaller, R.D.; Schlom, D.G.; Walko, D.A. Nanoscale Excitonic Photovoltaic Mechanism in Ferroelectric BiFeO3 Thin Films. APL Mater. 2018, 6, 084905. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jang, J.-W.; Youn, D.H.; Magesh, G.; Lee, J.S. A Stable and Efficient Hematite Photoanode in a Neutral Electrolyte for Solar Water Splitting: Towards Stability Engineering. Adv. Energy Mater. 2014, 4, 1400476. [Google Scholar] [CrossRef]
- Damodaran, A.R.; Liang, C.-W.; He, Q.; Peng, C.-Y.; Chang, L.; Chu, Y.-H.; Martin, L.W. Nanoscale Structure and Mechanism for Enhanced Electromechanical Response of Highly-Strained BiFeO3 Thin Films. arXiv 2011, arXiv:1105.4001. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yang, H.G.; Cheng, H.-M.; Lu, G.Q. (Max) Titania-Based Photocatalysts—Crystal Growth, Doping and Heterostructuring. J. Mater. Chem. 2010, 20, 831–843. [Google Scholar] [CrossRef]
- Pradhan, A.; Zhang, K.; Hunter, D.; Dadson, J.B.; Loiutts, G.B.; Bhattacharya, P.; Katiyar, R.; Zhang, J.; Sellmyer, D.; Roy, U.N.; et al. Magnetic and Electrical Properties of Single-Phase Multiferroic BiFeO3. J. Appl. Phys. 2005, 97, 093903. [Google Scholar] [CrossRef]
- Kusunose, T.; Sekino, T.; Niihara, K. Contact Damage of Silicon Carbide/Boron Nitride Nanocomposites. J. Am. Ceram. Soc. 2007, 90, 3341–3344. [Google Scholar] [CrossRef]
- Chandel, S.; Thakur, P.; Tomar, M.; Gupta, V.; Thakur, A. Investigation of Structural, Optical, Dielectric and Magnetic Studies of Mn Substituted BiFeO3 Multiferroics. Ceram. Int. 2017, 43, 13750–13758. [Google Scholar] [CrossRef]
- Gul, I.H.; Maqsood, A.; Naeem, M.; Ashiq, M.N. Optical, Magnetic and Electrical Investigation of Cobalt Ferrite Nanoparticles Synthesized by Co-Precipitation Route. J. Alloys Compd. 2010, 507, 201–206. [Google Scholar] [CrossRef]
- Leng, S.; Cheng, H.; Zhang, R.; Gao, C.; Li, Z. Electrical Properties of La-Mn-Codoped BaTiO3-(Bi0.5Na0.5)TiO3 Lead-Free PTCR Ceramics. Ceram. Int. 2021, 47, 30963–30968. [Google Scholar] [CrossRef]
- Sharma, A.D.; Hemanta, H.; Sharma, H.B. Effect of Mn Substitution on Structural and Dielectric Properties of Bismuth Ferrite. Ferroelectrics 2017, 519, 187–193. [Google Scholar] [CrossRef]
- Dhanalakshmi, B.; Pratap, K.; Rao, B.P.; Rao, P.S.V.S. Effects of Mn Doping on Structural, Dielectric and Multiferroic Properties of BiFeO3 Nanoceramics. J. Alloys Compd. 2016, 676, 193–201. [Google Scholar] [CrossRef]
- Jonscher, A.K. The ‘Universal’ Dielectric Response. Nature 1977, 267, 673–679. [Google Scholar] [CrossRef]
- Zhang, Z.; Hao, H.; Yang, H.; Hu, Y.; Min, J.; Zhang, G.; Bi, J.; Yan, S.; Li, H.; Hou, H. Direct Z-Scheme Ba0.8Sr0.2TiO3/Ag/Ag2O Heterostructural Nanotube with Pyroelectric and Photocatalytic Synergy for Enhanced Catalytic Performance. Mater. Sci. Eng. B 2022, 279, 115678. [Google Scholar] [CrossRef]
- Mazumdar, S.C.; Khan, M.N.I.; Islam, M.d.F.; Hossain, A.K.M.A. Tuning of Magnetoelectric Coupling in (1−y)Bi0.8Dy0.2FeO3–yNi0.5Zn0.5Fe2O4 Multiferroic Composites. J. Magn. Magn. Mater. 2016, 401, 443–454. [Google Scholar] [CrossRef]
- Rhaman, M.M.; Matin, M.A.; Hakim, M.A.; Islam, M.F. Dielectric, Ferroelectric and Ferromagnetic Properties of Samarium Doped Multiferroic Bismuth Ferrite. Mater. Res. Express 2019, 6, 125080. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).